Abstract
In addition to cancer and diabetes, inflammatory and ROS-related diseases represent one of the major health problems worldwide. Currently, several synthetic drugs are used to reduce oxidative stress; nevertheless, these approaches often have side effects. Therefore, to overcome these issues, the search for alternative therapies has gained importance in recent times. Natural bioactive compounds have represented, and they still do, an important source of drugs with high therapeutic efficacy. In the “synthetic” era, terrestrial and aquatic photosynthetic organisms have been shown to be an essential source of natural compounds, some of which might play a leading role in pharmaceutical drug development. Marine organisms constitute nearly half of the worldwide biodiversity. In the marine environment, algae, seaweeds, and seagrasses are the first reported sources of marine natural products for discovering novel pharmacophores. The algal bioactive compounds are a potential source of novel antioxidant and anticancer (through modulation of the cell cycle, metastasis, and apoptosis) compounds. Secondary metabolites in marine Algae, such as phenolic acids, flavonoids, and tannins, could have great therapeutic implications against several diseases. In this context, this review focuses on the diversity of functional compounds extracted from algae and their potential beneficial effects in fighting cancer, diabetes, and inflammatory diseases.
Keywords: marine bioactive compounds, secondary metabolites, algae, oxidative stress, ROS, cancer, diabetes, inflammation, apoptosis
1. Introduction
Epidemiological studies have evidenced the dangerous effects on human health of the ever-increasing intake of junk food, alcohol, and antibiotics. This bad behavior can increase the risk of oxidative stress which, in turn, can lead to accelerated aging and inflammatory diseases, such as cardiovascular and neurodegenerative disease and many types of cancer [1]. According to reports by the WHO, more than 200 types of lethal cancer accounted approximately for 9.6 million deaths per year in 2019 globally [2]. Similarly, diabetes mellitus, a metabolic disorder, has emerged as the third foremost cause of death worldwide (1.6 million deaths per year) with several associated ill-fated diseases, such as heart attack, stroke, kidney failure, high blood pressure, blindness, and lower limb amputation [3,4,5]. According to WHO, the world’s diabetic population will hike up to 592 million by 2035. In addition to cancer and diabetes, inflammatory diseases have tremendously increased in the recent past causing millions of deaths [6]. Unfortunately, current chemotherapeutic, anti-diabetic, and anti-inflammatory drugs often present several adverse effects, such as toxicity, drug tolerance, and metabolic impairments [1]. In this regard, natural products might provide alternative drugs with better characteristics [7]. Similarly, their regular uptake through diet or novel pharmacological formulations might help prevent oxidative stress-related diseases [8,9,10,11].
Approximately 70% of the Earth’s surface is covered by oceans and it hosts an immense variety of marine organisms which represent a rich source of natural products [1,12,13]. Marine algae are among the most promising sources of novel bioactive compounds with interesting biological effects, such as antioxidant, anticancer, antibacterial, antifungal, antidiabetic, and anti-inflammatory activities [1]. Marine algae are extensively used in diet and traditional medicine in Asian countries because of the presence of minerals, dietary fiber, lipids, omega-3 fatty acids, proteins, polysaccharides, and essential amino acids [14,15]. They also contain many vitamins, such as vitamins A, B, C, and E [16]. Few marine algae-derived bioactive compounds, such as phlorotannins, polysaccharides, fucoidans, alginic acid, tripeptides, pyropheophytin, and oxylipin, have been shown to reduce the risk of cancer, diabetes, and inflammatory diseases [15]. Hence, in this review we focused our attention on the diversity of marine algal bioactive compounds and the recent findings about their molecular mode of action in potentially fighting cancer, diabetes, and inflammation (Figure 1). Furthermore, algal extracts showed potential antimicrobial activity against aerobes, psychotropic, proteolytic, and lipolytic bacteria and act as natural preservatives. Additionally, they can prevent lipid oxidation [17,18].
Figure 1.
Potential beneficial effects of algal metabolites on human health. Secondary metabolites in marine algae could provide novel drug candidates for fighting various diseases, e.g., by reducing the α-amylase and α-glucosidase activity in diabetes; by reducing inflammation thanks to their antioxidant capacity; and inhibiting cellular proliferation in tumor cells.
2. Biological Activities of Marine Algae and Potential Health Benefits via Dietary Supplements
Diet plays an important role in disease prevention as more than 33% of diseases, such as cancer, diabetes, and inflammation-associated chronic diseases, could be avoided by changing lifestyle and food habits [19,20]. Nutritional supplements from natural sources could also play an important role in preventing diseases. Phytochemicals from marine algae, such as peptides, amino acids, lipids, fatty acids, sterols, polysaccharides, carbohydrates, polyphenols, photosynthetic pigments, vitamins, and minerals, some of which are represented in Figure 2, can act as potent antioxidants and have beneficial effects as anti-diabetic and chemotherapeutic drugs, as detailed below.
Figure 2.
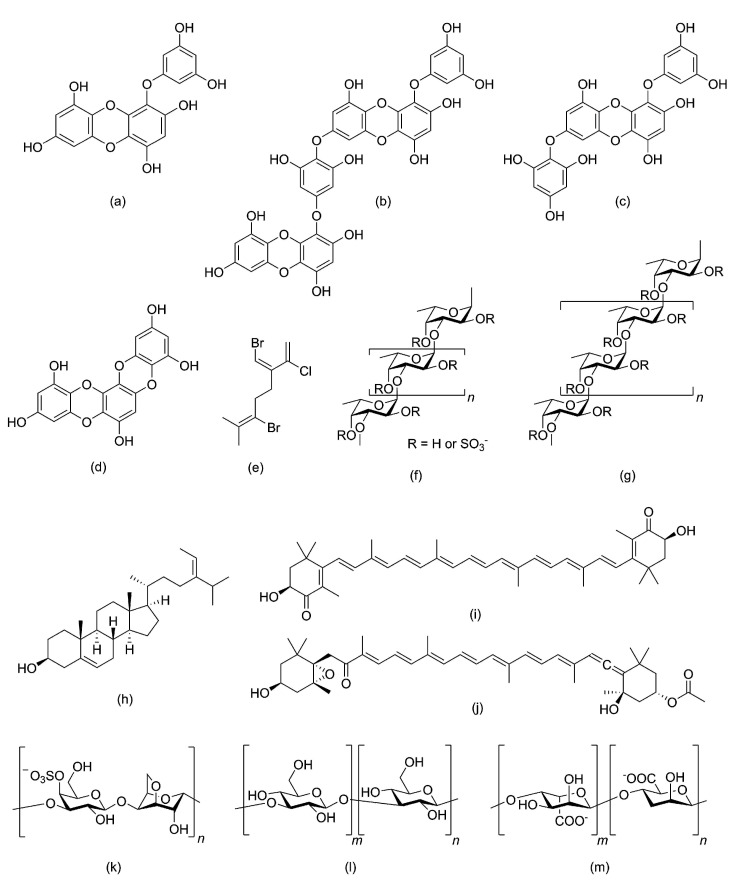
Chemical structure of several algal metabolites that can have beneficial health effects by acting as antioxidants: (a) eckol, (b) dieckol, (c) 7-phloroeckol, and (d) dioxinodehydroeckol, four phlorotannins; (e) 2-chloro-3-(bromomethylene)-6-bromo-7-methyl-1,6-octadiene, a halogenated monoterpene; (f) type I and (g) type II fucoidans; (h) fucosterol; (i) astaxanthin and (j) fucoxanthin; (k) k-carrageenan; (l) laminaran; and (m) alginate.
2.1. Peptides and Amino Acids
Hydrolysis of proteins can lead to bioactive peptides that can present beneficial health aspects and modulate the outcome of the disease. Bioactive phyto-peptides have 3 to 20 amino acid residues and display biological properties such as antioxidant, anticancer, anti-inflammation, and immunomodulation. For example, purified peptides from Chlorella vulgaris can prevent cellular damage and can act as potent anticancer agents [21,22].
The protein content in macro and micro algae comprises all essential amino acids which prevent cellular damage. The red alga Palmaria palmata is rich in Leu, Val, and Met, and their mean levels are similar to ovalbumin. Similarly, Ile and Thr concentrations are comparable to those in legume proteins. The green alga Ulva rigida contains Leu, Phe, and Val as major essential amino acids [23,24].
2.2. Lipids and Fatty Acids
The structural complexity of lipids and fatty acids are highly diverse and contribute to their therapeutic efficacy. It has been reported that small amounts of saturated fatty acids can help prevent cardiovascular diseases. Marine algae contain polyunsaturated fatty acids (PUFAs) and significant amounts of monounsaturated fatty acids which are beneficial to human health and could help reduce cardiovascular diseases [25].
2.2.1. Polyunsaturated Fatty Acids (PUFAs)
Humans are incapable of synthesizing PUFAs that, on the other hand, are abundant in both macro and microalgae. PUFAs in microalgae are mainly composed of omega-3 and omega-6 fatty acids (e.g., EPA and AA) [26]. PUFAs regulate blood clotting and blood pressure and modulate the function of the brain and nervous systems [27]. Moreover, they decrease the risk of several chronic diseases, such as diabetes and cancer. Additionally, they regulate inflammatory responses by producing eicosanoids, well-known inflammation mediators [27]. Omega-3 and omega-6 PUFA from macroalgae are already used as dietary supplements [28]. Red and brown algae have a high level of omega-3 fatty acids (e.g., EPA and GLA) and omega-6 fatty acids (e.g., AA and linoleic acid). Brown algae Laminaria ochroleuca and Undaria pinnatifida are a rich source of octadecatetraenoic acid, an omega-3 PUFA [29]. Green seaweed Ulva pertusa was found to be rich in hexadecatetraenoic (omega-3), oleic (omega-9), and palmitic acids (SFA). The lipid fraction of microalgae C. vulgaris contains oleic, palmitic, and linolenic acids. The green microalga Haematococcus sp. also contains short-chain fatty acids. Long-chain PUFAs are also used as nutritional supplements and food additives [30]. Spirulina sp. is a promising source of GLAs, a precursor of leukotrienes, prostaglandins, and thromboxans that regulate inflammatory, immunological, and cardiovascular disorders. Cyanobacteria and some green algae also contain bioactive fatty acids such as palmitic, oleic, and lauric acids along with DHA [22].
2.2.2. Sterols
Sterols are a class of lipids extensively found in both macro and microalgae. Sterols and some of their derivatives have potential biological, e.g., anti-inflammatory, activity. Sterols from Spirulina triggers the formation of the plasminogen-activating factor in vascular endothelial cells. Fucosterol, ergosterol, and chondrillasterol are found in brown algae and cholesterol has been found in red algae [22,31].
2.3. Polysaccharides and Carbohydrates
Polysaccharides are abundant in seaweeds and also found in microalgae. They generally comprise about 4% to 76% of the total dry weight of the alga. Polysaccharides are classified according to their chemical structure, such as sulfuric acid polysaccharides, sulfated xylans, and galactans (generally found in green algae). Moreover, alginic acid, fucoidan, laminarin, and sargassan are found in brown algae [32]. Agar, carrageenans, xylans, and floridean are generally found in red algae. Many algal polysaccharides present bioactivity and could become drug candidates with potential use in several human health disparities [33]. Carrageenans are sulfated galactans and they are extensively used in pharmaceutical and food industries. Soluble fibers such as fucans, alginates, and laminarans are found in brown seaweeds, whereas soluble fibers such as sulfated galactans (agars and carrageenans), xylans, and floridean starch are abundantly found in red seaweeds [34]. Green algae contain xylans, mannans, starch, and ionic sulfate group-containing polysaccharides in combination with uronic acids, rhamnose, xylose, galactose, and arabinose. Many of the polysaccharides can be regarded as dietary fibers and are classified into two groups, i.e., soluble and insoluble fibers [35,36]. Seaweeds contain about 25% to 75% dietary fibers in comparison to their dry weight, a higher percentage compared to that found in fruit and vegetables [37]. The algal dietary fiber consumption has several health benefits as they can be used as antitumor, anticancer, anticoagulant, and antiviral agents. Fucoidans are extensively found in the cell walls of brown macroalgae [38]. Fucoidans have several biological activities and act as antioxidant, antitumor, anti-inflammatory, antidiabetes, antiviral, anticoagulant, and antithrombotic agents. Additionally, they also modulate the human immune system [1]. Furthermore, laminarin is the second main source of glucan, abundantly found in brown algae, and it acts as a facilitator of intestinal metabolism [36].
Carbohydrates, such as glucose and starch, are abundantly found in microalgae [39]. Many biological functions of microalgal species are due to the presence of carbohydrates. Chlorella pyrenoidosa and Chlorella ellipsoidea contain glucose and a wide variety of combinations of galactose, mannose, rhamnose, N-acetylglucosamine, N-acetylgalactosamine, and arabinose that exert immune-modulatory and antiproliferative activity [40]. β-1,3-Glucans extracted from Chlorella have been shown to act as immunomodulators that can reduce blood lipids [41].
2.4. Polyphenolic Compounds
Marine algal bioactive compounds are potent antioxidant agents that protect from oxidative damage [42]. The antioxidant activity of bioactive compounds from marine algae is associated with protection against cancer, inflammatory, diabetes, and several ROS-related diseases [43]. Polyphenolic compounds are mainly found in both micro and macroalgae [42]. The phenolic components include hydroxycinnamic acids, phenolic acids, simple phenols, xanthones, coumarins, naphthoquinones, stilbenes, flavonoids, anthraquinones, and lignins [21]. The phlorotannins with potential antioxidant activity belong to polyphenolic compounds that have been screened from several brown algae. Phlorotannins are known for their chemopreventive, antibacterial, antiproliferative, and UV-protective properties [22].
2.5. Photosynthetic Pigments
Macroalgae contain chlorophylls and carotenoids as major photosynthetic pigments. Carotenoids are well known for their antioxidant properties and dietary carotenoids have high nutritional and therapeutic value [44]. Carotenoids are well known for their chemopreventive effect against several cancer subtypes. Microalgae are also the main source of antioxidants such as β-carotene and astaxanthin. β-Carotene is a natural colorant that has been conventionally used as food and drinks colorants and can act as dietary food supplements or additives with a high antioxidant capacity [45].
2.6. Vitamins and Minerals
Vitamins are micronutrients essential for human body growth and development. Seaweeds and microalgae are known to be a good source of vitamin B1, B2, and B12. Vitamin B12 (cobalamin) is extensively found in higher concentrations in green and red algae compared to brown algae [46]. Vitamin B12 is generally found in red macroalgae such as Palmaria longat and Porphyra tenera. The highest vitamin B12 content was found in red seaweed Porphyra sp. and green algae, such as Enteromorpha sp. and Spirulina. Cobalamin deficiency can cause health diseases, such as neuropsychiatric disorders and megaloblastic anaemia. Vitamin C (ascorbic acid) is present in all red, brown, and green seaweeds. Vitamin C has several health benefits, such as radical scavenging activity, antiaging, and immune stimulant activity. Vitamin E is a mixture of tocopherols. α-Tocopherol occurs in green, red, and brown seaweeds. Phaeophyceae also contain β- and γ-tocopherols and displayed outstanding antioxidant activity. Vitamins C and E were also found in Laminaria digitata and U. pinnatifida [22].
Seaweeds and macroalgae are rich in minerals, trace elements, and maintain inorganic atoms in seawater. Minerals and trace elements are required for the human diet [47]. Phaeophyceae, such as U. pinnatifida and Sargassum, and rhodophyta, such as Chondrus crispus and Gracilariopsis, are considered dietary supplement that meet the recommended daily intake of some of the major minerals, such as Na, K, Ca, and Mg, as well as trace minerals, such as Fe, Zn, Mn, and Cu. In addition, seaweeds are also important sources of Ca as they reduce Ca deficiency risk in pregnant women and adolescents and they inhibit preadolescent aging [48].
3. Marine Bioactive Metabolites and Their Therapeutic Efficacy
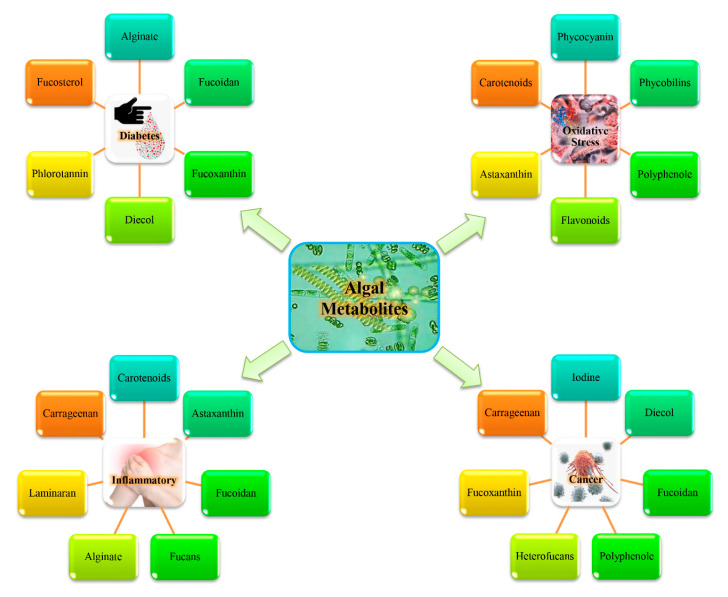
The marine ecosystem is a source of novel natural secondary metabolites with promising biomedical applications [49]. The impact of marine algae in the area of traditional medicine is huge and they have been used as Yunani hakim in many countries, such as China and Egypt. Marine algae produce diverse secondary metabolites and might be the most promising sources of proteins, vitamins, omega-3, carotenoids, phenolic acids, and flavonoids, as well as other natural antioxidants [50]. These marine bioactive compounds act as free radical scavengers and prevent oxyradical formation thus reducing oxidative stress and, as such, they have great importance in the prevention of cancer, diabetes, early aging, and several other inflammatory diseases (Figure 3).
Figure 3.
Potential effects of algal metabolites in different human disease.
Marine bioactive compounds, such as algal photosynthetic pigments, phycobiliproteins, carotenoids, polyphenols, terpenes, phlorotannins, and polysaccharides, have shown promising therapeutic activity in both in vitro and in vivo models [25,29,38,51].
3.1. Marine Bioactive Metabolites and Modulation of In Vitro Antioxidant Activity
Reactive oxygen species (ROS) comprise a group of oxygenic ions that are highly reactive and pose a serious threat to biological components, ultimately leading to serious disorders such as cancer, diabetes mellitus, neurodegenerative and inflammatory diseases [52]. The oxygen-containing radicals comprise of peroxyl (ROO•), hydroxyl (OH•), hydroperoxyl (HO2•), superoxide (O2•), alkoxyl (RO•), thiyl peroxyl (RSOO•), sulfonyl (ROS•), and nitric oxide (NO•) radical, as well as non-radical oxidizing agents such as singlet oxygen (1O2), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), and organic hydroperoxides (ROOH) [52,53,54,55]. Cells can detoxify ROS as they are furnished with antioxidant defense mechanisms to maintain cellular equilibrium. Antioxidants fight against ROS and exert a positive effect on human health by protecting macromolecules such as proteins, DNA, and membrane lipids [56]. The use of synthetic antioxidants used as food additives, such as butylated hydroxyanisole, butylated hydroxytoluene, tertiary-butylhydroquinone, and propyl gallate, might represent a threat because of their side effects [57]. Hence, the development of novel antioxidants from natural sources like marine flora can represent a promising approach. Marine algae could neutralize ROS because of their antioxidant compounds, such as phycobilins, phycocyanin, carotenoids, astaxanthin, polyphenols, and vitamins, which can act against cancer, diabetes, inflammation, aging, and immune responses. The antioxidant capacity of various marine algae, such as green, red, and brown algae species, has been already extensively reported in the literature.
Antioxidant activity of marine algal compounds have been determined by several methods, such as 2,2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging, ferric reducing antioxidant power (FRAP), lipid peroxide inhibition, ABTS radical scavenging, nitric oxide (NO) scavenging, hydrogen peroxide radical scavenging assays, superoxide radical and hydroxyl radical scavenging assays. The methanolic extract of blue-green algae has shown potent DPPH radical scavenging activity. In addition, phycocyanin from Spirulina platensis showed strong H2O2 scavenging activity [58]. Antioxidant properties in green algae Ulva fasciata and Ulva reticulate were characterized by free-radical-scavenging due to the presence of flavonoids [59,60,61]. In brown algae such as Ecklonia cava, Eisenia bicyclis, and Ecklonia kurome antioxidant activities were characterized by DPPH-radical scavenging [62]. Ethanolic extracts of Gracilaria tenuistipitata and Callophyllis japonica also have shown potential antioxidant activities [63,64].
3.2. Intricate Role of Algal Bioactive Metabolites as Anticancer Agents
Free radicals and ROS generally promote cancer initiation. Synthetic chemopreventive drugs often present several adverse side-effects to the tumor vicinity and bodily organs because of poor specificity and generalized biodistribution [65]. Several marine algal bioactive compounds have been designated as potent chemopreventives due to inhibition of cellular proliferation, modulation of the cell cycle, and induction of apoptosis [66,67].
3.2.1. Inhibition of Cell Proliferation
Several studies have reported that marine algal bioactive compounds have antiproliferative and inhibitory activity against several cancer subtypes in in vitro as well as in vivo [68]. Sulfated polysaccharides purified from brown seaweeds exhibited an antiproliferative effect on human leukemia and lymphoma cell lines [38]. They have also been reported to inhibit proliferation of breast (MCF-7) and cervical (HeLa) cancer cells [38]. Sulfated polysaccharides extracted from the brown seaweed Sargassum vulgare displayed inhibition of cell proliferation in HeLa and B16 cells without cytotoxicity in normal rabbit aortic endothelial cells [69]. Furthermore, sulfated polysaccharides from red seaweed Amansia multifidi inhibited the cellular viability of HeLa cells. The polysaccharides isolated from Gracilariopsis lemaneiformis, consisting of 3,6-anhydro-L-galactose and D-galactose and a linear structure of repeated disaccharide agarobiose units, hindered the viability of B16, A549, and MKN-28 cell lines [70]. Similarly, low-molecular-weight sulfated polysaccharides from green seaweed Gayralia oxysperma inhibited the cell viability of U87MG glioblastoma cells even at microgram-level concentrations (10, 100, and 1000 µg/mL) without any evident cytotoxicity [71]. Fucoidans isolated from Undaria pinnatifida also showed inhibition of cellular viability in SK-MEL-28, T-47, RPMI-7951, T47D, and DLD-1 cancer cell lines at microgram concentrations. Moreover, fucoidans isolated from the sporophyll of U. pinnatifida demonstrated to be able to inhibit cell growth in HeLa, A549, PC-3, and HepG2 cell lines, although at higher concentrations (treatment with 0.8 mg/mL for 24 h) [72]. Furthermore, in prostate cell lines (DU-145), fucoidans treatment marked a 90% reduction in cell viability [73]. Similarly, Fucus vesiculosus derived fucoidans were reported to reduce cell viability of human colorectal carcinoma (HCT116) cell line by 60% [74]. Fucoidan from Ecklonia cava have been also reported to inhibit proliferation of MDA-MB-231 cells. Moreover, the administration of fucoidan (20 mg/kg for 28 days) in a DU-145 cell-induced xenograft rat model has reduced the tumor growth by 50% [73]. Carrageenans from Kappaphycus alvarezii reduced the growth of liver, colon, breast, and osteosarcoma cell lines [75]. Similarly, treatment of phlorotannins (a type of polyphenol) isolated from E. cava has marked a reduction in cell viability in MDA-MB-231 and MCF-7 cells by 55% and 64%, respectively [76]. Similarly, phlorotannin-rich extracts from Ascophyllum nodosum reduced the cell viability of HT-29 colon cancer cells [77].
Halogenated monoterpenes isolated from red seaweeds Plocamium cornutum and Plocamium suhrii displayed potent antiproliferative activity as compared to anticancer drug cisplatin [78]. U. pinnatifida isolated fucoxanthins has a cytotoxic effect against LNCaP, DU145, PC-3, Caco-2, HT-29, DLD-1, HeLa, and Jurkat cell lines [79,80]. Moreover, fucoxanthinol displayed an anti-proliferative effect against drug-resistant HT-29-derived cells, and inhibited xenograft tumor development in a dose-dependent manner [81]. The guaiane sesquiterpene derivative guai-2-en-10-ol isolated from the green seaweed Ulva fasciata, reduced viability of breast cancer MDA-MB-231 cell line [82]. G. tenuistipitata aqueous extract counteracted the cellular proliferation in H1299 cells. Heterofucans from Sargassum filipendula exhibited anti-proliferative effects on cervical, prostate, and liver cancer cells [83]. Aqueous extracts of Sargassum oligocystum and Gracilaria corticata inhibited proliferation of human leukemic cell lines [84,85]. Ethanolic and methanolic extracts of Gracilaria tenuistipitata exhibited anti-proliferative effects against Ca9-22 oral cancer cells [86,87,88]. Several studies have reported that algae consumption modulates cancer prevention. The diets containing seaweeds decreased the growth of DU-145 human prostatic tumor cells in nude mice. Moreover, the administration of red algae Eucheuma cottonii extracts as dietary supplement to rats displayed tumor repression [89].
3.2.2. Cell Cycle Arrest and Inhibition of Angiogenesis
Inhibition of cell cycle hinders cancer cell proliferation for the subsequent exhibition of anticancer activity. Sulfated polysaccharides from G. oxysperma arrested the cell cycle [71]. Fucoidan from Fucus vesiculosus arrested the cell cycle at the G1 phase in HCT116 human colorectal carcinoma and HT-29 colon cancer cells [74]. Fucoxanthin arrested the cell cycle via downregulation of cyclin D1, D2, CDK4 and upregulation of p15INK4B and p27Kip1 expression [90]. Fucoxanthin from Laminaria japonica arrested the sub-G1 phase of the cell cycle in WiDr cancer cells [91]. Moreover, in LNCap prostate cancer cells, fucoxanthin arrested the G1 phase of the cell cycle via MAPK/ JNK and GDD45A pathways [92]. Pheophorbide a, from G. elliptica arrested the cell cycle in the G0/G1 phase in glioblastoma cells [93]. Aqueous extract of G. tenuistipitata induced G2/M arrest in the H1299 cell line [64].
Angiogenesis plays a key role in tumor growth and metastasis. Polysaccharides isolated from S. vulgare exhibited angiogenesis inhibitory activity. Fucoidans isolated from U. pinnatifida significantly reduced the expression of the angiogenesis factors VEGF-A and VEGF-162 [94]. Sulfated polysaccharides from brown seaweed Sargassum vulgare displayed antiangiogenic activity in HeLa and B16 cells without damage to the tumor vicinity [69]. Furthermore, dieckol decreased the expression of angiogenic markers such as PCNA, VEGF, COX-2, MMP-2, and MMP-9 to inhibit metastasis [95].
3.2.3. Induction of Apoptosis
Apoptosis (or programmed cell death, PCD) is the main goal of anticancer drugs. Several reports have demonstrated the role of algal bioactive compounds and polysaccharides as potent anticancer agents by modulating apoptosis, as schematized in Figure 4. Sulfated polysaccharides from Phaeophyceae act as novel chemopreventive drugs owing to their free-radical scavenging activity [1]. Sulfated polysaccharides induced apoptosis in human leukemic monocyte lymphoma cell line (U-937) [1]. Polysaccharides from Capsosiphon fulvescens induced apoptosis in gastric cancer cells via modulation of PI3K/Akt pathway [96]. Polysaccharides from U. lactuca increased the activity of antioxidant enzymes in a DMBA-induced breast cancer model via diminished lipid peroxidation as well as GSH-Px activity to restrain apoptosis [97]. Polysaccharides from red seaweed Champia feldmannii demonstrated in vivo antitumor effects in mice transplanted with sarcoma 180 tumors via modulation of apoptosis [98]. The polysaccharides isolated from sea lettuce U. lactuca displayed in vitro and in vivo anticancer activity in breast cancer via modulation of apoptosis. It also displayed a chemopreventive effect in DMBA-induced breast cancer in rat post-administration for 10 weeks and prevented breast-histological alterations and carcinogenic wounds. Additionally, it also amplified the p53 expression and inhibited the Bcl-2 expression to induce apoptosis in breast cancer cells [97].
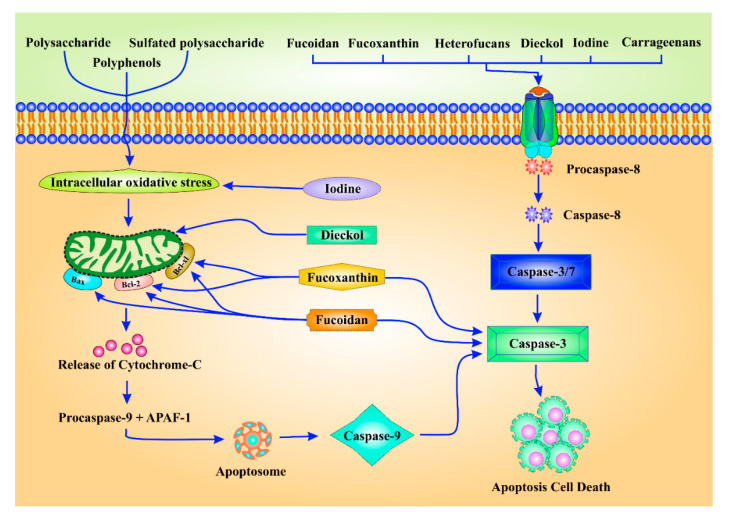
Figure 4.
Apoptosis modulation by algal metabolites in cancer prevention. Polysaccharides, sulfated polysaccharides, iodine, dieckol, fucoxanthine, fucoidan, and polyphenols downregulate the expression of anti-apoptotic protein Bcl-xl, Bcl-2. Similarly, they enhance the Bax expression to aid apoptosis. Fucoidan supports the intrinsic apoptosis via regulating the cytosolic release of cytochrome C. Fucoxanthine and fucoidan induces the expression of caspase 9 and caspase 3 to induce apoptotic cell death. Moreover, fucoidan, fucoxanthine, heterofucans, dieckol, iodine, and carrageenans induce apoptosis through modulation of caspase 3 activity via death receptor-mediated apoptotic cell death in several cancer cell lines. In addition to this, they also regulate caspase 8 activity inducing extrinsic apoptosis in different cancer cells.
Fucoidans isolated from the sporophyll of U. pinnatifida displayed apoptosis in DU-145 cell-induced xenograft rat model via inhibition of the JAK3/STAT pathway. In addition, fucoidan (IC50 530 ± 3.32 mg/mL) also reduced the viability of B16 melanoma cells via activating apoptosis [99]. Fucoidan from Fucus vesiculosus induced p53-independent apoptosis in HCT116 human colorectal carcinoma cell line [74]. Fucoidan from L. japonica induced apoptosis via activation of caspase-3, poly(ADP-ribose) polymerase (PARP), and DNA degradation in HT-29 cell line [100]. Moreover, fucoidan from E. cava induced apoptosis in MDA-MB-231 and MCF-7 cells via induction of p53 and activation of Bax, caspases 3 and 9, and PARP with inhibition of Bcl-2 [76]. Furthermore, fucoidan from Cladosiphon okamuranus displayed induction of apoptosis in MCF-7 cells via activation of caspase-3 and DNA fragmentation [101]. F. vesiculosus extracts enhanced mitochondria membrane permeability thus inducing apoptosis via cytoplasmic release of cytochrome C and the Smac/DIABLO pathway in human colon cancer cells [102]. Similarly, fucoidan from F. vesiculosus treatment induced apoptosis in HT-29 colon cancer cells via decreasing the expression of Bcl-xL, Bcl-2 and upregulation of Bax, pro-caspases 3, 7, and 9. An upregulation of Rb and E2 factor proteins and Fas-regulated extrinsic apoptosis was also evident post fucoidan treatment in HT-29 colon cancer cells [103].
Fucoxanthin from Laminaria japonica induced apoptosis via DNA fragmentation in human colon adenocarcinoma WiDr cells [91]. Fucoxanthin from Ishige okamurae exhibited anticancer activity in melanoma B16F10 cells both in vitro (B16F10 cell line) and in vivo (Balb/c mice implanted with B16F10 cells) via induction of apoptosis. Moreover, fucoxanthin induced apoptosis via caspases activation and reduction of BclxL and IAP expression [90]. Laminarin from Laminaria digitata induced apoptosis in human colon cancer (HT-29) cells and activated ErbB2 phosphorylation. Moreover, it also inhibited cell proliferation and induced apoptosis in prostate cancer (PC-3) cells and increased the expression of P27kip1 and PTEN [104]. Dieckol from E. cava daily administration (40 mg/kg for 15 weeks) decreased cancer cell proliferation in albino rats via induction of apoptosis [95]. The guaiane sesquiterpene derivative guai-2-en-10-ol from green seaweed Ulva fasciata induced apoptosis in MDA-MB-231 breast cancer cell line via direct interaction with the kinase site of EGFR [82]. The halogenated monoterpene, mertensene from red alga Pterocladiella capillacea induced apoptosis in HT-29 and LS174 via modulation of ERK1/2, Akt, and NF B pathways [78]. Ethanolic and methanolic solvent extracts of Gracilaria tenuistipitata displayed apoptosis in Ca9-22 oral cancer cells via DNA damage. Furthermore, methanol extract of Plocamium telfairiae induced caspase-dependent apoptosis in HT-29 colon cancer cells [88]. Iodine and polyphenols from L. japonica induced apoptosis via inhibiting SOD activity [105]. The red alga Porphyra yezoensis can induce cancer cell death via apoptosis in a dose-dependent manner in in vitro cancer cell lines without exhibiting cytotoxicity towards the normal cells. Moreover, Carrageenans, heterofucans, dieckol, and iodine can induce cancer cell death via apoptosis in a dose-dependent manner in in vitro cancer cell lines without exhibiting cytotoxicity towards the normal cells. Furthermore, L. japonica water extracts induced apoptosis in several human breast cancer cell lines. Moreover, Eucheuma cottonii extract displayed the upregulation of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) in cancer-induced rats [89]. Methanolic extracts of Fucus serratus and F. vesiculosus exhibited protection of DNA damage induced by H2O2 in Caco-2 cells [106]. Furthermore, Pelvetia canaliculata inhibited H2O2-induced superoxide dismutase depletion in Caco-2 cells [106]. C. japonica ethanol extracts inhibited H2O2-induced apoptosis via activating cellular antioxidant enzymes [63]. G. tenuistipitata aqueous extract enhanced the recovery of these cells from H2O2-induced DNA damage in the H1299 cell line [59]. Apoptosis modulation by algal metabolites in different cancerous cell lines with molecular pathways are summarized in Table 1.
Table 1.
Marine algal bioactive metabolites and their functional role in apoptosis.
| Bioactive Compounds | Algal Sources | Cell Lines/In Vivo Models Involved | Functional Involvement | Ref. | |
|---|---|---|---|---|---|
| 1 | Sulfated polysaccharides | Brawn algae | Human leukemic monocyte lymphoma cell line (U-937) | Inhibition of cell proliferation | [1] |
| 2 | Polysaccharides | Capsosiphon fulvescens | Gastric cancer cells | Modulation of PI3K/Akt pathway | [96] |
| 3 | Polysaccharides | U. lactuca | DMBA-induced breast cancer model | Diminished lipid peroxidation also GPx activity | [97] |
| 4 | Polysaccharides | Champia feldmannii | Mice transplanted with sarcoma 180 tumors | Reduction of tumor growth | [98] |
| 5 | Polysaccharides | U. lactuca | DMBA-induced breast cancer in rat | Prevented breast-histological alterations and carcinogenic wounds | [97] |
| 6 | Polysaccharides | U. lactuca | Breast cancer cells | p53 expression and inhibited the Bcl-2 expression | [97] |
| 7 | Fucoidan | Costaria costata | DLD-1 | 55% (100 μg/mL) | [107] |
| SK-MEL-28 | 20% (100 μg/mL) | [107] | |||
| 8 | Fucoidans | U. pinnatifida | DU-145 cell-induced xenograft rat model | Inhibition of the JAK3/STAT pathway | [99] |
| 9 | Fucoidan | Fucus vesiculosus | HCT116 human colorectal carcinoma cell line | p53-independent | [74] |
| 10 | Fucoidan | L. japonica | HT-29 cell line | Caspase-3, PARP, and DNA degradation | [100] |
| 11 | Fucoidan | E. cava | MDA-MB231 and MCF-7 cells | Induction of p53 and activation of Bax, caspases 3 and 9, and PARP with inhibition of Bcl-2 | [76] |
| 12 | Fucoidan | Cladosiphon okamuranus | MCF-7 cells | Activation of caspase-3 and DNA fragmentation | [101] |
| 13 | Extracts | F. vesiculosus | Human colon cancer cells | Cytoplasmic release of cytochrome C and the Smac/DIABLO pathway | [102] |
| 14 | Fucoidan | F. vesiculosus | HT-29 colon cancer cells | Decreased expression of Bcl-xL, Bcl-2 and upregulation of Bax, pro-caspases 3, 7, and 9 | [103] |
| 15 | Fucoidan | F. vesiculosus | HT-29 colon cancer cells | Upregulation of Rb and E2 factor proteins and Fas regulation | [103] |
| 16 | Fucoxanthin | Laminaria japonica | Human colon adenocarcinoma WiDr cells | DNA fragmentation | [91] |
| 17 | Fucoxanthin | Ishige okamurae | Melanoma B16F10 cells | Caspases activation and reduction of BclxL and IAP expression | [90] |
| 18 | Dieckol | E. cava | Albino rats | Decreased cancer cell proliferation | [95] |
| 19 | Guaiane sesquiterpene | Ulva fasciata | MDA MB-231 breast cancer cell line | Direct interaction with kinase site of EGFR | [82] |
| 20 | Halogenated monoterpene, mertensene | Pterocladiella capillacea | HT-29 and LS174 | ERK1/2, Akt, and NF B pathways | [78] |
| 21 | Ethanolic and methanolic extracts | Gracilaria tenuistipitata | Ca9-22 oral cancer cells | DNA damage | [88] |
| 22 | Methanolic extract | Plocamium telfairiae | HT-29 colon cancer cells | Induced caspase-dependent | [88] |
| 23 | Iodine and polyphenols | L. japonica | HT-29 colon cancer cells | Inhibition of SOD activity | [105] |
| 24 | Extract | Eucheuma cottonii | Cancer-induced rats | Upregulation of antioxidant enzymes, e.g., CAT, SOD, and GPx | [89] |
| 25 | Methanolic extracts | Fucus serratus and F. vesiculosus | Caco-2 cells | DNA damage | [106] |
| 26 | Methanolic extracts | Pelvetia canaliculata | Caco-2 cells | Inhibited H2O2-induced SOD depletion | [106] |
| 27 | Ethanol extracts | C. japonica | Caco-2 cells | Activating cellular antioxidant enzymes | [63] |
| 28 | Aqueous extract | G. tenuistipitata | H1299 cell line | Activating cellular antioxidant enzymes | [59] |
3.3. Anti-Inflammatory Activity of Marine Algal Bioactive Metabolites
Inflammation is a molecular marker of carcinogenesis. Marine natural products are well-known anti-inflammatory agents due to their potent antioxidant activity. Several anti-inflammatory compounds with potential pharmacological applications have been isolated from marine algal sources. Macroalgae contain several polysaccharides, such as fucoidan, fucans, alginates, laminarin, agar, and carrageenans, which are used as prebiotic compounds and that can have potential application as anti-inflammatory agents. Marine-derived carotenoids and astaxanthin exhibit potent anti-inflammatory activity [108,109].
The anti-inflammatory activity of marine algae is due to the presence of PUFAs (e.g., omega-3) that potentiate inhibition of inflammation [27]. Several studies have demonstrated that omega-3 to 6 fatty acids reduce inflammation when taken as dietary supplements [27]. The polysaccharide extracted from Turbinaria ornate, Delesseria sanguinea exhibited anti-inflammatory potential in several in vitro systems. Sulfated polysaccharide fraction from Gracilaria caudate, a galactan from Gelidium crinale, a mucin-binding agglutinin from Hypnea cervicornis, lectin from Pterocladiella capillacea, and sulfated galactofucan from Lobophora variegata also exhibited anti-inflammatory potency [110]. Oral administration of marine polysaccharide in an in vivo mouse model reduced the initiation of inflammation [111].
The alga Spirulina had demonstrated anti-inflammatory effects when assessed using a non-alcoholic steatohepatitis model [112]. C-phycocyanin from Spirulina platensis blocked inflammation via inhibiting the expressions of nitric oxide synthase, cyclooxygenase-2, and production of pro-inflammatory cytokines [113,114]. Methanolic extracts of Ulva lactuca and U. conglobate have shown anti-inflammatory effects in murine hippocampal HT22 cell line [115]. Lycopene from Chlorella marina demonstrated anti-inflammatory effects in an arthritic rat model [116]. Phytosterols from Dunaliella tertiolecta, aqueous and methanolic extracts of Caulerpa mexicana and lectin from Caulerpa cupressoides exhibited anti-inflammatory activities in several in vitro models [117]. Ethanolic extract of Ecklonia cava inhibited LPS-induced inflammation in human endothelial cells [118]. Furthermore, Ishige okamurae showed anti-inflammatory effects in a few in vitro models [119]. The astaxanthin isolated from Haematococcus pluvialis reduced gastric inflammation in Helicobacter pylori-infected mice via decreasing bacterial density [120]. Moreover, astaxanthin reduced the production of pro-inflammatory mediators and cytokines such as nuclear factor-κB (NF-κB), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), and suppresses T lymphocyte activation in asthma patients [120]. Fucans from Sargassum vulgare, Lobophora variegata, and Spatoglossum schroederi also displayed anti-inflammatory effects [121]. Furthermore, Alginic acid from Sargassum wightii exhibited anti-inflammatory effects in vivo in a rat model [121].
Methanolic extract of Bryothamnion triquetrum exhibited an anti-inflammatory effect in Swiss albino mice [122]. Two fatty acids of Gracilaria verrucosa such as (E)-10-oxooctadec-8-enoic acid and (E)-9-oxooctadec-10-enoic acid inhibited the production of inflammatory markers, such as nitric oxide, IL-6, and TNF-α [123]. The sulfoglycolipidic isolated from the red alga Porphyridium cruentum exhibited an anti-inflammatory effect due to the presence of AA (6.8%), palmitic acid (26.1%), and EPA (16.6%), and omega-9 fatty acid (10.5%) [124]. Pheophytin from Enteromorpha prolifera has superoxide radical (O2•_) reducing potential and inflammatory responses in mice [125,126]. A glycoprotein extracted from Porphyra yezoensis exhibited anti-inflammatory effects in LPS-stimulated macrophages [118]. Furthermore, phlorotannins (a polyphenol derived from Eisenia bicyclis, Ecklonia cava, and Ecklonia kurome), and sargachromanol G (derived from Sargassum siliquastrum) showed promising anti-inflammatory activity via inhibition of the production of inflammatory mediators in LPS-stimulated cells [127,128]. Moreover, methanolic extract of Neorhodomela aculeata inhibited ROS generation, H2O2-induced lipid peroxidation, and inducible nitric oxide synthase in neurological diseases via inhibition of inflammation [129].
3.4. Significance of Marine Algal Bioactive Metabolites as Anti-Diabetes Drugs
Diabetes mellitus is a chronic metabolic disorder which is characterized by high blood glucose levels that lead to renal dysfunction, cardiovascular diseases, and retinal damage [1]. Dietary management is a novel target for treating diabetes via maintaining the correct concentrations of both blood glucose and blood lipids [130]. Commercially available antidiabetic drugs exert several diseases-associated adverse side effects during treatments [131]. In this context, the identification of natural antidiabetic drugs with enhanced drug efficacy and lesser adverse effects has gained the attention of researchers in recent times. Marine algae-derived bioactive compounds exhibited antidiabetic properties furnished by regulation of various signaling pathways, such as inhibitory effect on enzymes such as α-amylase, α-glucosidase, aldose reductase, dipeptidyl peptidase-4, and protein tyrosine phosphatase 1B (PTP 1B) enzyme [132]. Enzymes like α-amylase and α-glucosidase play a significant role in the digestion of carbohydrates, leading to a delay in glucose absorption in blood and also to a reduction of glucose levels in blood plasma. Subsequently, these compounds may be exploited as potential functional food ingredients for preventing or diminishing insulin resistance and diabetes [132].
Marine algal compounds modulated the GLUT-4 and AMPK signaling pathways and triggered glucose tolerance [1]. Recent investigations displayed that fucoidan act as prebiotics and regulate the intercellular metabolism and blood sugar level [1]. Fucoidan isolated from S. fusiforme controlled the blood glucose level, recovered liver function, and inhibited oxidative stress in STZ-induced diabetic rats [133]. Fucoidan from Ecklonia maxima acted as a potent α-glucosidase inhibitor with a very low IC50 value (0.27–0.31 mg/mL) and regulated type II diabetes [134]. Fucoidan from Fucus vesiculosus displayed a robust α-glucosidase inhibitor in diabetes treatment [135]. Furthermore, low molecular weight fucoidan (LMWF) from S. hemiphyllum in combination with fucoxanthin displayed anti-diabetic properties in type II diabetes rat model (db/db). The oral administration of LMWF in combination with fucoxanthin decreased blood glucose and fasting blood sugar levels [135]. The synergistic drug effect was more effective in the in vivo model via reduction of urinal sugar level as compared to the LMWF treatment alone. LMWF enhanced the hepatic glycogen concentration and antioxidant enzymes which were assisted by lipid metabolism. The lipid metabolism displayed the regulation of glucose transporter (GLUT), insulin receptor substrate (IRS-1), peroxisome proliferator-activated receptor-gamma (PPARγ), and uncoupling protein (UCP)-1 level with the treatment of LMWF in combination with fucoxanthin [135]. Fucoidan from Cucumaria frondosa amplified the expression of insulin receptor substrate 1, Glut-4, and PI3K/Akt, glucose transporter protein in insulin-resistant rats [1]. Fucoidan from Saccarina japonica abridged blood sugar level too [1,136].
Moreover, sulfated fucoidan isolated from Undaria pinnatifida inhibited hyperglycemia by eliciting insulin sensitivity in a diabetic mouse (C57BL/KSJ/db/db) model [137]. Fucoidan from Sargassum wightii inhibited alpha-D-glucosidase that transport glucose into the blood and reduce glucose level in blood [138]. Dieckol, fucodiphloroethol G, 6,6′-Bieckol, 7-phloroeckol, phlorofucofuroeckol A from E. cava and phloroglucinol, dioxinodehydroeckol, eckol from E. stolonifera and E. bicyclis displayed robust α-glucosidase activity and reduced blood sugar level [139,140,141]. Furthermore, dieckol-rich extract from E. cava improved insulin sensitivity [142]. Polyphenolic-rich extract from I. okamurae improved insulin sensitivity [143]. Moreover, polyphenolic-rich extract from E. cava inhibited glucose uptake effect in skeletal muscle [141]. Fucosterol from Pelvetia siliquosa reduced serum glucose concentration and inhibited sorbitol accumulation in the lenses in Sprague–Dawley diabetic rats [144]. Phlorotannin components from Ascophyllum nodosum displayed potential inhibition of α-amylase and α-glucosidase activities in in vitro models [145]. Sodium alginate from Laminaria angustata inhibited the rising blood glucose and insulin levels in Wistar rats [146]. Fucoxanthin and fucosterol from Undaria pinnatifida and Ecklonia stolonifera displayed aldose reductase inhibition [147,148]. Furthermore, pheophorbide-A, pheophytin-A also displayed aldose reductase inhibition [149]. Algal bioactive metabolites and their functional role in diabetes are summarized in Table 2.
Table 2.
Marine algal bioactive metabolites and their functional role in diabetes.
| Algal Type | Algal Sources | Bioactive Metabolites | Functional Involvement | Ref. | |
|---|---|---|---|---|---|
| 1 | Red algae | Rhodomela confervoides | HPN analogues | Inhibition of PTP 1B | [150] |
| 2 | Brown alga | Ecklonia cava | Methanolic extract | Increases phosphorylation AMP-activated protein kinase; radical scavenging property |
[151] |
| 3 | Isochrysis galbana, Nannochloropsis oculata | DHA, EPA | Regulates glucose and lipid metabolism | [152] | |
| 4 | Palmaria, Ascophyllum, Alaria | Phenol rich extract | Inhibitory of α-amylase and α-glucosidase | [145] | |
| 5 | Brown algae | Polyphenols/Phlorotannins | Inhibition of α-glucosidase and α-amylase; increases skeletal muscle glucose uptake; inhibition of PTP 1B enzyme; increases insulin sensitivity. |
[153] | |
| 6 | Brown algae | E. cava | Dieckol | α-Glucosidase inhibitor [20]; postprandial hyperglycemia-lowering effect [7]; glucose uptake effect in skeletal muscle [40]; PTP 1B inhibition [10]; protective effect against diabetes complication | [139,140,154,155] |
| 7 | Brown algae | E. cava | Fucodiphloroethol G | α-Glucosidase inhibitor | [140] |
| 8 | Brown algae | E. cava | 6,6′-Bieckol | α-Glucosidase inhibitor | [140] |
| 9 | Brown algae | E. cava | 7-Phloroeckol | α-Glucosidase inhibitor; PTP 1B inhibition | [139,140] |
| 10 | Brown algae | E. cava | Phlorofucofuroeckol A | α-Glucosidase inhibitor; PTP 1B inhibition | [139,140] |
| 11 | Brown algae |
E. stolonifera
E. bicyclis |
Phloroglucinol | α-Glucosidase inhibitor; PTP 1B inhibition | [139] |
| 12 | Brown algae |
E. stolonifera
E. bicyclis |
Dioxinodehydroeckol | α-Glucosidase inhibitor; PTP 1B inhibition | [139] |
| 13 | Brown algae | I. okamurae | Diphlorethohydroxycarmalol | α-Glucosidase inhibitor; postprandial hyperglycemia-lowering effect; protective effect against diabetes complication | [156] |
| 14 | Brown algae |
E. stolonifera
E. bicyclis |
Eckol | α-Glucosidase inhibitor; PTP 1B inhibition | [139] |
| 15 | Brown algae | I. foliacea | Octaphlorethol A | Glucose uptake effect in skeletal muscle | [142] |
| 16 | Brown algae |
A. nodosum
F. vesiculosus |
Polyphenolic-rich extract | α-Glucosidase inhibitor; postprandial hyperglycemia-lowering effect | [145] |
| 17 | Brown algae | E. cava | Polyphenolic-rich extract | Glucose uptake effect in skeletal muscle | [141] |
| 18 | Brown algae | E. cava | Dieckol-rich extract | Improvement of insulin sensitivity | [142] |
| 19 | Brown algae | I. okamurae | Polyphenolic-rich extract | Improvement of insulin sensitivity | [143] |
| 20 | Ulva rigida | Ethanolic extract | Decreased blood glucose concentrations in Wistar diabetic rats | [157] | |
| 21 | Pelvetia siliquosa | Fucosterol | Reduction of serum glucose levels and inhibition of sorbitol accumulation in the lenses in Sprague–Dawley diabetic rats | [144] | |
| 22 | Brown algae | Ecklonia cava | Methanolic extract | Reduction in plasma glucose levels and increased insulin concentration; activation of AMPK/ACC and PI3/Akt signaling pathways in Sprague–Dawley diabetic rats | [141] |
| 23 | Green algae and Diatoms | Chlorella sp., Nitzschia laevis | Microalgal extracts | Inhibition of advanced glycation endproducts (AGEs) formation in in vitro models | [158] |
| 24 | Brown algae | Ascophyllum nodosum | Phlorotannin components | Inhibition of α-amylase and α-glucosidase activities in vitro | [145] |
| 25 | Brown algae | Laminaria angustata | Sodium alginate | Inhibition of rising blood glucose and insulin levels in Wistar rats | [146] |
| 26 | Brown algae | Eisenia bicyclis | Dioxinodehydroeckol | α-Glucosidase inhibitor | [159] |
| 27 | Brown algae | Eisenia bicyclis | 7-Phloroeckol | PTP 1B inhibition; α-glucosidase inhibitor | [159] |
| 28 | Brown algae | Eisenia bicyclis | Fucoxanthin | Aldose reductase inhibition | [148] |
| 29 | Brown algae | Ecklonia cava | Dieckol | α-Glucosidase inhibitor | [160] |
| 30 | Brown algae | Ecklonia cava | Fucodiphloroethol G | α-Amylase inhibitor | [160] |
| 31 | Brown algae | Ecklonia cava | 6,6′-Bieckol | PTP 1B inhibition | [160] |
| 32 | Brown algae | Ecklonia cava | 7-Phloroeckol | ACE inhibitor | [160] |
| 33 | Brown algae | Ecklonia cava | 2-Phloroeckol | α-Glucosidase inhibitor; α-glucosidase inhibitor; α-glucosidase inhibitor; PTP 1B inhibition; aldose reductase inhibition; aldose reductase inhibition |
[139,161] |
| 34 | Brown algae | Ecklonia cava | Phlorofucofuroeckol A | α-glucosidase inhibitor; PTP 1B inhibition; ACE inhibitor; AGEs inhibition; Aldose reductase inhibition | [139,162] |
| 35 | Brown algae | Ecklonia stolonifera | Phloroglucinol | α-glucosidase inhibitor | [153] |
| 36 | Brown algae | Ecklonia stolonifera | Eckol | PTP 1B inhibition α-glucosidase inhibitor | [153] |
| 37 | Brown algae | Ecklonia stolonifera | Dieckol | α-Amylase inhibitor; ACE inhibitor; PTP 1B inhibition; α-glucosidase inhibitor |
[144,160] |
| 38 | Brown algae | Ecklonia stolonifera | Phlorofucofuroeckol A | α-Amylase inhibitor; PTP 1B inhibition; ACE inhibitor; α-glucosidase inhibitor |
[144,160] |
| 39 | Brown algae | Ecklonia stolonifera | Fucosterol | PTP 1B inhibition; aldose reductase inhibition | [147] |
| 40 | Brown algae | Ishige okamurae | Diphlorethohydroxycarmalol | α-Glucosidase inhibitor; α-amylase inhibitor | [163] |
| 41 | Brown algae | Myagropsis myagroides | Eckol | α-Glucosidase inhibitor; α-amylase inhibitor | [153] |
| 42 | Brown algae | Sargassum serratifolium | Sargahydroquinoic acid | ACE inhibitor; PTP 1B inhibition | [164] |
| 43 | Brown algae | Ascophyllum nodosum | Methanol extract | α-Glucosidase inhibitor; α-amylase inhibitor | [145] |
| 44 | Brown algae | Saccharina japonica | Pheophorbide-A | Aldose reductase inhibition | [149] |
| 45 | Brown algae | Saccharina japonica | Pheophytin-A | Aldose reductase inhibition | [149] |
| 46 | Brown algae | Undaria pinnatifida | Fucoxanthin | Aldose reductase inhibition | [148] |
4. Algal Metabolites as Prebiotics for Human Health with Special References to Fucoidan
Algal metabolite consumption, such as polysaccharides, sulfated polysaccharides, fucoidans, chlorophylls, phycobilins, fucoxanthins, carotenoids, polyphenols, and omega-3 fatty acids, decreases blood pressure and sugar level, and it can have antiviral, anti-inflammatory, anticancer, and neuroprotective effects, as well as act as immune boost-up. The immunomodulatory potential of prebiotics modulates immune fitness via several metabolic processes and interactions with the gut microbiota in humans [165]. The gut microbiota produces short-chain fatty acids (SCFA) such as propionate, acetate and butyrate by breaking down prebiotics and modulating the immune response [165]. Intravenous use of acetate amplified the activity of NK cells in cancer patients. In addition, it activated G protein-coupled receptors (GPR41 and GPR43) in rats, thus triggering mitogen-activated protein kinase (MAPK) signaling and modulating the transcription factors activity [166]. Acetate also increased the production of IL-10 in rats and prevented the inhibitory activity of butyrate on IL-2 production [167]. Daily administration of fucoidans from A. nodosum increased in Lactobacillus and Ruminococcus in the intestine of mice [168].
Fucoidan possesses a wide range of immune-modulation effects by stimulating activation of natural killer (NK) cells, dendritic cells (DCs), and T cells and increasing anti-tumor and anti-viral responses [169]. Fucoidan enhanced immune modulation via activation of macrophage facilitated by membrane receptors, such as TLR4, cluster of differentiation 14 (CD14), competent receptor-3 (CR-3), and scavenging receptor (SR). This led to signal transduction via MAPK and activation of transcription factors, and it also induced cytokines production, which regulates activation of NK cells and T lymphocytes [170]. In this regard, treatment of C57BL/6 rats with fucoidan extracted from Fucus vesiculosus up-regulated pro-inflammatory cytokines (IL-6, IL-12, and TNF-α) in serum and spleenocytes after 3 h of administration [171]. Furthermore, fucoidan from L. cichorioides, L. Japonica, and F. evanescens served as TLR ligands and their interaction with TLR-2 and TLR-4 receptors in vitro activated NF-jB. Furthermore, it also controlled the expression of the defense mechanisms of intrinsic immunity, such as secretion of chemokines, cytokines, and manifestation of MHC class I and II particles [1]. These are essential for the defense against foreign attackers and for activating adaptive immune systems. A clinical trial based on the diet supplementation of 1 g/day of fucoidan from Undaria pinnatifida on adult male and female volunteers for 24 weeks showed modulation of the immunity to seasonal influenza vaccine by antibody production [172]. Based on these reports, there are several evidences that fucoidan acts as a potent prebiotic and that it is able to modulate immunity. This is achieved by interacting with intestinal cells of the gut microbiota and direct motivation of immune cells through TLRs.
5. Conclusions and Future Perspectives
Natural extracts have been used since ancient times for treating various illnesses. Products from natural products have also provided a large number of pharmaceuticals, or their prototypes, in recent times. Among the many natural sources, marine algae can still play a pivotal role in human health and disease because of the need for novel drug candidates. Their extracts are already well-known in traditional medicine and more recent studies investigated the many beneficial effects of their secondary metabolites, such as reduction of oxidative stress and modulation of apoptosis, and the main findings of these researches are summarized in this review. The exploitation of these results might lead to the development of novel algal dietary supplements and pharmaceuticals for preventing and treating chronic malfunctions and other age-associated chronic diseases. For example, sulfated fucoidans have been shown to be potential candidates as new pharmaceuticals in fighting cancer and diabetes. However, despite the extensive use of algae-derived compounds and extracts in the food industry, there are still no FDA-approved anticancer, antioxidant, anti-inflammatory, and antidiabetic drugs. Technology transfer from the pre-clinical results to the clinical application of secondary metabolites extracted from marine algae is still in its infancy and not fully exploited and more clinical studies are needed to really evaluate the pharmaceutical efficacy algal compounds.
In conclusion, marine algae offer a great variety of bioactive molecules with potential health benefits. Several types of marine algae are already consumed as food additives and nutritional supplements, potentially exerting their beneficial effects through diet. There is, however, an impelling necessity of considering the algal bioactive compounds in new drug discovery programs and to investigate their biological effects in deeper detail in order to find new pharmaceuticals with preventive and therapeutic efficacy.
Acknowledgments
The authors are thankful to Berhampur University for providing the necessary facilities to carry out this work. We are also thankful to Pradyot Kumar Behera for helping to draw the molecular structure of algal metabolites.
Author Contributions
Writing—original draft preparation, B.P., R.N., and S.P.; visualization, B.P. and B.P.J.; review and editing, A.R. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by “Tecnopolo per la medicina di precisione” (TecnoMed Puglia)—Regione Puglia: DGR n.2117 del 21/11/2018, CUP: B84I18000540002 and “Tecnopolo di Nanotecnologia e Fotonica per la medicina di precisione” (TECNOMED)—FISR/MIUR-CNR: delibera CIPE n.3449 del 7-08-2017, CUP: B83B17000010001.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pradhan B., Patra S., Nayak R., Behera C., Dash S.R., Nayak S., Sahu B.B., Bhutia S.K., Jena M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020;164:4263–4278. doi: 10.1016/j.ijbiomac.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Status Report on Alcohol and Health 2018. World Health Organization; Geneve, Switzerland: 2018. [Google Scholar]
- 3.Balakumar P., Maung-U K., Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016;113:600–609. doi: 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 4.Adeloye D., Ige J.O., Aderemi A.V., Adeleye N., Amoo E.O., Auta A., Oni G. Estimating the prevalence, hospitalisation and mortality from type 2 diabetes mellitus in Nigeria: A systematic review and meta-analysis. BMJ Open. 2017;7:e015424. doi: 10.1136/bmjopen-2016-015424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niemeijer M.N., van den Berg M.E., Leening M.J., Hofman A., Franco O.H., Deckers J.W., Heeringa J., Rijnbeek P.R., Stricker B.H., Eijgelsheim M. Declining incidence of sudden cardiac death from 1990–2010 in a general middle-aged and elderly population: The Rotterdam Study. Heart Rhythm. 2015;12:123–129. doi: 10.1016/j.hrthm.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 6.Wepner B., Giesecke S. Drivers, trends and scenarios for the future of health in Europe. Impressions from the FRESHER project. Eur. J. Futures Res. 2018;6:2. doi: 10.1007/s40309-017-0118-4. [DOI] [Google Scholar]
- 7.Azab A., Nassar A., Azab A.N. Anti-Inflammatory Activity of Natural Products. Molecules. 2016;21:1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragusa A., Centonze C., Grasso M.E., Latronico M.F., Mastrangelo P.F., Fanizzi F.P., Maffia M. Composition and Statistical Analysis of Biophenols in Apulian Italian EVOOs. Foods. 2017;6:90. doi: 10.3390/foods6100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ragusa A., Centonze C., Grasso M.E., Latronico M.F., Mastrangelo P.F., Sparascio F., Fanizzi F.P., Maffia M. A Comparative Study of Phenols in Apulian Italian Wines. Foods. 2017;6:24. doi: 10.3390/foods6040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragusa A., Centonze C., Grasso M.E., Latronico M.F., Mastrangelo P.F., Sparascio F., Maffia M. HPLC Analysis of Phenols in Negroamaro and Primitivo Red Wines from Salento. Foods. 2019;8:45. doi: 10.3390/foods8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zafar M.S., Quarta A., Marradi M., Ragusa A. Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations. Pharmaceutics. 2019;11:505. doi: 10.3390/pharmaceutics11100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patra S., Praharaj P.P., Panigrahi D.P., Panda B., Bhol C.S., Mahapatra K.K., Mishra S.R., Behera B.P., Jena M., Sethi G., et al. Bioactive compounds from marine invertebrates as potent anticancer drugs: The possible pharmacophores modulating cell death pathways. Mol. Biol. Rep. 2020;47:7209–7228. doi: 10.1007/s11033-020-05709-8. [DOI] [PubMed] [Google Scholar]
- 13.Maharana S., Pradhan B., Jena M., Misra M.K. Diversity of Phytoplankton in Chilika Lagoon, Odisha, India. Environ. Ecol. 2019;37:737–746. [Google Scholar]
- 14.Suleria H.A.R., Osborne S., Masci P., Gobe G. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs. 2015;13:6336–6351. doi: 10.3390/md13106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanna B., Mishra A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018;17:1613–1624. doi: 10.1111/1541-4337.12396. [DOI] [PubMed] [Google Scholar]
- 16.Mohanty S., Pradhan B., Patra S., Behera C., Nayak R., Jena M. Screening for nutritive bioactive compounds in some algal strains isolated from coastal Odisha. J. Adv. Plant Sci. 2020;10:1–8. [Google Scholar]
- 17.Miranda J.M., Trigo M., Barros-Velázquez J., Aubourg S.P. Effect of an icing medium containing the alga Fucus spiralis on the microbiological activity and lipid oxidation in chilled megrim (Lepidorhombus whiffiagonis) Food Control. 2016;59:290–297. doi: 10.1016/j.foodcont.2015.05.034. [DOI] [Google Scholar]
- 18.Barros-Velázquez J., Miranda J.M., Ezquerra-Brauer J.M., Aubourg S.P. Impact of icing systems with aqueous, ethanolic and ethanolic-aqueous extracts of alga Fucus spiralis on microbial and biochemical quality of chilled hake (Merluccius merluccius) Int. J. Food Sci. Technol. 2016;51:2081–2089. doi: 10.1111/ijfs.13182. [DOI] [Google Scholar]
- 19.Conlon M.A., Bird A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patra S., Pradhan B., Nayak R., Behera C., Rout L., Jena M., Efferth T., Bhutia S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Ibañez E., Herrero M., Mendiola J.A., Castro-Puyana M. Marine Bioactive Compounds. Springer; Boston, MA, USA: 2012. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates; pp. 55–98. [Google Scholar]
- 22.Lordan S., Ross R.P., Stanton C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs. 2011;9:1056–1100. doi: 10.3390/md9061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bocanegra A., Bastida S., Benedí J., Rodenas S., Sanchez-Muniz F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food. 2009;12:236–258. doi: 10.1089/jmf.2008.0151. [DOI] [PubMed] [Google Scholar]
- 24.Taboada C., Millán R., Míguez I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2010;90:445–449. doi: 10.1002/jsfa.3836. [DOI] [PubMed] [Google Scholar]
- 25.Ruxton C., Reed S.C., Simpson M., Millington K. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004;17:449–459. doi: 10.1111/j.1365-277X.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 26.Zheng J.-S., Hu X.-J., Zhao Y.-M., Yang J., Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706. doi: 10.1136/bmj.f3706. [DOI] [PubMed] [Google Scholar]
- 27.Wall R., Ross R.P., Fitzgerald G.F., Stanton C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 28.Fleurence J., Gutbier G., Mabeau S., Leray C. Fatty acids from 11 marine macroalgae of the French Brittany coast. J. Appl. Phycol. 1994;6:527–532. doi: 10.1007/BF02182406. [DOI] [Google Scholar]
- 29.Sánchez-Machado D., López-Cervantes J., Lopez-Hernandez J., Paseiro-Losada P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004;85:439–444. doi: 10.1016/j.foodchem.2003.08.001. [DOI] [Google Scholar]
- 30.Rodríguez-Meizoso I., Jaime L., Santoyo S., Señoráns F.J., Cifuentes A., Ibáñez E. Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. J. Pharm. Biomed. Anal. 2010;51:456–463. doi: 10.1016/j.jpba.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Bouzidi N., Viano Y., Ortalo-Magne A., Seridi H., Alliche Z., Daghbouche Y., Culioli G., El Hattab M. Sterols from the brown alga Cystoseira foeniculacea: Degradation of fucosterol into saringosterol epimers. Arab. J. Chem. 2019;12:1474–1478. doi: 10.1016/j.arabjc.2014.11.004. [DOI] [Google Scholar]
- 32.Menshova R.V., Ermakova S.P., Anastyuk S.D., Isakov V.V., Dubrovskaya Y.V., Kusaykin M.I., Um B.-H., Zvyagintseva T.N. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydr. Polym. 2014;99:101–109. doi: 10.1016/j.carbpol.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 33.Pereira L., Bahcevandziev K., Joshi N.H. Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder. CRC Press; Boca Raton, FL, USA: 2019. [Google Scholar]
- 34.Mohamed S., Hashim S.N., Rahman H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012;23:83–96. doi: 10.1016/j.tifs.2011.09.001. [DOI] [Google Scholar]
- 35.Lahaye M. Marine algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some ‘sea vegetables’. J. Sci. Food Agric. 1991;54:587–594. doi: 10.1002/jsfa.2740540410. [DOI] [Google Scholar]
- 36.Mišurcová L., Škrovánková S., Samek D., Ambrožová J., Machů L. Advances in Food and Nutrition Research. Volume 66. Elsevier; Amsterdam, The Netherlands: 2012. Health benefits of algal polysaccharides in human nutrition; pp. 75–145. [DOI] [PubMed] [Google Scholar]
- 37.Jiménez-Escrig A., Sánchez-Muniz F. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000;20:585–598. doi: 10.1016/S0271-5317(00)00149-4. [DOI] [Google Scholar]
- 38.Wijesinghe W., Jeon Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012;88:13–20. doi: 10.1016/j.carbpol.2011.12.029. [DOI] [Google Scholar]
- 39.Becker W. Microalgae in human and animal nutrition. In: Richmond A., editor. Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Wiley Online Library; Hoboken, NJ, USA: 2004. pp. 312–351. [Google Scholar]
- 40.Pugh N., Ross S.A., ElSohly H.N., ElSohly M.A., Pasco D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001;67:737–742. doi: 10.1055/s-2001-18358. [DOI] [PubMed] [Google Scholar]
- 41.Shao B., Wang Z., Liu X., Yu J., Lan J., Wang J., Ma L., Chen Z. Breeding of a Chlorella strain with high yield of polysaccharide and its effect on growth and immunoregulation of Litopenaeus vannamei. J. Nucl. Agric. Sci. 2013;27:168–172. [Google Scholar]
- 42.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 43.Fernando I.S., Kim M., Son K.-T., Jeong Y., Jeon Y.-J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food. 2016;19:615–628. doi: 10.1089/jmf.2016.3706. [DOI] [PubMed] [Google Scholar]
- 44.Faulks R.M., Southon S. Challenges to understanding and measuring carotenoid bioavailability. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2005;1740:95–100. doi: 10.1016/j.bbadis.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Matos J., Cardoso C., Bandarra N., Afonso C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017;8:2672–2685. doi: 10.1039/C7FO00409E. [DOI] [PubMed] [Google Scholar]
- 46.Kim S.-K., Taylor S. Marine Medicinal Foods: Implications and Applications, Macro and Microalgae. Volume 64 Academic Press; Cambridge, MA, USA: 2011. [Google Scholar]
- 47.Circuncisão A.R., Catarino M.D., Cardoso S.M., Silva A. Minerals from macroalgae origin: Health benefits and risks for consumers. Mar. Drugs. 2018;16:400. doi: 10.3390/md16110400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupérez P. Mineral content of edible marine seaweeds. Food Chem. 2002;79:23–26. doi: 10.1016/S0308-8146(02)00171-1. [DOI] [Google Scholar]
- 49.Kini S., Divyashree M., Mani M.K., Mamatha B.S. Advances in Cyanobacterial Biology. Elsevier; Amsterdam, The Netherlands: 2020. Algae and cyanobacteria as a source of novel bioactive compounds for biomedical applications; pp. 173–194. [Google Scholar]
- 50.Shahidi F. Functional foods: Their role in health promotion and disease prevention. J. Food Sci. 2004;69:R146–R149. doi: 10.1111/j.1365-2621.2004.tb10727.x. [DOI] [Google Scholar]
- 51.Sharma N., Khanra A., Rai M.P. Oxidative Stress: Diagnostic Methods and Applications in Medical Science. Springer; Berlin, Germany: 2017. Potential applications of antioxidants from algae in human health; pp. 153–168. [Google Scholar]
- 52.Bayr H. Reactive oxygen species. Crit. Care Med. 2005;33:S498–S501. doi: 10.1097/01.CCM.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 53.Halliwell B. Free radicals and other reactive species in disease. eLS. 2001 doi: 10.1038/npg.els.0003913. [DOI] [Google Scholar]
- 54.Storz P. Reactive oxygen species in tumor progression. Front. Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 55.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gechev T.S., Van Breusegem F., Stone J.M., Denev I., Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro J.S., Santos M.J.M.C., Silva L.K.R., Pereira L.C.L., Santos I.A., da Silva Lannes S.C., da Silva M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019;148:181–188. doi: 10.1016/j.meatsci.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Estrada J.P., Bescós P.B., Del Fresno A.V. Antioxidant activity of different fractions of Spirulina platensis protean extract. IL Farmaco. 2001;56:497–500. doi: 10.1016/S0014-827X(01)01084-9. [DOI] [PubMed] [Google Scholar]
- 59.Chakraborty K., Paulraj R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010;122:31–41. doi: 10.1016/j.foodchem.2010.02.012. [DOI] [Google Scholar]
- 60.Meenakshi S., Gnanambigai D.M., Mozhi S.T., Arumugam M., Balasubramanian T. Total flavanoid and in vitro antioxidant activity of two seaweeds of Rameshwaram coast. Glob. J. Pharm. 2009;3:59–62. [Google Scholar]
- 61.Balaji Raghavendra Rao H., Sathivel A., Devaki T. Antihepatotoxic nature of Ulva reticulata (Chlorophyceae) on acetaminophen-induced hepatoxicity in experimental rats. J. Med. Food. 2004;7:495–497. doi: 10.1089/jmf.2004.7.495. [DOI] [PubMed] [Google Scholar]
- 62.Shibata T., Ishimaru K., Kawaguchi S., Yoshikawa H., Hama Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In: Borowitzka M.A., Critchley A.T., Kraan S., Peters A., Sjøtun K., Notoya M., editors. Proceedings of the Nineteenth International Seaweed Symposium; Kobe, Japan. 26–31 March 2007; Amsterdam, The Netherlands: Springer; 2009. pp. 255–261. [Google Scholar]
- 63.Kang K.A., Bu H.D., Park D.S., Go G.M., Jee Y., Shin T., Hyun J.W. Antioxidant activity of ethanol extract of Callophyllis japonica. Phytother. Res. 2005;19:506–510. doi: 10.1002/ptr.1692. [DOI] [PubMed] [Google Scholar]
- 64.Yang J.-I., Yeh C.-C., Lee J.-C., Yi S.-C., Huang H.-W., Tseng C.-N., Chang H.-W. Aqueous extracts of the edible Gracilaria tenuistipitata are protective against H2O2-induced DNA damage, growth inhibition, and cell cycle arrest. Molecules. 2012;17:7241–7254. doi: 10.3390/molecules17067241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Avendaño C., Menendez J.C. Medicinal Chemistry of Anticancer Drugs. Elsevier; Amsterdam, The Netherlands: 2015. [Google Scholar]
- 66.Kalimuthu S., Se-Kwon K. Cell survival and apoptosis signaling as therapeutic target for cancer: Marine bioactive compounds. Int. J. Mol. Sci. 2013;14:2334–2354. doi: 10.3390/ijms14022334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pradhan B., Patra S., Behera C., Nayak R., Patil S., Bhutia S.K., Jena M. Enteromorpha compressa extract induces anticancer activity through apoptosis and autophagy in oral cancer. Mol. Biol. Rep. 2020 doi: 10.1007/s11033-020-06010-4. [DOI] [PubMed] [Google Scholar]
- 68.Talero E., García-Mauriño S., Ávila-Román J., Rodríguez-Luna A., Alcaide A., Motilva V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs. 2015;13:6152–6209. doi: 10.3390/md13106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dore C.M.P.G., Alves M.G.C.F., Santos N.D., Cruz A.K.M., Câmara R.B.G., Castro A.J.G., Alves L.G., Nader H.B., Leite E.L. Antiangiogenic activity and direct antitumor effect from a sulfated polysaccharide isolated from seaweed. Microvasc. Res. 2013;88:12–18. doi: 10.1016/j.mvr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Kang Y., Wang Z.-J., Xie D., Sun X., Yang W., Zhao X., Xu N. Characterization and potential antitumor activity of polysaccharide from Gracilariopsis lemaneiformis. Mar. Drugs. 2017;15:100. doi: 10.3390/md15040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ropellato J., Carvalho M.M., Ferreira L.G., Noseda M.D., Zuconelli C.R., Gonçalves A.G., Ducatti D.R., Kenski J.C., Nasato P.L., Winnischofer S.M. Sulfated heterorhamnans from the green seaweed Gayralia oxysperma: Partial depolymerization, chemical structure and antitumor activity. Carbohydr. Polym. 2015;117:476–485. doi: 10.1016/j.carbpol.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 72.Synytsya A., Kim W.-J., Kim S.-M., Pohl R., Synytsya A., Kvasnička F., Čopíková J., Park Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010;81:41–48. doi: 10.1016/j.carbpol.2010.01.052. [DOI] [Google Scholar]
- 73.Rui X., Pan H.-F., Shao S.-L., Xu X.-M. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: Possible JAK-STAT3 pathway. BMC Complement. Altern. Med. 2017;17:378. doi: 10.1186/s12906-017-1885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park H.Y., Park S.-H., Jeong J.-W., Yoon D., Han M.H., Lee D.-S., Choi G., Yim M.-J., Lee J.M., Kim D.-H. Induction of p53-independent apoptosis and G1 cell cycle arrest by fucoidan in HCT116 human colorectal carcinoma cells. Mar. Drugs. 2017;15:154. doi: 10.3390/md15060154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suganya A.M., Sanjivkumar M., Chandran M.N., Palavesam A., Immanuel G. Pharmacological importance of sulphated polysaccharide carrageenan from red seaweed Kappaphycus alvarezii in comparison with commercial carrageenan. Biomed. Pharmacother. 2016;84:1300–1312. doi: 10.1016/j.biopha.2016.10.067. [DOI] [PubMed] [Google Scholar]
- 76.Kong C.-S., Kim J.-A., Yoon N.-Y., Kim S.-K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009;47:1653–1658. doi: 10.1016/j.fct.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 77.Corona G., Coman M., Spencer J., Rowland I. Digested and fermented seaweed phlorotannins reduce DNA damage and inhibit growth of HT-29 colon cancer cells. Proc. Nutr. Soc. 2014;73:E31. doi: 10.1017/S0029665114000457. [DOI] [Google Scholar]
- 78.Antunes E.M., Afolayan A.F., Chiwakata M.T., Fakee J., Knott M.G., Whibley C.E., Hendricks D.T., Bolton J.J., Beukes D.R. Identification and in vitro anti-esophageal cancer activity of a series of halogenated monoterpenes isolated from the South African seaweeds Plocamium suhrii and Plocamium cornutum. Phytochemistry. 2011;72:769–772. doi: 10.1016/j.phytochem.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Hosokawa M., Kudo M., Maeda H., Kohno H., Tanaka T., Miyashita K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2004;1675:113–119. doi: 10.1016/j.bbagen.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 80.Ishikawa C., Tafuku S., Kadekaru T., Sawada S., Tomita M., Okudaira T., Nakazato T., Toda T., Uchihara J.N., Taira N. Antiadult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int. J. Cancer. 2008;123:2702–2712. doi: 10.1002/ijc.23860. [DOI] [PubMed] [Google Scholar]
- 81.Terasaki M., Maeda H., Miyashita K., Tanaka T., Miyamoto S., Mutoh M. A marine bio-functional lipid, fucoxanthinol, attenuates human colorectal cancer stem-like cell tumorigenicity and sphere formation. J. Clin. Biochem. Nutr. 2017:16–112. doi: 10.3164/jcbn.16-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lakshmi T.P., Vajravijayan S., Moumita M., Sakthivel N., Gunasekaran K., Krishna R. A novel guaiane sesquiterpene derivative, guai-2-en-10α-ol, from Ulva fasciata Delile inhibits EGFR/PI3K/Akt signaling and induces cytotoxicity in triple-negative breast cancer cells. Mol. Cell. Biochem. 2018;438:123–139. doi: 10.1007/s11010-017-3119-5. [DOI] [PubMed] [Google Scholar]
- 83.Lee J.-C., Hou M.-F., Huang H.-W., Chang F.-R., Yeh C.-C., Tang J.-Y., Chang H.-W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013;13:1–7. doi: 10.1186/1475-2867-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zandi K., Tajbakhsh S., Nabipour I., Rastian Z., Yousefi F., Sharafian S., Sartavi K. In vitro antitumor activity of Gracilaria corticata (a red alga) against Jurkat and molt-4 human cancer cell lines. Afr. J. Biotechnol. 2010;9:6787–6790. [Google Scholar]
- 85.Zandi K., Ahmadzadeh S., Tajbakhsh S., Rastian Z., Yousefi F., Farshadpour F., Sartavi K. Anticancer activity of Sargassum oligocystum water extract against human cancer cell lines. Eur. Rev. Med Pharmacol. Sci. 2010;14:669–673. [PubMed] [Google Scholar]
- 86.Yeh C.-C., Tseng C.-N., Yang J.-I., Huang H.-W., Fang Y., Tang J.-Y., Chang F.-R., Chang H.-W. Antiproliferation and induction of apoptosis in Ca9-22 oral cancer cells by ethanolic extract of Gracilaria tenuistipitata. Molecules. 2012;17:10916–10927. doi: 10.3390/molecules170910916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeh C.-C., Yang J.-I., Lee J.-C., Tseng C.-N., Chan Y.-C., Hseu Y.-C., Tang J.-Y., Chuang L.-Y., Huang H.-W., Chang F.-R. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement. Altern. Med. 2012;12:142. doi: 10.1186/1472-6882-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J.-Y., Yoon M.-Y., Cha M.-R., Hwang J.-H., Park E., Choi S.-U., Park H.-R., Hwang Y.-I. Methanolic extracts of Plocamium telfairiae induce cytotoxicity and caspase-dependent apoptosis in HT-29 human colon carcinoma cells. J. Med. Food. 2007;10:587–593. doi: 10.1089/jmf.2007.002. [DOI] [PubMed] [Google Scholar]
- 89.Namvar F., Mohamed S., Fard S.G., Behravan J., Mustapha N.M., Alitheen N.B.M., Othman F. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012;130:376–382. doi: 10.1016/j.foodchem.2011.07.054. [DOI] [Google Scholar]
- 90.Kim K.-N., Ahn G., Heo S.-J., Kang S.-M., Kang M.-C., Yang H.-M., Kim D., Roh S.W., Kim S.-K., Jeon B.-T. Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Environ. Toxicol. Pharmacol. 2013;35:39–46. doi: 10.1016/j.etap.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Das S.K., Hashimoto T., Shimizu K., Yoshida T., Sakai T., Sowa Y., Komoto A., Kanazawa K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta (BBA) Gen. Subj. 2005;1726:328–335. doi: 10.1016/j.bbagen.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Satomi Y. Fucoxanthin induces GADD45A expression and G1 arrest with SAPK/JNK activation in LNCap human prostate cancer cells. Anticancer Res. 2012;32:807–813. [PubMed] [Google Scholar]
- 93.Cho M., Park G.-M., Kim S.-N., Amna T., Lee S., Shin W.-S. Glioblastoma-specific anticancer activity of pheophorbide a from the edible red seaweed Grateloupia elliptica. J. Microbiol. Biotechnol. 2014;24:346–353. doi: 10.4014/jmb.1308.08090. [DOI] [PubMed] [Google Scholar]
- 94.Koyanagi S., Tanigawa N., Nakagawa H., Soeda S., Shimeno H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003;65:173–179. doi: 10.1016/S0006-2952(02)01478-8. [DOI] [PubMed] [Google Scholar]
- 95.Sadeeshkumar V., Duraikannu A., Ravichandran S., Kodisundaram P., Fredrick W.S., Gobalakrishnan R. Modulatory efficacy of dieckol on xenobiotic-metabolizing enzymes, cell proliferation, apoptosis, invasion and angiogenesis during NDEA-induced rat hepatocarcinogenesis. Mol. Cell. Biochem. 2017;433:195–204. doi: 10.1007/s11010-017-3027-8. [DOI] [PubMed] [Google Scholar]
- 96.Xue M., Ji X., Xue C., Liang H., Ge Y., He X., Zhang L., Bian K., Zhang L. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro. Biomed. Pharmacother. 2017;94:898–908. doi: 10.1016/j.biopha.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 97.Abd-Ellatef G.-E.F., Ahmed O.M., Abdel-Reheim E.S., Abdel-Hamid A.-H.Z. Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer Targets Ther. 2017;9:67. doi: 10.2147/BCTT.S125165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lins K.O., Bezerra D.P., Alves A.P.N., Alencar N.M., Lima M.W., Torres V.M., Farias W.R., Pessoa C., de Moraes M.O., Costa-Lotufo L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer) J. Appl. Toxicol. 2009;29:20–26. doi: 10.1002/jat.1374. [DOI] [PubMed] [Google Scholar]
- 99.Wang Z.-J., Xu W., Liang J.-W., Wang C.-S., Kang Y. Effect of fucoidan on B16 murine melanoma cell melanin formation and apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017;14:149–155. doi: 10.21010/ajtcam.v14i4.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang Y., Li H., Wu J., Xu X., Sun X., Zhao X., Xu N. Transcriptome profiling reveals the antitumor mechanism of polysaccharide from marine algae Gracilariopsis lemaneiformis. PLoS ONE. 2016;11:e0158279. doi: 10.1371/journal.pone.0158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teruya T., Konishi T., Uechi S., Tamaki H., Tako M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladosiphon okamuranus TOKIDA in U937 cells. Int. J. Biol. Macromol. 2007;41:221–226. doi: 10.1016/j.ijbiomac.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 102.Kim E.J., Park S.Y., Lee J.-Y., Park J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010;10:96. doi: 10.1186/1471-230X-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim I.H., Kwon M.J., Nam T.J. Differences in cell death and cell cycle following fucoidan treatment in high-density HT-29 colon cancer cells. Mol. Med. Rep. 2017;15:4116–4122. doi: 10.3892/mmr.2017.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park H.-K., Kim I.-H., Kim J., Nam T.-J. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int. J. Mol. Med. 2013;32:291–295. doi: 10.3892/ijmm.2013.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jung H.A., Jung H.J., Jeong H.Y., Kwon H.J., Ali M.Y., Choi J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia. 2014;92:260–269. doi: 10.1016/j.fitote.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 106.O’sullivan A., O’callaghan Y., O’grady M., Queguineur B., Hanniffy D., Troy D., Kerry J., O’brien N. In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chem. 2011;126:1064–1070. doi: 10.1016/j.foodchem.2010.11.127. [DOI] [Google Scholar]
- 107.Ermakova S., Sokolova R., Kim S.-M., Um B.-H., Isakov V., Zvyagintseva T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011;164:841–850. doi: 10.1007/s12010-011-9178-2. [DOI] [PubMed] [Google Scholar]
- 108.Abad M.J., Bedoya L.M., Bermejo P. Natural marine anti-inflammatory products. Mini Rev. Med. Chem. 2008;8:740–754. doi: 10.2174/138955708784912148. [DOI] [PubMed] [Google Scholar]
- 109.D’Orazio N., Gammone M.A., Gemello E., De Girolamo M., Cusenza S., Riccioni G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar. Drugs. 2012;10:812–833. doi: 10.3390/md10040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaves L.d.S., Nicolau L.A.D., Silva R.O., Barros F.C.N., Freitas A.L.P., Aragão K.S., Ribeiro R.d.A., Souza M.H.L.P., Barbosa A.L.d.R., Medeiros J.-V.R. Antiinflammatory and antinociceptive effects in mice of a sulfated polysaccharide fraction extracted from the marine red algae Gracilaria caudata. Immunopharmacol. Immunotoxicol. 2013;35:93–100. doi: 10.3109/08923973.2012.707211. [DOI] [PubMed] [Google Scholar]
- 111.Ananthi S., Raghavendran H.R.B., Sunil A.G., Gayathri V., Ramakrishnan G., Vasanthi H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga) Food Chem. Toxicol. 2010;48:187–192. doi: 10.1016/j.fct.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 112.Ku C.S., Pham T.X., Park Y., Kim B., Shin M.S., Kang I., Lee J. Edible blue-green algae reduce the production of pro-inflammatory cytokines by inhibiting NF-κB pathway in macrophages and splenocytes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013;1830:2981–2988. doi: 10.1016/j.bbagen.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Romay C., Armesto J., Remirez D., Gonzalez R., Ledon N., Garcia I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998;47:36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- 114.Shih C.-M., Cheng S.-N., Wong C.-S., Kuo Y.-L., Chou T.-C. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth. Analg. 2009;108:1303–1310. doi: 10.1213/ane.0b013e318193e919. [DOI] [PubMed] [Google Scholar]
- 115.Jin D.-Q., Lim C.S., Sung J.-Y., Choi H.G., Ha I., Han J.-S. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci. Lett. 2006;402:154–158. doi: 10.1016/j.neulet.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 116.Renju G., Muraleedhara Kurup G., Saritha Kumari C. Anti-inflammatory activity of lycopene isolated from Chlorella marina on Type II Collagen induced arthritis in Sprague Dawley rats. Immunopharmacol. Immunotoxicol. 2013;35:282–291. doi: 10.3109/08923973.2012.742534. [DOI] [PubMed] [Google Scholar]
- 117.Caroprese M., Albenzio M., Ciliberti M.G., Francavilla M., Sevi A. A mixture of phytosterols from Dunaliella tertiolecta affects proliferation of peripheral blood mononuclear cells and cytokine production in sheep. Vet. Immunol. Immunopathol. 2012;150:27–35. doi: 10.1016/j.vetimm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 118.Shin E.-S., Hwang H.-J., Kim I.-H., Nam T.-J. A glycoprotein from Porphyra yezoensis produces anti-inflammatory effects in liposaccharide-stimulated macrophages via the TLR4 signaling pathway. Int. J. Mol. Med. 2011;28:809–815. doi: 10.3892/ijmm.2011.729. [DOI] [PubMed] [Google Scholar]
- 119.Kim M.M., Rajapakse N., Kim S.K. Anti-inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF-κB transcription factor in RAW 264.7 cells. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009;23:628–634. doi: 10.1002/ptr.2674. [DOI] [PubMed] [Google Scholar]
- 120.Kim S.H., Lim J.W., Kim H. Astaxanthin inhibits mitochondrial dysfunction and interleukin-8 expression in Helicobacter pylori-infected gastric epithelial cells. Nutrients. 2018;10:1320. doi: 10.3390/nu10091320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dore C.M.P.G., Alves M.G.d.C.F., Will L.S.E.P., Costa T.G., Sabry D.A., de Souza Rêgo L.A.R., Accardo C.M., Rocha H.A.O., Filgueira L.G.A., Leite E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013;91:467–475. doi: 10.1016/j.carbpol.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 122.Cavalcante-Silva L.H.A., Barbosa Brito da Matta C., De Araújo M.V., Barbosa-Filho J.M., Pereira de Lira D., de Oliveira Santos B.V., De Miranda G.E.C., Alexandre-Moreira M.S. Antinociceptive and anti-inflammatory activities of crude methanolic extract of red alga Bryothamnion triquetrum. Mar. Drugs. 2012;10:1977–1992. doi: 10.3390/md10091977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee H.-J., Kang G.-J., Yang E.-J., Park S.-S., Yoon W.-J., Jung J.H., Kang H.-K., Yoo E.-S. Two enone fatty acids isolated from Gracilaria verrucosa suppress the production of inflammatory mediators by down-regulating NF-κB and STAT1 activity in lipopolysaccharide-stimulated Raw 264.7 cells. Arch. Pharmacal Res. 2009;32:453–462. doi: 10.1007/s12272-009-1320-0. [DOI] [PubMed] [Google Scholar]
- 124.Bergé J., Debiton E., Dumay J., Durand P., Barthomeuf C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J. Agric. Food Chem. 2002;50:6227–6232. doi: 10.1021/jf020290y. [DOI] [PubMed] [Google Scholar]
- 125.Song W., Wang Z., Zhang X., Li Y. Ethanol extract from Ulva prolifera prevents high-fat diet-induced insulin resistance, oxidative stress, and inflammation response in mice. Biomed Res. Int. 2018;2018:1374565. doi: 10.1155/2018/1374565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pangestuti R., Kim S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods. 2011;3:255–266. doi: 10.1016/j.jff.2011.07.001. [DOI] [Google Scholar]
- 127.Yoon W.-J., Heo S.-J., Han S.-C., Lee H.-J., Kang G.-J., Kang H.-K., Hyun J.-W., Koh Y.-S., Yoo E.-S. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch. Pharmacal Res. 2012;35:1421–1430. doi: 10.1007/s12272-012-0812-5. [DOI] [PubMed] [Google Scholar]
- 128.Kim M.-M., Kim S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010;48:2925–2933. doi: 10.1016/j.fct.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 129.Lim C.S., Jin D.-Q., Sung J.-Y., Lee J.H., Choi H.G., Ha I., Han J.-S. Antioxidant and anti-inflammatory activities of the methanolic extract of Neorhodomela aculeate in hippocampal and microglial cells. Biol. Pharm. Bull. 2006;29:1212–1216. doi: 10.1248/bpb.29.1212. [DOI] [PubMed] [Google Scholar]
- 130.Riccardi G., Rivellese A.A. Effects of dietary fiber and carbohydrate on glucose and lipoprotein metabolism in diabetic patients. Diabetes Care. 1991;14:1115–1125. doi: 10.2337/diacare.14.12.1115. [DOI] [PubMed] [Google Scholar]
- 131.Gupta P., Bala M., Gupta S., Dua A., Dabur R., Injeti E., Mittal A. Efficacy and risk profile of anti-diabetic therapies: Conventional vs traditional drugs—A mechanistic revisit to understand their mode of action. Pharmacol. Res. 2016;113:636–674. doi: 10.1016/j.phrs.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 132.Unnikrishnan P.S., Jayasri M.A. Marine algae as a prospective source for antidiabetic compounds–a brief review. Curr. Diabetes Rev. 2018;14:237–245. doi: 10.2174/1573399812666161229151407. [DOI] [PubMed] [Google Scholar]
- 133.Cheng Y., Sibusiso L., Hou L., Jiang H., Chen P., Zhang X., Wu M., Tong H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019;131:1162–1170. doi: 10.1016/j.ijbiomac.2019.04.040. [DOI] [PubMed] [Google Scholar]
- 134.Daub C.D., Mabate B., Malgas S., Pletschke B.I. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. Macromol. 2020;151:412–420. doi: 10.1016/j.ijbiomac.2020.02.161. [DOI] [PubMed] [Google Scholar]
- 135.Kim K.-T., Rioux L.-E., Turgeon S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. 2014;98:27–33. doi: 10.1016/j.phytochem.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 136.Wang D., Zhao X., Liu Y. Hypoglycemic and hypolipidemic effects of a polysaccharide from flower buds of Lonicera japonica in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2017;102:396–404. doi: 10.1016/j.ijbiomac.2017.04.056. [DOI] [PubMed] [Google Scholar]
- 137.Kim K.-J., Yoon K.-Y., Lee B.-Y. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+ db and C57BL/KSJ db/db mice. Fitoterapia. 2012;83:1105–1109. doi: 10.1016/j.fitote.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 138.Kumar T.V., Lakshmanasenthil S., Geetharamani D., Marudhupandi T., Suja G., Suganya P. Fucoidan–A α-d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Macromol. 2015;72:1044–1047. doi: 10.1016/j.ijbiomac.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 139.Moon H.E., Islam M.N., Ahn B.R., Chowdhury S.S., Sohn H.S., Jung H.A., Choi J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Biosci. Biotechnol. Biochem. 2011;75:1472–1480. doi: 10.1271/bbb.110137. [DOI] [PubMed] [Google Scholar]
- 140.Lee S.H., Karadeniz F., Kim M.M., Kim S.K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 2009;89:1552–1558. doi: 10.1002/jsfa.3623. [DOI] [Google Scholar]
- 141.Kang C., Jin Y.B., Lee H., Cha M., Sohn E.-T., Moon J., Park C., Chun S., Jung E.-S., Hong J.-S. Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food Chem. Toxicol. 2010;48:509–516. doi: 10.1016/j.fct.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 142.Lee S.-H., Min K.-H., Han J.-S., Lee D.-H., Park D.-B., Jung W.-K., Park P.-J., Jeon B.-T., Kim S.-K., Jeon Y.-J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012;50:575–582. doi: 10.1016/j.fct.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 143.Min K.-H., Kim H.-J., Jeon Y.-J., Han J.-S. Ishige okamurae ameliorates hyperglycemia and insulin resistance in C57BL/KsJ-db/db mice. Diabetes Res. Clin. Pract. 2011;93:70–76. doi: 10.1016/j.diabres.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 144.Lee Y.S., Shin K.H., Kim B.-K., Lee S. Anti-Diabetic activities of fucosterol fromPelvetia siliquosa. Arch. Pharmacal Res. 2004;27:1120–1122. doi: 10.1007/BF02975115. [DOI] [PubMed] [Google Scholar]
- 145.Nwosu F., Morris J., Lund V.A., Stewart D., Ross H.A., McDougall G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011;126:1006–1012. doi: 10.1016/j.foodchem.2010.11.111. [DOI] [Google Scholar]
- 146.Kimura Y., Watanabe K., Okuda H. Effects of soluble sodium alginate on cholesterol excretion and glucose tolerance in rats. J. Ethnopharmacol. 1996;54:47–54. doi: 10.1016/0378-8741(96)01449-3. [DOI] [PubMed] [Google Scholar]
- 147.Jung H.A., Yoon N.Y., Woo M.-H., Choi J.S. Inhibitory activities of extracts from several kinds of seaweeds and phlorotannins from the brown alga Ecklonia stolonifera on glucose-mediated protein damage and rat lens aldose reductase. Fish. Sci. 2008;74:1363–1365. doi: 10.1111/j.1444-2906.2008.01670.x. [DOI] [Google Scholar]
- 148.Peng J., Yuan J.-P., Wu C.-F., Wang J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs. 2011;9:1806–1828. doi: 10.3390/md9101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jung H.A., Islam M.N., Lee C.M., Oh S.H., Lee S., Jung J.H., Choi J.S. Kinetics and molecular docking studies of an anti-diabetic complication inhibitor fucosterol from edible brown algae Eisenia bicyclis and Ecklonia stolonifera. Chem. Biol. Interact. 2013;206:55–62. doi: 10.1016/j.cbi.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 150.Shi D., Guo S., Jiang B., Guo C., Wang T., Zhang L., Li J. HPN, a synthetic analogue of bromophenol from red alga Rhodomela confervoides: Synthesis and anti-diabetic effects in C57BL/KsJ-db/db mice. Mar. Drugs. 2013;11:350–362. doi: 10.3390/md11020350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yamazaki H., Nakazawa T., Sumilat D.A., Takahashi O., Ukai K., Takahashi S., Namikoshi M. Euryspongins A–C, three new unique sesquiterpenes from a marine sponge Euryspongia sp. Bioorg. Med. Chem. Lett. 2013;23:2151–2154. doi: 10.1016/j.bmcl.2013.01.102. [DOI] [PubMed] [Google Scholar]
- 152.Nuño K., Villarruel-López A., Puebla-Pérez A., Romero-Velarde E., Puebla-Mora A., Ascencio F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J. Funct. Foods. 2013;5:106–115. doi: 10.1016/j.jff.2012.08.011. [DOI] [Google Scholar]
- 153.Lee S.-H., Jeon Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia. 2013;86:129–136. doi: 10.1016/j.fitote.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 154.Guan J., Cui Z., Lee D., Lee Y., Park D. Effect of dieckol from Ecklonia cava on glucose transport in L6 muscle cells. Planta Med. 2011;77:PH3. doi: 10.1055/s-0031-1282585. [DOI] [Google Scholar]
- 155.Lee S.-H., Park M.-H., Heo S.-J., Kang S.-M., Ko S.-C., Han J.-S., Jeon Y.-J. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2010;48:2633–2637. doi: 10.1016/j.fct.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 156.Heo S.-J., Hwang J.-Y., Choi J.-I., Han J.-S., Kim H.-J., Jeon Y.-J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009;615:252–256. doi: 10.1016/j.ejphar.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 157.Celikler S., Tas S., Vatan O., Ziyanok-Ayvalik S., Yildiz G., Bilaloglu R. Anti-hyperglycemic and antigenotoxic potential of Ulva rigida ethanolic extract in the experimental diabetes mellitus. Food Chem. Toxicol. 2009;47:1837–1840. doi: 10.1016/j.fct.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 158.Sun Z., Peng X., Liu J., Fan K.-W., Wang M., Chen F. Inhibitory effects of microalgal extracts on the formation of advanced glycation endproducts (AGEs) Food Chem. 2010;120:261–267. doi: 10.1016/j.foodchem.2009.10.018. [DOI] [Google Scholar]
- 159.Abdelsalam S.S., Korashy H.M., Zeidan A., Agouni A. The role of protein tyrosine phosphatase (PTP)-1B in cardiovascular disease and its interplay with insulin resistance. Biomolecules. 2019;9:286. doi: 10.3390/biom9070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee J.Y., Kim S.M., Jung W.-S., Song D.-G., Um B.-H., Son J.-K., Pan C.-H. Phlorofucofuroeckol-A, a potent inhibitor of aldo-keto reductase family 1 member B10, from the edible brown alga Eisenia bicyclis. J. Korean Soc. Appl. Biol. Chem. 2012;55:721–727. doi: 10.1007/s13765-012-2169-3. [DOI] [Google Scholar]
- 161.Gunathilaka M., Ranasinghe P., Samarakoon K., Peiris L. In-vitro anti-diabetic activity of polyphenole-rich extract from marine brown algae Choonospora minima (Hering 1841); Proceedings of the 12th International Conference of KDU; General Sri John Kotelawala University, Kandawala, Sri Lanka. 11–12 September 2019; p. 185. [Google Scholar]
- 162.Son Y.K., Jin S.E., Kim H.-R., Woo H.C., Jung H.A., Choi J.S. Inhibitory activities of the edible brown alga Laminaria japonica on glucose-mediated protein damage and rat lens aldose reductase. Fish. Sci. 2011;77:1069–1079. doi: 10.1007/s12562-011-0406-z. [DOI] [Google Scholar]
- 163.Yang H.-W., Fernando K., Oh J.-Y., Li X., Jeon Y.-J., Ryu B. Anti-obesity and anti-diabetic effects of Ishige okamurae. Mar. Drugs. 2019;17:202. doi: 10.3390/md17040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ali M., Kim D.H., Seong S.H., Kim H.-R., Jung H.A., Choi J.S. α-Glucosidase and protein tyrosine phosphatase 1B inhibitory activity of plastoquinones from marine brown alga Sargassum serratifolium. Mar. Drugs. 2017;15:368. doi: 10.3390/md15120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lopez-Santamarina A., Miranda J.M. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules. 2020;25:1004. doi: 10.3390/molecules25041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim M.H., Kang S.G., Park J.H., Yanagisawa M., Kim C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406.e1-10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 167.Cavaglieri C.R., Nishiyama A., Fernandes L.C., Curi R., Miles E.A., Calder P.C. Differential effects of short-chain fatty acids on proliferation and production of pro-and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–1690. doi: 10.1016/S0024-3205(03)00490-9. [DOI] [PubMed] [Google Scholar]
- 168.Sun Y., Liu Y., Ai C., Song S., Chen X. Caulerpa lentillifera polysaccharides enhance the immunostimulatory activity in immunosuppressed mice in correlation with modulating gut microbiota. Food Funct. 2019;10:4315–4329. doi: 10.1039/C9FO00713J. [DOI] [PubMed] [Google Scholar]
- 169.Zhang W., Oda T., Yu Q., Jin J.-O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs. 2015;13:1084–1104. doi: 10.3390/md13031084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ale M.T., Maruyama H., Tamauchi H., Mikkelsen J.D., Meyer A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011;49:331–336. doi: 10.1016/j.ijbiomac.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 171.Jin J.-O., Zhang W., Du J.-Y., Wong K.-W., Oda T., Yu Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE. 2014;9:e99396. doi: 10.1371/journal.pone.0099396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Negishi H., Mori M., Mori H., Yamori Y. Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J. Nutr. 2013;143:1794–1798. doi: 10.3945/jn.113.179036. [DOI] [PMC free article] [PubMed] [Google Scholar]