Abstract
This study aimed to elucidate nationwide trends in reperfusion therapy utilization and subsequent 30-day mortality in acute ischemic stroke patients in Japan. The analysis focused on intravenous recombinant tissue plasminogen activator (IV rt-PA) and endovascular thrombectomy (EVT).
Using health insurance claims data, we calculated the age- and sex-adjusted monthly number of acute ischemic stroke patients who received IV rt-PA and/or EVT in Japan from April 2010 to March 2016, and investigated the 30-day all-cause mortality rates after undergoing these therapies. Through an interrupted time-series analysis, we examined the
-
(1)
trends prior to extension of the IV rt-PA therapeutic time window from 3 hours to 4.5 hours in September 2012,
-
(2)
changes that occurred immediately after the extension, and
-
(3)
differences in trends between the pre- and post-extension periods.
During the study period, 69,920 patients with acute ischemic stroke (mean age ± standard deviation: 74.9 ± 12.0 years; 41.4% women) received IV rt-PA and/or EVT. The age- and sex-adjusted number of patients receiving IV rt-PA monotherapy increased immediately after the time window extension (<rk-italic > P < .001), but did not change during the pre- (P = .90) and post-extension (P = .58) periods. In contrast, the number of patients receiving EVT with or without IV rt-PA continuously increased during the pre-extension period (P < .001), and further increased during the post-extension period (P <.001); however, this number decreased immediately after the extension (P < .001). There were no significant changes in 30-day all-cause mortality during the pre- (P = .40) and post-extension (P = .64) periods, as well as immediately after the extension (P = .53).
The extension of the IV rt-PA therapeutic time window and progressively widespread use of EVT in Japan have increased the number of acute ischemic stroke patients eligible for reperfusion therapy. These trends were not accompanied by a higher risk of post-reperfusion mortality.
Keywords: stroke, reperfusion therapy, endovascular therapy, thrombolysis
1. Introduction
Stroke is a leading cause of disability and death in many countries.[1,2] Despite advances in stroke care, patients with stroke may still experience functional impairments that reduce their quality of life.[3,4] Accordingly, preventive care and the reduction of post-stroke disabilities are imminent global issues.[2,5]
Over the past few decades, the development of various treatments has dramatically improved clinical outcomes after acute ischemic stroke.[6–8] Among these, reperfusion therapy has played a central role in the improvement of post-stroke outcomes. In the late 1990s, intravenous recombinant tissue plasminogen activator (IV rt-PA) emerged as an effective treatment for acute ischemic stroke within 3 hours of onset.[9] The efficacy and safety of this treatment is highly time-dependent, which precipitated a paradigm shift in acute stroke care. In 2008, the European Cooperative Acute Stroke Study III demonstrated that the efficacy and safety of IV rt-PA were still preserved 3.0 to 4.5 hours after acute ischemic stroke onset,[10,11] leading to the extension of the therapeutic time window to 4.5 hours worldwide.[12–14] Endovascular thrombectomy (EVT) is a newer option for acute care of ischemic stroke, with recent trials demonstrating its benefits in improving post-stroke outcomes in patients with anterior circulation large vessel occlusion strokes.[15–20] Newly developed devices for EVT have further increased the recanalization rate and improved the balance between the benefits and safety of this therapy.[21] EVT is currently indicated for patients who fulfill the exclusion criteria for IV rt-PA or cases where IV rt-PA therapy was unable to recanalize large vessel occlusions.[7,8,22]
As a result of these developments, the acute stroke care systems in many countries are undergoing major revisions to provide reperfusion therapy to a wider range of ischemic stroke patients in the safest, most effective, and most efficient manner possible.[23] However, there is scarce information regarding how the use of IV rt-PA and EVT has spread across individual countries in recent years, and whether the increased use of these reperfusion therapies has led to changes in case fatality rates.
Therefore, this study aimed to elucidate the nationwide trends of IV rt-PA and EVT use for acute ischemic stroke and the concomitant changes in 30-day mortality associated with these therapies in Japan. Using a nationwide health insurance claims database, we examined patients who were treated with IV rt-PA and/or EVT in acute care hospitals between 2010 and 2015.
2. Methods
2.1. Study design, study setting, and data source
We retrospectively investigated nationwide health insurance claims data on the use of IV rt-PA and EVT to treat acute ischemic stroke patients. The data of stroke patients who were hospitalized in Japan between April 2010 and March 2016 were obtained from the National Database of Health Insurance Claims and Specific Health Checkups (NDB). The claims data of more than 95% of all patients treated in Japan are formatted and stored in the NDB via an electronic claims system.
Our research project was proposed to the Ministry of Health, Labour and Welfare of Japan, and had been designed in accordance with the Act on Assurance of Medical Care for Elderly People. The Sage Committee, a government advisory committee, reviewed our proposal and recommended that the Ministry grant us permission to use the required data and perform the study (No. 0326-26). This study was approved by the Institutional Review Board of Kyushu University (No. 28-70). The need for informed consent was waived as all data were anonymized before being received by the authors.
2.2. Hospitals and patients
In Japan, acute care hospitals can be categorized according to their reimbursement systems. Hospitals that use the Diagnostic Procedure Combination (DPC) system (ie, a bundled payment system based on diagnosis-related groups) are referred to as DPC hospitals, whereas other hospitals that use the older fee-for-service system are referred to as non-DPC hospitals. The number (percentage) of DPC hospitals was 1390 (16.0%) in 2010, 1449 (16.8%) in 2011, 1505 (17.6%) in 2012, 1496 (17.5%) in 2013, 1585 (18.7%) in 2014, and 1580 (18.6%) in 2015.
From the NDB, we identified the claims data of stroke patients treated in DPC hospitals using the DPC diagnostic codes for acute stroke (DPC codes: 010020 [subarachnoid hemorrhage], 010040 [brain hemorrhage], 010060 [brain infarction], and 010070 [other cerebrovascular diseases]) and those treated in non-DPC hospitals using 243 Japanese diagnostic codes associated with stroke (corresponding to International Classification of Diseases, 10th revision codes: I60–I69). Through this process, we collected 1.5 billion and 0.9 billion claims records of stroke patients treated in DPC hospitals and non-DPC hospitals, respectively, from April 2010 to March 2016. For this study, each year was analyzed as a Japanese fiscal year, i.e., the 12-month period from April to March of the following year.
Among all the stroke patients, we excluded patients with hemorrhagic stroke and included only those with acute ischemic stroke who were treated with IV rt-PA and/or EVT during hospitalization in this study. Age was categorized in 5-year increments in accordance with the NDB's regulations for patient anonymity, and the mean age was calculated using the median age of each age category. To calculate the age- and sex-adjusted numbers of patients per 100,000 populations, the patient numbers were standardized to the population of Japan in April 2010.
Each hospital was also classified as an EVT hospital or non-EVT hospital according to whether EVT was performed or not each year. Hospital patient volume was defined as the number of patients treated with the target reperfusion therapies in each hospital during the study period.
2.3. Reperfusion therapy
For this study, reperfusion therapy referred to the use of IV rt-PA and/or EVT. IV rt-PA was defined as alteplase administered to patients with ischemic stroke. IV administration of alteplase was first approved in Japan in October 2005 for patients with acute ischemic stroke if it can be started within 3 hours of onset. The new labeled indication for IV rt-PA that extended the time window to 4.5 hours after onset was approved on 31 August 2012. In September 2012, the Japan Stroke Society released a statement indicating that the therapeutic time window for IV alteplase was extended to 4.5 hours after onset. Thus, we defined the period before and including August 2012 as the pre-extension period (29 months) and the period from September 2012 onward as the post-extension period (43 months).
EVT was defined as the use of percutaneous thrombectomy (Japanese medical service code: K178-4) or any type of thrombectomy catheter for ischemic stroke. Four types of percutaneous thrombectomy devices were approved in Japan from 2010 to 2014: the Merci Retrieval System (Concentric Medical Inc., Mountain View, CA) in 2010, Penumbra System (Penumbra Inc., Alameda, CA) in 2011, Solitaire FR (Covidien, Mansfield, MA) in 2014, and Trevo ProVue (Stryker Neurovascular, Fremont, CA) in 2014.
2.4. Clinical outcomes
The primary outcome was 30-day mortality, which was defined as in-hospital death from any cause within 30 days of the index ischemic stroke. In addition, we also sought to examine the occurrence of unfavorable events after reperfusion therapy, but claims data do not allow the direct identification of such events. Therefore, we used the following neurosurgical treatments as indicators of unfavorable events after reperfusion therapy: decompressive craniotomy (medical service code: K1492); surgical evacuation (K1643), stereotactic aspiration (K164-4), and endoscopic evacuation (K164-5) for intracerebral hematoma; and shunting (K145) for hydrocephalus. We also evaluated a composite outcome comprising death and post-reperfusion neurosurgical treatments. From April 2010 to March 2012, the NDB included information on the months, but not the exact dates, in which patients underwent neurosurgical procedures. During that period, the procedures performed in the month after reperfusion therapy was analyzed as surrogates.
2.5. Statistical methods
A trend analysis was performed to assess the temporal trends in the age and sex of patients treated with reperfusion therapy. We analyzed the monthly trends in the age- and sex-adjusted number of patients treated with reperfusion therapy, as well as the rates of all-cause mortality, neurosurgical treatments, and their composite within 30 days after reperfusion therapy. Using an interrupted time-series analysis, we evaluated the time trends in reperfusion therapy and unfavorable clinical outcomes before and after the IV rt-PA time window extension in September 2012.[24]
The single-group analysis was performed using the following regression equation:
Where Yt is the aggregated outcome at time t (month), Tt is the time (months) since the start of the study period, Xt is the dummy variable representing the extended time window, XtTt is the interaction term, β0 is the intercept, β1 is the slope prior to the time window extension, β2 is the change in level in the period immediately following the time window extension, and β3 is the difference between the pre- and post-extension slopes.
The multiple-group analysis was performed using the following regression equation:
Where Yt is the aggregated outcome at time t (month), Tt is the time (months) since the start of the study period, Xt is the dummy variable representing the extended time window, XtTt is the interaction term, Z is the dummy variable representing subgroup 1 or subgroup 2, β0 is the intercept, β1 is the slope prior to the time window extension, β2 is the change in level in the period immediately following the time window extension, β3 is the difference between the pre- and post-extension slopes, β4 is the difference in level between subgroup 1 and subgroup 2 (reference) prior to the time window extension, β5 is the difference in the slope between subgroup 1 and subgroup 2 (reference) prior to the time window extension, β6 is the difference in level between subgroup 1 and subgroup 2 (reference) in the period immediately following the time window extension, and β7 is the difference between subgroup 1 and subgroup 2 (reference) in the post-extension slope relative to the pre-extension slope.
All analyses were performed using Stata Statistical Software v14.0 (StataCorp LP, College Station, TX). Two-tailed P values below .05 were regarded as statistically significant.
3. Results
3.1. Demographic characteristics of patients receiving reperfusion therapy
During the study period, we identified 69,920 patients with acute ischemic stroke (mean age ± standard deviation: 74.9 ± 12.0 years; women: 41.4%) who underwent IV rt-PA and/or EVT therapy in hospitals throughout Japan during the study period (please see Table S1 (http://links.Iww.com/MD/F521), Supplemental Content). Over time, the patients who received reperfusion therapy tended to be older and comprise a higher proportion of women.
3.2. Temporal trends in hospitals and hospital volume for reperfusion therapy
Table 1 shows the annual proportions of hospitals that used EVT (EVT hospitals) and hospitals that used only IV rt-PA without EVT (non-EVT hospitals) between 2010 and 2015. There was an increase in the proportion of EVT hospitals and a decrease in the proportion of non-EVT hospitals during the pre-extension period. In contrast, the proportion of EVT hospitals decreased while the proportion of non-EVT hospitals increased immediately after the time window extension.
Table 1.
Temporal changes in the proportion of hospitals performing reperfusion therapy.
| EVT hospitals | Non-EVT hospitals | P valuea | |
| Proportion of hospitals, % | |||
| 2010, n = 8670 | 1.3 | 11.6 | |
| 2011, n = 8605 | 3.0 | 10.3 | |
| 2012, n = 8565 | 4.0 | 9.6 | |
| 2013, n = 8540 | 4.5 | 9.8 | |
| 2014, n = 8493 | 6.3 | 8.3 | |
| 2015, n = 8480 | 7.8 | 7.1 | |
| Pre-extension period | |||
| B1 (SE) | 0.08 (0.01) | −0.06 (0.01) | <.001 |
| P value | <.001 | <.001 | |
| Immediately after extension | |||
| B2 (SE) | −0.39 (0.10) | 0.85 (0.18) | <.001 |
| P value | <.001 | <.001 | |
| Post-extension period | |||
| B3 (SE) | 0.00 (0.01) | 0.02 (0.01) | 0.25 |
| P value | .84 | .14 | |
Next, we evaluated the trends in hospital volume for reperfusion therapy in EVT and non-EVT hospitals (Table 2). In both hospital types, hospital volume for reperfusion therapy increased immediately after the time window extension, but no significant changes were observed during the pre- and post-extension periods. There were no significant differences in these trends between EVT and non-EVT hospitals, indicating that hospital volume for reperfusion therapy underwent similar transitions irrespective of the ability to perform EVT.
Table 2.
Temporal changes in the number of patients receiving reperfusion therapy in each hospital.
| EVT hospitals | Non-EVT hospitals | P valuea | |
| Hospital volume, mean ± SD | |||
| 2010 | 13.6 ± 9.6 | 6.8 ± 7.3 | |
| 2011 | 14.2 ± 9.5 | 6.5 ± 6.9 | |
| 2012 | 15.3 ± 11.9 | 6.4 ± 6.7 | |
| 2013 | 17.1 ± 13.0 | 6.9 ± 7.7 | |
| 2014 | 17.5 ± 14.8 | 6.1 ± 7.3 | |
| 2015 | 18.4 ± 16.2 | 5.5 ± 6.8 | |
| Pre-extension period | |||
| B1 (SE) | −0.004 (0.006) | −0.003 (0.002) | .97 |
| P value | .55 | .09 | |
| Immediately after extension | |||
| B2 (SE) | 0.18 (0.08) | 0.10 (0.04) | .32 |
| P value | .02 | .009 | |
| Post-extension period | |||
| B3 (SE) | 0.011 (0.006) | 0.001 (0.002) | .16 |
| P value | .10 | .59 | |
3.3. Temporal trends in patients receiving reperfusion therapy
We assessed the monthly numbers of patients who underwent IV rt-PA alone, EVT alone, or EVT combined with IV rt-PA. Figure 1 shows the temporal trends in the monthly numbers of patients who received each therapy type.
Figure 1.
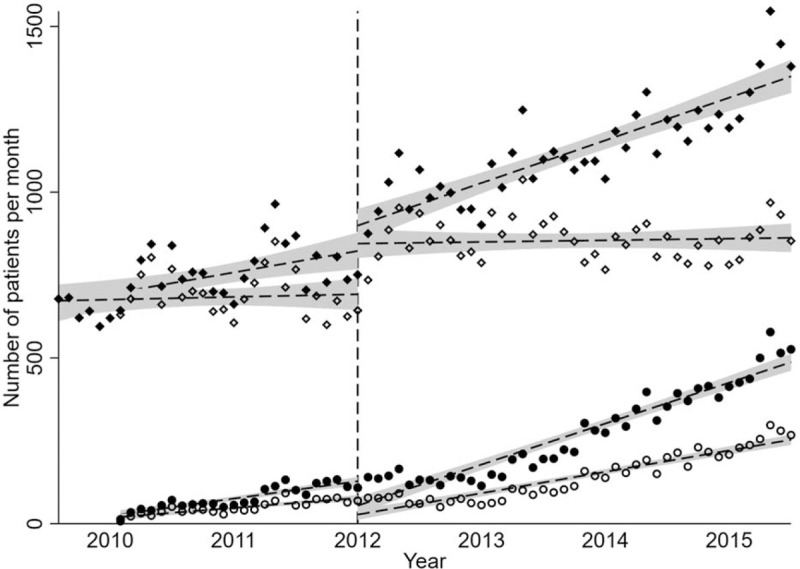
Temporal trends in IV rt-PA and EVT use for acute ischemic stroke. Each point indicates the monthly number of patients treated with EVT alone (open circles), EVT alone or combined with IV rt-PA (closed circles), IV rt-PA alone (open diamonds), or reperfusion therapy involving EVT and/or IV rt-PA (closed diamonds). The dashed lines and shaded areas represent the regression lines and 95% confidence intervals, respectively, for each group. The therapeutic time window was extended from 3.0 to 4.5 hours in September 2012 (vertical dashed line). Each year indicates the Japanese fiscal year from April to March.
Although there were no significant changes in the age- and sex-adjusted monthly number of patients receiving IV rt-PA alone during the pre- and post-extension periods, this number increased significantly immediately after the time window extension (Table 3). In contrast, the number of patients receiving EVT generally increased during the pre-extension period, and further increased during the post-extension period irrespective of concomitant IV rt-PA; however, this number decreased immediately following the time window extension. Overall, the number of patients receiving any reperfusion therapy did not significantly change immediately after the time window extension, but continuously increased during the pre- and post-extension periods at a relatively consistent pace.
Table 3.
Temporal changes in the number of patients receiving each reperfusion therapy procedure.
| EVT | |||||
| Overall | IV rt-PA alone | Overall | EVT alone | EVT and IV rt-PA | |
| Number of patients per 100,000 population | |||||
| 2010 | 6.5 | 6.3 | 0.2 | 0.1 | 0.1 |
| 2011 | 7.2 | 6.5 | 0.7 | 0.4 | 0.3 |
| 2012 | 7.8 | 6.7 | 1.1 | 0.6 | 0.5 |
| 2013 | 9.0 | 7.7 | 1.3 | 0.7 | 0.7 |
| 2014 | 9.7 | 7.2 | 2.5 | 1.3 | 1.2 |
| 2015 | 10.8 | 7.0 | 3.7 | 2.0 | 1.8 |
| Pre-extension period | |||||
| B1 (SE) | 0.004 (0.001) | −0.000 (0.001) | 0.003 (0.000) | 0.002 (0.000) | 0.002 (0.000) |
| P value | .004 | .90 | <.001 | <.001 | <.001 |
| Immediately after extension | |||||
| B2 (SE) | 0.05 (0.03) | 0.11 (0.03) | −0.05 (0.01) | −0.04 (0.01) | −0.02 (0.00) |
| P value | .11 | <.001 | <.001 | <.001 | .001 |
| Post-extension period | |||||
| B3 (SE) | 0.003 (0.001) | −0.001 (0.001) | 0.004 (0.001) | 0.002 (0.000) | 0.002 (0.000) |
| P value | .08 | .58 | <.001 | <.001 | <.001 |
3.4. Temporal trends in mortality and neurosurgical treatments after reperfusion therapy
Finally, we investigated the clinical outcomes after reperfusion therapy. The interrupted time-series analysis demonstrated that the rates of all-cause mortality, neurosurgical treatments, and the composite outcome within 30 days after reperfusion therapy did not significantly change during the pre-extension period, post-extension period, or immediately after the time window extension (Table 4).
Table 4.
Temporal changes in all-cause mortality and neurosurgical treatments after reperfusion therapy.
| Death | Neurosurgical treatments | Composite outcome | |
| Frequency, % | |||
| 2010 | 8.8 | 3.2 | 11.6 |
| 2011 | 8.0 | 2.9 | 10.4 |
| 2012 | 8.3 | 2.9 | 10.7 |
| 2013 | 7.9 | 3.0 | 10.4 |
| 2014 | 8.0 | 2.7 | 10.2 |
| 2015 | 6.9 | 2.5 | 9.0 |
| Pre-extension period | |||
| B1 (SE) | −0.02 (0.02) | −0.01 (0.01) | −0.04 (0.02) |
| P value | .40 | .39 | .14 |
| Immediately after extension | |||
| B2 (SE) | 0.34 (0.54) | 0.20 (0.31) | 0.55 (0.53) |
| P value | 0.53 | 0.53 | 0.30 |
| Post-extension period | |||
| B3 (SE) | −0.01 (0.03) | −0.00 (0.02) | −0.01 (0.03) |
| P value | .64 | .96 | .71 |
4. Discussion
The major findings of this study are as follows:
During the study period, the proportion of Japanese hospitals that used EVT continuously increased, but hospital volume for reperfusion therapy showed similar trends irrespective of the ability to perform EVT.
The use of IV rt-PA monotherapy (without EVT) increased immediately after the therapeutic time window was extended from 3.0 hours to 4.5 hours, but there were no similar increasing trends during the pre- and post-extension periods.
The use of EVT alone or combined with IV rt-PA continuously increased before the time window extension, and further increased after the extension; however, it decreased immediately following the extension.
Despite the transitions in IV rt-PA and EVT use, no significant changes were observed in the rates of all-cause mortality, neurosurgical treatments for unfavorable events, and their composite outcome within 30 days after reperfusion therapy. These findings provide new insight into the recent trends in reperfusion therapy (IV rt-PA and EVT) in Japan.
Previous studies have reported on the trends in IV rt-PA use for acute ischemic stroke over specific time periods in various countries: 2003 to 2006,[25] 2003 to 2010,[26] and 2007 to 2010[27] in Sweden; 2000 to 2012[28] and 2003 to 2011[29,30] in the US; 2007-2009 in Germany[31]; 2005 to 2012 in the Netherlands[32]; 2009 to 2014 in Taiwan[33]; 2014 to 2018 in South Korea[34]; and 2006 to 2018 in Austria.[35] These studies have observed continuous increases in IV rt-PA use over the study periods in their respective countries. In contrast, our present study found no similar trends in IV rt-PA monotherapy between 2010 and 2015 in Japan. However, the number of patients receiving IV rt-PA monotherapy had risen immediately following the time window extension, which is consistent with the findings of other studies.[26,29–31] A recent study conducted in Japan using an inpatient DPC claims database similarly reported no increasing trends in IV rt-PA use between 2010 and 2014, although patients treated with EVT were excluded from that analysis.[36] In Japan, IV rt-PA monotherapy has been utilized as a first-line reperfusion therapy throughout the country after its approval for acute ischemic stroke in 2005. Although an expanded time window for IV rt-PA increased the number of patients with the labeled indication, there are no expectations for the further dissemination of IV rt-PA monotherapy for acute ischemic stroke patients under Japan's current stroke care system.
Four thrombectomy devices were approved in Japan during our study period, and have been progressively used in clinical practice.[37] Our study showed that the use of these devices increased continuously from the period before the IV rt-PA time window extension, and further increased during the post-extension period. A recent increasing trend in EVT use has also been described in the US.[38,39] Our study additionally showed that EVT use generally decreased immediately after the IV rt-PA time window was extended from 3.0 hours to 4.5 hours. This transient decrease may indicate that EVT had been conventionally performed in patients between 3.0 and 4.5 hours after stroke onset in the pre-extension period, but was replaced by IV rt-PA for similar patients in the post-extension period. Thus, our study sheds new light on the trends in reperfusion therapy in Japan: EVT has been an alternative or additional therapy to IV rt-PA, which resulted in a continuous increase in reperfusion therapy for acute ischemic stroke between 2010 and 2015. The increased availability and use of EVT may facilitate the spread of reperfusion therapy for eligible patients throughout Japan.
Asian patients are at a higher risk of hemorrhagic complications following reperfusion therapy than other ethnic groups.[40,41] Accordingly, the increased use of reperfusion therapy in Japan has been met with concerns about the elevated risk of potentially lethal intracranial hemorrhagic events. However, our study found no increases in 30-day mortality or neurosurgical treatments for unfavorable events following the IV rt-PA time window extension and increased use of EVT. The Get With The Guidelines-Stroke program also noted a significant increase in the use of t-PA between 3.0 and 4.5 hours after stroke onset in the US, but found no difference in in-hospital mortality following the publication of the European Cooperative Acute Stroke Study III results.[30] Therefore, the extension of the IV rt-PA time window together with the increased use of EVT is not expected to elevate the risks of mortality and severe adverse events after reperfusion therapy in Japan. Nevertheless, the continued monitoring of trends in reperfusion therapy and post-reperfusion events is needed to inform and guide the dissemination of these therapies safely, effectively, and efficiently for acute ischemic stroke patients across the country.
There remain several barriers to providing all potentially eligible patients with reperfusion therapy.[42–44] Moreover, regional differences may exist in the use of IV rt-PA and/or EVT. The present study showed that EVT was increasingly utilized along with a rising number of hospitals performing this procedure. A recent study in the US similarly reported a steady increase in the number of centers performing EVT, although a greater number of procedures was still performed at lower-volume centers.[45] Because the benefits of these reperfusion therapies are time-dependent, the location and size of stroke centers that perform EVT should be optimized to implement IV rt-PA and EVT appropriately and expeditiously throughout Japan. In this way, there is an urgent need to reorganize stroke care systems in the EVT era.
4.1. Limitations
This study has several limitations. First, the analysis could not adjust for baseline comorbidities and neurological severity at stroke onset. Second, key outcomes—such as 90-day functional outcomes, symptomatic intracranial hemorrhage, and cause of death—could not be examined due to a lack of relevant data in the NDB. Third, the NDB did not contain data on whether study patients met the eligibility criteria for IV rt-PA or EVT. Therefore, the analysis was conducted under the assumption that study patients were indicated for reperfusion therapy in accordance with guidelines. Fourth, the 4 thrombectomy devices were approved at different times during the study period, which may have affected the EVT trend analysis. Finally, the interrupted time-series analysis did not consider other factors besides time, and unmeasured confounders may have affected the results.
4.2. Future directions
Our nationwide analysis included almost all patients with acute ischemic stroke who received IV rt-PA and/or EVT in the country, which enabled us to elucidate the overall trends in these therapies among more than 120 million people throughout Japan. Thus far, no significant changes have been detected in all-cause mortality and severe adverse events after reperfusion therapy following the IV rt-PA time window extension and increased use of EVT. Nevertheless, these trends may be dependent on acute stroke care guidelines and healthcare systems, and further studies are needed to understand these relationships. Effective and efficient stroke care systems should be established to provide IV rt-PA and EVT for eligible acute ischemic stroke patients throughout the country along with the continued surveillance of national trends.
5. Conclusions
The present study found that the number of acute ischemic stroke patients who were treated with IV rt-PA increased after the extension of the IV rt-PA therapeutic time window, and that EVT use has become progressively widespread in recent years in Japan. Despite these shifts in treatment patterns, post-reperfusion death did not increase during the study period. Further studies are warranted to elucidate how the use of reperfusion therapy and post-reperfusion outcomes change according to time and circumstances in real-world settings.
Acknowledgments
We thank Sadanobu Yamakawa and Sadao Kondo (Deno Laboratory, Tokyo, Japan) for their assistance with programing to extract the data.
Author contributions
Conceptualization: Megumi Maeda, Haruhisa Fukuda, Ryu Matsuo, Fumi Kiyuna, Tetsuro Ago, Takanari Kitazono, Masahiro Kamouchi.
Data curation: Megumi Maeda, Haruhisa Fukuda, Ryu Matsuo, Fumi Kiyuna, Masahiro Kamouchi.
Formal analysis: Megumi Maeda, Ryu Matsuo, Fumi Kiyuna, Masahiro Kamouchi.
Funding acquisition: Masahiro Kamouchi.
Investigation: Megumi Maeda, Haruhisa Fukuda, Ryu Matsuo, Fumi Kiyuna, Takanari Kitazono, Masahiro Kamouchi.
Methodology: Megumi Maeda, Ryu Matsuo, Fumi Kiyuna, Tetsuro Ago, Takanari Kitazono, Masahiro Kamouchi.
Project administration: Ryu Matsuo, Tetsuro Ago, Takanari Kitazono, Masahiro Kamouchi.
Resources: Masahiro Kamouchi.
Supervision: Haruhisa Fukuda, Ryu Matsuo, Tetsuro Ago, Takanari Kitazono, Masahiro Kamouchi.
Validation: Megumi Maeda, Fumi Kiyuna, Tetsuro Ago, Masahiro Kamouchi.
Visualization: Megumi Maeda, Ryu Matsuo, Fumi Kiyuna, Masahiro Kamouchi.
Writing – original draft: Megumi Maeda.
Writing – review & editing: Haruhisa Fukuda, Ryu Matsuo, Tetsuro Ago, Takanari Kitazono, Masahiro Kamouchi.
Footnotes
Abbreviations: DPC = diagnosis procedure combination, EVT = endovascular thrombectomy, IV rt-PA = intravenous recombinant tissue plasminogen activator, NDB = National Database of Health Insurance Claims and Specific Health Checkups.
How to cite this article: Maeda M, Fukuda H, Matsuo R, Kiyuna F, Ago T, Kitazono T, Kamouchi M. Nationwide temporal trend analysis of reperfusion therapy utilization and mortality in acute ischemic stroke patients in Japan. Medicine. 2021;100:1(e24145).
This study was supported by JSPS KAKENHI Grant Numbers JP15K08849, JP17H04143; MHLW AC Program Grant Number JPMH19196406; and AMED under Grant Numbers JP16hk0102038h0001, JP19213182.
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
EVT = endovascular thrombectomy; SE = standard error. Each hospital was classified as an EVT hospital or non-EVT hospital according to whether it performed EVT each year. The proportion of hospitals is presented as the percentage of all Japanese hospitals. Each year indicates the Japanese fiscal year; i.e., 12 months from April to March. The therapeutic time window of IV rt-PA was extended from 3.0 to 4.5 hours in September 2012, and the study period was divided into a pre-extension period (Apr 2010 − Aug 2012) and a post-extension period (Sep 2012 − Mar 2016). B1, B2, and B3 indicate the slope prior to extension, change in level immediately following the extension, and difference between the pre- and post-extension slopes, respectively.
Subgroup differences were assessed using the difference between the 2 groups for the slope during the pre- (B1) or post-extension periods (B3) and the level in the period immediately following the extension (B2).
EVT = endovascular thrombectomy; SD = standard deviation, SE: standard error. Each hospital was classified as an EVT hospital or non-EVT hospital according to whether it performed EVT each year. Hospital volume is presented as the number of patients who were treated with reperfusion therapy in each hospital each year. Each year indicates the Japanese fiscal year; i.e., 12 months from April to March. The therapeutic time window of IV rt-PA was extended from 3.0 to 4.5 hours in September 2012, and the study period was divided into a pre-extension period (Apr 2010 − Aug 2012) and a post-extension period (Sep 2012 − Mar 2016). B1, B2, and B3 indicate the slope prior to extension, change in level immediately following the extension, and difference between the pre- and post-extension slopes, respectively.
Subgroup differences were assessed using the difference between the two groups for the slope during the pre- (B1) or post-extension periods (B3) and the level in the period immediately following the extension (B2).
IV rt-PA = intravenous recombinant tissue plasminogen activator; EVT = endovascular thrombectomy; SE = standard error. The number of patients within the population are expressed as the age- and sex-adjusted number of patients who were treated with each procedure each year per 100,000 population. Each year indicates the Japanese fiscal year; that is, 12 months from April to March. The therapeutic time window of IV rt-PA was extended from 3.0 to 4.5 hours in September 2012, and the study period was divided into a pre-extension period (Apr 2010 − Aug 2012) and a post-extension period (Sep 2012 − Mar 2016). B1, B2, and B3 indicate the slope prior to extension, change in level immediately following the extension, and difference between the pre- and post-extension slopes, respectively.
SE = standard error. The frequency of unfavorable events is presented as the percentage of patients who experienced the event of interest after reperfusion therapy. All-cause mortality was defined as death from any cause within 30 days. Neurosurgical treatments included decompressive craniotomy, hematoma evacuation, and shunting for hydrocephalus. The composite outcome comprised all-cause mortality and neurosurgical treatments. Each year indicates the Japanese fiscal year; i.e., 12 months from April to March. The therapeutic time window of IV rt-PA was extended from 3.0 to 4.5 hours in September 2012, and the study period was divided into a pre-extension period (Apr 2010 − Aug 2012) and a post-extension period (Sep 2012 − Mar 2016). B1, B2, and B3 indicate the slope prior to extension, change in level immediately following the extension, and difference between the pre- and post-extension slopes, respectively.
References
- [1].Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017;120:439–48. [DOI] [PubMed] [Google Scholar]
- [2].Hankey GJ. Stroke. Lancet 2017;389:641–54. [DOI] [PubMed] [Google Scholar]
- [3].Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–69. [DOI] [PubMed] [Google Scholar]
- [4].Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim AS, Cahill E, Cheng NT. Global stroke belt: geographic variation in stroke burden worldwide. Stroke 2015;46:3564–70. [DOI] [PubMed] [Google Scholar]
- [6].Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- [7].Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–110. [DOI] [PubMed] [Google Scholar]
- [8].Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344–418. [DOI] [PubMed] [Google Scholar]
- [9].National Institute of Neurological Disorders Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–7. [DOI] [PubMed] [Google Scholar]
- [10].Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29. [DOI] [PubMed] [Google Scholar]
- [11].Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008;372:1303–9. [DOI] [PubMed] [Google Scholar]
- [12].Del Zoppo GJ, Saver JL, Jauch EC, et al. American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 2009;40:2945–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davis SM, Donnan GA. 4.5 hours: the new time window for tissue plasminogen activator in stroke. Stroke 2009;40:2266–7. [DOI] [PubMed] [Google Scholar]
- [14].Campbell BC, Meretoja A, Donnan GA, Davis SM. Twenty-year history of the evolution of stroke thrombolysis with intravenous alteplase to reduce long-term disability. Stroke 2015;46:2341–6. [DOI] [PubMed] [Google Scholar]
- [15].Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- [16].Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. [DOI] [PubMed] [Google Scholar]
- [17].Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. [DOI] [PubMed] [Google Scholar]
- [18].Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. [DOI] [PubMed] [Google Scholar]
- [19].Jovin TG, Chamorro, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. [DOI] [PubMed] [Google Scholar]
- [20].Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAM 2016;316:1279–88. [DOI] [PubMed] [Google Scholar]
- [21].Goyal M, Yu AY, Menon BK, et al. Endovascular therapy in acute ischemic stroke: challenges and transition from trials to bedside. Stroke 2016;47:548–53. [DOI] [PubMed] [Google Scholar]
- [22].Fischer U, Kaesmacher J, Mendes Pereira V, et al. Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke: a topical review. Stroke 2017;48:2912–8. [DOI] [PubMed] [Google Scholar]
- [23].Smith EE, Schwamm LH. Endovascular clot retrieval therapy: implications for the organization of stroke systems of care in North America. Stroke 2015;46:1462–7. [DOI] [PubMed] [Google Scholar]
- [24].Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- [25].Eriksson M, Jonsson F, Appelros P, et al. Dissemination of thrombolysis for acute ischemic stroke across a nation: experiences from the Swedish stroke register, 2003 to 2008. Stroke 2010;41:1115–22. [DOI] [PubMed] [Google Scholar]
- [26].Asplund K, Glader EL, Norrving B, et al. Effects of extending the time window of thrombolysis to 4.5 hours: observations in the Swedish stroke register (Riks-Stroke). Stroke 2011;42:2492–7. [DOI] [PubMed] [Google Scholar]
- [27].Stecksen A, Asplund K, Appelros P, et al. Thrombolytic therapy rates and stroke severity: an analysis of data from the Swedish stroke register (Riks-Stroke) 2007-2010. Stroke 2012;43:536–8. [DOI] [PubMed] [Google Scholar]
- [28].Domino JS, Baek J, Meurer WJ, et al. Emerging temporal trends in tissue plasminogen activator use: results from the BASIC project. Neurology 2016;87:2184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes 2013;6:543–9. [DOI] [PubMed] [Google Scholar]
- [30].Messe SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With The Guidelines-Stroke. Circ Cardiovasc Qual Outcomes 2012;5:321–6. [DOI] [PubMed] [Google Scholar]
- [31].Minnerup J, Wersching H, Ringelstein B, et al. Impact of the extended thrombolysis time window on the proportion of recombinant tissue-type plasminogen activator-treated stroke patients and on door-to-needle time. Stroke 2011;42:2838–43. [DOI] [PubMed] [Google Scholar]
- [32].Scherf S, Limburg M, Wimmers R, et al. Increase in national intravenous thrombolysis rates for ischaemic stroke between 2005 and 2012: is bigger better? BMC Neurol 2016;16:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cheng TJ, Peng GS, Jhao WS, et al. Nationwide "hospital emergent capability accreditation by level-stroke" improves stroke treatment in Taiwan. J Stroke 2017;19:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jeong HY, Jung KH, Mo H, et al. Characteristics and management of stroke in Korea: 2014-2018 data from Korean Stroke Registry. Int J Stroke 2020;15:619–26. [DOI] [PubMed] [Google Scholar]
- [35].Marko M, Posekany A, Szabo S, et al. Trends of r-tPA (recombinant tissue-type plasminogen activator) treatment and treatment-influencing factors in acute ischemic stroke. Stroke 2020;51:1240–7. [DOI] [PubMed] [Google Scholar]
- [36].Miyamoto Y, Aso S, Iwagami M, et al. Expanded indication for recombinant tissue plasminogen activator from 3 to 4.5 h after onset of stroke in Japan. J Stroke Cerebrovasc Dis 2020;29:105341. [DOI] [PubMed] [Google Scholar]
- [37].Hayakawa M, Matsumaru Y, Yamagami H, et al. Trends in endovascular reperfusion therapy for acute stroke after introduction of mechanical thrombectomy devices: Japanese Registry of NeuroEndovascular Therapy (JR-NET)3. Neurol Med Chir (Tokyo) 2020;60:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Smith EE, Saver JL, Cox M, et al. Increase in endovascular therapy in Get With The Guidelines-Stroke after the publication of pivotal trials. Circulation 2017;136:2303–10. [DOI] [PubMed] [Google Scholar]
- [39].Atchaneeyasakul K, Liaw N, Lee RH, Liebeskind DS, Saver JL. Patterns of mechanical thrombectomy for stroke before and after the 2015 pivotal trials and US national guideline update. J Stroke Cerebrovasc Dis 2020;29:105292. [DOI] [PubMed] [Google Scholar]
- [40].Mehta RH, Cox M, Smith EE, et al. Race/Ethnic differences in the risk of hemorrhagic complications among patients with ischemic stroke receiving thrombolytic therapy. Stroke 2014;45:2263–9. [DOI] [PubMed] [Google Scholar]
- [41].Toyoda K, Koga M, Hayakawa M, et al. Acute reperfusion therapy and stroke care in Asia after successful endovascular trials. Stroke 2015;46:1474–81. [DOI] [PubMed] [Google Scholar]
- [42].Messe SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology 2016;87:1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Paul CL, Ryan A, Rose S, et al. How can we improve stroke thrombolysis rates?. A review of health system factors and approaches associated with thrombolysis administration rates in acute stroke care. Implement Sci 2016;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gonzales S, Mullen MT, Skolarus L, et al. Progressive rural-urban disparity in acute stroke care. Neurology 2017;88:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Saber H, Navi BB, Grotta JC, et al. Real-world treatment trends in endovascular stroke therapy. Stroke 2019;50:683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


