Introduction
In 1981, Ikeda and coworkers published the first paper on the Wistar fatty diabetic rat. Because this was the first report of an adult-onset non-insulin-dependent diabetes mellitus (NIDDM) rat model with sustained hyperglycemia, it was of great interest to the Diabetes Research Training Center (DRTC) Animal Core in Indianapolis. Interest was stimulated by the fact that diabetes had been regularly observed in the DRTC (Drt) and Lilly (L) colonies of Zucker rats (Clark and Palmer, 1981, 1982; Clark et al., 1983), the stock in which the mutation fa (fatty) first arose.
In the initial report, Ikeda et al. (1981) used the nomenclature WKY to refer to a stock of rats into which the fa gene was backcrossed. Although this stock was not the same as the WKY/N rat, it was interpreted by our group as being identical or closely related to it, which led to a project at the DRTC Animal Core of backcrossing the fa gene from the Zucker rats into the WKY/N background to make the congenic strain WKY/NDrt-fa (N10). Some of the WKY/NDrt-fa rats used in this study were backcrossed for 8 generations (N8); others were backcrossed for 10 generations (N10).
In this short paper, we will present some of the comparison data we have obtained during development and testing of this model. After the model was backcrossed 10 generations, the colony was made pathogen free by delivering litters by cesarian section and placing them with pathogen-free foster mothers. Rats in this colony were subsequently kept in filter-top cages that were changed in a laminar flow hood. Male WKY/NDrt-fa/fa rats that were tested before derivation remained normoglycemic; those that were followed after derivation by cesarean delivery became hyperglycemic. This was an unexpected result which emphasizes the need to do comparison studies between different strains under similar conditions, including the infectious agents that the rats carry. Studies done on the same strain in different locations and under varying conditions may not give the same results.
Materials and Methods
Since 1978, we have maintained a Zucker rat colony that was originally obtained from the breeding stock of Walter Shaw at Lilly Research Laboratories, Indianapolis. In 1982, breeding stock from this colony was crossed with WKY/NHsd obtained from Harlan Industries. Subsequently, we backcrossed offspring through successive generations of WKY/N rats from either Harlan Industries or Laboratory Supply Company, selecting animals carrying the fa gene. The data presented in this paper are from male pre-cesarean-derived WKY/N-fa rats (N8 or 99.6 percent WKY/N, and N10 or 99.9 percent WKY/N) and from male post-cesarean-derived WKY/N-fa (N10) rats from this colony.
Male WKY/NDrt-fa/fa fatty rats were followed up to 20 weeks of age. They were kept in plastic cages with aspen chips and given food (Purina 5008) and water ad libitum. Blood was obtained by tail vein collections that took place at the same time early each morning. Blood glucose levels were measured using a Beckman Glucose II Analyzer. Weights were recorded just prior to blood collection. Data were analyzed using Student’s t-test.
Results
Figure 1 shows comparative weight gains between WKY/NDrt-fa male rats that were followed before and after cesarean derivation. There is a significant difference in weight between these two groups at each time period, with the post-cesarean-derived rats gaining more weight than the pre-cesarean-derived group.
Figure 1.
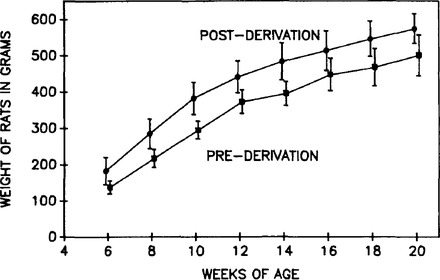
Comparative weight gains between pre-cesareanderived and post-cesarean-derived WKY/NDrt-fa/fa rats. The precesarean derivation data are from a combined group of N8 and N10 backcrossed rats, and the post-cesarean derivation data are from N10 rats. There are from 12 to 16 rats represented at each data point in the pre-cesarean derivation data and 5 to 12 in the post-cesarean derivation curve. There is an obvious delay in weight gain for the pre-cesarean-derived group of rats, with a significant difference between the two groups of rats at each time point. Data are presented as mean ± SD.
Figure 2 shows the difference in blood glucose levels over time comparing rats pre- and post-cesarean derivation. Some pre-cesarean-derived rats were backcrossed 8 times (N8) and some were backcrossed 10 times (N10). All of the rats that were followed before cesarean derivation failed to show any significant rise in blood glucose over time, whereas the rats that were cesarean derived showed a significant increase in blood glucose levels starting at about 7 weeks of age.
Figure 2.
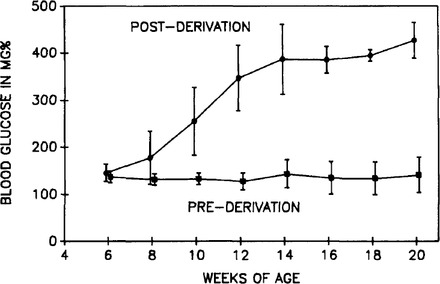
Blood glucose levels over time for pre- and postcesarean-derived WKY/NDrt-fa/fa rats fed ad libitum. These data are from the same groups of rats represented in Figure 1. The precesarean-derived rats do not show any rise in blood glucose, whereas the post-cesarean-derived rats show a very significant rise in blood glucose level over time. Data are presented as mean ± SD.
The infectious agents consistently carried by the rats before cesarean derivation were Mycoplasma (M. pulmonis) and pinworms. The colony also was exposed to rat coronavirus, sialodacryoadenitis virus, and Sendai virus.
After cesarean derivation, all rats were maintained in filter-top cages, and all cage changes were made in a laminar flow hood. Under these conditions, the rats did not demonstrate the presence of any pathogen, as measured by viral antibody testing and other observations.
Discussion
When fed ad libitum, the Wistar fatty male rat has been shown to become hyperglycemic in several studies from H. Ikeda’s laboratory (Ikeda et al., 1981; Matsuo et al., 1984) and from our laboratory (Berti-Mattera et al., 1989, Peterson et al., 1988a,b). This rat model was originally developed by Ikeda (Ikeda et al., 1981) from a partially inbred stock of Wistar Kyoto rats that were being developed and maintained at Takeda Chemical Industries, Ltd. and that were referred to as WKY. We originally began backcrossing the fa gene into the WKY/N strain in an attempt to duplicate this model, not realizing that the Takeda WKY rat was different from the WKY/N strain.
Our initial studies on the pre-cesarean-derived WKY/NDrt-fa/fa rats (N8 and N10) showed that they did not become diabetic when fed a 6-percent-fat diet (Purina 5008). More recent data from these rats, subsequent to cesarean derivation of the colony, have shown that this rat does develop hyperglycemia when fed the same diet. Although we have not attempted to reintroduce disease agents into this strain to see if we can suppress the development of hyperglycemia, we feel that the difference in the blood glucose level is probably a reflection of the microbial status of this animal model. Alternatively, some other more subtle conditions that we have not defined may be involved.
One of the original reasons we became involved in backcrossing the fa gene into WKY/N was our observation of diabetes in our colony of Zucker rats (Clark and Palmer, 1981, 1982; Clark et al., 1983). The data from these animals were inconsistent, and thus, we felt that putting the fa gene into a defined strain that was susceptible to diabetes would be a worthwhile project. About the time we completed the backcrossing of the fa gene into the WKY/N strain, we also started inbreeding the Zucker colony, selecting for the diabetic trait. These recent studies using strict inbreeding practices have shown that diabetes is now consistent in this model (Peterson et al., 1990). These recent studies have also been done on disease-free animals, which may explain in part the consistency of these data.
A second mutation called cp (corpulent), is also an obesityproducing gene in the rat. This finding was originally described by Koletsky (1973, 1975). Subsequent studies by Yen et al. (1977) showed that crosses between heterozygous corpulent (+/cp) and heterozygous fatty (+/fa) rats produced obese offspring with the genotype fa/cp. This information supports the hypothesis that the two mutations are on the same genetic locus. More recent studies of defined congenic rat strains backcrossed with the cp gene show that some of them also become diabetic under certain conditions (Michaelis et al., 1984, 1986a,b).
The similarity of the cp and fa genes is still somewhat in question. Studies should now be done to compare the physiological effects of the two genes in the same and different genetic backgrounds. To do these kinds of comparison studies, the conditions under which the rats are kept would have to be identical. The cp and fa genes are both in the WKY/N rat, making this strain a good candidate for such a study.
The WKY/NDrt-fa rat appears to be a good model for obesity and diabetes. In general it appears to be similar to the WDF/Ta-fa (Wistar fatty) rat that was developed by Ikeda (Ikeda et al., 1981). It also seems to have similar features to the WKY/N-cp model (O. E. Michaelis IV, Beltsville Human Nutrition Research Center, U.S. Department of Agriculture, personal communication, 1989). Indeed, this would be expected if the two genes are in the same locus causing the same disfunction. The WKY/NDrt-fa colony has now been contributed to Carl Hansen at the National Institutes of Health so that the model can be maintained and compared to the corpulent model in the same background strain.
References
- Berti-Mattera L. N., Lowery J., Peterson R. G., Eichberg J. 1989. Alteration of phosphoinositide metabolism, protein phosphorylation and carbohydrate levels in sciatic nerve from Wistar fatty diabetic rats. Diabetes 38:373–378. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Palmer C. J. 1981. The diabetic Zucker rat—a new model for non-insulin dependent diabetes. Diabetes 30:126A. [Google Scholar]
- Clark J. B., Palmer C. J. 1982. The spontaneously diabetic Zucker fatty rat. Fed. Proc. 41:857A. [Google Scholar]
- Clark J. B., Palmer C. J., Shaw W. N. 1983. The diabetic Zucker fatty rat. Proc. Soc. Exp. Biol. Med. 173:68–75. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Shino A., Matsuo T. 1981. A new genetically obesehyperglycemic rat (Wistar fatty). Diabetes 30:1045–1050. [DOI] [PubMed] [Google Scholar]
- Koletsky S. 1973. Obese spontaneously hypertensive rats—a model for study of atherosclerosis. Exp. Mol. Pathol. 19:53–60. [DOI] [PubMed] [Google Scholar]
- Koletsky S. 1975. Pathologic findings and laboratory data in a new strain of obese hypertensive rats. Am. J. Pathol. 80:129–142. [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Ikeda H., Iwatsuka H., Suzuoki Z. 1984. Role of sucrose diet in the development of hyperglycemia in female Wistar fatty rats. Pp. 261–263 in Lessons from Animal Diabetes, Shafrir E., Renold A. E., eds. London: John Libbey. [Google Scholar]
- Michaelis O. E., IV, Ellwood K. C., Judge J. M., Schoene N. W., Hansen C. T. 1984. Effect of dietary sucrose on the SHR/N-corpulent rat: A new model for insulin-independent diabetes. Am. J. Clin. Nutr. 39:612–618. [DOI] [PubMed] [Google Scholar]
- Michaelis O. E., IV, Ellwood K. C., Tulp O. L., Greenwood M. R. C. 1986. Effect of feeding sucrose or starch diets on parameters of glucose tolerance in the LA/N-corpulent rat. Nutr. Res. 6:95–99. [Google Scholar]
- Michaelis O. E., IV, Patrick D. H., Hansen C. T., Canary J. J., Werner R. M., Carswell N. 1986. Animal model of human disease, insulin-independent diabetes mellitus (type II). Am. J. Path. 123:398–400. [PMC free article] [PubMed] [Google Scholar]
- Peterson R. G., Neel M.-A., Little L. A. 1988. Comparison of metabolic data from the WKY/N and Wistar fatty (WDF) rat. Pp. 113–119 in New Models of Genetically Obese Rats for Studies in Diabetes, Heart Disease, and Complications of Obesity, Hansen C. T., Michaelis O. E., IV, eds. Bethesda, Md.: National Institutes of Health. [Google Scholar]
- Peterson R. G., Sharma A. K., Little L. A., Neel M., Potter C. G., Eichberg J. 1988. Peripheral nerve abnormalities in Wistar fatty diabetic rats, Pp. 488–491 in Frontiers in Diabetes Research. Lessons from Animal Diabetes II, Shafrir E., Renold A. E., eds. London: John Libbey. [Google Scholar]
- Peterson R. G., Shaw W. N., Neel M.-A., Little L. A., Eichberg J. 1990. Zucker diabetic fatty rat as a model for NIDDM. ILAR News 32(3):16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. T., Shaw W. N., Yu P. L. 1977. Genetics of obesity in Zucker rats and Koletsky rats. Heredity 38:373–377. [DOI] [PubMed] [Google Scholar]


