Abstract
Background
Microbiologic results are critical to optimal management of patients with lower respiratory tract infection, but standard methods may take several days. The multiplex polymerase chain reaction BioFire Pneumonia (PN) panel detects 15 common bacterial species semiquantitatively as copy number/mL, 8 viral species, and 7 resistance genes in about an hour within the clinical laboratory.
Methods
We tested 396 unique endotracheal or bronchoalveolar lavage specimens with the BioFire Pneumonia panel and compared the bacterial detections to conventional gram stain and culture results.
Results
Of the 396 patients, 138 grew at least 1 bacterium that had a target on the PN panel, and 136/138 (98.6%) were detected by the panel. A total of 177 isolates were recovered in culture and the PN panel detected 174/177 (98.3%). A further 20% of patients had additional targets detected that were not found on standard culture (specificity 69%, positive predictive value 63%, and negative predictive value 98.9%). Copy number was strongly related to standard semiquantitative growth on plates reported by the laboratory (eg, 1+, 2+, 3+ growths) and was significantly higher in those specimens that grew a potential pathogen. Both higher copy number and bacterial detections found by the PN panel, but not found in culture, were strongly positively related to the level of white blood cells reported in the initial gram stain.
Conclusions
Higher copy number and bacterial detections by the PN panel are related to the host respiratory tract inflammatory response. If laboratories can achieve a rapid turnaround time, the PN panel should have a significant impact both on patient management and on antibiotic stewardship.
Keywords: BioFire Pneumonia panel, gram stain white blood cells, multiplex bacterial PCR, standard clinical microbiology
Microbiologic results are critical to optimal management of patients with lower respiratory tract infection. Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guidelines recommend noninvasive semiquantitative microbiology cultures if possible but follow conventional colony count quantitation from protected specimen brushing (PSB; PSB ≥103 colony-forming units [CFU]/mL), and bronchoalveolar lavage (BAL; BAL ≥104 CFU/mL) if bronchoscopy is performed [1]. The diagnosis of hospital-, community-, or ventilator-associated pneumonia (HAP, CAP, and VAP) generally requires clinical variables such as white blood cell count, temperature, purulent sputum, and radiographic evidence of a new or persistent infiltrate in addition to microbiologic data. Because results from the microbiology laboratory may take 2–3 days for identification and susceptibility testing, empiric antibiotic treatment may be needed and can be based in part on initial gram stain results, but these often have a poor correlation with final culture results [1].
Rapid methods for the diagnosis of bacterial pneumonia have been primarily urine antigen testing for Streptococcus pneumoniae and Legionella pneumophila type 1, along with several rapid molecular methods available for viral respiratory diagnosis. The recently FDA-cleared BioFire FilmArray Pneumonia (PN) panel (BioFire Diagnostics, Salt Lake City, UT, USA) tests for 15 conventional bacterial species, 3 agents of atypical pneumonia, 7 antibiotic resistance genes, and 8 viruses (Table 1). The test also reports the conventional bacterial species results in a semiquantitative manner as genome copies/mL from 104 to 107/mL sample. In contrast to conventional cultures, these results can be obtained in ~1 hour in the laboratory, essentially in real time after the performance of a BAL, for example. Several recent studies have shown that virtually all bacterial isolates (>98%) recovered in culture were also detected by the PN panel [2–4]. However, these studies have also shown that a large number of targets were found by the PN panel but not found in standard culture, as much as doubling the total number of bacterial targets detected [4]. For these results to be useful and understood by physicians and other care providers, (1) these additional detections must be true, that is, truly present in the patient specimen, and (2) they have to be clinically relevant, understandable, and either show or not show a relationship with host factors, for example, host immune response or outcome variable such as length of stay.
Table 1.
Targets of the BioFire Pneumonia Panel
| Bacteria (Semiquantitative) |
|---|
| Acinetobacter calcoaceticus-baumannii complex |
| Enterobacter cloacae complex |
| Escherichia coli |
| Haemophilus influenzae |
| Klebsiella aerogenes |
| Klebsiella oxytoca |
| Klebsiella pneumoniae group |
| Moraxella catarrhalis |
| Proteus spp. |
| Pseudomonas aeruginosa |
| Serratia marcescens |
| Staphylococcus aureus |
| Streptococcus agalactiae |
| Streptococcus pneumoniae |
| Streptococcus pyogenes |
| Atypical bacteria (qualitative) |
| Legionella pneumophila |
| Mycoplasma pneumoniae |
| Chlamydia pneumoniae |
| Viruses |
| Influenza A |
| Influenza B |
| Adenovirus |
| Coronavirus |
| Parainfluenza virus |
| Respiratory syncytial virus |
| Human rhinovirus/enterovirus |
| Human metapneumovirus |
| Antimicrobial resistance genes |
| CTX-M |
| KPC |
| NDM |
| Oxa48-like |
| VIM |
| IMP |
| mecA/mecC and MREJ |
We compared the PN panel results with conventional microbiologic species identification and semiquantitation in BAL or endotracheal aspirates from 396 consecutive unique patients. We demonstrated that the copy number/mL reported by the PN panel is related both to the semiquantitative levels of the same organisms found in standard culture and to the reported level of white blood cells described in the initial gram stain. We also analyzed our data in relation to “noninformative” cultures, that is, no growth or growth of normal respiratory flora only, and found that extra PN panel detections within this group are also associated with a higher level of white blood cells (WBCs) in the initial gram stain. Understanding these issues will be key to the clinical application of the PN panel and the utility of the results for patient management.
METHODS
Patients
This study was performed in the clinical microbiology laboratory at the University of Florida Health Shands Hospital, Gainesville, Florida, a 1000+-bed tertiary care hospital serving North Central Florida. All patients underwent a bronchoscopy or were intubated and had endotracheal suction specimens submitted for culture and susceptibility as part of their routine standard of care from June to September 2018. The study was approved by our institutional review board (IRB# 2018-01834). Consecutive unique BAL fluids and endotracheal aspirates were frozen at –70°C within 18 hours of receipt in the laboratory until tested on the PN panel. A total of 396 unique patient specimens, having both culture results from the microbiology laboratory and polymerase chain reaction (PCR) results from the PN panel, were analyzed. Clinical data were obtained from the patient’s electronic medical record (Epic Systems, Madison, WI, USA). All patients had microbiological data available, but nondemographic clinical data (eg, length of stay, mortality) were only available for a subset of 270 patients, as the rest were either outpatients or in a long-term acute care hospital that uses our microbiology laboratory but has a separate medical record.
Microbiologic Methods
Cultures were performed by standard methods, and all isolates were identified and antimicrobial susceptibility testing performed by VITEK MS mass spectrometry and by Vitek II (AST GP 72, AST GP 78, AST GN 73, and AST XN06; bioMerieux, Durham NC, USA). All specimens were run on the PN panel per the manufacturer’s instructions using the FilmArray 2.0 instrument (BioFire Diagnostics, Salt Lake City, UT, USA) in our research laboratory. As all specimens were collected from the lower respiratory tract, evaluation for contamination by epithelial cells was not performed. All cultures were plated using the standard quadrant technique onto blood, chocolate, and MacConkey agar (Becton Dickinson, Sparks, MD, USA), incubated in 5% CO2 at 36°C, and examined for growth over the next 24–48 hours. Growth from endotracheal aspirates and nonquantitative BAL specimens was reported semiqualitatively based on the following criteria: <10 colonies “few,” 1+ for growth in the first quadrant; 2+ for growth in the second quadrant, and so forth. Potential pathogens with growth on plates were only reported if they were a sole pathogen or if their growth was at least 2+ or exceeded the growth of normal respiratory flora. Normal respiratory flora were reported as “normal flora,” “normal oral flora,” or “normal respiratory flora,” along with the growth of potential pathogens. Direct gram stains were performed on all specimens, and the presence of WBCs was reported semiquantitatively as No WBC, few, moderate, and many or 1+, 2+, 3+, and 4+, respectively, depending on the technologist. Under laboratory policy, 1+ or “rare” corresponds to <1 WBC per low power field (lpf), 2+ or “few” is equivalent to 1–9 WBC/lpf, 3+ or “moderate” corresponds to 10–25 WBC/lpf, and 4+ or “many” corresponds to >25 WBC/lpf. The presence of bacteria in the gram stain was reported in a similar manner, along with appropriate morphologic characteristics.
Gram stains for bacteria along with WBCs from unconcentrated BALs were reported as described above for endotracheal aspirates. Specimens ordered for quantitative counts were inoculated onto standard media (see above) using a 0.001-mL calibrated loop and were incubated at 36°C in 5% CO2. Quantitative growth was reported as CFU/mL and ranged from no growth to <1000 CFU/mL, 1000 to 10 000 CFU/mL, and the actual colony count/mL for those with growth of ≥104. All potential pathogens with growth of ≥104 were identified as described above. Those that were not ordered quantitatively were plated using standard quadrant plating and reported as no growth, few, 1+, 2+, 3+, and 4+, as described above.
Molecular Methods
PCR assays were developed to confirm discordant results for S. aureus, E. coli, K. pneumoniae, and P. aeruginosa, using primers based on reports in the literature (Supplementary Table 1). It was beyond the scope of the study to develop independent confirmatory molecular methods for all discordant species. For Staphylococcus aureus, 400 µL of the original specimen was digested with Lysostaphin (Sigma-Aldrich St. Louis, MO, USA, 100 µg/mL at 37°C × 30 minutes in TE buffer, sample diluted 1:4), followed immediately by incubation with Proteinase K (Sigma-Aldrich St. Louis, MO, USA) at 200 µg/mL at 37°C × 30 minutes (sample diluted 1:2 in same buffer) followed by bead beating with 100-µm zirconium beads for 30 seconds × 2, then purified with the Zymo Quick DNA kit (Irvine, CA, USA). DNA was eluted in 65 µL, and 2 µL was amplified with the Thermonuclease primer set (418 BP). For E. coli, Klebsiella, and Pseudomonas, 400 µL was directly extracted using the same Zymo Quick-DNA kit buffers and spin columns as for S. aureus. For the species-specific primer sets, 2 µL was added to a 25-µL reaction volume using the TaKaRa Ex Taq Hot Start Kit (RR006A, Mountain View, CA, USA). For the 16S-based primers sets, the TaKaRa Taq was replaced with that from Molzym (Oasis Diagnostics, Portland, OR, USA). PCRs were run on a Bioer XP Cycler (Hangzhou Bioer Technology Co. Ltd., Hangzhou, China). Cycling parameters were as follows: initial cycle: 94°C × 5 minutes, 55°C × 30 seconds, 72°C × 30 seconds; cycles 2–40: 94°C × 20 seconds, 55°C × 20 seconds, and 72°C × 30 seconds, 72°C × 5 minutes, 4°C until stopped manually (max of 60 minutes). The PCR (5 µL) product was then run on a flash gel (Lonza Scientific), and bands of the appropriate base pair size were considered positive.
Data Analysis
Data were analyzed by chi-square for an association between the PN panel bacterial target detection results and the corresponding growth in culture. PN panel results were considered “concordant” with culture results if at least 1 target detected by the PN panel was also recovered in culture. The highest PN panel copy number was compared with maximum reported quantitative (CFU/mL) or semiquantitative bacteriology (ie, no growth, few, 1+, 2+ growths, etc.) by 2 × N chi-square.
The highest PN panel copy number was also compared with the description of WBCs reported on the gram stain result. Statistics were calculated using Social Science Statistics (https://www.socscistatistics.com/tests/).
RESULTS
The patients had a mean age of 53.6 ± 21.5 years (median [interquartile range, range], 60 [42–69, 0–97] years), and were 59.2% male. Of the bacterial isolates recovered in conventional cultures, the PN panel detected 173/176 (98.3%). One patient had 3 colonies of Pseudomonas aeruginosa that were not found by the PN panel, and a second patient grew Serratia marcescens, Streptococcus pneumoniae, and Haemophilus influenzae, but only the Serratia was detected by the PN panel. Repeat PN panel testing was the same as the original result. Assuming culture as the gold standard, the sensitivity by specimen was 98.55% (95% CI, 94.86%–99.82%), specificity was 69% (62.9%–74.6%), positive predictive value was 63% (58.6%–67.1%), and negative predictive value was 98.9% (95.7%–99.7%) (Table 2). These data were essentially the same when broken down into BAL and endotracheal aspirate specimens (data not shown). The PN panel detected 1 or more bacterial targets in an additional 20% of patients (216/396, 55%) vs conventional culture methods (138/396, 35%; chi-square P < .00001). The PN panel detected a total of 409 bacterial targets in the 396 patient specimens. We were able to confirm a subset of PN panel–positive/culture-negative results from 39 patients, specifically 11/11 P. aeruginosa, 9/10 K. pneumoniae, 10/10 S. aureus, and 7/8 E. coli by an independent PCR (see the “Methods” and Supplementary Table 1 for more detail). If these adjudicated results were included, the sensitivity would be 97.8% (95% CI, 94.3%–99.4%), specificity 80.4% (95% CI, 74.5%–85.4%), positive predictive value 80% (95% CI, 75.5%–84%), and negative predictive value 97.8% (95% CI, 94.3%–99.1%).
Table 2.
Pneumonia Panel Detection Compared With Growth in Standard Culture by Patient Specimen
| Pneumonia Panel | Culture | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 136 | 80 | 216 |
| Negative | 2 | 178 | 180 |
| 138 | 258 | 396 | |
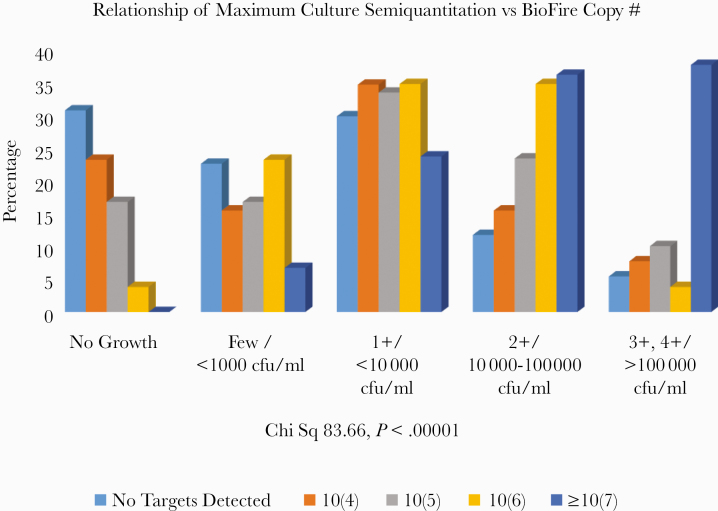
Table 3 shows all the species recovered in culture that are targets on the PN panel and the distribution of copy numbers for each. The general pattern was that the copy number was higher when the organism was recovered in culture. It should be noted that this table only refers to the concordance or nonconcordance for the species listed. Thus a patient with Staphylococcus aureus found by the PN panel but not grown in culture could have had other targets, for example, E. coli detected that were recovered in culture. We considered a PN panel result concordant for at least 1 target with culture results as “concordant” for a patient (see the “Methods”). Nine K. oxytoca and 4 S. pyogenes were only detected by the PN panel and not recovered in culture. The K. oxytoca patients in general had relatively low copy numbers (2 with 10 [6]/mL and the rest ≤10 [5] copies/mL) in the presence of high levels of other pathogens and/or >100 000 normal flora/mL. Two were unexplained. The S. pyogenes patients had all received antibiotics. We also studied the relationship of conventional microbiological laboratory quantitative (eg, <1000 CFU/mL, 10 000–100 000 CFU/mL, >100 000 CFU/mL) and semiquantitative reports (eg, 1+, 2+, 3+, 4+) to the PN panel copy number semiquantitation. For this purpose, we combined “few” with <1000 CFU/mL, 1+ with <10 000 CFU/mL, 2+ with 10 000–100 000 CFU/mL, and 3+ or 4+ with >100 000 CFU/mL. Figure 1 shows the distribution of the maximum PN panel copy number for any target detected on the PN panel vs the maximum semiquantitative growth for any bacteria recovered in culture. Overall, there was a highly statistically significant relationship between standard microbiological reporting and the PN panel semiquantitative copy number (4 × 4 chi-square 83.66, P < .00001). Notably, when the PN panel copy number/mL was ≥107/mL, 73.5% of cultures grew ≥2+ or ≥10 000 CFU/mL, and, conversely, when the PN panel detected no targets, 82.8% of cultures had no growth, <1+ growth, or <10 000 CFU/mL.
Table 3.
Distribution of Copy Number by Growth/No Growth of the Specified Organism in Culture
| Copy Number | |||||
|---|---|---|---|---|---|
| Organism | 104 | 105 | 106 | 107 | Total |
| Acinetobacter calcoaceticus-baumannii complex | |||||
| Culture pos | 1 | 3 | 5 | 9 | |
| Culture neg | 1 | 2 | 3 | 6 | |
| Enterobacter aerogenes | |||||
| Culture pos | 1 | 1 | 2 | 4 | |
| Culture neg | 1 | 2 | 3 | ||
| Enterobacter cloacae complex | |||||
| Culture pos | 1 | 3 | 4 | ||
| Culture neg | 7 | 1 | 3 | 2 | 13 |
| Escherichia coli | |||||
| Culture pos | 4 | 6 | 10 | ||
| Culture neg | 10 | 6 | 1 | 17 | |
| Haemophilus influenzae | |||||
| Culture pos | 10 | 10 | |||
| Culture neg | 11 | 6 | 6 | 5 | 28 |
| Klebsiella oxytoca | |||||
| Culture neg | 4 | 3 | 2 | 9 | |
| Klebsiella pneumoniae | |||||
| Culture pos | 2 | 2 | 9 | 13 | |
| Culture neg | 8 | 7 | 5 | 1 | 21 |
| Moraxella catarrhalis | |||||
| Culture pos | 2 | 2 | |||
| Culture neg | 4 | 1 | 2 | 3 | 10 |
| Proteus spp. | |||||
| Culture pos | 1 | 1 | 2 | ||
| Culture neg | 5 | 3 | 3 | 2 | 13 |
| Pseudomonas aeruginosa | |||||
| Culture pos | 8 | 42 | 50 | ||
| Culture neg | 6 | 6 | 5 | 4 | 21 |
| Serratia marcescens | |||||
| Culture pos | 15 | 15 | |||
| Culture neg | 2 | 3 | 3 | 8 | |
| Staphylococcus aureus | |||||
| Culture pos | 1 | 3 | 41 | 45 | |
| Culture neg | 19 | 23 | 4 | 6 | 52 |
| Streptococcus agalactiae | |||||
| Culture pos | 1 | 1 | |||
| Culture neg | 3 | 7 | 3 | 2 | 15 |
| Streptococcus pneumoniae | |||||
| Culture pos | 1 | 7 | 8 | ||
| Culture neg | 5 | 4 | 3 | 4 | 16 |
| Streptococcus pyogenes | |||||
| Culture neg | 1 | 1 | 1 | 1 | 4 |
| Grand total | 87 | 80 | 65 | 177 | 409 |
Abbreviations: neg, negative; pos, positive.
Figure 1.
There is a strong relationship between maximum semiquantitative culture results and corresponding copy number for the same bacterial target. The percentage of each copy number classification is shown as it is distributed across the semiquantitative classifications of bacterial plate growth from the standard clinical microbiology laboratory report. Abbreviation: Chi Sq, Chi Square.
WBCs were described on initial gram stain for 238/270 patients for whom clinical data were available. The remaining patient reports had no mention of WBC count, but we could not assume it meant there was none.
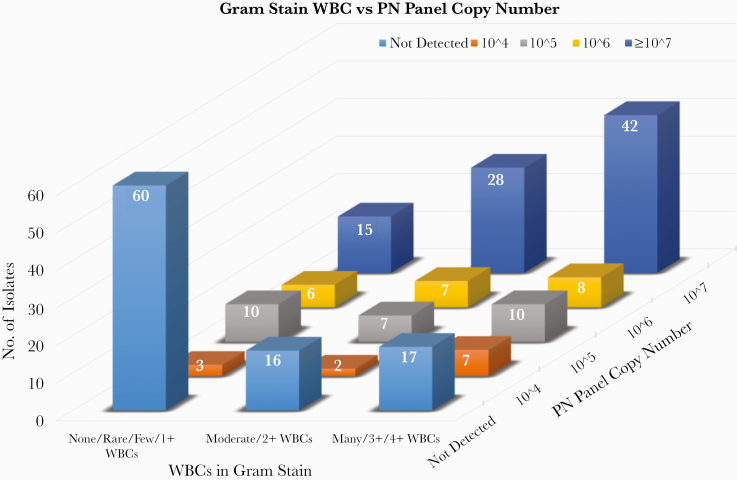
The PN panel maximum copy number was related to the description of WBC on the initial gram stain, as shown in Figure 2. For patients with ≥107 copies/mL for a bacterial target on the PN panel, 70/85 (82.4%) had Moderate (2+) or Many (3+ or 4+) WBCs described on the gram stain, while only 16/93 (17.2%) were reported as having no WBCs (P < .00001, 5 × 3 chi-square). Considering just the “noninformative” cultures (ie, no growth or normal respiratory flora only), 36/51 (70.6%) had Moderate (2+) or Many (3+ or 4+) WBCs described on gram stain when the PN panel detected targets compared with 25/74 (33.8%) when no targets were detected (P = .000112, chi-square with Yates correction) (data not shown).
Figure 2.
WBC level on gram stain was grouped as shown (described in detail in the “Methods” and the “Results”) and plotted against PN panel maximum copy number. As can be seen visually, there is a strong relationship between the semiquantitative copy number and gram stain–reported WBC. For example, for patients with ≥107 copies/mL for a bacterial target on the PN panel, 70/85 (82.4%) had Moderate (2+) or Many (3+ or 4+) WBCs described on the gram stain, while only 15/85 (17.2%) were reported as having no WBCs. For Figure 2 as a whole, P < .00001, 5 × 3 chi-square. Abbreviations: PN panel, BioFire FilmArray Pneumonia panel; WBCs, white blood cells.
To further understand the patient specimens with targets detected by the PN panel but not found in culture, we analyzed the extra targets detected on the PN panel based on the following 4 categories, as shown in Table 4: (1) no growth in culture; (2) growth reported only as “normal flora,” “normal oral flora,” or “normal respiratory flora”; (3) growth of at least 1 organism on the PN panel; and (4) only growth of ≥1 organism not on the PN panel, for example, Stenotrophomonas spp. or Candida with or without normal flora. There was a major difference in the percentage of patient specimens with targets detected by the PN panel between those with no growth, growth of normal flora only, and growth of a pathogen (with or without normal flora also) and the group that grew a pathogen that was also a target on the PN panel (98.6% positive for the latter vs 21%, 33%, and 38% positive, respectively, for the other groups; P < .00001, chi-square). Viruses (primarily rhino/enterovirus) were detected in 11%–15% of each group (P = NS) (data not shown).
Table 4.
Relationship of Copy Number of Any PN Panel Detection to Growth in Culture
| Copy No. Distribution* | |||||
|---|---|---|---|---|---|
| All bacterial targets (n = 409) culture result | 104 | 105 | 106 | ≥107 | |
| No growth | n = 18 | 8 | 9 | 1 | 0 |
| Normal flora only | n = 91 | 30 | 34 | 16 | 11 |
| Growth of ≥1 bacterial target | n = 281 | 43 | 29 | 44 | 165 |
| Only growth of organism(s) not on panel (w/w/out normal flora) | n = 19 | 6 | 9 | 4 | 0 |
Abbreviations: No, Number; PN, PN panel, BioFire FilmArray Pneumonia panel.
*P < .00001, chi-square.
A total of 64 isolates not on the PN panel from 61 patients were recovered in culture as follows: yeast 35, Stenotrophomonas 12, Achromobacter 5, Morganella spp. 2, C. striatum 2, Streptococcus sp. not Group A or B 2, Pseudomonas sp. 1, Burkholderia cepacia complex 1, Providencia 1, Aspergillus spp. 1, Cunninghamella 1, and Paecilomyces 1. Considering bacterial isolates only, 26/176 (14.8%) isolates not on the PN panel were identified in culture from 24/136 (17.6%) different patients.
Resistance genes were found in 63 patients: 46 mecA/C and MREJ, 14 CTX-M (5 K. pneumoniae, 4 E. coli, 4 P. aeruginosa), 2 KPC (1 K. pneumoniae, 1 P. aeruginosa), and 1 NDM in K. pneumoniae. The MRSA culture results were compared with PN panel mecA/C-MREJ results, as shown in Table 5. Of the 46 mecA/C- and MREJ-positive specimens, 25 had S. aureus recovered in culture, of which 5 had only methicillin-susceptible Staphylococcus aureus (MSSA) in culture. Considering culture of MRSA as the gold standard, the sensitivity was 80%, specificity 50%, PPV 50%, and NPV 80%. None of the 20 MSSA isolates recovered in culture had the mecA gene detected.
Table 5.
PN Panel Detection of MRSA vs Culture for MRSA
| mecA/C and MREJ | ||
|---|---|---|
| Culture Result | Positive | Negative |
| MRSA | 20 | 0 |
| MSSA | 5a | 20 |
Abbreviations: mecA, Staphylococcus aureus methcillin resistance gene cassette; MREJ, mec (SCCmec)-orfX right-extremity junction (MREJ).
aThese 5 patients showed mecA/C and MREJ by the PN panel, but only MSSA was recovered in culture.
Antibiotic treatment data were available for the 270 patients whose charts could be reviewed (please refer to the “Methods” section). Of the patients whose cultures had no growth, 91% had received antibiotics on the day of the culture, compared with 86% and 78% whose cultures grew normal flora only or grew a potential pathogen that was a target on the PN panel, respectively (chi-square 5.129, P = .07696). However, the use of common broad-spectrum antibiotics (cefepime, piperacillin/tazobactam, ceftriaxone, and levofloxacin) as a percentage of those on antibiotics was equally distributed across the 3 categories: 63.9%, 62.5%, and 63.3% respectively. We were able to review the subset of patients with 10(6) and 10(7) copies/mL in the PN panel who did not have a matching isolate in culture, and 16/21 (76.2%) had received antibiotics that would be considered active against the PN panel target found.
DISCUSSION
The ability to detect 15 conventional bacterial species semiquantitatively, as well as agents of atypical pneumonia, resistance genes, and common respiratory viruses within hours in the clinical laboratory, has the potential to impact treatment and outcomes in critically ill patients. To do so, accurate results that reasonably equate to those of conventional microbiology are critical, and this study illustrates many of the issues that will arise in applying this technology. The most apparent of these is the finding that many potentially pathogenic bacterial targets found by the PN panel were not found in culture. Recently, Webber et al. [4] reported a very high overall percentage agreement between the PN panel and standard culture and described at least 73 targets detected by the PN panel alone out of 200 patients tested. The majority of these were S. aureus and H. influenzae. In a study of 259 BALs, Buchan et al. [3] also found very high overall agreement between the PN panel and standard culture results (96.2% positive agreement and 98.1% negative agreement). In addition to the 75 bacterial targets that had the same species identified in culture, the PN panel detected a further 74 targets that were not found in standard culture. In a large multicenter study, Murphy et al. [2] found additional targets in 875 sputum and lower respiratory tract specimens from 1764 valid PN panel tests. Of these, about 25.1% were considered to have been true positives but with colony counts below their quantitative plating method cutoff of 3.5 × 103 CFU/mL, and the vast majority of the rest confirmed (74.5%) were confirmed as true positives by alternative molecular test methods. In their study, there was an excellent linear relationship between the PN panel copy number and actual colony counts in spiked samples. Several smaller studies have also reported similar observations using both the PN panel [5, 6] and independently developed multiplex molecular methods [7–10]. Lee et al. [5] found 7 additional bacterial targets among 29 culture-negative specimens (24.1%), consistent with our finding of bacterial targets in 21%. They did not break their data down into those cultures that had no growth and normal respiratory flora only. In Gatsby et al.’s study [7] using research-developed primers for 8 common pneumonia-causing bacteria, 12/20 (60%) sputum samples with no growth in culture had detection of 1 or more molecular targets. Among BALs, an additional 1/8 specimens with no growth had molecular targets detected, as did 4/6 with nonsignificant growth (essentially normal respiratory flora). In another comparative study of respiratory cultures compared with molecular detection of selected targets, Ozlem et al. [8] found significant pathogens by culture in 62/197 (31.5%) patients, but they found significant pathogens by molecular testing in 125/197 (63.5%). Using multiplex bacterial PCR compared with standard microbiologic culture, Baudet et al. [9] found that 66% of BALs had a pathogen by molecular methods, whereas only 40% grew a potential pathogen. The difference was particularly seen among patients who were gram stain negative. Sircar et al. [10] found similar results, also studying BALs.
Our data are in agreement with the studies reviewed above and raise a number of questions. First, we were able to validate 37/39 extra targets (S. aureus, E. coli, K. pneumoniae, and P. aeruginosa) that were not recovered in culture by an independent PCR developed in our research laboratory, although these assays were not standardized to determine limit of detection and absolute specificity. Nonetheless, based on these data and the studies discussed above [2], it seems reasonable to conclude that the additional molecular targets found by molecular tests are in fact true. Second, it is notable that 33% of our specimens with only normal respiratory flora reported in culture had a potential pathogen detected by the PN panel. Standard practice in clinical microbiology laboratories for nonsterile sites, such as those from the respiratory tract, is not to report low levels of pathogens, if present in quantities less than that of the normal respiratory flora, for example, if 2+ normal flora are present (ie, growth in the second quadrant), gram-negative organisms would not be reported if present only in the first quadrant mixed with the normal flora. This practice would explain some of the discordant results, but not the finding of many more molecular targets among the specimens that are culture positive for pathogens. In this group, an additional 108 targets were detected beyond the 173 targets corresponding to growth in culture, of which 17 had a copy number ≥107/mL (S. pneumoniae 4, Enterobacteriaceae 4, H. influenzae 3, S. aureus 3, S. agalactiae 2, Acinetobacter 1). With copy numbers at this level, it is surprising that S. aureus and the Enterobacteriaceae, which could have treatment implications, were not recovered in culture. One explanation could be the utilization of antibiotics that suppressed the growth of these organisms.
Resistance genes were detected in 63 patients, of which were mecA concordant with culture. It is well known that depending on the specifics of the molecular assay, the Staphylococcal Cassette Chromosome may lack the mecA gene, or there may be insertions, deletions, or mutations that alter the phenotypic susceptibility, in addition to other chromosomal genes, for example, femA/B [11–15]. In this limited sample, there were no false susceptibility results, that is, mecA/C and MREJ negative but phenotypically methicillin resistant. As noted in the “Results,” there were only 3 carbapenem-resistant genes detected, reflecting the overall low prevalence of these strains in our institution at the time of the study. And although the absence of these genes does not guarantee phenotypic susceptibility to the carbapenems, their detection in the rapid test format would be a highly important result.
In this study, viruses were detected in 11%–15% of patient groups and were not related to the growth or lack of growth of bacterial pathogens. As would be expected since the study was conducted over the summer months, influenza viruses were not detected. As the focus of the present study is to compare the detection of bacterial targets with conventional microbiological culture results, the clinical relevance would be an excellent opportunity for future research.
As the PN panel reports bacterial copy number semiquantitatively, it is important to determine if this quantitation corresponds to conventional reports of bacterial growth reported from microbiology laboratories, particularly because molecular methods can detect nonviable organisms. We found that when PN panel copy number/mL was ≥107/mL, 73.5% of cultures grew ≥2+/≥10 000 CFU/mL, and, conversely, when the PN panel detected no targets, 82.8% of cultures had no growth or <1+/<10 000 CFU/mL. The studies by Murphy [2] and Buchan [3] confirmed a strong relationship between the PN panel copy number/mL and quantitation by standard semiquantitative conventional culture results. The explanations for discrepant findings in the remaining samples could include detection of bacterial DNA from nonviable organisms, as well as a degree of subjectivity and variation from person to person in reading and interpreting bacterial growth on plates.
The PN panel maximum copy number was also related to the description of WBC on the initial gram stain, as shown in Figure 2. For patients with ≥107 copies/mL in the PN panel, 70/100 (70%) had Moderate (2+) or Many (3+ or 4+) WBCs described on the gram stain, while only 4/100 (4%) had No WBCs. Reports such as 1+, 2+, and Many vs Moderate are of course subject to variability in individual interpretation, but even so they show a strong statistical relationship with bacterial copy number. White blood cells in sputum, especially polymorphonuclear leukocytes, have long been considered an important criterion for judging sputum quality. To this point, Choi et al. [16] reported that total WBC in BAL fluid had 83.3% sensitivity, slightly better than the 79.2% sensitivity of BAL polymorphonuclear leukocytes in differentiating between bacterial and viral pneumonia.
The limitations of this study include being a single-institution study, so applicability to other patient populations cannot automatically be assumed. The study was not designed to be a randomized controlled trial of the PN panel to determine if the more rapid turnaround time would lead to improvement in clinical outcomes or antibiotic management, nor was the study designed to apply the PN panel to the “diagnosis” of bacterial pneumonia. The study was retrospective in nature, and it is possible that test results with the PN panel could have been different if performed in real time, rather than after frozen storage.
It is also important to note that the PN panel cannot and is not intended to replace standard culture for a number of reasons. Most critically, phenotypic susceptibility testing is necessary to confirm the empiric choice of antibiotics and to provide additional agents in case of toxicity, allergy, or other adverse reactions. Additionally, potential pathogens not included in the panel could be identified by culture; for example, of the 396 patients in addition to 35 Candida spp. isolates, there were 12 Stenotrophomonas, 5 Achromobacter, 5 miscellaneous bacteria, and 3 molds recovered. While Candida are usually considered nonpathogenic when isolated in the lungs and Stenotrophomonas pathogenicity is debated, other potential pathogen identifications could have significant consequences.
In summary, the findings of this study show that the PN panel detected >98% of targets on the panel that grew in standard culture, and consistent with other studies, the PN panel detected a great many targets that were not recovered in traditional cultures. The PN panel semiquantitative copy numbers were strongly related both to the WBC report on initial gram stain and to conventional bacterial semiquantitation, as reported in the microbiology laboratory. Uninformative cultures (either no growth or growth of only normal respiratory flora) positive for molecular targets on the PN panel had significantly higher levels of WBC on gram stain, suggesting a host response and potential pathogenicity of the bacterial targets detected. We conclude that if laboratories can provide a sufficiently rapid turnaround time, PN panel results should lead to improvements in the management of antibiotic treatment and stewardship, presumably leading to better-quality outcomes.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We gratefully acknowledge the support of the staff in the UF Health Shands Hospital clinical microbiology laboratory and the UF Health Department of Pathology, Immunology and Laboratory Medicine at the University of Florida (Gainesville, FL, USA).
Financial support. This work was supported by a grant from BioFire Diagnostics, Inc. (Salt Lake City, UT, USA).
Potential conflicts of interest. Dr. Rand has received speaker fees from BioFire Diagnostics. Drs. Rand and Beal have received unrelated grant funding from BioFire Diagnostics. Brianne Couterier, Beth Lingenfelter, Cory Rindlisbacher, and Jay Jones are or were employed by BioFire Diagnostics. To the best of our knowledge, there are otherwise no authors with a potential conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy CN, Fowler R, Balada-Llasat JM, et al. Multicenter evaluation of the BioFire® FilmArray® pneumonia/pneumonia plus panel for the detection and quantification of agents of lower respiratory tract infection. J Clin Microbiol 2020; 58:e00128-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buchan BW, Windham S, Balada-Llasat JM, et al. Practical comparison of the BioFire® FilmArray® Pneumonia Panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. J Clin Microbiol 2020; 58:e00135-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Webber DM, Wallace MA, Burnham CA, Anderson NW. Evaluation of the BioFire® FilmArray® pneumonia panel for detection of viral and bacterial pathogens in lower respiratory tract specimens in the setting of a tertiary care academic medical center. J Clin Microbiol 2020; 58:e00343-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee SH, Ruan SY, Pan SC, et al. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J Microbiol Immunol Infect 2019; 52:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoo IY, Huh K, Shim HJ, et al. Evaluation of the BioFire® FilmArray® Pneumonia Panel for rapid detection of respiratory bacterial pathogens and antibiotic resistance genes in sputum and endotracheal aspirate specimens. Int J Infect Dis 2020; 95:326–31. [DOI] [PubMed] [Google Scholar]
- 7. Gadsby NJ, McHugh MP, Russell CD, et al. Development of two real-time multiplex PCR assays for the detection and quantification of eight key bacterial pathogens in lower respiratory tract infections. Clin Microbiol Infect 2015; 21:788.e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aydemir O, Aydemir Y, Ozdemir M. The role of multiplex PCR test in identification of bacterial pathogens in lower respiratory tract infections. Pak J Med Sci 2014; 30:1011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baudel JL, Tankovic J, Dahoumane R, et al. Multiplex PCR performed of bronchoalveolar lavage fluid increases pathogen identification rate in critically ill patients with pneumonia: a pilot study. Ann Intensive Care 2014; 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sircar M, Ranjan P, Gupta R, et al. Impact of bronchoalveolar lavage multiplex polymerase chain reaction on microbiological yield and therapeutic decisions in severe pneumonia in intensive care unit. J Crit Care 2016; 31:227–32. [DOI] [PubMed] [Google Scholar]
- 11. Ikonomidis A, Michail G, Vasdeki A, et al. In vitro and in vivo evaluations of oxacillin efficiency against mecA-positive oxacillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2008; 52:3905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denys GA, Collazo-Velez V, Young S, et al. Multicenter evaluation of the portrait staph ID/R blood culture panel for rapid identification of staphylococci and detection of the mecA gene. J Clin Microbiol 2017; 55:1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altun O, Almuhayawi M, Ullberg M, Ozenci V. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J Clin Microbiol 2013; 51:4130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blanc DS, Basset P, Nahimana-Tessemo I, et al. High proportion of wrongly identified methicillin-resistant Staphylococcus aureus carriers by use of a rapid commercial PCR assay due to presence of staphylococcal cassette chromosome element lacking the mecA gene. J Clin Microbiol 2011; 49:722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pournaras S, Sabat AJ, Grundmann H, et al. Driving forces of mechanisms regulating oxacillin-resistance phenotypes of MRSA: truly oxacillin-susceptible mecA-positive Staphylococcus aureus clinical isolates also exist. Curr Pharm Des 2015; 21:2048–53. [DOI] [PubMed] [Google Scholar]
- 16. Choi SH, Hong SB, Hong HL, et al. Usefulness of cellular analysis of bronchoalveolar lavage fluid for predicting the etiology of pneumonia in critically ill patients. PLoS One 2014; 9:e97346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.