Background.
Frailty has emerged as a critical determinant of mortality in patients with cirrhosis. Currently, the United Network for Organ Sharing registry only includes the Karnofsky Performance Status (KPS) scale, which captures a single component of frailty. We determined the associations between frailty, as measured by the Liver Frailty Index (LFI), and KPS with waitlist mortality.
Methods.
Included were 247 adult patients with cirrhosis listed for liver transplantation without hepatocellular carcinoma from February 2014 to June 2019, who underwent outpatient assessments using the LFI and KPS within 30 days of listing. “Frail” was defined using the established LFI cutoff of ≥4.4. Competing risk models assessed associations between the LFI and KPS with waitlist mortality (death/delisting for sickness).
Results.
At a median 8 months follow-up, 25 (10%) patients died/were delisted. In this cohort, median Model for End-Stage Liver Disease-Sodium was 17, LFI was 3.9 (interquartile range 3.4–4.5), and KPS was 80 (interquartile range 70–90). In multivariable analysis, LFI (sub-hazard ratio 1.07, per 0.1 unit; 95% confidence interval, 1.01-1.12) was associated with waitlist mortality while KPS was not (sub-hazard ratio 1.00, per 10 units; 95% confidence interval, 0.78-1.29).
Conclusions.
Our data suggest that frailty, as measured by the LFI, may be more appropriate at capturing mortality risk than KPS and provide evidence in support of using the LFI more broadly in clinical transplant practice in the outpatient setting.
Frailty is a well-established determinant of mortality in patients with cirrhosis.1 Currently, the only metric available in the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network that approximates the concept of frailty is the Karnofsky Performance Status (KPS) score. The KPS score is a subjective assessment of a patient’s overall performance status that is assigned on a scale of 0–100 in increments of 10 (where a score of 0 is considered moribund and 100 is considered excellent health).2 In patients awaiting liver transplantation, functional status has been associated with mortality both before and after liver transplantation.3,4 Severe functional impairment at the time of liver transplantation, as assessed by KPS, has been shown to be associated with a markedly increased risk of mortality and/or having graft failure at 1-year posttransplantation.5-7 However, while the KPS metric has the advantage of being simple and quick to use, it represents only one component of the multi-dimensional construct of frailty—functional status and is subjective, making it susceptible to bias.8
Recently, we developed the Liver Frailty Index (LFI) to assess frailty in patients with cirrhosis.9 The LFI consists of 3 tests to represent 3 major components of the multi-dimensional construct of frailty: grip strength (malnutrition), chair stands (muscle weakness), and balance (altered neuromotor coordination). The frailty tests chosen to be included in the LFI were originally established in the geriatric population10-13 and have demonstrated broad prognostic utility in non-geriatric,14,15 surgical,16,17 and non-liver transplant18-20 populations. We have shown in several studies that the LFI is strongly associated with outcomes in patients with cirrhosis.21-25 However, it is a performance-based metric that must be administered in person, raising concerns about its ability to be incorporated into the national UNOS/Organ Procurement and Transplantation Network registry.
Given the standardized acceptance of the KPS metric to approximate frailty in the UNOS registry—and the recent availability of a more comprehensive, liver-specific assessment of frailty with the LFI—we aimed to determine the associations between the LFI and KPS with waitlist mortality in patients with cirrhosis in the ambulatory setting.
MATERIALS AND METHODS
Study Population
We analyzed data available from adult patients with cirrhosis listed for liver transplantation at a single center from February 1, 2014 to June 1, 2019 who underwent outpatient assessments of frailty (using the LFI) and performance status (using the KPS scale) within 30 days of listing for liver transplantation [see Study Procedures below]. Excluded were patients with hepatocellular carcinoma listed with Model for End-Stage Liver Disease (MELD) exception points given their differential wait time. Patients were followed until they experienced an outcome including death, deactivation for being too sick, liver transplantation, or removal from the waitlist for other reasons.
Study Procedures
Frailty was measured using the LFI, which consists of 3 performance-based tests administered by trained study personnel: (1) grip strength, (2) timed chair stands, and (3) balance. The LFI was calculated using the following equation (calculator available at http://liverfrailtyindex.ucsf.edu):
“Frail” was defined using the previously established LFI cutoff of ≥4.4 from the study by Kardashian et al26 In this study, the optimal cutoff was determined using the area under the curve in an analysis of waitlist mortality by 3, 6, and 12 months. The optimal (highest area under the curve) LFI cutoff for waitlist mortality was 4.4 at 3 months and 4.2 at 6 and 12 months. On the same day as the frailty assessment, KPS was assessed by trained transplant coordinators independent of the LFI assessment. Patients were assigned a score from 0 to 100, where a score of 0 is considered moribund and 100 is considered perfect health.
Additional data were collected from the electronic health record. These included age, sex, ethnicity, body mass index, MELD-Sodium (MELDNa), albumin, the presence of ascites, dialysis dependence, hepatic encephalopathy at time of listing, and co-morbidities (diabetes, hypertension, and coronary artery disease). Cause of liver disease was categorized as chronic hepatitis C, hepatitis B, alcohol, nonalcoholic steatohepatitis, autoimmune or cholestatic, and other.
Statistical Analysis
Baseline demographics were presented as a median (interquartile range [IQR]) for continuous variables or frequency for categorical variables. Differences in KPS by group were compared by frailty status using χ2 test. Correlation between the LFI and KPS was evaluated using Spearman’s rank-order correlation test.
The primary outcome was waitlist mortality, defined as the combined outcome of death before transplantation or deactivation for being too sick for liver transplantation. Uni- and multivariable analyses assessed associations between the LFI and KPS with waitlist mortality using competing risk models, with liver transplantation as the competing risk. Variables with P < 0.2 in univariable analysis were considered for inclusion in multivariable models. Variables with P < 0.05 were retained in the final multivariable models.
This study was approved by the institutional review board at the University of California, San Francisco (IRB approval number: 11-07513). Statistical analyses were performed using STATA 15 (StataCorp, College Station, TX).
RESULTS
Characteristics of the Patient Population
Baseline characteristics of the 247 patients with cirrhosis listed for transplantation are displayed in Table 1. Fifty-nine percent of the patients were male and median (IQR) age was 57 (50–63). The 2 most common primary etiologies of liver disease were alcoholic cirrhosis (36%) and nonalcoholic steatohepatitis (28%). Median (IQR) laboratory MELDNa score was 17 (14–20), LFI was 3.9 (3.4–4.5), and KPS was 80 (70–90).
Table 1.
Characteristics of the 247 patients with cirrhosis in this study categorized by frailty status
| Characteristics | All (N = 247) | By frailty status | P | |
|---|---|---|---|---|
| Not frail (LFI < 4.4) (N = 181; 73%) | Frail (LFI ≥ 4.4) (N = 66; 27%) | |||
| Age (years) | 57 (50–63) | 56 (49–63) | 60 (54–65) | 0.03 |
| Female | 102 (41%) | 76 (42%) | 26 (39%) | 0.71 |
| Race | ||||
| Non-Hispanic white | 213 (86%) | 154 (85%) | 59 (89%) | 0.03 |
| Black | 6 (2%) | 6 (3%) | — | |
| Asian or Pacific Islander | 17 (7%) | 15 (8%) | 2 (3%) | |
| Native American | 3 (1%) | 3 (2%) | — | |
| Other | 8 (3%) | 3 (2%) | 5 (8%) | |
| Body mass index (kg/m2) | 27.9 (24.6–32.3) | 27.9 (25.2–32.3) | 27.8 (23.2–34.1) | 0.22 |
| Cause of liver disease | ||||
| Chronic HCV | 33 (13%) | 23 (13%) | 10 (15%) | 0.44 |
| Alcohol | 90 (36%) | 67 (37%) | 23 (35%) | |
| Nonalcoholic steatohepatitis | 69 (28%) | 46 (25%) | 23 (35%) | |
| Autoimmune or cholestatic | 32 (13%) | 25 (14%) | 7 (11%) | |
| HBV | 4 (2%) | 4 (2%) | — | |
| Other | 19 (8%) | 16 (9%) | 3 (5%) | |
| Hypertension | 71 (29%) | 49 (27%) | 22 (33%) | 0.34 |
| Diabetes | 113 (46%) | 83 (46%) | 30 (45%) | 0.96 |
| Coronary artery disease | 7 (3%) | 4 (2%) | 3 (5%) | 0.33 |
| KPS | ||||
| 80–100 | 163 (66%) | 130 (72%) | 33 (50%) | 0.003 |
| 50–70 | 81 (33%) | 50 (28%) | 31 (47%) | |
| 10–40 | 3 (1%) | 1 (1%) | 2 (3%) | |
| MELDNa score | 17 (14–20) | 16 (14–20) | 18 (14–21) | 0.27 |
| Total bilirubin (mg/dL) | 2.9 (1.9–4.8) | 2.9 (2.0–4.8) | 2.8 (1.6–4.8) | 0.77 |
| Creatinine (mg/dL) | 0.86 (0.68–1.1) | 0.84 (0.67–1.03) | 0.96 (0.76–1.25) | 0.01 |
| INR | 1.5 (1.3–1.7) | 1.5 (1.3–1.7) | 1.5 (1.3–1.7) | 0.94 |
| Sodium (mEq/L) | 135 (132–137) | 135 (133–138) | 133 (129–136) | <0.001 |
| Albumin (g/dL) | 3.0 (2.6–3.4) | 3.0 (2.6–3.4) | 3.1 (2.6–3.4) | 0.82 |
| Dialysis | 11 (4%) | 7 (4%) | 4 (6%) | 0.46 |
| Ascites | ||||
| Absent | 165 (67%) | 129 (71%) | 36 (55%) | 0.01 |
| Mild/moderate | 64 (26%) | 44 (24%) | 20 (30%) | |
| Severe | 18 (7%) | 8 (4%) | 10 (15%) | |
| Hepatic encephalopathy | 114 (46%) | 76 (42%) | 38 (58%) | 0.01 |
| Outcome | ||||
| Waiting | 108 (44%) | 84 (46%) | 24 (36%) | 0.18 |
| Death or delisted for being too sick | 25 (10%) | 15 (8%) | 10 (15%) | |
| Transplanted | 72 (29%) | 55 (30%) | 17 (26%) | |
| Other | 42 (17%) | 27 (15%) | 15 (23%) | |
All continuous variables are expressed as median (interquartile range) or percentage.
KPS, Karnofsky Performance Status; LFI, Liver Frailty Index; MELDNa, Model for End-Stage Liver Disease-Sodium.
Assessments of and Correlations Between Frailty and Performance Status
Among the 247 outpatients, 66 (27%) met criteria for frailty. One hundred sixty-three (66%) patients had a KPS of 80–100, 81 (33%) had a KPS of 50–70, and 3 (1%) had a KPS of 10–40 (Table 1). Among the 66 patients who were categorized as frail, median (IQR) KPS was 75 (60–90) and 33 (50%) were considered impaired by the KPS with scores in the ranges of 10–40 and 50–70. On the other hand, among patients who were not frail, 51 (28%) were categorized as having some level of impairment by the KPS. The correlation between the LFI and KPS by Spearman’s rank-order test was −0.32 (P < 0.001) (Figure 1).
FIGURE 1.
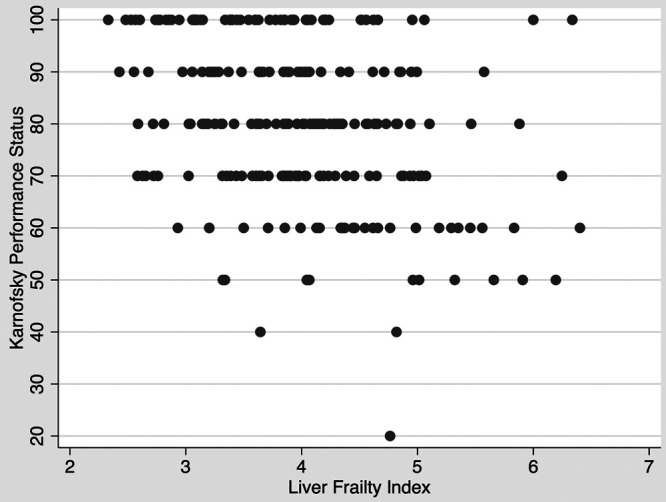
Correlation between the Liver Frailty Index and Karnofsky Performance Status scores (Spearman’s P = −0.32, P < 0.001)
Associations Between the LFI and the KPS With Waitlist Mortality
At a median of 8 months follow-up, 25 (10%) patients died or were deactivated for being too sick for liver transplantation. In univariable competing risk analysis, the LFI was associated with a 9% increased risk of waitlist mortality per 0.1 unit (95% confidence interval [CI], 1.03-1.14) whereas KPS was not (sub-hazard ratio [sHR] 0.92, per 10 units; 95% CI, 0.70-1.22). These associations did not change for either variable in bivariable analysis including both the LFI and KPS (Table 2). A sensitivity analysis evaluating KPS by group (10–40, 50–70, 80–100), did not qualitatively change the association between the LFI, KPS, and waitlist mortality (data not shown). We then performed forward stepwise regression to determine whether adjustment for other factors changed these associations. After adjustment for other factors that were associated with waitlist mortality in univariable analysis (MELDNa, age, ascites, and albumin), the LFI remained significantly associated with waitlist mortality (sHR 1.07, per 0.1 unit; 95% CI, 1.01-1.12) while KPS was not (sHR 1.00, per 10 units; 95% CI, 0.78-1.29) (Table 2).
Table 2.
Univariable and stepwise additive multivariable models using competing risks models (with liver transplantation as competing risk)
| Sub-hazard ratio (95% CI), P | ||||||
|---|---|---|---|---|---|---|
| Univariable analysis | Stepwise multivariable analyses | |||||
| LFI, per 0.1 unit | 1.09 (1.03-1.14), P = 0.001 | 1.09 (1.04-1.14), P = 0.001 | 1.08 (1.03-1.13), P = 0.001 | 1.07 (1.02-1.12), P = 0.003 | 1.07 (1.02-1.11), P = 0.01 | 1.07 (1.01-1.12), P = 0.01 |
| KPS, per 10 units | 0.92 (0.70-1.22), P = 0.57 | 1.02 (0.79-1.31), P = 0.90 | 1.02 (0.79-1.31), P = 0.90 | 1.00 (0.78-1.27), P = 0.98 | 1.00 (0.78-1.29), P = 0.99 | 1.00 (0.78-1.29), P = 0.99 |
| MELDNa, per 1 unit | 1.04 (0.98-1.10), P = 0.25 | 1.03 (0.97-1.09), P = 0.40 | 1.04 (0.97-1.10), P = 0.28 | 1.03 (0.97-1.10), P = 0.36 | 1.03 (0.96-1.10), P = 0.44 | |
| Age, per year | 1.07 (0.99-1.15), P = 0.08 | 1.06 (0.98-1.14), P = 0.15 | 1.06 (0.98-1.14), P = 0.14 | 1.06 (0.98-1.14), P = 0.15 | ||
| Ascites | 2.21 (1.02-4.80), P = 0.05 | 1.75 (0.81-3.79), P = 0.16 | 1.73 (0.79-3.79), P = 0.17 | |||
| Albumin (g/dL) | 0.60 (0.30-1.20), P = 0.146 | 0.68 (0.35-1.33), P = 0.26 | ||||
CI, confidence interval; KPS, Karnofsky Performance Status; LFI, Liver Frailty Index; MELDNa, Model for End-Stage Liver Disease-Sodium.
DISCUSSION
The process of evaluating patients for transplant candidacy is one that is very complex and depends on more than just the severity of liver disease. Frailty is becoming increasingly recognized as a key determinant of outcomes in patients with cirrhosis, which calls for standardized tools that are able to capture its multi-dimensionality. In this study, we examined 2 available indices that have been studied independently in the transplant setting. We sought to ascertain the relationship between the LFI and KPS, which are measurements of frailty and performance status (a component of frailty), respectively, and found that there was a modest correlation. While the LFI was predictive of waitlist mortality in our outpatient cohort of liver transplant candidates with a median MELDNa of 17, KPS was not. Our study suggests that frailty, as measured by the LFI, may be more appropriate to assess mortality risk than performance status alone.
While the KPS has the benefit of being a very rapid test that does not require specialized equipment, the LFI has several advantages over the KPS. Because it consists of 3 components, it is better able to capture the dominant contributors to physical frailty in patients with cirrhosis: malnutrition (grip strength), muscle weakness (chair stands), and altered neuromotor coordination (balance). By measuring grip strength, the LFI also accounts for sarcopenia, which has been recognized as an independent risk factor of mortality on the transplant waitlist and after liver transplantation.27-29
In our study, among the patients who were categorized as frail by the objective LFI, only half were considered impaired by the KPS scale. Conversely, among those who were not frail by the objective LFI, more than one-quarter were classified as having at least some impairment in performance status. This highlights the subjective nature of the KPS and raises the possibility that subjective assessments of performance status may be influenced by factors that are not accurately tied to physical performance. The fact that the LFI was associated with waitlist mortality and not KPS in our cohort suggests that these subjective factors may not be important in risk prediction. Thus, the LFI is especially useful in the ambulatory setting to identify frail patients who may have subjectively higher KPS scores.
Although we did not observe an association between KPS with waitlist mortality in our study, there are several studies that demonstrate its prognostic value in this context. Both Orman et al3 and McCabe et al30 showed that worse functional status as measured by the KPS was significantly associated with waitlist mortality in cirrhotic patients. Thuluvath et al7 found a significant association between lower KPS scores and with mortality pre- and post-liver transplantation. However, in a study examining the relationship between KPS and another more objective assessment, the 6-minute walk test, only the 6-minute walk test was associated with waitlist mortality.31 Despite its subjectivity, KPS has been shown to have good inter-observer reliability at least among oncology patients, but it is less well-validated amongst patients with cirrhosis.32,33 In comparison, the LFI is specific to patients with liver disease and has external validity in non-cirrhotic patients with excellent inter-observer reliability.34
We acknowledge the following limitations to our study. Our cohort was restricted to patients seen in clinic as outpatients, as the LFI has only been validated in the ambulatory setting. As a result, there were few patients with low KPS scores in this population. Therefore, these findings are not generalizable to the entire liver transplant waitlist as a whole; KPS may indeed be equally prognostic among inpatients. We also restricted our analyses to patients who had assessments of frailty and performance status within 30 days of listing to create a more homogeneous cohort of patients who were just beginning their time on the waitlist. However, we did not evaluate changes in either frailty or performance status over time, which may be prognostically valuable.7,25,35 A recent multi-center study by Lai et al25 showed that patients who experienced improvements in frailty over time had a lower risk of death/delisting than patients who experienced worsening frailty, regardless of their baseline frailty and disease severity.
Despite these limitations, our study adds to the growing body of evidence that frailty should be incorporated into transplant decision-making. Frailty, as measured by the LFI, is a significant predictor of waitlist mortality. While we acknowledge the advantages of using the KPS as a rapid screening tool, we strongly advocate for the LFI to be used more broadly in clinical transplant practice in the outpatient setting as it offers a more comprehensive assessment of frailty and accurate estimation of mortality risk.
Footnotes
Published online 7 January, 2021.
This study was funded by NIH K23AG048337 (J.C.L.) and NIH R01AG059183 (J.C.L.). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
The authors declare no conflicts of interest.
C.Q.X. performed the acquisition of data, statistical analysis, interpretation of data, drafting of the manuscript, and critical revision of the manuscript; F.Y., Y.M., R.W., D.K., S.S., and Y.S. performed the acquisition of data and review of the manuscript; J.C.L. performed the study concept and design, acquisition of data, interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
REFERENCES
- 1.Lai JC, Sonnenday CJ, Tapper EB, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019; 19:1896–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky Performance Status. Cancer. 1980; 45:2220–2224 [DOI] [PubMed] [Google Scholar]
- 3.Orman ES, Ghabril M, Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016; 14:1189–1195.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perito ER, Bucuvalas J, Lai JC. Functional status at listing predicts waitlist and posttransplant mortality in pediatric liver transplant candidates Am J Transplant. 2019; 19:1388–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolgin NH, Movahedi B, Anderson FA, et al. Impact of recipient functional status on 1-year liver transplant outcomes. World J Transplant. 2019; 9:145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolgin NH, Martins PN, Movahedi B, et al. Functional status predicts postoperative mortality after liver transplantation. Clin Transplant. 2016; 30:1403–1410 [DOI] [PubMed] [Google Scholar]
- 7.Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky Performance Status before and after liver transplantation predicts graft and patient survival J Hepatol. 2018; 69:818–825 [DOI] [PubMed] [Google Scholar]
- 8.Wang CW, Lai JC. Reporting functional status in UNOS: the weakness of the Karnofsky Performance Status Scale. Clin Transplant. 2017; 31:e13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017; 66:564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 11.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995; 332:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011; 305:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 14.Leong DP, Teo KK, Rangarajan S, et al. ; Prospective Urban Rural Epidemiology (PURE) Study investigators. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015; 386:266–273 [DOI] [PubMed] [Google Scholar]
- 15.Pavasini R, Guralnik J, Brown JC, et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016; 14:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010; 210:901–908 [DOI] [PubMed] [Google Scholar]
- 17.Lin HS, Watts JN, Peel NM, et al. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016; 16:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015; 15:149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jha SR, Hannu MK, Chang S, et al. The prevalence and prognostic significance of frailty in patients with advanced heart failure referred for heart transplantation. Transplantation. 2016; 100:429–436 [DOI] [PubMed] [Google Scholar]
- 20.Singer JP, Diamond JM, Gries CJ, et al. Frailty phenotypes, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med. 2015; 192:1325–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai JC, Rahimi RS, Verna EC, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology. 2019; 156:1675–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JC, Dodge JL, McCulloch CE, et al. Frailty and the burden of concurrent and incident disability in patients with cirrhosis: a prospective cohort study. Hepatol Commun. 2020; 4:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai JC, Segev DL, McCulloch CE, et al. Physical frailty after liver transplantation. Am J Transplant. 2018; 18:1986–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fozouni L, Mohamad Y, Lebsack A, et al. Frailty is associated with increased rates of acute cellular rejection within 3 months after liver transplantation. Liver Transpl. 2020; 26:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai JC, Dodge JL, Kappus MR, et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol. 2020; 73:575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kardashian A, Ge J, McCulloch CE, et al. Identifying an optimal Liver Frailty Index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology. [Epub ahead of print. June 3, 2020]. doi: 10.1002/hep.31406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo SZ, Ahmad M, Dunn MA, et al. Sarcopenia predicts post-transplant mortality in acutely ill men undergoing urgent evaluation and liver transplantation. Transplantation. 2019; 103:2312–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012; 18:1209–1216 [DOI] [PubMed] [Google Scholar]
- 29.Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, et al. Inclusion of sarcopenia within MELD (MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015; 6:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCabe P, Galoosian A, Wong RJ. Patients with alcoholic liver disease have worse functional status at time of liver transplant registration and greater waitlist and post-transplant mortality which is compounded by older age. Dig Dis Sci. 2020; 65:1501–1511 [DOI] [PubMed] [Google Scholar]
- 31.McNally BB, Carey EJ. Objective versus subjective assessment of functional status in candidates for liver transplantation. Transplant Proc. 2018; 50:3508–3512 [DOI] [PubMed] [Google Scholar]
- 32.Mor V, Laliberte L, Morris JN, et al. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984; 53:2002–2007 [DOI] [PubMed] [Google Scholar]
- 33.Schag CC, Heinrich RL, Ganz PA. Karnofsky Performance Status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984; 2:187–193 [DOI] [PubMed] [Google Scholar]
- 34.Wang CW, Lebsack A, Chau S, et al. The range and reproducibility of the Liver Frailty Index. Liver Transpl. 2019; 25:841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the functional assessment in liver transplantation (FrAILT) study. Hepatology. 2016; 63:574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]


