Abstract
Background
sarcopenia is the loss of muscle mass and quality and is diagnosed using measures of muscle strength, size and mass. We evaluated the literature on whether sarcopenia measures are predictive of motor outcomes in older people in clinical settings.
Methods
electronic databases (MEDLINE Ovid, EMBASE, CINAHL and Web of Science) were searched for articles on measures of muscle mass, volume, thickness or strength, in older people in clinical settings, which reported cross-sectional or longitudinal associations with motor outcomes. Clinical cohorts included geriatric medical inpatients and outpatients, patients with hip fracture, geriatric rehabilitation and care home residents. Motor outcomes were mobility, falls, balance and activities of daily living (ADL). Due to high study heterogeneity, standardised mean differences were used to compare strength of associations.
Results
in total, 83 articles were identified. The most frequently studied measures were grip strength (47 studies), knee extension strength (21 studies) and bioelectrical impedance analysis (18 studies). Handgrip strength (HGS) had evidence for cross-sectional associations with mobility (14 of 16 studies, 2,088 participants), balance (6 of 6 studies, 1,177 participants) and ADL independence (10 of 11 studies, 3,228 participants), and evidence of longitudinal associations with mobility (3 of 3 studies, 883 participants) and ADL independence (7 of 10 studies, 1,511 participants). There was no conclusive evidence for association with falls.
Conclusions
HS was the most studied measure and was associated with mobility, balance and ADL outcomes. There was a paucity of studies, particularly with longitudinal follow-up, measuring muscle mass, volume or thickness using gold-standard approaches.
Keywords: sarcopenia, muscle strength, systematic review, older people, clinical settings
Key points
HGS associated with mobility, balance and ADL outcomes in clinical settings.
No measure of muscle mass or strength clearly associated with falls.
There was a paucity of studies measuring muscle mass, volume or thickness using gold-standard approaches.
Introduction
Sarcopenia is the progressive age-related decline in muscle mass, quality, strength and performance. It is thought to underpin declining physical function with increasing age [1] and growing evidence suggests it can be modified through exercise and nutritional interventions [2, 3]. Therapies targeting sarcopenia can potentially improve physical function, dependency, hospitalisation and mortality [4].
In clinical settings, diagnosis of sarcopenia is limited by accuracy and feasibility of measures in those with frailty and functional deficits [5]. Muscle size and strength measures used hitherto in sarcopenia research have predominantly been validated in community-dwelling volunteers, who are characteristically more robust than patients presenting to healthcare. To be clinically useful, measures need to have been used and validated in patients recruited from, and representative of, those seen in clinical settings.
A clinically useful measure could act as a proxy measure of sarcopenia, when a person is unable to perform gold-standard assessments such as magnetic resonance imaging (MRI) or dual-energy X-ray absorptiometry (DXA)—this could be established through cross-sectional studies. Alternatively, a measure might act as a predictive biomarker, enabling anticipation of future adverse outcomes such as poor mobility and falls—this would be established through longitudinal studies.
This review aimed to describe the measures of muscle mass and strength that have been studied in clinical settings and the degree to which they have been associated—both cross-sectionally and longitudinally—with motor outcomes.
Objective
To conduct a systematic review of the literature on clinical usefulness of measures used to diagnose sarcopenia in older people in clinical settings.
Methods
Study protocol and registration
The study protocol was published online in October 2017 on the PROSPERO database registration number CRD42017079957: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017079957
Eligibility criteria
Population
Primary studies of clinical populations aged ≥65 or with a mean age ≥70 years were included. Studies conducted in settings known to routinely care for older people with frailty, including acute, subacute and long-term care, specialist services for people with dementia or hip fractures and geriatric medicine outpatients were included. To avoid studies where associations between sarcopenia and motor outcomes could be confounded, we excluded studies of patients with cancer, osteoarthritis, heart failure, stroke, neurological injury, lung, liver or renal disease, obesity, mood disorders and those with postabdominal surgery or in critical care. Studies that considered nutritional interventions for undernutrition or malnutrition were excluded, because the mechanisms of muscle loss and dysfunction in this context are different [1,6].
Measures of sarcopenia
Studies that measured muscle mass, thickness or volume, measuring strength of either the upper or lower extremities, were included. Studies using peak expiratory flow as a measure were excluded because this is influenced by factors other than sarcopenia, including pulmonary compliance and lung disease. Masseter muscle tension was excluded as it is not a standard strength measure. Studies assessing chair stand repetitions for strength were excluded as this was considered a motor outcome measure. Studies assessing muscle power were excluded because of the wide variation in testing velocities and need for expensive equipment.
Outcomes
The clinical outcomes of interest were those directly or strongly related to motor outcomes: mobility, balance, falls and activities of daily living (ADL). Mobility outcomes were defined as any timed walking or composite mobility test.
Study design
The review included observational and intervention studies. Case-control studies and case reports were excluded. Studies with <10 participants were excluded due to small effect size.
Search strategy and information sources
A systematic literature search was conducted in the MEDLINE Ovid, EMBASE, CINAHL and Web of Science databases on 5 February 2020. Supplementary Material A2 shows an example of search strategy. No forward citation search was done.
Study selection and data extraction
Two authors (E.L. and A.L.G.) independently screened retrieved titles and abstracts to identify potentially relevant studies. Disagreement was resolved by discussion and consensus. Full-text reviews were conducted by two authors (drawn from E.L., T.O. and A.L.G.) working independently using a standardised data extraction template. Studies that simultaneously assessed different types of muscle measure for one outcome or assessed one measure with different outcomes were included, and each association was analysed separately.
Data extracted from each article comprised: first author, publication year, country of study, study design, healthcare setting or clinical group, sample size, average age, muscle mass or strength technique and motor outcome. Correlation coefficients, odds ratios, mean differences, P values and linear regression statistics were extracted from the full-text studies for inclusion in the synthesis. Studies with no data to support the claim of association were excluded. To compare the magnitude of effect sizes across studies with different metrics, extracted statistics were converted into the standardised mean difference (Cohen’s d) as a common index [7]. Associations were defined as strong (d ≥ 0.80), moderate (0.5 ≤ d > 0.8), weak (0.2 ≤ d > 0.5) or none (d < 0.2).
Quality assessment
Methodological quality of the included studies was assessed using the Appraisal tool for Cross-Sectional Studies (AXIS) [8]. This comprises 20 questions appraising methods and risk of bias in cross-sectional studies. The score was converted into a percentage for comparability. AXIS rating ≥75, 50–74% and <50% were considered good, moderate and poor quality, respectively. Conference abstracts that did not provide sufficient information for appraisal were included but not rated. Results are displayed in Supplementary Material A4.
Results
Study selection
The search identified 5,634 articles, of which 989 were duplicates. Title and abstract screening excluded 4,452 articles, leaving 193 articles for full-text review. In total, 110 of these were excluded; the reasons and process are summarised in Figure 1.
Figure 1.
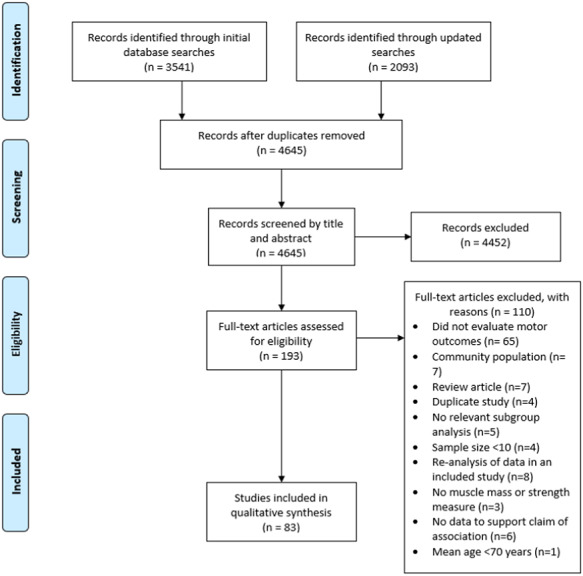
Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram for the selection of studies on measures of muscle strength, muscle mass and motor outcomes.
Study characteristics
Of the 83 studies included in the review, 50 were cross-sectional, 30 longitudinal and 3 described baseline data of interventional trials. Publication dates ranged from 2000 to 2019 and articles came from 26 countries. Patients were recruited from inpatient wards (n = 19), geriatric medicine outpatient clinics (n = 10), rehabilitation wards (n = 5), care homes (n = 26) and patients with hip fractures (n = 23).
Muscle measures and clinical outcomes
Six measures of muscle mass and four measures of muscle strength were studied in clinical populations (Table 1). The most studied muscle measures were grip strength (47 studies), knee extension strength (KES) (21 studies) and bioelectrical impedance analysis (BIA, 18 studies). Outcomes studied were mobility (39 studies), balance (8 studies), falls (17 studies) and ADLs (33 studies). Other measures evaluated by a small number of studies were included in the analysis and summary tables but not discussed further [9–16], mainly for brevity. An overview of cross-sectional associations between measures and outcomes is provided in Table 2 and evidence from longitudinal studies is summarised in Table 3.
Table 1.
Number of studies of each measure and outcome by health care setting
| Inpatient | Outpatient | Rehabilitation | Care home | Hip fracture | Total number of studies | ||
|---|---|---|---|---|---|---|---|
| Measures for muscle strength | HGS | 15 | 6 | 2 | 13 | 11 | 47 |
| KES | 2 | 4 | 2 | 9 | 4 | 21 | |
| Lower extremity strength (excluding KES) | 1 | 1 | 2 | 6 | 5 | 15 | |
| Upper extremity strength (excluding HGS) | 0 | 0 | 0 | 2 | 0 | 2 | |
| Measures for muscle mass or size | BIA | 8 | 3 | 2 | 4 | 1 | 18 |
| Anthropometry | 0 | 0 | 0 | 5 | 2 | 7 | |
| Ultrasound | 1 | 0 | 1 | 1 | 0 | 3 | |
| DXA | 0 | 0 | 0 | 1 | 7 | 8 | |
| CT | 0 | 0 | 0 | 1 | 0 | 1 | |
| MRI | 0 | 0 | 0 | 1 | 0 | 1 | |
| Types of motor outcomes | Mobility (timed walking test, TUG, chair rises, SPPB and De Morton Mobility Index) | 10 | 6 | 3 | 14 | 6 | 39 |
| Balance (Berg balance scale, timed standing balance, CTSIB) | 0 | 2 | 2 | 4 | 0 | 8 | |
| Falls | 4 | 4 | 0 | 6 | 3 | 17 | |
| ADLs (Katz Index, Barthel Index, FIM, Mobility items of ADLs) | 10 | 1 | 1 | 6 | 15 | 33 |
TUG, timed-up and go test; SPPB, Short Physical Performance Battery; CTSIB, Clinical Test of Sensory Interaction for Balance; FIM, functional Independence Measure.
Table 2.
Summary of cross-sectional evidence, with number of studies and strength of associations between measure and outcome
| Measure | Outcome: No. = number of studies evaluating measure and outcome Strength of association: s = strong/m = medium/w = weak/n = no association | |||
|---|---|---|---|---|
| Mobility | Balance | Falls | ADL | |
| No.: s/m/w/n | No.: s/m/w/n | No.: s/m/w/n | No.: s/m/w/n | |
| HGS | 16a: 5/6/3/2 | 6: 3/2/1/0 | 6: 0/0/2/4 | 11: 5/5/0/1 |
| KES | 10: 6/1/0/3 | 2: 0/1/0/1 | 4: 0/2/0/2 | 2: 1/0/1/0 |
| Lower extremity strength | 8: 5/1/0/2 | 2: 1/0/0/1 | 2: 0/1/0/1 | 1: 0/0/0/1 |
| Upper extremity strength (excluding HGS) | 1: 1/0/0/0 | 0: 0/0/0/0 | 0: 0/0/0/0 | 1: 0/1/0/0 |
| BIA | 8: 0/1/3/4 | 1: 0/0/0/1 | 0: 0/0/0/0 | 7: 0/2/2/3 |
| Anthropometry | 0: 0/0/0/0 | 0: 0/0/0/0 | 0: 0/0/0/0 | 2: 1/0/0/1 |
| DXA | 1: 0/0/0/1 | 0: 0/0/0/0 | 0: 0/0/0/0 | 0: 0/0/0/0 |
| Ultrasound | 3a: 1/2/0/0 | 1: 0/0/0/1 | 0: 0/0/0/0 | 0: 0/0/0/0 |
| CT | 1: 0/0/0/1 | 0: 0/0/0/0 | 0: 0/0/0/0 | 0: 0/0/0/0 |
| MRI | 0: 0/0/0/0 | 0: 0/0/0/0 | 0: 0/0/0/0 | 0: 0/0/0/0 |
aStrength of association dependent on mobility measure in one study.
Table 3.
Summary of longitudinal evidence for each muscle size or strength measure at baseline and motor outcome at follow-up
| Measure | Outcome: No. = number of studies evaluating measure and outcome | ||
|---|---|---|---|
| Direction of association: p = positive/ng = negative/nu = neutral | |||
| Mobility | Falls | ADL | |
| No.: p/ng/nu | No.: p/ng/nu | No.: p/ng/nu | |
| HGS | 3: 3/0/0 | 5: 0/2/3 | 10: 7/0/3 |
| KES | 2: 1/0/1 | 3: 0/2/1 | 0 |
| Lower extremity strength | 0 | 2: 0/0/2 | 1: 0/0/1 |
| BIA | 0 | 3: 0/1/2 | 2: 1/0/1 |
| Anthropometry | 0 | 2: 0/1/1 | 3: 0/0/3 |
| DXA | 1: 0/0/1 | 0 | 7: 1/0/6 |
| Ultrasound | 0 | 0 | 1: 0/0/1 |
| MRI | 0 | 0 | 1: 0/0/1 |
Zero is given alone when there were no longitudinal studies between that measure and outcome.
Mobility
Cross-sectional studies reported positive associations between handgrip strength (HGS) and mobility in 14 of 16 studies, between KES and mobility in 7 of 10 studies, between lower extremity strength and mobility in 6 of 8 studies and between BIA and mobility in 4 of 8 studies.
The strength of associations from 14 cross-sectional studies between HGS and mobility were strong (5 studies) [17–21], moderate (6 studies) [22–27] and weak (3 studies) [28–30]. They included 2,088 participants and were undertaken across the full range of clinical settings. However, in two studies, the strength of association was dependent on the mobility measure used [21, 30], in a third on the hand dominance used for HGS [28] and, in a fourth on gender, with a strong association for men and a moderate association for women [23]. There was no link between HGS and mobility in two cross-sectional studies. One was small (n = 22) and published as a conference abstract [31], whereas the other (n = 163) reported a univariate association that was lost when KES was adjusted for [32]. The studies were rated good or moderate quality.
For KES and mobility, 7 of 10 cross-sectional studies reported medium to strong associations (total n = 459) [18, 19, 33–37]. Three cross-sectional studies (total n = 67) found no association with mobility [31, 38, 39]. All studies were of good or moderate quality.
Six of eight cross-sectional studies reported medium to strong associations between lower extremity strength and mobility (total n = 291) [27, 34, 35, 40–42], although two of these studies reported mixed association strengths, depending on which lower limb muscle group was tested [34, 35]. Two cross-sectional studies found no association (total n = 41) [38, 43]. No consistent technique was used to measure lower extremity strength. All studies were of good or moderate quality.
Four of eight cross-sectional studies reported weak to medium association between BIA and mobility (total n = 1,427) [24, 44–46]. No association was reported in four studies (n = 718) [23, 26, 40, 47]. The studies were of good or moderate quality.
Mobility was used as an outcome in longitudinal studies of three measures: HGS (three studies), KES (two studies) and DXA (one study). For HGS, three of three longitudinal studies (n = 883) found an association with mobility at discharge from hospital [48, 49] or 3 months following discharge [50]. For KES, one study reported a positive association with mobility at 3-year follow-up (n = 95) [51], whereas the other study (n = 137) reported no association with mobility at discharge from hospital [52]. For DXA, one study (n = 123) reported no association with mobility at discharge [48]. Quality rating was good or moderate for all studies.
In summary HGS had the strongest associations with mobility, both cross-sectionally and longitudinally. There was limited or inconclusive evidence for other measures.
Balance
All studies examining balance were cross-sectional. HGS was the most studied measure with six of six studies reporting positive associations with balance. The associations were strong (three studies, n = 439) [21, 53, 54], medium (two studies, n = 529) [28, 55] and weak (one study n = 209) [30]. The six studies were rated good or moderate quality.
Falls
Cross-sectional studies of HGS (two of six studies), KES (two of four studies) and lower limb strength (one of two studies) found that lower strength was associated with a history of falls.
For HGS, two cross-sectional studies (n = 454) reported weak associations [56, 57]. Four cross-sectional studies reported no link (n = 309) [32, 58–60], particularly when results were adjusted for confounders, namely lower limb strength [32, 58], suggesting HGS may not be independently associated with falls history. For KES and falls history, two cross-sectional studies found a medium association with historical falls (total n = 114) [58, 61], whereas two cross-sectional studies reported no association (n = 40) [31, 62]. All studies were of good or moderate quality.
The most studied measures predicting incident falls in longitudinal studies were HGS (two of five studies), KES (two of three studies) and BIA (one of three studies).
Two longitudinal studies (n = 758) reported lower HGS was weakly associated with increased falls risk at 1 year [63, 64]. Three studies found no association between HGS and falls at 3 months (n = 297) [57] or at 1 year (n = 691) [65, 66]. Studies were of good or moderate quality.
Lower KES predicted falls risk in two studies, one at 6 months (n = 69) [67] and one at 1 year (n = 171) [68]. A third study found no association at 1 year (n = 565) [63]. Studies were rated as good or moderate quality.
One longitudinal study found that lower muscle mass measured with BIA weakly predicted falls occurrence at 3 months (n = 297) [57], whereas two longitudinal studies (n = 365) reported no association [46, 66]. Studies were rated as good or moderate quality.
ADL
The most studied cross-sectional associations with ADL ability were HGS (11 studies) and BIA (7 studies).
Higher HGS had medium to strong associations with higher ADL scores in 10–11 studies (n = 3,228) [14, 25, 59, 69–75]. The associations were regardless of ADL measure used and were seen in all clinical settings except outpatients [32].
Four of seven cross-sectional studies reported weak to medium associations between BIA and ADL ability (total n = 1,663) [70, 73, 76, 77], although two of these assessed only mobility ADLs [70, 77] rather than full ADL performance. Three studies showed no association (total n = 355) [45, 72, 78]. The studies were of good or moderate quality.
Longitudinal associations for ADL outcomes were seen for HGS (10 studies) and DXA (7 studies).
In total, 7 of 10 longitudinal studies reported higher HGS associated with ADL ability (n = 1,511). These associations were sustained to hospital discharge [79, 80], 3-month [74, 81], 6-month [74, 79, 82, 83] and 12-month [84] follow-up. Three longitudinal studies reported no association after adjustment for other variables (total n = 775) at 3 weeks [85], 3 months [75] or 1 year [86]. All the studies were conducted in inpatient or hip fracture settings and the studies were of good or moderate quality.
Longitudinal studies of DXA measured muscle mass and ADL scores reported an association in only one of six studies measured at the point of discharge [87]. It was small (n = 27) and only included male patients. Six studies found no link between DXA and ADL ability, either at discharge or after 1 year [48, 84, 88–91]. These studies only included participants following a hip fracture (n = 911). All studies were moderate quality. However, six of the seven studies were from the same author [48, 87–91] with two studies including the same cohort of participants assessed at different time points [48, 90].
Discussion
The review identified 83 studies investigating associations between measures of muscle mass or strength and measures of motor function in clinical populations of older adults. It found evidence of cross-sectional associations between of handgrip and motor outcomes measuring mobility, balance and ADL ability. Longitudinal associations were found between HGS and motor outcomes around mobility and ADL performance. For associations between other measures and outcomes, evidence was either absent or inconclusive.
Our findings about HGS match findings in healthy older volunteers, where it is a predictor of mobility, disability and mortality [92–95]. In contrast, lower limb strength, in particular KES, predicts mobility and ADL outcomes in healthy adults [96–98] but not in the clinical populations included here. This could, in part, be explained by the differing strength measurements and different combinations of muscle groups assessed between studies included in this review. Adopting a standardised approach to measuring lower limb strength in older adults in clinical settings would make these data easier to analyse and understand in the future.
It was unexpected to find no link between muscle strength or mass, and falls, given that these are frequently reported to be associated [99, 100]. Difficulties with measuring lower limb strength in populations with frailty may have contributed, as may the difficulty of accurately recording falls in patients with cognitive impairment. Possibly measures of muscle size and strength are not good biomarkers of falls. Dynamic measures, such as balance and muscle power, may be more predictive.
Our findings reinforce the focus of EWGSOP2 [101] on muscle strength as opposed to gait speed when screening for sarcopenia. Included studies in the review reported only 3–6% were unable to perform HGS [44, 45] compared with 25–41% of participants unable to perform gait speed [28, 44, 45], and 66% of participants in one study unable to perform chair stand repetitions [28]. Although the review found insufficient evidence for the use of other measures of muscle mass and strength to predict motor outcomes, it highlighted current gaps in the literature.
The review highlighted a lack of studies using measures of muscle mass, size or thickness that could be viewed as ‘gold standard’ for assessing sarcopenia. Computed tomography (CT), MRI and DXA are in widespread clinical use and it is surprising that these measures have had little evaluation in clinical populations for sarcopenia diagnosis. Publication bias, with a tendency not to publish negative findings, may have contributed to this; however, this is unlikely, given that 9 of the 10 studies of these measures reported no association with motor outcomes. Challenges to clinical research with CT, MRI or DXA might include higher costs, radiation exposure and impracticality for bedside use. Although there were eight studies of DXA measures, seven were in hip fracture patients where pain and discomfort may have influenced functional and motor outcome measures. Further studies in other clinical settings are needed.
Ultrasound has shown promise for measuring muscle size in patient cohorts [102, 103]. It has good validity against MRI, but more evidence is needed around use in older people. Novel methods including isotope tracers correlate well with MRI-measured muscle volume [104] and predict physical function and falls rates in community-dwelling older men [105], but evidence for their in clinical situations is limited.
Strengths and limitations of the study
The review had a broad focus that enabled a wide range of measurement methods to be included. The search strategy was robust and reproducible, using comprehensive search terms in multiple electronic databases, and methodological quality was assessed using a standardised checklist. The results were not restricted by a particular sarcopenia definition to include studies that pre-dated published criteria, and trends were identified independent of contentious threshold values. However, the wide inclusion criteria and substantial heterogeneity between the eligible studies limited comparisons and precluded meta-analysis. Transforming the extracted statistics into a common index to compare effect sizes made assumptions about measurement similarities. It is possible that these assumptions did not hold universally true. The designation of ‘clinical’ settings mixed both acute and stable populations. Muscle mass and strength values have been known to change rapidly in acute populations, which may introduce some greater heterogeneity into findings that could have been negated if only stable outpatient populations had been addressed.
Implications for practice and research
This study supports the use of HGS in clinical settings both for its ability to act as a cross-sectional proxy for gold-standard sarcopenia measures where these are not feasible, and as a predictor of future adverse outcomes related to predict mobility and independence with ADL in older people. It reinforces the focus of EWGSOP2 [101] on muscle strength as opposed to gait speed when screening for sarcopenia in clinical settings.
Supplementary Material
A full reference list is provided in Supplementary Material A1.
Declaration of Conflicts of Interest
None. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute of Health Research or the Department of Health and Social Care.
Declaration of Sources of Funding
National Institute for Health Research Nottingham Biomedical Research Centre.
References
- 1. Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010; 39: 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz-Jentoft AJ, Landi F, Schneider SM et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing 2014; 43: 748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaudart C, Dawson A, Shaw SC et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017; 28: 1817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beaudart C, Zaaria M, Pasleau F et al. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One 2017; 12: e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borenstein MH, Hedges LV, Higgins JPT, Rothstein HR. Converting among effect sizes In: Introduction to Meta-Analysis. Chichester, UK: L. John Wiley & Sons, 2009. [Google Scholar]
- 8. Downes MJ, Brennan ML, Williams HC et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016; 6: e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen PJ, Lin M-H, Peng L-N et al. Predicting cause-specific mortality of older men living in the veterans home by handgrip strength and walking speed: a 3-year, prospective cohort study in Taiwan. J Am Med Dir Assoc 2012; 13: 517–21. [DOI] [PubMed] [Google Scholar]
- 18. Martien S, Delecluse C, Boen F et al. Is knee extension strength a better predictor of functional performance than handgrip strength among older adults in three different settings? Arch Gerontol Geriatr 2015; 60: 252–8. [DOI] [PubMed] [Google Scholar]
- 21. Caballer VB, Lisón JF, Rosado-Calatayud P et al. Factors associated with the 6-minute walk test in nursing home residents and community-dwelling older adults. J Phys Ther Sci 2015; 27: 3571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuesta F, Formiga F, Lopez-Soto A et al. Prevalence of sarcopenia in patients attending outpatient geriatric clinics: the ELLI study. Age Ageing 2015; 44: 807–9. [DOI] [PubMed] [Google Scholar]
- 28. Bergland A, Narum I, Grönstedt H et al. Evaluating the feasibility and intercorrelation of measurements on the functioning of residents living in Scandinavian nursing homes. Phys Occup Ther Geriatr 2010; 28: 154–69. [Google Scholar]
- 44. Bianchi L, Abete P, Bellelli G et al. Prevalence and clinical correlates of sarcopenia, identified according to the EWGSOP definition and diagnostic algorithm, in hospitalized older people: the GLISTEN study. J Gerontol A Biol Sci Med Sci 2017; 72: 1575–81. [DOI] [PubMed] [Google Scholar]
- 45. Rossi AP, Fantin F, Micciolo R et al. Identifying sarcopenia in acute care setting patients. J Am Med Dir Assoc 2014; 15: 303.e7–12. [DOI] [PubMed] [Google Scholar]
- 53. Lauretani F, Maggio M, Ticinesi A et al. Muscle weakness, cognitive impairment and their interaction on altered balance in elderly outpatients: results from the TRIP observational study. Clin Interv Aging 2018; 13: 1437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Söke F, K. HK, Ilgin D, Yuksel E, Ozcan O, Arslan T. Relationship between postural control and hand function in the subjects aged 65 years and over. Turk J Physiother Rehabil 2018; 29: 33–8. [Google Scholar]
- 55. Bijlsma AY, Pasma JH, Lambers D et al. Muscle strength rather than muscle mass is associated with standing balance in elderly outpatients. J Am Med Dir Assoc 2013; 14: 493–8. [DOI] [PubMed] [Google Scholar]
- 63. Buckinx F, Croisier J-L, Reginster J-Y et al. Prediction of the incidence of falls and deaths among elderly nursing home residents: the SENIOR study. J Am Med Dir Assoc 2018; 19: 18–24. [DOI] [PubMed] [Google Scholar]
- 70. Maeda K, Shamoto H, Wakabayashi H et al. Sarcopenia is highly prevalent in older medical patients with mobility limitation. Nutr Clin Pract 2017; 32: 110–5. [DOI] [PubMed] [Google Scholar]
- 92. Al Snih S, Markides KS, Ottenbacher KJ et al. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin Exp Res 2004; 16: 481–6. [DOI] [PubMed] [Google Scholar]
- 93. Rijk JM, Roos PR, Deckx L et al. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int 2016; 16: 5–20. [DOI] [PubMed] [Google Scholar]
- 94. Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther 2008; 31: 3–10. [DOI] [PubMed] [Google Scholar]
- 95. Alexandre Tda S, Oliveira Duarte YA, Santos JLF et al. Sarcopenia according to the European working group on sarcopenia in older people (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutr Health Aging 2014; 18: 751–6. [DOI] [PubMed] [Google Scholar]
- 97. Lauretani F, Russo CR, Bandinelli S et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003; 95: 1851–60. [DOI] [PubMed] [Google Scholar]
- 99. Landi F, Liperoti R, Russo A et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012; 31: 652–8. [DOI] [PubMed] [Google Scholar]
- 100. Moreland JD, Richardson JA, Goldsmith CH et al. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 2004; 52: 1121–9. [DOI] [PubMed] [Google Scholar]
- 101. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Franchi MV, Longo S, Mallinson J et al. Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand J Med Sci Sports 2018; 28: 846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nijholt W, Scafoglieri A, Jager-Wittenaar H et al. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017; 8: 702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Clark RV, Walker AC, O'Connor-Semme RL et al. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985) 2014; 116: 1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cawthon PM, Orwoll ES, Peters KE et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci 2019; 74: 844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


