Abstract
Background
Current guidelines target low-density lipoprotein cholesterol (LDL-C) concentrations to reduce atherosclerotic cardiovascular disease (ASCVD) risk, and yet clinical trials demonstrate persistent residual ASCVD risk despite aggressive LDL-C lowering.
Content
Non–LDL-C lipid parameters, most notably triglycerides, triglyceride-rich lipoproteins (TGRLs), and lipoprotein(a), and C-reactive protein as a measure of inflammation are increasingly recognized as associated with residual risk after LDL-C lowering. Eicosapentaenoic acid in statin-treated patients with high triglycerides reduced both triglycerides and ASCVD events. Reducing TGRLs is believed to have beneficial effects on inflammation and atherosclerosis. High lipoprotein(a) concentrations increase ASCVD risk even in individuals with LDL-C < 70 mg/dL. Although statins do not generally lower lipoprotein(a), proprotein convertase subtilisin/kexin type 9 inhibitors reduce lipoprotein(a) and cardiovascular outcomes, and newer approaches are in development. Persistent increases in C-reactive protein after intensive lipid therapy have been consistently associated with increased risk for ASCVD events.
Summary
We review the evidence that biochemical assays to measure TGRLs, lipoprotein(a), and C-reactive protein are associated with residual risk in patients treated to low concentrations of LDL-C. Growing evidence supports a causal role for TGRLs, lipoprotein(a), and inflammation in ASCVD; novel therapies that target TGRLs, lipoprotein(a), and inflammation are in development to reduce residual ASCVD risk.
Introduction
Based on the well-established relation between increased blood cholesterol, particularly cholesterol in low-density lipoproteins (LDL-C), and atherosclerotic cardiovascular disease (ASCVD) morbidity and mortality, current treatment guidelines focus on reducing LDL-C concentration to reduce ASCVD risk (1, 2). However, numerous clinical trials of statin, nonstatin, and combination therapy have shown persistent residual ASCVD risk despite aggressive LDL-C lowering (3–5), leading to efforts to identify determinants of residual ASCVD risk, including those that can be measured by clinical chemistry methods. Recently, the multidisciplinary consensus panel of the European Atherosclerosis Society and the European Federation of Clinical Chemistry and Laboratory Medicine published their recommendations on the quantification of atherogenic lipoproteins beyond LDL-C for lipid-lowering strategies (6).
We will review the evidence for triglycerides (TGs) and triglyceride-rich lipoproteins (TGRLs), lipoprotein(a) [Lp(a)], and inflammation as residual risk factors. In particular, we discuss the association of measurements obtained by available biochemical assays for these factors (Table 1) with ASCVD risk in statin-treated patients, the increasing data supporting a causal role for these factors in ASCVD, and novel therapies in development that target these factors to reduce residual ASCVD risk.
Table 1.
Laboratory tests for triglyceride-rich lipoprotein measures, lipoprotein(a), and inflammatory markers.
| Measures | Assay features | Pros | Cons |
|---|---|---|---|
| Total TGs | Measures circulating concentrations of total TGs based on quantification of glycerol following lipolytic conversion of TGs |
Automated chemistry assay Standardized methodology via traceable SRMs High-throughput/low-complexity method |
Does not measure TG concentrations of individual lipoprotein classes/subfractions Non–glycerol blanked TG assays can result in a free glycerol error |
| Calculated RC | Total cholesterol – ( HDL-C + directly measured LDL-C) |
Cost effective: can be calculated using standard lipid profile High-throughput/low-complexity method |
May be inaccurate when calculated LDL-C is used instead of directly measured LDL-C Does not measure cholesterol concentrations of lipoprotein classes/subfractions |
| RLP-C by homogeneous assay (Denka Seiken) | Measures cholesterol concentration in remnant lipoproteins using specific surfactants and enzymes |
Automated chemistry assay Standardized against density gradient ultracentrifugation method Directly measures cholesterol concentration in remnant lipoproteins High-throughput/low-complexity method |
Novel assay with limited data available from clinical studies |
| LDL-TG by homogeneous assay (Denka Seiken) | Measures TG concentration in LDL using specific surfactants and enzymes |
Automated chemistry assay Standardized against density gradient ultracentrifugation method Directly measures TG concentration in LDL High-throughput/low-complexity method |
Novel assay with limited data available from clinical studies |
| Density gradient ultracentrifugation/ precipitation (beta quantification) | Can be used to measure cholesterol and TG concentrations in VLDL, IDL, LDL, HDL, and Lp(a) by density ultracentrifugation/precipitation and subsequent enzymatic methods for cholesterol and TGs | Gold standard reference method for LDL-C and HDL-C | High-complexity method/low-throughput method |
| NMR (LipoScience/ LabCorp) | Measures lipoprotein particle concentrations (VLDL, IDL, LDL, HDL) and size in 11 lipoprotein subfractions |
LDL-P assay is US FDA approved High-throughput assay Directly measures lipoprotein concentrations and sizes |
High-complexity method Does not measure cholesterol or TG concentration in lipoprotein subfractions Issues regarding standardization of lipoprotein subfractions and instrument sensitivity |
| NMR (Nightingale Health) | Measures lipoprotein particle concentrations (VLDL, IDL, LDL, HDL) and size in 14 lipoprotein subfractions as well as their TGs, cholesterol (free and esterified), phospholipids, and fatty acids |
High-throughput assay Directly measures lipoprotein concentrations and sizes in addition to their lipid constituents |
High-complexity method Specific software and data analysis tools required Issues regarding standardization of lipoprotein subfractions and instrument sensitivity |
| ES-DMA (Quest) | Measures lipoprotein particle concentrations (VLDL, IDL, LDL, HDL) and size in lipoprotein subfractions in the range of 17.2–540.0 Å based on ion mobility |
High-throughput assay Directly measures lipoprotein concentrations and sizes |
High-complexity method Does not measure cholesterol or TG concentration in lipoprotein subfractions Issues regarding standardization against other methodologies |
| Lp(a) (immuno turbidimetric assays; Denka Seiken, Roche, Randox) | Measures circulating Lp(a) concentration using antibodies directed against apo(a) |
Automated immunoturbidimetric assay Assay standardization possible via traceable SRMs (WHO/IFCC) High-throughput/low-complexity method Assays using monoclonal antibodies that are kringle IV type 2 repeat insensitive allow for assay standardization (values expressed in nmol/L) |
Lp(a) cut off values for ASCVD risk unresolved (older guidelines suggest >30 mg/dL or >50 mg/dL) Global assay standardization issues related to general acceptance of nmol/L units of measurement Most currently available Lp(a) assays are affected by apo(a) size heterogeneity |
| hs-CRP (immuno turbidimetric assays) | Widely accepted surrogate marker for chronic low-grade inflammation |
Automated immunoturbidimetric/nephelometric assay Assay standardization via traceable SRMs (ERM/IFCC) High-throughput/low-complexity method Cost-effective |
Large intraindividual biological variability requires serial measurements for accurate risk classification |
Abbreviations: apo, apolipoprotein; ASCVD, atherosclerotic cardiovascular disease; ERM, European Reference Material; ES-DMA, electrospray-differential mobility analysis; FDA, U.S. Food and Drug Administration; HDL, high-density lipoprotein; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; IDL, intermediate-density lipoprotein; IFCC, International Federation of Clinical Chemistry and Laboratory Medicine; LDL, low-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; LDL-TG, low-density lipoprotein triglycerides; Lp(a), lipoprotein(a); NMR, nuclear magnetic resonance; RC, remnant cholesterol; RLP-C, remnant-like particle cholesterol; SRM, U.S. Standard Reference Material; TG, triglyceride; VLDL, very-low-density lipoprotein; WHO, World Health Organization.
Role of Triglycerides and Remnant Lipoproteins in Atherosclerotic Cardiovascular Disease
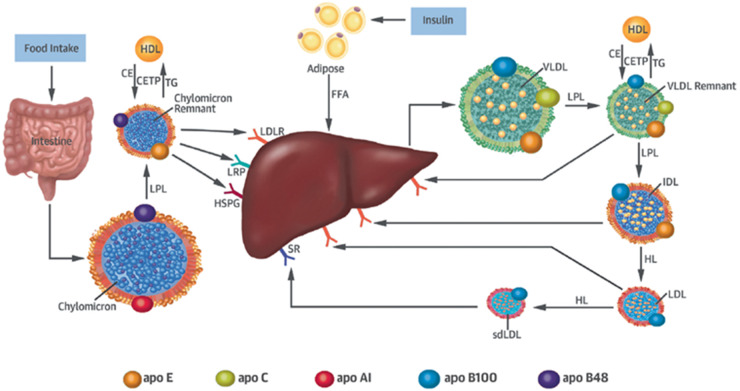
In recent years, growing evidence suggests that TGs and TGRLs may contribute to residual ASCVD risk. TGRLs are a heterogeneous population of lipoprotein particles that consist of remnant lipoproteins derived from either chylomicrons synthesized in the intestine or very-low-density lipoproteins (VLDL) synthesized in the liver (Fig. 1). Increased production and delayed catabolism of TGRLs lead to increased TG-enriched remnant lipoproteins, with increased concentrations of remnant-like particle cholesterol (RLP-C). In hypertriglyceridemia, cholesteryl ester transfer protein–mediated transfer of TGs from chylomicrons and VLDL to LDL and high-density lipoprotein (HDL) in exchange for cholesteryl esters from LDL and HDL leads to TG-enriched VLDL remnants, intermediate-density lipoprotein (IDL), LDL, and small dense LDL. Although all remnant particles have a relatively high TG content, it is not fully understood to what extent the TGs and cholesterol of remnant lipoproteins each contribute to ASCVD risk. The cholesterol content of remnant lipoproteins, broadly defined as remnant cholesterol (RC) and sometimes described as RLP-C, is highly atherogenic. Numerous studies have focused on the atherogenic potential of remnant lipoproteins and RLP-C (8, 9). However, few data describe the association between TGs in LDL (LDL-TG) and future ASCVD risk.
Fig. 1.
Remnant lipoprotein metabolism. Chylomicrons secreted from the intestine and very-low-density lipoprotein (VLDL) secreted from the liver are lipolyzed by lipoprotein lipase (LPL), leading to triglyceride-rich lipoprotein (TGRL) remnants. Chylomicron secretion is largely regulated by food intake, whereas VLDL secretion is controlled by insulin. Remnant particles undergo remodeling via the enzymatic action of cholesteryl ester transfer protein (CETP) with high-density lipoprotein (HDL), hepatic lipase (HL), and the exchange of soluble apolipoproteins such as E, C-I, C-II, and C-III. TGRL remnants are cleared from the circulation via receptor-mediated uptake involving the low-density lipoprotein (LDL) receptor (LDLR), LDL receptor–like protein (LRP), and heparan sulfate proteoglycans (HSPG). Chylomicron remnants and VLDL remnants compete for the same lipolytic pathway, a process mediated by apoE. While chylomicron remnant clearance may be mediated by LDLR, LRP, or HSPG, VLDL remnants are believed to be cleared predominantly via LDLR. Individuals with apoE2 isoforms have reduced remnant clearance and are postulated to have compensatory upregulation of cellular LDLR expression that may lead to decreased LDL-TG and LDL-C concentrations. The purported role of HL in the lipolytic conversion of IDL to LDL may at least partly explain why individuals with decreased HL activity due to genetic variation in the LIPC gene (e.g., rs 2070895) have high LDL-TG concentrations. Reproduced with permission from Saeed et al. (7).
Triglyceride-Rich Lipoproteins and Inflammation
Both LDL-TG (10) and RLP-C, but not cholesterol in LDL (LDL-C) (11), are related to systemic low-grade inflammation and vascular damage, and it has been proposed that the TG content of LDL may be a marker for delayed remnant particle catabolism (10). Consistent with these observations (7), individuals with increased LDL-TG and RLP-C concentrations also had increased concentrations of inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and white blood cell count (7), which may reflect an adverse impact on inflammation.
Laboratory Tests for Triglyceride-Rich Lipoprotein Measurements
It is generally believed that the circulating concentration of TGs serves as a surrogate measure for TGRLs. Plasma TG concentration predominantly reflects the TG content of hepatic-derived VLDL remnants (i.e., VLDL-TG, IDL-TG, and LDL-TG) in the fasting state but also includes the TG content of intestinal-derived chylomicron remnants in the nonfasting state or in the altered fasting state in hypertriglyceridemic individuals. Plasma TGs are most commonly measured using automated chemistry methods and involve the quantification of glycerol upon lipolytic conversion of TGs (12). Methods for the measurement of plasma TG concentrations are standardized through the distribution of commercially available standardized reference materials and are governed by clinical laboratory accreditation and certification agencies. A variety of methods have been used to measure the TG content of specific lipoprotein fractions, particularly LDL-TG content, to investigate the efficacy of these TGRL measures for ASCVD risk prediction. These methodologies generally use some form of conventional equilibrium density-gradient ultracentrifugation to isolate the lipoprotein fraction of interest (e.g., LDL), combined with an enzymatic method for TG quantification (10). More recently, an automated detergent-based homogeneous assay for LDL-TG was developed, which was validated against the standard sequential density ultracentrifugation method (13).
To determine the cholesterol content of TGRLs, a number of different methods have been described. The simplest method, which has been most commonly used, involves a formula for calculating RC by simply subtracting LDL-C and HDL-C from total cholesterol. Calculated RC is a more accurate estimate if directly measured LDL-C is used instead of LDL-C calculated based upon the Friedewald equation, as the Friedewald equation becomes unreliable when TG concentrations are 400 mg/dL or higher (14). It is important to note that calculated RC simply equals TG/5 (i.e., a fixed factor for the TG-to-VLDL-C ratio in mg/dL) when Friedewald-estimated LDL-C is used, and in this case it does not add clinical information beyond TG concentration (15). A newer method for estimating LDL-C using an adjustable factor for the TG-to-VLDL-C ratio based on TG and non–high-density lipoprotein cholesterol (non-HDL-C) concentrations (16) may more accurately estimate calculated RC, particularly in hypertriglyceridemic individuals. Laboratory methods for direct measurement of RC typically involve separation of remnant lipoprotein fractions based on density-gradient ultracentrifugation followed by measurement of their cholesterol content. Several studies, including the VLDL-3 Study, Jackson Heart Study, and Framingham Offspring Cohort Study, have used this method, in which RC was determined by the sum of cholesterol in the densest VLDL subfraction (VLDL3‐C) and IDL (9, 17). Other laboratory methods for direct measurement of RC are based on immunoseparation of apolipoprotein (apo) E–enriched remnant particles by removing lipoproteins containing apo A-I and some apo B-100–containing lipoproteins and subsequent determination of their cholesterol content (18). More recently, a fully automated homogeneous assay was developed for the direct measurement of RLP-C (19). Although the lack of standardization of methods for the measurements of TGRLs hampers their use in ASCVD risk assesment, the recent availability of validated fully automated procedures for measuring LDL-TG and RLP-C should facilitate widespread use in routine clinical chemistry laboratories and may aid in evaluating the use of TGRLs in ASCVD risk assesment.
Nuclear magnetic resonance (NMR) spectroscopy has been used since the early 1990s to obtain quantitative lipoprotein measurements. Although several research teams have applied NMR spectroscopy for lipoprotein quantification, mostly on smaller numbers of study subjects, the development of commercial NMR-based lipoprotein quantification methods pioneered by Otvos and colleagues at LipoScience (now LabCorp, Raleigh, NC) allowed for a broader application of this method in large population-based studies and clinical trials. Studies using NMR-derived lipoprotein quantification focus on characterization of both particle size and concentration rather than lipoprotein lipid composition. The concentration of each lipoprotein particle subfraction is calculated and particles are classified based on size as VLDL (VLDL-P, including 5 subclasses), LDL (LDL-P, including 3 subclasses), and HDL (HDL-P, including 3 subclasses). Several studies have shown that NMR-derived lipoprotein measures are associated with future ASCVD events (20, 21) and diabetes (22).
More recently, the Nightingale Health Company has developed a NMR spectroscopy method to measure >200 metabolic plasma biomarkers, including lipoprotein particle size and 14 lipoprotein subclasses. In addition, for each lipoprotein subclass, total, free, and esterified cholesterol, triglycerides, phospholipids, fatty acids, apolipoproteins, and particle concentration are measured (23). The Nightingale Health NMR methodology has been used to measure directly the contribution of cholesterol content in remnant lipoproteins to plasma total cholesterol concentrations in the general population (24). Lipoprotein particle concentrations in clinical laboratories are also measured using electrospray differential mobility analysis, also known as ion mobility, which can be used for the direct quantification of lipoprotein particles in the range of 17.2–540.0 Å, spanning from small, dense HDL to large buoyant VLDL and providing information on both particle size and concentration (25). The method is based on the principle that particles of a given size and uniform charge behave in a predictable manner when carried in a laminar air flow and subjected to an electrical field. The use of ion mobility for quantification of lipoprotein particle subfractions has been validated against other methods such as NMR and gradient density (vertical auto profile) ultracentrifugation; small dense LDL particles were consistently associated with greater coronary atherosclerosis progression independent of standard lipid measurements (26).
Although commercially available high-throughput NMR spectroscopy and ion mobility are promising methods for quantifying lipoprotein subclasses, including remnant lipoproteins, several obstacles prevent their broad application in routine clinical chemistry laboratories, including lack of standardization for lipoprotein subclasses, proprietary software, issues related to sensitivity (variability in 600- to 800-MHz NMR instruments used), high costs for instruments, and complexity of the methods.
Epidemiologic Studies of Triglycerides and Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease Risk
Large epidemiologic studies provide accumulating evidence for the role of TG in ASCVD risk (27). Few data are available on clinical utility of LDL-TG concentrations in ASCVD risk prediction, possibly because of the complexity of measuring LDL-TG (28). In a cross-sectional study of cases with stable coronary heart disease (CHD), in which LDL-TG was measured after fractionation of LDL by equilibrium density-gradient centrifugation, altered LDL metabolism characterized by high LDL-TG was correlated with prevalent CHD and systemic low-grade inflammation independent of LDL-C (10). The Atherosclerosis Risk in Communities (ARIC) study, was the first to demonstrate a significant association of LDL-TG concentrations with incident CHD and stroke in middle-aged adults (mean age 63 years) followed up to 16 years (7). Similarly, the Ludwigshafen Risk and Cardiovascular Health (LURIC) study in 3316 subjects (mean age 63 years) also showed that high concentrations of LDL-TG were associated with increased risk for cardiovascular mortality, independent of LDL-C, during approximately 10-year follow-up (29). In a study of older ARIC participants (mean age 76 years, half of whom were taking statin therapy, LDL-TG and RLP-C, markers of TGRL, were associated with TG, and higher LDL-TG concentration was associated with higher risk for CHD and ASCVD independent of traditional risk factors, indicating a role of LDL-TG in residual ASCVD risk in individuals on lipid-lowering therapy and suggesting additional targets for ASCVD risk reduction. In a biracial cohort from the Jackson Heart Study and Framingham Offspring Cohort Study, RLP-C was positively associated with incident CHD in unadjusted models (9). The ARIC study showed that high concentrations of small dense LDL-C, but not large buoyant LDL-C, were associated with increased risk of ASCVD even in individuals considered to be at low cardiovascular risk based on their LDL-C concentrations (30). The Copenhagen General Population Study recently showed that individuals with high concentrations of small dense LDL-C had higher risks for myocardial infarction (MI) and ASCVD (31). The Women’s Health Study recently found that TGRL-C was strongly associated with future MI and peripheral artery disease events, whereas small dense LDL-C was associated with MI alone (32). The authors suggested that the cholesterol content of TGRLs and small dense LDL might therefore influence atherogenesis with potentially different potency across vascular beds.
Genetic Studies of Triglycerides and Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease Risk
Genetic studies, in particular Mendelian randomization studies, have been used to investigate the causal relationship between a biomarker and disease risk because they capture the lifetime effect of an exposure without the possibility of reverse causality. Genetic studies provide additional evidence of the association of TGRL with ASCVD (33). Among genes involved in TG and TGRL metabolism, variants of those encoding lipoprotein lipase (LPL) and hepatic lipase (LIPC) and their inhibitors (apo C-III [APOC3], angiopoietin-like protein 3 [ANGPTL3; ANGPTL3]) have been associated with ASCVD risk.
A gain-of-function variant of LPL (rs328) was strongly associated with lower RLP-C and LDL-TG concentrations in ARIC (7) and has been associated with decreased TG and increased HDL-C concentrations (34) and reduced CHD (35). The LIPC variant rs2070895, in the promoter region of the gene, has been associated with decreased hepatic lipase activity (36) and increased LDL-TG concentrations (7). LIPC variants were associated with increased risk for ischemic heart disease risk in the Copenhagen City Heart Study (37), and complete deficiency of hepatic lipase leads to increases in RLP-C and LDL-TG (36).
The inhibitory effect of apo C-III on lipoprotein lipase leads to delayed clearance of TGRLs (38). APOC3 loss-of function variants are associated with decreased concentrations of TGs and small dense LDL-C, increased HDL-C concentration, and reductions in postprandial lipemia and CHD risk (39). In ARIC, APOC3 loss-of-function variants were associated with decreased RLP-C and LDL-TG concentrations.
ANGPTL3 also inhibits lipoprotein lipase and decreases clearance of VLDL-TG (40). Loss-of-function variants in ANGPTL3 have been associated with decreased TG and LDL-C concentrations and reduced ASCVD risk (41).
Evidence from Clinical Trials of Lowering Triglycerides and Triglyceride-Rich Lipoproteins to Reduce Atherosclerotic Cardiovascular Disease Risk
Eicosapentaenoic acid (EPA) reduces TG and RLP-C concentrations (42). In the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) (43), patients with high TGs and either ASCVD or high ASCVD risk who were treated with EPA had significant ASCVD risk reduction compared with placebo. In contrast, Statin Residual Risk Reduction with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH; NCT02104817), in which patients with ASCVD, high TG, and low HDL-C were treated with omega-3 carboxylic acids (Epanova; EPA + docosahexaenoic acid) or placebo, was stopped earlier than planned on the recommendation of an independent data monitoring committee because of low likelihood of benefit; trial results have not yet been published.
Fibrates activate peroxisome proliferator-activated receptor–α to downregulate APOC3 expression and upregulate LPL expression, which leads to reductions in TG and TGRL and improvements in LDL phenotype from small dense to more-buoyant particles (44). The ongoing Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides In Patients with Diabetes (PROMINENT; NCT03071692) is examining the clinical efficacy of pemafibrate added to statin therapy for ASCVD risk reduction in high-risk patients with diabetes, high TG concentrations, and low HDL-C concentrations.
Emerging therapies use gene silencing that targets APOC3 and ANGPTL3 as potential new approaches for treating hypertriglyceridemia (45). Antisense oligonucleotides in development inhibit APOC3 (APOCIII-LRx) (46) and hepatic ANGPTL3 (ANGPTL3-LRx) (47), and targeted delivery of small interfering RNAs against APOC3 (ARO-APOC3) (48) and ANGPTL3 (ARO-ANG3) (49) to hepatocytes provides efficient gene silencing. In early-phase clinical trials, both approaches to both targets demonstrated dramatic and durable TG reductions.
Role of Lipoprotein(a) in Atherosclerosis
Genetic, epidemiologic, and clinical studies have established that high concentrations of Lp(a) are associated with increased risk for ASCVD. Lp(a) was first described in 1963 and has an apo B lipoprotein moiety with covalent bonding to apo(a), which has homology to plasminogen (50). Apo(a) contains multiple repeating kringle motifs, and the differing number of kringle IV type 2 repeats in apo(a) leads to Lp(a) isoform size heterogeneity. There is a general inverse correlation between apo(a) isoform size and plasma Lp(a) concentration, which is believed to be largely due to less efficient secretion of the larger apo(a) isoforms from hepatocytes. Lp(a) is thought to have pleiotropic effects on ASCVD: promoting atherosclerosis due to its LDL moiety, promoting thrombosis due to the homology with plasminogen, and promoting vascular inflammation due to the oxidized phospholipids (51) which mediate arterial wall inflammation. Targeting Lp(a) may therefore be of incremental value beyond lowering LDL-C, even in individuals without increased LDL-C (52).
Evidence from Epidemiologic and Genetic Studies for a Causal Role of Liprotein(a) in Atherosclerotic Cardiovascular Disease
Numerous epidemiologic studies have shown a relationship between Lp(a) concentrations and MI, stroke, and aortic stenosis (53), and high Lp(a) concentrations have been shown to improve risk assessment (54). Lp(a) concentrations are primarily determined by genetics, and the ability to combine genomic data with epidemiologic data in Mendelian randomization studies has provided evidence that increased concentrations of Lp(a) are causally related to both ASCVD and aortic stenosis (55, 56). The accumulating evidence that Lp(a) is an independent ASCVD risk factor has led to the recent consensus statement from HEART UK, which provides recommendations for its measurement in clinical practice and reviews therapeutic strategies to reduce ASCVD risk in individuals with high Lp(a) concentrations (57).
Lipoprotein(a) Assay Standardization and Cutoffs for Atherosclerotic Cardiovascular Disease Risk Assessment
Measurement of Lp(a) is typically performed with autoanalyzers that use immunological methods usually based on polyclonal antibodies directed against apo(a). Historically, most labs have reported Lp(a) concentrations in mg/dL with target values of the assay calibrators in terms of total Lp(a) mass, which consists of apo(a), apo B, and lipids. The preferred approach is to report values in nmol/L using calibrators traceable to the World Health Organization/International Federation of Clinical Chemistry and Laboratory reference materials (58). There is no conclusive evidence for specific cutpoints based on age, sex, and race. Risk in primary prevention has been documented to increase at Lp(a) concentrations >30 mg/dL or >75 nmol/L in some studies, but the most recent guidelines in the United States and Europe consider a concentration ≥50 mg/dL or ≥125 nmol/L to be at high risk (2, 59).
Efficacy of Lipid-Lowering Therapies in Patients with High Lipoprotein(a) Concentrations
In the past, niacin was frequently used in patients with high concentrations of Lp(a) because it had been shown to reduce Lp(a) by 20%–25%. However, as recently reviewed, both the AIM-HIGH and HPS2-THRIVE trials failed to show any benefit of adding niacin to statin therapy for reducing ASCVD events but showed increased risk for adverse events such as new-onset diabetes and infections (60). Although statins are highly effective therapies for reducing LDL-C and apo B concentrations and reducing ASCVD events, statins do not lower Lp(a), and high-efficacy statin doses may lead to increases in Lp(a) concentrations. Statin therapy has been shown to reduce ASCVD events in patients with high concentrations of Lp(a) in both primary and secondary prevention studies (52, 61).
The approved proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab and alirocumab, reduce Lp(a) concentration by 23%–27% (5, 62). In patients with higher baseline Lp(a), PCSK9 inhibitor provided greater absolute Lp(a) reduction and greater absolute risk reduction for cardiovascular events (63, 64). In a subgroup analysis from Odyssey Outcomes, Lp(a) reduction with alirocumab independently contributed to ASCVD reduction beyond the effects of reductions in LDL-C and non-HDL-C (63).
Emerging Therapies to Reduce High Lipoprotein(a) Concentrations
Because of the limited efficacy of current therapies to lower Lp(a) concentrations, novel approaches are in development that use newer technologies to lower Lp(a) by inhibiting synthesis of apo(a). APO(a)-LRx is a GalNAc-conjugated second-generation antisense oligonucleotide that targets apo(a) by reducing the synthesis of apo(a) in the liver. Dose-dependent and sustained Lp(a) reductions of up to 80% were observed in a phase 2 clinical trial, in which the agent was well tolerated, with the major side effect being increased frequency of injection-site erythema reported in those receiving drug compared with placebo (65). The Assessing the Impact of Lipoprotein (a) Lowering With TQJ230 on Major Cardiovascular Events in Patients With CVD (Lp(a)HORIZON; NCT04023552) is a phase 3 outcomes trial enrolling 7860 high-risk secondary prevention patients with Lp(a) concentrations ≥70 mg/dL.
AMG 890 (formerly ARO-LPA) is a GalNAc-conjugated small interfering RNA designed to inhibit Lp(a) production. A phase 1 single ascending dose clinical trial to evaluate the safety, pharmacokinetics, and pharmacodynamics in patients with high Lp(a) (n = 80) has been completed (NCT03626662), and a phase 2 clinical trial to determine dosing is ongoing in patients with high Lp(a) (n = 240; NCT04270760).
Inflammation and Residual Atherosclerotic Cardiovascular Disease Risk with Low Concentrations of Low-Density Lipoprotein Cholesterol
Although numerous biochemical measures related to inflammation have been shown to be associated with increased risk for ASCVD in both epidemiologic studies and clinical trials, hs-CRP is the most widely studied and has the greatest evidence as a useful marker in patients who have been treated to low concentrations of LDL-C. Although hs-CRP has been a useful marker of increased risk, Mendelian randomization studies investigating the association of genetic variants in the CRP gene with risk for CHD suggest that CRP concentration itself is unlikely to be a causal factor in CHD (66). Other Mendelian randomization studies have demonstrated a causal relationship between genetic variation in cytokine signaling, such as interleukin-6 receptor and the risk for CHD (67).
In a post hoc analysis of 10 major statin trials (68) and a prospective analysis the JUPITER study, the greatest benefit of therapy was observed in those who achieved the lowest concentrations of LDL-C and hs-CRP (69). This has also been observed when nonstatin therapies, such as ezetimibe (70) or PCSK9 inhibitors, were added to statin therapy (71). A recent prespecified post hoc secondary analysis of the Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High Risk for Vascular Outcomes (ACCELERATE) trial showed that increased Lp(a) concentrations with high-risk vascular disease are related to cardiovascular death, MI, and stroke when hs-CRP concentrations are ≥2 mg/L but not <2 mg/L, suggesting a potential benefit of lowering Lp(a) in patients with residual systemic inflammation despite optimal medical therapy (72).
Canakinumab is a monoclonal antibody against interleukin-1β. In CANTOS, a phase 3 randomized, placebo-controlled clinical trial in 10 061 participants with a history of MI and hs-CRP concentrations >2.0 mg/L, canakinumab 150 mg resulted in a 15% reduction in the primary composite endpoint of MI, stroke, and cardiovascular death (hazard ratio [HR] 0.85, 95% confidence interval [CI] 0.74–0.98) compared with placebo (73). Key secondary endpoints including MI, unstable angina requiring urgent revascularization, and any coronary revascularizations were also reduced. There was no significant reduction in cardiovascular mortality or stroke. CANTOS was a landmark trial because it was the first to show that a therapy specifically targeting inflammation provided clinical benefit independent of lowering lipoproteins. Although canakinumab therapy was associated with increased risk for fatal infection, it was also associated with reduced risk for death from cancer, with a reduction in lung cancer. Furthermore, participants who achieved hs-CRP concentrations <2 mg/L after a single dose of canakinumab had greater reduction in cardiovascular outcomes (by 25%) and all-cause mortality (by 31%) (74). These findings have important clinical implications, as they may help identify a subset of patients in whom canakinumab or other anti-inflammatory therapy may prove particularly clinically effective.
Beyond residual cholesterol risk, these results support the concept of residual inflammatory risk. Residual inflammatory risk is not uncommon; in both Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE IT) (75) and IMPROVE-IT (4), almost one-third of statin-treated patients had hs-CRP >2 mg/L. More than one-third of the patients in the PCSK9 mAb cardiovascular outcomes trials met the eligibility criteria for CANTOS despite achieving very low LDL-C (71).
Summary/Conclusions
Accumulating evidence from epidemiologic and genetic studies, as well as randomized clinical trials, suggest that remnant lipoproteins, Lp(a), and inflammation are causally related to risk of ASCVD in individuals already treated with statin therapy. Novel therapies to reduce circulating concentrations of TGRL, Lp(a), and inflammatory markers in these individuals show promising results, although their efficacy in reducing residual cardiovascular risk is still under investigation with several clinical trials currently ongoing. Issues related to the lack of standardization of various clinical tests to measure TGRLs and Lp(a) remain an obstacle to their widespread adoption in clinical practice.
Nonstandard Abbreviations:
- AIM-HIGH
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High
- Triglycerides
Impact on Global Health Outcomes;
- ANGPTL3
angiopoietin-like protein 3;
- apo
apolipoprotein;
- ARIC
Atherosclerosis Risk in Communities;
- ASCVD
atherosclerotic cardiovascular disease;
- CHD
coronary heart disease;
- EPA
eicosapentaenoic acid;
- HDL
high-density lipoprotein;
- HPS2-THRIVE
Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events;
- hs-CRP
high-sensitivity C-reactive protein;
- IDL
intermediate-density lipoprotein;
- LDL-C
low-density lipoprotein cholesterol;
- LDL-TG
low-density lipoprotein triglycerides;
- Lp(a)
lipoprotein (a);
- MI
myocardial infarction;
- NMR
nuclear magnetic resonance;
- non-HDL-C
non–high-density lipoprotein cholesterol;
- PCSK9
proprotein convertase subtilisin/kexin type 9;
- PROMINENT
Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides In Patients with Diabetes;
- RC
remnant cholesterol;
- REDUCE-IT
Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial;
- RLP-C
remnant-like particle cholesterol;
- STRENGTH
Statin Residual Risk Reduction with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia;
- TG
triglyceride;
- TGRL
triglyceride-rich lipoprotein;
- VLDL
very-low-density lipoproteins
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
R. C. Hoogeveen, Denka Seiken; C. M. Ballantyne, Abbott Diagnostics, Akcea, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Corvidia, Denka Seiken, Esperion, Intercept, Janssen, Matinas BioPharma Inc., Merck, Novartis, Novo Nordisk, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
R. C. Hoogeveen has received research grants (to institution) from Denka Seiken; C. M. Ballantyne has received National Institutes of Health grant R01HL134320 and has received grants or research support (to institution) from Abbott Diagnostic, Akcea, Amgen, Esperion, Novartis, Regeneron, and Roche Diagnostics.
Expert Testimony
None declared.
Patents
None declared.
References
- 1. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–50. [DOI] [PubMed] [Google Scholar]
- 2.Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. [DOI] [PubMed] [Google Scholar]
- 3. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. [DOI] [PubMed] [Google Scholar]
- 5. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22. [DOI] [PubMed] [Google Scholar]
- 6. Langlois MR, Nordestgaard BG, Langsted A, Chapman MJ, Aakre KM, Baum H, et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from EAS and EFLM. Clin Chem Lab Med 2020;58:496–517. [DOI] [PubMed] [Google Scholar]
- 7. Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, et al. Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol 2018;72:156–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG.. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 2013;61:427–36. [DOI] [PubMed] [Google Scholar]
- 9. Joshi PH, Khokhar AA, Massaro JM, Lirette ST, Griswold ME, Martin SS.. Remnant lipoprotein cholesterol and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. J Am Heart Assoc 2016;5:e002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MäRz W, Scharnagl H, Winkler K, Tiran A, Nauck M, Boehm BO, et al. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health study. Circulation 2004;110:3068–74. [DOI] [PubMed] [Google Scholar]
- 11. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG.. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013;128:1298–309. [DOI] [PubMed] [Google Scholar]
- 12. Nägele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, et al. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem 1984;22:165–74. [DOI] [PubMed] [Google Scholar]
- 13. Ito Y, Ohta M, Ikezaki H, Hirao Y, Machida A, Schaefer EJ, et al. Development and population results of a fully automated homogeneous assay for LDL triglyceride. Jrnl App Lab Med 2018;2:746–56. [DOI] [PubMed] [Google Scholar]
- 14. Martin SS, Blaha MJ, Elshazly MB, Brinton EA, Toth PP, McEvoy JW, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol 2013;62:732–9. [DOI] [PubMed] [Google Scholar]
- 15. Langlois MR, Chapman MJ, Cobbaert C, Mora S, Remaley AT, Ros E, et al. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and very low concentrations of LDL cholesterol. A consensus statement from EAS and EFLM. Clin Chem 2018;64:1006–33. [DOI] [PubMed] [Google Scholar]
- 16. Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013;310:2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lupton JR, Faridi KF, Martin SS, Sharma S, Kulkarni K, Jones SR, et al. Deficient serum 25-hydroxyvitamin D is associated with an atherogenic lipid profile: the Very Large Database of Lipids (VLDL-3) study. J Clin Lipidol 2016;10:72–81.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakajima K, Saito T, Tamura A, Suzuki M, Nakano T, Adachi M, et al. Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apo B-100 and anti apo A-I immunoaffinity mixed gels. Clin Chim Acta 1993;223:53–71. [DOI] [PubMed] [Google Scholar]
- 19. Hirao Y, Nakajima K, Machida T, Murakami M, Ito Y.. Development of a novel homogeneous assay for remnant lipoprotein particle cholesterol. J Appl Lab Med 2018;3:26–36. [DOI] [PubMed] [Google Scholar]
- 20. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM.. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, et al. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study–implications for LDL management. J Clin Lipidol 2007;1:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dugani SB, Akinkuolie AO, Paynter N, Glynn RJ, Ridker PM, Mora S.. Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol 2016;1:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soininen P, Kangas AJ, Wurtz P, Tukiainen T, Tynkkynen T, Laatikainen R, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 2009;134:1781–5. [DOI] [PubMed] [Google Scholar]
- 24. Balling M, Langsted A, Afzal S, Varbo A, Davey Smith G, Nordestgaard BG.. A third of nonfasting plasma cholesterol is in remnant lipoproteins: lipoprotein subclass profiling in 9293 individuals. Atherosclerosis 2019;286:97–104. [DOI] [PubMed] [Google Scholar]
- 25. Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, et al. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem 2008;54:1307–16. [DOI] [PubMed] [Google Scholar]
- 26. Williams PT, Zhao XQ, Marcovina SM, Otvos JD, Brown BG, Krauss RM.. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis 2014;233:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A.. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 28. Albers JJ, Slee A, Fleg JL, O’Brien KD, Marcovina SM.. Relationship of baseline HDL subclasses, small dense LDL and LDL triglyceride to cardiovascular events in the AIM-HIGH clinical trial. Atherosclerosis 2016;251:454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silbernagel G, Scharnagl H, Kleber ME, Delgado G, Stojakovic T, Laaksonen R, et al. LDL triglycerides, hepatic lipase activity, and coronary artery disease: an epidemiologic and Mendelian randomization study. Atherosclerosis 2019;282:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol 2014;34:1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balling M, Nordestgaard BG, Langsted A, Varbo A, Kamstrup PR, Afzal S.. Small dense low-density lipoprotein cholesterol predicts atherosclerotic cardiovascular disease in the Copenhagen General Population Study. J Am Coll Cardiol 2020;75:2873–5. [DOI] [PubMed] [Google Scholar]
- 32. Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD.. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol 2020;75:2122–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013;45:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bentley AR, Chen G, Shriner D, Doumatey AP, Zhou J, Huang H, et al. Gene-based sequencing identifies lipid-influencing variants with ethnicity-specific effects in African Americans. PLoS Genet 2014;10:e1004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khera AV, Won H-H, Peloso GM, O’Dushlaine C, Liu D, Stitziel NO, et al. Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA 2017;317:937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deeb SS, Zambon A, Carr MC, Ayyobi AF, Brunzell JD.. Hepatic lipase and dyslipidemia: interactions among genetic variants, obesity, gender, and diet. J Lipid Res 2003;44:1279–86. [DOI] [PubMed] [Google Scholar]
- 37. Andersen RV, Wittrup HH, Tybjærg-Hansen A, Steffensen R, Schnohr P, Nordestgaard BG.. Hepatic lipase mutations, elevated high-density lipoprotein cholesterol, and increased risk of ischemic heart disease: the Copenhagen City Heart Study. J Am Coll Cardiol 2003;41:1972–82. [DOI] [PubMed] [Google Scholar]
- 38. Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008;322:1702–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng C, Khoo C, Furtado J, Sacks FM.. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 2010;121:1722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shimizugawa T, Ono M, Shimamura M, Yoshida K, Ando Y, Koishi R, et al. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J Biol Chem 2002;277:33742–8. [DOI] [PubMed] [Google Scholar]
- 41. Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol 2017;69:2054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ballantyne CM, Bays HE, Philip S, Doyle RT Jr, Braeckman RA, Stirtan WG, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis 2016;253:81–7. [DOI] [PubMed] [Google Scholar]
- 43. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 44. Chapman MJ. Fibrates in 2003: therapeutic action in atherogenic dyslipidaemia and future perspectives. Atherosclerosis 2003;171:1–13. [DOI] [PubMed] [Google Scholar]
- 45. Nordestgaard BG, Nicholls SJ, Langsted A, Ray KK, Tybjærg-Hansen A.. Advances in lipid-lowering therapy through gene-silencing technologies. Nat Rev Cardiol 2018;15:261–72. [DOI] [PubMed] [Google Scholar]
- 46. Alexander VJ, Xia S, Hurh E, Hughes SG, O’Dea L, Geary RS, et al. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J 2019;40:2785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–32. [DOI] [PubMed] [Google Scholar]
- 48. Ballantyne CM. RNA Interference Targeting Apolipoprotein C-III Results in Deep and Prolonged Reductions in Plasma Triglycerides American Heart Assocation Scientific Sessions 2020. Philadelphia, USA. [Google Scholar]
- 49. Watts GF. RNA Interference Targeting Hepatic Angiopoietin-Like Protein 3 Results in Prolonged Reductions in Plasma Triglycerides and LDL-C in Human Subjects. American Heart Association Scientific Sessions; 2020. Philadelphia, USA. [Google Scholar]
- 50. Berg K. A new serum type system in man–the Lp system. Acta Pathol Microbiol Scand 2009;59:369–82. [DOI] [PubMed] [Google Scholar]
- 51. Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res 2008;49:2230–9. [DOI] [PubMed] [Google Scholar]
- 52. Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation 2014;129:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG.. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol 2013;61:1146–56. [DOI] [PubMed] [Google Scholar]
- 55. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG.. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331–9. [DOI] [PubMed] [Google Scholar]
- 56. Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG.. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol 2014;63:470–7. [DOI] [PubMed] [Google Scholar]
- 57. Cegla J, Neely RDG, France M, Ferns G, Byrne CD, Halcox J, et al. HEART UK consensus statement on lipoprotein(a): a call to action. Atherosclerosis 2019;291:62–70. [DOI] [PubMed] [Google Scholar]
- 58. Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN, Koschinsky ML, Marcovina SM, et al. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol 2018;71:177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–209. [DOI] [PubMed] [Google Scholar]
- 60. Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374–92. [DOI] [PubMed] [Google Scholar]
- 61. Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet 2018;392:1311–20. [DOI] [PubMed] [Google Scholar]
- 62. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–107. [DOI] [PubMed] [Google Scholar]
- 63. Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol 2020;75:133–44. [DOI] [PubMed] [Google Scholar]
- 64. O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 2019;139:1483–92. [DOI] [PubMed] [Google Scholar]
- 65. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 2020;382:244–55. [DOI] [PubMed] [Google Scholar]
- 66.C Reactive Protein Coronary Heart Disease Genetics Collaboration. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ 2011;342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Interleukin 6 Receptor Mendelian Randomisation Analysis Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379:1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol 2013;168:5126–34. [DOI] [PubMed] [Google Scholar]
- 69. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 70. Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation 2015;132:1224–33. [DOI] [PubMed] [Google Scholar]
- 71. Bohula EA, Giugliano RP, Leiter LA, Verma S, Park JG, Sever PS, et al. Inflammatory and cholesterol risk in the FOURIER trial. Circulation 2018;138:131–40. [DOI] [PubMed] [Google Scholar]
- 72. Puri R, Nissen SE, Arsenault BJ, St John J, Riesmeyer JS, Ruotolo G, et al. Effect of C-reactive protein on lipoprotein(a)-associated cardiovascular risk in optimally treated patients with high-risk vascular disease: a prespecified secondary analysis of the ACCELERATE Trial. JAMA Cardiol 2020;5:1136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 74. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018;391:319–28. [DOI] [PubMed] [Google Scholar]
- 75. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Comparison of intensive and moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. [DOI] [PubMed] [Google Scholar]