Abstract
Background and Objectives:
18F-FDG-PET/CT parameters may help distinguish malignant from benign adrenal tumors, but few have been externally validated or determined based on definitive pathological confirmation. We determined and validated a threshold for 18F-FDG-PET/CT maximum standard uptake value (SUVmax) in patients who underwent adrenalectomy for a nonfunctional tumor.
Methods:
Database review identified patients with 18F-FDG-PET/CT images available (training cohort), or only SUVmax values (validation cohort). Discriminative accuracy was assessed by area under the curve (AUC), and optimal cutoff value estimated by maximally selected Wilcoxon rank statistics.
Results:
Of identified patients (n = 171), 86 had adrenal metastases, 20 adrenal cortical carcinoma, and 27 adrenal cortical adenoma. In the training cohort (n = 96), SUVmax was significantly higher in malignant vs. benign tumors (median 8.3 vs. 3.0, p < 0.001), with an AUC of 0.857. Tumor size did not differ. The optimal cutoff SUVmax was 4.6 (p < 0.01). In the validation cohort (n = 75), this cutoff had a sensitivity of 75%, and specificity 55%.
Conclusions:
18F-FDG-PET/CT SUVmax was associated with malignancy. Validation indicated that SUVmax ≥ 4.6 was suggestive of malignancy, while lower values did not reliably predict benign tumor.
Keywords: predictive tool, incidentaloma, adrenal metastasis, adrenal cortical carcinoma, adrenal cortical adenoma
Introduction
Incidental discovery of adrenal masses, termed adrenal incidentalomas, has increased in recent years because of greater use of cross-sectional imaging for diagnostic and surveillance purposes.(1-3) Patients with an adrenal incidentaloma that autonomously secretes cortisol, aldosterone, or catecholamines are typically offered adrenal resection.(4) Whether non-hormone-secreting masses should be surgically resected depends on the risk of malignancy. The American Association of Clinical Endocrinologists (AACE) and the American Association of Endocrine Surgeons (AAES) clinical practice guidelines for the management of adrenal incidentalomas (excluding masses found in patients being imaged as part of workup for another cancer) recommends that any adrenal mass with concerning radiographic characteristics, and most lesions ≥ 4 cm, should be resected because of increased risk of adrenal cancer.(4) Concerning radiographic characteristics include nonhomogeneous enhancement, irregular borders, high enhancement (> 10 Hounsfield units), and slow contrast-washout kinetics.(4, 5) The incidence of adrenal masses in cancer patients with other primaries is also increasing along with the number of patients living with cancer in the US.(6) No guidelines exist regarding the management of adrenal masses in this patient group, only half of which are estimated to represent metastases.(5, 7) Further, data to suggest when surgery is necessary are scarce, and predicting the benefit of surgery remains difficult for some patients.
18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) combined with computed tomography (CT) has been shown to be highly sensitive and predictive for differentiating between malignant and benign adrenal tumors in two recent meta-analyses in mixed patient populations with and without a cancer history.(8, 9) However, the method by which 18F-FDG-PET/CT images are interpreted varies widely, from visual inspection to cutoffs for quantitative values such as maximum standardized uptake value (SUVmax) or adrenal-to-liver SUV ratio. All studies included in the meta-analyses relied on biopsy and/or imaging and follow-up for definitive diagnosis, except for one performed in adrenalectomy patients with definitive pathologic evaluation available.(10) While sensitivity and specificity estimates were all > 70%, none of the studies tested the diagnostic accuracy of their optimal cutoff value in an external validation cohort.
As quantitative interpretation is most replicable and SUVmax is the most consistently recorded value, we sought to identify an optimal 18F-FDG-PET/CT SUVmax cutoff value and externally evaluate its accuracy in discriminating between benign and malignant adrenal tumors in patients who underwent adrenalectomy for a nonfunctional adrenal tumor.
Methods
After obtaining IRB approval, adult patients who underwent 18F-FDG-PET/CT prior to adrenal resection for an adrenal tumor during the period 1996 to 2018 were identified from a prospective institutional database. Patients were excluded if an adrenal was resected without the preoperative finding of an adrenal mass, if preoperative clinical or biochemical evaluation showed adrenal hormonal secretion, or if no postoperative pathology report was available.
Patients for whom 18F-FDG-PET/CT images were available served as the training cohort; images were reviewed by a nuclear medicine physician (SCG or RKG) to determine SUVmax. Patients who underwent 18F-FDG-PET/CT at an outside institution and did not have reviewable images served as the validation cohort, and SUVmax was taken from the originally issued nuclear medicine/radiology reports.
18F-FDG-PET/CTs at our institution were performed on a dedicated PET/CT scanner (GE Healthcare) approximately 60 min following intravenous injection of 10 mCi ± 10% of 18F-fluorodeoxyglucose (18F-FDG). A low-dose CT scan (80 mA) was performed from mid-skull to mid-thigh, followed by an emission scan (3 min/field of view). Images were reconstructed using iterative reconstruction and attenuation correction in PET volume computer-assisted reading (VCAR) software (GE Healthcare). Regions of interest (ROIs) were drawn on a homogeneous liver region and the adrenal tumor. Activity counts in the ROIs were normalized to injected doses per kilogram of patient body weight to determine the SUVmax.
Malignant tumors included adrenal cortical carcinoma, metastasis, neuroblastoma, and B cell lymphoma. Benign tumors included adrenal cortical adenoma, schwannoma, and other benign adrenal findings (i.e., cyst, hematoma, and normal tissue with malignant tumor outside the adrenal gland). Nonfunctional pheochromocytoma was classified as benign or malignant based on pathological evaluation.
Statistical analysis
Wilcoxon rank-sum test was used to evaluate the significance of difference between the distributions of SUVmax between malignant and benign tumors. A logistic regression model was constructed to examine the association between SUVmax and disease type. The diagnostic ability of binary classification systems of 18F-FDG-PET/CT SUVmax was assessed by quantifying the area under the curve (AUC). The optimal cutoff was determined by systematic search using maximally selected Wilcoxon rank statistics via the minimum p-value approach,(11, 12) and its accuracy was tested in the validation cohort. Sociodemographic characteristics were summarized using the frequency and percentage for categorical variables and median and interquartile range (IQR) for continuous variables, and compared between training and validation cohorts using Fisher’s exact test and Wilcoxon rank-sum test. Sensitivity, specificity, positive predictive value, and negative predictive value, along with exact 95% confidence intervals (CIs), were estimated. All statistical analyses were performed using SPSS for Windows, version 25.0 (IBM, Armonk, NY), SAS 9.3 (SAS Institute, INC., Cary, NC, USA) or R version 3.0.1 (R Foundation for Statistical Computing, Vienna Austria). All p-values were two-sided. P-values of < 0.05 were considered to indicate statistical significance.
Results
Training cohort
Review of the prospectively maintained database identified 171 patients who underwent 18F-FDG-PET/CT prior to adrenal resection between 1996 and 2018 at Memorial Sloan Kettering Cancer Center. In 96 (56%) patients, 18F-FDG-PET/CT images were available for review; these patients constituted the training cohort. Of these, 22 (23%) patients had a benign tumor and 74 (77%) had a malignant tumor as determined by histopathology of the resected specimen (Table 1). Of the benign tumors, 17 (77%) were adrenal cortical adenoma, 2 ganglioneuroma, 1 pheochromocytoma, 1 hematoma, and 1 benign adrenal gland with focal medullary fibrosis. Of the malignant tumors, 63 (85%) were metastatic, 9 (12%) were adrenal cortical carcinoma, and 2 (2.7%) were neuroblastoma.
Table 1.
Patient and tumor characteristics of total study population (n = 171). Categorical data presented as n (%) and continuous as median (IQR).
| Training cohort (n = 96) | Validation cohort (n = 75) | |||
|---|---|---|---|---|
| Malignant (n = 74) |
Benign (n = 22) |
Malignant (n = 64) |
Benign (n = 11) |
|
| Female | 34 (46%) | 14 (64%) | 34 (53%) | 6 (55%) |
| Age (years) | 61 (53-68) | 60 (51-65) | 64 (54-70) | 59 (47-68) |
| Year of 18F-FDG-PET | ||||
| 1996-2003 | 7 (9.5%) | 3 (14%) | 26 (41%) | 3 (27%) |
| 2004-2010 | 34 (46%) | 5 (23%) | 17 (27%) | 5 (46%) |
| 2011-2018 | 33 (45%) | 14 (64%) | 21 (33%) | 3 (27%) |
| Time between 18F-FDG-PET and surgery (months) | 1 (0-4) | 1 (0-3) | 1 (0-3) | 1 (0-1) |
| Tumor size (cm) | 4.1 (2.6-6.1) | 4.1 (3.0-5.6) | 5.0 (3.6-7.4) | 4.4 (3.9-5.0) |
| Tumor type | ||||
| Metastasis | 63 (85%) | - | 51 (80%) | - |
| Adrenal cortical adenoma | - | 17 (77%) | - | 6 (55%) |
| Adrenal cortical carcinoma | 9 (12%) | - | 11 (17%) | - |
| Other | 2 (2.7%) | 5 (23%) | 2 (3.1%) | 5 (46%) |
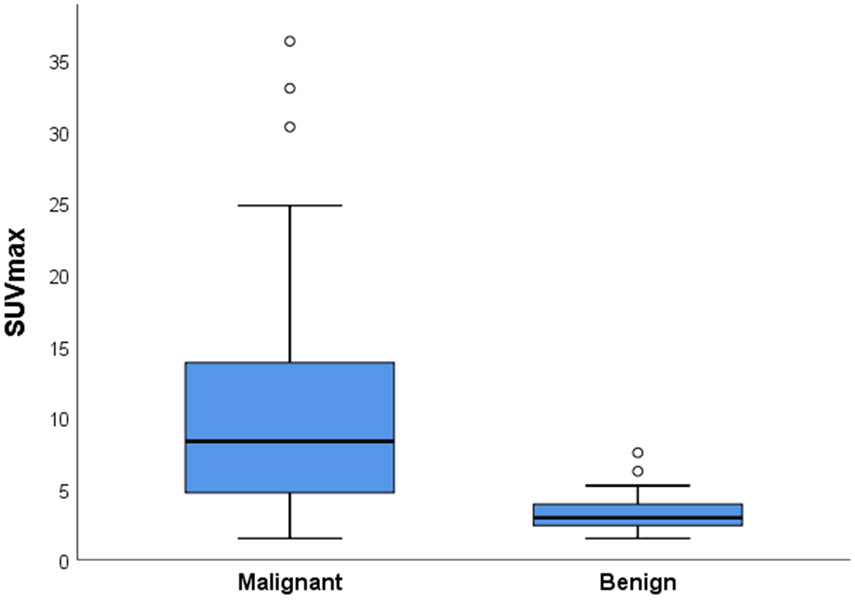
In the training cohort, median SUVmax was significantly higher in malignant tumors, at 8.3 (interquartile range [IQR] 4.7–13.7) vs. 3.0 (IQR 2.4–3.8) for benign tumors (p < 0.001; Fig. 1). Median tumor size was similar in malignant and benign adrenal tumors: 4.1 (IQR 2.6–6.1) vs. 4.1 cm (IQR 3.0–5.6) (p = 0.976). Compared with adrenal cortical adenomas, adrenal cortical carcinomas had a significantly higher median SUVmax (14.5 [IQR 11.3–17.2] vs. 3.0 [2.5–3.8]; p < 0.001) and were significantly larger (9.5 cm [IQR 4.8–12.1] vs. 3.9 cm [IQR 3.0–5.3]; p = 0.004). In a logistic regression model, SUVmax was significantly associated with malignancy, with an odds ratio per 1 unit increase in SUVmax of 1.72 (95% CI 1.27–2.34; p < 0.001) and AUC of 0.857. Selection of maximal Wilcoxon rank statistics with minimum p-values identified an optimal cutoff point for SUVmax of ≥ 4.6 (p < 0.01). The sensitivity and specificity of this SUVmax cutoff value in the training cohort were 76% (95% CI 64–85%) and 82% (95% CI 60–95%), respectively. To reach a sensitivity of 95%, an SUVmax cutoff value of ≥ 2.2 was required, lowering specificity to 18%.
Figure 1.
Distribution of 18F-FDG-PET SUVmax values of benign (n = 22) and malignant (n = 74) adrenal tumors in the training cohort.
Validation cohort
The remaining 75 patients’ 18F-FDG-PET/CT was performed elsewhere, so images were not available for review, but reports were available in the medical record; these patients represented the external validation cohort. Among these, 11 (15%) patients had a benign tumor and 64 (85%) had a malignant tumor. Of the benign tumors, 6 (50%) were adrenal cortical adenoma, 2 schwannoma, 1 ganglioneuroma, 1 necrotic tissue consistent with adrenal cortical infarction, and 1 medullary hemorrhage with focal fibrin deposition. Of the malignant tumors, 51 (81%) were metastases, 11 (18%) adrenal cortical carcinoma, 1 B cell lymphoma, and 1 was a pathologically normal adrenal gland abutting a leiomyosarcoma. Gender, age, time between 18F-FDG-PET/CT and surgery, and prevalence of malignancy did not differ between the training and validation cohorts. However, median tumor size was different, at 5.0 cm (IQR 3.9–6.8) in the validation cohort vs. 4.1 cm (2.8–6.0) in the training cohort (p = 0.022), as was the date of imaging, with patients in the validation cohort having been evaluated earlier in the review period (p < 0.001). In the validation cohort, tumor size was again similar between malignant and benign adrenal tumors: 5.0 cm (IQR 3.6–7.4) vs. 4.4 cm (IQR 3.9–5.0) (p = 0.124). Tumor size did not differ between adrenal cortical carcinomas and adrenal cortical adenomas, with median tumor sizes of 5.2 cm (IQR 4.8–8.6) vs. 4.7 cm (4.1–5.1) (p = 0.086).
The sensitivity, specificity, positive predictive value, and negative predictive value of the SUVmax cutoff of ≥ 4.6 in differentiating between malignant and benign tumors were 75% (95% CI 64–85%), 55% (95% CI 27–81%), 91% (95% CI 0.81–0.97%), and 27% (95% CI 0.12–0.48%), respectively. Table 2 shows the number of true positives, false positives, false negatives, and true negatives. Among the 5 patients with a false positive result (benign adrenal mass with SUVmax > 4.6) in the validation cohort, final diagnoses were adrenal cortical adenoma (n=2) with SUVmax of 4.6 and 39, myelolipoma (n=1) with SUVmax of 4.6, necrotic tissue consistent with adrenal cortical infarction (n=1) with SUVmax of 4.7, and schwannoma (n=1) with SUVmax of 4.9. Among the 16 patients with a false negative result (malignancy with SUVmax < 4.6), patients had metastasis from renal cell carcinoma (n=3), lung cancer (n=3), liver cancer (n=2), melanoma (n=2), colorectal cancer (n=1), cholangiocarcinoma (n=1), bladder cancer (n=1), and three with adrenal cortical carcinoma. The majority (12 of 16) of false negative SUVmax values in the validation cohort ranged between 3.0 and 4.4. The AUC was 0.648 (95% CI 0.46–0.83). SUVmax did not differ between adrenal cortical carcinomas and adrenal cortical adenomas (9.0 [IQR 3.3–12.8] vs 4.1 [IQR 3.1–13.3]; p = 0.393). The SUVmax cutoff of ≥ 2.2 resulted in a sensitivity of 97%, but lowered specificity to 0% in this cohort.
Table 2.
Performance characteristics of 18F-FDG PET SUVmax cutoff value of 4.6 in differentiating between benign and malignant non-secreting adrenal tumors in the validation cohort (n = 75).
| Malignant | Benign | Total | |
|---|---|---|---|
| SUVmax ≥ 4.6 | True positive 48 | False positive 5 | 53 |
| SUVmax < 4.6 | False negative 16 | True negative 6 | 22 |
| Total | 64 | 11 | 75 |
Discussion
In the training cohort of 96 resected adrenal tumors, 18F-FDG-PET/CT SUVmax was significantly associated with malignancy, with 8.3 versus 3.0 in benign tumors. The optimal cutoff value for distinguishing malignant from benign tumors was ≥ 4.6 with a sensitivity of 76%, specificity of 83%, and AUC of 0.857. Validation of the optimal cutoff value of ≥ 4.6 in an external cohort of 75 resected adrenal tumors (for which imaging was performed at other institutions) resulted in a sensitivity of 75%, specificity of 55%, and low AUC (0.648).
The estimated specificity in the validation cohort indicates that among benign tumors, 55% had a SUVmax below 4.6 and hence a low SUVmax value is not informative. The specificity in the training cohort was considerably higher (82%), reflecting fewer false positives. In the validation cohort, 4 of the 5 false positives had an SUVmax just above the cutoff value, between 4.6 and 4.9. The estimated sensitivity indicates that in case of malignancy, 75% of SUVmax values are ≥ 4.6 and 25% < 4.6. There are several reasons why malignant adrenal tumors may present with low SUVmax values, including prior chemotherapy treatment, generally low metabolic activity, tumor necrosis, and hemorrhage. As 81% of malignant adrenal tumors in this study were metastases from other malignancies, many had received chemotherapy.(13) Low metabolic activity is characteristic of renal cell carcinoma due to the physiological excretion of FDG from the kidneys,(14) which accounted for 3 of the 16 false negatives in the validation cohort. The positive predictive value suggests that 91% of patients with high SUVmax have a malignant tumor. However, because positive predictive value increases with increasing incidence of malignancy, the high value may be biased by the current cohort’s inclusion of only patients who underwent adrenal resection and thus likely displayed more concerning characteristics (i.e., higher risk for malignancy) than other series.
The accuracy of our SUVmax cutoff in the validation cohort was low compared with the literature. A recent systematic review of the diagnostic accuracy of 18F-FDG-PET SUVmax for adrenal tumors calculated a pooled sensitivity and specificity of 0.95 (95% CI 0.86–0.98) and 0.91 (95% CI 0.81–0.96) from 6 different studies, respectively.(9) However, each study estimated accuracy using the same cohort in which the optimal SUVmax cutoff was determined, and did not validate the cutoff in a separate cohort. This lack of reproducibility testing may have led to over-interpretation and prevents the application of their accuracy estimates to other cohorts.(15) Also, all studies relied on biopsy or imaging and follow-up for definitive diagnosis, likely leading to misclassification bias. Previously estimated optimal SUVmax cutoff values ranged between 2.4 and 5.2.(10, 16-22)
Given our large patient cohort with definitive pathologic confirmation of diagnoses, we took advantage of this unique opportunity to validate the performance characteristics of previously published optimal SUVmax cutoff values (Table 3). The cutoff values ranged between 2.5 and 5.2, and the optimal SUVmax likely lies within this range. However, this range is still very wide, as 53 of the 171 (31%) tumors in both our cohorts had an SUVmax between 2.5–5.2. The one study that included only patients who underwent adrenal resection found an optimal SUVmax cutoff value of 5.2 and had the best overall performance in our cohort, with a sensitivity of 71%, specificity of 88%, PPV of 97%, NPV of 43%, and AUC of 0.810.(10) The performance characteristics calculated in our validation cohort are considerably lower than the performance characteristics in each of the original study populations, emphasizing the importance of external validation for reproducibility. The discrepancy in performance could also be caused by inherent differences in study populations. As our institution is a tertiary referral center, our cohort might include more patients with indeterminate tumor characteristics on imaging.
Table 3.
Performance characteristics of previously published SUVmax cutoff values for malignant versus benign adrenal lesions in our total cohort (n = 171).
| Reference | SUVma x cutoff |
No. patients with adrenal masses |
Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|---|---|
| Perri et al. 2001 (18) | > 2.8 | 93 | 126/138 (91%) | 11/33 (33%) | 126/148 (85%) | 11/23 (48%) | 0.623 |
| Jana et al. 2006 (16) | ≥ 3.4 | 74 | 119/138 (86%) | 16/33 (48%) | 119/136 (88%) | 16/35 (46%) | 0.674 |
| Metser et al. 2006 (17) | ≥ 3.1 | 150 | 124/138 (90%) | 14/33 (42%) | 124/143 (87%) | 14/28 (50%) | 0.661 |
| Brady et al. 2009 (21) | > 3.1 | 147 | 122/138 (88%) | 15/33 (45%) | 122/140 (87%) | 15/31 (48%) | 0.669 |
| Oczan Kara et al. 2011 (19) | ≥ 4.2 | 81 | 109/138 (79%) | 22/33 (67%) | 109/120 (91%) | 22/51 (43%) | 0.728 |
| Kunikowska et al. 2014 (10) | > 5.2 | 85 | 98/138 (71%) | 30/33 (88%) | 98/101 (97%) | 30/70 (43%) | 0.810 |
| Launay et al. 2015 (20) | ≥ 3.7 | 66 | 117/138 (85%) | 19/33 (58%) | 117/131 (89%) | 19/40 (48%) | 0.712 |
| Kim et al. 2015 (22) | ≥ 2.5 | 24 | 128/138 (93%) | 7/33 (21%) | 128/154 (83%) | 7/17 (41%) | 0.570 |
| Current study | ≥ 4.6 | 171 | 104/138 (75%) | 24/33 (73%) | 104/113 (92%) | 24/58 (41%) | 0.740 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AUC, area under the receiver operating characteristic curve
Number with adrenal masses
The use of SUV values has limitations. Even within the same tumor type, SUV depends on patient-related and technical factors including plasma glucose concentration, body size and composition, time between 18F-FDG injection and scanning, image reconstruction accuracy, and properties of the PET/CT scanner. Although one would seldom question the interpretation of high SUV values, uncorrected errors could artifactually lower SUV values.(23) The fact that all 18F-FDG-PET/CT scans of the training cohort were reviewed prior to the SUVmax cutoff analysis decreases the likelihood of such errors and thus increases our confidence in the calculated cutoff. However, this data may guide surgical decision making to distinguish patients who can be watched (those with SUVmax ≤ 2.2) by serial imaging from those with almost certain malignancy (those with SUVmax ≥ 4.6) who can then be assessed for suitability for surgery or other risk-mitigating procedure such as radio-frequency ablation or cryoablation.
The AACE/AAES guidelines advise resecting tumors larger than 4 cm, based on a retrospective study in which tumor size was highly correlated with malignancy.(24) We found that tumor size was similar between malignant and benign adrenal tumors, though this may reflect differences in patient population. Whereas the guideline-informing study included only true incidentalomas, the majority (83%) of lesions in ours, similar to most studies of PET parameters in adrenal lesions were metastases of other cancers.(10, 16-19, 21, 25) Size thus appears to be less relevant in the distinction of benign from malignant lesions when affected patients are known to have other cancers.
This is the first study to test the diagnostic accuracy of a SUVmax cutoff value in a validation cohort. Other strengths of our study were the selection of patients who underwent adrenal resection and thus had definitive pathology to avoid misclassification, and the large, uniform study population, which to our knowledge is the largest yet evaluated to determine a diagnostic SUVmax cutoff value for adrenal tumors.
Conclusions
In this series of 171 resected adrenal tumors in a mixed population of patients with and without a cancer history, 18F-FDG-PET SUVmax was associated with malignancy, but tumor size was not. The diagnostic accuracy of the optimal cutoff value of ≥ 4.6 was tested in a validation cohort and found to be suggestive of malignancy with a sensitivity of 75%. However, low values did not reliably predict a benign tumor. An SUVmax cutoff of ≥ 2.2 yielded sensitivity of ≥ 95%, but severely limited specificity because very few adrenal tumors present with such a low SUVmax.
Synopsis: Analysis of 96 resected adrenal tumors determined the optimal cutoff for predicting malignancy by 18F-FDG-PET/CT maximum standard uptake value to be ≥ 4.6. This cutoff had a sensitivity of 75% but low specificity (50%) in a validation cohort of 75 tumors.
Acknowledgments
The authors thank Jessica Moore, MS, for editing the manuscript. We also thank Murray F. Brennan, MD, for his intellectual contributions.
Disclosure: This study was supported in part by the MSKCC Cancer Center Support Grant P30 CA008748.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Young WF Jr. Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am. 2000;29(1):159–85, x. [DOI] [PubMed] [Google Scholar]
- 2.Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16(4):460–84. [DOI] [PubMed] [Google Scholar]
- 3.Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298–302. [DOI] [PubMed] [Google Scholar]
- 4.Zeiger M, Thompson G, Duh Q-Y, et al. AACE/AAES Adrenal Incidentaloma Guidelines. Endocr Pract. 2009;15:1–20. [DOI] [PubMed] [Google Scholar]
- 5.Young WF Jr., Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–10. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 7.Lenert JT, Barnett CC Jr., Kudelka AP, et al. Evaluation and surgical resection of adrenal masses in patients with a history of extra-adrenal malignancy. Surgery. 2001;130(6):1060–7. [DOI] [PubMed] [Google Scholar]
- 8.Boland GW, Dwamena BA, Jagtiani Sangwaiya M, et al. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology. 2011;259(1):117–26. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Lee SW, Pak K, Kim IJ, Kim K. Diagnostic accuracy of (18)F-FDG PET or PET/CT for the characterization of adrenal masses: a systematic review and meta-analysis. Br J Radiol. 2018;91(1086):20170520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunikowska J, Matyskiel R, Toutounchi S, Grabowska-Derlatka L, Koperski L, Krolicki L. What parameters from 18F-FDG PET/CT are useful in evaluation of adrenal lesions? Eur J Nucl Med Mol Imaging. 2014;41(12):2273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torsten H, Berthold L. On the exact distribution of maximally selected rank statistics. Comp Stat Data Anal. 2003;43(2):121–37. [Google Scholar]
- 12.Lausen B, Schumacher M. Maximally selected rank statistics. Biometrics. 1992;48:73–85. [Google Scholar]
- 13.Lastoria S, Piccirillo MC, Caraco C, et al. Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J Nucl Med. 2013;54(12):2062–9. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y The Place of FDG PET/CT in Renal Cell Carcinoma: Value and Limitations. Front Oncol. 2016;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EW S A practical approach to development, validation, and updating. . New York: Springer; 2009. [Google Scholar]
- 16.Jana S, Zhang T, Milstein DM, Isasi CR, Blaufox MD. FDG-PET and CT characterization of adrenal lesions in cancer patients. Eur J Nucl Med Mol Imaging. 2006;33(1):29–35. [DOI] [PubMed] [Google Scholar]
- 17.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47(1):32–7. [PubMed] [Google Scholar]
- 18.Perri M, Erba P, Volterrani D, et al. Adrenal masses in patients with cancer: PET/CT characterization with combined CT histogram and standardized uptake value PET analysis. AJR Am J Roentgenol. 2011;197(1):209–16. [DOI] [PubMed] [Google Scholar]
- 19.Ozcan Kara P, Kara T, Kara Gedik G, et al. The role of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiating between benign and malignant adrenal lesions. Nucl Med Commun. 2011;32(2):106–12. [DOI] [PubMed] [Google Scholar]
- 20.Launay N, Silvera S, Tenenbaum F, et al. Value of 18-F-FDG PET/CT and CT in the Diagnosis of Indeterminate Adrenal Masses. Int J Endocrinol. 2015;2015:213875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: a proposal for an efficient diagnostic algorithm. Radiology. 2009;250(2):523–30. [DOI] [PubMed] [Google Scholar]
- 22.Kim BS, Lee JD, Kang WJ. Differentiation of an adrenal mass in patients with non-small cell lung cancer by means of a normal range of adrenal standardized uptake values on FDG PET/CT. Ann Nucl Med. 2015;29(3):276–83. [DOI] [PubMed] [Google Scholar]
- 23.Mah K, Caldwell CB. Biological Target Volume In: Paulino AC, editor. PET-CT in Radiotherapy Treatment Planning. Philadelphia, PA: Saunders (Elsevier); 2008. p. 52–92. [Google Scholar]
- 24.Angeli A, Osella G, Ali A, Terzolo M. Adrenal incidentaloma: an overview of clinical and epidemiological data from the National Italian Study Group. Horm Res. 1997;47(4–6):279–83. [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Carrasquillo JA. Molecular imaging of adrenal neoplasms. J Surg Oncol. 2012;106(5):532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.