Abstract
The tetraploid Avena species in the section Pachycarpa Baum, including A. insularis, A. maroccana, and A. murphyi, are thought to be involved in the evolution of hexaploid oats; however, their genome designations are still being debated. Repetitive DNA sequences play an important role in genome structuring and evolution, so understanding the chromosomal organization and distribution of these sequences in Avena species could provide valuable information concerning genome evolution in this genus. In this study, the chromosomal organizations and distributions of six repetitive DNA sequences (including three SSR motifs (TTC, AAC, CAG), one 5S rRNA gene fragment, and two oat A and C genome specific repeats) were investigated using non-denaturing fluorescence in situ hybridization (ND-FISH) in the three tetraploid species mentioned above and in two hexaploid oat species. Preferential distribution of the SSRs in centromeric regions was seen in the A and D genomes, whereas few signals were detected in the C genomes. Some intergenomic translocations were observed in the tetraploids; such translocations were also detected between the C and D genomes in the hexaploids. These results provide robust evidence for the presence of the D genome in all three tetraploids, strongly suggesting that the genomic constitution of these species is DC and not AC, as had been thought previously.
Introduction
The cultivated hexaploid oat, Avena sativa L. (2n = 6x = 42, genomes AACCDD), is the sixth most important cereal crop cultivated worldwide [1]. The superior nutraceutical properties of the oat grain have attracted considerable attention from both breeders and consumers [2]. The genus Avena L. comprises a number of closely related species with a basic chromosome number of seven, and includes diploids, tetraploids, and hexaploids [3]. A. sativa is an allohexaploid species displaying disomic inheritance, and is closely related to the weedy species A. sterilis L. [4]. It is believed that A. sativa was domesticated from A. sterilis somewhere in Northwest Asia [4, 5].
The evolutionary history of the hexaploid oat A, C, and D genomes has been under intense scrutiny [6–13]. The identities of its genome donors, however, remain inconclusive. It has been assumed that one of the species in the section Pachycarpa Baum, which includes A. insularis Ladiz., A. maroccana Gdgr. (synonym, A. magna Murphyi et Terr.), and A. murphyi Ladiz., has been involved in the formation of the hexaploids [13–15]. These tetraploid species have all been designated as having AC genomes (2n = 4x = 28, genomes AACC) based on genomic in situ hybridization [16] and C-banding analyses [17]. The most challenging mystery has concerned the origin of the D genome donor, since no natural diploid with a D genome has been identified. Because of the high homology between the A and D genomes in A. sativa [9], the D genome in the hexaploid has been considered to be a variant of the A genome, suggesting that it originated from one of the A genome diploids [9, 18]. Furthermore, the AC genome designations of the three tetraploids in the section Pachyacarpa have been challenged by evidence from both cytogenetic [19] and molecular studies [13, 20]. Fluorescence in situ hybridization (FISH) with an A genome-specific probe did not produce hybridization signals in the chromosomes of the AC genome tetraploids, and this absence was also observed in the D and C genome chromosomes of the hexaploids [19]. Another previous study, which used high-density genotyping-by-sequencing (GBS) markers, also showed that the hexaploid D genome, rather than the hexaploid A genome, has stronger matches with the A genome of these tetraploids [13]. Thus, more evidence is needed to confirm the genomic composition of these tetraploids.
Repetitive DNA elements are major components of plant genomes, including those of the Avena species, where more than 70% of the genome is predominated by repetitive DNA sequences [21]. Generally, repetitive DNA sequences evolve more rapidly than genic sequences, and play essential roles in genome structuring and evolution [22]. Their organization, distribution, and density can be specific for a species, a genome, or even a chromosome [23]. Therefore, extensive examination of the organization and distribution of repetitive DNA sequences could assist our understanding of the organization and evolution of genomes [24, 25], provide valuable information in taxonomic and phylogenetic studies [26], and help develop diagnostic markers for identifying specific chromosomes and chromosome regions [27–29].
Fluorescence in situ hybridization (FISH) is one of the most routinely-used tools to study the physical organization and distribution of repetitive DNA sequences. Indeed, FISH techniques using repetitive DNA sequences have been shown to be powerful tools in cytogenetic studies of Avena species. For instance, FISH using oat A and C genome-specific repetitive DNA sequences as probes clearly differentiated the A, C, and D genomes of hexaploid oat [19]. However, conventional FISH analysis is time-consuming because of the preparation and labeling of probe sequences and the denaturing of probes and chromosomes [29]. In recent years, a new FISH labeling procedure, non-denaturing FISH (ND-FISH), has been developed. ND-FISH uses synthesized oligonucleotide sequences as probes, and performs FISH analysis under non-denaturing conditions, thus substantially simplifying the procedure [30]. It has been widely used for cytogenetic studies in barley [27, 31], wheat [28], and rye [28], but less often for oat [32–35].
In this study, we used ND-FISH analysis to analyze the chromosomal organization of three tri-nucleotide SSR motifs (TTC, AAC, and CAG) and three oligonucleotide sequences (oligo-Am1, oligo-As120a, and oligo-Ta794) in Avena spp. The latter probes were derived from the oat A and C genome-specific repetitive DNA sequences Am1 and As120a and the wheat 5S rRNA gene. The relationships amongst the genomes from three tetraploid species in the section Pachycarpa, as well as two hexaploid oat species, were determined.
Materials and methods
Plant materials
Table 1 shows the plant materials used in this study, which comprised two hexaploid species (A. sativa and A. sterilis) and three AC(DC) genome tetraploid species (A. insularis, A. marrocana, and A. murphyi). Seeds of these materials were obtained from either the United States Department of Agriculture (USDA), or Plant Gene Resources of Canada (PGRC), with the exception of A. insularis, for which the seeds were kindly provided by Dr. Rick Jellen, Brigham Young University, Provo, UT, USA.
Table 1. List of materials used in this study, including species name, accession number, haplome and origin.
| Species | Accession number | Haplome a | Country of origin |
|---|---|---|---|
| A. sativa L. | CN 64226 | ACD | Brazil |
| A sterilis L. | PI 411503 | ACD | Algeria |
| A. insularis Ladiz. | SN | AC(DC) | Italy |
| A. maroccana Gdgr. | CIav 8331 | AC(DC) | Morocco |
| A. murphyi Ladiz. | CN 21989 | AC(DC) | Spain |
a Genomic compositions of A. insularis, A. maroccana, and A. murphyi are based on Yan et al. [13]
ND-FISH probes
Three SSR motifs ((TTC)5, (AAC)5, (CAG)5), two oligonucleotides derived from oat A and C genome specific repeats, and a wheat 5S rRNA gene fragment were used as ND-FISH probes. TTC, AAC, and CAG are tri-nucleotides that are highly abundant and widely distributed throughout the barley, wheat, and rye genomes [36, 37]. The other three probes are: (1) oligo-As120a, a 71bp fragment derived from the A-genome specific repetitive DNA sequence, As120a, a 114 bp fragment identified from the As genome diploid A. strigosa by southern blot [19]; (2) oligo-Am1 [33], a 51bp fragment derived from the C genome-specific repetitive DNA sequence Am1, a 467bp fragment that was isolated from the tetraploid A. muphyi by southern blot [38]; and (3) oligo-Ta794, a 41bp 5S rRNA sequence fragment that was isolated from T. aestivum [39]. Both Am1 and As120a are the only available C and A genome specific probes at present to our knowledge. All of these probes were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The synthesized oligonucleotides were 5’-end labeled with either 6-carboxytetramethylrhodamine (TAMRA) or 6-carboxyfluorescein (FAM). Further details concerning the probes, including their names, DNA sequences, and fluorochromes, are listed in Table 2.
Table 2. Oligonucleotide probes used for fluorescence in situ hybridization (FISH) analysis.
| Probe | Oligonucleotide |
|---|---|
| oligo-As120a | TAMRA-5'-ACTACAACGGAATGGCTAAATAAAACTGCCAACAACTGTGTGTTTGGTTTATCACTTACGATCTGTACCT-3' |
| oligo-Am1 | FAM-5'-GATCCATGTGTGGTTTGTGGAAAGAACACACATGCAATGACTCTAGTGGTT-3' |
| oligo-Ta794 | FAM-5'-TCAGAACTCCGAAGTTAAGCGTGCTTGGGCGAGAGTAGTAC -3' |
| oligo-(TTC)5 | TAMRA-5'-TTCTTCTTCTTCTTC-3' |
| oligo-(AAC)5 | TAMRA-5'-AACAACAACAACAAC-3' |
| oligo-(CAG)5 | FAM-5'-CAGCAGCAGCAGCAG-3' |
Preparation of metaphase spreads
Metaphase chromosome preparation paralleled that of previous experiments with some modifications [35]. In brief, seeds were imbibed in distilled water for 18 h at 25°C in the dark, and then placed in petri dishes lined with a layer of moist filter paper. To synchronize cell division and accumulate metaphase plates, the germinated seeds were transferred to a 4°C growth cabinet for 48 h, then to one at 25°C for 24 h. Root tips were harvested from germinated seeds, pre-treated in 1.0 MPa nitrous oxide gas for 3.5 h, then fixed in glacial acetic acid for 5 to 20 min before being fixed in 70% ethanol. Apical meristems were extruded from the fixed root tips and enzymatically digested with 2% cellulose and 1% pectinase. After being squashed in a drop of 60% acetic acid, each suspension was dropped onto a clean glass slide. The slides were air-dried prior to ND-FISH analysis.
ND-FISH analysis
ND-FISH was performed as described by Fu et al. [28]. Briefly, air-dried, pre-treated slides were fixed for 10 min with 4% (w/v) paraformaldehyde, and then immersed in 2×saline sodium citrate (SSC) for 10 min. After dehydration in an ice-cold ethanol series of 75%, 95%, and 100% for 5 min each, they were air-dried These air-dried slides were then denatured at 80°C for 2 min in deionized formamide (60 μL per slide), followed by dehydration in 75%, 95%, and 100% alcohol at -20°C for 5 min each before air drying. A 10 μL aliquot of a hybridization mixture containing 0.5 μL FISH probe, 4.75 μL 2×SSC, and 4.75 μL 1×TE was applied to each slide, then the slides were incubated for 2 h at 37°C. The slides were counterstained with DAPI and Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA). Sequential FISH analyses were performed as described in Fominaya et al. [35]. Digital images were captured using an Olympus BX-51 epifluorescence microscope equipped with a Photometric SenSys Olympus DP80 CCD camera (Olympus, Tokyo), and processed using Photoshop V7.0 (Adobe Systems Incorporated, San Jose, CA). After capturing each image, the slides were washed as described by Komuro et al. [37]. To make results reliable, at least 3 slides for each accession were performed FISH analysis, and for each slide, images for at least 30 cells were captured.
Results
The first hexaploid oat reference genome has been released recently (available at https://wheat.pw.usda.gov/GG3/node/922), which named the 21 chromosomes following Triticeae system (i.e. 1-7A, 1-7C, and 1-7D). However, the correspondences between the newest nomenclature system and that used in Sanz et al. [40] were not clear. Hence, the assignments of the chromosomes of the hexaploids were based on Sanz et al. [40]. Because the homologous relationships between the tetraploids and the hexaploids were not confirmed, thus, the assignments of each chromosome of A. maroccana and A. murphyi were based on the work of Fominaya et al. [35], which numbered the 14 chromosomes by combination of the chromosome ratio, relative length, and FISH patterns. The karyotype of A. maroccana was used as the reference to assign the chromosomes of A. insularis because of their high similarity in chromosome morphology. The A and C genome-specific probes oligo-Am1 and olligo-As120a, as well as the 5S rRNA probe oligo-pTa794, were used to assist with chromosome identification, enabling the A and C genome chromosomes to be distinguished from the D genome chromosomes.
Chromosomal organization of repeats in two hexaploid species
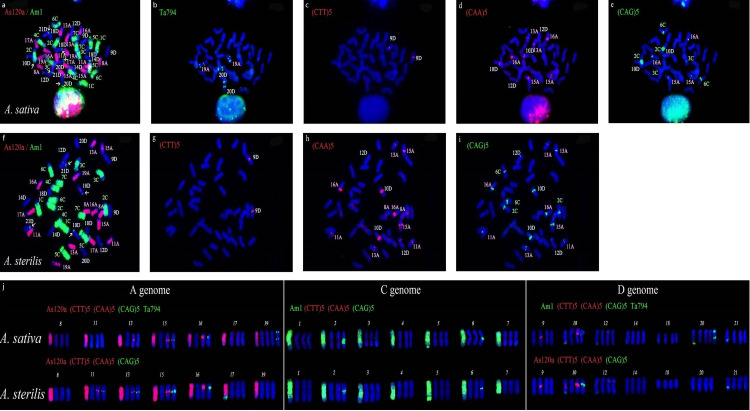
In the two hexaploids, the oligo-Am1 and oligo-As120a probes produced multiple signals that were evenly distributed along 14 chromosomes each, identifying these chromosomes as belonging to the the C and A genomes, while indicating that the remaining 14 chromosomes belong to the D genome (Fig 1A and 1F). Three minor C/D translocations, on chromosomes 10D, 20D, and 21D, were detected in A. sativa (Fig 1A and 1J), whereas two minor C/D translocations, on 10D and 21D, were observed in A. sterilis (Fig 1F and 1J). Two 5S sites were detected in A. sativa, on chromosomes 19A and 20D (Fig 1B and 1J).
Fig 1.
FISH performed on mitotic metaphase plates of hexaploid oats (a) A. sativa and (f) A. sterilis. The nomenclature system of Sanz et al. [40] was used to assign the chromosomes. (a) Simultaneous FISH of TAMRA-labeled As120a (red) and FAM-labeled Am1 (green). (b-e) The same cell as in panel ‘a’ after sequential FISH with FAM-labeled Ta794 (green), TAMRA-labeled (TTC)5 (red), TAMRA-labeled (AAC)5, and FAM-labeled (CAG)5 (green). (f) Simultaneous FISH of TAMRA-labeled As120a (red) and FAM-labeled Am1 (green). (g-i) The same cell as in panel ‘f’ after simultaneous FISH with TAMRA-labeled (TTC)5 (red), TAMRA-labeled (AAC)5, and FAM-labeled (CAG)5 (green). (j) Karyotypes showing a single chromosome of each homologous group chosen from the metaphases in panels ‘a’ and ‘f’. The white arrows indicate the C/D genome translocations.
All three SSR probes produced detectable signals in the two hexaploid oats. Similar to what was found with the tetraploids, one pair of strong signals produced by the oligo-(TTC)5 probe was detected in the centromeric region of 9D in both hexaploids (Fig 1C, 1G and 1J). In the hexaploids, oligo-(AAC)5 once again produced more signals than oligo-(TTC)5 did. Signals from (TTC)5 were detected on five (13A, 15A, 16A, 10D, 12D) (Fig 1D and 1J) and six (11A, 15A, 16A, 10D, 12D) (Fig 1H and 1J) chromosomes in A. sativa and A. sterilis, respectively. All of these signals were located in centromeric or petricentromeric regions, but differed in intensity. For (CAG)5, the signal intensities between A. sativa and A. sterilis were similar, but differed in number. In A. sativa, oligo-(CAG)5 produced signals on five chromosomes, including 15A, 16A, 2C, 6C, and 10D (Fig 1E and 1J). All of these signals were located in centromeric regions. In A. sterilis, the number of chromosomes with hybridization signals was eight, including four A genome chromosomes (11A, 13A, 15A, 16A), two C genome chromosomes (2C and 6C), and two D genome chromosomes (10D and 12D) (Fig 1I and 1J).
Chromosomal organization of repeats in three tetraploid species
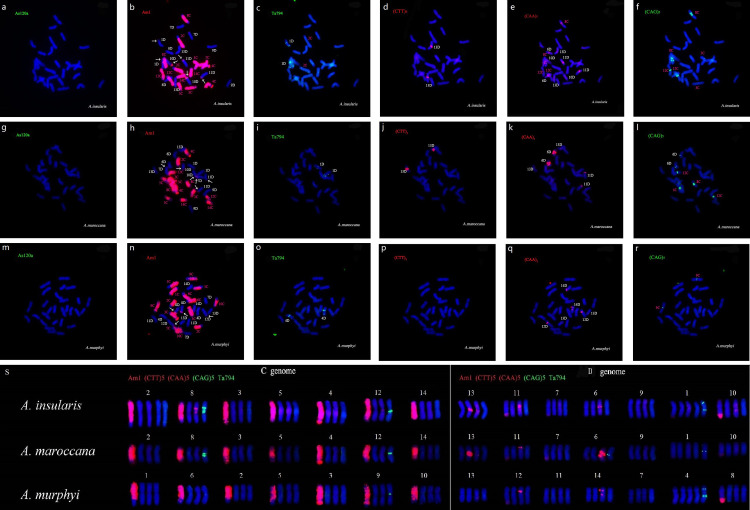
As expected, the oligo-Am1 probe produced multiple strong signals all along half of the chromosomes in all three tetraploids (Fig 2B, 2H and 2N). These chromosomes were identified as being the C genome chromosomes, meaning the remaining chromosomes should belong to the A(D) genome. Six C/A(D) translocations were observed in A. insularis (Fig 2B and 2S) and A. maroccana (Fig 2H and 2S), while only four were found in A. murphyi (Fig 2N and 2S). The oligo-Ta794 probe produced two pairs of bright signals on chromosome 1 A(D) in A. insularis (Fig 2C and 2S) and A. maroccana (Fig 2I and 2S), and on chromosome 4A(D) in A. murphyi (Fig 2O and 2S). In addition, a pair of weak signals was detected on chromosome 2C of A. insularis (Fig 2C and 2S). No discernable hybridization signals were detected in any of the three tetraploids using the oligo-As120a probe (Fig 2A, 2G and 2M).
Fig 2.
Karyotypes of the tetraploid species A. insularis (a-f), A. maroccana (g-l), and A. murphyi (m-r) after sequential FISH analysis. The chromosome assignments were based on work of Fominaya et al. [35]. The probes included FAM-As120a (green), TAMRA-Am1 (red), FAM-Ta794 (green), TAMRA-(TTC)5 (red), TAMRA-(AAC)5 (red), and FAM-(CAG)5 (green). (s) Karyotype of single metaphase chromosomes of these species. The white arrows indicate the intergenomic translocations.
For the SSR probes, the oligo-(TTC)5 probe produced strong signals on chromosome 13A(D) in A. insularis (Fig 2D and 2S) and A. maroccana (Fig 2J and 2S), covering a large region around the centromeres. However, no such signals were detected in A. murphyi (Fig 2P and 2S). Many more signals were produced by oligo-(AAC)5 than by oligo-(TTC)5. In A. insularis, oligo-(AAC)5 produced strong signals on chromosomes 8C, 6A(D), 10 A(D), 11 A(D), and13 A(D), with faint signals on 12C and the remaining three A(D) genome chromosome pairs. These signals were found in centromeric regions, intercalary regions, or both (Fig 2E and 2S). In the other two tetraploids, chromosome pairs with (AAC)5 hybridization signals were reduced to three, all belonging to the A(D) genome, but the signal patterns were highly differentiated both in distribution and intensity. In A. maroccana, signals on 6A(D) were very strong and covered a large region around the centromere, while signals on 11A(D) and 13A(D) were weak and located in the centromeric and telomeric regions, respectively (Fig 2K and 2S). In A. murphyi, signals on 12A(D) were positioned in the centromeric region, while signals on 14A(D) were located in the centromeric region and on the short arm. Very weak signals on the long arm were observed on 13A(D) (Fig 2Q and 2S). The oligo-(CAG)5 probe produced signals on two (8C and 12C) (Fig 2F and 2S), three (8C, 12C and 6A(D)) (Fig 2L and 2S) and one (9C) chromosome (Fig 2R and 2S) in A. insularis, A. maroccana, and A. murphyi, respectively. All of these signals were located in centromeric regions, but differed in intensity.
Discussion
The chromosomal organization of SSR repeats in oat genomes
We have elucidated the chromosomal organization of three SSR motifs, (TTC)5, (AAC)5, and (CAG)5, in five Avena polyploids. All three SSR oligonucleotides produced detectable hybridization in mitotic metaphase chromosomes in these species. Most of the signals produced were located at the centrometic or petricentromeric regions (Figs 1 and 2). These results were consistent with previous studies [32–34], and implied that Avena genomes contain more repetitive sequences in the centromeric regions of the chromosome than in intercalary parts, as has been found in many other plants [24, 36, 41]. Signal numbers produced by these three SSR probes varied, with (AAC)5 producing the most hybridization signals, followed by (CAG)5. The (TTC)5 probe produced few discernable signals (Figs 1 and 2). For the tri-nucleotide repeats, A/T-rich repeats (e.g., AAG/CTT, AAC/GTT) have been shown to be predominant in dicot species [42], but that is not the case in the monocot species barley or rice. Previous studies showed that an AAT repeat gave poor hybridization signals in barley [43], and a GCC motif was dominant in the rice genome [44]. In this study, both of the A/T-rich tri-nucleotide motifs, TTC and AAC, gave poor hybridization signals in Avena genomes, unlike what was seen in barley and wheat, where these probes hybridized to many sites and usually appeared all along the chromosomes [31, 36]. Previous studies also showed that other tri-nucleotide motifs (AAG, TTG, ACT) hybridized poorly in Avena species [32–34]. Taken together, these results suggest that the tri-nucleotides, at least the A/T-rich ones, are not the predominant repeat types of SSRs in Avena genomes.
In Avena species, the C genome is highly diverged from both the A and D genomes, and the A and D genomes are of high homology [9, 45]. These differences could be observed by comparing the distribution of the SSR motifs used in this study. For instance, almost all of the signals produced by the (CAA)5 probe are observed in the A or D genome chromosomes, with the exception of two C genome A. insularis chromosomes that had detectable (CAA)5 signals (Figs 1J and 2S). The signal number produced by the three SSR probes also differed between the A and D genomes, with the A genome chromosomes having more signals than the D genome chromosomes (Fig 1J and 2S). The centromere plays an important role during mitosis and meiosis in higher eukaryotic organisms [41]. Centromeric sequences are the most rapidly evolved in the genome, and have been considered to generate the major differences between genomes [22]. In this study, most signals produced by the probes used were located in centromeric regions; hence, the differences in signal patterns among the A, C, and D genomes would reflect the structural differences between these genomes and support the essential role of the centromere in genome restructuring.
The genomic compositions of A. insularis, A. maroccana, and A. murphyi
It is well accepted that the tetraploid species A. insularis, A. maroccana, and A. murphyi have been involved in the formation of hexaploid oats; however, the genomic constitutions of these species remain inconclusive. The AC genome designation was first assigned to A. maroccana after As and Cp genomic DNA used as probes for genomic in situ hybridization (GISH) each labeled half of the chromosomes in this species [16]. In addition, C-banding analysis showed high similarity between the chromosomes of A. insularis, A. maroccana, and A. murphyi [17], suggesting that they share the same genomic composition. However, these AC genome designations have been challenged by considerable evidence coming from both cytogenetic and molecular studies (summarized in Table 3). The strongest lines of evidence come from FISH analysis [19] and a GBS study [13]. An A genome-specific repetitive sequence isolated from the As diploid A. strigosa failed to hybridize with the genomes of the so-called AC tetraploids or the hexaploid D genome [19]. GBS markers revealed that half of the chromosomes of the tetraploids showed strong matches with the C genome chromosomes of the hexaploids, while the others showed strong matches with the D genome chromosomes [13]. However, evidence provided by the aforementioned studies couldn’t demonstrate the presence of the D genome in these tetraploid species perfectly. For example, the absence of the A genome-specific DNA repeat, As120a, in the three tetraploids could only demonstrate the absence of the A genome in these tetraploids since As120a signals were not observed in the Ac genome diploid A. canariensis and the Ad genome diploid A. damascena, and no robust evidence supports the latter two diploids are the D genome donors of the hexaploids. In previous GBS analysis [13], haplotype calling was based on the consensus hexaploid linkage map, but only a few linkage groups have been anchored to a certain chromosome. Therefore, there needs more evidence to confirm the genomic compositions of the three tetraploids. In the current study, the A genome-specific probe oligo-As120a failed to hybridize with the chromosomes of the three tetraploids or the D genome chromosomes of the hexaploids (Fig 2A, 2G and 2M), once again confirming the absence of the A genome in these tetraploids. Interestingly, some C/D genome translocations were observed in A. sativa and A. sterilis (Fig 1A and 1F), and these were also detected in the three tetraploids (Fig 2B, 2H and 2N). In addition, a pair of strong (TTC)5 signals was detected on one A(D) chromosome in A. insularis and A. maroccana (Fig 2D, 2J and 2P). Such signals were also observed on hexaploid chromosome 9D. Together with information from previous studies, the similarities between the hexaploid D genome and the A(D) genome in all three of the tetraploids observed in this study provide robust evidence for the presence of the D genome in these tetraploids, strongly suggesting that the three tetraploid species in the section Pachycarpa should be re-designated as having CD genomes, rather than AC genomes.
Table 3. Evidence for the DC genome assignment of the tetraploid species in the section Pachycarpa.
| Species | FISH | Molecular marker | Chromosome pairing behaviour |
|---|---|---|---|
| A. insularis | Linares et al. [19] | Yan et al. [13] | Loskutov [46] |
| A. maroccana | Fominaya et al. [35]; Linares et al. [19] | Oliver et al. [47, 48]; Chew et al. [18]; Yan et al. [13, 20] | Ladizinsky [49] |
| A. murphyi | Linares et al. [19] | Peng et al. [6, 7, 50]; Yan et al. [13, 20] |
The tetraploid progenitor of hexaploid oats
No conclusive agreement has been reached regarding which tetraploid species may have contributed to the hexaploid oat genome. Examining the existing literature, all of the three tetraploid species in the section Pachycarpa have been postulated to be the tetraploid ancestor of the hexaploids at one time or another [10, 13–15, 20]. In the current study, the signal patterns produced by the probes used revealed that the three tetraploids were closely related, but well differentiated from each other. For instance, the number of intergenomic translocations in A. murphyi (Fig 2N) differed from that in A. insularis (Fig 2B) and A. maroccana (Fig 2H), while the signal patterns produced by (TCC)5 allowed for the differentiation of A. maroccana (Fig 2J) from the other two tetraploids (Fig 2D and 2P). However, none of these tetraploids showed a FISH karyotype that is better matched to the hexaploids than the others in this study. One possibility is that it wasn’t one of the extant tetraploids that participated in the formation of the hexaploid oats, but, rather, a common ancestor that has not been identified or is now extinct. There is considerable evidence that all three tetraploids originated from the same tetraploid ancestor and then diverged from one another after several large chromosomal rearrangements and other changes in their chromosomes decreased their level of homology [17, 51, 52]. Another plausible explanation is that the genomes of the hexaploids may have experienced substantial restructuring after polyploidy took place. This hypothesis is supported by previous study, which showed significant genome downsizing after poyploidizations in genus Avena [53]. A similar phenomenon has been observed in wheat. Zhang et al. [54] observed distinct differences in multiple phenotypic traits and identified a large number of differentially expressed genes between the natural AB genome tetraploid wheat and a ploidy-reversed (from hexaploid to tetraploid) “extracted tetraploid wheat” which has AB genomes that are virtually identical to the AB sub-genomes of its bread wheat donor. In hexaploid oat, there exists a genetic mechanism that is similar to the Ph1 locus in hexaploid wheat, which ensures exclusive homologous chromosome paring in meiosis [55]. This attribute of hexaploid oat would make possible the reconstitution of its CD component by a simple backcrossing technique, and, therefore, could provide a unique opportunity to address whether and to what extend the CD component of hexaploid oat has been modified during its evolutionary history at the allohexaploid level, and provide more substantial evidence on the tetraploid ancestor of hexaploids.
Conclusions
FISH techniques with six repetitive DNA sequences showed a distinct hybridization patterns of Avena A, C and D genomes, confirming the substantial structural differences among these genomes, particular the large divergence between the C and A/D genomes. Several intergenomic translocations between the D and C genomes in hexaploid oats were detected, and such intergenomic translocations were also observed in all three tetraploids in the section Pachycarpa, providing good evidence for the presence of the D genome in these tetraploids, hence supporting a final re-designation of these tetraploids as CD genomes.
Supporting information
Acknowledgments
We gratefully acknowledge the fellows in Triticeae Research Institute who gave us professional and technical assistance during the experiment, and we thank Dr. Eric N. Jellen, Brigham Young University, Provo, Utah, USA, for kindly providing the seeds of A. insularis.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (Grant No. 32072025, 31801430), Sichuan International (Hong Kong/Macao/Taiwan) Innovation Cooperation in Science and Technology (Grant No. 2019YFH0125) and Jilin Scientific and Technological Development Program (Grant No. 20200402034NC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Food and agriculture organization of the United Nations—statistics division. 2020 [cited 2020.7.15]. http://www.fao.org/faostat/zh/#data/QC [Internet]. Available from: http://faostat.fao.org/.
- 2.Zimmer CM, Ubert IP, Pacheco MT, Federizzi LC. Molecular and comparative mapping for heading date and plant height in oat. Euphytica. 2018;214(6): 101 [Google Scholar]
- 3.Baum BR. Oats: wild and cultivated. A monograph of the genus Avena L. (Poaceae). Ottawa, Canada: Minster of Supply and Services; 1977. [Google Scholar]
- 4.Coffman FA. Oat history, identification and classfication. Washington D. C, USA: Agricultural Research Service, United States Department of Agriculture; 1977. [Google Scholar]
- 5.Zhou P, Yan H, Peng Y. Hexaploid ancestor of cultivated hexaploid oats inferred from high throughput GBS-SNP markers. Acta Agronomica Sinica. 2019;45: 1604–1612. In Chinese with English abstract. [Google Scholar]
- 6.Peng Y, Wei Y, Baum BR, Yan Z, Lan X, Dai S, et al. Phylogenetic inferences in Avena based on analysis of FL intron2 sequences. 2010;121: 985–1000. 10.1007/s00122-010-1367-9 [DOI] [PubMed] [Google Scholar]
- 7.Peng Y, Wei Y, Baum BR, Zheng Y. Molecular diversity of the 5S rRNA gene and genomic relationships in the genus Avena (Poaceae: Aveneae). Genome. 2008;51(2): 137–154. 10.1139/g07-111 [DOI] [PubMed] [Google Scholar]
- 8.Leggett J, Thomas H. Oat evolution and cytogenetics In: Welch RW, editor. The oat crop: production and utilization. Dordrecht: Springer; 1995. [Google Scholar]
- 9.Jellen E, Gill B, Cox T. Genomic in situ hybridization differentiates between A/D- and C-genome chromatin and detects intergenomic translocations in polyploid oat species (genus Avena). Genome. 1994;37: 613–618. 10.1139/g94-087 [DOI] [PubMed] [Google Scholar]
- 10.Li C, Rossnagel B, Scoles G. Tracing the phylogeny of the hexaploid oat Avena sativa with satellite DNAs. Crop Sci. 2000;40: 1755–1763. [Google Scholar]
- 11.Fu Y. Oat evolution revealed in the maternal lineages of 25 Avena species. Sci Rep. 2018;8: 4245 10.1038/s41598-018-20586-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Lin L, Zhou X, Peterson P, Wen J. Unraveling the evolutionary dynamics of ancient and recent polyploidization events in Avena (Poaceae). Sci Rep. 2017;7: 41944 10.1038/srep41944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, Bekele W, Wight C, Peng Y, Langdon T, Latta R, et al. High‑density marker profiling confirms ancestral genomes of Avena species and identifies D‑genome chromosomes of hexaploid oat. Theor Appl Genet. 2016;129: 2133–2149. 10.1007/s00122-016-2762-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladizinsky. A new species of Avena from Sicily, possibly the tetraploid progenitor of hexaploid oats. Genet Resour Crop Evol. 1998;45: 263–269. [Google Scholar]
- 15.Ladizinsky G, Zohary D. Notes on species delimination, species relationships and polyploidy in Avena L. Euphytica. 1971;20(3): 380–395. [Google Scholar]
- 16.Leggett JM, Thomas HM, Meredith MR, et al. , Intergenomic translocations and the genomic composition of Avena maroccana Gdgr. revealed by FISH. Chromosome Res. 1994;2(2): 163–164. 10.1007/BF01553495 [DOI] [PubMed] [Google Scholar]
- 17.Shelukhina OY, Badaeva ED, Loskutov IG, Pukhal’sky VA. A comparative cytogenetic study of the tetraploid oat species with the A and C genomes: Avena insularis, A. magna, and A. murphyi. Russ J Genet. 2007;43(6): 747–761. [PubMed] [Google Scholar]
- 18.Chew P, Meade K, Hayes A, Harjes C, Bao Y, Beattie AD, et al. A study on the genetic relationships of Avena taxa and the origins of hexaploid oat. Theor Appl Genet. 2016;129(7): 1405–1415. 10.1007/s00122-016-2712-4 [DOI] [PubMed] [Google Scholar]
- 19.Linares C, Ferrer E, Fominaya A. Discrimination of the closely related A and D genomes of the hexaploid oat Avena sativa L. Proc Natl Acad Sci. 1998;95: 12450–12455. 10.1073/pnas.95.21.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan H, Baum BR, Zhou P, Wei Y, Ren C, Xiong F, et al. Phylogenetic analysis of the genus Avena based on chloroplast intergenic spacer psbA–trnH and single-copy nuclear gene Acc1. Genome. 2014;57: 267–277. 10.1139/gen-2014-0075 [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Li X, Zhou X, Li M, Zhang F, schwarzacher T, et al. The repetitive DNA landscape in Avena (Poaceae): chromosome and genome evolution defined by major repeat classes in whole-genome sequence reads. BMC Plant Biol. 2019;19: 226 10.1186/s12870-019-1769-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehrotra S, Goyal V. Repetitive sequences in plant nuclear DNA: types, distribution, evolution and function. Genom, Proteom Bioinf. 2014;12(4): 164–171. 10.1016/j.gpb.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao X, Lu J, Zhang Z, Hu J, Huang S, Jin W. Comparison of the distribution of the repetitive DNA sequences in three variants of Cucumis sativus reveals their phylogenetic relationships. J Genet Genomics. 2011;38: 39–45. 10.1016/j.jcg.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Sun C, S Z, Hou X, Bonnema G. Cytogenetic diversity of simple sequences repeats in morphotypes of Brassica rapa ssp. chinensis. Front Plant Sci. 2016;7: 1049 10.3389/fpls.2016.01049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Z, Liu H, He Q, Pu M, Chen J, Lai J, et al. Differential genome evolution and speciation of Coix lacryma-jobi L. and Coix aquatica Roxb. hybrid guangxi revealed by repetitive sequence analysis and fine karyotyping. BMC Genomics. 2014;15(1): 1025 10.1186/1471-2164-15-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolano B, Gardunia BW, Michalska M, Bonifacio A, Fairbanks DJ, Maughan PJ, et al. Chromosomal localization of two novel repetitive sequences isolated from the Chenopodium quinoa Willd. genome. Genome. 2011;54: 710–717. 10.1139/g11-035 [DOI] [PubMed] [Google Scholar]
- 27.Carmona A, Friero E, de Bustos A, Jouve N, Cuadrado A. Cytogenetic diversity of SSR motifs within and between Hordeum species carrying the H genome: H. vulgare L. and H. bulbosum L. Theor Appl Genet. 2013;126: 949–961. 10.1007/s00122-012-2028-y [DOI] [PubMed] [Google Scholar]
- 28.Fu S, Chen L, Wang Y, Li M, Yang Z, Qiu L, et al. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci Rep. 2015;5(1): 10552 10.1038/srep10552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi W, Tang Z, Tang S, Yang Z, Luo J, Fu S. New ND-FISH-positive oligo probes for identifying thinopyrum chromosomes in wheat backgrounds. Int J Mol Sci. 2019;20(8): 2031 10.3390/ijms20082031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuadrado A, Jouve N. Chromosomal detection of simple sequence repeats (SSRs) using nondenaturing FISH (ND-FISH). Chromosoma. 2010;119(5): 495–503. 10.1007/s00412-010-0273-x [DOI] [PubMed] [Google Scholar]
- 31.Dou Q, Liu R, Yu F. Chromosomal organization of repetitive DNAs in Hordeum bogdanii and H. brevisubulatum (Poaceae). Comp Cytogenet. 2016;10(4): 465–481. 10.3897/CompCytogen.v10i4.9666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo X, Tinker NA, Zhou Y, Liu J, Wan W, Chen LJAPP. A comparative cytogenetic study of 17 Avena species using Am1 and (GAA)6 oligonucleotide FISH probes. Acta Physiol Plant 2018;40(8): 145. [Google Scholar]
- 33.Luo X, Tinker NA, Zhou Y, Liu J, Wan W, Chen LJGR, et al. Chromosomal distributions of oligo-Am1 and (TTG)6 trinucleotide and their utilization in genome association analysis of sixteen Avena species. Genet Resour Crop Evol. 2018;65(6): 1625–1635. [Google Scholar]
- 34.Luo X, Tinker NA, Zhou Y, Wight CP, Liu J, Wan W, et al. Genomic relationships among sixteen Avena species based on (ACT)6 trinucleotide repeat FISH. Genome. 2018;61(1): 63–70. 10.1139/gen-2017-0132 [DOI] [PubMed] [Google Scholar]
- 35.Fominaya A, Loarce Y, Montes A, Ferrer E. Chromosomal distribution patterns of the (AC)10 microsatellite and other repetitive sequences, and their use in chromosome rearrangement analysis of species of the genus Avena. Genome. 2017;60(3): 216–227. 10.1139/gen-2016-0146 [DOI] [PubMed] [Google Scholar]
- 36.Cuadrado A, Schwarzacher T. The chromosomal organization of simple sequence repeats in wheat and rye genomes. Chromosoma. 1998;107(8):587–594. 10.1007/s004120050345 [DOI] [PubMed] [Google Scholar]
- 37.Komuro S, Endo R, Shikata K, Kato A. Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome. 2013;56(3): 131–137. 10.1139/gen-2013-0003 [DOI] [PubMed] [Google Scholar]
- 38.Solano R, Hueros G, Fominaya A, Ferrer E. Organization of repeated sequences in species of the genus Avena. Theor Appl Genet. 1992;83(5): 602–607. 10.1007/BF00226904 [DOI] [PubMed] [Google Scholar]
- 39.Gerlach W, Dyer T. Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Res. 1980;8(21): 4851–4865. 10.1093/nar/8.21.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanz MJ, Jellen EN, Irigoyen ML, Ferrer E, Fominaya A. A new chromosome nomenclature system for oat (Avena sativa L. and A. byzantina C. Koch) based on FISH analysis of monosomic lines. Theor Appl Genet. 2010;121(8): 1541–1552. 10.1007/s00122-010-1409-3 [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Zhang W, Cao Y, Zhang Z, Zheng D, Zhou B, et al. Localization of high level of sequence conservation and divergence regions in cotton. Theor Appl Genet. 2012;124(7): 1173–1182. 10.1007/s00122-011-1777-3 [DOI] [PubMed] [Google Scholar]
- 42.Sonah H, Deshmukh R, Sharma A, Singh V, Gupta DK, Gacche RN, et al. Genome-wide distribution and organization of microsatellites in plants: an insight into marker development in Brachypodium. Plos One. 2011;6: e21298 10.1371/journal.pone.0021298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuadrado A, Jouve N. The nonrandom distribution of long clusters of all possible classes of trinucleotide repeats in barley chromosomes. Chromosome Res. 2007;15(6): 711–720. 10.1007/s10577-007-1156-8 [DOI] [PubMed] [Google Scholar]
- 44.Morgante M, Hanafey MK, Powell W. Microsatellites are preferentially associated with nonrepetitive DNA in plant genomes. Nat Genet. 2002;30(2): 194–200. 10.1038/ng822 [DOI] [PubMed] [Google Scholar]
- 45.Jellen EN, Phillips RL, Rines HW. C-banded karyotypes and polymorphisms in hexaploid oat accessions (Avena spp.) using Wright's stain. Genome. 1993;36(6): 1129–1137. 10.1139/g93-151 [DOI] [PubMed] [Google Scholar]
- 46.Loskutov IG. Interspecific crosses in the genus Avena L. Russ J Genet. 2001;37: 467–475. [PubMed] [Google Scholar]
- 47.Loskutov IG. Interspecific crosses in the genus Avena L. Russ J Genet. 2001;37: 467–475.Oliver RE, Jellen EN, Ladizinsky G, Korol AB, Kilian A, Beard JL, et al. New Diversity Arrays Technology (DArT) markers for tetraploid oat (Avena magna Murphy et Terrell) provide the first complete oat linkage map and markers linked to domestication genes from hexaploid A sativa L. Theor Appl Genet. 2011;123(7): 1159. [DOI] [PubMed] [Google Scholar]
- 48.Oliver RE, Tinker NA, Lazo GR, Chao S, Jellen EN, Carson ML, et al. SNP discovery and chromosome anchoring provide the first physically-anchored hexaploid oat map and reveal synteny with model species. Plos One. 2013;8(3): e58068 10.1371/journal.pone.0058068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ladizinsky. Studies in oat evolution: a man's life with Avena. Heidelberg, Germany: Springer; 2012. [Google Scholar]
- 50.Peng Y, Zhou P, Zhao J, Li J, Lai S, Tinker NA, et al. Phylogenetic relationships in the genus Avena based on the nuclear Pgk1 gene. Plos One. 2018;13(11): e0200047 10.1371/journal.pone.0200047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fominaya A, Vega C, Ferrer E. C-banding and nucleolar activity of tetraploid Avena species. Genome. 1988;30(5): 633–638. [Google Scholar]
- 52.Jellen EN, Ladizinsky G. Giemsa C-banding in Avena insularis Ladizinsky. Genet Resour Crop Evol. 2000;47(3): 227–230. [Google Scholar]
- 53.Yan H, Martin SL, Bekele WA, Latta RG, Diederichsen A, Peng Y, et al. Genome size variation in the genus Avena. Genome. 2016;59(3):209–220. 10.1139/gen-2015-0132 [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Zhu B, Qi B, Gou X, Dong Y, Xu C, et al. Evolution of the BBAA component of bread wheat during its history at the allohexaploid level. Plant Cell. 2014;26(7): 2761–2776. 10.1105/tpc.114.128439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajhathy T, Thomas H. Genetic control of chromosome pairing in hexaploid oats. Nat New Biol. 1972;239: 217–219. 10.1038/newbio239217a0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.