Abstract
Background
Type 2 myocardial infarction (T2MI) is frequently encountered in clinical practice and associated with adverse outcomes.
Content
T2MI occurs most frequently due to noncoronary etiologies that alter myocardial oxygen supply and/or demand. The diagnosis of T2MI is often confused with acute nonischemic myocardial injury, in part because of difficulties in delineating the nature of symptoms and misunderstandings about disease categorization. The use of objective features of myocardial ischemia using electrocardiographic (ECG) or imaging abnormalities may facilitate more precise T2MI diagnosis. High-sensitivity cardiac troponin (hs-cTn) assays allow rapid MI diagnosis and risk stratification, yet neither maximum nor delta values facilitate differentiation of T2MI from T1MI. Several investigational biomarkers have been evaluated for T2MI, but none have robust data. There is interest in evaluating risk profiles among patients with T2MI. Clinically, the magnitude of maximum and delta cTn values as well as the presence and magnitude of ischemia on ECG or imaging is used to indicate disease severity. Scoring systems such as GRACE, TIMI, and TARRACO have been evaluated, but all have limited to modest performance, with substantial variation in time intervals used for risk-assessment and endpoints used.
Summary
The diagnosis of T2MI requires biomarker evidence of acute myocardial injury and clear clinical evidence of acute myocardial ischemia without atherothrombosis. T2MIs are most often caused by noncoronary etiologies that alter myocardial oxygen supply and/or demand. They are increasingly encountered in clinical practice and associated with poor short- and long-term outcomes. Clinicians require novel biomarker or imaging approaches to facilitate diagnosis and risk-stratification.
Acute myocardial infarction (MI) is a clinical syndrome that requires biomarker evidence of acute myocardial injury and clinical evidence of acute myocardial ischemia (1). While the term acute MI is often equated with acute coronary atherothrombosis, it has long been recognized that acute MI can occur in the absence of atherothrombosis (2). Specific nomenclature for the latter was formulated in 2007 when the Task Force for the Universal Definition of Myocardial Infarction (UDMI) introduced the term type 2 myocardial infarction (T2MI) (3). In this document, we summarize evolving concepts about T2MI, particularly in relation to its diagnosis and risk stratification. We will not expand on issues specific to management, for which there is paucity of data.
Definition, Etiologies, and Epidemiology
T2MI occurs most often secondary to another illness or trigger. Tachyarrhythmias are often identified as the most common mechanism, but profound hypoxia and hypotension are also commonly noted (4–6). T2MI may also occur due to acute coronary pathology including coronary spasm, embolism, spontaneous coronary artery dissection (SCAD), aortic dissection with coronary involvement, and endothelial dysfunction; but the frequency of these conditions is much lower, and as compared to noncoronary T2MI, such diagnoses are often made after coronary angiography is performed in patients with initially suspected type 1 MI (T1MI) (7).
The T2MI definition has broadly remained the same throughout iterations of the UDMI (online Supplemental Table 1) (1, 3, 8). While nonatherothrombotic T2MI and atherothrombotic T1MI have distinct pathophysiology, both diagnoses require clinical evidence of acute myocardial ischemia. Notably, despite recognizing that several acute nonatherothrombotic coronary processes can cause T2MI, the Fourth UDMI did not explicitly include coronary angiography as a criterion to support the diagnosis of T2MI (1) (online Supplemental Table 2). Coronary angiography is not mandatory to establish any MI diagnosis (1). Most studies addressing T2MI indicate that most patients do not undergo angiography (7). If performed, however, the information obtained may facilitate diagnosis. It helps to exclude acute atherosclerotic plaque disruption and atherothrombosis, with greater certainty if intracoronary imaging is available. It also helps with the diagnosis of other acute coronary pathologies such as spasm, embolism, SCAD, or endothelial dysfunction.
While some have argued the T2MI definition is confusing and have challenged the present UDMI nomenclature (9), the fact that acute MI can occur without acute coronary atherothrombosis is unequivocal (2). The incidence of T2MI varies widely (1.6% to 74% of MIs) (7). This heterogeneity is in part due to variations in the approach to cTn testing that influence the study population (10). Investigations addressing only those with chest discomfort or that apply rigorous exclusion criteria are likely to report a higher incidence of T1MI, whereas studies addressing all-comers report that T2MI is more common. Variations in adjudication processes and definitions, such as the use of strict supply-demand mismatch criteria (4), differences in cardiac troponin assays and thresholds, and study design influence the reported T2MI incidence. Critically, epidemiological data indicates an evolution in the type of MI occurring in the community, with the incidence of T2MI now similar to T1MI (11). The incidence of T2MI is expected to further increase with the transition to hs-cTn assays (12, 13).
Distinguishing T2MI from Myocardial Injury: The Case for Objective Ischemia
The clinical context and initial diagnostic evaluation including history, 12-lead electrocardiogram (ECG), and serial cTn measurements are often sufficient to inform whether the likely reason for cTn increases above the 99th percentile upper-reference limit (URL) is due to nonischemic myocardial injury (acute or chronic), T1MI, or T2MI. Distinguishing acute non-ischemic myocardial injury from T1MI or T2MI, however, is often challenging in certain clinical circumstances, particularly in those with critical illness, postoperative, or with chronic comorbidities such as those with established coronary artery disease or ischemic cardiomyopathy.
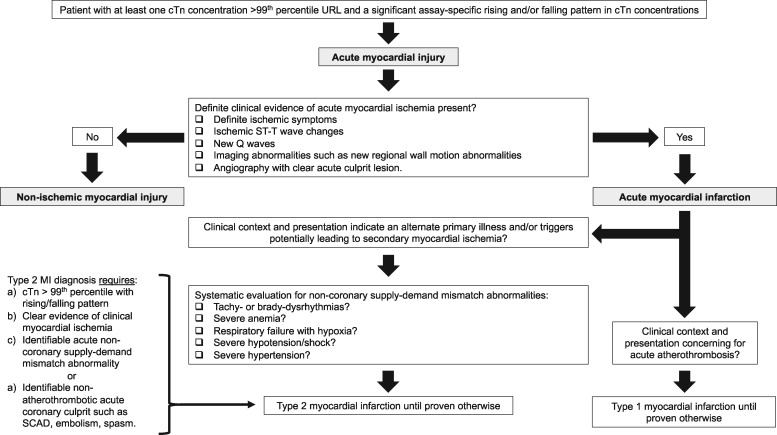
In the absence of tools that reliably differentiate T2MI from T1MI or myocardial injury, present ‘best’ practice relies on delineating whether clear, overt evidence of acute clinical myocardial ischemia is present or not. Those without clear ischemia are categorized as having nonischemic myocardial injury. Those with ischemia are categorized as acute MI and the distinction between T1MI or T2MI is largely based on evaluation of the clinical context and patient’s presentation, including a systematic evaluation of whether clear evidence of clinical supply-demand mismatch abnormalities are present (Fig. 1).
Fig. 1.
Type 2 myocardial infarction flowchart. The diagnosis of type 2 myocardial infarction applies to those with at least one cTn concentration above the 99th percentile in whom there is presence of a rising and/or falling pattern in cTn concentrations, clinical evidence of acute myocardial ischemia, and either a clear identifiable acute noncoronary supply-demand mismatch abnormality or a nonatherothrombotic acute coronary culprit.
For T2MI there are no prospective studies that specifically inform on the role and timing of noninvasive and invasive imaging. However, in patients with acute myocardial injury, particularly those in whom symptoms and ECG findings are equivocal, noninvasive imaging can facilitate diagnosis. Echocardiography can help identify regional wall motion abnormalities, as well as evaluate left ventricular function. Cardiac magnetic resonance imaging can help assess for acute MI but also evaluate for other common mimickers of acute MI such as myocarditis. For those with large cTn increases and/or deltas, significant symptoms, or concerning ECG changes, consideration of more timely cardiac evaluation is reasonable. The role for coronary angiography is being evaluated in the Appropriateness of Coronary investigation in myocardial injury and Type 2 myocardial infarction (ACT-2) (ACTRN12618000378224) trial. If there is a high pre-test probability for obstructive coronary artery disease (CAD) and there are high-risk features such as marked cTn increases/deltas or significant imaging or ECG abnormalities, coronary angiography should be considered in selected patients without contraindications.
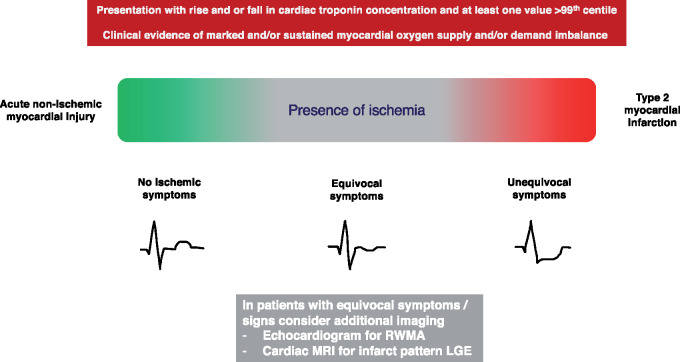
Challenges in MI classification can be amplified in research studies that rely on patient records and available clinical data for diagnosis. The agreement rates reported in T2MI studies involving adjudicators familiar with the UDMI illustrate this well. Gard et al. evaluated 1159 patients with acute MI and noted that the two initial reviewers agreed in 86% of T1MI diagnoses, but only 64% of T2MIs (14). Likewise, data from UTROPIA (Use of TROPonin I in ACS) demonstrated excellent agreement rates of 95% for T1MI, but moderate agreement of 72% and 71% for T2MI and nonischemic myocardial injury, respectively (15). The latter data suggest that a potential important source of disagreement stems from the interpretation of ischemic symptoms (15). This is important because the diagnosis of MI may be established using symptoms alone, without the need for additional corroborative evidence of ischemia (1). In the context of biomarker evidence of acute myocardial injury, the presence of unequivocal ischemic symptoms is sufficient to support the diagnosis of acute MI. For patients with T2MI, however, the evaluation of symptoms is subjective and can be much more challenging. Patients with T2MI due to conditions such as arrhythmias, anemia, or respiratory failure can manifest various symptoms, such as dyspnea or atypical chest discomfort, which may be due to the primary disease process rather than myocardial ischemia. Further, some of these patients may have other acute or chronic respiratory illnesses such as pneumonia or chronic obstructive pulmonary disease that further challenge the interpretation of symptoms. Given these limitations, when in doubt and if clinically indicated, clinicians should consider additional evaluation, for example, using imaging to determine if imaging abnormalities consistent with acute infarction are present (Fig. 2).
Fig. 2.
Clinical distinction of acute nonischemic myocardial injury and type 2 myocardial infarction. Clinical distinction between acute nonischemic myocardial injury and type 2 myocardial infarction is based upon the presence of myocardial ischemia. Such distinction is challenging in practice and some patients will have equivocal symptoms or signs, in which case further noninvasive testing may be of benefit. RWMA: regional wall motion abnormality. MRI: magnetic resonance imaging. LGE: late gadolinium enhancement.
Cardiac Troponin: Maximum and Delta Troponin
The preferred cardiac biomarker for the diagnosis of acute myocardial infarction is cardiac troponin (cTn) (1). Similar threshold and delta biomarker criteria are needed to support either a T1MI or T2MI diagnosis (1). While high-sensitivity (hs) cTn assays permit more rapid diagnosis and risk-stratification (16), they cannot distinguish MI subtypes based on their underlying pathophysiology (6, 17, 18). Several studies indicate that patients with T1MI have higher maximum cTn concentrations and larger deltas than patients with T2MI (4–6, 11, 12). The cTn distributions, however, overlap sufficiently that such information cannot be used to reliably and consistently determine whether a patient has a T1MI or T2MI (6, 17, 18).
Investigational Approaches for Prediction and MI Differentiation
Natriuretic Peptides
Data suggest that natriuretic peptides (NP) are predictive of T2MI. In the Catheter Sampled Blood Archive in Cardiovascular Diseases (CASABLANCA) study (19), Gaggin et al. measured N-terminal pro-B-type natriuretic peptide (NT-proBNP) in patients undergoing coronary or peripheral angiography that were followed for incident T1MI and T2MI. In addition to higher baseline hs-cTnI, patients with incident T2MI had higher NT-proBNP than patients without T2MI. As compared to T1MI, those with incident T2MI also had higher baseline NT-ProBNP. Multivariable Cox regression analyses identified NT-proBNP as a significant predictor of first T2MI.
For diagnostic purposes, studies addressing NP in patients with T1MI and T2MI show conflicting findings (5, 6, 20, 21). Paiva et al. showed that patients with T2MI had significantly higher NT-proBNP concentrations than T1MI (20). Likewise, Meigher and colleagues showed higher BNP in T2MI than T1MI (21). In UTROPIA, no significant differences were observed between T1MI and T2MI but those with nonischemic myocardial injury had much higher concentrations (5). Overall, in isolation, NP concentrations overlap sufficiently between T1MI and T2MI patients, with similar or higher increases also observed in those with nonischemic myocardial injury, that the information does not facilitate T2MI diagnosis.
A small analysis from the Rapid Evaluation of ACuTe myocardial infarction in the United States (REACTION-US) study addressed the use of a NT-proBNP/5th Gen cTnT ratio among 18 T2MI patients and showed that the ratio was higher at all time points in patients with T2MI than T1MI (22). The analyses, however, involved few T2MI patients and specific etiologies were not recorded, with modest diagnostic performance and wide confidence intervals (22). A potential important limitation to all such studies is that investigations addressing the UDMI often inappropriately include acute heart failure as T2MI (23). Unless unequivocal overt evidence of myocardial ischemia is present to qualify as a T1MI or T2MI, patients with heart failure are best categorized as having acute or chronic myocardial injury (7).
Inflammatory Markers: C-Reactive Protein
T2MI may be a higher inflammatory state than T1MI. Data from the DEF-AMI (Consequences of the universal 2007 DEFinition of Acute Myocardial Infarction studied in a Danish consecutive hospital population) study, for which infection was categorized as nonischemic myocardial injury, showed that patients with strictly defined T2MI had significantly higher median C-reactive protein (CRP) concentrations than patients with T1MI (21 vs. 5 ng/L, P < 0.001) (4). These concentrations were similar to those observed for myocardial injury (28 ng/L) (24). Bormann et al. showed significant increases in CRP in patients with T2MI (n = 55) as compared to those with T1MI (n = 199) (0.6 vs. 0.3, P = 0.02) (25). Likewise, Putot et al. found that patients with T2MI were more likely to have CRP concentrations >3 mg/L (88% vs. 62%, P < 0.001) (26). In this latter study, CRP and cTnI (Siemens Dimension Vista) were compared to a CRP/cTnI ratio among patients with a history of coronary artery disease (CAD) and the ratio had the best predictive values for T2MI diagnosis with an AUC of 0.84 as compared to 0.74 for CRP and 0.77 for cTnI, with higher ratio cutoffs associated with a higher specificity for T2MI (26). In patients with CAD, CRP >3 mg/L was the strongest factor associated with T2MI on multivariate analysis (OR 3.53, 95% CI 2.17-5.75, P < 0.001). For this dataset, however, 36% of all T2MIs had concomitant infection (27), mostly pulmonary source, which could influence results. More data are needed on whether an increased inflammatory response exists in T2MI patients without infection.
Myeloid-Related Protein 8/14
Myeloid-related protein 8/14 complex (MRP 8/14) is a marker of phagocyte activation that is involved in plaque destabilization (28). It was evaluated among 254 patients, including 55 T2MIs, in which higher MRP-8/14 concentrations were observed at both 0- and 3-h, as well as a higher delta, in T2MI than T1MI (25). This study evaluated the diagnostic potential of MRP 8/14 and hs-cTnI (Abbott) to identify T2MI and showed poor area under the receiver operating characteristic (AUROC) for hs-cTnI (0.69) and MRP-8/14 (0.62), with a small improvement using both together (0.72). Independent of hs-cTnI, MRP-8/14 was shown to be predictive of T2MI (OR 2.15, 95% CI 1.2-4.2, P = 0.01). MRP-8/14 has also been evaluated as part of a ‘plaque disruption index’ along with myeloperoxidase, high-sensitivity interleukin-6, and pregnancy-associated plasma protein–A to identify patients with T1MI (29), for which reason it has been suggested that it may facilitate T1MI/T2MI distinction, but no data exist to support such.
Other Approaches
The use of CK-MB is not advised in contemporary practice because of its inferior analytical and diagnostic performance and lack of cardiac-specificity, as compared to cTn assays (30). Its clinical use, however, persists. In this context, Pandey et al. evaluated the use of a contemporary cTnT (Roche 4th Gen) and CK-MB ‘peak’ ratio to distinguish T1MI from T2MI. The study cohort included 103 T2MIs, amongst which 25% and 24% were thought to be from decompensated heart failure and sepsis, respectively (23), conditions that are often best classified as nonischemic myocardial injury rather than T2MI unless unequivocal evidence of overt myocardial ischemia exists (7). A higher but not statistically significant increase in the ratio of peak cTnT to peak CK-MB was observed for T2MI as compared to T1MI (0.09 vs. 0.06, P = 0.06). This study has several limitations, including reservations about T2MI adjudication, use of the older and less sensitive cTnT assays, as well as of CK-MB that has analytical and diagnostic limitations (30), and the advocacy for trending until ‘peak’ which is not advised nor cost-effective with the use of hs-cTn assays.
DeFilippis et al. have probed various biomarkers and metabolites for T1MI/T2MI distinction in a series of exploratory analyses addressing 22 individuals with T1MI and 12 with T2MI (31–33). Baseline plasminogen and oxidized phospholipids bound to plasminogen were found significantly lower in T1MI than T2MI, potentially reflecting reduced fibrinolytic capacity (31). There was, however, overlap in confidence intervals and the diagnostic performance is unknown. Metalloproteinases (MMPs) 2, 3, and 9 were unable to differentiate between T1MI and T2MI (32). Using the same biorepository, 1032 metabolites were evaluated using module enrichment analysis to determine differences between atherothrombotic vs. nonatherothrombotic MI and identified that a decrease in plasma amino acids was associated with thrombotic but not nonthrombotic MI, potentially due to increases in activated platelets protein synthesis (33).
Thus far, none of the above approaches have robust data to endorse routine clinical use, and several limitations exist in the majority of studies including smaller datasets, uncertainty regarding T2MI classification, and/or lack of external validation.
Prognosis
T2MI is associated with poor short- and long-term clinical outcomes (7). Patients with multiple triggers do worse than those with a single etiology, and prognosis appears to be associated with the provoking factor, with those with arrhythmias having a more favorable prognosis than those caused by hypoxia, hypotension, or anemia (11). Cause-specific mortality analyses indicate that as compared to patients with T1MI, patients with T2MI have a clear excess in noncardiovascular death (11, 34). Despite this competing risk, data from the High-STEACS trial indicates that crude rates and the future hazard of MI or cardiovascular death were broadly comparable irrespective of myocardial injury or MI subtype (34). Similar findings were observed in the Mayo Clinic Olmsted cohort, in which rates of cardiovascular mortality were similar after either T1MI or T2MI (11). This observation indicates that a proportion of patients with T2MI may potentially benefit from investigation to identify and treat risk factors for cardiovascular disease, and there may be an opportunity to modify clinical outcomes.
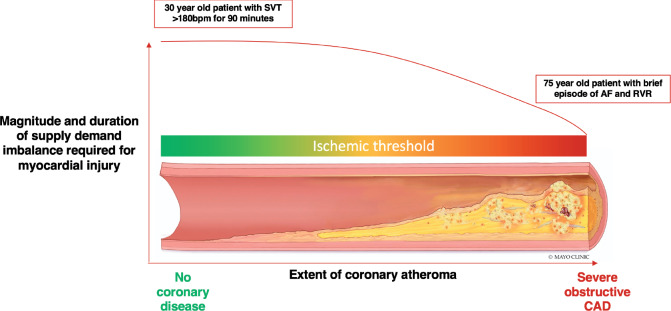
Observational studies suggest the presence of CAD to be an important predictor of short- and long-term adverse outcomes (34, 35). Such studies are limited as the decision to investigate for CAD has been guided by clinician assessment and a higher pre-test probability that may overestimate the prevalence of CAD. Substantial heterogeneity exists between studies in the use of coronary angiography and prevalence of CAD (7). Even if CAD is present, T2MI patients are less likely to be on secondary prevention relative to those with atherothrombotic T1MI (34, 35). Importantly, the presence and extent of CAD will likely influence an individual’s ischemic threshold and likelihood of T2MI (Fig. 3).
Fig. 3.
Magnitude of myocardial oxygen supply and/or demand imbalance and variation in ischemic threshold based on presence and extent of coronary artery disease. The magnitude and duration of myocardial oxygen supply and/or demand imbalance required to cause myocardial ischemia is heterogenous and related to the presence and extent of coronary artery disease. A young patient with no coronary disease will require a significant physiological stressor to trigger myocardial ischemia. Conversely, an older patient with established coronary artery disease may only need a minor trigger. Individualized assessment is paramount.
Patients with T2MI are older than those with T1MI, with a higher prevalence of co-morbidities. The extent to which future risk is driven by age and co-morbidity is uncertain. Analyses from the YOUNG-MI registry restricted to those under the age of 50 years with an adjudicated diagnosis of T1MI and T2MI demonstrated those with T2MI have a lower prevalence of diabetes, hypertension, hyperlipidemia and smoking, but worse renal function and longer length of hospitalization (36). Despite this, T2MI patients had the highest absolute rate of death from future cardiovascular disease at a median follow up of 10.2 years (36).
Risk Stratification
For T1MI there are various risk-stratification tools such as TIMI and GRACE that have been evaluated within clinical trials and are used to prognosticate and guide care (37, 38). For T2MI, no prospective data on risk-stratification exists. Clinicians rely on the magnitude of cTn increases (11) and deltas, as well the presence/magnitude of ischemia on ECG or imaging for risk stratification (15). The development of T2MI risk-stratification tools is of interest as the determination of short- and long-term risk can inform the level of care and follow-up that T2MI patients need.
The Global Registry of Acute Coronary Events (GRACE) 2.0 score was derived in patients with confirmed acute coronary syndrome to improve consistency in clinical decision making, aid prognostication, and identify those that might benefit from an early invasive strategy (39). The score applies clinical co-variates, ECG, and cardiac biomarkers to estimate risk of future all-cause mortality and MI. Whilst GRACE was not intended to be used in patients with T2MI, it does have moderate discriminatory ability. In a multi-center analysis of the GRACE 2.0 algorithm in patients with T2MI managed in the Karolinska Institute in Sweden and the High-STEACS trial in Scotland, the AUC was 0.73 (95%CI 0.70-0.77) and 0.73 (95%CI 0.66-0.81) for the prediction of all-cause death, respectively (38). Prediction of future death MI was less robust at 0.70 (95%CI 0.67-0.74) and 0.72 (95%CI 0.65-0.80), respectively (38).
Efforts have been made to derive bespoke risk stratification tools in patients with T2MI. The TARRACO clinical score was derived in patients with T2MI and myocardial injury (40). This score combines contemporary cTnI concentrations in addition to patient age, blood pressure, dyspnea, anemia, and the absence of chest pain. It had moderate discrimination for future major adverse cardiovascular events (AUC 0.74, 95%CI 0.70-0.79). In a recent external validation and direct comparison, only the GRACE score was predictive of all-cause mortality at 90 days (AUC 0.70, 95%CI 0.63-0.77 for GRACE versus AUC 0.52, 95%CI 0.46-0.58 for TARRACO, respectively) (37).
Studies addressing scoring systems for risk-stratification vary in the outcomes evaluated and timing of endpoint assessment (Table 1). Further, risk stratification performance can vary given the heterogeneity of the T2MI and variations in study designs and populations. Ideally, prospective multi-center trials or registries with uniform T2MI diagnostic criteria adhering to the 4th UDMI are needed.
Table 1.
Risk stratification scoring data for T2MI.
| Risk Score | GRACE ( 37 ) | GRACE 2.0 (38) | TIMI ( 37 ) | TARRACO ( 37, 40) |
|---|---|---|---|---|
| Variables |
Age Heart rate Systolic blood pressure CHF Killip class Creatinine ST-segment deviation Elevated troponin Cardiac arrest at admission |
Age Heart rate Systolic blood pressure CHF Killip class Creatinine ST-segment deviation Elevated troponin Cardiac arrest at admission Diuretic use* Renal failure* |
Age >=65 >=3 CAD risk factors Known CAD (stenosis >=50%) Aspirin use in past 7 days Severe angina (>=2 episodes in 24 hours) ECG ST changes >=0.5 mm Positive cardiac marker |
Age Arterial hypertension. Absence of chest pain. Dyspnea. Anemia. Troponin >5 URL. |
| Original endpoint |
At admission: in-hospital/to 6 months At discharge (to 6 months) |
Death: in-hospital, to 6 months, 1 year, and 3 years. Death/MI: 1 year. |
All-cause mortality, new or recurrent MI, or severe recurrent ischemia requiring urgent revascularization through 14 days | Post-discharge major adverse cardiac events at 180-days including all-cause death and readmission for congestive heart failure or acute MI |
| T2MI performance (AUC) |
All-cause mortality: In-hospital: 0.66 30-day: 0.69 90-day: 0.70 CV death In-hospital: 0.69 30-day: 0.72 90-day: 0.74 CV death, stroke, recurrent T1MI or T2MI In-hospital: 0.52 30-day: 0.58 90-day: 0.57 |
All-cause mortality * In-hospital: Scottish: 0.67 Swedish: 0.82 * 1 ear: Scottish: 0.73 Swedish: 0.73 Death or MI * 1 ear: Scottish: 0.70 Swedish: 0.72 |
All-cause mortality: In-hospital: 0.55 30-day: 0.43 90-day: 0.54 CV death In-hospital: 0.75 30-day: 0.67 90-day: 0.68 CV death, stroke, recurrent T1MI or T2MI In-hospital: 0.61 30-day: 0.61 90-day: 0.58 |
180-day all-cause death and readmission for CHF or acute MI Cediel et al. Derivation: 0.75 Validation: 0.74 All-cause mortality: In-hospital: 0.51 30-day: 0.51 90-day: 0.52 CV death In-hospital: 0.49 30-day: 0.46 90-day: 0.47 CV death, stroke, recurrent T1MI or T2MI In-hospital: 0.46 30-day: 0.49 90-day: 0.47 |
GRACE 2.0 recalibrated the original score using nonlinear associations for continuous variables to improve discrimination and calibration and provides an individual percentage risk. The algorithm was simplified by incorporating diuretic use and renal failure as categorical surrogates where creatinine or Killip class was not recorded. Abbreviations: CHF: congestive heart failure; AUC: Area Under the Curve; CAD: coronary artery disease; ECG: electrocardiogram; URL: upper reference limit; MI: myocardial infarction; CV: cardiovascular.
Future Directions
Diagnosis: educational efforts are required that emphasize the need for clear, clinical evidence of acute myocardial ischemia; for example, definite ECG or imaging abnormalities; to make a T2MI diagnosis. More studies are needed to inform the role and timing of advanced imaging.
Biomarkers: hs-cTn assays permit rapid MI diagnosis and risk stratification, but not T1MI/T2MI differentiation, which has therapeutic implications. Newer biomarker approaches, such as those probing markers specific for atherothrombosis, are needed.
Risk stratification: scoring systems evaluated to date do not provide robust short- and long-term risk stratification. Ideal approaches would provide individualized information about short (i.e., in-hospital and/or 30-day) and long-term (6-12 months) risk. Studies to date have varied in the assessed outcomes, but evaluation of the risk of death and subsequent MI is essential.
Mechanisms: DEMAND-MI (NCT03338504) is an observational T2MI cohort study in which patients underwent invasive coronary angiography with optical coherence tomography to determine if plaque rupture was present, and an assessment of the functional consequences of coronary stenosis using a coronary pressure wire and fractional flow reserve technique. Those unable to undergo coronary angiography had a CT coronary angiogram with CT-FFR where suitable. All patients underwent cardiac MRI to determine the pattern and extent of myocardial injury using the late gadolinium enhancement technique. The primary outcome is the prevalence of obstructive CAD (>50% in the left main stem or 70% in other major epicardial vessel) and study results are pending.
Phenotypes: T2MI is a heterogeneous clinical syndrome, for which data indicate that outcomes differ by both the number and nature of cause (11), as well as by the presence or absence of CAD. Recognizing this diversity, more phenotype-specific data are needed.
Clinical trials and management: The ACT-2 trial has been designed to investigate the prevalence of CAD and the effect of intervention on clinical outcomes. Patients with a clinical diagnosis of T2MI will be randomized to invasive or CT coronary angiography within 5 days of index diagnosis, with revascularization and secondary prevention at the discretion of the attending cardiologist. The control arm of conservative care permits functional testing. The primary outcome is all-cause mortality which will be evaluated at 30 days, 12 months, and 2 years.
Conclusions
The clinical syndrome of nonatherothrombotic MI caused by an acute and/or sustained supply-demand mismatch is frequently encountered in clinical practice and is associated with poor clinical outcomes. Its incidence is expected to further increase with the transition to hs-cTn assays. Clinicians require novel approaches that facilitate T2MI distinction and short- and long-term risk stratification.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved .
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
Y. Sandoval, Roche Diagnostics (past) and Abbott Diagnostics without personal compensation.
Stock Ownership
None declared.
Honoraria
Y. Sandoval, speaker without personal financial compensation for Abbott Diagnostics.
Research Funding
A.R. Chapman, a Starter Grant for Clinical Lecturers from the Academy of Medical Sciences, which is supported by the Wellcome Trust, the Medical Research Council, the British Heart Foundation, Versus Arthritis, Diabetes UK and the British Thoracic Society [SGL021\1075].
Expert Testimony
None declared.
Patents
None declared.
References
- 1. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231–64. [DOI] [PubMed] [Google Scholar]
- 2. Friedberg CK, Horn H.. Acute myocardial infarction not due to coronary artery occlusion. JAMA 1939;112:1675–9. [Google Scholar]
- 3. Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173–95. [DOI] [PubMed] [Google Scholar]
- 4. Saaby L, Poulsen TS, Hosbond S, Larsen TB, Pyndt Diederichsen AC, Hallas J, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med 2013;126:789–97. [DOI] [PubMed] [Google Scholar]
- 5. Sandoval Y, Smith SW, Sexter A, Thordsen SE, Bruen CA, Carlson MD, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med 2017;130:1431–9. [DOI] [PubMed] [Google Scholar]
- 6. Nestelberger T, Boeddinghaus J, Badertscher P, Twerenbold R, Wildi K, Breitenbücher D, et al. Effect of definition on incidence and prognosis of type 2 myocardial infarction. J Am Coll Cardiol 2017;70:1558–68. [DOI] [PubMed] [Google Scholar]
- 7. Sandoval Y, Jaffe AS.. Type 2 myocardial infarction: JACC review topic of the week. J Am Coll Cardiol 2019;73:1846–60. [DOI] [PubMed] [Google Scholar]
- 8. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–98. [DOI] [PubMed] [Google Scholar]
- 9. Nagele P. A simplified proposal to redefine acute myocardial infarction versus acute myocardial injury. Circulation 2020;141:1431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah ASV, Sandoval Y, Noaman A, Sexter A, Vaswani A, Smith SW, et al. Patient selection for high sensitivity cardiac troponin testing and diagnosis of myocardial infarction: prospective cohort study. BMJ 2017;359:j4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raphael CE, Roger VL, Sandoval Y, Singh M, Bell M, Lerman A, et al. Incidence, trends, and outcomes of type 2 myocardial infarction in a community cohort. Circulation 2020;141:454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chapman AR, Adamson PD, Shah ASV, Anand A, Strachan FE, Ferry AV,. et al. High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation 2020;141:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandoval Y, Wells JW, Newman JS, Clements CM, Grube E, Ola O, et al. Implementing high-sensitivity cardiac troponin T in a United States Regional Healthcare System. Circulation 2020;141:1937–9. [DOI] [PubMed] [Google Scholar]
- 14. Gard A, Lindahl B, Batra G, Hadziosmanovic N, Hjort M, Szummer KE, Baron T.. Interphysician agreement on subclassification of myocardial infarction. Heart 2018;104:1284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandoval Y, Smith SW, Sexter A, Schulz K, Apple FS.. Use of objective evidence of myocardial ischemia to facilitate the diagnostic and prognostic distinction between type 2 myocardial infarction and myocardial injury. Eur Heart J Acute Cardiovasc Care 2020;9:62–9. [DOI] [PubMed] [Google Scholar]
- 16. Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J, IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem 2017;63:73–81. [DOI] [PubMed] [Google Scholar]
- 17. Consuegra-Sánchez L, Martínez-Díaz JJ, de Guadiana-Romualdo LG, Wasniewski S, Esteban-Torrella P, Clavel-Ruipérez FG, et al. No additional value of conventional and high-sensitivity cardiac troponin over clinical scoring systems in the differential diagnosis of type 1 vs. type 2 myocardial infarction. Clin Chem Lab Med 2018;56:702–9. [DOI] [PubMed] [Google Scholar]
- 18. Sandoval Y, Thordsen SE, Smith SW, Schulz KM, Murakami MM, Pearce LA, Apple FS.. Cardiac troponin changes to distinguish type 1 and type 2 myocardial infarction and 180-day mortality risk. Eur Heart J Acute Cardiovasc Care 2014;3:317–25. [DOI] [PubMed] [Google Scholar]
- 19. Gaggin HK, Liu Y, Lyass A, van Kimmenade RR, Motiwala SR, Kelly NP, et al. Incident type 2 myocardial infarction in a cohort of patients undergoing coronary or peripheral arterial angiography. Circulation 2017;135:116–27. [DOI] [PubMed] [Google Scholar]
- 20. Paiva L, Providência R, Barra S, Dinis P, Faustino AC, Gonçalves L.. Universal definition of myocardial infarction: clinical insights. Cardiology 2015;131:13–21. [DOI] [PubMed] [Google Scholar]
- 21. Meigher S, Thode HC, Peacock WF, Bock JL, Gruberg L, Singer AJ.. Causes of elevated cardiac troponins in the emergency department and their associated mortality. Acad Emerg Med 2016;23:1267–73. [DOI] [PubMed] [Google Scholar]
- 22. Nowak RM, Jacobsen G, Christenson RH, Moyer M, Hudson M, McCord J.. Differentiating type 1 and 2 acute myocardial infarctions using the N-terminal pro B-type natriuretic peptide/cardiac troponin T ratio. Am J Emerg Med 2018;36:1849–54. [DOI] [PubMed] [Google Scholar]
- 23. Pandey AK, Duong T, Swiatkiewicz I, Daniels LB.. A comparison of biomarker rise in type 1 and type 2 myocardial infarction. Am J Med 2020;S0002-9343(20)30211-4. [DOI] [PubMed] [Google Scholar]
- 24. Sarkisian L, Saaby L, Poulsen TS, Gerke O, Jangaard N, Hosbond S, et al. Clinical characteristics and outcomes of patients with myocardial infarction, myocardial injury, and nonelevated troponins. Am J Med 2016;129:446.e5-446–e21. [DOI] [PubMed] [Google Scholar]
- 25. Bormann J, Psyrakis DA, von Jeinsen B, Grün D, Elsner LK, Wolter JS, et al. Myeloid-related protein 8/14 and high-sensitivity cardiac troponin I to differentiate type 2 myocardial infarction. Int J Cardiol 2020;304:144–7. [DOI] [PubMed] [Google Scholar]
- 26. Putot A, Jeanmichel M, Chagué F, Avondo A, Ray P, Manckoundia P, et al. Type 1 or type 2 myocardial infarction in patients with a history of coronary artery disease: data from the emergency department. J Clinc Med 2019;8:2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Putot A, Derrida SB, Zeller M, Avondo A, Ray P, Manckoundia P, Cottin Y.. Short-term prognosis of myocardial injury, type 1, and type 2 myocardial infarction in the emergency unit. Am J Med 2018;131:1209–19. [DOI] [PubMed] [Google Scholar]
- 28. Dekker MS, Mosterd A, van 't Hof AW, Hoes AW.. Novel biochemical markers in suspected acute coronary syndrome: systematic review and critical appraisal. Heart 2010;96:1001–10. [DOI] [PubMed] [Google Scholar]
- 29. Al-Mohaissen MA, Carere RG, Mancini GB, Humphries KH, Whalen BA, Lee T, et al. A plaque disruption index identifies patients with non-STE-type 1 myocardial infarction within 24 hours of troponin positivity. PLoS One 2016;11:e0164315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saenger AK, Jaffe AS.. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008;118:2200–6. [DOI] [PubMed] [Google Scholar]
- 31. DeFilippis AP, Chernyavskiy I, Amraotkar AR, Trainor PJ, Kothari S, Ismail I, et al. Circulating levels of plasminogen and oxidized phospholipids bound to plasminogen distinguish between atherothrombotic and non-atherothrombotic myocardial infarction. J Thromb Thrombolysis 2016;42:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Owolabi US, Amraotkar AR, Coulter AR, Singam NSV, Aladili BN, Singh A, et al. Change in matrix metalloproteinase 2, 3, and 9 levels at the time of and after acute atherothrombotic myocardial infarction. J Thromb Thrombolysis 2020;49:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trainor PJ, Hill BG, Carlisle SM, Rouchka EC, Rai SN, Bhatnagar A, DeFilippis AP.. Systems characterization of differential plasma metabolome perturbations following thrombotic and non-thrombotic myocardial infarction. J Proteomics 2017;160:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapman AR, Shah ASV, Lee KK, Anand A, Francis O, Adamson P, et al. Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation 2018;137:1236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baron T, Hambraeus K, Sundström J, Erlinge D, Jernberg T, Lindahl B, TOTAL-AMI study group. Impact on long-term mortality of presence of obstructive coronary artery disease and classification of myocardial infarction. Am J Med 2016;129:398–406. [DOI] [PubMed] [Google Scholar]
- 36. Singh A, Gupta A, DeFilippis EM, Qamar A, Biery DW, Almarzooq Z, et al. Cardiovascular mortality after type 1 and type 2 myocardial infarction in young adults. J Am Coll Cardiol 2020;75:1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy SP, McCarthy CP, Cohen JA, Rehman S, Jones-O'Connor M, Olshan DS, et al. Application of the GRACE, TIMI, and TARRACO risk scores in type 2 myocardial infarction. J Am Coll Cardiol 2020;75:344–5. [DOI] [PubMed] [Google Scholar]
- 38. Hung J, Roos A, Kadesjo E, McAllister DA, Kimeina DM, Shah AS, et al. Performance of the GRACE 2.0 score in patients with type 1 and 2 myocardial infarction. Eur Heart J 2020;as doi: 10.1093/eurheartj/ehaa375 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fox KA, Fitzgerald G, Puymirat E, Huang W, Carruthers K, Simon T, et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014;4:e004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cediel G, Sandoval Y, Sexter A, Carrasquer A, González-Del-Hoyo M, Bonet G, et al. Risk estimation in type 2 myocardial infarction and myocardial injury: The TARRACO Risk Score. Am J Med 2019;132:217–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.