Abstract
Background
Failure of a small surgical aortic bioprosthesis represents a challenging clinical scenario with valve-in-valve (ViV) transcatheter aortic valve implantation (TAVI) often resulting in patient-prosthesis mismatch. Bioprosthetic valve fracture (BVF) performed as a part of the ViV TAVI has recently emerged as an alternative approach with certain types of surgical bioprostheses.
Case summary
An 81-year-old woman with a history of three surgical aortic valve procedures presented with heart failure. Aortic bioprosthesis degeneration with severe stenosis and moderate regurgitation was found. The patient was deemed a high-risk surgical candidate and the heart team decided that ViV TAVI was the preferred treatment option. Due to the very small 19 mm stented surgical aortic bioprosthesis Mitroflow 19 mm (Sorin Group, Italy) we decided to perform BVF as a part of ViV TAVI to prevent patient-prosthesis mismatch. Since this was the first BVF procedure in our centre, an ex vivo BVF of the same kind of bioprosthetic valve was performed first. Subsequently, successful BVF with implantation of Evolut R 23 mm (Medtronic, USA) self-expandable transcatheter valve was performed. Excellent haemodynamic result was achieved and no periprocedural complications were present. The patient had an immediate major improvement in clinical status and remains asymptomatic after 6 months.
Discussion
Bioprosthetic valve fracture together with ViV TAVI is a safe and effective emerging technique for treatment of small surgical aortic bioprosthesis failure. Bioprosthetic valve fracture allows marked oversizing of implanted self-expandable transcatheter aortic valves, leading to excellent haemodynamic and clinical results. An ex vivo BVF can serve as an important preparatory step when introducing the new method.
Keywords: Bioprosthetic valve fracture, Case report, Transcatheter aortic valve implantation, Valve-in-valve, Aortic bioprosthesis failure
Learning points
Bioprosthetic valve fracture (BVF) together with valve-in-valve transcatheter aortic valve implantation is a safe and effective emerging technique for treatment of failure of certain types of surgical aortic bioprostheses.
Bioprosthetic valve fracture allows marked oversizing of implanted self-expandable transcatheter aortic valves, leading to excellent haemodynamic and clinical results.
An ex vivo BVF can serve as an important preparatory step when introducing the new method.
Introduction
Valve-in-valve (ViV) transcatheter aortic valve implantation (TAVI) is an established treatment method for failed surgical aortic bioprostheses.1 However, small surgical aortic bioprostheses represent a challenging and increasingly common clinical scenario with ViV TAVI often resulting in high residual gradients and patient-prosthesis mismatch.2 This results in increased late mortality.3,4 High implantation of TAVI prostheses and use of valves with supra-annular design have been associated with decreased post-procedural gradients.5 Furthermore, bioprosthetic valve fracture (BVF) performed as a part of the ViV TAVI has recently emerged as an alternative approach with certain types of surgical bioprostheses.6 With BVF the operator ‘cracks’ the ring of the surgical bioprosthesis by means of non-compliant transcatheter balloons, either before or after implanting the transcatheter valve. This allows implantation of larger TAVI prostheses with better haemodynamic performance.7
In the present article, we present a case of a successful ViV TAVI with BVF of a degenerated 19 mm stented surgical aortic bioprosthesis and implantation of a markedly oversized 23 mm self-expandable transcatheter valve. We also demonstrate an ex vivo BVF, which served as a preparatory step for the procedure, since it was the first TAVI with BVF in our centre.
Timeline
| 2007 | First surgical aortic valve replacement due to severe stenosis |
| 2007 | First reoperation due to dehiscence of aortic bioprosthesis |
| 2012 | Second reoperation due to degeneration of aortic bioprosthesis |
| April 2019 | Percutaneous coronary intervention of the right coronary artery |
| 2 August 2019 | Hospitalization in our institution due to heart failure |
| 21 August 2019 | Transcatheter aortic valve implantation with fracturing of aortic bioprosthesis |
| March 2020 | Feeling well, excellent functional status (New York Heart Association I) |
Case presentation
In August 2019, an 81-year-old female was admitted due to progressive worsening of heart failure symptoms. She had dyspnoea at rest, orthopnoea, and had gained weight. Physical examination revealed loud systolic ejection murmur radiating to the neck, diminished breath sounds in lower pulmonary fields on both sides suggesting pleural effusion and bilateral leg oedema.
The patient had a surgical aortic valve replacement due to severe aortic stenosis in 2007 and a reoperation due to valve dehiscence in the same year (Sorin Freedom SOLO 23 mm, Sorin Group, Italy). In 2012, a second reoperation was performed due to severe stenosis of the degenerated aortic bioprosthesis. At that time, a stented aortic bioprosthesis Mitroflow 19 mm (Sorin Group, Italy) was implanted. At discharge, left ventricle size and ejection fraction (EF) were normal (end-diastolic volume index 56 mL/m2, EF 53%), mean transaortic gradient was 17 mmHg, and there was no aortic regurgitation. The patient also had a history of coronary artery disease with percutaneous coronary intervention of the right coronary artery in April 2019, arterial hypertension, hyperlipidaemia, persistent atrial fibrillation, and hypothyroidism following Hashimoto thyroiditis. She was treated with bisoprolol, ramipril, spironolactone, furosemide, rosuvastatin, warfarin, digoxin, and levothyroxine.
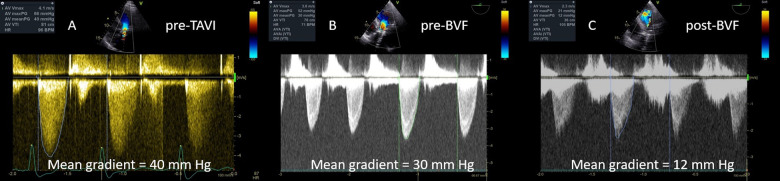
After admission, echocardiography was performed. Aortic bioprosthesis degeneration with severe stenosis (maximum velocity 4.1 m/s, mean gradient 40 mm Hg, Figure 1A) and moderate regurgitation was diagnosed. Additionally, moderate primary mitral regurgitation (effective regurgitant orifice area 20 mm2), moderate secondary tricuspid regurgitation, and moderate post-capillary pulmonary hypertension (estimated systolic pulmonary artery pressure 57 mm Hg) were present. Left ventricle was dilated (end-diastolic volume index 71 mL/m2) with preserved EF 66% and the right ventricle was dilated (basal diameter of 4.4 cm) with reduced systolic function (fractional area change 29%). Coronary angiography showed non-obstructive coronary atherosclerosis with no restenosis of the stent in the right coronary artery. Laboratory tests showed greatly elevated pro brain natriuretic peptide (BNP) levels (30 986 ng/L, normal values for patients age < 738 ng/L), stable chronic kidney disease with a glomerular filtration rate of 35 mL/min, normocytic anaemia due to intravascular haemolysis (haemoglobin 84 g/L, mean corpuscular volume 101 fL), and pathologic hepatic enzymes attributed to liver congestion.
Figure 1.
Evaluation of bioprosthesis function with continuous-wave Doppler echocardiography before transcatheter aortic valve implantation (A), before bioprosthetic valve fracture (B), and after bioprosthetic valve fracture (C).
With EuroSCORE 2 of 21.98% and STS risk score of 16.77%, the patient was deemed a high-risk surgical candidate and the combined team of cardiovascular surgeons and cardiologists decided that ViV TAVI was the preferred treatment option. In order to prevent patient-prosthesis mismatch due to a very small aortic bioprosthesis (true inner diameter of the Mitroflow 19 mm valve reported by industry is 15.4 mm8) we decided to perform BVF as a part of TAVI procedure.
Since this was the first BVF procedure in our centre, to get hands-on experience we first performed an ex vivo simulation of the BVF. We used a Mitroflow 23 mm bioprosthesis (the smallest available size in our centre at that time) and a 22 mm non-compliant Atlas Gold balloon (Bard, USA). Bioprosthetic valve fracture was achieved at a pressure of 14 atm (Supplementary material online, Video S1) and could be felt by the operator as a sudden drop in the balloon pressure. The inspection of the prosthesis revealed a single fracture of the silicone ring. We also determined the burst pressure for the Atlas Gold balloon, which was achieved at a pressure of 32 atm (Supplementary material online, Video S2). Rupturing caused a formation of a tiny hole in the distal part of the balloon.
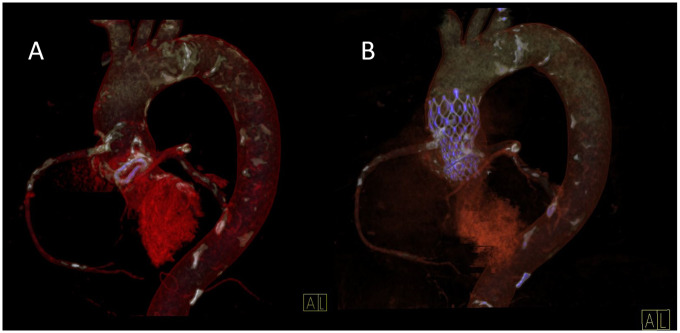
We proceeded with TAVI using right femoral approach. Without pre-dilation, an Evolut R 23 mm (Medtronic, USA) transcatheter valve was implanted in the degenerated 19 mm Mitroflow bioprosthesis. We targeted high implantation to enhance supra-annular position of the leaflets. Mean transvalvular pressure gradient with transoesophageal echocardiography immediately after transcatheter valve deployment was 30 mmHg due to suboptimal valve leaflet opening (Figure 1B). To fracture the Mitroflow silicone ring and achieve optimal prosthesis deployment we performed a post-dilation using Atlas Gold 22 mm balloon. At a 12 atm of pressure, a sudden drop of pressure was felt by the operator and an increase of annular size was observed on fluoroscopy (Supplementary material online, Videos S3 and S4). Mean transvalvular gradient dropped to 12 mmHg (Figure 1C). Minimal paravalvular regurgitation was observed (Supplementary material online, Video S5). We confirmed good position of the TAVI prosthesis with computed tomography 4 days after the procedure (Figure 2). The post-procedural stay was uneventful and the patient left the hospital 7 days later with major improvement in clinical status. Six months after procedure the patient was feeling good and reported no symptoms of heart failure (Class I functional capacity according to New York Heart Association). Serum proBNP concentration dropped to 8847 ng/L.
Figure 2.
Computed tomography reconstructions of aortic bioprostheses. (A) A Mitroflow 19 mm annulus is painted in violet. (B) An Evolut R 23 mm frame inside of a Mitroflow 19 mm annulus (both painted in violet).
Discussion
In the presented case, we have demonstrated a successful ViV TAVI in a failed small surgical aortic bioprosthesis. By combining BVF with implantation of an oversized supra-annularly positioned self-expandable transcatheter valve, we have achieved excellent haemodynamic and clinical result.
Recently, BVF has been increasingly used to overcome patient-prosthesis mismatch after ViV TAVI in small surgical aortic bioprostheses.9 According to the bench testing, all bioprostheses without metal frame and some with metal frame are feasible for BVF.6 Successful BVF and ViV TAVI in a degenerated Mitroflow bioprosthesis has shown to be feasible with balloon- and self-expandable transcatheter valves.6,7,10–14 However, most authors have reported to implant a moderately oversized transcatheter valve (1–2 mm larger than the outer diameter of the surgical bioprosthesis).6,9–12 In contrast, we have chosen a prosthesis with a 4 mm larger outer diameter and 7.6 mm larger (49% device annular sizing ratio) than the true inner diameter of the surgical bioprosthesis. We had extensive discussion in the heart team prior to the procedure about the optimal transcatheter bioprosthesis size. Despite the possibility of potential annular damage, the arguments for the Evolut R 23 mm bioprosthesis prevailed. First, we tried to avoid the potential patient-prosthesis mismatch with a smaller valve. Furthermore, the first implanted surgical aortic bioprosthesis in 2007 was a 23 mm size, which reassured us that the patient could tolerate greater transcatheter valve. Finally, by being a high-volume TAVI centre we have substantial experience with ViV procedures. The supra-annular design of the self-expandable valve as well as high implantation was crucial to allow successful implantation with adequate leaflet opening and closure. Importantly, our case is one of the first in the literature to show that BVF of a Mitroflow 19 mm surgical aortic bioprosthesis and ViV TAVI with markedly oversized self-expandable transcatheter valve is feasible.13
While the optimal timing of BVF is unknown recently published bench testing showed that BVF performed after vs. before TAVI is associated with superior prosthesis expansion and lower residual transvalvular gradients with certain balloon- and self-expandable transcatheter valves.10 However, in the same study the Evolut R transcatheter valve showed similar hydrodynamic performance irrespective of the BVF timing.10 We decided to first implant the transcatheter valve and subsequently performed BVF mainly to reduce the chance of intermittent severe aortic regurgitation.
Finally, we have demonstrated how BVF can be safely and successfully introduced as a new method in a centre. The ex vivo BVF simulation was a very important step for gaining operator hands-on experience with the technique and we recommend it as an essential step when introducing the new method.
Conclusions
The presented case demonstrates that BVF together with ViV TAVI is a safe and effective emerging technique for treatment of failure of certain types of small surgical aortic bioprostheses. Bioprosthetic valve fracture allows marked oversizing of implanted self-expandable transcatheter aortic valves, leading to excellent haemodynamic and clinical results. An ex vivo BVF can serve as an important preparatory step when introducing the new method.
Lead author biography
Matjaz Bunc is an internationally renowned expert in the field of cardiology, research, and education. He is a head of interventional cardiology UKC Ljubljana, president of the working group for interventional cardiology of Slovenian Society of Cardiology. He is an expert in the structural heart disease (TAVI, Mclip, PV leaks, CFR) and coronary interventions (CTO). He is a faculty, chair/co-chair in EuroPCR, ICI Tel-Aviv, TCT, and plenty of other scientific meetings. He has published more than 450 scientific papers (more than 110 in SCI journals) and presentations and has actively participated in numerous international congresses. He is a reviewer of ESC Guidelines for Valvular disease, STEMI, NSTEMI, Stable coronary disease.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We would like to thank all of the medical and administrative staff who contributed to the treatment of the patient.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Webb JG, Mack MJ, White JM, Dvir D, Blanke P, Herrmann HC. et al. Transcatheter aortic valve implantation within degenerated aortic surgical bioprostheses: PARTNER 2 valve-in-valve registry. J Am Coll Cardiol 2017;69:2253–2262. [DOI] [PubMed] [Google Scholar]
- 2. Zenses AS, Dahou A, Salaun E, Clavel MA, Rodés-Cabau J, Ong G. et al. Haemodynamic outcomes following aortic valve-in-valve procedure. Open Heart BMJ 2018;5:e000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S. et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014;312:162. [DOI] [PubMed] [Google Scholar]
- 4. Faerber G, Schleger S, Diab M, Breuer M, Figulla HR, Eichinger WB. et al. Valve-in-valve transcatheter aortic valve implantation: the new playground for prosthesis-patient mismatch J Interv Cardiol 2014;27:287–292. [DOI] [PubMed] [Google Scholar]
- 5. Simonato M, Webb J, Kornowski R, Vahanian A, Frerker C, Nissen H. et al. Transcatheter replacement of failed bioprosthetic valves: large multicenter assessment of the effect of implantation depth on hemodynamics after aortic valve-in-valve Circ Cardiovasc Interv 2016;9:e003651. [DOI] [PubMed] [Google Scholar]
- 6. Allen KB, Chhatriwalla AK, Cohen DJ, Saxon JT, Aggarwal S, Hart A. et al. Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation Ann Thorac Surg 2017;104:1501–1508. [DOI] [PubMed] [Google Scholar]
- 7. Chhatriwalla AK, Allen KB, Saxon JT, Cohen DJ, Aggarwal S, Hart AJ. et al. Bioprosthetic valve fracture improves the hemodynamic results of valve-in-valve transcatheter aortic valve replacement. Circ Cardiovasc Interv 2017;10:e005216. [DOI] [PubMed] [Google Scholar]
- 8. Bapat VN, Attia R, Thomas M.. Effect of valve design on the stent internal diameter of a bioprosthetic valve. JACC Cardiovasc Interv 2014;7:115–127. [DOI] [PubMed] [Google Scholar]
- 9. Allen KB, Chhatriwalla AK, Saxon JT, Cohen DJ, Nguyen TC, Webb J, Loyalka P. et al. Bioprosthetic valve fracture: technical insights from a multicenter study. J Thorac Cardiovasc Surg 2019;158:1317–1328.e1. [DOI] [PubMed] [Google Scholar]
- 10. Sathananthan J, Fraser R, Hatoum H, Barlow AM, Stanová V, Allen KB. et al. Bioprosthetic valve fracture performed before versus after valve-in-valve intervention: insights from bench testing. EuroIntervention 2020;15:1409–1416. [DOI] [PubMed] [Google Scholar]
- 11. Nielsen-Kudsk JE, Christiansen EH, Terkelsen CJ, Nørgaard BL, Jensen KT, Krusell LR. et al. Fracturing the ring of small Mitroflow bioprostheses by high-pressure balloon predilatation in transcatheter aortic valve-in-valve implantation Circ Cardiovasc Interv 2015;8:e002667. [DOI] [PubMed] [Google Scholar]
- 12. Nielsen-Kudsk JE, Andersen A, Therkelsen CJ, Christensen EH, Jensen KT, Krusell LR. et al. High-pressure balloon fracturing of small dysfunctional Mitroflow bioprostheses facilitates transcatheter aortic valve-in-valve implantation. EuroIntervention 2017;13:e1020–e1025. [DOI] [PubMed] [Google Scholar]
- 13. Singh M, Reitknecht F, Rogers G, Kaluski E.. Transcatheter valve–in–valve aortic valve replacement with bioprosthetic valve fracture/stretching technique in a degenerated Mitroflow valve. J Cardiol Cases 2020;21:189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sathananthan J, Sellers S, Barlow AM, Stanová V, Fraser R, Toggweiler S. et al. Valve-in-valve transcatheter aortic valve replacement and bioprosthetic valve fracture comparing different transcatheter heart valve designs: an ex vivo bench study. JACC Cardiovasc Interv 2019;12:65–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.