Abstract
Background:
The pelvic floor muscle (PFM) is associated with respiratory function. We investigated the effects of PFM training by pelvic floor electrical stimulation (PFES) on PFM strength, diaphragm excursion, and upper rib cage movement during tidal and forceful breathing and coughing in women with stress urinary incontinence (SUI).
Methods:
In total, 33 participants with SUI were divided into PFES and control groups. The two groups were measured pre- and post-8 weeks of training. Diaphragm excursion and upper rib cage movement during tidal and forceful breathing and coughing and PFM strength were measured using sonography, electromagnetic sensors, and perineometry.
Results:
There were significant difference of main effect between pre- and post-training and between groups in PFM strength (between groups: P = .001, between time: P < .001) and diaphragm excursion during forceful breathing (between groups: P = .015, between time: P = .026) and coughing (between groups: P = .035, between time: P = .006). There were significant differences in diaphragm excursion during tidal (P = .002) and forceful breathing (P = .005) and coughing (P < .001) between pre- and post-training in the PFES group. Elevation of the upper rib cage during tidal (P < .001) and forceful breathing (P = .001) was significantly decreased after 8 weeks of training in the PFES group. Widening in the horizontal plane in the upper rib cage during forceful breathing (P < .001) was significantly increased after 8 weeks of training in the PFES group. PFM strength (P < .001) was significantly increased after 8 weeks of training in the PFES group.
Conclusions:
Pelvic floor muscles training by electrical stimulation can improve diaphragm excursion and breathing patterns in women with SUI.
Keywords: Breathing, cough, diaphragm, pelvic floor muscle
1. Introduction
Stress urinary incontinence (SUI) has been defined by the International Continence Society as “the complaint of involuntary leakage on exertion, coughing or sneezing”.[1] Pelvic floor muscle (PFM) training is the first line treatment in SUI treatment, increasing PFM strength and urethral pressure, and decreasing urethral hypermobility.[2,3] Pelvic floor electrical stimulation (PFES), as a form of PFM training, could improve urinary loss and increase the strength of PFM contractions by identifying the PFM or facilitating the ability to volitionally contract the PFM.[4–6]
An abnormal breathing pattern can be activated in patients with SUI and PFM dysfunction;[7–9] during inspiration, the rib cage elevates more and the diaphragm descends less than in healthy individuals.[7] There is often less abdominal wall excursion and more rib cage elevation in patients with SUI.[7] During expiration, the rib cage drops down, the abdominal wall is forced out, and the PFM is forced down.[7]
In normal breathing, a costodiaphragmatic breathing pattern is monitored when the lateral costal and abdominal expansion is predominant over the superior thoracic expansion during inspiration at rest, to allow maximum lung expansion and gas exchange.[7,10,11] An abnormal breathing pattern occurs when the superior thoracic expansion exceeds the lateral costal abdominal and lateral costal expansion during inspiration at rest, known as the upper costal breathing type.[7,10,11] Previous studies suggested theoretically that this abnormal breathing type produces a smaller expansion of the rib cage.[10,11] Although the pectoralis major, pectoralis minor, latissimus dorsi, and upper trapezius are considered more accessary respiratory muscle than postural function and contribute to upper costal breathing pattern in the dysfunctional or paradoxical breather.[12] An inhibited diaphragmatic movement could impair the coordination between the diaphragm, transverse abdominis muscle and PFM during tasks or functional movement.[13–15]
The PFM is associated with respiratory function.[16–19] It contracts eccentrically during inspiration and contracts concentrically together with the abdominal muscles during forced expiratory maneuvers and coughing, thereby reducing the volume of the abdominal cavity and increasing intra-abdominal pressure (IAP), which forces the diaphragm upwards and increases expiratory effort.[14,16,18,19] The PFM works in synergy with the diaphragm, to control and respond to changes in IAP, provide trunk stability, and contribute to continence while breathing and coughing.[14,16,18,20,21] The core muscle training included PFM contraction affects breathing movements measured by the respiratory inductive plethysmography.[22] Because of the association between the PFM and breathing, as an alternative treatment for urinary incontinence, not only PFM training but also corrective training for breathing patterns and diaphragmatic breathing training have been suggested[7] and applied.[23] However, although previous studies suggested association PFM and breathing,[16–19] causality is still unclear for relationship between PFM and breathing and there are lack of study for effect of PFM training on breathing pattern. Thus, it is necessary to study whether PFM training affects diaphragm contraction and breathing patterns.
We investigated the effects of PFM training by PFES on PFM strength, diaphragm excursion, upper rib cage movement during tidal and forceful breathing, and coughing in women with SUI.
2. Methods
2.1. Subjects and design
The study design was a single blinded randomization of all participants into PFES and control group in a laboratory setting, from August to December 2018. G∗Power (version 3.1.3; University of Trier, Trier, Germany) was used to calculate the sample size for a power of 0.95, an α level of 0.05, partial η2 of 0.387 and an effect size f of 0.794, as determined by pilot data (3 participants each group) on variables of diaphragm excursion during forceful breathing. The sample size required at least 12 subjects per group. Participants were recruited by advertisements that provided a telephone contact; all participants were asked to visit the Urogynecology Clinic in Seoul, Korea, for diagnosis of SUI, and were evaluated regarding the inclusion and exclusion criteria.
Table 1 shows the inclusion/exclusion criteria. In total, 33 subjects were allocated using a random numbers generated by www.randomization.com into PFES and control group (Fig. 1). All subjects gave written informed consent in a form approved by the Institutional Review Board of Yonsei University Mirae Campus (approval no. 1041849–202002-BM-014-01), before the study. The study protocol was registered with the Clinical Research information Service (KCT0003357).
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
| Age between 30 and 60 years |
| Body mass index < 30 kg/m2 |
| Leakage episode recorded more than once a week |
| SUI diagnosed by a urogynecologist |
| Successful completion of the medical screening questionnaire |
| Not addicted to alcohol or drugs |
| Exclusion criteria |
| Not meeting the inclusion criteria |
| Concomitant treatment for SUI during the trial period |
| Cardiac pacemaker or metal materials implanted |
| Pelvic or abdominal surgery within the last 6 months |
| Pregnant/planning to become pregnant |
| Aversion to electrical stimulation |
| Urinary tract infection |
| Urogenital prolapse of grade III or higher |
| Neurological or psychiatric disease |
| Cognitive impairment: perception problem of understanding of the experimental procedure |
Figure 1.
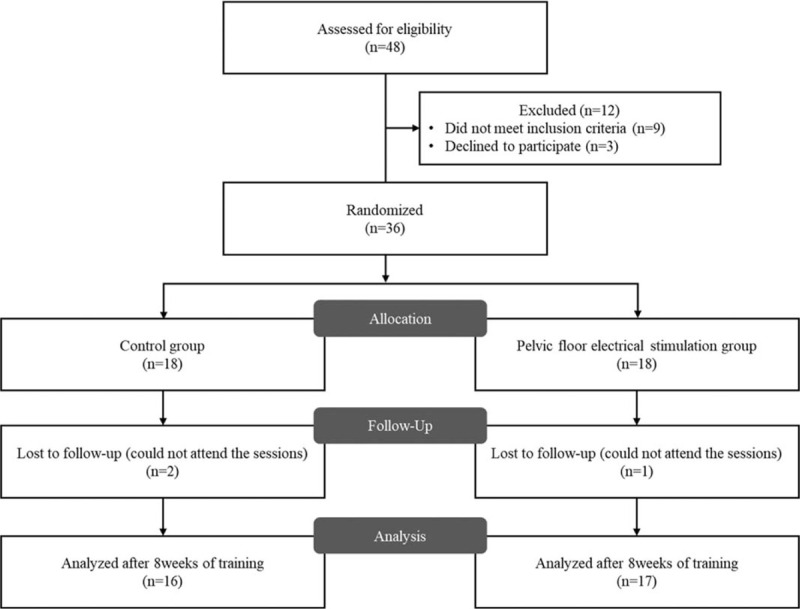
Flow diagram of participant recruitment.
2.2. Pelvic floor electrical stimulation
The PFES device (EasyK7, Alphamedic Co., Ltd., Daegu, Korea) applied 3 surface electrodes [positioned in both the sacral (1 electrode) and perivaginal regions (2 electrodes)] for stimulating the PFM and surrounding structures. These electrodes create the electromagnetic field that stimulates the PFM when the user sits on the device. For neuromuscular electrical stimulation, high-frequency (50–75 Hz) fatigue generates excessive loss of force, which could be due to failure of electrical propagation with a rapid decreasing in the evoked action potential amplitude.[24,25] Thus, we decided to use electrical stimulation at 25 Hz. The PFES was applied as biphasic and asymmetric impulses at 25 Hz;[26,27] the pulse and resting durations were 11 s each. A PFES session was 15 minutes in duration.
2.3. Intervention
The PFES participants were each given a PFES device, and were taught how to use, apply, and manage the device in our laboratory setting. We asked participants to use the PFES device once a day (15-minutes session), 5 days a week for 8 weeks.[28] Also, all subjects underwent training sessions exploring possible increases in PFES amplitude (as individually tolerated). Adherence to this schedule was confirmed by phone twice each week and we encouraged participants to perform PFES at least >5 days each week.
The control group walked for >20 minutes daily. Both groups were assessed at baseline and after 8 weeks.
3. Outcome measures
3.1. Pelvic floor muscle strength
PFM strength was measured in the hook-lying by a urogynecologist, using a VVP-3000 perineometer (QLMED Ltd, Gyeonggi-do, Korea), and a vaginal probe 115 mm in length (vaginal insertion surface measurement length 66 mm) and 24 mm in diameter.[28,29] The initial pressure of the vaginal probe was controlled at 40 mm Hg, because of differences in the vaginal volume of each subject. The baseline without voluntary PFM contraction was recorded in mmHg and then the device was zeroed during rest. The PFM strength was measured from the baseline to that of peak effort for 5 s and recorded (in mm Hg) as the mean increase in vaginal pressure during 2 maximal voluntary contractions.
3.2. Diaphragm excursion by M-mode ultrasonography
Subjects were positioned supine and diaphragm excursions were recorded in the M-Mode. The ultrasound device (A35; Samsung Medison, Seoul, Korea) was used to measure ultrasonographic indices of the diaphragm with a sector transducer (3.5 MHz). The probe was placed on the abdominal region just below the lowest right rib, between the mammillary and midaxillary lines in the longitudinal plane in the superior direction, with the liver as an acoustic window.[30] The angle of the probe was adjusted so that the ultrasound beam was perpendicular to the posterior third of the right hemidiaphragm.[30] The diaphragm excursion was assessed from the M-mode image between the end of expiration and end of inspiration during tidal and forceful breathing and coughing and reported in cm. The averages of 3 values were taken for each tidal and forceful breath and coughing.
3.3. Kinematics measurement for upper rib cage
The Liberty system (Polhemus, VT, USA) was used to investigate upper rib cage movement at 120 Hz and to monitor superoinferior and anteroposterior translation and pump-handle movement (anteroposterior rotation) during tidal and forceful breathing and coughing. The electromagnetic motion sensor was attached at the center of the manubrium below the jugular notch. Metal objects were removed to avoid interference. The sensor and wire were firmly fixed with adhesive tape in the same position to prevent motion artifacts. The transmitter was placed in the same position and orientation during tidal and forceful breathing and coughing. The electromagnetic tracker system was aligned with the orientation of breathing in the supine position, with +x directed along the superoinferior axis, +y directed along the mediolateral axis, and +z directed along the anteroposterior axis (Fig. 2). The sensor in the center of the manubrium was used for superoinferior and anteroposterior translation (cm) and anteroposterior rotation data (°) of the upper rib cage. Upper rib cage movements (translation and rotation) were measured based on the difference between the end of expiration and end of inspiration during tidal and forceful breathing and coughing. The averages of 3 values were taken for each tidal and forceful breath and coughing.
Figure 2.
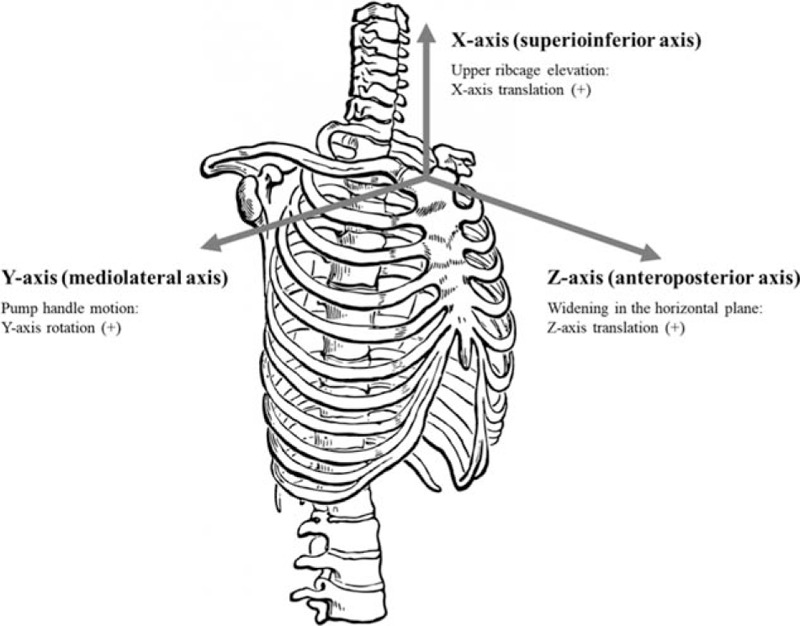
Axes of upper rib cage movement.
3.4. Statistical analyses
The Kolmogorov–Smirnov Z-test was applied to verify the assumption of data normality. Initially, an independent t-test was performed for each dependent variable (PFM strength, diaphragm excursion, upper rib cage movement during tidal and forceful breathing and coughing) to determine whether there were differences pre-measurement between the groups. When statistically significant differences were found, analysis of covariance (ANCOVA) was performed with the baseline measurement as a covariate. When no statistically significant differences were found, two-way analysis of variance (ANOVA) with repeated measures was conducted to compare measurements of each dependent variable between groups, as well as the interaction effect (time × group). Whenever a significant interaction was observed, a paired t-test was used to determine within-group differences, and an independent t-test was used to determine between-group differences. All statistical analyses were performed using SPSS ver. 18.0 software (SPSS Inc., Chicago, IL, USA). A P value of .05 was taken to indicate statistical significance.
4. Results
The characteristics of the subjects are shown in Table 2. There were no adverse events during performing the present study. The mean intensity among all participants was 19.13 ± 5.47 mA (range: 2.5 to 30 mA). Compliance to the intervention was to perform PFES for 5 days a week. Three participants (2 participants in control group and 1 participant in PFES group) did not perform compliance to the intervention (Fig. 1). Table 3 shows the post-intervention changes in PFM strength, diaphragm excursion, X- and Z-axis translation, and Y-axis rotation in upper rib cage kinematics during tidal and forceful breathing and coughing relative to baseline for each group.
Table 2.
Characteristics of the participants.
| Control group (n = 16) | PFES group (n = 17) | P value | |
| Age (y) | 41.1 ± 7.2 | 42.1 ± 8.8 | .726 |
| BMI (kg/m2) | 22.8 ± 3.5 | 22.6 ± 2.7 | .869 |
| Duration of symptoms (y) | 7.8 ± 6.0 | 5.9 ± 3.6 | .229 |
| Deliveries (n) | 1.5 ± 0.9 | 1.8 ± 0.8 | .385 |
| Vaginal deliveries (n) | 1.5 ± 0.9 | 1.4 ± 1.0 | .799 |
Table 3.
PFM strength, diaphragm excursion and upper rib cage kinematics values pre- and post-training for both groups (means ± SD).
| Group | Pre | Post | P | ||
| Pelvic floor muscle strength | PFES | 24.21 ± 7.14 | 39.14 ± 11.88 | .000∗ | |
| Control | 33.19 ± 13.18 | 29.31 ± 11.89 | .072 | ||
| Diaphragm excursion (cm) | Tidal breathing | PFES | 1.79 ± 0.81 | 2.31 ± 0.83 | .002∗ |
| Control | 1.82 ± 0.79 | 1.70 ± 0.73 | .393 | ||
| Forceful breathing | PFES | 6.26 ± 0.95 | 6.73 ± 1.05 | .005∗ | |
| Control | 5.68 ± 0.98 | 5.65 ± 0.91 | .829 | ||
| Coughing | PFES | 4.25 ± 1.01 | 5.04 ± 0.91 | .000∗ | |
| Control | 4.05 ± 0.98 | 3.83 ± 0.94 | .057 | ||
| X-axis translation in upper rib cage kinematics (cm) | Tidal breathing | PFES | 0.09 ± 0.03 | 0.06 ± 0.02 | .000∗ |
| Control | 0.14 ± 0.17 | 0.18 ± 0.19 | .204 | ||
| Forceful breathing | PFES | 1.02 ± 0.58 | 0.77 ± 0.44 | .001∗ | |
| Control | 1.07 ± 0.42 | 1.16 ± 0.38 | .358 | ||
| Coughing | PFES | 2.25 ± 0.99 | 2.03 ± 0.78 | .400 | |
| Control | 2.38 ± 0.77 | 2.27 ± 0.85 | .610 | ||
| Z-axis translation in upper rib cage kinematics (cm) | Tidal breathing | PFES | 0.09 ± 0.05 | 0.16 ± 0.23 | .218 |
| Control | 0.15 ± 0.20 | 0.11 ± 0.06 | .513 | ||
| Forceful breathing | PFES | 0.81 ± 0.35 | 1.03 ± 0.35 | .000∗ | |
| Control | 0.93 ± 0.20 | 0.86 ± 0.38 | .075 | ||
| Coughing | PFES | 1.08 ± 0.41 | 0.97 ± 0.45 | .337 | |
| Control | 1.16 ± 0.41 | 1.18 ± 0.37 | .866 | ||
| Y-axis rotation in upper rib cage kinematics (°) | Tidal breathing | PFES | 0.73 ± 0.46 | 0.78 ± 0.43 | .751 |
| Control | 0.87 ± 0.64 | 0.86 ± 0.86 | .983 | ||
| Forceful breathing | PFES | 4.92 ± 3.48 | 4.99 ± 3.58 | .932 | |
| Control | 4.75 ± 2.23 | 4.69 ± 2.67 | .936 | ||
| Coughing | PFES | 11.52 ± 4.24 | 12.42 ± 4.75 | .461 | |
| Control | 10.67 ± 3.37 | 9.48 ± 4.77 | .054 | ||
4.1. Pelvic floor muscle strength
PFM strength was significantly different between groups at baseline. The baseline measurement was used as a covariate in the subsequent analyses. ANCOVA showed a statistically significant main effect between pre- and post-training (95% CI: 2.394–8.726, P < .001) and between groups (95% CI: 4.267–12.508, P = .001). There were also statistically significant interactions between time and groups (P < .001) but no difference between time and the covariate (P = .134). The PFES group showed significantly greater PFM strength than the control group (95% CI: −18.277.267–−1.388, P = .024) post-training. PFM strength (95% CI: −20.070–−9.794, P < .001) was significantly increased after training in the PFES group, while the control group showed no significant difference between pre- and post-training (CI: −0.398–8.156, P = .072).
4.2. Diaphragm excursion
There were no significant differences between groups at baseline for diaphragm excursion during tidal and forceful breathing and coughing. ANOVA showed a statistically significant main effect between pre- and post-training during tidal (95% CI: 0.007–0.394, P = .043) and forceful breathing (95% CI: 0.028–0.414, P = .026) and coughing (95% CI: 0.088–0.479, P = .006) and between groups during forceful breathing (95% CI: 0.172–1.500, P = .015) and coughing (95% CI: 0.053–1.361, P = .035). However, there were significant time × group interactions for diaphragm excursion during each action. The PFES group had significantly greater excursion during each action than the control group post-training. There were also significant differences in each action between pre- and post-training, while the control group did not show any significant difference between pre- and post-training.
4.3. Upper rib cage kinematics
There were no significant differences between groups at baseline for X- and Z-axis translation, and Y-axis rotation in the upper rib cage during tidal and forceful breathing and coughing. For X-axis translation, ANOVA did not showed a statistically significant main effect between pre- and post-training during tidal and forceful breathing and coughing and between groups forceful breathing and coughing. However, there was significantly difference between groups during tidal breathing (95% CI: 0.001–0.172, P = .048). However, there were significant time × group interactions for X-axis translation during tidal (P = .022) and forceful breathing (P = .005). The PFES group had significantly less X-axis translation during tidal (95% CI: 0.026–0.217, P = .014) and forceful breathing (95% CI: 0.096–0.680, P = .011) than the control group at post-training. X-axis translation during tidal (95% CI: 0.019–0.043, P < .001) and forceful breathing (95% CI: 0.111–0.384, P = .001) significantly decreased after 8 weeks of training, while the control group showed no significant differences between pre- and post-training.
For Z-axis translation, ANOVA did not showed a statistically significant main effect between pre- and post-training and between groups during tidal and forceful breathing and coughing. There were significant time × group interactions for Z-axis translation in the upper rib cage during forceful breathing (P < .001), but no significant difference during tidal breathing (P = .177) or coughing (P = .461). The PFES group had significantly greater translation than the control group during forceful breathing (95% CI: −0.499–−0.041, P = .022) at post-training. This significantly increased after 8 weeks of training, while the control group showed no significant differences between pre- and post-training.
For Y-axis rotation, ANOVA did not showed a statistically significant main effect between pre- and post-training and between groups during tidal and forceful breathing and coughing. There were no significant time × group interactions for Y-axis rotation during tidal and forceful breathing and coughing. No significant differences were found for main effect in Y-axis rotation in the upper rib cage during tidal and forceful breathing and coughing.
5. Discussion
PFM training by PFES significantly increased PFM strength and diaphragm excursion during tidal and forceful breathing and coughing in women with SUI. In addition, there were significant differences in X-axis translation in the upper rib cage during tidal and forceful breathing and Z-axis translation at this location during forceful breathing between pre-and post-PFM training by PFES. Although the cause and effect relationship between altered breathing pattern and PFM weakness is controversial, PFM training by PFES could affect diaphragm excursion and breathing patterns in women with SUI. Also, although we did not measure expiratory function, such as forced vital capacity and forced expiratory flows, and gas change, increasing diaphragm excursion and decreasing elevation of upper ribcage movement may help improve to optimal breathing pattern in women with SUI.
A previous study reported diaphragm excursion (tidal breathing: 18.4 ± 7.6 and forceful breathing: 78.8 ± 13.3 mm) in healthy subjects, assessed via sonography.[30] The diaphragm excursions of subjects with SUI in our study (tidal breathing: 18.1 ± 7.7 and forceful breathing: 59.7 ± 9.7 mm) were smaller than those in that previous study, although it is difficult to compare our data directly to those of the previous study.[30] Movement of the diaphragm and the PFM are synchronous cranio-caudal movements.[18] Similar basic movement patterns of the diaphragm and PFM occur during coughing and breathing.[18] PFM activity increases during inspiration, because of the postulated synergistic coactivation of PFM and anterolateral abdominal muscles.[14] In one study, an incremental positive correlation was found between voluntary PFM contraction strength and forced expiratory flow at 25% (r = 0.320), 50% (r = 0.388), and 75% (r = 0.432) of the forced vital capacity in healthy nulliparous women.[16] Sapsford[7] applied a treatment approach to urinary incontinence using diaphragmatic breathing and motor re-learning of functional expiratory patterns. In addition, retraining diaphragmatic, deep abdominal, and PFM-coordinated function involving diaphragm breathing and functional expiratory pattern training improves symptoms of urinary incontinence as an alternative intervention.[22,23] Otherwise, specific motor learning intervention involving PFM contraction for subjects with sacroiliac joint pain can positively change diaphragm kinematics and patterns of respiration.[9]
The cause and effect relationship between improved PFM contraction and improved diaphragm excursion has been controversial. However, our study demonstrated that improved PFM strength by PFES increased diaphragmatic excursion during tidal and forceful breathing and coughing, which may be explained as follows. First, it may increase to work synergistically with the PFM and diaphragm.[16,18,19] Because of a synchronous parallel movement of the diaphragm and PFM during tidal and forceful breathing as well as during coughing,[18,21] PFM training could help the diaphragm co-contract with the PFM. Second, improved PFM strength could withstand the greater IAP during inspiration than before PFM training. The PFM contracts eccentrically during inspiration and contracts concentrically together with the abdominal muscles during coughing, sneezing and forceful expiration, thereby decreasing the abdominal cavity volume and increasing the IAP, which forces the diaphragm upwards and enhances expiratory effort.[13,14,31] Thus, diaphragmatic excursion could be increased by improved PFM strength, because the PFM could maintain a higher urethral than vesical pressure with the urethral sphincter during tidal and forceful breathing and coughing. Third, reduction of the fear or stress of urine leakage could increase diaphragmatic breathing. Women with SUI have expressed their concern about body odor from leaking urine.[32] Emotional states such as stress or fear could cause shallow and rapid breathing patterns.[33] PFES has demonstrated improvement of both subjective and objective symptoms in women with SUI.[28] Thus, subjects may experience more diaphragmatic breathing than before training.
The abnormal breathing pattern of lifting the sternum vertically during inspiration, instead of widening the thorax in the horizontal plane and pump handle motion, occurs due to bilateral overactivity in the scalene, trapezius, and levator scapulae musculature.[10] For the abnormal breathing pattern, excessive use of accessory respiratory muscles may be required to compensate for insufficient gas exchange, which could lead to chronic cervical and shoulder overstrain, decreased rib movement, decreased intercostal muscle activation, and an inhibited diaphragm movement.[10] Our study measured superoinferior (X-axis) and anteroposterior (Z-axis) translation (cm) and anteroposterior (Y-axis) rotation data (°) of the upper rib cage for detecting elevation of the upper rib cage, widening in the horizontal plane, and pump handle motion, respectively. Elevation of the upper rib cage was significantly decreased during tidal and forceful breathing, and widening in the horizontal plane was significantly increased during forceful breathing after PFM training. If recruitment of the abdominal muscles and consequently the PFM during breathing is decreased, the inspiratory effort requires upper rib cage elevation.[7] In addition, an improved diaphragm excursion could lead to improvement of the abnormal breathing pattern. This is possibly the reason why PFM training may help improve to optimal breathing pattern.
5.1. Methodological limitations
The principal limitations of this study were the lack of electromyography data to assess changes in PFM and accessory respiratory muscle activation during tidal and forceful breathing and coughing. In addition, we did not measure the vital capacity and expiratory flow rates during tidal and forceful breathing. Further study is needed to determine if PFES training can affect the vital capacity and expiratory flow rates during tidal and forceful breathing in women with SUI. Further studies are also needed to determine the change in respiratory-related cranio-caudal movement of the diaphragm and PFM using real-time dynamic magnetic resonance imaging before and after PFM training by PFES. In addition, although we included women of a wide range of ages with SUI, and both pre- and postmenopausal women, studies with larger sample sizes are required.
6. Conclusions
Despite these limitations, pelvic floor muscles training by electrical stimulation can improve diaphragmatic excursion during tidal and forceful breathing and coughing in women with stress urinary incontinence, and the upper rib cage movement pattern during tidal and forceful breathing. The results of this study may be useful for developing guidelines for improving diaphragm excursion and decreasing elevation of upper ribcage movement in patients with stress urinary incontinence. Thus, pelvic floor muscles training could be recommended for improving abnormal breathing pattern in women with stress urinary incontinence.
Acknowledgments
We wish to thank all of the subjects for their time and commitment to the study.
Author contributions
Conceptualization: Ui-jae Hwang.
Formal analysis: Min-seok Lee, Sung-hoon Jung, Sun-hee Ahn.
Funding acquisition: Oh-yun Kwon.
Investigation: Ui-jae Hwang, Min-seok Lee, Sung-hoon Jung, Sun-hee Ahn.
Methodology: Min-seok Lee, Sung-hoon Jung, Sun-hee Ahn.
Project administration: Oh-yun Kwon.
Supervision: Oh-yun Kwon.
Validation: Sung-hoon Jung, Sun-hee Ahn.
Visualization: Sun-hee Ahn.
Writing – original draft: Ui-jae Hwang, Oh-yun Kwon.
Footnotes
Abbreviations: IAP = intra-abdominal pressure, PFES = pelvic floor electrical stimulation, PFM = pelvic floor muscle, SUI = stress urinary incontinence.
How to cite this article: Hwang Uj, Lee Ms, Jung Sh, Ahn Sh, Kwon Oy. Effect of pelvic floor electrical stimulation on diaphragm excursion and rib cage movement during tidal and forceful breathing and coughing in women with stress urinary incontinence: a randomized controlled trial. Medicine. 2021;100:1(e24158).
Yonsei University Research Fund (grant numbers: 2018–51–0213 and 2020–52–0016) provided funding for this study (to O.Y.K). The funders had no role in the study design, data collection and analysis, preparation of the manuscript, or the submission process.
The authors have no conflicts of interests.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
BMI = body mass index, PFES = pelvic floor electrical stimulation.
PFES = pelvic floor electrical stimulation.
References
- [1].Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodynamics 2002;21:167–78. [DOI] [PubMed] [Google Scholar]
- [2].Syan R, Brucker BM. Guideline of guidelines: urinary incontinence. BJU Intern 2016;117:20–33. [DOI] [PubMed] [Google Scholar]
- [3].Zubieta M, Carr RL, Drake MJ, et al. Influence of voluntary pelvic floor muscle contraction and pelvic floor muscle training on urethral closure pressures: a systematic literature review. Intern Urogynecol J 2016;27:687–96. [DOI] [PubMed] [Google Scholar]
- [4].Castro RA, Arruda RM, Zanetti MR, et al. Single-blind, randomized, controlled trial of pelvic floor muscle training, electrical stimulation, vaginal cones, and no active treatment in the management of stress urinary incontinence. Clinics 2008;63:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Correia GN, Pereira VS, Hirakawa HS, et al. Effects of surface and intravaginal electrical stimulation in the treatment of women with stress urinary incontinence: randomized controlled trial. Europ J Obstetrics Gynecol Reproduct Biol 2014;173:113–8. [DOI] [PubMed] [Google Scholar]
- [6].Bø K, Talseth T, Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ 1999;318:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sapsford R. Rehabilitation of pelvic floor muscles utilizing trunk stabilization. Manual Therapy 2004;9:3–12. [DOI] [PubMed] [Google Scholar]
- [8].Thompson JA, O'Sullivan PB, Briffa NK, et al. Altered muscle activation patterns in symptomatic women during pelvic floor muscle contraction and Valsalva manouevre. Neurourol Urodynamics 2006;25:268–76. [DOI] [PubMed] [Google Scholar]
- [9].O'Sullivan PB, Beales DJ. Changes in pelvic floor and diaphragm kinematics and respiratory patterns in subjects with sacroiliac joint pain following a motor learning intervention: a case series. Manual Therapy 2007;12:209–18. [DOI] [PubMed] [Google Scholar]
- [10].Perri MA, Halford E. Pain and faulty breathing: a pilot study. J Bodywork Movement Therapies 2004;8:297–306. [Google Scholar]
- [11].Ha S-m, Kwon O-y, Kim S-j, et al. The importance of a normal breathing pattern for an effective abdominal-hollowing maneuver in healthy people: an experimental study. J Sport Rehabilit 2014;23:12–7. [DOI] [PubMed] [Google Scholar]
- [12].Hruska RJ., Jr Dysfunctional, respiratory mechanics on orofacial pain. Dental Clin North Am 1997;41:211. [PubMed] [Google Scholar]
- [13].Hodges PW, Gandevia SC. Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J Applied Physiol 2000;89:967–76. [DOI] [PubMed] [Google Scholar]
- [14].Hodges P, Sapsford R, Pengel L. Postural and respiratory functions of the pelvic floor muscles. Neurourol Urodynamics 2007;26:362–71. [DOI] [PubMed] [Google Scholar]
- [15].Bradley H, Esformes JD. Breathing pattern disorders and functional movement. Intern J Sports Physical Therapy 2014;9:28. [PMC free article] [PubMed] [Google Scholar]
- [16].Talasz H, Kofler M, Kalchschmid E, et al. Breathing with the pelvic floor? Correlation of pelvic floor muscle function and expiratory flows in healthy young nulliparous women. Intern Urogynecol J 2010;21:475–81. [DOI] [PubMed] [Google Scholar]
- [17].Talasz H, Kremser C, Kofler M, et al. Proof of concept: differential effects of Valsalva and straining maneuvers on the pelvic floor. Europ J Obstetrics Gynecol Reproduct Biol 2012;164:227–33. [DOI] [PubMed] [Google Scholar]
- [18].Talasz H, Kremser C, Kofler M, et al. Phase-locked parallel movement of diaphragm and pelvic floor during breathing and coughing—a dynamic MRI investigation in healthy females. Intern Urogynecol J 2011;22:61–8. [DOI] [PubMed] [Google Scholar]
- [19].Neumann P, Gill V. Pelvic floor and abdominal muscle interaction: EMG activity and intra-abdominal pressure. Intern Urogynecol J 2002;13:125–32. [DOI] [PubMed] [Google Scholar]
- [20].Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Intern J Sports Physical Therapy 2013;8:62. [PMC free article] [PubMed] [Google Scholar]
- [21].Deffieux X, Hubeaux K, Porcher R, et al. Pelvic floor muscle activity during coughing: altered pattern in women with stress urinary incontinence. Urology 2007;70:443–7. [DOI] [PubMed] [Google Scholar]
- [22].Szczygieł E, Blaut J, Zielonka-Pycka K, et al. The impact of deep muscle training on the quality of posture and breathing. J Motor Behav 2018;50:219–27. [DOI] [PubMed] [Google Scholar]
- [23].Hung H-C, Hsiao S-M, Chih S-Y, et al. An alternative intervention for urinary incontinence: retraining diaphragmatic, deep abdominal and pelvic floor muscle coordinated function. Manual Therapy 2010;15:273–9. [DOI] [PubMed] [Google Scholar]
- [24].Moritani T, Muro M, Kijima A. Electromechanical changes during electrically induced and maximal voluntary contractions: electrophysiologic responses of different muscle fiber types during stimulated contractions. Exp Neurol 1985;88:471–83. [DOI] [PubMed] [Google Scholar]
- [25].Hasegawa S, Kobayashi M, Arai R, et al. Effect of early implementation of electrical muscle stimulation to prevent muscle atrophy and weakness in patients after anterior cruciate ligament reconstruction. J Electromyography Kinesiol 2011;21:622–30. [DOI] [PubMed] [Google Scholar]
- [26].Brubaker L. Electrical stimulation in overactive bladder. Urology 2000;55:17–23. [DOI] [PubMed] [Google Scholar]
- [27].Wyndaele J-J. Study on the influence of the type of current and the frequency of impulses used for electrical stimulation on the contraction of pelvic muscles with different fibre content. Scandinavian J Urol 2016;50:228–33. [DOI] [PubMed] [Google Scholar]
- [28].Hwang U-j, Lee M-s, Jung S-h, et al. Which pelvic floor muscle functions are associated with improved subjective and objective symptoms after 8 weeks of surface electrical stimulation in women with stress urinary incontinence? Europ J Obstetrics Gynecol Reproduct Biol 2020;247:16–21. [DOI] [PubMed] [Google Scholar]
- [29].Hwang U-j, Lee M-s, Jung S-h, et al. Pelvic floor muscle parameters affect sexual function after 8 weeks of transcutaneous electrical stimulation in women with stress urinary incontinence. Sexual Med 2019;7:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Testa A, Soldati G, Giannuzzi R, et al. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol 2011;37:44–52. [DOI] [PubMed] [Google Scholar]
- [31].Sapsford R, Hodges P, Richardson C, et al. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol Urodynamics 2001;20:31–42. [DOI] [PubMed] [Google Scholar]
- [32].Kinchen KS, Burgio K, Diokno AC, et al. Factors associated with women's decisions to seek treatment for urinary incontinence. JWomen's Health 2003;12:687–98. [DOI] [PubMed] [Google Scholar]
- [33].Lee DG, Lee L-J, McLaughlin L. Stability, continence and breathing: the role of fascia following pregnancy and delivery. J Bodywork Movement Therapies 2008;12:333–48. [DOI] [PubMed] [Google Scholar]


