Abstract
Introduction
Cannabis use is known to be associated with significant cardiovascular morbidity. We describe three cases of cannabis-related malignant arrhythmias, who presented to the cardiac department at our institution within the last 2 years. All three patients were known to smoke cannabis on daily basis.
Case summaries
Case 1: A 30-year-old male, presented with recent onset of palpitations. A 12-lead electrocardiogram (ECG), transthoracic echocardiogram (TTE), and blood tests were all normal. During an inpatient exercise treadmill test (ETT) he developed polymorphic ventricular tachycardia (VT), which converted spontaneously to supraventricular tachycardia (SVT) in the recovery phase of the test. Subsequent risk stratification with cardiac magnetic resonance imaging and coronary angiography showed no abnormalities and an electrophysiological study was negative for sustained VT, however, SVT was easily induced with rapid conversion to atrial fibrillation. The patient successfully stopped smoking all tobacco products including cannabis and was treated with beta-blockers, with no further episodes of arrhythmia. Case 2: A 30-year-old male presented to the Emergency Department with palpitations, chest pain, and dizziness that improved during exertion. His initial ECG demonstrated complete atrioventricular block (AVB). Subsequent traces showed Mobitz Type I and second-degree AVB, which converted to atrial flutter after exertion. Routine blood tests, TTE, and an ETT were all normal and he was discharged home with no conduction abnormalities. Case 3: A 24-year-old male presented with two episodes of syncope. Baseline examination was normal, with an ECG showing a low atrial rhythm. Interrogation of his implantable loop recorder showed episodes of early morning bradycardia episodes with no associated symptoms.
Discussion
Cannabis-related arrhythmia can be multiform regarding their presentation. Therefore, ambiguous combinations of arrhythmia should raise suspicion of underlying cannabis abuse, where clinically appropriate. Although causality with regards to cannabis use cannot be proven definitively in these cases, the temporal relationship between drug use and the onset of symptoms suggests a strong association.
Keywords: Cannabis, Atrial fibrillation, Atrioventricular block, Ventricular tachycardia, Electrophysiology study, Cardiac arrhythmia, Case report
Learning points
Serious adverse effects associated with cannabis use are rare and include combination of arrhythmias and conduction disturbances.
A combination of different types of arrhythmias in young patient with normal heart should raise a suspicion of cannabis use. Cessation of cannabis use is crucial to cure those arrhythmias.
In times when cannabis use is increasing worldwide health care professionals should be familiar with its proarrhythmic effects.
Introduction
Cannabis is one of the oldest plants described in ancient Greek literature for its mind-altering properties and later on for its health benefits such as achievement of local haemostasis and tapeworm eradication using the plants leaves, anti-inflammatory and analgesic effects of seeds extract.1 Currently, the usage of cannabis as a recreational drug is increasing worldwide and in recent years there have been concerted efforts in many countries, to campaign for its legalization. However, there is a rising concern regarding cannabis-related adverse effects such as psychosis,2 increased incidence of acute myocardial infarction (MI),3 uncontrolled hypertension,4 and cardiac rhythm disturbances.
In our study, we will focus on the proarrhythmic effects of cannabis, discuss its possible mechanisms, and present several cases from our clinical practice.
According to the study of Johnson and Domino5 which was performed on healthy volunteers in the early 70s, the proarrhythmic effect of cannabis is mediated via autonomic nervous system and begins within a few minutes of smoking, reaching its peak within half an hour, but can last longer than 90 min. This is mediated by a biphasic and dose-dependent effect with sympathetic activation leading to tachycardia and an increase in cardiac output and blood pressure at low or moderate doses of the drug; however, parasympathetic activation with bradycardia and hypotension can both occur with higher doses of drug exposure.4 Epidemiological data indicate high rates of palpitations caused by dose-related sinus tachycardia in cannabis users;6 few case reports describe cannabis-induced atrial fibrillation (AF)7 with both sympathetic and parasympathetic mechanisms implicated. In addition, a number of case reports exist on cannabis-induced sinus bradycardia, and even second- and third-degree atrioventricular block (AVB).8,9 We reviewed the electrophysiological data in our hospital from November 2017 to November 2019 for cannabis-related conduction disturbances and arrhythmias. Three cannabis-related cases were identified and are described as follows.
Timeline
| Patient number | Events |
|---|---|
| 1 | A 30-year-old male presented to our institution with palpitations. His electrocardiogram (ECG), transthoracic echocardiogram (TTE), and blood tests were all normal. During the recovery stage of a previously unremarkable exercise treadmill test (ETT), he developed polymorphic ventricular tachycardia (VT) that converted spontaneously to supraventricular tachycardia (SVT) and subsequent atrial fibrillation (AF). Whilst in AF he developed further haemodynamically stable VT, which finally reverted back to sinus rhythm without intervention |
| Coronary angiography demonstrated normal coronary arteries. Cardiac MRI excluded the presence of underlying structural heart disease. An electrophysiological study confirmed the absence of scar tissue; AF and SVT were both induced during the study but not VT | |
| Following selective beta-blocker therapy with cessation of cannabis use, a subsequent exercise test was normal without recurrence of inducible arrhythmia | |
| 2 | A 30-year-old male patient presented with palpitations, chest discomfort, and dizziness. His initial ECG showed complete atrioventricular block (AVB) followed by atrial flutter with variable conduction. Routine blood tests and TTE were all normal |
| During his ETT he developed asymptomatic Mobitz I, second-degree AVB with long Wenckebach cycling; of note his target heart rate was achieved | |
| Following admission and cessation of marijuana use, his symptoms resolved and his ECG reverted back to normal sinus rhythm | |
| 3 | A 24-year-old male patient presented with two episodes of malignant syncope associated with head injury. His index ECG showed a low atrial rhythm with a heart rate of 50 b.p.m. Investigations including blood tests, TTE, stand-up tilt test, carotid sinus massage, and 24-h Holter monitoring were all normal |
| The patient underwent internal loop recorder implantation, with no interval evidence of further syncopal episodes despite the occurrence of multiple, significant bradycardic episodes in the early morning hours. Following regular smoking cessation counselling, the patient continued to smoke cannabis |
Case presentation
Case 1
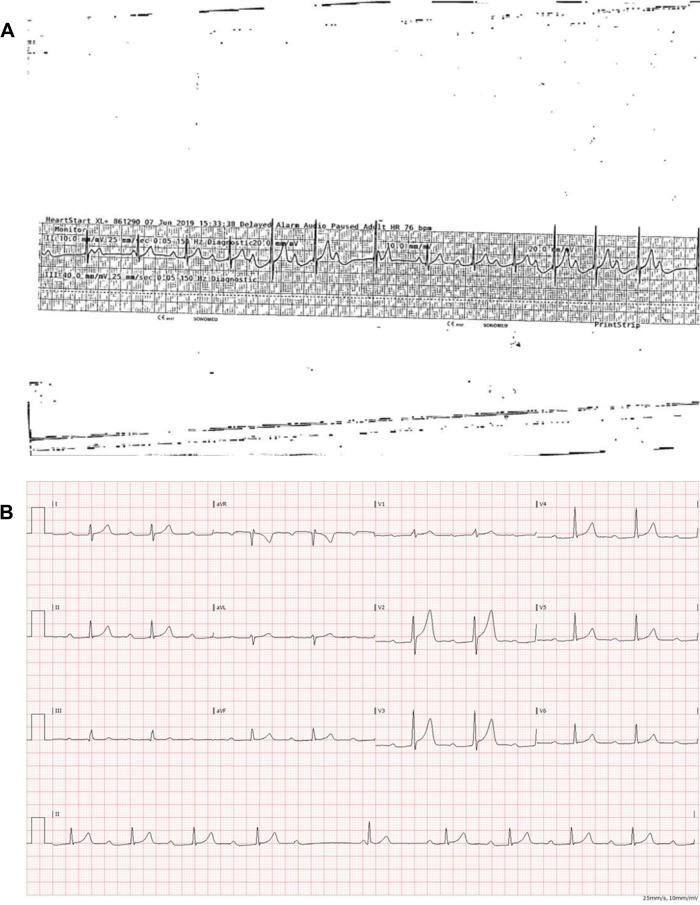
A 30-year-old healthy male, known to smoke marijuana on a daily basis for the last 5 years, presented with a 3-day history of palpitations. The patient recalled no associated chest pain or dizziness and had no family history of sudden cardiac death (SCD) or premature coronary artery disease. His past medical history was remarkable for unexplained sudden-onset syncope without prodrome, 5 years prior to his index admission, as well as a 5-year history of frequent episodes of palpitations occurring at the end of physical activity. At the time of presentation to our clinic his resting 12-lead electrocardiogram (ECG) was unremarkable apart from an early repolarization pattern. The QTc was normal with no epsilon wave. Blood tests confirmed normal electrolytes and thyroid hormone levels. Transthoracic echocardiogram demonstrated non-dilated ventricles with a normal, systolic left ventricular ejection fraction and no evidence of valvular heart disease. The left atrial (LA) size was normal (LA diameter 38 mm, LA area 13.8 cm2, LA volume 31 mL). Pulmonary artery pressures were within normal limits. The patient underwent an exercise test, which showed no evidence of either inducible myocardial ischaemia or arrhythmias during 13 min of exercise. After 45 s of recovery, however, his ECG changed to a wide complex tachycardia, 190 b.p.m., suspected to be polymorphic ventricular tachycardia (VT), starting with an outflow tract premature ventricular contraction (Figure 1). At this point, the patient was haemodynamically stable and asymptomatic, nevertheless, following the initiation of a Valsalva manoeuver, he reverted to a regular narrow complex tachycardia of 226 b.p.m. with associated symptoms of palpitations (Figure 2). Subsequently, he developed AF before converting spontaneously to haemodynamically stable VT (Figure 3). After 5 min within the recovery stage, his VT spontaneously converted to normal sinus rhythm. A second exercise test induced similar sustained monomorphic outflow tract VT (left bundle inferior axis with transition in V2–V3 leads). Coronary angiography demonstrated normal coronary arteries. Cardiac magnetic resonance (CMR) confirmed normal LA dimensions, non-dilated ventricles, and no evidence of late gadolinium enhancement ruling out previous MI, myocarditis, cardiac sarcoidosis, or arrhythmogenic right ventricular cardiomyopathy (ARVC).
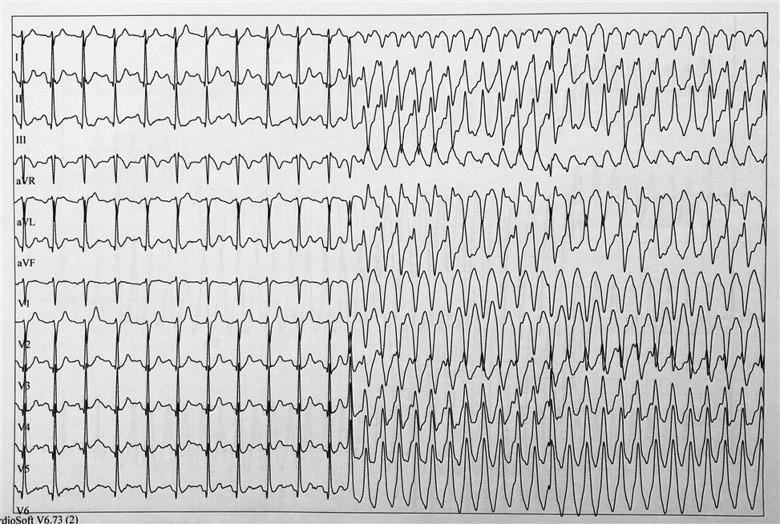
Figure 1.
Patient 1: Exercise test, early recovery phase. Sinus rhythm followed by polymorphic ventricular tachycardia starting with ventricular premature beat.
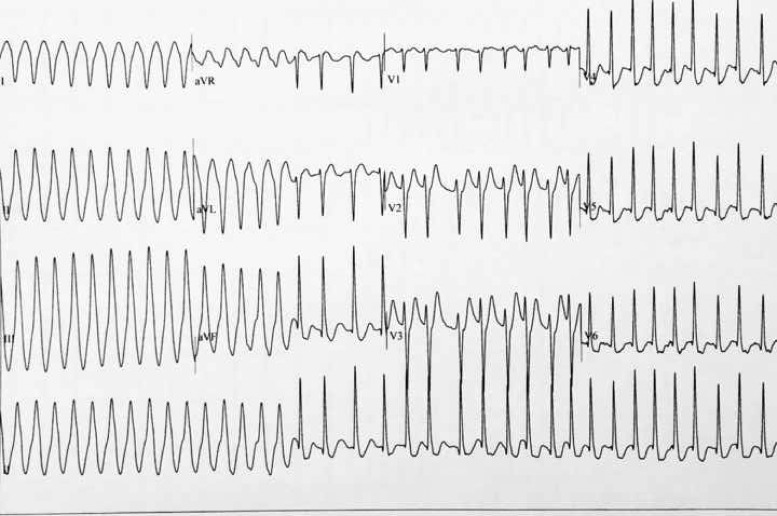
Figure 2.
Patient 1: Exercise test, recovery phase. Conversion of ventricular tachycardia to supraventricular tachycardia during Valsalva manoeuver.
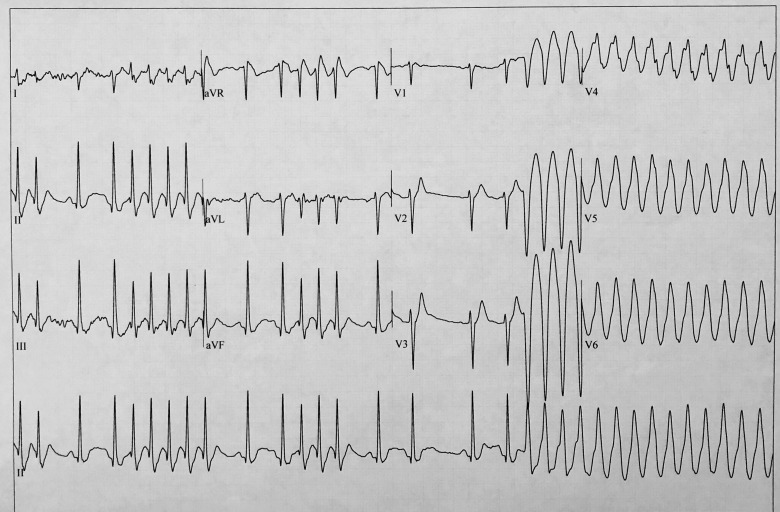
Figure 3.
Patient 1: Exercise test, recovery phase. Conversion of atrial fibrillation to sustained monomorphic ventricular tachycardia.
The patient was then referred for electrophysiological study. Voltage mapping using three-dimensional electroanatomical mapping, Carto® system (Biosense Webster), showed no evidence of scar tissue within the right ventricle and its outflow tract. A subsequent VT study with aggressive ventricular programmed stimulation, with and without isoprenaline and in washout, was performed, with no induction of VT demonstrated. We were unable to reveal dual atrioventricular (AV) node physiology. During ventricular pacing, supraventricular tachycardia (SVT) was induced with concentric atrial activation (Figure 4A). It was not possible to finish the study due to incessant, differential arrhythmias. During episodes of atrial and ventricular pacing the patient developed several episodes of AF with a fast ventricular response, requiring recurrent electrical cardioversion in order to continue the study (Figure 4B). Once completed, the patient was instructed to withhold from marijuana use and was started on a non-selective beta-blocker (Propranolol SR titrated to 120 mg daily). A subsequent exercise test confirmed no evidence of inducible VT/SVT, following which he was changed to a selective beta-blocker (Bisoprolol 5 mg daily) and a repeat exercise test confirmed the absence of inducible cardiac arrhythmia, hence the decision was taken not to implant an implantable cardioverter-defibrillator. He was seen in our clinic 2 months after discharge, where a third exercise stress test was undertaken again without any abnormality at maximum workload. In addition, 24-h ambulatory ECG monitoring was unremarkable. Eighteen months after his arrhythmic presentation the patient remains well and has not smoked cannabis since.
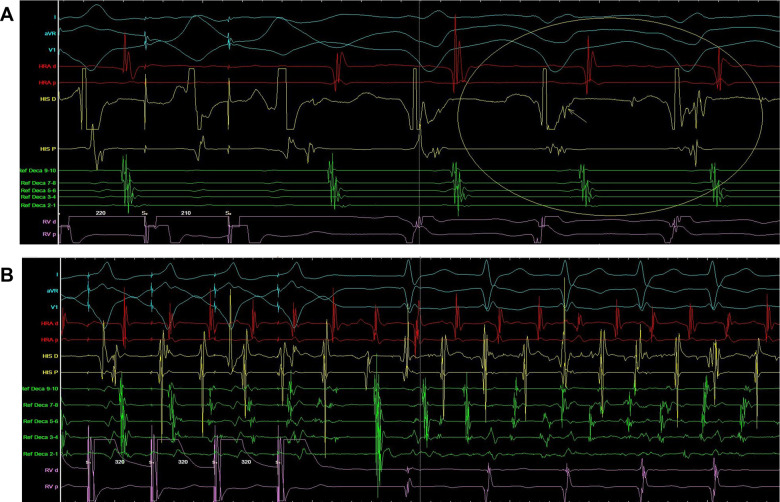
Figure 4.
(A) Electrophysiological study of patient 1. Supraventricular tachycardia induced during ventricular pacing (arrow shows concentric activation). (B) Electrophysiological study of patient 1. Supraventricular tachycardia deteriorating to atrial fibrillation during ventricular overdrive pacing.
Case 2
A 30-year-old healthy male with a history of smoking tobacco/marijuana, occasional cocaine use (last reported use a week prior to his index admission) presented with a 1-day history of palpitations and chest discomfort. The symptoms were temporarily relieved by cycling and other physical activities. The following morning, in addition to the aforementioned symptoms, he recalled episodes of dizziness. He was initially assessed in his local urgent care centre, where his examination revealed normal vital signs, however, a 12-lead ECG confirmed sinus rhythm with complete AVB. A repeat ECG demonstrated Type 1, second-degree AVB (Wenckebach conduction) with a single junctional escape beat with AV conduction block (Figure 5A). A subsequent ECG showed atrial flutter with variable conduction, resulting in an urgent referral to our institution. On admission, he was haemodynamically stable with an entirely normal examination. Blood tests, including electrolytes and thyroid function tests, were within normal range. Electrocardiograms on admission showed Mobitz Type I AVB (Figure 5B) followed by atrial flutter with a ventricular rate of 140 b.p.m. after the patient had been instructed to perform squatting exercises. Transthoracic echocardiogram confirmed normal chamber dimensions with good biventricular systolic function with no evidence of valvular heart disease. During a subsequent exercise test, in which the patient achieved a heart rate of 180 b.p.m., there was no evidence of inducible ischaemia or worsening of the baseline first-degree AVB.
Figure 5.
(A) Patient 2: Type 1 second-degree atrioventricular block (Wenckebach conduction) with a single junctional escape beat every time when atrioventricular conduction is blocked. (B) Patient 2. Type I second-degree atrioventricular block.
Three days after hospitalization the patient was discharged home, asymptomatic with a normal ECG. He was strongly advised to stop all illicit drug use and counselled accordingly. Unfortunately, following discharge he was lost to follow-up.
Case 3
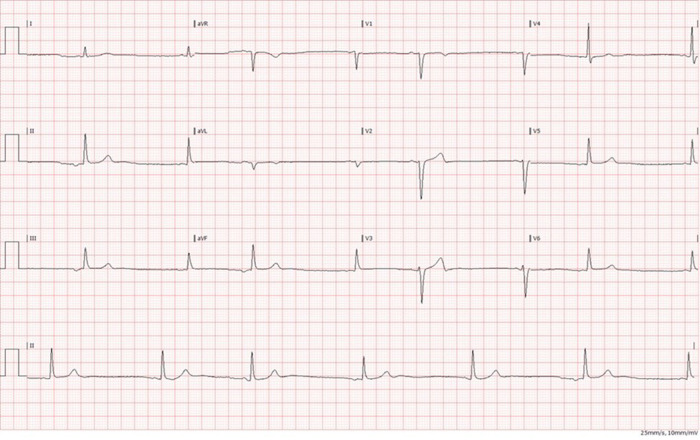
A healthy 24-year-old male, known to be a regular smoker of cannabis, was admitted to our arrhythmia clinic with a history of two, identical episodes of malignant syncope within the past year, both associated with head injury. On each occasion, whilst he was having his hair cut, he became symptomatic with dizziness leading to transient loss of consciousness. His past history was remarkable for asymptomatic sinus bradycardia. His baseline examination and blood tests were entirely normal. Urine toxicology was positive for cannabis but no other recreational drugs were detected. A 12-lead ECG showed inverted P-waves in leads II, III, and AVF and upright P-waves in aVR-consistent with retrograde sinoatrial node activation from an ectopic site (Figure 6).
Figure 6.
Patient 3: Retrograde sinoatrial node activation from the ectopic site.
Stand-up tilt test, carotid sinus massage, and 24-h Holter ECG monitoring were all normal. A computed tomography brain scan revealed no evidence of intracerebral haemorrhage or infarction and no cerebral masses. His echo study was entirely normal. The patient underwent further assessment with an implantable loop recorder (ILR) and was counselled with regards to smoking cessation including marijuana. During a follow-up visit he reported to smoking less marijuana (single episode per week) with no further episodes of syncope despite ongoing evidence of nocturnal and early morning sinus bradycardia, as recorded by his ILR.
Discussion
The global increase in the recreational use of cannabis remains a significant concern within the wider medical community, with recent acknowledgement of the potential to cause malignant heart rhythm disturbances. There has, however, been conflicting evidence about the association between cannabis use and arrhythmia. There is evidence to suggest that chronic cannabis use increases the incidence of important cardiovascular risk factors such as insulin resistance, dyslipidaemia,10 and hypertension in young to middle-aged adults.11
Cannabis use may increase the risk of acute MI. Potential mechanisms may include an increase in heart rate and blood pressure, increase in serum carboxyhaemoglobin levels, which together result in a mismatch of augmented oxygen demand and decreased oxygen supply.3
In patient’s post-MI, the risk of VT/ventricular fibrillation (VF) has not been shown to differ in reported marijuana users vs. non-users. However, a lower risk of in-hospital mortality and a trend towards a lower risk of AF was demonstrated.12 In younger patients without a history of acute coronary syndrome, cannabis use was shown to be associated with an increased risk of VF, AF, atrial flutter, pre-excitation syndromes, and long-QT syndrome.13 Of note an arrhythmogenic potential of cannabis smoking in patients without underlying cardiovascular disease and risk factors has rarely been reported.
In this case series we have highlighted the variant, clinical presentations of cardiac arrhythmia in the setting of significant cannabis use. Our patients had no relevant past medical history with CHA2DS2-VASc scores of 0. Although causality, with regards to cannabis use and the onset of arrhythmia cannot be proven definitively in these cases, the temporal relationship suggests a strong association.
Regarding Case 1 (combination of idiopathic VT, SVT, and AF) alternative diagnoses include ARVC,14 catecholaminergic polymorphic ventricular tachycardia (CPVT),15 and myocarditis.16 There were no echocardiographic or CMR features of ARVC or myocarditis. Catecholaminergic polymorphic ventricular tachycardia often presents during exercise in patients with a family history of SCD; in this case VT occurred after exercise. Whilst there are case reports describing SVT within the post-exercise period,17 our patient had no family history of SCD with symptom onset aged 30 as opposed to 7–12 years, commonly seen in CPVT.
With regards to Case 2 (AVB and atrial flutter) alternative diagnoses included cardiac sarcoidosis,18 Lamin A/C cardiomyopathy,19 and Brugada syndrome,20 however, the patient had no clinical, ECG, or imaging features consistent with any of these conditions.
Regarding Case 3 (recurrent syncope, extreme bradycardia with retrograde sinoatrial node activation from an ectopic site), the differential diagnosis included sinus node dysfunction, increased vagal tone, carotid sinus hypersensitivity, and orthostatic syncope. The majority of these conditions were excluded by his baseline examination and preliminary tests during the index hospitalization; furthermore, there was no correlation between the patient’s symptoms and his episodes of nocturnal/early morning bradycardia, as demonstrated by interrogation of the ILR device.
The exact mechanisms mediating cardiac arrhythmias in marijuana smokers are unknown although several theories exist. The active psychotropic component of marijuana, delta-9-tetrahydrocannabinol (THC), acts mainly via G protein and cannabinoid receptors CB1 and CB2. As shown in animal models, activation of CB1 receptor leads to sympathetic inhibition and enhanced vagal tone, bradycardia, and hypotension.21 Tachycardia following marijuana use is attributed to direct sympathetic stimulation22 and can also occur as a direct consequence of parasympathetic vasodilatation also induced by THC. Possible mechanisms of cannabis-related cardiac arrhythmias presented in Figure 7.
Figure 7.
Possible mechanisms of cannabis-related cardiac arrhythmia. Shown is a diagram of possible mechanisms of arrhythmias in cannabis users. BP, blood pressure; CB1, cannabinoid receptor type 1; COHb, carboxyhaemoglobin; GPCRs, G protein-coupled receptors; HR, heart rate.
Conclusion
Cannabis-related arrhythmias tend to be heterogeneous regarding their presentation. Given the increasing abuse of recreational cannabis, the potential for life-threatening, arrhythmia, even in patients with structurally normal hearts, is a growing clinical concern.
Within this case series, two patients stopped smoking in entirety (one of whom was lost to follow-up), whilst the last patient continued to smoke cannabis but at a significantly reduced amount. This is an important aspect of this condition, regarding cannabis use.
Although causality with regards to cannabis use and the onset of arrhythmia cannot be proven definitively in these cases, their temporal relationship suggests a strong association.
Lead author biography

Ella Yahud is a Senior Resident, Cardiology Division, Assuta Ashdod University Hospital, Ha Refuah Street, Ashdod, Israel.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Butrica JL. The medical use of cannabis among the Greeks and Romans. J Cannabis Ther 2002;2:51–70. [Google Scholar]
- 2. Gage SH, Hickman M, Zammit S.. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry 2016;79:549–556. [DOI] [PubMed] [Google Scholar]
- 3. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE.. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805–2809. [DOI] [PubMed] [Google Scholar]
- 4. Fisher BAC, Ghuran A, Vadamalai V, Antonios TF.. Cardiovascular complications induced by cannabis smoking: a case report and review of the literature. Emerg Med J 2005;22:679–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson S, Domino EF.. Some cardiovascular effects of marihuana smoking in normal volunteers. Clin Pharmacol Ther 1971;12:762–768. [DOI] [PubMed] [Google Scholar]
- 6. Petronis KR, Anthony JC.. An epidemiologic investigation of marijuana- and cocaine-related palpitations. Drug Alcohol Depend 1989;23:219–216. [DOI] [PubMed] [Google Scholar]
- 7. Singh GK. Atrial fibrillation associated with marijuana use. Pediatr Cardiol 2000;21:284. [DOI] [PubMed] [Google Scholar]
- 8. Akins D, Awdeh MR.. Marijuana and second-degree AV block. South Med J 1981;74:371–373. [DOI] [PubMed] [Google Scholar]
- 9. Van Keer JM. Cannabis-induced third-degree AV block. Case Rep Emerg Med 2019;2019:5037356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muniyappa R, Sable S, Ouwerkerk R, Mari A, Gharib AM, Walter M, Courville A. et al. Metabolic effects of chronic cannabis smoking. Diabetes Care 2013;36:2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alshaarawy O, Elbaz H.. Cannabis use and blood pressure levels: United States National Health and Nutrition Examination Survey, 2005-2012. J Hypertens 2016;34:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson-Sasso CP, Kao DP, Walker LA, Tompkins C. Arrhythmic effects of marijuana following acute myocardial infarction. Presented at HRS 2018. Boston, MA.
- 13. Ramphul DK, Joynauth DJ.. Cardiac arrhythmias among teenagers using cannabis in the United States. Am J Cardiol 2019;124:1966. [DOI] [PubMed] [Google Scholar]
- 14. Ermakov S, Scheinman M.. Arrhythmogenic right ventricular cardiomyopathy—antiarrhythmic therapy. Arrhythm Electrophysiol Rev 2015;4:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leenhardt A, Denjoy I, Guicheney P.. Catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 2012;5:1044–1052. [DOI] [PubMed] [Google Scholar]
- 16. Baksi AJ, Kanaganayagam GS, Prasad SK.. Arrhythmias in viral myocarditis and pericarditis. Card Electrophysiol Clin 2015;7:269–281. [DOI] [PubMed] [Google Scholar]
- 17. Else SDN, Potts JE, Sanatani S.. Postexertional supraventricular tachycardia in children with catecholaminergic polymorphic ventricular tachycardia. Case Rep Cardiol 2012;2012:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R.. Cardiac sarcoidosis: state-of-the-art review. Cardiovasc Diagn Ther 2016;6:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP. et al. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J 2018;39:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuka Mizusawa Y, Wilde AAM.. Brugada syndrome. Circ Arrhythm Electrophysiol 2012;5:606–616. [DOI] [PubMed] [Google Scholar]
- 21. Niederhoffer N, Schmid K, Szabo B.. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch Pharmacol 2003;367:434–443. [DOI] [PubMed] [Google Scholar]
- 22. Beaconsfield P, Ginsburg J, Rainsbury R.. Marihuana smoking: cardiovascular effects in man and possible mechanisms. N Engl J Med 1972;287:209–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.