Abstract
Background
Mosquito-borne diseases remain a significant public health problem in tropical regions. Housing improvements such as screening of doors and windows may be effective in reducing disease transmission, but the impact remains unclear.
Objectives
To examine whether housing interventions were effective in reducing mosquito densities in homes and the impact on the incidence of mosquito-borne diseases.
Methods
In this systematic review and meta-analysis, we searched 16 online databases, including NIH PubMed, CINAHL Complete, LILACS, Ovid MEDLINE, and Cochrane Central Register of Controlled Trials for randomized trials published from database inception to June 30, 2020. The primary outcome was the incidence of any mosquito-borne diseases. Secondary outcomes encompassed entomological indicators of the disease transmission. I2 values were used to explore heterogeneity between studies. A random-effects meta-analysis was used to assess the primary and secondary outcomes, with sub-group analyses for type of interventions on home environment, study settings (rural, urban, or mixed), and overall house type (traditional or modern housing),
Results
The literature search yielded 4,869 articles. After screening, 18 studies were included in the qualitative review, of which nine were included in the meta-analysis. The studies enrolled 7,200 households in Africa and South America, reporting on malaria or dengue only. The type of home environmental interventions included modification to ceilings and ribbons to close eaves, screening doors and windows with nets, insecticide-treated wall linings in homes, nettings over gables and eaves openings, mosquito trapping systems, metal-roofed houses with mosquito screening, gable windows and closed eaves, and prototype houses using southeast Asian designs. Pooled analysis depicted a lower risk of mosquito-borne diseases in the housing intervention group (OR = 0.68; 95% CI = 0.48 to 0.95; P = 0.03). Subgroup analysis depicted housing intervention reduced the risk of malaria in all settings (OR = 0.63; 95% CI = 0.39 to 1.01; P = 0.05). In urban environment, housing intervention was found to decrease the risk of both malaria and dengue infections (OR = 0.52; 95% CI = 0.27 to 0.99; P = 0.05).Meta-analysis of pooled odds ratio showed a significant benefit of improved housing in reducing indoor vector densities of both Aedes and Anopheles (OR = 0.35; 95% CI = 0.23 to 0.54; P<0.001).
Conclusions
Housing intervention could reduce transmission of malaria and dengue among people living in the homes. Future research should evaluate the protective effect of specific house features and housing improvements associated with urban development.
Introduction
Mosquito-borne diseases represent a major contributor to the burden of infectious disease globally, resulting in adverse financial, health, and societal impacts [1]. The incidence and geographical distribution of many mosquito-borne diseases are projected to grow with infections emerging in new areas or re-emerging in regions from which they have previously been eliminated [2–4]. Malaria and dengue are the most common mosquito-borne diseases in humans, with an estimated 212 million cases and 96 million cases reported respectively each year [5]. Annually, dengue illness costs approximately US$9 billion [6], whereas malaria spending is approximately US$5 billion [7, 8].
Insecticide-treated bednets and indoor residual spraying have been widely used to prevent the mosquito-borne disease transmission [9, 10]. However, the widespread insecticide resistance has significantly compromised the effectiveness of such interventions [11], suggesting a need for additional approaches. In tandem with the global call for action to make human settlements inclusive, safe, resilient, and sustainable by 2030 [12], large-scale investments can have a positive impact on various health and wellbeing outcomes, including but not limited to chronic respiratory tract diseases, mental health conditions, and infectious diseases such as enteric diseases, respiratory infections, malaria, and dengue fever [13, 14].
Historical records from Greek (circa 550 B.C.) and Roman era depicted large drainage schemes could reduce plague and fever. Other mechanical vector control methods such as sleeping in high buildings, use of bednets, and installation of bed curtains were noted in the 13th century [15]. The first malaria intervention of house screening was implemented among railway workers in Italy. Only 4% of those in the intervention group contracted malaria as compared to 92% in the non-intervened group [15, 16]. Since then, public health scientists have continued to reveal that simple changes in house design have the potential for protecting people against mosquito-borne diseases [16].
An existing systematic review evaluating the evidence for housing improvements to reduce malaria included 90 studies, of which only five were randomized trials [17]. A multi-country analysis of 29 survey data indicated that improved housing reduced malaria and had similar protective effect as insecticide-treated bednets [18]. More recently, a Cochrane review illuminated some evidence that screened houses may reduce malaria infection based on two published trials [19]. In view of randomized controlled trials remain the most robust method to provide reliable evidence on the real effect of an exposure or intervention [20], we performed a systematic review and meta-analysis to summarize the findings from randomized trials examining approaches related to both primary construction and modification of homes, and assessed the benefits of various housing interventions on prevention of mosquito-borne diseases.
Methods
Search strategy
We searched for articles indexed in 16 electronic databases, including PubMed, CINAHL Plus, LILACS, Ovid MEDLINE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Cochrane Clinical Answers, ASCE Civil Engineering Database, ACP Journal Club, NHS Economic Evaluation Database, Allied and Complementary Medicine, Database of Abstracts of Reviews of Effects, Wiley Online Library, Emerald Insight, IEEE Xplore, and ICONDA®CIBlibrary Database from database inception to June 30, 2020. No search restrictions were applied on study population, setting, or language. Search strings included terms related to mosquito-borne diseases (“malaria” or “Plasmodium” or “dengue” or “Zika”), housing interventions (“house” or “building” or “window*” or “door”), and randomized trials (“randomised trial” or “randomized trial” or “randomly”). Further detailed search was specified in the S1 Appendix. The bibliographies of recent reviews and all relevant articles were scrutinized for additional studies.
Study selection, inclusion, and exclusion criteria
Titles and abstracts were independently screened by two reviewers, followed by the retrieval and screening of full-text articles using the eligibility criteria described below. Any disagreements between the two reviewers were resolved by consensus and consultation of an external researcher whenever necessary.
Randomized trials of any mosquito-borne diseases were eligible for inclusion if they were published in English and evaluated one of the following type of housing interventions [21]:
| Type of interventions | Examples |
| Primary construction | |
| Construction materials | Wall, roof, door, and eave |
| Design | House built above ground level on stilts, or fewer or smaller windows |
| Modifications to existing houses (Non‐insecticidal) | |
| Screening | Covering of potential entry points (ceilings, eaves, doors, windows, or gable ends) |
| Eaves | Filling in of eaves |
| Wall maintenance | Filling in of cracks and crevices |
| Modifications to existing houses (Insecticidal) | |
| Eave tubes | Insecticide‐treated netting fitted into tubes inserted into closed eaves Eaves are closed and tubes with insecticide‐treated netting |
| Insecticidal screening | Screening potential entry points of house with insecticidal-treated netting |
The numerous structural housing interventions could be divided into three categories:
Design and material specifications for primary construction
Modifications or additions to the physical structure of existing houses
Incorporation of non-insecticidal or insecticidal systems into existing house structures to reduce indoor mosquito densities
Studies employing insecticide-treated bednets or curtains as a single intervention were excluded because the evidence has been reviewed extensively and has long been recommended by the World Health Organization and the United States Centers for Disease Control and Prevention as a core intervention for the disease control [22–29]. Studies available only as an abstract (e.g., conference abstracts) or non-English research articles were also excluded.
Data collection
Two reviewers independently extracted information from included studies using a standardized, predesigned table in Microsoft Word 2016, including (1) study population, number of participants enrolled, mean age, percentage of participants, and baseline clinical characteristics; (2) intervention details; (3) outcome measures; and (4) study results. Primary outcome of interest was the incidence of any mosquito-borne diseases defined as the occurrence of the infection cases in a study population for the whole study period. Secondary outcomes encompassed entomological indicators of the disease transmission, including indoor or outdoor mosquito density quantified by the numbers and characteristics of mosquitoes caught using techniques such as baits, light traps, knockdown catches, aspirators, or other methods.
Risk of bias assessment
Two reviewers independently assessed the risk of bias of each included trial using RoB 2.0 [30]. Risk domains include randomization process, timing of identification, and recruitment of individual participants in relation to timing of randomization (cluster-randomized trials only), deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. The overall risk of bias was classified as low if all domains were at low risk of bias, as high if at least one domain was at high risk of bias or multiple domains were judged to have some concerns, or as some concerns if at least one domain was judged to have some concerns. Any discrepancies between the authors were resolved through consensus.
Statistical analysis
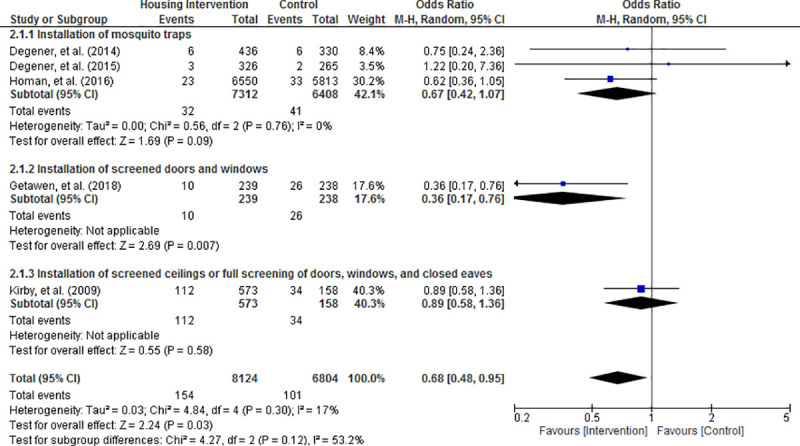
Narrative and tabular synthesis of data was performed for all included studies. For studies which were insufficient for a meta-analysis, the findings were only systematically reviewed. When the primary articles had adequate similarities in terms of study outcomes, a random-effects meta-analysis was carried out to generate a pooled odds ratio for mosquito vector densities and mosquito-borne diseases, specifically malaria and dengue. Stratified analyses were also conducted based on illness prevented (malaria or dengue), type of housing interventions (installation of mosquito traps—incorporation of systems into existing house structures; installation of screened doors and windows or installation of screened ceilings or full screening of doors, windows, or closed eaves—modifications or additions to the physical structure of existing houses), study settings (entirely rural, entirely urban, or mixed), and overall house type (traditional or modern housing).
We analyzed quantitative data using random-effects meta-analyses to generate a pooled odds ratio and 95% confidence interval for each dichotomous outcome or a weighted mean difference, and 95% confidence interval for each continuous outcome, if any. Forest plots were presented for each meta-analysis along with the I2 statistic which is used to quantify heterogeneity [31]. Funnel plots were checked for publication bias using Egger’s test for asymmetry [32]. We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system with GRADEpro GDT software to judge the quality of evidence of the meta-analyzed outcome [33]. Analyses were undertaken using RevMan for Windows (version 5.3) and Stata (version 14.0).
Ethics statement
The study was a systematic review and did not require approval from an ethics committee.
Results
Of 4,869 studies identified, 18 were eligible for qualitative synthesis and nine for meta-analysis (Fig 1). Fourteen of the randomized trials were conducted in Africa [34–47], whilst four in South America [48–51]. The studies were published between 2003 and 2019, and enrolled approximately 7,200 households (Table 1). Four trials examined housing intervention on controlling Aedes and preventing the transmission of dengue, whereas fourteen trials evaluated on Anopheles and malaria transmission.
Fig 1. Flow diagram of study selection.
Table 1. Characteristics of included studies.
| Study (year), country | Recruitment and baseline sample size | Intervention(s) | Control condition(s) | Duration of intervention | Outcome measures | Main findings |
|---|---|---|---|---|---|---|
| Entomological (Aedes) | ||||||
| Che-Mendoza, et al. (2015), Mexico [48] | 20 clusters of 100 households each | Duranet® screens (0.55% w/w alpha-cypermethrin-treated non-flammable polyethylene netting) were mounted in aluminium frames custom-fitted to doors and windows of residential houses (n = 780 houses) | No intervention (n = 1,000 houses) | 24 months | Indoor adult mosquitos collected using modified CDC backpack aspirators | At 5-month, significantly fewer houses of intervention group were infested with Ae. aegypti adult females (OR = 0.38; 95% CI = 0.21 to 0.69), blood-fed females (OR = 0.36; 95% CI = 0.21 to 0.60), and males (OR = 0.39; 95% CI = 0.19 to 0.77). Significant impact was still observed at 12-month post-intervention for adult females (OR = 0.41; 95% CI = 0.25 to 0.68) and males (OR = 0.41; 95% CI = 0.27 to 0.64). |
| Che-Mendoza, et al. (2018), Mexico [49] | 20 clusters of 100 households each | Duranet® screens (0.55% w/w alpha-cypermethrin-treated non-flammable polyethylene netting) were mounted in aluminium frames custom-fitted to doors and windows of residential houses (n = 844 houses) | No intervention (n = 1,000 houses) | 24 months | Indoor adult mosquitos collected using Prokopack aspirators | Significant reductions in the indoor presence and abundance of Ae. aegypti adults (OR = 0.48; IRR = 0.45; P<0.05 respectively) and Ae. aegypti female mosquitoes (OR = 0.47; IRR = 0.44; P<0.05 respectively) were observed in intervention group compared to control group. |
| Entomological (Anopheles) | ||||||
| Atieli, et al. (2009), Western Kenya [34] | 20 houses | Houses were modified with ceilings of papyrus mats to close eaves and small insecticide-treated nettings were incorporated in sleeping room ceilings and windows (n = 10 houses) | No intervention (n = 10 houses) | 4 months | Indoor-resting mosquito densities determined based on pyrethrum spray collection method | 84% An. gambiae reduction (OR = 0.16; 95% CI = 0.07 to 0.38) and 87% An. funestus reduction (OR = 0.13; 95% CI = 0.03 to 0.5) were observed in intervened houses compared to controls. |
| Jatta, et al. (2018), Gambia [47] | 5 houses | (1) Modified modern house was built with mosquito screening and increased ventilation, including metal roof with ventilation in gables, closed eaves, complete mosquito screening, and well-fitted doors (n = 1 house) | Traditional house was built with thatched roof, open eaves, and poorly fitted doors (n = 1 house) | 2 months | Indoor mosquitoes collected with a CDC light trap and mean indoor temperature for house | Closing the eaves of thatched houses resulted in 94% decrease in An. gambiae house entry (95% CI = 89 to 97) and increase in indoor temperature by 0.5°C (95% CI = 0.3 to 0.6) compared to thatched-roofed houses with open eaves. Metal-roofed houses with poorly fitted doors had three times more An. gambiae (Mean ratio = 2.99; 95% CI = 1.96 to 4.57) and were 1.5°C (95% CI = 1.3 to 1.7) hotter, and had 25% higher levels of carbon dioxide than thatched-roofed houses. In metal- roofed houses with closed eaves, mosquito numbers indoors were decreased by 96% with well-fitted screened doors. Improved ventilation with gable windows in metal-roofed houses made them as cool as thatched houses with open eaves. |
| (2) Traditional house was built with metal roof, closed eaves, and poorly fitted doors (n = 1 house) | ||||||
| (3) Traditional house was built with thatched roof, closed eaves, complete mosquito screening, and well-fitted doors (n = 1 house) | ||||||
| (4) Traditional house was built with thatched roof, closed eaves, and poorly fitted doors (n = 1 house) | ||||||
| Jawara, et al. (2018), Gambia [38] | 37 houses | Screened doors and windows were constructed to prevent mosquito entry, provide security and privacy, and increase airflow, held in place with an aluminum frame (n = 24 houses) | No intervention (n = 6 houses) | 10 weeks | Indoor mosquitoes sampled using light traps | All prototype doors and windows of intervention reduced the number of house-entering mosquitoes by 59 to 77% in comparison with the control houses (P<0.001). |
| Kampango, et al. (2013), Southern Mozambique [39] | 16 houses | Netting materials (mosquito bednets, locally purchased untreated shade cloth or deltamethrin-impregnated shade cloth) against mosquito entry inside houses were applied over gables and eaves openings (n = 12 houses) | No intervention (n = 4 houses) | 3 weeks | Mosquito entry rates assessed by light-trap collection | Entry rates of An. funestus were significantly reduced when the netting material was fitted over the gables of houses (IRR = 0.75; 95% CI = 0.62 to 0.91) and that extending the intervention over eaves did not enhance the protective effect (IRR = 0.80; 95% CI = 0.64 to 1.01). The netting materials significantly reduced entry of An. gambiae when applied over the gables (IRR = 0.17; 95% CI = 0.11 to 0.27) and both gables and eaves (IRR = 0.25; 95% CI = 0.17 to 0.37). |
| Kruger, et al. (2015), South Africa [46] | 40 houses | (1) Western-style houses were built with brick and cement, and corrugated iron roofs or tiled roofs were incorporated with deltamethrin 0.52% w/w brown color lining, deltamethrin 0.85% w/w purple color lining, alpha-cypermethrin 0.29% w/w green color lining, or alpha-cypermethrin 0.47% w/w orange color lining (n = 16 houses)(2) Traditional mud huts were installed with deltamethrin 0.52% w/w brown color lining, deltamethrin 0.85% w/w purple colour lining, alpha-cypermethrin 0.29% w/w green color lining or alpha-cypermethrin 0.47% w/w orange color lining (n = 16 houses) | Western-style houses and traditional mud huts with no intervention on wall lining (n = 8 houses) | 6 months | Knockdown and mortality rates of mosquitoes through WHO-recommended laboratory-scale contact or cylinder test and questionnaire-based data collection that included observations on the numbers of mosquitoes in the home | All four insecticide‑treated wall linings showed 100% knockdown and mortality of mosquitoes throughout 6-month post-installation in study homes. Thatch roofs and absence of ceiling in traditional mud huts increased mosquito access to dwellings. Gaps between roofs and tops of the walls (eave gaps) that were larger than 2 centimeters were present in 95% (19/20) of the traditional huts and 15% (3/20) of the modern houses, causing more mosquitoes in the homes. 65% (13/20) of participants in houses and 95% (19/20) in huts experienced irritation by mosquitoes while sleeping. Use of insecticides and repellents was higher among residents of huts. |
| Lindsay, et al. (2003), Gambia [41] | 6 experimental huts (128 participants) | (1) Plywood ceiling (n = 1 hut) | No intervention (n = 1 hut) | 6 weeks | Indoor mosquitos caught by traps | There were significantly fewer An. gambiae in huts with ceilings compared with controls: plywood (59% reduction), synthetic-netting (79%), insecticide-treated synthetic-netting (78%), plastic insect-screen (80%; P< 0.001 in all ceilings), and closed eaves (37%; P = 0.057). Likewise, netting and insect-screen ceilings reduced the number of Mansonia spp. by 70 to 72% (P<0.001) compared with controls. |
| (2) Synthetic-netting ceiling (n = 1 hut) | ||||||
| (3) Insecticide-treated synthetic-netting ceiling (n = 1 hut) | ||||||
| (4) Plastic insect-screen ceiling (n = 1 hut) | ||||||
| (5) Eaves closed with mud blocks (n = 1 hut) | ||||||
| All ceilings were installed below the open eaves | ||||||
| Massebo, et al. (2013), South-west Ethiopia [42] | 40 houses | Doors and windows were screened by metal mesh and openings in the walls, and eaves were closed with mud. Any openings in the wall for ventilation purpose were closed by metal mesh only. Timber-framed was used for screening doors. Screened doors were fixed on the frame of the main door externally using hinges and were removed by rolling to enter or leave the houses. Windows were permanently fixed externally by metal mesh (n = 20 houses) | No intervention (n = 20 houses) | 2 months | Indoor mosquitoes collected using CDC light trap | Mean number of An. arabiensis was 7.9 per light trap per night in control houses compared to 4.8 in houses with screened doors and windows, resulting in 40% fewer An. arabiensis in houses with intervention (P = 0.006). |
| Njie, et al. (2009), Gambia [43] | 12 houses | Doors were screened and eaves were completely closed with a mixture of sand, rubble, and cement (n = 6 houses) | Screened doors with open eaves (n = 6 houses) | 8 weeks | Indoor mosquitoes sampled using light traps | A 65% reduction in An. gambiae caught indoors (Mean number of An. gambiae per trap per night = 6.1 versus 2.1; OR = 0.34; 95% CI = 0.20 to 0.57) was reported in intervention group compared to controls. |
| Swai, et al. (2019), Southeastern Tanzania [44] | 24 huts | Transfluthrin-treated eave ribbons were installed along eaves spaces (n = 12 huts) | Untreated eave ribbons (n = 12 huts) | 7 weeks | Indoor and outdoor mosquito collections using light traps and carbon dioxide-baited BG® malaria traps | Intervention group showed decreased indoor densities of An. arabiensis by 77%, An. funestus by 60%, Culex spp. by 84%, and Mansonia spp. by 98% (P<0.001) compared to controls. Reductions in outdoor mosquito densities was also significant between intervention and control groups. |
| von Seidlein, et al. (2017), Northeastern Tanzania [45] | 22 houses (40 participants) | Prototype houses of southeast Asian design were constructed with walls made of lightweight permeable materials (bamboo, shade net, or timber) with bedrooms elevated from the ground and with screened windows (n = 7 participants) | Modified and unmodified traditional African houses, wattle-daub or mud-block constructions, were built on the ground with poor ventilation (n = 33 participants) | 9 months | Indoor mosquitos collected using Furvela tent traps during rainy season | There were fewer mosquitoes in prototype houses compared with traditional African houses, with double-storey houses showed the highest reduction in mosquito densities (96%; 95% CI = 92 to 98), followed by single-storey houses (77%; 95% CI = 72 to 82), and lowest in the modified reference houses (43%; 95% CI = 36 to 50) and traditional homes (23%; 95% CI = 18 to 29). |
| Clinical (Aedes) | ||||||
| Degener, et al. (2014), Brazil [51] | 1,487 households (6,300 participants) | BG-Sentinel® traps were installed in peridomestic area of houses such as verandas, kitchens, backyards, or indoors (n = 444 houses) | No intervention (n = 753 houses) | 73 weeks | Mosquitos collected using BG-Sentinel® traps and cases of dengue virus IgM-seropositivity among residents | The intervention group had significantly less Ae. aegypti females captured during rainy seasons. The frequency of dengue virus IgM-seropositivity was marginally lower in intervention households compared with controls (Fisher’s exact test: P = 0.0624; OR = 4.97). |
| Degener, et al. (2015), Brazil [50] | 775 houses | MosquiTRAP sticky traps were installed in peridomestic area of houses (n = 403 houses) | No intervention (n = 372 houses) | 73 weeks | Mosquitos collected using BG-Sentinel® traps and cases of dengue virus IgM-seropositivity among residents | A higher abundance of female Ae. aegypti was collected in the intervention group (P = 0.008). There was no significant difference of mosquito abundance between intervention and control groups during the first rainy season (P = 0.141) and significantly higher abundance of female Ae. aegypti in the intervention arm during the dry season (P = 0.01) and second rainy season (P = 0.003). The frequency of dengue virus IgM-seropositivity was similar between houses in the intervention arm and the control arm (Fisher’s exact test: P = 1; OR = 0.59). |
| Clinical (Anopheles) | ||||||
| Corbel, et al. (2012), West Africa [35] | 28 villages (1,677 children) | Long-lasting insecticidal mosquito netting-universal coverage of sleeping units and full coverage of carbamate-treated plastic sheeting were lined up to the upper part of the household walls (n = 415 children) | (1) Long-lasting insecticidal mosquito netting-targeted coverage was given to pregnant women and children younger than 6 years (n = 429 children) | 18 months | Incidence density rates of clinical malaria | There were no significant differences in incidence density of Plasmodium falciparum clinical malaria (Adjusted incidence density ratio = 1.05; 95% CI = 0.75 to 1.48), parasite densities of Plasmodium falciparum (Adjusted multiplicative coefficient = 0.98; 95% CI = 0.92 to 1.04) and prevalence of asymptomatic infections in children younger than 6 years (Adjusted OR = 0.81; 95% CI = 0.61 to 1.07) between the study groups. |
| (2) Long-lasting insecticidal mosquito netting-universal coverage was given to all sleeping units (n = 413 children) | ||||||
| (3) Long-lasting insecticidal mosquito netting-targeted coverage was given to pregnant women and children younger than 6 years plus full coverage of carbamate- indoor residual spraying applied every 8 months (n = 420 children) | ||||||
| Getawen, et al. (2018), South-western Ethiopia [36] | 98 houses (477 participants) | Doors and windows of eligible houses were screened with wire-meshes. Screened doors were fixed on frame of main door externally using hinges. Windows screening was permanently fixed externally (n = 46 houses comprising 239 participants) | No intervention (n = 46 houses comprising 238 participants) | 6 months | Mosquitos collected using CDC light traps and incidence of clinical malaria | There was an overall 48% reduction in indoor density of An. arabiensis (Mean ratio = 0.52) in intervention arm. The incidence of clinical malaria among residents of intervention group was significantly lower compared with control group (IRR = 0.39; 95% CI = 0.20 to 0.80). |
| Homan, et al. (2016), Western Kenya [37] | 50 to 51 houses | Solar-powered odour-baited mosquito trapping systems were installed in households (n = 6,550 participants) | No intervention (n = 5,813 participants) | 100 weeks | Incidence of clinical malaria and mosquito densities | Intervened clusters had 23 clinical malaria episodes, whereas non-intervened clusters had 33 episodes. Malaria prevalence measured by rapid diagnostic test was 29.8% (95% CI = 20.9 to 38.0) lower in clusters with intervention than in non-intervened clusters. The density of An. funestus was significantly lower in the intervention group compared to control group (Adjusted effectiveness = 69.2%; 95% CI = 29.1 to 87.4). |
| Kirby, et al. (2009), Gambia [40] | 500 houses | (1) In homes with full screening, timber-framed doors and windows were constructed and covered with polyvinyl chloride-coated fibreglass netting (1.2-meter wide for doors, 2.4-meter wide for ceilings, and 1.0-meter wide for windows), with a mesh size of 42 holes per cm2. The gap between the top of the wall and roof (eaves) was filled with a mixture of sand, rubble, cement, and water (n = 200 houses) | No intervention (n = 100 houses) | 12 months | Mosquitos collected using CDC light traps and incidence of malaria parasitemia | Mean number of An. gambiae caught in houses without screening was 37.5 per trap per night (95% CI = 31.6 to 43.3) versus 15.2 in houses with full screening (95% CI = 12.9 to 17.4) and 19.1 in houses with screened ceilings (95% CI = 16.1 to 22.1). Frequency of microscopically detectable malaria parasitemia was slightly higher in the control group than in either of the intervention groups (Full screening: OR = 0.79; 95% CI = 0.53 to 1.66; Screened ceilings: OR = 0.91; 95% CI = 0.54 to 1.70), although this was not statistically significant. There were no differences in the prevalence of high parasitemia (≥5000 parasites/μL): 6.3% in the control group, 4.2% in the full screening group, and 3.8% in the screened ceiling group. |
| (2) In homes with screened ceilings, netting was stretched across the room below the eaves, fixed to the walls with wooden battens and any small holes were filled with mortar (n = 200 houses) | ||||||
Methodological quality
11 out of 18 included studies (61.1%) were judged to have low risk of bias [34–37, 40, 43–45, 47–49]. Some concerns of risk of bias were identified with regard to randomization process in five randomized trials [38, 39, 41, 42, 46] and timing of recruitment of participants in two cluster randomized trials [50, 51] (S1 Fig). Due to the nature of the studies, it was not possible to blind the study participants and outcome assessors to intervention status.
Housing interventions
Numerous housing modifications and designs were studied which included installation of ceilings to close eaves [41], closed eaves with modified ceiling and nettings in sleeping room ceilings and windows [34], screened doors and windows [36, 38, 42, 48, 49], nettings in sleeping room with carbamate-treated plastic sheeting lined up to walls [35], monofilament polyethylene insecticide-treated wall linings in traditional mud huts and modern type brick houses [46], nettings over gables and eaves openings [39], mosquito trapping systems in house [37, 50, 51], full screening of windows, doors, and closing eaves [40, 43], transfluthrin-treated eave ribbons to close eaves spaces [44], metal-roofed house with closed eaves, mosquito screening, and increased ventilation [47], and prototype houses of southeast Asian design built with walls made of lightweight permeable materials (bamboo, shade net, or timber) with bedrooms elevated from the ground and with screened windows [45].
Design and material specifications for primary construction
In one study, modern prototype houses of southeast Asian design were constructed. Single-storey houses were elevated on stilts whereas double-storey houses had upstairs bedrooms. The houses utilized permeable materials (cladding) for the construction of walls to maximize air ventilation and had a concrete or timber floor that was elevated from the ground for sleeping areas, a reinforced storage area that could be locked, an outdoor cooking area with chimney connected to the main building and covered by a roof, an outdoor latrine, and a water harvesting system that facilitated the collection of rain water through gutters and storage of water in a plastic container [45].
Another study evaluated a novel design of metal-roofed house that was screened and ventilated, consisting of screened and well-fitted doors, closed eaves, and triangular screened windows constructed with wooden frames and mosquito screening and were positioned in the gable ends of the building [47].
Modifications or additions to the physical structure of existing houses
The study by Njie and co-authors involved the installation of improved doors made of softwood, strengthened at the corners with polyvinyl chloride-coated fibreglass netting and had eave gaps closed thoroughly with a mixture of sand, rubble, and cement [43]. In the study by Massebo and co-workers, wooden framed doors and windows were screened by metal mesh, whereas openings on eaves and walls were closed with mud [42]. Similarly, another study fixed wire-mesh screening on the frame of main doors and windows externally [36]. Likewise, the study by Jawara and colleagues designed state-of-the-art screened doors and windows using a modular system, held in place using an aluminium frame [38]. In the research conducted by Kirby et al., houses with full screening were designed to have timber-framed doors and windows covered with polyvinylchloride-coated fibreglass netting, with gaps between the top of the wall and roof (eaves) filled with a mixture of sand, rubble, cement, and water [40].
In terms of insecticidal interventions, one study installed transfluthrin-treated eave ribbons along eaves spaces of houses [44]. Another study utilized modified ceilings such as plywood ceiling, synthetic-netting ceiling, deltamethrin-treated synthetic-netting ceiling, and plastic insect-screen ceiling for closing eaves [41]. Moreover, the study performed by Kampango and co-workers covered gables and eaves openings with bednets, untreated shade cloth, or deltamethrin-impregnated durable lining [39]. In two studies, the researchers mounted alpha-cypermethrin-treated non-flammable polyethylene netting in aluminium frames custom-fitted to doors and windows [48, 49]. The study by Atieli et al. modified houses with ceilings of papyrus mats to close eaves and fixed permethrin-impregnated netting in ceiling openings above sleeping room [34]. Another study utilized long-lasting insecticidal mosquito netting for coverage of sleeping units plus full coverage of carbamate-treated plastic sheeting lined up to the upper part of household walls [35]. The next study evaluated four insecticidal pyrethroid-impregnated polymer linings of different colors installed on inner walls of traditional mud huts and Western-style houses [46].
Incorporation of non-insecticidal or insecticidal systems into existing house structures to reduce indoor mosquito densities
Three studies have installed mass trapping systems in households. The study by Homan and colleagues incorporated a solar-powered odour-baited mosquito trap in each household. For two adjacent single-roomed households, one trap was being shared [37]. In the second study, three non-electrical sticky traps were installed in peridomestic areas (covered yard area, laundry area, or veranda) of each household, positioned at least five meters apart from one another [50]. The third study incorporated an electric-powered mass trapping system inside of home or in peridomestic areas such as veranda, kitchen, or backyard [51].
Study findings
Entomological
Of 17 studies evaluating entomological outcomes [34, 36–51], 16 reported that housing intervention was effective in reducing the density of vector mosquitoes [34, 36–49, 51] (Table 1). One study utilized pyrethrum spray method that collected dead mosquitoes from a sheet and kept in a cooler box for laboratory quantification [34]. In two studies, mosquitoes were collected with aspirators for a 15-minute period per house [48, 49]. In addition, mosquitoes were caught using a light or carbon dioxide-baited trapping system in eleven studies [36–40, 42–44, 47, 50, 51]. A further study collected mosquitos using Furvela tent traps [45], whereas another study caught mosquitoes in the room and window traps [41]. Mosquitoes were also sampled using human landing catches technique in one study [35]. A study quantified residents’ perceptions based on observed number of dead mosquitoes on the floor, furniture, during cleaning, bites, and irritation, in addition to a laboratory-based analysis of knockdown and mortality rates of mosquitoes [46]. The most frequently caught mosquitoes in the studies were Ae. aegypti (dengue), An. gambiae (malaria), and An. arabiensis (malaria).
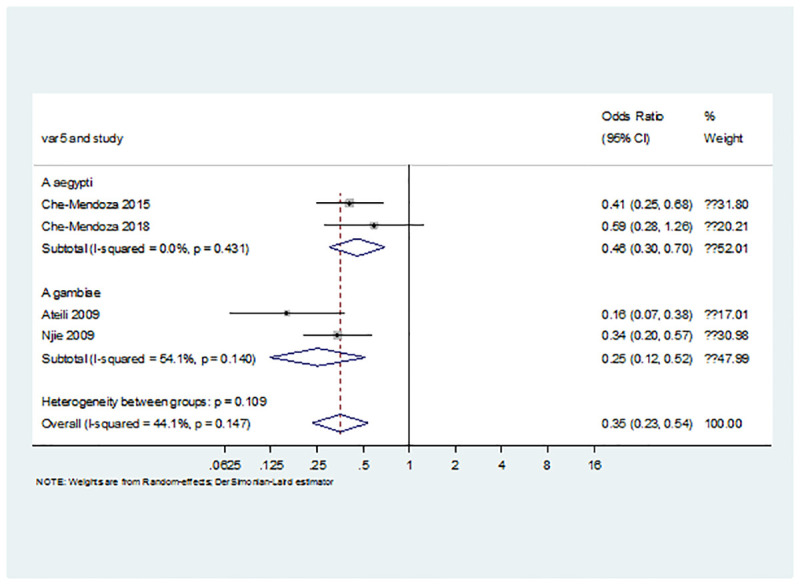
In the pooled analysis of odds ratio reported in the primary studies, there was a significant benefit for improved housing on indoor vector densities of both Aedes and Anopheles (OR = 0.35; 95% CI = 0.23 to 0.54; P<0.001; Fig 2).
Fig 2. Pooled odds ratio for the effect of housing intervention in reducing mosquito vector densities.
Clinical
Of three studies on malaria [35–37], two reported that housing intervention was effective in reducing the incidence (Table 1) [36, 37]. One study examined on parasitemia and found no evidence of an effect of housing intervention on the prevalence of malaria infection [40]. Two studies of dengue infection reported no statistically significant difference in the frequency of dengue virus IgM-seropositivity between houses in the intervention group than the control arm [50, 51].
Random effects meta-analysis of the results revealed that the risk of acquiring mosquito-borne diseases was significantly reduced in housing intervention group compared with control condition (OR = 0.68; 95% CI = 0.48 to 0.95; P = 0.03; Figs 3–6). Visual inspection of the funnel plots did not show any sign of publication bias (S1 File). Subgroup analysis found that housing intervention had a significant benefit in reducing the risk of malaria in all settings (OR = 0.63; 95% CI = 0.39 to 1.01; P = 0.05; Fig 3) and the risk of both malaria and dengue in urban environment (OR = 0.52; 95% CI = 0.27 to 0.99; P = 0.05; Fig 5).
Fig 3. Effect of housing intervention on the risk of mosquito-borne diseases stratified by type of mosquito-borne diseases.
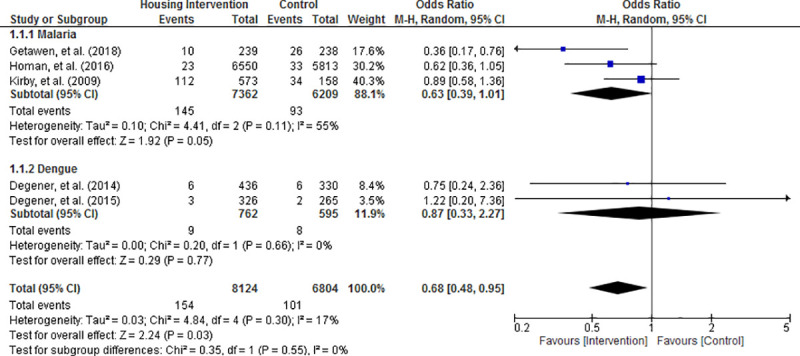
Fig 6. Effect of housing intervention on the risk of mosquito-borne diseases stratified by type of houses.
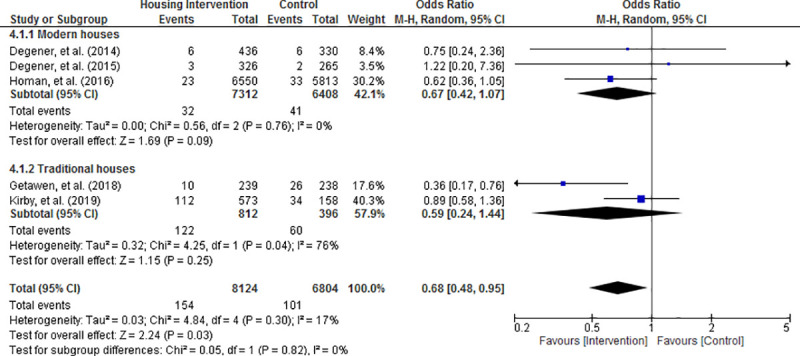
Fig 5. Effect of housing intervention on the risk of mosquito-borne diseases stratified by urbanicity.
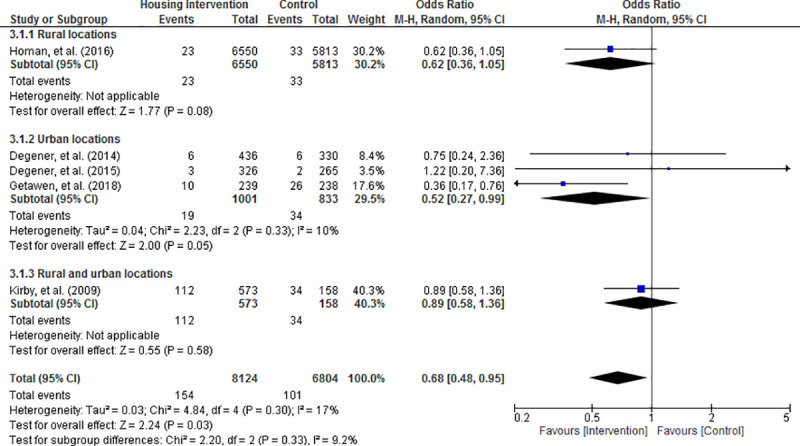
Fig 4. Effect of housing intervention on the risk of mosquito-borne diseases stratified by type of housing interventions.
Quality of evidence in meta-analysis
The certainty of retrieved evidence through GRADE assessments is presented in S1 Table. The incidence of mosquito borne diseases was rated as moderate, due to the serious imprecision from the wide confidence intervals. The certainty of evidence for the subgroup analyses varied; it was low for the incidence of malaria, moderate for incidence of dengue, and low to high with respect to incidence of mosquito-borne diseases under the subgroups of type of housing interventions, urbanicity, and overall house type.
Community acceptability
In all eleven studies that evaluated community acceptance of housing improvements, positive responses were received. More than 90% of study participants cited satisfaction toward the installation of mosquito trapping system in houses and it was comfortable to use [50, 51]. Most community members believed that house screening improved privacy and prevented mosquitoes from entering [36, 38, 40]. Furthermore, modified ceilings were perceived to be essential in vector control and could improve the functionality and beauty of houses [34, 41]. Over 90% of study participants reported that they would use and pay for transfluthrin-treated eave ribbons installed along the eaves spaces as a means of mosquito prevention [44]. In the study by Kruger and colleagues, all of the study subjects indicated that they were pleased with the appearance, including color, position, and attachment method of the wall linings and agreed that the intervention resulted in decrease of indoor mosquitoes and other insects [46]. For prototype houses of southeast Asian design, residents expressed satisfaction with the new design, especially double-storey buildings because the bedrooms had more privacy, cooler indoor temperature, and were safer from insects and crawling animals. The community showed a preference for timber building material which they regarded as secure, durable, and protective of privacy [45]. In metal-roofed houses, ventilated houses were considered more comfortable compared to unventilated houses during the night when people retired to bed, nonetheless, those with closed eaves were deemed more uncomfortable than thatched-roofed houses because of the higher temperatures [47].
Discussion
In this systematic review, we synthesized evidence from randomized studies conducted in malaria and dengue endemic tropical regions in Africa and South America, and depicted that housing intervention may offer protection against malaria and dengue. The results from our meta-analysis showed significant benefits overall for reducing the densities of the malaria and dengue vectors in homes, and reducing the incidence of clinical malaria but no significant effect on the incidence of dengue. Moreover, findings from individual studies reported that modified ceiling to close eaves, mosquito trapping systems, screened windows and doors, netting barriers to cover gable ends and eaves as well as prototype southeast Asian homes may reduce malaria or dengue transmission as depicted in the entomological outcomes.
Malaria and dengue are long-standing public health problems, particularly in the tropics. About a third of the world's population lives in regions where the climate is suitable for the transmission. To date, there is no effective dengue-specific prophylaxis or therapeutic. As such, an integrated vector management remains the only recommended approach for the disease prevention [52]. Space-spraying of insecticide to kill adult vectors in and around households is a popular approach, but, such method has not been eliciting a sustained positive impact on vector control [53]. It is noteworthy that malaria is mainly transmitted by mosquitoes indoor at night, hence, house modifications that decline mosquitoes from the indoor environment could lead to malaria incidence reduction. Dengue however is transmitted by the bite of the Aedes mosquito that typically attacks during daytime both inside and outside of the house. Therefore, the environment surrounding the house (waterbodies) rather than the house itself is likely to be the most important. It is very plausible and justifiable that malaria cases can be reduced by improved housing as indicated in our meta-analysis, nevertheless, dengue infections are more likely to be reduced by both housing and environmental management. Day-biting mosquitoes (Aedes), particularly females, are attracted to light during the day regardless of spectra. Their biting activity correlates with the time when people are also active outdoors, resulting in little protection against this mosquito species. Night-biting mosquitoes (Anopheles) specifically avoid ultraviolet and blue light during the day. Such behavioral attraction to and avoidance of light in both species change with time of day and show distinct sex and circadian neural circuit differences [54]. Genetic complexity and ecosystem diversity may cause behavioral changes and resistance in the mosquitoes, contributing to diminished effectiveness of insecticide-treated materials [55].
Modification of houses is a long-term, sustainable solution to control and eliminate of mosquito-borne diseases. Research suggest that this is achieved through two postulated mechanisms [17, 56]. Firstly, house entry by mosquito vectors is deterred by specific features in the homes such as closed eaves, the presence of ceilings, tiled, or metal roofs [18, 57]. These designs, which result in a higher daytime indoor temperature may impair mosquito survival and parasite development [58–61]. The architectural design of houses and choice of building materials equally play an important role. They may influence the existence of holes as routes of mosquito entry and the changes in indoor temperature and humidity [62]. In São Tomé of Africa, elevating house structure above the ground has been proven to reduce mosquito biting and indoor vector density [63]. More unscreened windows and open eaves or gables are likely to increase mosquito entry, hence, culminating in a higher risk of mosquito-borne infections [47]. While the design of openings in house structures is indispensable for ventilation and light, feasible intervention involves screening of the openings using appropriate building materials of which the effectiveness depends on the size and frequency of openings [38]. It is also possible to incorporate insecticides into housing materials albeit some concerns have been raised about photo-degradation of the quality upon prolonged exposure to sunlight as well as increase in the environmental levels of the chemical metabolites and their negative impact on the environment and the inhabitants of the house [64]. Our review identified a broad set of house improvements, consisting of chemical interventions (such as insecticides embedded in building materials) and physical interventions (such as screened doors and windows). Chemical interventions last much shorter than any physical interventions. Due to limited number of studies, our meta-analysis of clinical endpoint (incidence of illness) was able to pool data from studies of physical interventions. The pooled odds ratio of entomological endpoint showed a less pronounced effect associated with the two studies of chemical interventions [48, 49], demonstrating the interventions may be temporal compared to permanent (physical) interventions. Our review has indicated that eave ribbons can confer peridomestic protections against bites of malaria vectors. Such intervention is very useful for reducing the disease transmission risks in communities where people spend time outdoors during the day as well as early-night hours before going indoors or sleep under the bednets [65]. Likewise, mass trapping systems installed in peridomestic areas may have the benefit as well.
Our systematic review provides a timely contribution to the existing evidence base about the effects of housing interventions on all mosquito-borne diseases. To the best of our knowledge, it is the only review that focuses exclusively on randomized studies of malaria and dengue. The addition of this contemporary publication serves to address a paucity of such research design in housing interventions. Many of the included trials were conducted in some of the poorest communities, where often national health and development policies may not reach [66]. This can serve as a baseline for many countries in their future policy development planning to ensure that any public housing development can take these suggestions into consideration. Importantly, studies have shown that people with low socioeconomic status tend to spend more time at home [67–69]. This highlights the importance of housing improvements for the poor and socially disadvantaged groups who would be likely to spend more time at home. While housing quality is recognized as a prominent risk factor for a range of transmission settings [70, 71], our subgroup analysis could not find any discernible differences in effectiveness of interventions between residents of modern and traditional houses. A recent publication has similarly identified inconsistent correlation patterns between house type and prevalence of mosquito-borne disease within sub-Saharan African countries and suggested that it was caused by variations in the definitions of housing quality and conduct of surveys [18]. While a more pronounced advantage is observed in urban environments plausibly due to better access to health and social services, our data highlights the necessity of tailoring of the interventions for populations with different socioeconomic positions whose risk factor pattern and disease burden vary considerably.
Despite considerable variations in the complexity of housing interventions of the included studies, clinical and entomological evidence appeared to be consistently positive across the studies, thus highlighting the importance of comprehensive housing interventions as part of the epidemiological prevention of the infectious diseases. Our current study found that installation of screened doors and windows had a significant effect in reducing the risk of transmission of mosquito-borne diseases. Residents of both urban and rural settings would benefit from improved homes. Moreover, the potential health benefits of modern houses would go far beyond those built using traditional materials or designs. Further research is needed to investigate how different building elements contributed to clinically meaningful reduction in mosquito-borne illness. Reliance on a single intervention to control mosquito-borne diseases has often been ineffective, thus, systematic application of different interventions in combination and in synergy is anticipated to be a strategy with great promise [72]. While much remains a rather perplexing clinical puzzle, the effectiveness of existing multidisciplinary, comprehensive community-targeted intervention for various disease prevention would reasonably support the operation of future randomized clinical trials to evaluate housing as a strategic, long-term intervention for preventing mosquito-borne diseases. This is especially true taking into consideration that currently available or investigational malaria and dengue vaccines do not confer 100% rates of protective efficacy against the infections [73, 74]. At present, an increasing number of professionals and international organizations appreciate the strategies that feature “housing as a vaccine” to eliminate illness and disability. Stakeholders from both worlds of health and housing should be engaged in real-world case management, community-based counseling, and home-based health support services to ensure that the homes robustly meet the needs of people [75].
Our review findings possess global health implications. Mosquito-borne diseases will continue to exert healthcare and socioeconomic burden on numerous low- and middle-income countries across the world [76]. There is some evidence of the benefits of housing intervention on the prevention of mosquito-borne pathogens. However, the investments required for the construction of novel housing are markedly higher compared to indoor residual spraying and insecticide-treated bednets that cost less than USD$10 [77]. The health impact of novel designs in housing can go far beyond that to include decreased indoor mosquito density in tandem with a more comfortable environment for bednets to reduce mosquito-borne diseases, improved air quality, and availability of safe water and latrines to prevent other infectious diseases. The interventions might possibly translate into substantial improvements in morbidity, mortality and family health as well as social and economic impact attributable to the diseases. Government policies and current private housing expenditure determine the extent to which improved housing can be regarded as providing value for money. It is appropriate for government to promote public-private partnerships and deliver tax cuts to businesses that deliver healthy housing projects for community benefits [78]. Banks can offer microloan services for owners to make housing modifications. While the public sphere may exhibit a degree of scepticism with regard to provision of decent homes for political or humanitarian reasons, it is indeed crucial to collect more evidence along the lines of housing as a social determinant of health in the context of public expenditure. Integration of housing interventions within health and social care systems may improve physical, mental, and social wellbeing as well as reduce public health risks for infectious diseases and disability [79]. To glean a maximally precise picture concerning this nascent area of research, the approach would prove sufficient if it is bundled with clinical and cost-effectiveness findings from adequately powered, well-designed, and well-executed trials that can be applied to a diverse population, thereby garnering source of financial support from industry, public sector, and philanthropic organizations.
Several limitations of this review are worth noting. The sparse number of randomized trials published precluded our analysis for assessing a diverse range of building materials and architectural designs in the market. Most of the housing intervention research conducted thus far were observational studies [17]. It is interesting to note that evaluation of housing quality that was already present in the communities would yield a weaker evidence base compared to randomized trials that administered a direct intervention targeting communities who were known to be afflicted by mosquito-borne diseases. Overall, our findings broadly concurred with a recent Cochrane review that showed malaria infection may be reduced through improved house features [19]. Furthermore, majority of the included studies (61.1%) had comparatively short-term follow-up of 6 months or shorter. The effectiveness of such interventions on subjective outcomes such as quality of life, functional status, social or family wellbeing, and participants' satisfaction in housing conditions might require longer follow-up period to ascertain any differences and facilitate a thorough realist evaluation to determine what works, for whom and under what conditions [80, 81]. As such, it may present a spectrum of new challenges to be addressed in future research. While one of our included studies has involved migratory farmers, thus far, there have been very sparse number of community-level randomized controlled trials on mitigating mosquito-borne disease burden in humanitarian emergencies such as refugees, slums, and migratory communities, including fishermen, pastoralist, and forest workers who live in a poorly constructed house with no deployment of vector program. The unpredictable and volatile nature of these settings can often be restrictive pertaining to designing experimental studies [82]. In addition, all included studies were conducted in low- and middle-income countries. There has been a remarkable transformation of housing in urban and rural sub-Saharan Africa between 2000 and 2015, with the prevalence of improved housing has surged twofold from 11% to 23%, nonetheless, housing need is still acute given the rapid population growth [83]. Caution should be exercised in generalizing these interventions to other high-income countries with significantly different political, welfare, health, and socioeconomic systems. Effectiveness and cost-effectiveness of these interventions may vary across countries, nevertheless, further confirmation research is warranted in the settings based upon the concept of providing physical and chemical barriers to prevent mosquito entry.
Results of this review will hopefully encourage the development of mainstream policy discourse for the design of high-quality residential buildings to yield health benefits. It also resonates with the breath of interest of holistic sustainable development agenda to account for and remediate the incongruity of perpetuating substandard housing conditions and the attributable health inequities in resource-poor parts the world [14]. From a scientific, economic, and ethical perspective, appropriate housing interventions for implementation should be location and community-specific, effective, inclusive, acceptable, and affordable. Hence, the selection of the most appropriate housing interventions, combinations or enhanced design-led innovations must undergo initial pilot trials to provide a solid foundation for successive sizable scale-up of the project. Community-based housing interventional research that supports a collaboration between business, academia, and the public sector should be undertaken in multiple countries to give accurate, clear, location-specific, authoritative, scientifically sound, and economically viable policy recommendations.
Conclusions
Housing intervention offers significant protection against malaria and dengue. Interventions such as screened doors and windows, improvements to roofs, ceilings, gables, eaves, or walls, mosquito trapping systems, and novel design houses hold promise for reducing dengue and malaria transmission.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This paper was written during the first author's stay at the Oxford Institute of Population Ageing, University of Oxford as a Leslie Kirkley Visitor.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Chapter 2—Mosquito-borne siseases In: Qureshi AI, editor. Zika virus disease: Academic Press; 2018. p. 27–45. [Google Scholar]
- 2.Paixão ES, Teixeira MG, Rodrigues LC. Zika, chikungunya and dengue: the causes and threats of new and re-emerging arboviral diseases. BMJ Glob Health. 2018;3(Suppl 1):e000530 Epub 2018/02/13. 10.1136/bmjgh-2017-000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(6):712–23. Epub 2016/02/15. 10.1016/S1473-3099(16)00026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grobbelaar AA, Weyer J, Moolla N, Jansen van Vuren P, Moises F, Paweska JT. Resurgence of yellow fever in Angola, 2015–2016. Emerg Infect Dis. 2016;22(10):1854–5. Epub 2016/08/19. 10.3201/eid2210.160818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklinos LHV, Jones KE, Redding DW, Abubakar I. The effect of global change on mosquito-borne disease. Lancet Infect Dis. 2019;19(9):e302–e12. 10.1016/S1473-3099(19)30161-6 [DOI] [PubMed] [Google Scholar]
- 6.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16(8):935–41. Epub 2016/04/20. 10.1016/S1473-3099(16)00146-8 . [DOI] [PubMed] [Google Scholar]
- 7.Haakenstad A, Harle AC, Tsakalos G, Micah AE, Tao T, Anjomshoa M, et al. Tracking spending on malaria by source in 106 countries, 2000–16: an economic modelling study. Lancet Infect Dis. 2019;19(7):703–16. Epub 2019/05/01. 10.1016/S1473-3099(19)30165-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micah AE, Su Y, Bachmeier SD, Chapin A, Cogswell IE, Crosby SW, et al. Health sector spending and spending on HIV/AIDS, tuberculosis, and malaria, and development assistance for health: progress towards Sustainable Development Goal 3. Lancet. 2020;396(10252):693–724. 10.1016/S0140-6736(20)30608-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okumu FO, Moore SJ. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malaria Journal. 2011;10(1):208 10.1186/1475-2875-10-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinder M, Jawara M, Jarju LBS, Salami K, Jeffries D, Adiamoh M, et al. Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: a cluster-randomised controlled trial. Lancet. 2015;385(9976):1436–46. 10.1016/S0140-6736(14)61007-2 [DOI] [PubMed] [Google Scholar]
- 11.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32(3):187–96. 10.1016/j.pt.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 12.Fullman N, Barber RM, Abajobir AA, Abate KH, Abbafati C, Abbas KM, et al. Measuring progress and projecting attainment on the basis of past trends of the health-related Sustainable Development Goals in 188 countries: an analysis from the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1423–59. 10.1016/S0140-6736(17)32336-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards RT, Bray N. The Warm Homes for Health project: exploring the cost-effectiveness of improving population health through better housing. Lancet. 2014;384:S80 10.1016/S0140-6736(14)62206-6 [DOI] [Google Scholar]
- 14.von Seidlein L, Wood H, Brittain OS, Tusting L, Bednarz A, Mshamu S, et al. Knowledge gaps in the construction of rural healthy homes: a research agenda for improved low-cost housing in hot-humid Africa. PLoS Med. 2019;16(10):e1002909 10.1371/journal.pmed.1002909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14(1):e0007831 10.1371/journal.pntd.0007831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsay SW, Emerson PM, Charlwood JD. Reducing malaria by mosquito-proofing houses. Trends Parasitol. 2002;18(11):510–4. 10.1016/s1471-4922(02)02382-6 [DOI] [PubMed] [Google Scholar]
- 17.Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malaria Journal. 2015;14(1):209 10.1186/s12936-015-0724-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tusting LS, Bottomley C, Gibson H, Kleinschmidt I, Tatem AJ, Lindsay SW, et al. Housing improvements and malaria risk in Sub-Saharan Africa: a multi-country analysis of survey data. PLoS Med. 2017;14(2):e1002234 10.1371/journal.pmed.1002234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furnival-Adams J, Olanga EA, Napier M, Garner P. House modifications for preventing malaria. Cochrane Database Syst Rev. 2020;10:CD013398 Epub 2020/10/16. 10.1002/14651858.CD013398.pub2 . [DOI] [PubMed] [Google Scholar]
- 20.Bauchner H, Golub RM, Fontanarosa PB. Reporting and interpretation of randomized clinical trials. JAMA. 2019;322(8):732–5. 10.1001/jama.2019.12056 [DOI] [PubMed] [Google Scholar]
- 21.Furnival‐Adams J, Olanga EA, Napier M, Garner P. Housing interventions for preventing malaria. Cochrane Database Syst Rev. 2019;(8):CD013398 10.1002/14651858.CD013398 PubMed Central PMCID: PMC6691978. [DOI] [Google Scholar]
- 22.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2):CD000363 Epub 2004/04/24. 10.1002/14651858.CD000363.pub2 . [DOI] [PubMed] [Google Scholar]
- 23.Lengeler C. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Database Syst Rev. 2000;(2):CD000363 Epub 2000/05/05. 10.1002/14651858.CD000363 . [DOI] [PubMed] [Google Scholar]
- 24.Pryce J, Richardson M, Lengeler C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst Rev. 2018;11(11):CD000363 Epub 2018/11/07. 10.1002/14651858.CD000363.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson AL, Dhiman RC, Kitron U, Scott TW, van den Berg H, Lindsay SW. Benefit of insecticide-treated nets, curtains and screening on vector borne diseases, excluding malaria: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(10):e3228 Epub 2014/10/10. 10.1371/journal.pntd.0003228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wangdi K, Furuya-Kanamori L, Clark J, Barendregt JJ, Gatton ML, Banwell C, et al. Comparative effectiveness of malaria prevention measures: a systematic review and network meta-analysis. Parasit Vectors. 2018;11(1):210 10.1186/s13071-018-2783-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamble C, Ekwaru JP, ter Kuile FO. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane Database Syst Rev. 2006;2006(2):Cd003755 Epub 2006/04/21. 10.1002/14651858.CD003755.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamble C, Ekwaru PJ, Garner P, ter Kuile FO. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med. 2007;4(3):e107–e. 10.1371/journal.pmed.0040107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisele TP, Larsen DA, Anglewicz PA, Keating J, Yukich J, Bennett A, et al. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis. 2012;12(12):942–9. Epub 2012/09/22. 10.1016/S1473-3099(12)70222-0 . [DOI] [PubMed] [Google Scholar]
- 30.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 31.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334(7584):94–6. 10.1136/bmj.39057.406644.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Akl EA, Schünemann HJ. Using systematic reviews in guideline development: the GRADE approach. Res Synth Methods. 2018. Epub 2018/07/15. 10.1002/jrsm.1313 . [DOI] [PubMed] [Google Scholar]
- 34.Atieli H, Menya D, Githeko A, Scott T. House design modifications reduce indoor resting malaria vector densities in rice irrigation scheme area in western Kenya. Malaria Journal. 2009;8(1):108 10.1186/1475-2875-8-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbel V, Akogbeto M, Damien GB, Djenontin A, Chandre F, Rogier C, et al. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: a cluster randomised controlled trial. Lancet Infect Dis. 2012;12(8):617–26. Epub 2012/06/12. 10.1016/S1473-3099(12)70081-6 . [DOI] [PubMed] [Google Scholar]
- 36.Getawen SK, Ashine T, Massebo F, Woldeyes D, Lindtjørn B. Exploring the impact of house screening intervention on entomological indices and incidence of malaria in Arba Minch town, southwest Ethiopia: a randomized control trial. Acta Trop. 2018;181:84–94. Epub 2018/02/17. 10.1016/j.actatropica.2018.02.009 . [DOI] [PubMed] [Google Scholar]
- 37.Homan T, Hiscox A, Mweresa CK, Masiga D, Mukabana WR, Oria P, et al. The effect of mass mosquito trapping on malaria transmission and disease burden (SolarMal): a stepped-wedge cluster-randomised trial. Lancet. 2016;388(10050):1193–201. 10.1016/S0140-6736(16)30445-7 [DOI] [PubMed] [Google Scholar]
- 38.Jawara M, Jatta E, Bell D, Burkot TR, Bradley J, Hunt V, et al. New prototype screened doors and windows for excluding mosquitoes from houses: a pilot study in rural Gambia. Am J Trop Med Hyg. 2018;99(6):1475–84. Epub 2018/10/24. 10.4269/ajtmh.18-0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kampango A, Bragança M, Sousa Bd, Charlwood JD. Netting barriers to prevent mosquito entry into houses in southern Mozambique: a pilot study. Malaria Journal. 2013;12(1):99 10.1186/1475-2875-12-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirby MJ, Ameh D, Bottomley C, Green C, Jawara M, Milligan PJ, et al. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet. 2009;374(9694):998–1009. 10.1016/S0140-6736(09)60871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsay SW, Jawara M, Paine K, Pinder M, Walraven GE, Emerson PM. Changes in house design reduce exposure to malaria mosquitoes. Trop Med Int Health. 2003;8(6):512–7. Epub 2003/06/07. 10.1046/j.1365-3156.2003.01059.x . [DOI] [PubMed] [Google Scholar]
- 42.Massebo F, Lindtjørn B. The effect of screening doors and windows on indoor density of Anopheles arabiensis in south-west Ethiopia: a randomized trial. Malaria Journal. 2013;12(1):319 10.1186/1475-2875-12-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Njie M, Dilger E, Lindsay SW, Kirby MJ. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol. 2009;46(3):505–10. Epub 2009/06/06. 10.1603/033.046.0314 . [DOI] [PubMed] [Google Scholar]
- 44.Swai JK, Mmbando AS, Ngowo HS, Odufuwa OG, Finda MF, Mponzi W, et al. Protecting migratory farmers in rural Tanzania using eave ribbons treated with the spatial mosquito repellent, transfluthrin. Malaria Journal. 2019;18(1):414 10.1186/s12936-019-3048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Seidlein L, Ikonomidis K, Mshamu S, Nkya TE, Mukaka M, Pell C, et al. Affordable house designs to improve health in rural Africa: a field study from northeastern Tanzania. Lancet Planet Health. 2017;1(5):e188–e99. 10.1016/S2542-5196(17)30078-5 [DOI] [PubMed] [Google Scholar]
- 46.Kruger T, Sibanda MM, Focke WW, Bornman MS, de Jager C. Acceptability and effectiveness of a monofilament, polyethylene insecticide-treated wall lining for malaria control after six months in dwellings in Vhembe District, Limpopo Province, South Africa. Malaria Journal. 2015;14(1):485 10.1186/s12936-015-1005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jatta E, Jawara M, Bradley J, Jeffries D, Kandeh B, Knudsen JB, et al. How house design affects malaria mosquito density, temperature, and relative humidity: an experimental study in rural Gambia. Lancet Planet Health. 2018;2(11):e498–e508. Epub 2018/11/07. 10.1016/S2542-5196(18)30234-1 . [DOI] [PubMed] [Google Scholar]
- 48.Che-Mendoza A, Guillermo-May G, Herrera-Bojórquez J, Barrera-Pérez M, Dzul-Manzanilla F, Gutierrez-Castro C, et al. Long-lasting insecticide-treated house screens and targeted treatment of productive breeding-sites for dengue vector control in Acapulco, Mexico. Trans R Soc Trop Med Hyg. 2015;109(2):106–15. Epub 2015/01/22. 10.1093/trstmh/tru189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Che-Mendoza A, Medina-Barreiro A, Koyoc-Cardeña E, Uc-Puc V, Contreras-Perera Y, Herrera-Bojórquez J, et al. House screening with insecticide-treated netting provides sustained reductions in domestic populations of Aedes aegypti in Merida, Mexico. PLoS Negl Trop Dis. 2018;12(3):e0006283–e. 10.1371/journal.pntd.0006283 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Degener CM, de Ázara TMF, Roque RA, Rösner S, Rocha ESO, Kroon EG, et al. Mass trapping with MosquiTRAPs does not reduce Aedes aegypti abundance. Mem Inst Oswaldo Cruz. 2015;110(4):517–27. Epub 2015/04/28. 10.1590/0074-02760140374 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Degener CM, Eiras AE, Azara TM, Roque RA, Rösner S, Codeço CT, et al. Evaluation of the effectiveness of mass trapping with BG-sentinel traps for dengue vector control: a cluster randomized controlled trial in Manaus, Brazil. J Med Entomol. 2014;51(2):408–20. Epub 2014/04/15. 10.1603/me13107 . [DOI] [PubMed] [Google Scholar]
- 52.Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future. Lancet Infect Dis. 2014;14(12):1271–80. 10.1016/S1473-3099(14)70834-5 [DOI] [PubMed] [Google Scholar]
- 53.Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010;15(5):619–31. 10.1111/j.1365-3156.2010.02489.x [DOI] [PubMed] [Google Scholar]
- 54.Baik LS, Nave C, Au DD, Guda T, Chevez JA, Ray A, et al. Circadian regulation of light-evoked attraction and avoidance behaviors in daytime- versus nighttime-biting mosquitoes. Curr Biol. 2020;30(16):3252–9.e3. 10.1016/j.cub.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sokhna C, Ndiath MO, Rogier C. The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria. Clin Microbiol Infect. 2013;19(10):902–7. 10.1111/1469-0691.12314 [DOI] [PubMed] [Google Scholar]
- 56.Degroote S, Zinszer K, Ridde V. Interventions for vector-borne diseases focused on housing and hygiene in urban areas: a scoping review. Infect Dis Poverty. 2018;7(1):96 Epub 2018/09/04. 10.1186/s40249-018-0477-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seidahmed OME, Lu D, Chong CS, Ng LC, Eltahir EAB. Patterns of urban housing shape dengue distribution in Singapore at neighborhood and country scales. GeoHealth. 2018;2(1):54–67. 10.1002/2017GH000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murdock CC, Sternberg ED, Thomas MB. Malaria transmission potential could be reduced with current and future climate change. Sci Rep. 2016;6(1):27771 10.1038/srep27771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindsay SW, Wilkins HA, Zieler HA, Daly RJ, Petrarca V, Byass P. Ability of Anopheles gambiae mosquitoes to transmit malaria during the dry and wet seasons in an area of irrigated rice cultivation in The Gambia. J Trop Med Hyg. 1991;94(5):313–24. Epub 1991/10/01. . [PubMed] [Google Scholar]
- 60.Siraj AS, Oidtman RJ, Huber JH, Kraemer MUG, Brady OJ, Johansson MA, et al. Temperature modulates dengue virus epidemic growth rates through its effects on reproduction numbers and generation intervals. PLoS Negl Trop Dis. 2017;11(7):e0005797 10.1371/journal.pntd.0005797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alto BW, Bettinardi D. Temperature and dengue virus infection in mosquitoes: independent effects on the immature and adult stages. Am J Trop Med Hyg. 2013;88(3):497–505. Epub 2013/02/04. 10.4269/ajtmh.12-0421 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindsay SW, Jawara M, Mwesigwa J, Achan J, Bayoh N, Bradley J, et al. Reduced mosquito survival in metal-roof houses may contribute to a decline in malaria transmission in sub-Saharan Africa. Sci Rep. 2019;9(1):7770 10.1038/s41598-019-43816-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charlwood JD, Pinto J, Ferrara PR, Sousa CA, Ferreira C, Gil V, et al. Raised houses reduce mosquito bites. Malaria Journal. 2003;2(1):45 Epub 2003/12/12. 10.1186/1475-2875-2-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kayedi MH, Lines JD, Haghdoost AA, Vatandoost MH, Rassi Y, Khamisabady K. Evaluation of the effects of repeated hand washing, sunlight, smoke and dirt on the persistence of deltamethrin on insecticide-treated nets. Trans R Soc Trop Med Hyg. 2008;102(8):811–6. 10.1016/j.trstmh.2008.05.025 [DOI] [PubMed] [Google Scholar]
- 65.Mmbando AS, Ngowo H, Limwagu A, Kilalangongono M, Kifungo K, Okumu FO. Eave ribbons treated with the spatial repellent, transfluthrin, can effectively protect against indoor-biting and outdoor-biting malaria mosquitoes. Malaria Journal. 2018;17(1):368 10.1186/s12936-018-2520-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fitzpatrick C, Bangert M, Mbabazi PS, Mikhailov A, Zouré H, Polo Rebollo M, et al. Monitoring equity in universal health coverage with essential services for neglected tropical diseases: an analysis of data reported for five diseases in 123 countries over 9 years. Lancet Glob Health. 2018;6(9):e980–e8. 10.1016/S2214-109X(18)30307-3 [DOI] [PubMed] [Google Scholar]
- 67.American Lung Association. Urban air pollution and health inequities: a workshop report. Environ Health Perspect. 2001;109 Suppl 3(Suppl 3):357–74. . [PMC free article] [PubMed] [Google Scholar]
- 68.Gibson M, Hearty W, Craig P. The public health effects of interventions similar to basic income: a scoping review. Lancet Public Health. 2020;5(3):e165–e76. 10.1016/S2468-2667(20)30005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steenhuis IH, Nooy SB, Moes MJ, Schuit AJ. Financial barriers and pricing strategies related to participation in sports activities: the perceptions of people of low income. J Phys Act Health. 2009;6(6):716–21. Epub 2010/01/28. 10.1123/jpah.6.6.716 . [DOI] [PubMed] [Google Scholar]
- 70.Rek JC, Alegana V, Arinaitwe E, Cameron E, Kamya MR, Katureebe A, et al. Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced transmission: a cohort study. Lancet Planet Health. 2018;2(2):e83–e94. 10.1016/S2542-5196(18)30010-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snyman K, Mwangwa F, Bigira V, Kapisi J, Clark TD, Osterbauer B, et al. Poor housing construction associated with increased malaria incidence in a cohort of young Ugandan children. Am J Trop Med Hyg. 2015;92(6):1207–13. Epub 2015/04/13. 10.4269/ajtmh.14-0828 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ. Integrated vector management for malaria control. Malaria Journal. 2008;7(1):S4 10.1186/1475-2875-7-S1-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, et al. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med. 2016;374(26):2519–29. 10.1056/NEJMoa1515257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biswal S, Reynales H, Saez-Llorens X, Lopez P, Borja-Tabora C, Kosalaraksa P, et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med. 2019;381(21):2009–19. 10.1056/NEJMoa1903869 [DOI] [PubMed] [Google Scholar]
- 75.Koh HK, Restuccia R. Housing as health. JAMA. 2018;319(1):12–3. 10.1001/jama.2017.20081 [DOI] [PubMed] [Google Scholar]
- 76.Katzelnick LC, Coloma J, Harris E. Dengue: knowledge gaps, unmet needs, and research priorities. Lancet Infect Dis. 2017;17(3):e88–e100. Epub 2017/02/12. 10.1016/S1473-3099(16)30473-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scates SS, Finn TP, Wisniewski J, Dadi D, Mandike R, Khamis M, et al. Costs of insecticide-treated bed net distribution systems in sub-Saharan Africa. Malaria Journal. 2020;19(1):105–. 10.1186/s12936-020-03164-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butler SM. Housing as a step to better health. JAMA. 2018;320(1):21–2. Epub 2018/07/05. 10.1001/jama.2018.7736 . [DOI] [PubMed] [Google Scholar]
- 79.Aubry T, Bloch G, Brcic V, Saad A, Magwood O, Abdalla T, et al. Effectiveness of permanent supportive housing and income assistance interventions for homeless individuals in high-income countries: a systematic review. Lancet Public Health. 2020;5(6):e342–e60. 10.1016/S2468-2667(20)30055-4 [DOI] [PubMed] [Google Scholar]
- 80.Gandhi GY, Murad MH, Fujiyoshi A, Mullan RJ, Flynn DN, Elamin MB, et al. Patient-important outcomes in registered diabetes trials. JAMA. 2008;299(21):2543–9. Epub 2008/06/05. 10.1001/jama.299.21.2543 . [DOI] [PubMed] [Google Scholar]
- 81.Wickham S. Effective interventions for homeless populations: the evidence remains unclear. Lancet Public Health. 2020;5(6):e304–e5. 10.1016/S2468-2667(20)30120-1 [DOI] [PubMed] [Google Scholar]
- 82.Messenger LA, Furnival-Adams J, Pelloquin B, Rowland M. Vector control for malaria prevention during humanitarian emergencies: protocol for a systematic review and meta-analysis. medRxiv. 2020:2020.11.16.20232306. 10.1101/2020.11.16.20232306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tusting LS, Bisanzio D, Alabaster G, Cameron E, Cibulskis R, Davies M, et al. Mapping changes in housing in sub-Saharan Africa from 2000 to 2015. Nature. 2019;568(7752):391–4. 10.1038/s41586-019-1050-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


