Abstract
Immune check-point inhibitors (ICIs) have changed our view on how to treat cancer. Despite their approval in treatment of many different cancers, efficacy of immune check-point inhibitors (ICI) in neuroendocrine neoplasia is limited and poorly understood. Established treatment options of neuroendocrine tumors (NET) and neuroendocrine carcinomas (NECs) are based on surgery, tumor-targeted medical treatments, Peptide Receptor Radionuclide Therapy (PRRT), and locoregional therapies. However, in many patients these treatments lose efficacy over time, and novel therapies are urgently needed. We report on 8 patients diagnosed with neuroendocrine neoplasms (NEN) that were treated with ICI (pembrolizumab, avelumab, nivolumab plus ipilimumab) as salvage therapy. In this cohort, we observed tumor response with partial remission in 3 patients and stable disease in 1 patient. Four patients showed progressive disease. Of note, responses were observed both in PD-L1 positive and PD-L1 negative patients. Here, we discuss clinical courses of these patients in the context of available literature to highlight limitations and drawbacks currently preventing the use of ICI in routine management of patients with NEN.
Keywords: immune check-point inhibitors, neuroendocrine tumor, PD-L1, response, survival
1. Introduction
Neuroendocrine neoplasms (NEN) represent an uncommon disease group that originates from the diffuse neuroendocrine system with an incidence of 7/100,000 and a prevalence of 40/100,000 in most countries.[1] According to the World Health Organization (WHO), NEN are subclassified based on their Ki-67 proliferation index and histological differentiation into low (grade 1; G1), intermediate (grade 2; G2), and high (G3) grade NEN.[2] The latter are further subdivided into neuroendocrine tumors (NET G3) and NEC based on differences in cell morphology, proliferation, response to chemotherapy, as well as patients’ outcome.[3] In most cases, abdominal NEN are localized in ileum, pancreas, or stomach, extrabdominal locations (e.g., thoracic, genitourinary) are comparatively rare. Patients display a 5-years and 10-years survival of 90% and 63% (ileum), 69% and 62% (pancreas), or 85% and 56% (stomach), respectively.[4]
Current guidelines for the treatment of advanced NET recommend antiproliferative therapy with somatostatin analogues, Peptide Receptor Radionuclide Therapy (PRRT), or systemic therapies including streptozotocin, temozolomide, everolimus, or sunitinib.[5,6] In case of advanced NEC, platinum-based chemotherapeutic regimens represent the standard of care.[7,8] Despite the fact that patients with NET or NEC in almost all cases display an initial response to therapy, many patients develop resistance at early time-points during the course of disease and have only limited prognosis. Hence, alternative therapies are needed.
The recent introduction of immune checkpoint inhibitors has changed treatment algorithms for many patients, including many types of gastrointestinal cancers.[9] PD-1/PD-L1 is the best studied immune check-point in the context of cancer.[10] PD-1 represents an inhibitory receptor, which is found on different immune cells including T- and B-cells as well as natural killer cells.[10] Binding of PD-1 to PD-L1 activates the receptor complex, leading to downregulation of immune cell activation, proliferation, survival, and cytokine production.[11] While, under physiological conditions, PD-1/PD-L1 protects normal tissue against recognition by the immune system, tumor cells upregulate expression of PD-L1 as a mechanism to evade the immune response, allowing the tumor to grow and to develop metastases in the course of disease.[12,13] Recently, specific inhibitors of the PD-1/PD-L1 system have been successfully used for the treatment of different malignancies including lung cancer, malignant melanoma, and hepatocellular carcinoma, just to name a few.[14] However, in the context of NEN, only very few data are available. Pembrolizumab and spartalizumab have been tested in patients with NEN, but revealed an overall limited antiproliferative activity at least in non-selected patients.[15–18] Thus, many authors concluded that a better identification of patients with a high likelihood for tumor response before treatment will be mandatory before immune check-point inhibitors can be used regularly in the clinical management of patients with NET.[19] Avelumab is an agent that has been largely studied in Merkel cell neuroendocrine tumors being approved as first- and second-line treatment of adult Merkel cell carcinoma since September 2017.[20,21]
Here we report a real-life study of 8 NEN-patients that have been treated at our outpatient unit with immune check-point inhibitors (ICI) (pembrolizumab, avelumab, nivolumab plus ipilimumab) as salvage therapy and discuss these cases in the context of available literature.
2. Patients and methods
2.1. Study design and study population
The retrospective study was conducted in a tertiary health care center that provides advanced specialty care to patients with NEN. Our NEN database comprises 612 patients with histologically proven diagnosis of NEN from 2008 to 2019 for cases of neuroendocrine tumors and neuroendocrine carcinoma treated with PD-L1 inhibitors. The database includes information on primary and metastatic tumor localizations, histology, including mitotic rate or Ki67 proliferation index, diagnostic methods used for detection of primary and metastatic tumors, classification according to staging and grading as recently described.[22] We could identify 8 patients fulfilling our inclusion criteria (patients characteristics are given in Table 1, individual treatment schedules are summarized in Table 2, the individual course of disease is summarized in supplement Tables 1–8). Patients included in our study had to fulfill the following criteria:
Table 1.
Clinical, immunohistochemical, and molecular features of 8 patients with NEN with ICI.
| No. | Sex | Age | Date of diagnosis | Diagnosis | Distant metastases | Ki67 | Immunohistochemistry | Molecular analysis |
| 1 | m | 49 | 04/2010 | NET G2, larynx | Lymph node, pulmonal cerebral, orbital, cutaneus, testicular, osseus | 12% (04/10)15% (09/16)10% (01/20) | Synaptophysin +, chromogranin A+, CEA+, EMA+, pan-cytokeratin+, SMA−, S100−, CT31−, PD-L1 < 1% | Several SNV's in POLE, BRCA1, BRACA2, CHEK2, HRAS |
| 2 | f | 63 | 01/2005 | NET G2/G3, kidney | Liver, lymph node, bone | 5% (01/05)7% (08/07)20% (04/10)25% (08/19) | Synaptophysin+, chromogranin A +, NSE+, S 100+, vimentin+, CK7−, PD-L1 1% | No mutation in MSH2, MSH6, PMS2, MLH1 |
| 3 | m | 55 | 08/2012 | NET G2/NEC G3, pancreas tail | Liver, lymph node, spleen, kidney, adrenal, peritoneal carcinomatosis | 20% (08/12)50% (10/16)35% (06/17) | Synaptophysin+, CD56+, CK20+, PD-L1 30% | No mutation in MSH2, MSH6, PMS2, MLH1 |
| 4 | m | 68 | 09/2010 | Functional, NET G2/NEC G3, gastric | Liver, lymph nodes, peritoneal carcinomatosis, bone | 5% (09/10)50% (11/13)>70% (10/14) | Synaptophysin+, chromogranin A+, pan-cytokeratin+, CD 74+, VMAT2+>EP-CAM+, CDX2−, TTF1−, ISL1−, serotonin−, PD-L1 5% | No mutation of HER2NEU, BRAF, K-RAS, NRASMUC1 10% |
| 5 | f | 44 | 04/2015 | NET G3, pancreas head | Liver, lymph node | 26% | Synaptophysin+, chromogranin A+, SSTR2- CDX2+, SSTR2−, PD-L1−, RET+ | No mutation in MSH2, MSH6, PMS2, MLH1 |
| 6 | m | 46 | 07/2013 | NET G1, pancreas head | Liver, lymph node | 2–3% | Synaptophysin+, CD 56+, PDX1+, CKMNF+, SSTR2 +, PD-L1− | SNV in MSH2 06/18 |
| 7 | m | 44 | 04/2012 | Mixed neuroendocrine-non-neuroendocrine neoplasm (MINEN) | Diaphragm, pleural, liver, lymph node | Synaptophysin+, CD56+, PD-L1− | SNV in MSH2, MSH6, POLE NR-21, BAT-26, BAT-25, NR-24, Mono-27 | |
| 8 | m | 69 | 03/2017 | NEC G3, duodenum | Liver | 80% | Synaptophysin+, chromogranin A+, CDX2+, CD56−, TTF−, insulin−, serotonin−, CD3−, CD20−, PD-L1 5% | No mutation in EGRF, KRAS, NRAS, BRAF, PIK3CA, HER2NEU, EML4, ALK, RET, ROS |
Table 2.
Treatment schedules and follow-up of eight patients with NEN treated with ICI.
| No. | Surgery | Locoregional procedures/Radiotherapy/PRRT | Chemotherapy/targeted therapy/SSA | ICI therapy agent | Outcome and duration of therapy | ICI-related-side effects | Follow-up 02/2020 |
| 1 | 04/2010 local resection of left ary cartilage08/2012 right neck dissection with lymph node resection02/15 right orchiectomy09/16 resection of cutaneous metastasis | 11/16 stereotactic radiation of orbital metastasis (54 Gy)06/17 stereotactic radiation of cerebral metastases (49,4 Gy)11/17 stereotactic radiation of cerebellar metastasis (25,6 Gy)10/18 stereotactic radiation of retrotracheal lymph node | 11/12-02/14 temozolomide12/14-09/15 FOLFOX06-09/16 everolimus10/16-03/17 CAP/TEM | 04/17-01/20 pembrolizumab | PR, 31 months | No | Alive, PD after 32 months PR |
| 2 | 01/05 right nephrectomy07/07 lymphadenectomy04/10 retrocaval tumor with partial resection of the inferior vena cavalymphadenectomy | 04/13-01/16 PRRT (3cycles)11/14- 01/18 TAE (5 cycles)05/18-06/18 SIRT01/19 brachytherapyin afterloading technique | 09/10-03/13 SSA05/12-12/12 everolimus + SSA06/16-09/17 TEM/CAP | Since 10/19 pembrolizumab | PR, at least 3 months | No | Alive, PR |
| 3 | None | 10/16 Brachytherapy in afterloading technique | 08/12-06/13 FOLFOX 06/13-01/17 CAP/TEM 01-04/17 Everolimus04-05/17 FOLFIRI | Since 07/17 pembrolizumab | PR at least 30 months | Recurrent pneumonitis | Alive, PR |
| 4 | 11/10 atypical partial gastric resection, resection of multiple liver metastasis12/11 anterior rectal resection with appendectomy, splenectomy, parietal peritonectomy with omentectomy and HIPEC11/13 relaparotomy with tumor debulking05/14 resection of pancreatic lymph node09/14 resection of metastases of the skin03/16 resection of peritoneal metastases, HIPEC02/17 robot-assisted mediastinal lymphadenectomy | 01/11-11/11 TACE (3 cycles)03-09/11 PRRT01/13-12/16Brachytherapyin afterloading technique (5 cycles)04/16 radiosurgery of metastasis of sacral bone (20Gy) | 10/10 sandostatin01-04/12 CAP/TEM (12cycles)02-04/12 ipilimumab04-07/13 peginterferon alpha2b08/13-12/14 immunotherapy including vaccination of transfected autologous tumor03/16 HIPEC08/16 – 04/17 immunotherapy including vaccination of transfected autologous tumor03/17 sunitinib06/17 everolimus | 09-11/14 pembrolizumab11/14-06/16 pembrolizumab + ipilizumab08/16 atezolizumab04/17-10/19 pembrolizumab + ipilizumab | SD at least 35 months | Recurrent pneumonitis | Alive, SD |
| 5 | 04/15 partial pancreaticoduodenectomy with partial resection of the stomach08/17 liver wedge resection | None | 02-04/16 STU/5FU06-09/09 CAP/TEM09-12/16 FOLFOX-IV01-07/17 everolimus12/17-03/18 sunitinib | 03-05/18 pembrolizumab | PD, 3 months | No | Death (06/18) |
| 6 | None | 04-10/16 PRRT (3 cycles) | 01/14-12/14 CAP/TEM 11/15 STZ/5-FU 02-05/17 FOLFOX 05/17 FOLFIRI 12/17-02/18 everolimus 03-05/18 sunitinib 06-10/18 CAP/TEM | 09-11/18 avelumab | PD, 3 months | No | Death (12/18) |
| 7 | 04/12 right and left s hemicolectomy09/15 resection of diaphragm metastasis12/17 diagnostic laparoscopy | 11/2018 radiotherapy of pleural metastases | 04-08/12 cisplatin/etoposide09/16-01/17 FOLFOX01-10/17 FOLFIRI11/18-06/19 CAP/TEM | 03-09/18 pembrolizumab | PD, after 6 months SD | Diarrhea, asthma, sleep disorder | Alive, PD |
| 8 | None | None | 03/17- 01/18 carboplatin /etoposide02-05/2018 FOLFOX605-07/2018 FOLFIRI | 09/18-11/18 pembrolizumab | PD, 3 months | No | Death (02/19) |
diagnosis of NEN according to WHO classification 2010,[2]
failure of standard treatment, which is defined as failure after guideline-based chemotherapy, PRRT radio- and/or brachytherapy,[6,23]
ICI treatment at our institution between 2008 and 2019.
Data were extracted from electronic medical charts into a standardized case report form. The study protocol was reviewed and approved by the institutional ethics committee (ethical approval number EA1/229/17) and was done in accordance with the Declaration of Helsinki.
For classification of NEN the grading system according to WHO classification 2010 was used.[2] Assessment of disease progression was performed according to radiological imaging modalities (computed tomography (CT), MR) and evaluated according to objective response evaluation criteria (RECIST 1.1). In some cases, we used quantitative functional imaging evaluation (FDG-PET-CT, DOTATOC PET-CT, somatostatin receptor scintigraphy). Adverse events were evaluated and graded according to Common Terminology Criteria for Adverse Events (CTCAE).[24] Quality of life (QoL) was assessed regularly (at least every 6 months) based on standardized questionnaires of the European Organization for Research and Treatment of Cancer (EORTC).
For literature review, PUBMED and ClinicalTrials.gov were searched using a combination of the following keywords: neuroendocrine neoplasia, carcinoid, neuroendocrine carcinoma, mutational load, PD-1, PD-L1, nivolumab, pembrolizumab, ipilimumab, avelumab. Published literature was reviewed with respect to demographic data (age, sex) as well as clinical features including metastases, symptoms, complications, treatment, and diagnostic methods.
3. Individual case presentation
We present a series of 8 patients with histologically confirmed diagnosis of neuroendocrine neoplasms, who were treated at our outpatient unit with ICI after failure of standard therapies (as defined in Section 2). Patient characteristics and therapy schedules (including therapy response and therapy-associated side effects) are provided in Tables 1 and 2. The individual course of disease is summarized in supplement Tables 1–8.
3.1. Case 1
A 49-year-old male patient was diagnosed with a NET G2 of the larynx in April 2010.
The patient initially presented with hoarseness to his otorhinolarnygologist, who detected a tumor of the left ary cartilage during laryngoscopy. Subsequently, local resection of the left ary cartilage was performed. After more than 2 years of tumor free survival, a relapse of the primary tumor with synchronous lymph node (cervical, hilar, mediastinal) and pulmonary metastases became apparent in August 2012. Two weeks later, the patient was admitted to right neck dissection with lymph node resection. Histological and immunohistochemical analysis of the resected tumor and lymph nodes revealed a moderately-differentiated NET with expression of synaptophysin, chromogranin A, CK7, pan-cytokeratin, and negativity for TTF-1, CK5/6. Ki-67 was up to 12%. Surgery was followed by 15 cycles temozolomide monotherapy, which resulted in stable disease according to RECIST until follow-up revealed progressive disease with appearance of testicular metastases in November 2014. Subsequently, the patient underwent right orchiectomy, followed by FOLFOX-based chemotherapy,[25,26] which had to be discontinued after 12 cycles due to peripheral motor neuropathy Grade 2 according to CTCAE.[24] In October 2016, after failure of everolimus (10 mg/day, given between June and September 2016), combination chemotherapy with capecitabine/temozolomide (CAP/TEM[27]) was initiated. In parallel, stereotactic radiation therapy of a newly diagnosed orbital metastasis and resection of a small cutaneous metastasis on the right upper arm were performed. Molecular analysis of resected tumor tissue showed several single nucleotide variants (SNV) within the DNA polymerase epsilon (POLE) gene, several mutations in DNA repair genes such as BRCA1, BRACA2, CHEK2, as well as HRAS mutations. Since POLE gene mutations are associated with hypermutations, which are a positive predictive factor for ICI treatment response,[28–32] we initiated therapy with pembrolizumab (2 mg/kg, initially 150 mg intravenously (i.v.) every 21 days) in April 2017. Notably, this treatment resulted in a partial remission according to RECIST 1.1 of pulmonary, osseous, cutaneous, and cerebral metastases (Fig. 1a and b). However, 3 months later a newly diagnosed cerebellar metastasis was treated with stereotactic radiation therapy. Since all other lesions showed enduring response to ICI, this therapy was continued concomitantly. In the following year, the patient showed stable disease. In October 2018, isolated progression of a retrotracheal lymph node was detected and treated with stereotactic radiation therapy. Based on the long lasting tumor response of most of the metastases,[33] therapy with pembrolizumab was continued. When the patient presented with hemoptysis in November 2019, a bronchoscopy was performed, revealing a tumor stenosis of the right main bronchus, due to metastatic infiltration. The patient also developed pneumonitis Grade 2 according to CTCAE criteria as a side effect of ICI and was treated successfully with systemic steroids. In this clinical setting, we decided to discontinue treatment with pembrolizumab in January 2020 and to switch the therapy regimen to CAP/TEM.[27] Overall QoL during ICI treatment was reported between good and excellent.
Figure 1.
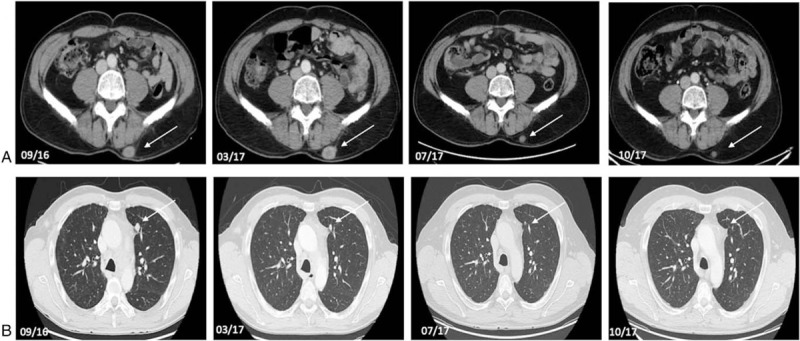
Axial contrast-enhanced CT-scan of abdomen and chest of a 49-year-old male patient (#1) with a NET G2 of the larynx with pulmonary, osseous, cerebral, cutaneous, and subcutaneous metastases. Axial contrast enhanced CT-scans between September 2016 and October 2017 (09/16, 03/17, 07/17, 10/17) show regression of subcutaneous (a) and pulmonary metastases (b) during therapy with pembrolizumab. Tumor lesions are indicated by white arrows.
3.2. Case 2
A 63-year-old female patient was diagnosed with a NET G2 of the right kidney in January 2005, which progressed into a NET G3 with a Ki-67 up to 25% during course of disease.
The patient initially presented for a routine check-up to her family practitioner, when a mass of the right kidney was detected in sonography. Further clinical work-up including biopsy and a DOTATOC/PET-CT revealed a somatostatin receptor (SSR) positive moderately-differentiated NET of the right kidney. No distant metastases could be found. Subsequently, a right nephrectomy (in curative intention) was performed. Histological and immunohistochemical analysis of the resected tumor showed positive expression of synaptophysin, chromogranin A, vimentin, neuron specific enolase (NSE), S100 protein, and negativity for CK 7. Initially, Ki-67 was up to 5%. In the following years recurrent lymph node metastases (07/07, 02/10) and a retrocaval local relapse were detected and followed by retrocaval tumor resection with partial resection of the inferior vena cava and lymphadenectomy in April 2010. Further immunohistochemical analysis revealed a Ki-67 up to 20%. DOTATOC/PET-CT displayed newly diagnosed SSR positive liver metastases. Thus, we initiated treatment with the somatostatin analogue lanreotide, using its antiproliferative effects. In the following months staging examinations showed stable disease until progression of liver metastases and newly diagnosed bone metastases were detected in April 2012. In this setting, we started treatment with everolimus (10 mg/day). Although therapy was initially well-tolerated, it had to be reduced (5 mg/day) and later discontinued due to 2nd grade mucositis according to CTCAE[24] and generalized pruritus. In the following months progressive disease was detected. Considering the positive SSR status, we performed PRRT Lut-177-DOTATATE (3 cycles), which led to stable disease, but also to a drop in leucocyte count as well as recurrent fever episodes. In the following months, the patient underwent 3 cycles of transarterial embolization (TAE) which resulted in further progressive disease with a hepatic tumor load of 15% in October 2015. In this clinical setting, further PRRT cycles were refused by the patient. Staging examinations in June 2016 detected further progression. Thus, we initiated chemotherapy with CAP/TEM[27] in June 2016, which had to be discontinued due to further progression according to RECIST 1.1. in September 2017. In the following months, we performed 2 more cycles of TAE, selective internal radiation therapy (SIRT) as well as 1 cycle of brachytherapy in afterloading technique in January 2019 which initially resulted in size reduction of liver metastases. Immunohistological analysis of a biopsy taken during afterloading in January 2019 displayed a Ki-67 of 25%, positive PD-L1 expression, and no deficiency in mismatch repair proteins (Fig. 2a–d). Nevertheless, in August 2019, further progressive disease with size increase of liver metastases according to RECIST 1.1 and newly diagnosed subphrenic lesions was detected. In this clinical setting, we initiated treatment with pembrolizumab (2 mg/kg, initially 150 mg i.v. every 21 days) in October 2019. Therapy was well-tolerated and staging examinations revealed partial remission with a hepatic tumor load reduction of 40% according to RECIST 1.1. after 3 months (Fig. 3a and b).
Figure 2.
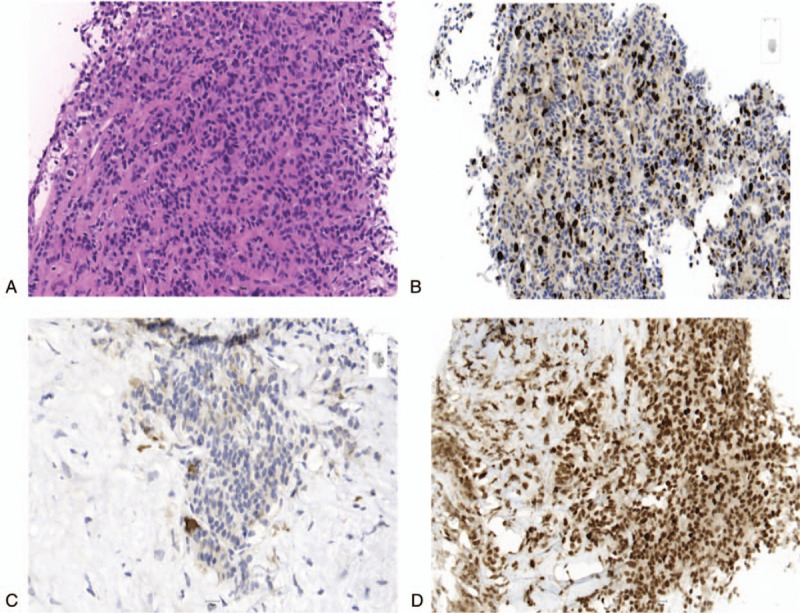
Immunohistochemical expression of tumor cells of hepatic metastases of a 63-year-old female patient (#2), who was initially diagnosed with a NET G2 of the right kidney, which progressed into a NET G3 during course of disease. H&E stain (20×) of the tumor cells of hepatic metastases shows cells with moderate nuclear pleomorphism, finely speckled chromatin and finely granular eosinophilic cytoplasm (a). Immunohistochemical staining (20×) reveals a Ki-67 proliferation index of 25% (b) few scattered cells with membranous PD-L1 expression (c) and no deficiency in mismatch repair proteins such as MSH6 (d).
Figure 3.
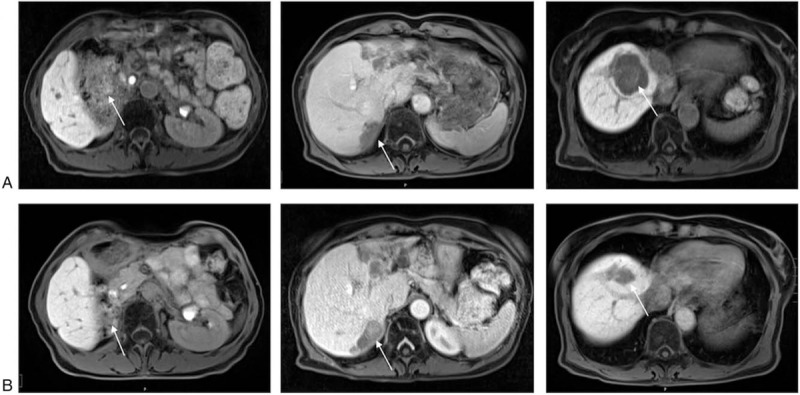
Axial contrast-enhanced MR-scans of the abdomen of a 63-year-old female patient (#2) with a NET G3 of the right kidney with liver and bone metastases. Axial contrast-enhanced MR-scans of the abdomen demonstrate hepatic metastases in segments 5, 7, and 8 (indicated by white arrows) before therapy initiation with pembrolizumab in October 2019 (a). Axial MR of the abdomen in February 2020 reveals hepatic tumor load reduction of 40% according to RECIST 1.1. Hepatic metastases in segments 5, 7, and 8 are indicated by white arrows (b).
In February 2020, the patient is in a very well general condition (ECOG 0), in partial remission and therapy is continuing. During ICI treatment overall QoL was reported between good and excellent.
3.3. Case 3
We have already reported[34] about a 55-year-old male patient, who was diagnosed with a non-functional SSR-positive NET G2 of the pancreatic tail with invasion of the spleen and synchronous SSR negative bilobar liver metastases, which progressed into a NEC (Ki-67 > 50%) with additional metastasis to kidney, adrenals, peritoneum, and lymph nodes.
After several treatment, attempts including first- and second-line chemotherapy regimens (FOLFOX,[25,26] FOLFIRI,[35] CAP/TEM[27]) as well as brachytherapy in afterloading technique, disease progression continued. Based on a 30% PD-L1 expression in tumor cells, we initiated an off-label immunotherapy with pembrolizumab (2 mg/kg, initially 150 mg i.v. every 21 days) in July 2017. In our previous case report, we demonstrated partial remission until April 2018 with a reduction in hepatic tumor size of at least 66% combined with an improvement of the Karnofsky score rising from 60% to 100%. In further follow-up, after administration of 13 cycles, a CT-scan of the chest indicated disseminated bilateral ground glass opacities suggesting pneumonitis. Clinically, the patient was free of any symptoms. Thus, we started oral steroid therapy and we temporarily discontinued treatment with pembrolizumab. In October 2018, the patient started to suffer from dyspnea and joint pain. Further clinical work-up revealed bilateral rales and a recurrent pneumonitis Grade 2 according to CTCAE[24] (Fig. 4a). Again, we discontinued pembrolizumab and started anti-inflammatory monotherapy with adalimumab (initial dose 160 mg, after 2 weeks 80 mg, after 4 weeks 40 mg). After the resolution of the pneumonitis (Fig. 4b), in January 2020 the 23rd cycle of pembrolizumab was carried out, the patient is still in good general health, being fully recovered from the therapy-related side effects and still in partial remission. During treatment overall QoL was rated good to excellent.
Figure 4.
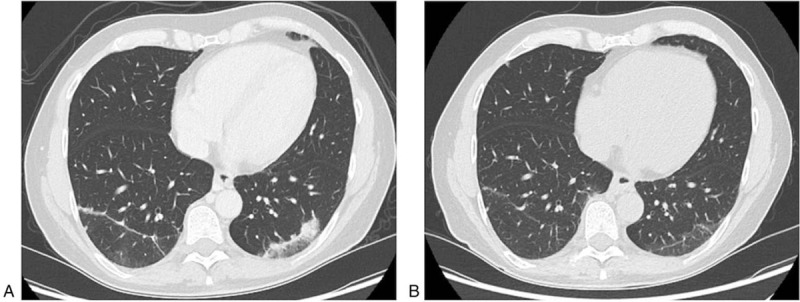
Axial contrast-enhanced CT-scans of a 50-year-old male patient (#3) with a non-functional NET G2 of the pancreatic tail, who developed pneumonitis during treatment with pembrolizumab. Axial contrast-enhanced CT-scan of the chest reveals disseminated bilateral ground glass opacities suggesting pneumonitis after several cycles of treatment with pembrolizumab in October 2018 (a). Axial contrast-enhanced CT-scan in December 2018 demonstrates regression of the ground glass opacities after initiation of treatment with adalimumab therapy and discontinuation of pembrolizumab therapy (b).
3.4. Case 4
We have already reported[36] about a 68-year-old male patient who was initially diagnosed with a functional, gastric NET G2 (Ki67 5%) with liver, bone, lymph node metastases, and peritoneal carcinomatosis in September 2010, which progressed into a NEC with a Ki-67 > 70% during the course of disease.
Between 2010 and 2016 a combination of multiple therapies such as octreotide, CAP/TEM,[27] hyperthermic intraperitoneal chemotherapy (HIPEC), PRRT, transarterial chemoembolization (TACE), and locoablative brachytherapy in afterloading technique were performed. Additionally, the patient underwent several abdominal surgeries on his own request (as demonstrated in Table 2). Despite the implementation of all possible therapies, the patient continued to show progressive disease. Considering the high mutational tumor load (2777 single nuclear variants and 21 indels), immunotherapy including vaccination with transfected autologous tumor RNA between August 2013 and December 2014 was started. Due to recurrent pneumonitis Grade 2 according to CTCAE,[24] therapy had to be stopped and was switched to atezolizumab (1200 mg every 28 days) accompanied by vaccination of transfected autologous tumor RNA in August 2016 leading to stable disease until April 2017 as we have already reported in our previous paper.[36]
In this clinical setting, combination therapy of ipilumimab and pembrolizumab was continued resulting in stable disease. In November 2017 a CT-scan of the chest indicated disseminated bilateral ground glass opacities suggesting pneumonitis. Clinical evaluation revealed pneumonitis Grade 2 according to CTCAE.[24] Therapy was temporarily discontinued and oral steroid therapy was initiated. Due to recurrent episodes of extensive hyperglycemia treatment with adalimumab rather than steroids (initial dose 80 mg, maintenance therapy 40 mg each 2 weeks) was successfully started. Despite stable disease of the NET, increasing thrombocythemia necessitated bone marrow analysis leading to the additional diagnosis of acute myeloid leukemia (AML) in 10/2019. The patient is currently receiving specific treatment leading to remission so far. During treatment QoL was reported to be between good and excellent.
3.5. Case 5
A 44-year-old female patient was diagnosed with a non-functional NET G3 of the pancreatic head in April 2015.
The patient initially presented to our outpatient unit with painless jaundice. Further clinical work-up including a multi-slice CT-scan revealed a tumor of the pancreatic head. No distant metastases were found. Subsequent surgery with partial duodenopancreatectomy and partial gastric resection was performed. Pathological work-up of the resected tumor revealed a poorly differentiated neuroendocrine tumor. Immunohistochemical analysis showed expression of synaptophysin, chromogranin, CDX2, while no SSTR2 was detected. Ki-67 was 25%. Follow-up examinations did not show any signs of recurrence until September 2015, when multiple hepatic metastases were detected. Despite administration of several chemotherapeutic regimens (STZ/5-FU,[37] CAP/TEM,[27] FOLFOX[25,26]) and later on everolimus (discontinued in July 2017) no sustained tumor response was achieved. PRRT was impossible since the tumor was negative for somatostatin receptor on imaging, this was later confirmed by expression immunohistochemical means.[38,39] Another tumor biopsy was taken and molecular characterization of the tumor was conducted, revealing strong expression of RET, no expression of PD-L1, while a loss of DNA mismatch repair genes (MSH2, MSH6, PMS2, MLH1) was not detected. Based on the overexpression of RET, treatment with sunitinib was initiated; however, not associated with tumor response. We, therefore, changed systemic therapy to salvage therapy with pembrolizumab (2 mg/kg, initially 150 mg i.v. every 21 days) in March 2018. Unfortunately, in the following weeks, the patient's general condition deteriorated, going along with progressive weight loss, increase in tumor pain, and loss of liver function, finally resulting in patient's death (June 2018).
3.6. Case 6
A 46-year-old male patient was diagnosed with a non-functional NET G1 of the pancreatic head and synchronous liver metastases in July 2013.
The patient initially presented for a routine check-up to his family practitioner, when multiple liver metastases were detected by liver sonography. The patient was referred to our outpatient unit. Multi-slice CT-scan displayed a tumor of the pancreatic head as well as bilobar liver metastases. A biopsy of the primary tumor revealed a well-differentiated neuroendocrine tumor (G1). Further immunohistochemical analysis showed expression of synaptophysin, CD 56, PDX1, and CKMNF. Ki-67 was 2% to 3%. In line with SSR2A expression by immunohistochemical analysis, DOTATOC/PET-CT imaging confirmed SSR positivity for the primary tumor and liver metastases. Curative resection rendered to be impossible due to extensive liver metastases. Cytoreductive therapy with CAP/TEM,[27] streptozotocin/5-fluorouracil (STZ/5FU),[37] PRRT, FOLFOX,[25,26] and finally FOLFIRI[35] was conducted within the next years. Moreover, targeted therapies with everolimus and sunitinib were performed without sustained tumor response. Based on the detection of a MSH2 mutation, treatment with avelumab (10 mg/kg) was initiated in September 2018. The patient did not suffer from any specific side effects, but his general condition deteriorated rapidly and the patient died from multiorgan failure in December 2018.
3.7. Case 7
We report about a patient diagnosed with a mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN) of the colon in April 2012.
A 44-year-old male patient presented initially with stool irregularities leading to a colonoscopy revealing 2 adenomas in the ascending and descending colon. The adenoma in the descending colon was diagnosed as a MiNEN with the neuroendocrine component graded as a NEC G3. The patient underwent right hemicolectomy combined with ileotransversostomy and left hemicolectomy. Immunohistochemical analysis showed positive expression of synaptophysin, CD56, and NSE in tumor cells. Surgery was followed by adjuvant chemotherapy with cisplatin/etoposide. Follow-up CT in June 2015 displayed an isolated diaphragmatic metastasis, which was surgically removed. Three months later, staging revealed further progressive disease with appearance of ileal lymph nodes as well as liver and pleural metastases. In September 2016, chemotherapy was started with FOLFOX[25,26] and later switched to FOLFIRI[35] due to severe peripheral neuropathy. In the following months, the patient was in a good general condition (ECOG 0), showing stable disease. As follow-up examinations revealed tumor progression, diagnostic laparoscopy was performed. Molecular analysis of obtained tissue samples revealed mutations in MSH2, MSH6, POLE, and in NR-21, BAT-26, BAT-25, NR-24, Mono-27. PD-L1 expression was negative. Due to further progression, we initiated treatment with pembrolizumab (2 mg/kg, initially 150 mg i.v. every 21 days) in March 2018. Follow-up examinations after 3 months revealed stable disease. However, after 6 months of stable disease, progressive disease was observed. Therefore, treatment with CAP/TEM[27] was initiated in November 2018. Additionally, we admitted the patient to radiotherapy of pleural metastases. In the following years, staging examinations revealed stable disease. In November 2019, progressive disease was detected. In February 2020, the patient was still alive. During treatment no relevant impairment to QoL was reported.
3.8. Case 8
A 69-year-old-male patient was diagnosed in March 2017 with a NEC G3 of the duodenum and synchronous liver metastases.
In March 2017, an upper endoscopy, which was performed as work-up of an iron deficiency anemia, showed a tumorous lesion in the duodenum. Several upper endoscopies between 2015 and 2017 had not revealed any pathological finding except gastroesophageal reflux disease (GERD). Further radiological work-up including multi-slice CT of chest and abdomen as well as an magnetic resonance imaging (MRI) displayed a tumorous lesion of the uncinate process extending to the horizontal portion of the duodenum as well as multiple bilobar liver metastases (initial tumor load 30%). Histological and immunohistochemical analysis of a biopsy from the duodenal tumor revealed a neuroendocrine carcinoma with strong expression of synaptophysin, chromogranin and a slightly weaker expression of CDX2, confirming the gastrointestinal origin of the primary tumor. Expression of CD56, TTF1, insulin, serotonin was negative. Ki-67 was up to 80% resulting in a NEC G3. Between March 2017 and January 2018, the patient received systemic therapy with carboplatin and etoposide, which was well-tolerated, but had to be changed to a second-line therapy with FOLFOX[25,26] because radiological follow-up revealed progressive disease. Due to polyneuropathy Grade 2 according to CTCAE,[24] treatment with FOLFOX was terminated and chemotherapy with FOLFIRI[35] was initiated. Under this treatment further tumor progression was observed. Since no “standard” third-line therapy is established for patients with NEC, we performed a new liver biopsy revealing PD-L1 expression in 5% of tumor cells and initiated an off-label therapy with pembrolizumab (2 mg/kg, initially 150 mg i.v. every 21 days). Further molecular analysis (comprising EGRF, KRAS, NRAS, BRAF, PIK3CA, HER2NEU, EML4, ALK, RET, ROS1) did not detect any targetable mutation. In November 2018, pembrolizumab was discontinued due to progressive deterioration in the patient's general condition. The patient succumbed to death due to multiorgan failure in February 2019.
4. Review of the literature and discussion of results
We report a case series of 8 patients with histologically confirmed diagnosis of NEN who were treated at our institution with ICI as salvage therapy. According to the 2012 WHO classification, 4 patients (#1,2,5,6) suffered from a well/moderately-differentiated NET and 4 patients (#3,4,7,8) suffered from a neuroendocrine carcinoma. Five patients displayed PD-L1 positive tumors. All patients described within this case series were lacking standard treatment options and were treated as “salvage” therapy with check-point inhibitors. Based on the efficacy of these substances in other malignant diseases, we hypothesized that at least a subgroup of patients with NEN might benefit from check-point inhibition in this clinical setting. Since no other treatment option existed, it seemed reasonable to initiate therapy, despite the lack of a reliable marker for identification of patients, who might particularly benefit from treatment. Analyzing the clinical course and response of patients treated with ICI, may help to identify parameters facilitating the selection of patients benefiting from the therapy.
All our patients received first- and second-line chemotherapies according to their primary tumor, which had to be discontinued due to tumor progression or therapy-associated side effects. Due to the extent of the primary tumor, curative surgery could not be performed in 3 cases (#3,6,8). Liver metastases of 3 patients (#2,3,4) were considered accessible for locoregional procedures. Radiation therapy was performed in 3 cases. Three patients (#2,4,6) with SSR positive tumors received PRRT. Importantly, we conducted molecular analysis in all patients before ICI treatment was initiated. Mutations in DNA Mismatch Repair Proteins (#6,7) and POLE gene (#1,7) were detected in 2 patients, respectively. We treated 6 patients with pembrolizumab monotherapy, 1 patient with avelumab (#6), and 1 patient with a combination therapy with pembrolizumab and ipilumimab accompanied by vaccination of transfected autologous tumor RNA. Three patients (#5,6,8) died due to tumor progression shortly after initiation of ICI treatment. In 4 patients (#1–4), ICI treatment led to partial remission or stable disease. Three patients suffered from therapy-related side effects such as recurrent pneumonitis Grade 2 according to CTCAE[24] (#3,4), diarrhea, sleep disorders, and asthma (#7) or joint pain/arthralgia (#3). Patients presenting with recurrent pneumonitis not only required steroid therapy but also therapy with adalimumab (#3,4).
We report about a highly selected population which had exhausted all standard treatments. Since the treatment options for progressive disease in NEN are limited and most patients still do not benefit from treatment with PD-L1 inhibitors, it is necessary to identify markers that could have predictive value for a clinical benefit. We demonstrate a clinical benefit in some patients with PD-L1 negative tumor. Thus, our case series suggest that a response to the PD-L1 inhibitor pembrolizumab in NEN is independent of PD-L1 expression, which is similar to the results from Keynote-158 but in contrast to small cell lung cancer, in which PD-L1 expression is already described as an approved marker for durable therapeutic response.[40] According to the latest update of NSCLC treatment guidelines of the American Society of Clinical Oncology (ASCO), monotherapy with pembrolizumab can be used in cases with PD-L1 expression over 50%, highlighting the importance of PD-L1 expression. In contrast, additional chemotherapy is needed in cases with lower PD-L1 expression.[41] Along with PD-L1 expression, mismatch-repair (MMR) deficiency and density of tumor infiltrating lymphocytes are reported to be associated with a better therapeutic outcome.[42] An expert group around Dung T. Le analyzed response to therapy in MMR-deficient cancers across 12 different tumor types (e.g., colorectal carcinoma, endometrial carcinoma, esophageal carcinoma, NEN) and found out that a large proportion of mutant neoantigens in MMR-deficient cancers make them sensitive to immune checkpoint blockade, regardless of the cancers’ tissue of origin.[43] Nevertheless, our patients with MMR-deficiency (#6,7) did not show any therapeutic response. According to Mehnert et al, the presence of a POLE mutation is associated with high mutational burden and elevated expression of several immune checkpoint genes in endometrial cancer.[32] Two of our case patients displayed SVP in POLE gene: whereas patient #1's gene analysis was correlated with PR to ICI treatment, patient #7 showed further progressive disease. Furthermore, we also noticed that all patients with response to therapy showed a Ki-67 of at least 15% (2 of them developed a NEC during course of disease (#3,4)), suggesting that a better response is associated with a higher proliferation rate. Obviously, our findings must be tested in larger populations.
The potential of immunological therapies has already been demonstrated by several studies using IFN in patients with neuroendocrine tumors. Administration of IFN, both as monotherapy and in combination with somatostatin analogue (SSA), led to both antiproliferative and anti-secretory effects. As an example, the expert group Arnold et al studied interferon plus octreotide (n = 54) versus octreotide alone (n = 51) in 105 gastroenteropancreatic tumor patients. The median survival was 54 months in the combination arm vs 32 months in the octreotide only arm. Despite this difference not reaching statistical significance, the study highlights that immune-oncological therapies might be used in patients with NEN.[44] Modern immunotherapy consists of check-point-inhibitors that might help to overcome mechanisms by which cancer cells evade immune response. Immune checkpoints represent a class of receptors or ligands, which once activated, repress T-cell response against cancer cells (Fig. 5). In turn, antibodies against these immune checkpoints might activate the immune system to re-recognize these tumor cells as foreign.
Figure 5.
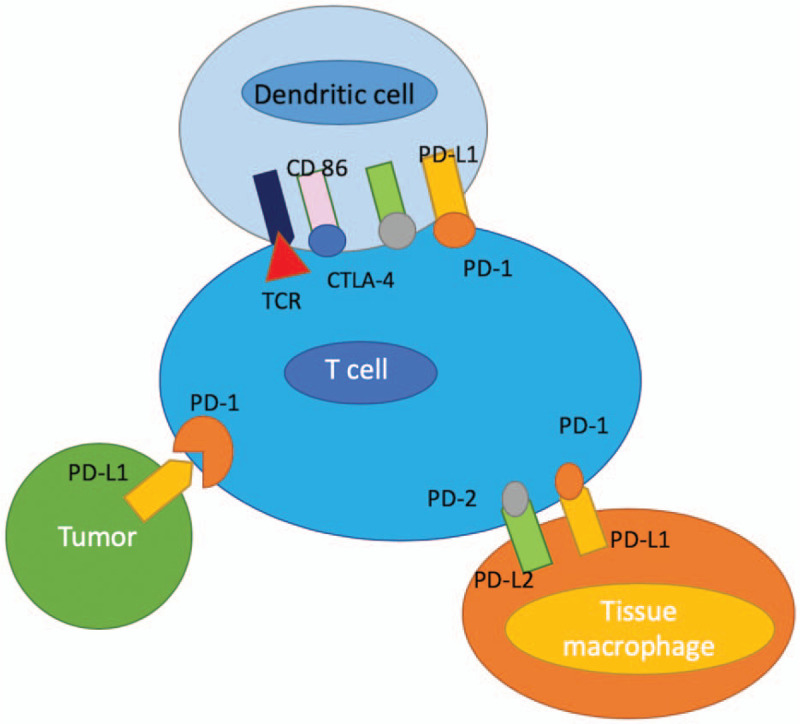
Expression of immune checkpoint inhibitors on T-cells and cancer cells. Immune checkpoints such as PD1/PD-L1 represent a class of receptors or ligands, which once activated, repress T-cell response against cancer cells (adapted from Fig. 1 in Ref.[13]).
The role of immune checkpoint inhibitors in the context of neuroendocrine neoplasms is currently poorly understood. Individual case reports provided the basis for the investigation of the efficacy and safety of this substance class also in NEN. Regarding NET, recently the results of the multi-cohort phase 1b KEYNOTE-028 study (NCT02054806) were reported, which enrolled small intestine/carcinoid- (n = 25) and pNET-patients (n = 16) with PD-L1 positive tumors after failure of standard therapy, respectively. Notably, out of 276 screened patients only 36% displayed PD-L1 positive tumors.[16] In contrast, our case series features both patients with PD-L1-positive and PD-L1-negative expression. Within Keynote-028, patients received pembrolizumab at a dose of 10 mg/kg every 2 weeks, which is very similar to the treatment used in our patients. The efficacy of the ICI treatment was moderate with 3 small intestine/carcinoid patients (12%) and 1 pNET patient (6%) displaying objective responses. Stable disease was achieved in 60% (n = 15) and 88% (n = 14) of the patients.[16] Interestingly, we observed tumor response with partial remission in 3 patients, in which PD-L1 staining revealed positive expression in 30%, 1%, and <1% (can be considered negative PD-L1 expression according to keynote-028 criteria) of tumor cells. Stable disease was detected in 1 patient with positive PD-L1 expression in 5% of tumor cells. Four patients showed progressive disease, only 1 of them presented with PD-L1 positivity in more than 5% of tumor cells. Based on data from KEYNOTE-028, the phase II basket KEYNOTE-158 (NCT02628067) study was launched to analyze antitumor activity of pembrolizumab in 107 patients with well- and moderately-differentiated NET of the lung, appendix, small intestine, colon, rectum, or pancreas that progressed on ≥1 line of standard therapy. After a median follow-up of 18.6 months, an ORR of 3.7% with 0 CR and 4 PR (3 pancreatic and 1 gastrointestinal) was achieved. 61 patients had SD as best response.[18] Strikingly, the 4 responses were observed in patients with PD-L1 negative tumors. Median PFS was 4.1 months and median OS had not been reached, the 6-months survival-rate was 84.6%, further underlining the note that activity of pembrolizumab monotherapy is rather limited and independent on the PD-L1 status. As mentioned before, also in our cohort no difference in tumor response was observed with regards to PD-L1 status.
In line with our report, Keynote-028 demonstrated that pembrolizumab was associated with very moderate toxicity. Nearly 70% (n = 11) of pNET patients in the study did not suffer from any therapy-related side effects. The most frequent side effects were fatigue (n = 6, 38%) and diarrhea (n = 4, 25%), followed by hypothyroidism (n = 2, 13%) in both cohorts (pNET and carcinoid). In contrast, 2 of our case patients suffered from recurrent pneumonitis (#3,4), which led to interruption of therapy, but could be treated successfully with steroid and adalimumab therapy (Fig. 4a and b).
Similar to neuroendocrine tumors, a potential role of immunotherapy was also analyzed in the context of neuroendocrine carcinoma. The potential role of ICI in NEC might be derived from small cell lung carcinoma (SCLC), which is widely regarded as a “model-disease” for NEC, where the PD-L1 inhibition demonstrated antitumor activity in terms of an increased response rate and increased overall survival compared to placebo. Regarding NEC, just recently Vijayvergia et al reported data from 21 patients with metastatic high-grade neuroendocrine neoplasms (Ki-67 > 20%) that were treated with pembrolizumab after failure of a first line platinum/etoposide doublet (NCT02939651). Pembrolizumab, though generally well-tolerated, showed limited activity as a single agent in this study (Disease Control Rate 19%, PFS 9.14 weeks, OS 15.4 weeks[45]). Similar results were found by Mulvey et al, who analyzed the efficacy of pembrolizumab in 14 patients with extrapulmonary poorly differentiated neuroendocrine carcinomas with progression on first-line chemotherapy (NCT03136055). The disease control rate was 21% (3/14) and median PFS 58 days.[17] Comparably unfavorable results were reported from 21 patients with GEP NEC, who had progressed on first-line of chemotherapy and were subsequently treated with spartalizumab (NCT02955069). In these patients, only very low response rates of 4.8% and stable disease rates of 14.3% were achieved,[15] highlighting the very limited activity of single agent immunotherapy in neuroendocrine carcinoma. In contrast to these data, 2 patients who showed positive response to monotherapy with pembrolizumab suffering from NEC. In case of patient #6, who was initially diagnosed with a NET G2 and developed a NEC Ki-67 > 70% during the course of the disease, stable disease was demonstrated during combined therapy with pembrolizumab, ipililumab, and vaccination of transfected autologous tumor RNA. Notably, our patient with DNA mismatch protein deficiency, but lack of PD-L1 expression did not respond to treatment with pembrolizumab.
Combination immunotherapies have demonstrated superior efficacy compared to single agent therapy across different cancers. Recently the combination of atezolizumab plus bevacizumab was tested in patients with well to moderate differentiated progressive NET. Interestingly, this combination was associated to improved response rates and survival compared to monotherapies, highlighting the potential of immunotherapies also in patients with NET.[46] The efficacy of a combination therapy consisting of nivolumab and ipilumumab was analyzed within the SWOG S1609, a phase II clinical basket trial in patients with high-grade neuroendocrine tumors (NCT02834013). The combination of ipilimumab plus nivolumab demonstrated a striking ORR of 42%. Six-months progression-free survival rate was 30% and the median overall survival 11 months, respectively.[47] In summary, these data clearly demonstrate, that while PD-L1 blocking antibodies given as single agents have only limited activity in patients with NEC, combination immunotherapies might significantly improve patients’ prognosis and warrant further investigation.
5. Conclusion and future perspectives
Neuroendocrine neoplasia might be successfully treated – in very selected cases – with check-point inhibitors such as pembrolizumab, avelumab, or a combination of nivolumab plus ipilimumab. In patients after failure of standard therapies, partial remissions as well as long lasting periods of stable disease combined with an improvement of quality of life can be observed. At present, molecular mechanisms leading to tumor response upon administration of ICI are only poorly understood. Although, the determination of PD-L1, MSI, and mutational load allows the identification of at least 3 specific subgroups, an unknown number of patients is missed possibly benefitting from ICI. The estimated percentages of patients who are eligible for and who respond to checkpoint inhibitor drugs are higher than reported estimates for drugs approved for genome-driven oncology but remain modest. Future research should explore biomarkers to maximize the benefit of immunotherapy among patients receiving it.
Acknowledgments
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
The authors thank all members of the “NET-team” for helpful discussions.
Author contributions
All authors were involved in patients’ treatment. BÖ, HJ, CR, and BW wrote the manuscript. All authors read the manuscript and approved the manuscript.
Conceptualization: Burcin Özdirik, Henning Jann, Christoph Roderburg, Bertram Wiedenmann.
Data curation: Burcin Özdirik, Henning Jann, Christoph Roderburg.
Methodology: Christoph Roderburg, Bertram Wiedenmann.
Resources: Henning Jann, Bertram Wiedenmann.
Supervision: Frank Tacke, Christoph Roderburg, Bertram Wiedenmann.
Validation: Christoph Roderburg, Bertram Wiedenmann.
Writing – original draft: Burcin Özdirik, Henning Jann, Philip Bischoff, Frank Tacke, Christoph Roderburg, Bertram Wiedenmann.
Writing – review & editing: Burcin Özdirik, Henning Jann, Philip Bischoff, Uli Fehrenbach, Frank Tacke, Christoph Roderburg, Bertram Wiedenmann.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AML = acute myeloid leukemia, ASCO = American Society of Clinical Oncology, CAP/TEM = capecitabin/temozolomide, CT = computed tomography, CTCAE = Common Terminology Criteria for Adverse Events, EORTC = European Organization for Research and Treatment of Cancer, GEP-NEN = gastroenteropancreatic neuroendocrine neoplasms, GERD = gastroesophageal reflux disease, i.v. = intravenously, ICI = immune check-point inhibitors, MiNEN = mixed neuroendocrine non-neuroendocrine neoplasm, MMR = mismatch-repair, MRI = magnetic resonance imaging, NEC = neuroendocrine carcinoma, NEN = neuroendocrine neoplasms, NET = neuroendocrine tumors, NSE = neuron specific enolase, POLE = DNA polymerase epsilon, PRRT = Peptide Receptor Radionuclide Therapy, QoL = quality of life, SCLC = small cell lung carcinoma, SIRT = selective internal radiation therapy, SSA = somatostatin analogue, SSR = somatostatin receptor, STZ/5FU = streptozotocin/5-fluorouracil, TACE = transarterial chemoembolization, TAE = transarterial embolization, WHO = World Health Organization.
How to cite this article: Özdirik B, Jann H, Bischoff P, Fehrenbach U, Tacke F, Roderburg C, Wiedenmann B. PD-L1 – inhibitors in neuroendocrine neoplasia: Results from a real-life study. Medicine. 2021;100:1(e23835).
BÖ and HJ share first authorship.
CR and BW share senior authorship.
The authors have no funding to disclose.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
The patients provided written consent for publication according to the Declaration of Helsinki.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
NET = neuroendocrine tumor (NET), NSE = neuron specific enolase, SNV = single nucleotide variants, SSTR2 = Somatostatin receptor 2.
CAP/TEM = capecitabin/temozolomide, HIPEC = Hyperthermic intraperitoneal chemotherapy, PD = Progressive disease, PR = Partial Remission, PRRT = Peptide Receptor Radionuclide Therapy, SD = Stable Disease, SSA = Somatostatin analogue, STZ/5FU = streptozocin/5-fluorouracil, TACE = transarterial chemoembolization, TAE = transarterial embolization.
References
- [1].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. Epub 2017/04/28. doi 10.1001/jamaoncol.2017.0589. PubMed PMID: 28448665; PubMed Central PMCID: PMCPMC5824320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bosman FTCF, Hruban RH, Theise ND. Classification of Tumours of the Digestive System. 4th ed.Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- [3].Lloyd RV, Osamura RY, Klöppel G, et al. WHO Classification of Tumours of Endocrine Organs. 4th ed. Lyon: International Agency for Research on Cancer; 2017. 355. [Google Scholar]
- [4].Pape UF, Berndt U, Müller-Nordhorn J, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2008;15:1083–97. [DOI] [PubMed] [Google Scholar]
- [5].Practice guideline neuroendocrine tumors – AWMF-Reg. 021-27. Z Gastroenterol 2018;56:583–681. [DOI] [PubMed] [Google Scholar]
- [6].Pavel M, O”Toole D, Costa F, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 2016;103:172–85. [DOI] [PubMed] [Google Scholar]
- [7].Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol: official journal of the European Society for Medical Oncology 2013;24:152–60. Epub 2012/09/13. doi 10.1093/annonc/mds276. PubMed PMID: 22967994. [DOI] [PubMed] [Google Scholar]
- [8].Heetfeld M, Chougnet CN, Olsen IH, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2015;22:657–64. DOI 10.1530/erc-15-0119. Epub 2015/06/27. PubMed PMID: 26113608. [DOI] [PubMed] [Google Scholar]
- [9].Lorenzen S, Lordick F, Loosen SH, et al. Current status of immunotherapy in gastrointestinal malignancies. Z Gastroenterol 2020;4:1071–8322. [DOI] [PubMed] [Google Scholar]
- [10].Kuol N, Stojanovska L, Nurgali K, et al. PD-1/PD-L1 in disease. Immunotherapy 2018;10:149–60. [DOI] [PubMed] [Google Scholar]
- [11].Baumeister SH, Freeman GJ, Dranoff G, et al. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol 2016;34:539–73. [DOI] [PubMed] [Google Scholar]
- [12].Alsharedi M, Katz H. Check point inhibitors a new era in renal cell carcinoma treatment. Med Oncol 2018;35:018–1147. [DOI] [PubMed] [Google Scholar]
- [13].Chauhan A, Horn M, Magee G, et al. Immune checkpoint inhibitors in neuroendocrine tumors: a single institution experience with review of literature. Oncotarget 2017;9:8801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shahid K, Khalife M, Dabney R, et al. Immunotherapy and targeted therapy-the new roadmap in cancer treatment. Ann Transl Med 2019;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yao JC, Strosberg J, Fazio N, et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Ann Oncol; ESMO; Munich 2018;29:viii467–78. [Google Scholar]
- [16].Mehnert JM, Rugo HS, O’Neil BHea. Pembrolizumab for patients with PD-L1-positive advanced carcinoid or pancreasesreatic neuroendocrine tumors: results from the keynote-028 study. Ann Oncol 2017;28: suppl_5: V142–57. [Google Scholar]
- [17].Mulvey C, Raj NP, Chan JA, et al. Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: results of Part A (pembrolizumab alone). Am Soc Clin Oncol 2019;37:363–70. [Google Scholar]
- [18].Strosberg JR, Mizuno N, Doi T, et al. Pembrolizumab treatment of advanced neuroendocrine tumors: results from phase II KEYNOTE-158 study; Gastrointestinal Cancers Symposium; Chicago. J Clin Oncol 2019;37:190.30523716 [Google Scholar]
- [19].Rindi G, Wiedenmann B. Neuroendocrine neoplasia goes molecular – time for a change. Nat Rev Clin Oncol 2019;16:149–50. doi 10.1038/s41571-018-0118-8. Epub 2018/11/06. PubMed PMID: 30390038. [DOI] [PubMed] [Google Scholar]
- [20].Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17:1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].D’Angelo SP, Russell J, Lebbe C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol 2018;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst 2012;104:764–77. [DOI] [PubMed] [Google Scholar]
- [23].Rinke A, Wiedenmann B, Auernhammer C, et al. S2k-Leitlinie Neuroendokrine Tumore. Z Gastroenterol 2018;56:583–681. doi: 10.1055/a-0604-2924. [DOI] [PubMed] [Google Scholar]
- [24].CTEP NCICTEP. Common Terminology Criteria for Adverse Events (CTCAE) Version v4.03 2009. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06 14_QuickReference_8.5x11.pdf. [Google Scholar]
- [25].Hadoux J, Malka D, Planchard D, et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer 2015;22:289–98. [DOI] [PubMed] [Google Scholar]
- [26].Spada F, Antonuzzo L, Marconcini R, et al. Oxaliplatin-based chemotherapy in advanced neuroendocrine tumors: clinical outcomes and preliminary correlation with biological factors. Neuroendocrinology 2016;103:806–14. [DOI] [PubMed] [Google Scholar]
- [27].Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268–75. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015;350:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov 2016;6:1230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mehnert JM, Panda A, Zhong H, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest 2016;126:2334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Blumenthal GM, Theoret MR, Pazdur R. Treatment beyond progression with immune checkpoint inhibitors-known unknowns. JAMA Oncol 2017;3:1473–4. [DOI] [PubMed] [Google Scholar]
- [34].Stuven AK, Wiedenmann B. Sustained partial remission of a metastatic NEN using off-label immunotherapy with pembrolizumab. Oncotarget 2019;10:3302–11. doi: 10.18632/oncotarget.26906. eCollection 2019 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hentic O, Hammel P, Couvelard A, et al. FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer 2012;19:751–7. [DOI] [PubMed] [Google Scholar]
- [36].Schmidt D, Wiedenmann B. Extremely long survival under combined immunotherapy in a metastatic functional neuroendocrine neoplasia patient. Neuroendocrinology 2018;106:381–8. [DOI] [PubMed] [Google Scholar]
- [37].Eriksson B, Annibale B, Bajetta E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: chemotherapy in patients with neuroendocrine tumors. Neuroendocrinology 2009;90:214–9. [DOI] [PubMed] [Google Scholar]
- [38].Pavel M, Baudin E, Couvelard A, et al. ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157–76. [DOI] [PubMed] [Google Scholar]
- [39].Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American neuroendocrine tumor society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 2017;46:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schmidt LH, Kümmel A, Görlich D. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PloS One 2015;10:e0136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nasser H. Schneider BJ. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2020;38:1608–32. [DOI] [PubMed] [Google Scholar]
- [42].Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 2017;17:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. New York. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Arnold R, Rinke A, Klose KJ, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol: the official clinical practice journal of the American Gastroenterological Association 2005;3:761–71. Epub 2005/10/20. PubMed PMID: 16234004. [DOI] [PubMed] [Google Scholar]
- [45].Vijayvergia N, Ross DA, Dotan EA, et al. Pembrolizumab (P) monotherapy in patients with previously treated metastatic high grade neuroendocrine neoplasms (HG-NENs). J Clin Oncol 2018;36:4104–410. [Google Scholar]
- [46].Venook APKA, Tempero MA, Uy J, et al. Phase II trial of FOLFOX plus bevacizumab in advanced, progressive neuroendocrine tumors. J Clin Oncol 2008;26:15545–50. [Google Scholar]
- [47].Patel S. Immune Checkpoint Inhibitor Combination Efficacious for Patients with High-grade Neuroendocrine Carcinoma. Atlanta: American Association for Cancer Research; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


