Abstract
Background
Cognitive health expectancy estimates the proportion of the lifespan that is lived in good cognitive health at the population level. A number of cardiovascular diseases have been identified to be risk factors for cognitive decline and dementia including diabetes, stroke, heart diseases and hypertension. The aim of this study was to examine how these cardiovascular conditions relate to cognitive health expectancy.
Methods
Longitudinal data were obtained from the US Health and Retirement Study. Multistate modelling was used to estimate total life expectancy (LE), cognitive impairment free life expectancy (CIFLE) and years spent with cognitive impairment (CILE) across self-reported diabetes, hypertension, heart problems and stroke. Individual and cumulative effects of multiple cardiovascular conditions were examined.
Results
The presence of cardiovascular disease was associated with a 5- to 9-year decrease in LE and 4- to 8-year decrease in CIFLE at age 55. The outcomes varied in a hierarchical fashion by cardiovascular condition. Relative to other conditions, individuals with stroke had the shortest LE and CIFLE. Analysis of multiple cardiovascular risk factors revealed that each additional cardiovascular condition was associated with an exponential decrease in LE and CIFLE.
Conclusions
Having a cardiovascular condition is associated with a lower CIFLE and higher proportion of life lived with cognitive impairment. However, the outcomes vary depending on the type of cardiovascular condition. Reducing incidence of stroke and minimising exposure to multiple cardiovascular risk factors may be beneficial in helping to improve population estimates of cognitive health expectancy.
Keywords: cognitive health expectancy, cognitive impairment, cardiovascular disease
Key points
Cardiovascular disease is associated with a shorter life expectancy and cognitive impairment free life expectancy.
Outcomes varied in a hierarchical fashion by cardiovascular condition.
Having multiple cardiovascular conditions was associated with an exponential decrease in cognitive health expectancy.
Educational attainment is protective, but the effect is reduced in individuals with stroke, heart disease and diabetes.
Introduction
As the global life expectancy (LE) in the older population increases, it has become progressively more important to understand whether the extra years gained are lived in good health. Health expectancy is a metric that combines information about both the mortality and morbidity of a population [1]. Cognitive health expectancy provides information about the number of years lived with and without cognitive impairment (CI) and thus provides information about both the quantity and quality of life lived at a population level [2].
There is presently a lack of immediate treatments for Alzheimer’s disease and other dementias. It is therefore important to try to mitigate risk or delay the onset of developing dementia through modifiable risk factors. Knowing how chronic diseases impact on cognitive health expectancy could help inform preventive health policies and individual health advice. A range of cardiovascular conditions have been identified as risk factors for dementia and cognitive decline including diabetes, hypertension, heart disease and stroke [3–6]. However, it is currently unknown how having cardiovascular disease can affect the number of years lived with CI.
The present study aims to explore the relationship between cardiovascular risk factors for dementia and cognitive health expectancy. Specifically, it will examine how the presence of hypertension, heart disease, diabetes and stroke relate to cognitive health expectancy. It will also investigate the relationship between multiple cardiovascular conditions and cognitive health expectancy.
Method
Data from the Health and Retirement Study (HRS) [7] were used in this study. The HRS is a US-based longitudinal household study that commenced data collection in 1992. Data collection involved a combination of in-person and telephone interviews with a nationally representative panel of individuals over the age of 50 and their spouses. The study collected 12 waves of data over ~24 years (N = 37,495, female = 56.2%). Individuals who were institutionalised during the follow-up period were retained in the study but were not included in the initial sample.
Cognition measures
Using HRS data, Langa and colleagues [8] derived a composite cognition score from the immediate and delayed recall tests, serial 7s and counting backwards test [9,10]. The composite score ranged from 0 to 27. The criteria for CI were generated using norms from the Aging, Demographics and Memory Study and was defined as a score between 0 and 11 [10,11].
It is worth noting that the recall word test in the HRS changed between wave 2 and 3 from a 20-word list to a 10-word list. The 10-word version was used for subsequent interview waves from the wave 3 onwards and is the version used to calculate the composite cognition score [10]. For this reason, only data from wave 3 (the 1996 interview wave) onwards was included for analysis in this study. From this point onwards, wave 3 will hereby be referred to as the baseline wave in this study.
Cardiovascular conditions
Diabetes, hypertension, stroke and heart disease status were recorded based on the participants’ self-report of ever having being diagnosed with the condition (i.e. ‘Has a doctor ever told you that you have [condition])’. Hypertension was defined as high blood pressure or hypertension, diabetes was defined as diabetes or high blood sugar, heart disease was defined as heart attack, coronary heart disease, angina, congestive heart failure or other heart problems and stroke was defined as stroke or transient ischemic attack [12,13].
Health expectancy calculations
A multistate Markov model was used in these analyses to model the transitions between the states of cognitively healthy, cognitively impaired and death for each of the cardiovascular conditions. Cognitive health expectancies were calculated using IMaCh software [14]. This program partitions time intervals between interview waves into shorter steps (e.g. months) and models the resulting transition probabilities between states using multinomial logistic regression and maximum likelihood. IMaCh then constructs multistate life tables based on these estimated transition probabilities. The results are separated into total LE, cognitive impairment free life expectancy (CIFLE) and cognitive impairment life expectancy (CILE). Version 0.99r17 of IMaCh was used in this analysis.
Diabetes, stroke, hypertension and heart disease statuses were entered as time-varying dummy covariates. This means that the algorithm takes into consideration participants who did not have the condition at baseline but went on to develop the condition in later waves. The cognitive states were also time-varying and defined based on the participants cognitive scores at each wave (to account for fluctuations in scores across waves). Sex, education and smoking status were entered as fixed dummy covariates in order to examine their influence on cognitive health expectancies within each cardiovascular condition. Education was categorised as ‘low’ (high school degree and below) and ‘high’ (college degree and above). Smoking status was categorised as ‘ever smoked’ and ‘never smoked’ cigarettes.
Participants who enrolled in the HRS postwave 3 (the baseline wave) were not included in the analysis. Participants were also not included in the analysis if they were under the age of 50 at time of enrolment into the HRS (this situation only applied to spouses).
The final data set included 16,753 participants.
Results
Demographics
In total, 68% of participants reported having hypertension, 42% heart problems, 27% diabetes and 19% a stroke at some point in their lives throughout duration of the HRS (Table 1). A total of 18% of participants reported never developing any of these conditions in the study, 33% reported having one cardiovascular condition during the study, 30% reported having two cardiovascular conditions and 19% reported having more than two cardiovascular conditions. See Supplementary Material A1 for the numbers of people for each combination of conditions. There were slightly more females enrolled in the study than males (56 vs 44%). One-third reported having a college degree or higher (32%). More than half of the participants reported having smoked cigarettes some time in their lives (59%).
Table 1.
Demographic information of participants in the study sample stratified by cardiovascular condition
| Stroke | Diabetes | Heart disease | Hypertension | No conditions | Total | ||
|---|---|---|---|---|---|---|---|
| N | Total | 3,217 | 4,432 | 7,102 | 11,318 | 2,931 | 16,753 |
| % in each condition | 19 | 27 | 42 | 68 | 18 | 100 | |
| Mean age | At baseline SD | 70.59 (10.15) | 65.64 (9.14) | 69.09 (10.29) | 67.09 (9.93) | 66.85 (10.84) | 67.61 (10.33) |
| Range | 53–103 | 53–100 | 53–102 | 53–100 | 53–103 | 53–105 | |
| Gender | Males % | 43 | 47 | 48 | 41 | 44 | 44 |
| Females % | 57 | 53 | 52 | 59 | 56 | 56 | |
| Education | High school and below % | 71 | 73 | 69 | 69 | 65 | 68 |
| College and above % | 29 | 27 | 31 | 31 | 35 | 32 | |
| Smoking | Never smoked % | 41 | 40 | 38 | 42 | 41 | 41 |
| Ever smoked % | 59 | 60 | 62 | 58 | 59 | 59 | |
| Cognition | Mean cognition score (SD) | 12.43 (4.32) | 13.43 (4.19) | 13.46 (4.23) | 13.73 (4.25) | 14.55 (4.53) | 13.84 (4.35) |
| No impairment (≥12) % | 37 | 42 | 44 | 44 | 55 | 47 | |
| CI (0–11) % | 63 | 58 | 56 | 56 | 45 | 53 | |
| Mortality | Number of deaths | 2,251 | 2,507 | 4,511 | 6,142 | 1,538 | 9,404 |
| % of deaths | 70 | 57 | 64 | 54 | 52 | 56 | |
| Multiple conditions | One condition (%) | 5 | 7 | 21 | 66 | — | 33 |
| Two conditions (%) | 20 | 33 | 59 | 88 | — | 30 | |
| Two+ conditions (%) | 58 | 72 | 90 | 98 | — | 19 |
SD, standard deviation.
Cognitive health expectancies across cardiovascular conditions
Compared with individuals with no reported cardiovascular conditions, having a cardiovascular condition was associated with reduced LE (by 5–9 years at age 55), reduced CIFLE (by 4–8 years at age 55) and increased the proportion of life lived with CI. However, the extent of these differences varied according to the cardiovascular condition. Individuals with stroke had the shortest LE and CIFLE, followed by diabetes, heart disease, and hypertension respectively (Fig. 1). This hierarchy remains generally consistent across the lifespan.
Figure 1.
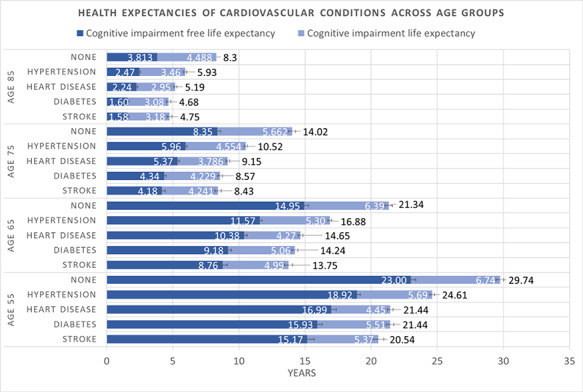
LE, CIFLE and CILE estimates in individuals with stroke, diabetes, heart disease, hypertension and no reported conditions across age groups. Error bars represent 95% confidence interval. LE is reported at the end of each bar.
Individuals with stroke also showed the highest proportion of life lived with CI (CILE/LE = 26.14% at age 55), whereas heart disease showed the lowest (CILE/LE = 20.74% at age 55). Individuals with heart disease also showed a lower proportion of life lived with CI compared with individuals who reported never having been diagnosed with any condition at age 55 (CILE/LE = 20.74 vs 22.66%). However, this effect disappeared post age 65. See Supplementary Material A2 for a tabular representation of the results.
Cognitive health expectancies and multiple cardiovascular conditions
When examining the additive effective of cardiovascular conditions on cognitive health expectancy, there appears to be an exponential increase in the reduction of LE and CIFLE per additional condition. The difference in LE and CIFLE between individuals with no reported conditions and one reported condition at age 55 was 2.33 and 1.82 years, between one and two reported conditions was 3.82 and 3.12 years and between two and more than two reported conditions was 5.02 and 4.08 years, respectively (Fig. 2). See Supplementary Material A3 for a tabular representation of the results.
Figure 2.
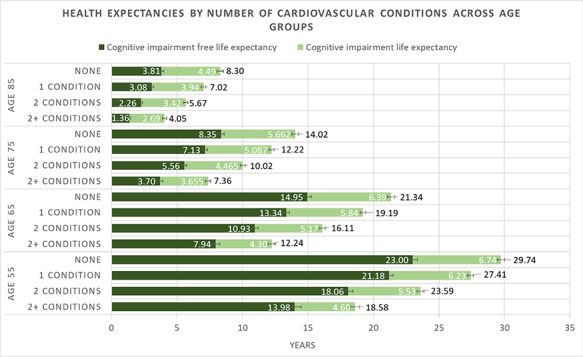
LE, CIFLE and CILE estimates in individuals with multiple cardiovascular conditions across age groups. Error bars represent 95% confidence interval. LE is reported at the end of each bar.
Covariate analysis
Sex differences
The results showed that within each cardiovascular condition, females had a longer absolute LE, CIFLE and CILE than males (Fig. 3a). Proportionally, women also lived longer in a cognitively impaired state relative to their total LE across all conditions (2–3% more than men at age 55). The magnitude of sex differences was similar across cardiovascular conditions.
Figure 3.
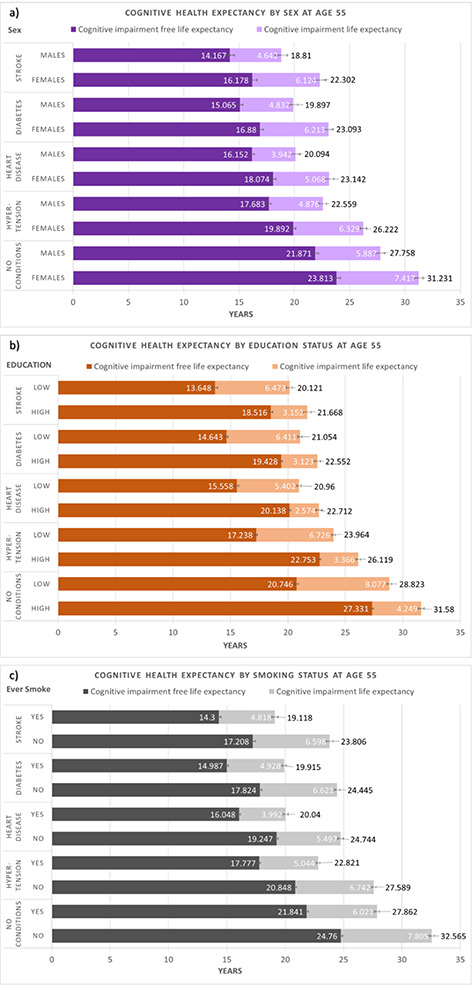
LE, CIFLE and CI estimates at age 55 in individuals with stroke, diabetes, heart disease, hypertension and no conditions by (a) sex, (b) education and (c) smoking status. Error bars represent 95% confidence interval. LE is reported at the end of each bar.
Education
Within each cardiovascular condition, on average, those who had a lower educational attainment (i.e. high school degree or below) had a lower LE, lower CIFLE and higher CILE compared with those who had higher educational attainment (i.e. college degree or above). Those who had lower education also had a higher CILE and lower CIFLE as a proportion of total LE compared with those with higher education (10–20% difference at age 55). This effect was consistent across all conditions (Fig. 3b).
Comparing between conditions, the magnitude of difference between the two education groups was greater in individuals with no reported conditions compared with individuals with stroke, diabetes and heart disease (by ~1 year in LE and 2 years in CIFLE at age 55).
Smoking
Within each cardiovascular condition, on average, those who had reported never smoking in their lives had a higher absolute LE, CIFLE and CILE compared with individuals who had reported smoking sometime in their lives. Those who had reported never smoking in their lives also had a relatively higher CILE and lower CIFLE as a proportion of total LE compared with individuals who had reported smoking sometime in their lives (1–3% difference at age 55). This effect was consistent across all conditions (Fig. 3c). The magnitude of differences was similar across cardiovascular conditions.
For a tabular representation of the data on covariates see Supplementary Material A4.
Discussion
Quantifying the relationship between cardiovascular disease and cognitive health expectancy
The results estimated that having a cardiovascular condition is associated with an ~5- to 9-year decrease in LE and a 4- to 8-year decrease in CIFLE at age 55. When comparing diabetes, hypertension, heart disease and stroke with each other, a hierarchy of LE and CIFLE emerged. The findings of a hierarchy of LE across cardiovascular conditions are consistent with previous studies [15]; however, this is the first study to show that this effect also occurs with CIFLE.
Stroke was associated with the shortest LE and CIFLE, followed by diabetes, heart disease, and then hypertension. Compared with people who have no reported conditions, a history of stroke was related to a 9-year reduction in LE and almost 8-year reduction in CIFLE at age 55. Having a history of stroke was also associated with a ~4% increase in the proportion of life lived with CI at age 55. This suggests that preferencing a reduction in the incidence of stroke could be beneficial in helping to reduce the overall population estimates of life spent lived with CI.
Individuals with a history of heart disease and diabetes had similar LE’s (8 years shorter than individuals with no reported conditions); however, individuals with heart disease had slightly better outcomes as they showed proportionally more years lived in good cognitive health (5% or 1 year more at age 55). Notably, individuals with heart disease were also found to have proportionally more life lived in good cognitive health and less life lived in a cognitively impaired state compared with people with no reported conditions at age 55. Future research should investigate whether these results could be attributed to the protective effects of specific heart disease medication use and/or is a reflection of the fact that the category for ‘heart disease’ in the HRS encompass a range of different heart conditions with varying severities and biological mechanisms, which may have differing relationships with CI.
Additive effect of cardiovascular conditions on cognitive health expectancy
Analysis of individuals with multiple cardiovascular conditions showed that the accumulation of each additional condition was associated with an exponential reduction in LE and CIFLE. These results are in line with recent findings on the dose–response effect of multiple risk factors in increasing dementia risk [16] and reinforce the need for individuals with pre-existing cardiovascular conditions to avoid exposure to additional cardiovascular risk factors in order to maintain cognitive health.
Covariate analysis
The effects of smoking, sex and education were largely in line with current literature. Previous research has found that smoking is related to risk of cardiovascular disease [17] and CI [18]. The results from this study showed that, on average, individuals who had never smoked had a longer LE, CIFLE and CILE compared with individuals who had a history of smoking. The results suggest that abstaining from cigarettes may delay the onset of CI, but also increasing years lived with CI. This is in line with previous findings on smoking and cognitive health [19]. Anstey et al. [19] found that while smoking reduced LE, it also ‘decreased’ years lived with CI due to the ‘longevity paradox’. This is because longevity (or increased age) itself increases the risk of CI. In a similar vein, women, on average, live longer than men [20]; however, as a result of their longer lifespan, they also live a larger proportion of their lives in a cognitively impaired state [2]. The results from this study showed that this ‘longevity paradox’ for both smoking and sex holds true in the context of cardiovascular disease and does not differ across cardiovascular conditions.
Consistent with the current literature [21], individuals with higher educational attainment had, on average, a longer LE and longer CIFLE compared with individuals with lower educational attainment. Higher educational attainment was also associated with a relatively larger proportion of life lived in good cognitive health (around 15% more at age 55 compared with those who had lower educational attainment) indicating that higher educational attainment is protective against CI. However, the results indicated that the protective effect of education is dampened slightly in individuals with stroke, heart disease and diabetes by ~2 years at age 55.
Limitations and future directions
This study drew on data from a nationally representative sample to quantify the relationship between cardiovascular disease and cognitive health expectancy. However, the results should be interpreted in the context of some limitations. Firstly, data on cardiovascular condition status in the HRS was obtained by self-report, which means that the prevalence of diabetes and hypertension may be underestimated due to undiagnosed cases [22,23]. In addition, there was no information on how well the conditions were managed. Secondly, we were not able to include ethnicity as a covariate in our study as 82% of the sample was White/Caucasian. This means that there was not enough data in each ethnic category to calculate the relevant LE’s for each cardiovascular condition. However, as incidences of cardiovascular disease, cognition scores, educational attainment and smoking are likely to differ depending on ethnic background, ethnicity is an important covariate that should be included in future studies when possible. Thirdly, the influence of lifestyle factors on cardiovascular conditions and cognitive healthy expectancy should be considered by future studies. A healthy diet and physical activity are likely to have an impact on both cognitive and cardiovascular health. Unfortunately we were unable to examine these factors as there was either no information collected (diet) or the coding was inconsistent across the waves (physical activity). Future studies should take into account the effects of cardiovascular risk factors in midlife versus late-life and their impact on cognitive health. Some cardiovascular conditions have been found to differentially relate to cognitive decline and dementia in midlife and late-life [24,25]. Future research should aim to separate cardiovascular risk factors by the stage of life they occur in, to better understand the relationship between age at exposure and cognitive health expectancies.
Conclusion
Cognitive health expectancy estimates allow us to quantify the impact that each health condition has on the quality of life lived. The results showed that having a cardiovascular condition is associated with a decrease in LE (by 5–9 years at age 55) and CIFLE (by 4–8 years at age 55). Individuals with stroke had the worst cognitive health expectancy outcomes, indicating that dementia risk reduction may be most beneficial for individuals with stroke compared with the other cardiovascular conditions. Each additional cardiovascular condition was found to be associated with an exponential decrease in LE and CIFLE, highlighting the need to keep multiple cardiovascular risk factor exposure to a minimum. Higher educational attainment helped to reduce years lived with CI; however, the protective effect was reduced in individuals with stroke, heart disease and diabetes. Overall, the results from this study provide a number of insights into the relationship between cardiovascular disease and cognitive health expectancy and highlight the areas that may prove most effective targets for dementia risk reduction from a population standpoint.
Supplementary Material
Declaration of conflicts of interest
None.
Declaration of sources of funding
The National Health and Medical Research Council Centre of Research Excellence in Cognitive Health (APP1100579). The financial sponsors played no role in the design, execution, analysis and interpretation of data or writing of the study. The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan.
References
- 1. Saito Y, Robine J-M, Crimmins EM. The methods and materials of health expectancy. Stat J IAOS 2014; 30: 209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suthers K, Kim JK, Crimmins E. Life expectancy with cognitive impairment in the older population of the United States. J Gerontol B 2003; 58: S179–86. [DOI] [PubMed] [Google Scholar]
- 3. Knopman D, Boland LL, Mosley T et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56: 42–8. [DOI] [PubMed] [Google Scholar]
- 4. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 2005; 48: 2460–9. [DOI] [PubMed] [Google Scholar]
- 5. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–18. [DOI] [PubMed] [Google Scholar]
- 6. Deckers K, Schievink SHJ, Rodriquez MMF et al. Coronary heart disease and risk for cognitive impairment or dementia: systematic review and meta-analysis. PloS One 2017; 12: e0184244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol 2014; 43: 576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langa KM, Kabeto M, Weir D. Alzheimer's disease facts and figures. Alzheimers Dement 2010; 6: 158–94. [DOI] [PubMed] [Google Scholar]
- 9. Díaz-Venegas C, Schneider DC, Myrskylä M, Mehta NK. Life expectancy with and without cognitive impairment by diabetes status among older Americans. PLOS ONE 2017; 12: e0190488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langa KM, Kabeto M, Weir D. Report on race and cognitive impairment using HRS in 2010 Alzheimer’s Dis Facts Figures, vol. 12, 2010; 2010. [Google Scholar]
- 11. Plassman BL, Langa KM, Fisher GG et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007; 29: 125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Health and Retirement Study, (RAND HRS Longitudinal File 2014) public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute of Aging (grant number NIA U01AG009740). Ann Arbor, Mi, 2018. [Google Scholar]
- 13. RAND HRS Longitudinal File 2014. Produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration. Santa Monica, CA, 2018. [Google Scholar]
- 14. Lièvre A, Brouard N, Heathcote C. The estimation of health expectancies from cross-longitudinal surveys. Math Popul Stud 2003; 10: 211–48. [Google Scholar]
- 15. Jia H, Zack MM, Thompson WW. The effects of diabetes, hypertension, asthma, heart disease, and stroke on quality-adjusted life expectancy. Value Health 2013; 16: 140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peters R, Booth A, Rockwood K, Peters J, D’Este C, Anstey KJ. Combining modifiable risk factors and risk of dementia: a systematic review and meta-analysis. BMJ Open 2019; 9: e022846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tonstad S, Johnston JA. Cardiovascular risks associated with smoking: a review for clinicians. Eur J Cardiovasc Prevention Rehab 2006; 13: 507–14. [DOI] [PubMed] [Google Scholar]
- 18. Anstey KJ, Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol 2007; 166: 367–78. [DOI] [PubMed] [Google Scholar]
- 19. Anstey KJ, Kingston A, Kiely KM, Luszcz MA, Mitchell P, Jagger C. The influence of smoking, sedentary lifestyle and obesity on cognitive impairment-free life expectancy. Int J Epidemiol 2014; 43: 1874–83. [DOI] [PubMed] [Google Scholar]
- 20. Hausmann R. The global gender gap report 2009. Geneva, Switzerland: World Economic Forum, 2009. [Google Scholar]
- 21. Matthews FE, Jagger C, Miller LL, Brayne C, Mrc C. Education differences in life expectancy with cognitive impairment. J Gerontol A Biol Sci Med Sci 2009; 64: 125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wall HK, Hannan JA, Wright JS. Patients with undiagnosed hypertension: hiding in plain sight patients with undiagnosed hypertension patients with undiagnosed hypertension. JAMA 2014; 312: 1973–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract 2014; 103: 150–60. [DOI] [PubMed] [Google Scholar]
- 24. Kloppenborg RP, Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol 2008; 585: 97–108. [DOI] [PubMed] [Google Scholar]
- 25. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement 2015; 11: 718–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


