Abstract
The regeneration of the muscles of the rotator cuff represents a grand challenge in musculoskeletal regenerative engineering. Several types of matrices have been proposed for skeletal muscle regeneration. However, biomimetic matrices to promote muscle regeneration and mimic native muscle tissue have not been successfully engineered. Besides topographical cues, an electrical stimulus may serve as a critical cue to improve interactions between materials and cells in scenarios fostering muscle regeneration. In this in vitro study, we engineered a novel stimuli-responsive conductive nanocomposite matrix, and studied its ability to regulate muscle cell adhesion, proliferation, and differentiation. Electroconductive nanocomposite matrices demonstrated tunable conductivity and biocompatibility. Under the optimum concentration of conductive material, the matrices facilitated muscle cell adhesion, proliferation, and differentiation. Importantly, conductive aligned fibrous matrices were effective in promoting myoblast differentiation by upregulation of myogenic markers. The results demonstrated promising potential of aligned conductive fibrous matrices for skeletal muscle regenerative engineering.
Keywords: Muscle regeneration, Nanofibrous matrices, Conductive material, Electrospinning
Lay summary
Around 40% of the human body’s mass consists of skeletal muscle. Musculoskeletal disorders such as muscle atrophy and fatty infiltration after rotator cuff injury lead to disability and pain and increase the rate of retear after rotator cuff surgery. The study showed the potential of novel engineered matrix to regenerate skeletal muscle by utilizing conductive material and nanofiber-based matrices. The incorporation of conductive material and aligned nanofibers as electrical and topographical cues significantly impacted cell viability and differentiation to support muscle regeneration.
Introduction
Skeletal muscle comprises 40% to 45% of adult body mass (1). These muscles are responsible for generating forces which facilitate voluntary movement and locomotion (2). Despite the highly regenerative nature of most skeletal tissue when minor injury occurs, chronic diseases and sports-related traumatic injuries of skeletal muscle are challenging to treat in primary care and sports medicine (3, 4). In addition, some muscles, such as those of the rotator cuff, have been found to be a challenge to regenerate (5). Currently, the standard approach being explored for volumetric muscle loss is to transfer and engraft healthy, vascularized, innervated autologous tissue (2). However, the use of muscle autografts presents a host of morbidity issues and does not lead to a total recovery of pre-injury muscle strength and functionality (2). To overcome the limitations of muscle autografts, regenerative engineering approaches have been suggested. For instance, biomimetic matrices with structures similar to native muscle tissue may provide a promising alternative for muscle regeneration (2).
Skeletal muscle tissue consists of highly oriented myofibers which are formed by multinucleated myotubes derived from the fusion of mononucleated myoblasts (2, 4). Nanofibrous matrices are favorable constructs because of their adjustable porosity, relatively large specific area, and high similarity in structure to native extracellular matrices (ECMs) (6). Electrospinning is the most popular strategy to fabricate nanofibrous mats due to the ability to control the morphology, orientation, and physical properties of the fabricated nanofibers (7). The technique, first applied to tissue regeneration applications by Laurencin and his colleagues (8), has shown that unidirectionally oriented nanofibers may better induce muscle cell alignment and myotube formation than randomly oriented nanofibers (9, 10).
Electrical stimulation as a biophysics cue is an important potential approach for stimulating tissue repair and regeneration (11, 12). The use of conductive polymers as electrical cues has been investigated for neuron regeneration, biosensors, neural implants, drug delivery devices, and regenerative engineering matrices (11). Tissue regenerative matrices consist of conductive polymers such as polyaniline (6, 11), and polypyrrole (13) and can be used for localized electrical signals to seeded cells. For example, polypyrrole (PPy) has been incorporated in multiple films to support myoblast adhesion/proliferation in varying degrees (13). The polymer has also been coated on PLGA (poly (lactic-co-glycolic acid)) nanofiber matrices to promote longer neurites (12). Direct co-electrospinning of the conductive polymer polyaniline (PANI) with poly(L-lactide-co-ε-caprolactone) (PLCL) has also showed a positive effect in promoting myoblast differentiation (6, 14).
Poly (3,4-ethylenedioxythiophene) (PEDOT), polythiophene (PTh) derivative, has emerged in recent decades. Widely used due to its high conductivity in organic solar cells (15), PEDOT is a promising conductive polymer for biomedical application such as biosensing and bioengineering applications (16), electrically controlled drug release (17), nerve grafts, and heart muscle patches (18). It has captured attention owing to its good electrical, chemical and environmental stability along with better conductivity and thermal stability than conventionally studied polypyrrole (PPy) (11).
The surface properties of a matrix are important for myoblast formation as well as myogenic differentiation and maturation (19, 20). Before coating nanofiber matrices with conductive polymers, matrices can be modified with dopamine self-polymerization to change the surface properties of the matrix (21). Poly(dopamine) deposition onto electrospun poly(ε-caprolactone) PCL has been reported to significantly promoted rat osteoblast adhesion and proliferation by Tsai et al. (22).
In this study, we sought to develop an effective method to fabricate a novel, stimuli-responsive, conductive nanocomposite matrix, which can regulate muscle regeneration in vitro. Our preliminary results showed positive data that supported the concept that matrix biocompatibility could be achieved with the development of an electroconductive polymer (23). In addition, we demonstrated that our novel electroconductive nanocomposite matrices could regulate the cellular microenvironment to promote electrical communication between cells, and ultimately provide a robust and functional structure for muscle regeneration. The matrix was achieved by the combination of poly (3,4ethylenedioxythiophene): poly (styrenesulfonate) (PEDOT: PSS), with a dopamine-polymerized biodegradable substrate made from poly (ε-caprolactone) (PCL) nanofibers. Here, we engineered a novel stimuli-responsive conductive nanocomposite matrix, and studied its ability to regulate muscle cell adhesion, proliferation, and differentiation. The goal of study was to elucidate the effects of topographical and electrical cues on muscle cellular growth and differentiation.
Results and discussion
Characterization of nanocomposite matrices
-Morphology study
Diluted PEDOT:PSS dispersions in water showed uniform particle size (Fig. S1). The average particle size of PEDOT:PSS was 384.9 ± 3.8 nm. Different percentages of PEDOT:PSS dispersion were then coated on dopamine polymerized nanofibrous mats. Fig. 2-a shows SEM images of PEDOT:PSS/DOPA/PCL nanocomposite matrices. The topography of the pure PCL nanofiber matrix showed a smooth surface while dopamine polymerized surface (DOPA/PCL) shows some surface roughness without a change in the diameter of fibers. The distribution of PEDOT:PSS was relatively uniform.
Fig. 2. Characterization of nanocomposite matrices.
A) SEM images showing nanotopography preservation and PEDOT:PSS distribution (8000x) (i) Pure PCL matrices (ii) Dopamine polymerized PCL matrices (DOPA/PCL) (iii) 1% PEDOT: PSS coated on dopamine polymerized PCL (1% PEDOT:PSS/DOPA/PCL) (iv)10% PEDOT: PSS coated on dopamine polymerized PCL (10% PEDOT:PSS/DOPA/PCL) (v) 33% PEDOT:PSS coated on dopamine polymerized PCL (33% PEDOT:PSS/DOPA/PCL) (vi) 100% PEDOT:PSS coated on dopamine polymerized PCL (100% PEDOT:PSS/DOPA/PCL). B) Contact angle analysis for (i) Pure PCL (ii) Dopamine polymerized PCL. C) Sheet Resistance of different concentration of PEDOT:PSS coating.
-XPS analysis
The surface composition of fabricated matrices were characterized using XPS. Fig. S2-a showed XPS results for DOPA/PCL matrices. The presence of N on the surface of DOPA/PCL matrices at 400 eV confirmed that dopamine treatment on the PCL matrices was successful which was consistent with previous literature (24). Consistently with the literature, there was no N peak in the PCL samples (6, 25). Fig. S2-b indicates the presence of sulfur in PSS and in PEDOT of PEDOT:PSS/DOPA/PCL matrices. Based on the XPS results, PEDOT:PSS coating onto the surface of modified PCL matrices was successful.
-Hydrophilicity behavior
The literature indicates that poor myoblast formation is found on hydrophobic surfaces such as pure PCL matrices (26). Based on contact angle measurements, there was a significant difference between DOPA/PCL and PCL matrices (Fig.2-b). However, no significant differences were found between DOPA/PCL and PEDOT:PSS/DOPA/PCL matrices. This finding implies that the changes in cellular differentiation could be attributed to surface conductivity. Measurements showed an average contact angle ranging from 125.3° for untreated PCL to 0° for DOPA/PCL matrices. Based on the results, the dopamine coating was found to be effective in changing hydrophobicity while minimally affecting fiber morphology (fiber diameter was unchanged from SEM analysis). Also, the further addition of PEDOT: PSS on the dopamine-polymerized group still showed super hydrophilicity as demonstrated in Movie S1.
-Conductivity measurements
The electrical properties of the matrices were studied by measuring the sheet resistance of surfaces with a four-point probe method. Sheet resistance is inversely proportional to conductivity. We measured changes in surface electrical conductivity with respect to different concentrations of PEDOT:PSS (Fig.2-c), which was consistent with SEM images in Fig 2-a. Pure PCL matrices have no detectable electrical conductivity. PCL coated with different concentrations of PEDOT:PSS demonstrated a wide range of electrical conductivity. The average sheet resistance of the samples were 5× 104 Ω/sq, 13× 105 Ω/sq, and 17× 106 Ω/sq for 100% PEDOT:PSS/DOPA/PCL, 33% PEDOT:PSS/DOPA/PCL, and 10% PEDOT:PSS/DOPA/PCL, respectively. The 1% PEDOT:PSS/DOPA/PCL showed relatively higher resistivity. In light of this, the conductivity of the matrices can be tailored for various applications.
Cellular biocompatibility evaluation
-Myoblast biocompatibility
As indicated in Fig 3-a and Fig.S3, the vast majority of cells were viable, and all groups showed more than 90% cell viability on day 3, indicating mild or minimal cytotoxicity. For 1% and 10% groups, the cellular density was similar to the control groups but relatively higher than the 100% and 33% groups. This suggested that high concentrations of PEDOT:PSS may have impact on cell proliferation but exhibit little toxicity.
Fig.3. Biocompatibility characterization of conductive matrices.
A) Representative fluorescent images of C2C12 myoblasts cultured on different groups on day 3 and stained using a Live/ Dead Cell Viability kit (green, viable cells; red, dead cells), (i) Pure PCL matrices (ii) Dopamine polymerized PCL matrices (DOPA/PCL) (iii) 1% PEDOT: PSS coated on dopamine polymerized PCL (1% PEDOT:PSS/DOPA/PCL) (iv)10% PEDOT: PSS coated on dopamine polymerized PCL (10% PEDOT:PSS/DOPA/PCL) (v) 33% PEDOT:PSS coated on dopamine polymerized PCL (33% PEDOT:PSS/DOPA/PCL) (vi) 100% PEDOT:PSS coated on dopamine polymerized PCL (100% PEDOT:PSS/DOPA/PCL). B) proliferation study on day 3, day 7 and day 14, Mean ± SD; n = 3–5 samples per group, *P < 0.05 between different matrix groups C) SEM images of cell-seeded matrices on day 3, (i) Pure PCL matrices (ii) Dopamine polymerized PCL matrices (DOPA/PCL) (iii) 1% PEDOT: PSS coated on dopamine polymerized PCL (1% PEDOT:PSS/DOPA/PCL) (iv)10% PEDOT: PSS coated on dopamine polymerized PCL (10% PEDOT:PSS/DOPA/PCL) (v) 33% PEDOT:PSS coated on dopamine polymerized PCL (33% PEDOT:PSS/DOPA/PCL) (vi) 100% PEDOT:PSS coated on dopamine polymerized PCL (100% PEDOT:PSS/DOPA/PCL).
To evaluate cell proliferation, 80K C2C12 myoblast cells were seeded on the matrices for all groups. After 3,7, and 14 days no toxicity was shown among all treatment and control groups (Fig. 3-b). Higher concentrations of PEDOT:PSS (33% and 100%) inhibited cell proliferation during the first week; however, a stimulatory effect on myoblast growth was observed in the second week.
Moreover, low and medium concentrations of PEDOT:PSS (1% and 10%) showed stimulatory effects on myoblast growth during the first and second weeks. In general, it was observed that low and medium concentrations of PEDOT:PSS (1% and 10%) promoted a higher level of cell growth than control groups on day 14 (p<0.001).
SEM images (Fig. 3-C) of cell-seeded matrices showed excellent spreading of cells on the matrices, confirmingthe cytocompatibility of nanocomposite matrices.
-Myogenic differentiation
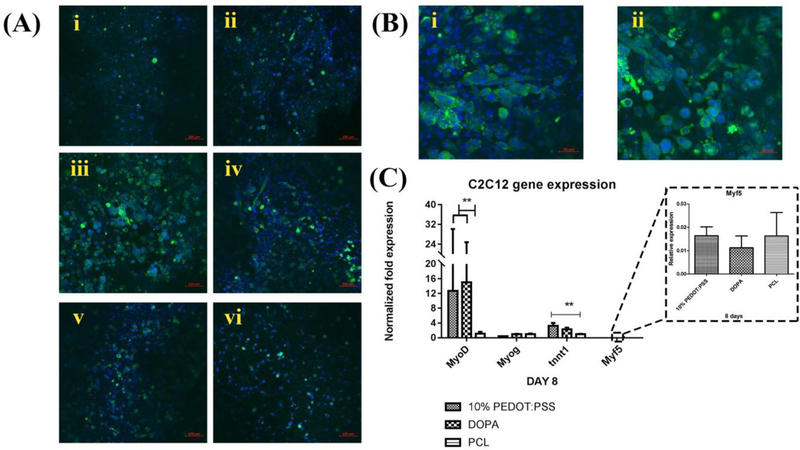
We investigated the effects of PEDOT:PSS/DOPA/PCL nanocomposite matrices on C2C12 myoblast myotube formation and expression of myogenic markers. Higher levels of myotube formation were observed for 1% and 10% concentrations of PEDOT:PSS. Our data indicated that under the optimum concentration of PEDOT:PSS, myoblasts expressed myosin heavy chain (MHC) (a marker for more mature skeletal muscle (27)) and fused into multinucleated myotubes. More multinucleated myotubes were observed on the electrically conductive nanocomposite matrices (Fig. 4-a and b).
Fig.4. Myogenic differentiation.
A) Representative fluorescent images of C2C12 cells cultured on day 14, (i)100% PEDOT: PSS/DOPA/PCL, (ii) 33% PEDOT: PSS/DOPA/PCL, (iii) 10% PEDOT: PSS/DOPA/PCL, (iv)1% PEDOT: PSS/DOPA/PCL, (v) DOPA/PCL, and (vi) PCL, sarcomeric-myosin heavy chain (green), nuclei (blue), scale bars indicate 100 μm. B) Representative images of C2C12 myotube formation on (i) 1% PEDOT: PSS/DOPA/PCL, (ii) 10% PEDOT: PSS/DOPA/PCL, scale bars indicate 50 μm. C) Impact of PEDOT: PSS on myogenic differentiation (gene expression) on day 8.
Our results demonstrated overall that myotube formation was significantly influenced by PEDOT: PSS coatings on day 14, as shown in Fig.4-a and b. We observed more myotube formation on 10% PEDOT: PSS/DOPA/PCL matrices. However, there was minimal myotube formation in the other experimental groups. The highest levels of myotube formation were found on 10% and 1% concentrations of PEDOT: PSS based on immunofluorescence images. However, the 1% concentration of PEDOT: PSS could not provide consistent conductivity. Therefore the 10% concentration of PEDOT: PSS was chosen for further study. To evaluate the differentiation of C2C12 cells to skeletal muscle quantitatively, RT-PCR analysis was performed (Fig.4-c). We found that troponin T, which is a muscle contraction marker, was over-expressed on the 10% PEDOT: PSS/DOPA/PCL matrices. In addition, 10% PEDOT: PSS/DOPA/PCL as well as the dopamine group (DOPA/PCL) expressed significantly higher MyoD than pure PCL matrices. There was no significant difference in the expression level of the Myf5 and Myog gene among the groups on day 8. However, other genes such as MyoD and troponin T, were over-expressed on the 10% PEDOT:PSS/DOPA/PCL matrices on day 8.
-Effect of topographical cue enhances myoblast differentiation
We further incorporated topographical cues into the conductive matrices to promote myoblast maturation. Aligned fiber matrices with fiber diameters in the same range as random fiber matrices were fabricated using the electrospinning technique (Fig. S3).
Fig.5-a shows F-actin and dapi staining results for the nanocomposite matrices on day 8. The results showed that conductive nanocomposite matrices supported cell proliferation, which was consistent with our previous observations. Alignment of nanofibers facilitated cellular alignment compared to random fiber matrices.
Fig.5. Effect of topographical on myoblast differentiation.
A) Representative images day 8 factin/dapi staining ofmyoblast cells (i) A/10% PEDOT: PSS/DOPA/PCL electrospun nanofibers (ii) R/10% PEDOT:PSS/DOPA/PCL electrospun nanofibers (iii) Pure A/PCL electrospun nanofibers (iv) Pure R/PCL electrospun nanofibers. B) Protein expression Tnnt1 D14 (random and alignment). C) Normalized protein expression Tnnt1 D14 (random and alignment).
To quantitatively assess the level of protein expression on different matrices, TNNT1 was measured on day 14. Similar protein expression levels were observed between the aligned and random DOPA/PCL groups as well as aligned and random PCL groups. Higher protein expression was observed for both aligned and random 10% PEDOT:PSS/DOPA/PCL groups (p<0.001) (Fig.5-b). Aligned 10% PEDOT:PSS/DOPA/PCL (A/10% PEDOT:PSS/DOPA/PCL) showed higher protein expression than randomly aligned 10% PEDOT:PSS/DOPA/PCL (R/10% PEDOT:PSS/DOPA/PCL) but no significant difference was detected.
Assessing the normalized data, a similar conclusion could be determined (Fig.5-c). The 10% PEDOT:PSS/DOPA/PCL matrix showed higher normalized protein expression but no significant difference was detected. This suggested a mild synergistic effect of topographical and electrical cues on the differentiation of myoblasts. By comparing the results of a single factor (electrical conductivity) with a dual factor (electrical conductivity and matrix alignment), electrical conductivity appeared to play a more important role in promoting myotube formation.
Observations of the length and width of the myotubes are commonly used to detect myogenic differentiation (14). Fig.6-a showed that conductive nanocomposite matrices (A/10% PEDOT:PSS/DOPA/PCL and R/10% PEDOT:PSS/DOPA/PCL) promoted more myotube formation than PCL nanofibers (A/PCL and R/PCL). In addition, the incorporation of nanofiber alignment of A/10% PEDOT:PSS/DOPA/PCL encouraged muscle cell alignment and also further enhanced myoblast differentiation compared with the R/10% PEDOT:PSS/DOPA/PCL. By counting myotube numbers, A/10% PEDOT:PSS/DOPA/PCL showed significantly higher (p<0.05) numbers of myotubes than R/10% PEDOT:PSS/DOPA/PCL (Fig.6-b). We observed a significant difference between A/10% PEDOT:PSS/DOPA/PCL and the other groups (p<0.005). As shown in Fig.6-b, A/10% PEDOT:PSS/DOPA/PCL showed an average length of 153.21 ± 56.56 μm, while R/10% PEDOT:PSS/DOPA/PCL, A/PCL, and R/PCL showed an average length of 95.97 ± 56.09 μm, 89.17± 56.09 μm, and 47.03 ± 20.86 μm, respectively. Based on our results, the conductive polymer was shown to promote higher amounts of myotube formation, but alignment helped the formation of longer and more mature myotubes. This indicated that matrices with both electrically conductive properties and alignment structure were effective in enhancing myotube formation.
Fig.6. Effect of topographical on myotube formation.
A) Representative images of MHC/PI staining of C2C12 cells on (i) A/10% PEDOT:PSS/DOPA/PCL (ii) R/10% PEDOT:PSS/DOPA/PCL (iii) A/PCL (iv) R/PCL. B) (i) Quantification of the myotube number and (ii) myotube length formed in (A) * p<0.05 significantly different.
Conclusion
We have successfully developed a novel method for the fabrication of electroconductive nanocomposite matrices. The changes in myoblast maturation can be attributed to differences in conductivity and topographies rather than surface wettability on the polymeric matrices. Low and medium concentrations (1% and 10%) of PEDOT: PSS coating were shown to induce myogenic gene expression. The results substantiate the important role of electrical conductivity in engineering functional and biomimetic skeletal muscle tissues.
Overall we found that the combination of electrically conductive materials and aligned electrospun nanofibers enhanced both myoblast differentiation and structural organization without the need for external electrical stimulation. These matrices could have an important therapeutic benefit in clinical settings where shoulder volumetric muscle loss, or atrophy as can occur with rotator cuff tears, are treated. Further investigations are needed to find the mechanism through which conductive materials influence cell behavior and what the functionality of this novel matrix may be in vivo.
Materials and Methods
Materials
Polycaprolactone, dopamine hydrochloride (average Mw 8000), poly (3,4-ethylenedioxythiophene)-polystyrene sulfonate (PEDOT: PSS 1:2.5) 1.3wt% dispersion in H2O, Ethanol, Methylene chloride, and Acetic acid were purchased from Sigma-Aldrich.
Fabrication of fibrous matrices
Aligned and random oriented nanofibrous matrices were fabricated using an electrospinning technique. Fig 1 shows the schematic illustration and fabrication process of cell seeded conductive fibrous matrix. We used optimized electrospinning parameters of 15 % (w/v) PCL solution in ethanol and methylene chloride (15:85 ratio) with a 2.5 mL/min flow rate, and 1 kV/cm-1 potential at ambient temperature and humidity to obtain bead-free nanofibers. To fabricate aligned structures, a rotating motor setup was optimized at 2300 rpm to produce aligned nanofibers. Following the electrospinning, dopamine (2mg/ml) was applied to the surface for 24 hours at pH 8.5 to polymerize onto the surface of the polycaprolactone (PCL) fibrous mats and formed a polydopamine coating (21). The modified DOPA/PCL surfaces were washed, dried, and subsequently coated with 30 μl of 1%, 10%, 33%, and 100% PEDOT: PSS solutions, where the 100% solution indicates a PEDOT: PSS 1.3wt% dispersion in H2O and 33%, 10%, 1% were further diluted with PBS. The dried matrices (1% PEDOT:PSS/DOPA/PCL, 10% PEDOT:PSS/DOPA/PCL, 33% PEDOT:PSS/DOPA/PCL, and 100% PEDOT:PSS/DOPA/PCL) were treated with mild 13.3% acetic acid, at pH 2 to enhance its conductivity. In this study, pure PCL served as control 1, and dopamine coated PCL (DOPA/PCL) served as control 2.
Fig. 1. Preparation of conductive nanocomposite matrices.
Schematic illustration of the preparation of conductive nanocomposite matrices for muscle regeneration.
Characterization of conductive matrices
-Scanning electron microscopy (SEM)
The fiber morphology and diameter of all groups were investigated by scanning electron microscopy (SEM) (FEI Nova NanoSEM 450). The fibrous mats were mounted onto the SEM sample studs and coated with gold using a sputter coater (Polaron E5100) for 3 minutes. The SEM images were analyzed with ImageJ software (National Institutes of Health, USA) to calculate average fiber diameters. The mean and standard deviation of the fiber diameters were calculated from 50 random fiber measurements per image.
-XPS spectroscopy
After fabrication, the scaffolds were carefully washed with distilled water three times and dried under vacuum. Chemical composition of pure PCL, DOPA/PCL, and PEDOT:PSS/DOPA/PCL matrices were then determined using X-ray photoelectron spectroscopy (XPS) (PHI 595 Multiprobe System, USA).
-Electrical conductivity
The conductivity of the different concentrations of conductive matrices (1 inch × 1 inch, n=3) was measured using a four-point probe technique using a Keithley 6000 picoammeter (Keithley; USA) at a voltage sweep of 1–10 V by applying a constant current of 2.5 mA between two alligator clips. The conductivity of the conductive nanocomposite matrices (n = 3) was calculated based on the below formula:
where σ is conductivity, 1 is the distance between reference electrodes, Rs is the bulk resistance of the matrix, and S is the cross-sectional area of the matrix (28).
-Surface Wettability:
The water contact angles of matrices were examined to study the surface wettability of the matrices in relation to surface coatings. A droplet of deionized water was added onto the dry matrices at room temperature and allowed to equilibrate for 10 s to determine the surface hydrophobicity of various matrices. To capture the images of the water droplet a charge-coupled device (CCD) camera was used, and the mean contact angles were calculated using ImageJ software analysis. The surface hydrophobicity of the nanocomposite matrices was determined by measurement of the contact angle of water using a contact angle and surface tension meter (KSV Instruments Ltd). The contact angle of each sample was taken as the average of three measurements at different points on the surface of the matrices.
In Vitro Tests
-Culture of murine skeletal muscle cells (C2C12)
A myoblast cell line (C2C12) (CRL-1772) (ATCC, USA) was used to study cell proliferation and differentiation on nanocomposite matrices. Cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (p/s) under standard culture conditions (37 °C, 5% CO2). For cell culture, circular matrices were punched out, approximately 1.9 cm2 and sterilized with 70% ethanol and UV for half an hour. Cells were seeded onto the different groups at a density of 100K per well or matrix and cultured with growth media for 3, 7, and 14 days. After 3 days, the media was replaced with differentiation media (DMEM supplemented with 2% horse serum and 1% p/s) and cultured for an additional 5 and 11 days to induce myotube formation.
-Live/dead cytotoxicity assay
Cytotoxicity of the 1% PEDOT:PSS/DOPA/PCL, 10% PEDOT:PSS/DOPA/PCL, 33% PEDOT:PSS/DOPA/PCL, and 100% PEDOT:PSS/DOPA/PCLDOPA/PCL and pure PCL matrices was determined with live/dead cytotoxicity assay (Thermo Fisher Scientific) on Day 3. matrices with cells seeded were incubated with ethidium homodimer-1 (4 μM; red fluorescence) and calcein-AM (2 μM; green fluorescence) in PBS for 10 min at 37°C in a 5% CO2 atmosphere incubator. Then, samples were immediately examined using confocal microscopy (ZEISS LSM 880, USA). The shown result is the mean values of three individual samples for each type of matrix.
-Cell proliferation:
Cell proliferation was determined by CellTiter 96® AQueous One solution [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS; Promega, USA] assay. 80 K cells were seeded on each of the matrices initially. The assays were performed after 3, 7, and 14 days of incubation according to the manufacturers’ instructions. The absorbance at 530nm/590 nm values were normalized by the background readings. A standard curve of cell number that corresponds to MTS activity was constructed for the specific myoblast cell line used in the current study.
-Immunohistochemistry:
Immunostaining with sarcomeric-MHC was used to determine myotube formation. The myoblast cells were cultured with differentiation media on pure PCL matrices and conductive nanocomposite matrices. The cells were washed with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich, USA) at room temperature for 15 min. Cells were washed with PBS again then were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, USA) in PBS for 5 min. 5% bovine serum albumin with a protein blocker solution (Sigma-Aldrich, USA) was used to block the cells at room temperature for 30 min. Following this, the samples were sequentially incubated with MF20 (1:200, Anti-MHC Alexa Fluor 488; Thermo Fisher Scientific, USA) at 4 °C overnight. The stained samples were mounted with Vectashield containing 4′, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories, USA) or Propidium Iodide (Thermo Fisher Scientific, USA) and images were captured with confocal microscopy.
-Quantitative RT-PCR:
Myogenic differentiation genes (MyoD, MyoG, Tnnt1) expressions were evaluated by quantitative real-time polymerase chain reaction (qRT-PCR). Total RNAs were extracted from the myoblasts cultured on various substrates (n = 3) after 8 days in differentiation medium using the RNeasy Minikit (Qiagen, USA). cDNA was synthesized from 1μg RNA using lyophilized master mix (RNA to cDNA EcoDry™ Premix (Double Primed, Clontech, USA). qPCR was performed using Power SYBR Green PCR Master Mix (Bio-Rad, USA) the MyiQ2 Real-Time PCR Detection System (Bio-Rad, USA). The expression levels of relative myogenic differentiation genes (MyoD, MyoG, Tnnt1) were was analyzed relative to the reference glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene using ΔΔCt method. The relative transcript levels were expressed as means ± SD (n = 3 for each group).
-ELISA analysis:
The expression of Mouse troponin I type 1 (skeletal, slow) (TNNI1) was assayed by ELISA Myoblast cells incubated with 10% PEDOT: PSS, dopamine and PCL were lysed after 14 days of culture. TNNI1 protein expression in cell lysates was obtained by the ELISA kit (myBiosource, USA) following the manufacturer’s instructions. Total protein of each matrix was determined using BCA protein assay kit (Pierce, USA). The amount of TNNT1 on each matrix was also normalized to the total protein amount.
Statistical Analyses:
All quantitative data were expressed as the mean ± standard deviation. Both one way and two-way ANOVA tests were used depending on the number of variables being tested. The Tukey pairwise comparison test was performed on samples to determine significant differences. The star (*) sign were used to indicate the statistically significant difference (p < 0.05).
Supplementary Material
Future work.
The study demonstrated that electroconductive nanocomposite matrix can favorably modulate myoblasts proliferation and differentiation. Future study will investigate the in vivo efficacy of the engineered matrix using a rat rotator cuff tear model to understand the ability of the engineered matrix in reducing the fatty infiltration.
Acknowledgments
Funding: The authors would like to acknowledge the NSF EFRI 1332329 and NIH DP1AR068147 and NIH RO1 AR063698 for funding this work.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Competing interests: No competing interests.
References
- 1.Juhas M, Bursac NJCoib. Engineering skeletal muscle repair. 2013;24(5):880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qazi TH, Mooney DJ, Pumberger M, Geissler S, Duda GNJB. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. 2015;53:502–21. [DOI] [PubMed] [Google Scholar]
- 3.Yang HS, Ieronimakis N, Tsui JH, Kim HN, Suh K-Y, Reyes M, et al. Nanopatterned muscle cell patches for enhanced myogenesis and dystrophin expression in a mouse model of muscular dystrophy. 2014;35(5):1478–86. [DOI] [PubMed] [Google Scholar]
- 4.Jiang T, Carbone EJ, Lo KW-H, Laurencin CTJPipS. Electrospinning of polymer nanofibers for tissue regeneration. 2015;46:1–24. [Google Scholar]
- 5.Sevivas N, Teixeira FG, Portugal R, Araújo L, Carriço LF, Ferreira N, et al. Mesenchymal stem cell secretome: a potential tool for the prevention of muscle degenerative changes associated with chronic rotator cuff tears. The American journal of sports medicine. 2017;45(1):179–88. [DOI] [PubMed] [Google Scholar]
- 6.Chen M-C, Sun Y-C, Chen Y-HJAb. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. 2013;9(3):5562–72. [DOI] [PubMed] [Google Scholar]
- 7.Saveh-Shemshaki N, S.Nair L, Laurencin CT. Nanofiber-based Matrices for Rotator Cuff Regenerative Engineering. Acta Biomaterialia. 2019. [DOI] [PubMed] [Google Scholar]
- 8.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2002;60(4):613–21. [DOI] [PubMed] [Google Scholar]
- 9.Aviss K, Gough J, Downes SJECM. Aligned electrospun polymer fibres for skeletal muscle regeneration. 2010;19(1):193–204. [DOI] [PubMed] [Google Scholar]
- 10.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJJB. The influence of electrospun aligned poly (epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. 2008;29(19):2899–906. [DOI] [PubMed] [Google Scholar]
- 11.Balint R, Cassidy NJ, Cartmell SHJAb. Conductive polymers: towards a smart biomaterial for tissue engineering. 2014;10(6):2341–53. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Bashur CA, Goldstein AS, Schmidt CEJB. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. 2009;30(26):4325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmore KJ, Kita M, Han Y, Gelmi A, Higgins MJ, Moulton SE, et al. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. 2009;30(29):5292–304. [DOI] [PubMed] [Google Scholar]
- 14.Jun I, Jeong S, Shin HJB. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. 2009;30(11):2038–47. [DOI] [PubMed] [Google Scholar]
- 15.Tait JG, Worfolk BJ, Maloney SA, Hauger TC, Elias AL, Buriak JM, et al. Spray coated high-conductivity PEDOT:PSS transparent electrodes for stretchable and mechanically-robust organic solar cells. Solar Energy Materials and Solar Cells. 2013;110:98–106. [Google Scholar]
- 16.Yang G, Kampstra KL, Abidian MR. High Performance Conducting Polymer Nanofiber Biosensors for Detection of Biomolecules. 2014;26(29):4954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abidian MR, Kim D-H, Martin DC. Conducting-Polymer Nanotubes for Controlled Drug Release. 2006;18(4):405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peramo A, Urbanchek MG, Spanninga SA, Povlich LK, Cederna P, Martin DC. In Situ Polymerization of a Conductive Polymer in Acellular Muscle Tissue Constructs. 2008;14(3):423–32. [DOI] [PubMed] [Google Scholar]
- 19.Lampin M, Warocquier-Clérout R, Legris C, Degrange M, Sigot-Luizard MF. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. 1997;36(1):99–108. [DOI] [PubMed] [Google Scholar]
- 20.Köunönen M, Hormia M, Kivilahti J, Hautaniemi J, Thesleff I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. 1992;26(10):1325–41. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. 2007;318(5849):426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai W-B, Chen W-T, Chien H-W, Kuo W-H, Wang M-J. Poly(dopamine) coating to biodegradable polymers for bone tissue engineering. 2014;28(6):837–48. [DOI] [PubMed] [Google Scholar]
- 23.Tang X, Khan Y, Laurencin C. Electroconductive nanofiber scaffolds for muscle regenerative engineering.
- 24.Jo S, Kang SM, Park SA, Kim WD, Kwak J, Lee H. Enhanced Adhesion of Preosteoblasts inside 3D PCL Scaffolds by Polydopamine Coating and Mineralization. 2013;13(10):1389–95. [DOI] [PubMed] [Google Scholar]
- 25.Hong KH, Oh KW, Kang TJ. Preparation of conducting nylon-6 electrospun fiber webs by the in situ polymerization of polyaniline. 2005;96(4):983–91. [Google Scholar]
- 26.Kim MS, Jun I, Shin YM, Jang W, Kim SI, Shin H. The Development of Genipin-Crosslinked Poly(caprolactone) (PCL)/Gelatin Nanofibers for Tissue Engineering Applications. 2010;10(1):91–100. [DOI] [PubMed] [Google Scholar]
- 27.Wakelam MJ. The fusion of myoblasts. The Biochemical journal. 1985;228(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y, Invernale MA, Sotzing GA. Conductivity Trends of PEDOT-PSS Impregnated Fabric and the Effect of Conductivity on Electrochromic Textile. ACS Applied Materials & Interfaces. 2010;2(6):1588–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.