Abstract
Background:
This study aimed to investigate the effects of dexmedetomidine (Dex) on hemodynamics and organ protection in congenital heart disease (CHD) children who underwent open-heart surgery under cryogenic cardiopulmonary bypass.
Methods:
Ninety children were randomly allocated to group C (0.9% saline 0.2 μg/kg/hour), group D1 (Dex 0.2 μg/kg/hour), and group D2 (Dex 0.4 μg/kg/hour) (n = 30 per group). All participants received fentanyl, propofol and 1% sevoflurane for anesthesia induction. Hemodynamic data were measured from T0 (before the induction) to T7 (30 minutes after extubation). The difference of arterial internal jugular vein bulbar oxygen difference and cerebral oxygen extraction ratio were calculated according to Fick formula. Enzyme-linked immunosorbent assay was performed to detect the serum myocardial, brain and kidney injury markers. The incidence of acute kidney injury (AKI) was calculated by serum creatinine level. Tracheal extubation time, postoperative pain score and emergence agitation score were also recorded.
Results:
Compared with group C, group D1, and D2 exhibited reduction in hemodynamic parameters, myocardial and brain injury indicators, and tracheal extubation time. There were no significant differences in blood urea nitrogen and neutrophil gelatinase-associated lipocalin or incidence of AKI among the 3 groups. Besides, the incidence of tachycardia, nausea, vomiting and moderate agitation, and the FLACC scale in group D1 and D2 were lower than those in group C. Moreover, Dex 0.4 g/kg/hour could further reduce the dosage of fentanyl and dopamine compared with Dex 0.2 g/kg/hour.
Conclusions:
Dex anesthesia can effectively maintain hemodynamic stability and diminish organ injuries in CHD children.
Keywords: cardiopulmonary bypass, congenital heart disease, dexmedetomidine, hemodynamic parameter, organ injury
1. Introduction
Congenital heart disease (CHD) is one of the most common congenital malformations, characterized by great intraoperative injury and the need of extracorporeal circulation.[1] In recent years, there has been a significant increase in the number of CHD surgeries in children in China, most of which are performed under general anesthesia combined with cryogenic cardiopulmonary bypass (CPB).[2] In addition, CHD patients may develop long QT syndrome after surgery due to pathological changes in cardiac action potential duration caused by defective cardiac slow delayed rectifier current channels.[3,4]
CPB is a key auxiliary means in open-heart surgery, providing guarantee for the success of heart surgery by injecting oxygen and blood into the bodys organs and tissues. However, most CHD children have obvious impaired cardiac function, while CPB often causes a strong stress response during open-heart surgery. This is because the ascending aorta is blocked during CPB, which greatly decreases the normal blood perfusion to various organs of the body. Meanwhile, the blood in CPB is directly exposed to extracorporeal circulation pipeline and oxygenator, resulting in generation of numerous inflammatory cytokines and cascade release, and even triggering systemic inflammatory response syndrome.[5] Besides, hyperglycemia and hyperlacticacidemia caused by stress response, as well as ischemia and reperfusion injury caused by hemodynamic instability, can lead to great functional damage to the heart, lung, brain and kidney.[6] Although the cure rate of CHD children has been remarkably improved,[7] the effect of surgical anesthesia on the function of important organs in perioperative period and long-term development of the postoperative nervous system remains a hot issue of concern. Therefore, it is urgent to find an effective and safe anesthesia sedation scheme in the perioperative period to reduce the stress response, and maintain the balance of oxygen supply and demand in the body of children.
Dexmedetomidine (Dex) is a highly selective α-adrenergic receptor agonist that acts as a sedative and hypnotic agent by activating presynaptic and postsynaptic α2 receptors in the locus coeruleus, thus inducing an unconscious state similar to natural sleep. Because of the wide presence of α receptor in many organs, such as liver, lung, kidney and brain, Dex plays a role in almost every important organ in the body.[8] Previous researches have clarified that Dex is capable of stabilizing hemodynamic changes,[9] reducing the usage of opioids and sedatives,[10] declining the incidence of postoperative delirium,[11] and having neuroprotective effects.[12] These characteristics enable Dex to be widely used as an anesthetic adjuvant in perioperative anesthesia and ICU anesthesia, especially cardiothoracic surgery. Nazir et al[13] have proved that Dex can control hypotension in patients undergoing lumbar spine decompression and fixation surgery. In addition, Goyal et al[14] have also believed that Dex can be served as suitable alternative for fentanyl in breast cancer surgery because of its better hemodynamic stability, anesthetic sparing efficacy and better recovery profile. Several animal studies have proposed that Dex may decrease renal inflammatory response and ischemia-reperfusion injury.[15,16] Jiang et al[17] have conducted a meta-analysis of 19 randomized controlled trials and concluded that Dex can diminish the release of inflammatory mediators and neuroendocrine hormones, and abate ischemic brain damage.
At present, Dex is still off-label in pediatric patients in China. By studying the pharmacokinetics of Dex in children in intensive care, Wiczling et al[18] predicted that the pharmacokinetics of Dex in children over 1 year of age were consistent with the results of adults. Maud et al[19] have believed that the effect of Dex in children older than 1 month was similar to adults. Dex has been widely recognized for its clinical value in adults, but its application in pediatric CHD surgery remains unclear. Herein, this study aimed to evaluate the efficacy of different dosages of Dex on hemodynamics and organ protection including heart, brain and kidney in CHD children.
2. Methods and materials
2.1. Ethics and informed consent
This study was approved by the Ethics Committee of Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, and written informed consents were obtained from all participants. This study was registered with the Chinese Clinical Study under the registration number ChiCTR18000171819.
2.2. Participants and study design
Children who were scheduled for repair of atrioventricular septal defect (AVSD) during CPB under elective general anesthesia were enrolled in this study from July 1st, 2017 to February 28th, 2018 at the Ruikang Hospital of Guangxi University of Traditional Chinese Medicine. Inclusion criteria: patients aged 1 to 6 years were diagnosed as CHD or AVSD, with American Society of Anesthesiologists (ASA) class II-III and preoperative New York Heart Association class I/II. Exclusion criteria:
-
1.
patients with severe malnutrition and cyanosis were premature and low birth weight;
-
2.
a previous cardiac surgery;
-
3.
allergic to narcotic drugs;
-
4.
pulmonary hypertension;
-
5.
combined with cerebral palsy, severe renal or hepatic disorders, metabolic dysfunction, or other congenital diseases affecting the brain, liver, and kidney, such as Trisomy 21 syndrome.
In a preliminary experiment, 20 CHD children were randomized into control group (0.9% saline 0.2 μg/kg/hour), low-dose Dex group (Dex 0.1 μg/kg/hour), medium-dose Dex group (Dex 0.2 μg/kg/hour) and high-dose Dex group (Dex 0.4 μg/kg/hour), with 5 patients per group. Dex and saline were continuously pumped intravenously from the onset of anesthesia until the end of the operation. There were no significant differences in terms of the hemodynamics, postoperative recovery quality and functional indexes of each organ after surgery between the low-dose Dex group and control group, which might be caused by the low dose and small sample size. Thus, Dex 0.2 and 0.4 μg/kg/hour were applied in the subsequent experiments.
A total of 127 children with CHD were recruited for the present study. After excluding 9 children who did not meet the inclusion criteria, 5 who dropped out of the study and 23 who refused to participate, 90 eligible participants were included in the final analysis. They were randomly divided into group C (0.9% saline 0.2 μg/kg/hour), group D1 (Dex 0.2 μg/kg/hour) and group D2 (Dex 0.4 μg/kg/hour), with 30 patients per group (Fig. 1). The random allocation table was generated by SPSS software, and the starting point was set to 20161201.
Figure 1.
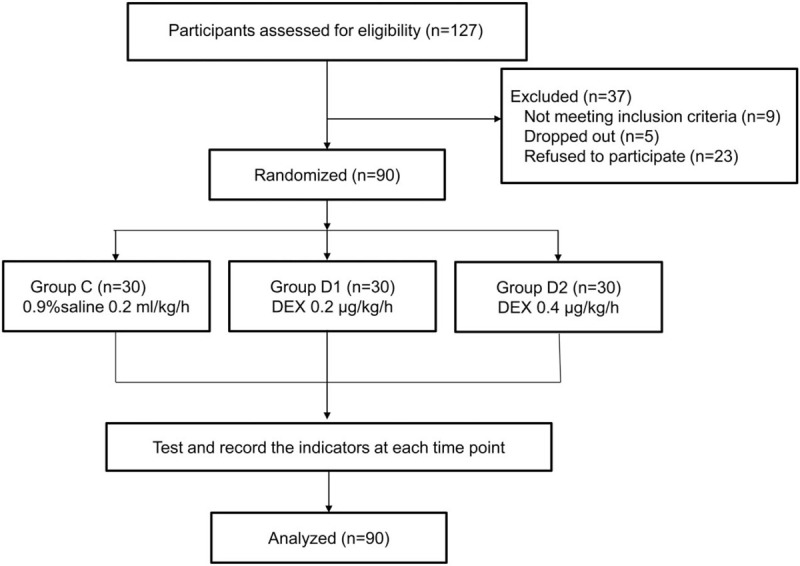
Flow chart of the children undergoing atrioventricular septal defect.
2.3. Anesthetic process
All children were fasted for 6 to 8 hours and were forbidden to drink for 2 to 4 hours before surgery. Preoperative skin test of cefazolin was performed. If no allergic reaction occurred, cefazolin was administered intravenously 30 minutes before surgery. Then, children were given intravenous injection of midazolam 0.1 mg/kg. After the loss of consciousness, their electrocardiogram, blood pressure, heart rate (HR), oxygen saturation and Bipolar Spectrum Index were closely monitored.
Intravenous injection of vecuronium bromide (0.1 mg/kg), fentanyl (3–8 g/kg), propofol (1–3 mg/kg) were performed. When Bipolar Spectrum Index reached 45 to 60, tracheal intubation was performed through the mouth, and then anesthesia machine was connected for mechanical ventilation (tidal volume: 6–8 ml/kg, respiratory rate 15–32 times/minutes, suction ratio 1:1.5), with an inspiratory oxygen concentration of 50% and maintenance of the end-tidal carbon dioxide at 35 to 45 mm Hg (1 mm Hg = 0.133 kPa). From the start of anesthesia, Dex continued to be administered intravenously at the rate of 0.2 ml/kg/hour until the end of the operation. The concentrations were Dex 0.2 g/kg/hour in group D1, Dex 0.4 g/kg/hour in group D2, and normal saline in group C.
2.4. Surgery and CPB management
CPB was conducted with Stockert SII artificial heart-lung machine, Dideco hollow fiber oxygenator (Sorin, Italy) and children extracorporeal circulation pipeline. All surgeries were performed by the same group of cardiothoracic surgeons. The sternum was sawed open from the median of the sternum, followed by intravenous injection of heparin 3.0 to 3.5 mg/kg, and then CPB was established by intubation of aorta, superior vena cava and descending venous cannula. After the aorta was blocked, 30 ml/kg of amino acid histidine-tryptophan-ketoglutaratesolution was injected into the aortic root, and cardiac malformation correction was performed after complete cardiac arrest. The moderate hypothermia was maintained at 30–34°C. Routine ultrafiltration was performed with blood ultrafilter (Bellco, Italy) to keep moderate blood dilution and hematocrit at 0.25 to 0.35 during CPB. After opening aorta, dopamine 3 to 6 g/kg/minutes and nitroglycerin 0.3 g/kg/minutes were given intravenously, and the dosage was adjusted according to blood pressure and central venous pressure. Protamine was intravenously injected to neutralize heparin (1:1) following CPB.
2.5. Hemodynamic index
Mean arterial pressure (MAP) and HR of each time period were investigated before the induction (T0), before the injection of Dex (T1), 30 minutes after the injection of Dex or saline (T2), after sternotomy (T3), the end of CPB (T4), the end of surgery (T5), extubation (T6), and 30 minutes after extubation (T7). The blood lactic acid (LA) level was determined before surgery, 30 minutes after the start of CPB, at the end of CPB, after surgery and 24 hours post-operation. The C-reaction protein (CRP) level was detected before surgery, at the end of surgery, 6 hours, 24 hours, and 48 hours post-operation.
2.6. Detection of organ injury markers
Blood of jugular vein bulb and radial artery were collected before surgery, 30 minutes after the start of CPB, at the end of CPB, after surgery and 24 hours post-operation for arterial blood gas analysis. The arterial-internal jugular venous oxygen concentration difference (Ca-jvO2) and cerebral oxygen extraction ratio (O2ER) were calculated according to Fick formula.
Venous blood (3–5 ml) of children was extracted before surgery, at the end of surgery, 6 hours, 24 hours, and 48 hours post-operation, followed by centrifugation at room temperature at 4000 r/minutes for 10 minutes. The supernatant was isolated and stored at −80°C. Subsequently, serum myocardial injury markers (creatine kinase isoenzymes, CK-MB; cardiac troponin I, cTnI; heart-type fatty acid binding protein, H-FABP), brain injury markers (S100β; neuron-specific enolase, NSE), and kidney injury marker (neutrophil gelatinase-associated lipocalin, NGAL) concentrations were detected by double antibody 1 step sandwich enzyme-linked immunosorbent assay in accordance with manufacturers instructions. Blood urea nitrogen (BUN) and serum creatine (Scr) tests were carried out in the laboratory department of the hospital.
The condition of acute kidney injury (AKI) was based on the diagnostic criteria established by Kidney Disease: Improving Global Outcomes in 2012. AKI occurred when the absolute value of Scr increased ≥26.5 umol/L within 48 hours, or Scr enhanced to more than 1.5 times of the basic value, or the urine volume was less than 0.5 ml/kg/hours with more than 6 hours of duration.
2.7. Emergence agitation score
Emergence agitation was assessed using a five-point scoring scale: 0 point, drowsiness; 1 point, awake and quiet; 2 point, crying and irritable; 3 point, fidgety and inconsolable crying; 4 point, severe restlessness.[20] Emergence agitation score 0 to 1 was considered as negative agitation, 2 as mild agitation, 3 as moderate agitation, and 4 as severe agitation. Observation and scoring were performed by the same uninformed physician in the ICU.
2.8. Postoperative pain score
The Face, Legs, Activity, Cry and Consolability (FLACC) scale was used to score postoperative pain 10 minutes after tracheal extubation.[21] 0, relaxed; 1–3, mild discomfort; 4–6, moderate pain; 7–10 severe pain and discomfort. Intravenous drip of tramadol (1 mg/kg) was administered with FLACC≥4 points for analgesia.
2.9. Adverse reactions
Adverse reactions, such as hypertension (SBP > 120 mm Hg), hypotension (SBP < 60 mm Hg), bradycardia (HR < 70 times/minutes) and tachycardia (HR >170 times/minutes) were recorded during the perioperative period. Besides, the occurrence of nausea and vomiting 3 days after surgery was also recorded.
2.10. Statistical analysis
Data analysis was performed using SPSS 23.0 statistical software. The measurement data were tested by Shapiro–Wilk test for normal distribution and Levene test for homogeneity of variance. Normality data were expressed as mean ± standard deviation (x̄±s); comparisons between groups were performed using analysis of variance (ANOVA), and comparisons at different points in the groups were performed using repeated measures analysis of variance. Skewness data were expressed as median and interquartile range, and comparisons between groups were performed via Kruskal–Wallis test. Enumeration data were expressed as a percentage, comparisons between groups were performed using χ2 test or Fisher exact test. P < .05 was considered statistically significant.
3. Results
3.1. Demographic and perioperative data
As shown in Table 1, there were no differences among the 3 groups in terms of gender, age, height, weight, ASA classification, type of surgery, duration of operation, duration of anesthesia, duration of CPB, duration of aortic occlusion, duration of HR recovery, intraoperative blood loss, intraoperative infusion volume, pretransition urine volume and intraoperative infusion volume.
Table 1.
Demographic data in the study groups.
| Index | Group C(n = 30) | Group D1(n = 30) | Group D2(n = 30) | F/X2 | P value |
| Age (years) | 2.5 ± 1.80 | 2.55 ± 1.61 | 3.15 ± 1.79 | 0.638 | .727 |
| Gender (Male/Female) | 16/14 | 19/11 | 17/13 | 0.875 | .422 |
| Height (cm) | 84.45 ± 16.79 | 85.50 ± 19.14 | 94.75 ± 17.89 | 1.922 | .146 |
| Weight (kg) | 12.25 ± 4.08 | 12.40 ± 4.99 | 13.46 ± 4.59 | 0.424 | .656 |
| ASA (II/III) | 21/9 | 23/7 | 20/10 | 0.757 | .685 |
| ASD/VSD/ASD+VSD | 9/16/5 | 7/15/8 | 6/17/7 | 1.461 | .833 |
| Duration of surgery (min) | 182.85 ± 24.00 | 185.65 ± 19.96 | 184.65 ± 21.57 | 0.084 | .920 |
| Duration of anesthesia (min) | 250.40 ± 31.91 | 256.95 ± 23.77 | 253.05 ± 24.65 | 0.297 | .744 |
| Duration of CPB (min) | 75.90 ± 21.43 | 76.40 ± 14.55 | 71.55 ± 15.63 | 0.472 | .626 |
| Duration of aortic occlusion (min) | 37.75 ± 16.15 | 37.75 ± 13.86 | 36.50 ± 13.04 | 0.050 | .951 |
| Heart recovery time (min) | 2.20 ± 0.83 | 1.65 ± 0.88 | 1.75 ± 1.07 | 1.977 | .148 |
| Intraoperative blood loss (mL) | 63.40 ± 19.73 | 52.80 ± 19.71 | 58.00 ± 13.90 | 1.737 | .185 |
| Intraoperative infusion volume (ml) | 113.50 ± 51.33 | 101.50 ± 28.89 | 103.00 ± 34.84 | 0.501 | .608 |
| Infusion of deleukocyte (U) | 1.23 ± 0.43 | 1.30 ± 0.47 | 1.27 ± 0.45 | 0.067 | .848 |
| Intraoperative fentanyl requirement (ug/kg) | 404.33 ± 50.36 | 364.00 ± 32.01 | 333.33 ± 45.66 | 20.214 | <.001 |
| Intraoperative propofol requirement (mg/kg) | 206.67 ± 39.42 | 180.00 ± 37.32 | 176.00 ± 35.29 | 5.966 | .04 |
| Adverse reaction | |||||
| Hypotension [n (%)] | 4 (13.3%) | 5 (16.7%) | 8 (26.7%) | 1.794 | .493 |
| Tachycardia [n (%)] | 7 (23.3%) | 1 (3.3%) | 0 | 11.799 | .003 |
| Bradycardia [n (%)] | 2 (6.7%) | 3 (10%) | 3 (10%) | 0.274 | .872 |
| Nausea and vomiting [n (%)] | 8 (26.7%) | 2 (6.7%) | 2 (6.7%) | 5.958 | .047 |
Bipolar Spectrum Index in all 3 groups was maintained at 45 to 60, and the dosage of anesthetic drugs was adjusted according to Bipolar Spectrum Index, mean arterial pressure and HR. Compared with group C, the intraoperative dosage of fentanyl and propofol in group D1 and group D2 was significantly diminished (P < .05). Besides, the dosage of fentanyl in group D2 was notably lower than that in group D1, while no significant difference was observed in the dosage of propofol (Table 1). As for adverse reactions, no hypertension was occurred among the 3 groups. The blood pressure and HR of children decreased to some extent after 30 minutes of dexmedetomidine, which might be dose-dependent. There were no significant differences in hypotension and bradycardia among the 3 groups. Whereas, compared with group C, the occurrence of tachycardia, nausea and vomiting was decreased significantly in experimental groups (P < .003 and P = .047, respectively).
All operations were successful, and intracardiac malformations of the AVSD children in the 3 groups were well corrected. Besides, no severe adverse effects were observed at the 1-year follow-up visit.
3.2. Hemodynamic data
From Figure 2A and 2B, MAP and HR were notably reduced after administration of Dex (P < .01 for MAP, P < .001 for HR), but were then slowly enhanced at T3 compared with T1. In the group D1 and D2, the only significant changes at T1-T3 compared with baseline (T0) were the changes of MAP at T1 and T2 and HR at T1, T2, and T3 (all P < .001). At T3, the MAP of group D2 and the HR of group D1 and D2 were significantly lower than those of group C (P < .05 for MAP, P < .001 for HR).
Figure 2.
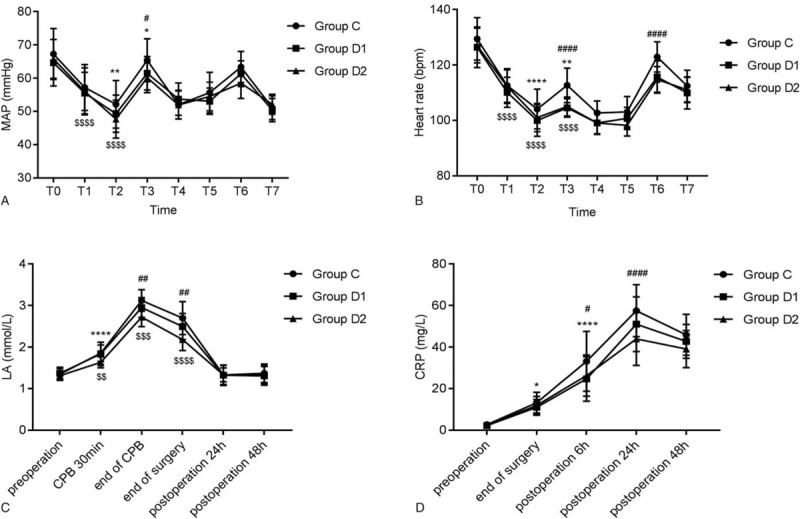
Effects of dexmedetomidine on hemodynamic changes. The comparison of (A) MAP, (B) HR, (C) LA, and (D) CRP among 3 groups at different time points. ∗P < .05, ∗∗P < .01, ∗∗∗∗P < .0001 vs T1; $P < .0001 vs T0; #P < .05, ####P < .0001 vs Group C. T0: before the induction, T1: before the injection of Dex, T2: 30 minutes after the injection of Dex (0.2 or 0.4 μg/kg/hour) or saline (0.2 μg/kg/h), T3: after sternotomy, T4: the end of CPB, T5: the end of surgery, T6: extubation, T7: 30 minutes after extubation. ∗∗∗∗P < .0001 vs. pre-operation; #P < .05, ##P < .01, ####P < .0001 vs group C; $$P < .01, $$$P < .001, $$$$P < .0001 vs group D1. MAP: mean arterial pressure; LA = lactic acid; CRP = C-reaction protein.
LA level reflects the microcirculation state, oxygen supply level and perfusion of brain and other important organs. LA level remarkably increased 30 minutes after the start of CPB, and reached a peak at the end of CPB (P < .001), then recovered to baseline levels 24 hours after operation in the 3 groups. Compared with group C, obvious decrease of LA levels in group D1 at the end of surgery and group D2 at the end of CPB and surgery were noted (both P < .01). LA level in group D2 was lower than that in the group D1 at 30 minutes after the start of CPB, at the end of CPB and operation (Fig. 2C). The perioperative CRP levels in the 3 groups augmented from the end of surgery to 24 hours after surgery and declined 48 hours after surgery, which were still significantly higher than those at pre-operation. CRP in group D1 at 6 hours after surgery (P < .05) and D2 at 24 hours after surgery (P < .001) were dramatically lower than that in group C (Fig. 2D).
3.3. Concentration changes of H-FABP, CK-MB, and cTnI
CK-MB, cTnI, and H-FABP are commonly used in the evaluation of perioperative myocardial injury. In this study, similar trends in CK-MB and cTnI were noticed among the 3 groups. After surgery, CK-MB and cTnI continued to increase from baseline until 24 hours post-operation, whereas H-FABP began to decline at 6 hours post-operation. There was no significant difference in CK-MB concentration between group D1 and group C or group D2 at each time point (Fig. 3A). CK-MB concentration in group D2 at 24 hours and 48 hours post-operation, cTnI at 6 hours, 24 hours, and 48 hours post-operation, as well as H-FABP at the end of surgery, 6 hours post-operation was significantly lower than that in group C. Significant differences of cTnI and H-FABP were observed at 6 hours post-operation between group D1 and D2 (P < .001 for cTnI, P < .01 for H-FABP) (Fig. 3B and 3C).
Figure 3.
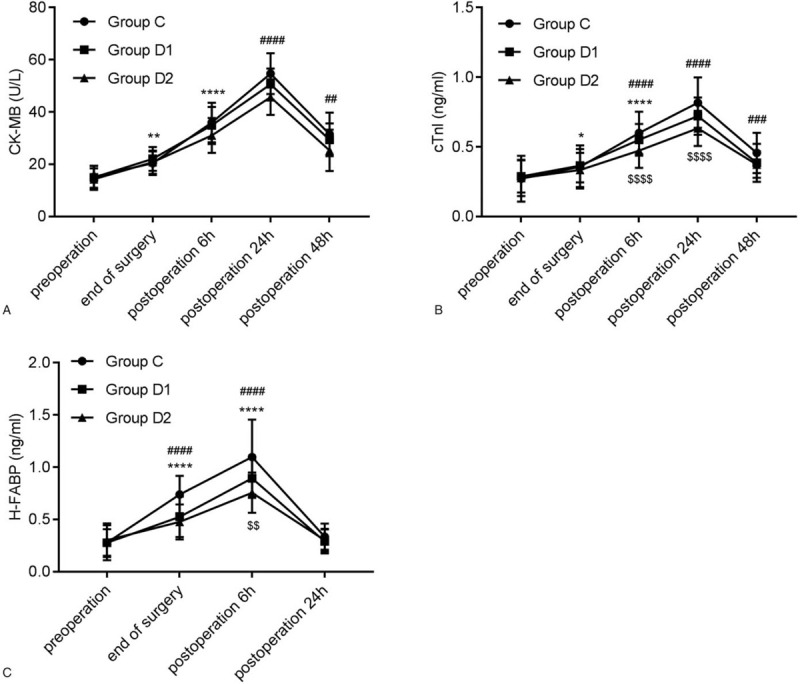
Effects of dexmedetomidine on myocardial damage. The comparison of (A) CK-MB, (B) cTnI, and (C) H-FABP among the 3 groups at different time points. ∗P < .05, ∗∗P < .01, ∗∗∗∗P < .0001 vs. pre-operation; ##P < .01, ###P < .001, ####P < .0001 vs. Group C; $$P < .01, $$$$P < .0001 vs. group D1. CK-MB = creatine kinase isoenzymes; cTnI = cardiac troponin I; H-FABP = heart-type fatty acid binding protein.
3.4. Levels of S100β, NSE, Ca-jvO2, and O2ER in the 3 groups
As shown in Figure 4A and 4B, compared with pre-operation, obvious improvements in S100β at the end of surgery, 6 hours post-operation, and NSE at 6 hours, 24 hours, and 48 hours post-operation were found among the 3 groups (all P < .001). At the end of surgery and 6 hours post-operation, S-100β in group D2 was lower than that in group C and D1. After 30 minutes of CPB in each group, Ca-jvO2 and O2ER were increased (P < .001); moreover, Ca-jvO2 and O2ER in group D2 were higher than those in group C (Fig. 4C and 4D).
Figure 4.
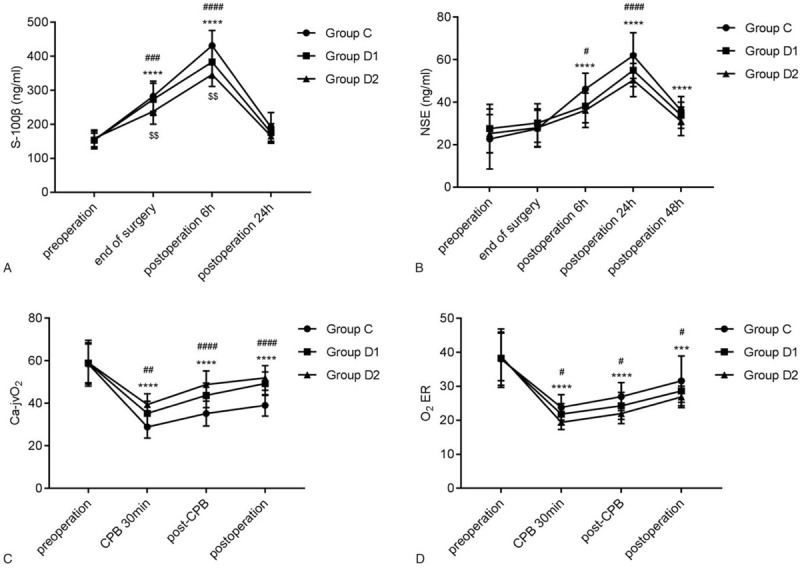
Effects of dexmedetomidine on brain injury. The comparison of (A) S-100β, (B) NSE, (C) Ca-jvO2 and (D) O2ER among the 3 groups at different time points. O2 ER: oxygen extraction ratio. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001 vs. pre-operation; #P < .05, ##P < .01, ###P < .001, ####P < .0001 vs group C; $$P < .01 vs group D1. NSE = neuron-specific enolase; Ca-jvO2 = arterial internal jugular vein bulbar oxygen difference; cerebral O2ER = oxygen extraction ratio.
3.5. Kidney injury parameters
NGAL, Scr and BUN levels were enhanced at the end of surgery, 6 hours and 24 hours post-operation when compared with pre-operation (Fig. 5). There was no statistical difference in NGAL and BUN among the 3 groups at each point. However, in group D2, Scr was remarkably lower than that in group C and D1. Besides, a total of 6 AKI patients (20.0%) were in group C, and 4 AKI patients (13.3%) were in group D1 and D2. There was no statistically significant difference in the incidence of AKI among the 3 groups (F = 0.677, P = .713).
Figure 5.
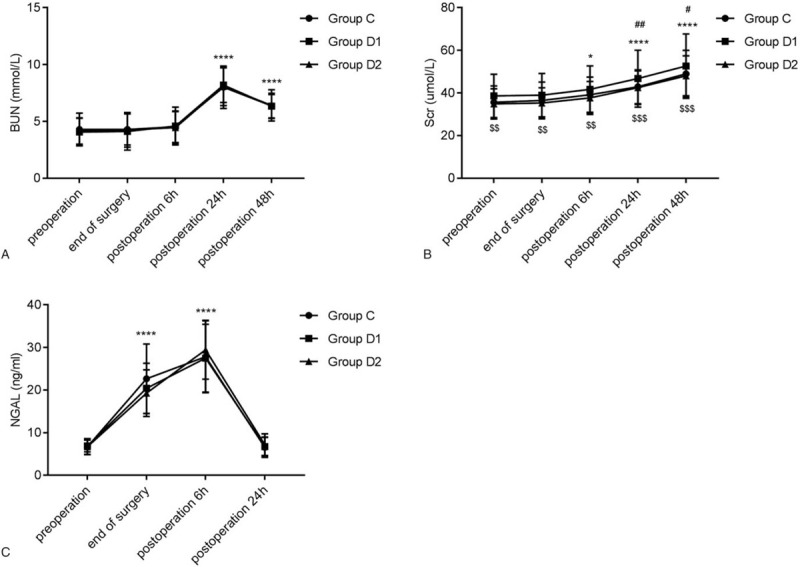
Effects of dexmedetomidine on kidney injury. The comparison of (A) BUN, (B) Scr, and (C) NGAL among the 3 groups at different time points. ∗P < .05, ∗∗∗∗P < .0001 vs. pre-operation; #P < .05, ##P < .01 vs group C; $$P < .01, $$$P < .001 vs group D1. BUN = blood urea nitrogen; Scr = serum creatine; NGAL = neutrophil gelatinase-associated lipocalin.
3.6. Results of emergence agitation score and postoperative pain score
From Table 2, no prominent differences in mild agitation, severe agitation and total occurrence of emergence agitation was observed among the 3 groups. However, the occurrence of moderate agitation in group D2 was dramatically lower than that in group C (P = .002).
Table 2.
Emergence agitation score and postoperative pain score.
| Index | Group C(n = 30) | Group D1(n = 30) | Group D2(n = 30) | F/X2 | P value |
| Emergence agitation score | |||||
| Mild agitation [n (%)] | 6 (20%) | 12 (40%) | 11 (36.7%) | 3.154 | .207 |
| Moderate agitation [n (%)] | 12 (40%) | 5 (16.7%) | 1 (3.3%) | 12.917 | .002 |
| Severe agitation [n (%)] | 2 (6.7%) | 0 | 0 | 4.091 | .129 |
| Total occurrence [n (%)] | 20 (66.7%) | 17 (56.7%) | 12 (40%) | 4.390 | .111 |
| Tracheal extubation time (min) | 167.17 ± 32.86 | 150.20 ± 24.70 | 152.30 ± 31.33 | 6.693 | .002 |
| FLACC score [median (IQR)] | 4 (3–6) | 3 (2–5) | 3 (2–3) | 9.298 | .01 |
| Frequency of tramadol use [n (%)] | 18 (60%) | 13 (43.3%) | 10 (33.3%) | 4.390 | .039 |
Tracheal extubation time in group D1 and group D2 was 150.20 ± 24.70 and 152.30 ± 31.33, respectively, which was noticeably shorter than that in group C (167.17 ± 32.86) (P = .002). In addition, FLACC scale in group D1 and group D2 was also lower than that in group C (P = .01). After tracheal extubation, 18 children in group C were given intravenous drip of tramadol for analgesia, 13 in group D1 and 10 in group D2.
4. Discussion
Open-heart surgery under CPB is still the main treatment for CHD.[2] The structure and function of organs in children are different from those in adults, and the response mechanism to surgical anesthesia injury is not exactly the same. So organ protection methods suitable for adults may not be equally effective for infants. In this study, the MAP and HR in each group were increased significantly at T3 (after sternotomy) and T6 (extubation), suggesting that operations at these time points had a great stimulus on the body, which could bring about hemodynamic instability in children. However, both MAP and HR in group D1 and D2 were lower than those in group C, suggesting that Dex inhibited stress response produced by sternum and extubation during CHD surgery anesthesia and had a stable hemodynamic effect, which was more obvious after Dex 0.4 g/kg/hour treatment. A dual-center study conducted by Petroz et al[22] demonstrated that hemodynamic response of children restrained with the enhancement of Dex dosage, which was consistent with the above conclusions.
As a valid adjunctive to general anesthesia, Dex is usually injected at 0.5 to 1.0 μg/ kg followed by continuous injection at 0.2 to 2.0 μg/kg/hour.[23] Su et al performed a pharmacokinetic study of Dex and found that continuous injection of Dex at a rate of 0.75 μg/kg/hour was well tolerated in children following open-heart surgery.[24] In the present report, Dex 0.2 μg/kg/hour (group D1) and 0.4 μg/kg/hour (group D2) were adopted, and no hypertension occurred in the 2 groups after infusion, revealing that the dosage of Dex was safe. Nevertheless, we noted that at the time of CPB establishment, there were 4 participants in group C suffered from hypotension, 5 in group D1 and 8 in group D2, which might be caused by the pulling or compression of the heart by surgeon. Thus, when hypotension appears, it is necessary to release the tension and compression in time, and most childrens blood pressure can be restored to normal within 1 minutes, and those who fail to return to normal levels needs to be given phenylephrine. Additionally, the occurrence of tachycardia, nausea and vomiting in experimental groups was significantly lower than that in group C, indicating that Dex effectively reduced the complications such as heart failure that might be induced by tachyarrhythmia. High LA is associated with postoperative cognitive dysfunction,[25] and the degree of elevation of CRP is often used to judge the size or activity of inflammation.[26] We found that after establishment of CPB, LA level and CRP concentration in each group showed an upward trend over time, which were notably attenuated after Dex infusion. High-dose opioids are the basis for the treatment of perioperative pain in pediatrics, but they also have several side effects, including respiratory depression, constipation, cognitive dysfunction and mental complications.[27,28] In a study of 142 children undergoing bronchoscopy, Dang et al found that Dex diminished midazolam dosage.[29] Our results indicated that Dex anesthesia decreased the dosages of fentanyl and propofol in a dose-dependently manner.
Emergence agitation is a typical complication after pediatric surgery, with an incidence of 10% to 50%.[30] Cohen et al. have confirmed that Dex can diminish the incidence of emergence agitation during maintenance of anesthesia or before surgery.[31] As expected, the occurrence of moderate agitation in group D2 in this study was dramatically lower than that in group C. The extubation time in group D1 and group D2 was shorter than that in group C, and the frequency of postoperative tramadol usage was also lower than that in group C, indicating that the application of Dex in CHD operation in children could shorten postoperative extubation time and abate postoperative pain. The reason may be because Dex plays an analgesic function to avoid severe pain by activating α2 receptor in the spinal dorsal horn, thereby attenuating postoperative agitation caused by pain.[32,33]
Dynamic changes of CK-MB are used to judge the condition of myocardial injury, and its continuous increase often indicates that myocardial injury is in a progressive state.[34] CTnI is a sensitive indicator for the prediction of neonatal death, with better sensitivity and specificity than CK-MB, and can predict myocardial injury degree in children at an early stage.[35] H-FABP is involved in the uptake, transport and metabolism of fatty acids in cardiomyocytes.[36] The levels of CK-MB, cTnI and H-FABP in participants were enhanced at the end of surgery and after surgery, and the increase in the observation group was significantly lower than that in the group C, suggesting that Dex had a protective effect on the myocardium after continuous administration of 0.2 g/kg/hour.
Brain tissue is particularly sensitive to ischemia and hypoxia, and hypoxemia facilitates the apoptosis and necrosis of nerve cells, leading to neurological defects.[37] In this study, Ca-jvO2 and O2ER were calculated by measuring blood gas in jugular bulb and combining with arterial blood gas to reflect cerebral oxygen metabolism. Results showed that Dex improved Ca-jvO2, but decreased ERO2, clarifying that Dex played an important role in reducing cerebral oxygen metabolism and brain damage in children undergoing CHD surgery. When brain tissue and neurons are damaged, the cell membranes of neurons are compromised, followed by S100β and NSE entering the blood circulation via blood-brain barrier.[38] Studies have shown that S100β and NSE levels in serum are positively correlated with the degree of brain injury.[39] So the combined detection can be used as a diagnostic indicator of brain injury.[40] The levels of S100β and NSE increased post-operation, whereas were obviously lower than those in group C, suggesting that continuous administration of Dex 0.4 μg/kg/hour could promote cerebral oxygen supply and demand balance, suppress neuronal discharge and abated neurotoxicity, thereby having a protective effect on nervous system.[41]
The levels of BUN, Scr, and NGAL were elevated at different time points after surgery. However, there were no statistically significant differences in levels of BUN and NGAL and AKI incidence among the 3 groups. The reason may be that various renal protection strategies were adopted for all subjects, such as strict control of infusion volume and ultrafiltration during CPB; additionally, duration of extracorporeal circulation was short in children over 1 year of age, greatly reducing the degree of renal damage in the participants; moreover, Dex 0.4 μg/kg/hour was lower than the dose selected in many clinical studies.
There were several limitations in this study. Firstly, postoperative agitation of the children was observed, with no long-term follow-up on the function of surgical organs. Since Dex has not been widely studied in CHD children at present, this study only selected AVSD children aged 1 to 6 years, and failed to evaluate the effect of Dex on CHD newborns less than 1-year old, which requires further study.
5. Conclusion
In CHD children undergoing CPB, Dex reduces the dosage of other anesthetic drugs, inhibited inflammatory stress response and maintain perioperative hemodynamic stability. Also, Dex downregulates the expression of myocardial and brain injury markers, lowered the incidence of tachycardia, nausea, vomiting and moderate agitation, and shortened tracheal extubation time. Moreover, Dex 0.4 g/kg/hour can further decrease the dosage of fentanyl and dopamine compared with Dex 0.2 g/kg/hour. In summary, Dex 0.4 μg/kg/hour is a clinically safe and effective anesthesia adjuvant for pediatric open-heart surgery in CHD children.
Author contributions
Shaopeng Ming and Yubo Xie contributed to the conception and design of this study and funding acquisition, and wrote the first draft of manuscript; Yongguo Xie and Xueke Du contributed to the experiment performance and data analysis; Haiqing Huang, Yue Fan and Qingxuan Liang contributed to the analysis and interpretation of the data. All authors critically revised the manuscript, and agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Conceptualization: Shaopeng Ming, Yubo Xie.
Data curation: Haiqing Huang, Yue Fan, Qingxuan Liang.
Formal analysis: Yongguo Xie, Xueke Du.
Funding acquisition: Shaopeng Ming, Yubo Xie.
Investigation: Haiqing Huang, Yue Fan, Qingxuan Liang.
Methodology: Yongguo Xie, Xueke Du.
Writing – original draft: Shaopeng Ming, Yubo Xie.
Writing – review & editing: Yongguo Xie, Haiqing Huang, Yue Fan, Qingxuan Liang.
Footnotes
Abbreviations: AKI = acute kidney injury, AKI = acute kidney injury, ASA = American Society of Anesthesiologists, AVSD = atrioventricular septal defect, BUN = blood urea nitrogen, Ca-jvO2 = carotid venous blood oxygen concentration, CHD = congenital heart disease, CK-MB = creatine kinase isoenzymes, CPB = cardiopulmonary bypass, CRP = C-reaction protein, cTnI = cardiac troponin I, Dex = Dexmedetomidine, FLACC = Face, Legs, Activity, Cry and Consolability, H-FABP = heart-type fatty acid binding protein, HR = heart rate, LA = lactic acid, MAP = mean arterial pressure, NGAL = neutrophil gelatinase-associated lipocalin, NSE = neuron-specific enolase, O2ER = cerebral oxygen extraction ratio.
How to cite this article: Ming S, Xie Y, Du X, Huang H, Fan Y, Liang Q, Xie Y. Effect of dexmedetomidine on perioperative hemodynamics and organ protection in children with congenital heart disease: a randomized controlled trial. Medicine. 2021;100:1(e23998).
This study was supported by the Guangxi Key Research and Development Program (No. AB18221031), the National Natural Science Foundation of China (No. 81373498), self-Foundation of Guangxi Health Commission (No. Z20181017), Guangxi Medical and Health Key Discipline Construction Project, and Guangxi Medical and Health Appropriate Technology Development and Popularization Application Project (No. S2020014).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data are mean ± standard deviation. ASA = American Society of Anesthesiology; ASD = atrial septal defect; VSD = ventricular septal defect; CPB = cardiopulmonary bypass.
Data are mean ± standard deviation. FLACC = Face, Legs, Activity, Cry and Consolability.
References
- [1].Bouma BJ, Mulder BJM. Changing landscape of congenital heart disease. Circ Res 2017;120:908–22. [DOI] [PubMed] [Google Scholar]
- [2].Caputo M, Pike K, Baos S, et al. Normothermic versus hypothermic cardiopulmonary bypass in low-risk paediatric heart surgery: a randomised controlled trial. Heart 2019;105:455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Song MK, Bae EJ, Kim GB, et al. Patients diagnosed with long QT syndrome after repair of congenital heart disease. Pacing Clin Electrophysiol 2018;41:1435–40. [DOI] [PubMed] [Google Scholar]
- [4].Dixit G, Dabney-Smith C, Lorigan GA. The membrane protein KCNQ1 potassium ion channel: Functional diversity and current structural insights. Biochim Biophys Acta Biomembr 2020;1862:183148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwartz LI, Twite M, Gulack B, et al. The perioperative use of dexmedetomidine in pediatric patients with congenital heart disease: an analysis from the congenital cardiac anesthesia society-society of thoracic surgeons congenital heart disease database. Anesth Analg 2016;123:715–21. [DOI] [PubMed] [Google Scholar]
- [6].Ozcan C, Dixit G, Li Z. Activation of AMP-activated protein kinases prevents atrial fibrillation. J Cardiovasc Transl Res 2020. [DOI] [PubMed] [Google Scholar]
- [7].Tsilimigras DI, Oikonomou EK, Moris D, et al. Stem cell therapy for congenital heart disease: a systematic review. Circulation 2017;136:2373–85. [DOI] [PubMed] [Google Scholar]
- [8].Behrle N, Birisci E, Anderson J, et al. Intranasal dexmedetomidine as a sedative for pediatric procedural sedation. J Pediatr Pharmacol Ther 2017;22:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Colin PJ, Hannivoort LN, Eleveld DJ, et al. Dexmedetomidine pharmacodynamics in healthy volunteers: 2. Haemodynamic profile. Br J Anaesth 2017;119:211–20. [DOI] [PubMed] [Google Scholar]
- [10].Liu Y, Liang F, Liu X, et al. Dexmedetomidine reduces perioperative opioid consumption and postoperative pain intensity in neurosurgery: a meta-analysis. J Neurosurg Anesthesiol 2018;30:146–55. [DOI] [PubMed] [Google Scholar]
- [11].Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA 2019;321:686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li F, Wang X, Deng Z, et al. Dexmedetomidine reduces oxidative stress and provides neuroprotection in a model of traumatic brain injury via the PGC-1α signaling pathway. Neuropeptides 2018;72:58–64. [DOI] [PubMed] [Google Scholar]
- [13].Nazir O, Wani MA, Ali N, et al. Use of dexmedetomidine and esmolol for hypotension in lumbar spine surgery. Trauma Mon 2016;21:e22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goyal S, Gupta KK, Mahajan V. A comparative evaluation of intravenous dexmedetomidine and fentanyl in breast cancer surgery: a prospective, randomized, and controlled trial. Anesth Essays Res 2017;11:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Inatomi O, Imai T, Fujimoto T, et al. Dexmedetomidine is safe and reduces the additional dose of midazolam for sedation during endoscopic retrograde cholangiopancreatography in very elderly patients. BMC Gastroenterol 2018;18:166–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Panchgar V, Shetti AN, Sunitha HB, et al. The effectiveness of intravenous dexmedetomidine on perioperative hemodynamics, analgesic requirement, and side effects profile in patients undergoing laparoscopic surgery under general anesthesia. Anesth Essays Res 2017;11:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cheng H, Li Z, Young N, et al. The effect of dexmedetomidine on outcomes of cardiac surgery in elderly patients. J Cardiothorac Vasc Anesth 2016;30:1502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wiczling P, Bartkowska-Śniatkowska A, Szerkus O, et al. The pharmacokinetics of dexmedetomidine during long-term infusion in critically ill pediatric patients. A Bayesian approach with informative priors. J Pharmacokinet Pharmacodyn 2016;43:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet 2017;56:893–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Peng YY, Jin YX, Chen CF, et al. Reduced emergence agitation with proparacaine hydrochloride eye drops after general anaesthesia for paediatric strabismus surgery. Intern J Nursing Sci 2015;2:58–60. [Google Scholar]
- [21].Crellin DJ, Harrison D, Santamaria N, et al. Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain 2015;156:2132–51. [DOI] [PubMed] [Google Scholar]
- [22].Petroz GC, Sikich N, James M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology 2006;105:1098–110. [DOI] [PubMed] [Google Scholar]
- [23].Cheng X, Zuo Y, Zhao Q, et al. Comparison of the effects of dexmedetomidine and propofol on hemodynamics and oxygen balance in children with complex congenital heart disease undergoing cardiac surgery. Congenit Heart Dis 2015;10:E123–30. [DOI] [PubMed] [Google Scholar]
- [24].Su F, Gastonguay MR, Nicolson SC, et al. Dexmedetomidine Pharmacology in Neonates and Infants After Open Heart Surgery. Anesth Analg 2016;122:1556–66. [DOI] [PubMed] [Google Scholar]
- [25].Zhang X, Yan X, Gorman J, et al. Perioperative hyperglycemia is associated with postoperative neurocognitive disorders after cardiac surgery. Neuropsychiatr Dis Treat 2014;10:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chalmers JD, Singanayagam A, Hill AT. C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med 2008;121:219–25. [DOI] [PubMed] [Google Scholar]
- [27].Szczepaniak A, Fichna J, Zielinska M. Opioids in cancer development, progression and metastasis: focus on colorectal cancer. Curr Treat Options Oncol 2020;21:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ayad S, Demitrack MA, Burt DA, et al. Evaluating the incidence of opioid-induced respiratory depression associated with oliceridine and morphine as measured by the frequency and average cumulative duration of dosing interruption in patients treated for acute postoperative pain. Clin Drug Investig 2020;40:755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dang X, Hu W, Yang Z, et al. Dexmedetomidine plus sufentanil for pediatric flexible bronchoscopy: A retrospective clinical trial. Oncotarget 2017;8:41256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hino M, Mihara T, Miyazaki S, et al. Development and validation of a risk scale for emergence agitation after general anesthesia in children: a prospective observational study. Anesth Analg 2017;125:550–5. [DOI] [PubMed] [Google Scholar]
- [31].Cohen IT, Finkel JC, Hannallah RS, et al. Rapid emergence does not explain agitation following sevoflurane anaesthesia in infants and children: a comparison with propofol. Paediatr Anaesth 2003;13:63–7. [DOI] [PubMed] [Google Scholar]
- [32].Ng KT, Shubash CJ, Chong JS. The effect of dexmedetomidine on delirium and agitation in patients in intensive care: systematic review and meta-analysis with trial sequential analysis. Anaesthesia 2019;74:380–92. [DOI] [PubMed] [Google Scholar]
- [33].Cho HK, Yoon HY, Jin HJ, et al. Efficacy of dexmedetomidine for perioperative morbidities in pediatric tonsillectomy: a metaanalysis. Laryngoscope 2018;128:E184–93. [DOI] [PubMed] [Google Scholar]
- [34].Chen YC, Wen Bin YU, Ning ZX, et al. Preliminary study on release of cardiac troponin I and creatine kinase-MB in sera of heart transplantation patients. J Fourth Military Med Univers 2002;23:2063–6. [Google Scholar]
- [35].Türker G, Babaoğlu K, Gökalp AS, et al. Cord blood cardiac troponin I as an early predictor of short-term outcome in perinatal hypoxia. Biol Neonate 2004;86:131–7. [DOI] [PubMed] [Google Scholar]
- [36].Carless DR, Wnęk M, Knox C, et al. Clinical and analytical evaluation of an immunoturbidimetric heart-type fatty acid-binding protein assay. Scand J Clin Lab Invest 2013;73:48–53. [DOI] [PubMed] [Google Scholar]
- [37].Jha NK, Jha SK, Sharma R, et al. Hypoxia-induced signaling activation in neurodegenerative diseases: targets for new therapeutic strategies. J Alzheimers Dis 2018;62:15–38. [DOI] [PubMed] [Google Scholar]
- [38].Wang Z, Chen Q, Guo H, et al. Effects of dexmedetomidine on H-FABP, CK-MB, cTnI levels, neurological function and near-term prognosis in patients undergoing heart valve replacement. Exp Ther Med 2017;14:5851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rodríguez-Rodríguez A, Egea-Guerrero JJ, Gordillo-Escobar E, et al. S100B and Neuron-Specific Enolase as mortality predictors in patients with severe traumatic brain injury. Neurol Res 2016;38:130–7. [DOI] [PubMed] [Google Scholar]
- [40].Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 2013;9:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sun L, Guo R, Sun L. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand 2014;58:642–50. [DOI] [PubMed] [Google Scholar]


