Abstract
Objective:
To systematically review the literature and synthesize the evidence for the effectiveness of botulinum toxin injection to the pelvic floor muscles for treating pelvic floor myofascial pain in female patients.
Methods:
This systematic literature search was performed in February 2018 and updated in September 2019. Articles were screened based on predefined criteria: 1) adult population, 2) female patients, 3) treatment of pelvic pain by transvaginal botulinum toxin injection into the pelvic floor, 4) published in English or English translation available, 5) study design including randomized controlled trials, cohort studies, and case series with more than 10 participants, and 6) quantitative report of pain scores. 9 studies were included in the primary analysis, and an unpublished study included in a sensitivity analysis. A random effects model with robust variance estimation was used to estimate the pooled mean difference in patient-reported pain scores after botulinum toxin injection.
Results:
A statistically significant reduction in patient-reported pain scores was noted at 6 weeks after botulinum toxin injection (mean difference 20.3, 95% CI 11.7 – 28.9) and continued past 12 weeks (mean difference 19.4, 95% CI 14.6 – 24.2). Significant improvement was noted in secondary outcomes including dyspareunia, dyschezia, and quality of life.
Conclusions:
This systematic review and meta-analysis supports the conduct of future, large-scale randomized controlled trials to determine the efficacy and optimize administration of botulinum toxin injections for treatment of pelvic floor myofascial pain and associated symptoms in women.
Keywords: Pelvic pain, botulinum toxin, pelvic floor myofascial pain
Introduction
Pelvic floor myofascial pain refers to pain arising in the muscles and connective tissue of the pelvic floor [levator ani (LA)] and internal hip [obturator internus (OI)]. It is characterized by the presence of trigger points, tender points, and local and referred pain that may progress to debilitating chronic pelvic pain and negatively influence quality of life.[1–3] Pelvic floor myofascial pain is more common in women than men, and has been associated with a history of fibromyalgia, depression, interstitial cystitis, chronic pelvic pain, prior pelvic surgery/trauma, obstetric trauma, and sexual abuse in several studies.[1, 4, 5] Prevalence estimates vary based on the population of interest, method of examination, and definition used. Estimates range from as low as 17% in pain-free controls[6] to up to 87% in patients with chronic pelvic pain and interstitial cystitis [7] with several authors reporting prevalence estimates within this range[8–11].
Treatment of pelvic floor myofascial pain typically involves pelvic floor physical therapy (PT), often with concurrent treatment of movement impairment disorders of the lumbopelvic region, for example impairments in lumbar flexion/extension, and/or myofascial release.[2, 12] Additional conservative treatment options, including nonsteroidal anti-inflammatory agents, muscle relaxants, and pelvic floor trigger point injections with local anesthetic with or without steroid may be added to the treatment regimen when pain does not resolve with PT alone.[3, 13] Refractory cases, however, can be challenging to treat.
Botulinum toxin injections have been used successfully to treat myofascial pain located elsewhere in the body. Through the inhibition of presynaptic acetylcholine release at the neuromuscular junction, injection of botulinum toxin results in reduced muscle tone and prevention of further muscle spasm.[14] Based on the success of this agent in treating myofascial pain in other musculoskeletal disorders,[15] some physicians have begun incorporating pelvic floor botulinum toxin injections into the treatment of pelvic floor myofascial pain, especially in cases refractory to conservative management strategies like PT. However, data on the use of botulinum toxin for treatment of pelvic floor myofascial pain are limited to case reports, case series, small observational studies, and two small randomized controlled trials (RCTs) that were likely underpowered to detect differences in pain scores. Considered individually, methodologic concerns in each of these studies have likely limited clinical adoption of botulinum toxin injections. However, viewed together, this small body of literature may still provide insight into the utility of botulinum toxin injections. Therefore, we systematically reviewed and synthesized data from all available studies to inform the effectiveness of botulinum toxin injections to the pelvic floor muscles for the treatment of pelvic floor myofascial pain in female patients.
Materials and Methods
Sources
A medical librarian searched the published literature using search strategies for the concepts of Botulinum Toxins Type A, Botox®, onabotulinumtoxinA, pelvic floor, pelvic pain and myofascial pain syndromes. These strategies were established using a combination of standardized terms and key words, and were implemented in PubMed/Medline 1946-, EMBASE 1947-, Scopus 1823-, Cumulative Index of Nursing and Allied Health Literature (CINAHL) 1937-, Web of Science 1900-, PEDro 1929- Cochrane Library and Clinicaltrials.gov. References of the identified studies were reviewed to add any additional relevant studies (one author, MM). All searches were completed in February 2018 and updated in September 2019. The full strategies for Ovid Medline, Embase, Cochrane Library, and Scopus are available in the supplemental material.
Study Selection
We used the following criteria for inclusion: 1) adult, 2) female, 3) treatment of pelvic pain by transvaginal botulinum toxin injection into the pelvic floor, 4) published in English or English translation available, 5) study design including RCTs, cohort studies, and case series with more than 10 participants, and 6) quantitative report of pain scores. Due to the limited literature on use of botulinum toxin injections for treatment of pelvic floor myofascial pain, we included a variety of study designs and studies with any duration of follow-up in order to synthesize all available data.
Titles and abstracts resulting from the systematic search were screened by one author (MM), and articles found to be potentially relevant were retrieved for full-text review. Review of the full texts was performed independently by two authors (MM and AB). Studies were included if they met the aforementioned inclusion criteria. Abstracts, reviews, commentaries, and case series with fewer than 10 patients were excluded. Studies were also excluded if they did not include a quantitative assessment of pain scores after treatment. After abstract and full text screening, the decision was made to include an RCT with results published online on ClinicalTrials.gov, even though the results were not yet published in a peer-reviewed journal. Because this study did not meet our predefined inclusion criteria, it was not included in the primary meta-analysis, but was incorporated in sensitivity analyses.
Data were extracted independently by two authors (MM and AB). Extracted data included study design, inclusion and exclusion criteria, number of patients, demographic data, site of injection, duration of follow-up, and primary and secondary outcomes. Details on the botulinum toxin intervention including total dose received, dose per injected site, method of dilution and final concentration, as well as concomitant and comparative treatments were also collected. Outcomes reported at all study time points (4 weeks, 6 weeks, 12 weeks, and final time points up to 120 weeks post-injection) were collected. In some cases, outcomes were reported in figures only without corresponding text. The relevant scores were estimated from these figures and consensus reached between two authors (MM and AB).
As most studies did not include a comparison group, our primary outcome of interest was mean difference in pain scores after botulinum toxin injection in the botulinum toxin arm of each study. When not reported directly, this was calculated based on pre- and post-treatment pain scores. Included studies used a variety of scales to report pain scores including 0–4, 0–10, and 0–100. Pain scores were converted to a 100-point scale if not originally reported in this manner by multiplying the reported score by 25 or 10, as appropriate. We used a random effects model with robust variance estimation[16] to calculate pooled mean differences, 95% confidence intervals (CIs), and p-values for linear trends. This approach was used to account for inclusion of multiple estimates per study and to estimate between-study variation robustly because I2 can be falsely low with a small number of studies.[17] Due to variability in time points for outcome reporting among the included studies, the primary outcome was assessed at pre-determined time points: less than or equal to 6 weeks, 7–12 weeks, and greater than 12 weeks in order to include and compare data from all studies that met our inclusion criteria. An overall estimate was also calculated using the study endpoint defined by each study.
To investigate the influence of intervention characteristics and study methodology on our findings, we performed several stratified analyses. These included analyses stratified by time since botulinum toxin injection, total dose, method of location of injection site, and use of concomitant therapies. This latter stratified analysis was performed to investigate the independent influence of botulinum toxin on pain scores. To further investigate the independent influence (i.e., independent of a possible placebo effect), we also assessed the difference in the change in pain scores between the botulinum toxin injection and comparator arms (placebo[18, 19] or Kenalog[20]) in the two published and one unpublished RCT. A random effects model with robust variance estimation was used to account for heterogeneity between studies.
Finally, secondary analyses were performed to evaluate changes in outcomes of dysmenorrhea, dyspareunia, dyschezia, SF-12 quality of life scores, and manometry pressure readings at rest and with maximum contraction when reported. Secondary outcomes were assessed at less than 4 weeks, 4–12 weeks, and greater than 12 weeks.
We assessed for the presence of publication bias through visual inspection of a funnel plot at 6 weeks or less following treatment in order for all studies to be included in the assessment of publication bias.
REDCap electronic data capture tool hosted at Washington University in St. Louis[21] was used for data extraction. Statistical analyses were performed in STATA version 15 using the ROBUMETA add-on program (StataCorp LP, College Station, TX). This systematic review and meta-analysis followed guidelines established by Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines and was prospectively registered with PROSPERO (CRD42018093636). This study was exempt from IRB review.
The initial search identified 4448 publications, of which 2016 were duplicates and were removed (Figure 1). The remaining 2432 abstracts were screened by one author (MM) and a further 2315 abstracts were excluded based on predefined inclusion and exclusion criteria. During screening, an additional 2 relevant articles were identified through reference search, resulting in 119 full text articles reviewed. After completion of full text review, 7 studies met criteria for inclusion (Table 1) with 214 patients. The updated search identified an additional 643 studies. After removal of duplicates, there were 417 abstracts for review, which were again screened by one author (MM), and an additional 386 articles were excluded based on predefined inclusion and exclusion criteria, leaving 30 full text articles for review. After completion of full text review, an additional 2 studies met inclusion criteria for a total of 257 participants. One additional RCT was identified on ClinicalTrials.gov that included updated results for all primary and secondary outcomes. Although this study was not published at the time of literature review, the data were included in sensitivity analyses (n=10 participants).
Figure 1.
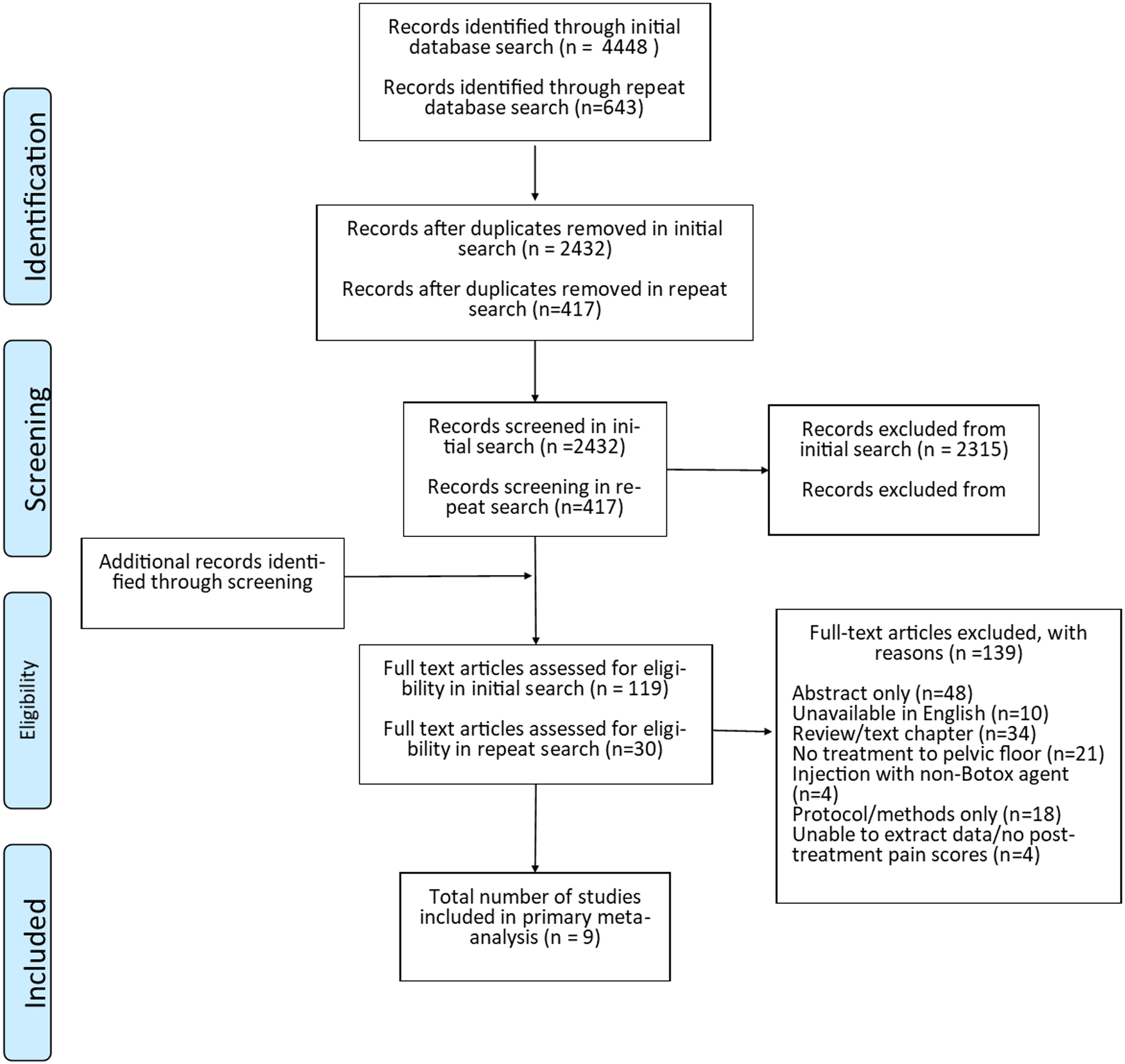
PRISMA flow diagram.
Table 1. Characteristics of studies that evaluated the effectiveness of botulinum toxin injections to the pelvic floor for treatment of pelvic floor myofascial pain in female patients.
CPP, chronic pelvic pain; PFM, pelvic floor muscle; CS, case series; NRS, numeric rating scale; PC, prospective cohort; PT, physical therapy; RC retrospective cohort; RCT, randomized controlled trial; VAS, visual analog scale; VPRS, verbal pain rating scale.
| Study | Study design | Indications | Concomitant Treatment | Comparison Treatment | Pain Score (scale) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CPP | PFM spasm | PFM pain | Vaginismus | Prior treatment failure | PFM trigger points | |||||
| Jarvis, 2004 | PC | √ | √ | None | None | VAS (0–100) | ||||
| Abbott, 2006 | RCT | √ | √ | √ | None | Placebo | VAS (0–100) | |||
| Bertolasi, 2009 | PC | √ | √ | √ | PT | None | VAS (0–100) | |||
| Adelowo, 2013 | RC | √ | √ | √ | None | None | NRS (0–10) | |||
| Nesbitt-Hawes, 2013 | PC | √ | √ | None | Multiple injections | VAS (0–100) | ||||
| Morrissey, 2015 | PC | √ | √ | √ | Pudendal block | None | VPRS (0–4) | |||
| Halder, 2017 | CS | √ | √ | √ | PT | None | NRS (0–10) | |||
| Tandon, 2019 | CS | √ | √ | None | None | VAS (0–10) | ||||
| Dessie, 2019 | RCT | √ | √ | PT | Placebo | VAS (0–10) | ||||
| Bartley, 2019* | RCT | √ | None | Kenalog | VAS (0–10) | |||||
data unpublished; study excluded from primary meta-analysis results
Results
Of the 9 studies included in the primary analysis, there were two randomized placebo-controlled trials,[18, 19] 4 prospective cohort studies,[22–25] 1 retrospective cohort study,[26] and 2 case series with more than 10 participants[27, 28] (Table 1). An additional, unpublished RCT was also identified and included in sensitivity analyses[20]. Chronic pelvic pain was the most common indication for botulinum toxin injection (7/9, 78%),[18–20, 22, 24, 25, 27, 28] and many studies enrolled patients who had failed prior therapies (5/9, 56%).[23, 25–28] Four studies involved concomitant treatment, 3 with pelvic floor physical therapy [19, 23, 27] and 1 with concomitant pudendal block.[25] Two studies compared botulinum toxin injections to a placebo control,[18, 19] and 1 investigated single vs multiple injections.[24] Patients in the multiple-injection arm (i.e. those who had received botulinum toxin injections on more than one occasion) were enrolled from participants in 2 other included studies;[18, 22] thus only patients in the single-botulinum toxin injection arm from this study were included in the analysis to avoid including patients more than once in the meta-analysis. The additional, unpublished RCT was designed for women with chronic pelvic pain and compared botulinum toxin injections to an active control (Kenalog). [20]
Total botulinum toxin dose ranged from 20 international units (IU) to 300 IU in the 9 published studies, with most authors reporting a dose of 20–30 IU per site (5/9, 56%)[18, 24–26, 28] at concentrations ranging from 10 IU/ml to 30 IU/ml (Table 2). Dose per site was not reported in 2 studies.[23, 27] Location for injection was identified solely by digital palpation in the majority of studies, (6/9, 67%)[18, 19, 22, 24, 26, 27] by electromyography (EMG) alone in 1 study (10%),[23] and by combined digital palpation and EMG in two studies (22%).[25, 28] Most studies used botulinum toxin injection into the levator ani muscles (7/9, 78%),[18, 19, 22–26] while 2 studies reported injection into pelvic floor trigger points without specifying muscle groups.[27, 28] The unpublished RCT used a total dose of 200 IU, a dose per site of 33.3 IU, a concentration of 33.3 units/ml, and digital palpation to identify the location of injection into unspecified pelvic floor sites.[20]
Table 2. Botulinum toxin treatment details in studies that evaluated the effectiveness of botulinum toxin injections to the pelvic floor for treatment of pelvic floor myofascial pain in female patients.
C, coccygeus; EMG electromyogram; IC, iliococcygeus; LA, levator ani; NR, not reported; NOS, not otherwise specified; OI, obturator internus; PC, pubococcygeus; PR, puborectalis;
| Study | N | Dose (total units) |
Dose (units/site) | Concentration (units/ml) | Method of detection | Location of injection |
|---|---|---|---|---|---|---|
| Jarvis, 2004 | 12 | 40 | 10 | 10 | Digital palpation | PC, PR |
| Abbott, 2006 | 30 | 80 | 20 | 20 | Digital palpation | PC, PR |
| Bertolasi, 2009 | 39 | 20 | NR | NR | EMG | LA (NOS) |
| Adelowo, 2013 | 29 | 251 | 20 | 10 | Digital palpation | Trigger points, OI, PC, PR, IC, C, pyriformis |
| Nesbitt-Hawes, 2013 | 26 | 100 | 25 | 25 | Digital palpation | PC, PR |
| Morrissey, 2015 | 28 | 300 | 30 | 30 | Digital palpation, EMG | OI, PC, IC, C |
| Halder, 2017 | 50 | 200 | NR | 10 | Digital palpation | Trigger points |
| Tandon, 2019 | 13 | 100 | 25 | 25 | Digital palpation, EMG | Palpable muscle spasm |
| Dessie, 2019 | 30 | 200 | 10 | 10 | Digital palpation | PC, PR, IC, C, OI, pyriformis |
| Bartley, 2019* | 10 | 200 | 33.3 | 33.3 | Digital palpation | Pelvic floor** |
data not published, therefore study not included in primary meta-analysis results.
injections placed at pre-defined sites (1, 3, 5, 7, 9, and 11 o’clock).
Visual analogue scale (VAS) ranging from 0–100 was the most common method of quantifying pain (4/9, 44%; Table 1).[18, 22–24] Two studies used VAS ranging from 0–10[19, 28], 2 studies used numeric rating scales (NRS) ranging from 0–10,[26, 27] and 1 study used a 0–4 visual pain rating scale.[25] The unpublished RCT used a VAS ranging from 0–10.[20] Overall, average pre-treatment pain scores ranged from 45–90 when measured on a 0–100 scale, 5–9.5 on a 0–10 point scale, and 2.8 on a 0–4 point scale. Patients were assessed at a variety of time points in the individual studies with final follow up ranging from 2 to 120 weeks after injection.
Primary Outcome
Pelvic floor botulinum toxin injection was associated with an overall 25.0-point reduction in pain scores (95% CI 13.7 – 36.3), consistent with the observed improvement in pain in each of the individual studies. When pain scores were analyzed at predetermined time points, there was a 20.3-point reduction in pain scores at ≤6 weeks (95% CI 8.85 – 31.7), 27.5-point reduction at 7–12 weeks (95% CI 31.6 – 41.4), and 19.4-point reduction at >12 weeks (95% CI 0.55 – 38.2; Figure 2). These reductions in pain did not differ significantly over time (p=0.833). There was moderate statistical heterogeneity with I2 of 45.7% overall, and there was minimal evidence of publication bias (Figure 3).When we included data from one additional unpublished RCT, we observed similar estimates as in our primary analysis (overall: 23.8 point-reduction, [95% CI 13.9–33.7]; ≤6 weeks: 20.1-point reduction [95% CI 9.1–31.1]; 7–12 weeks: 26.0-point reduction [95% CI 14.1–37.8]; and >12 weeks: 19.1-point reduction [95% CI 4.5–33.7]; Supplemental Figure).
Figure 2. Meta-analysis of pooled results from published studies that evaluated the effectiveness of botulinum toxin injections to the pelvic floor for treatment of pelvic floor myofascial pain in female patients.
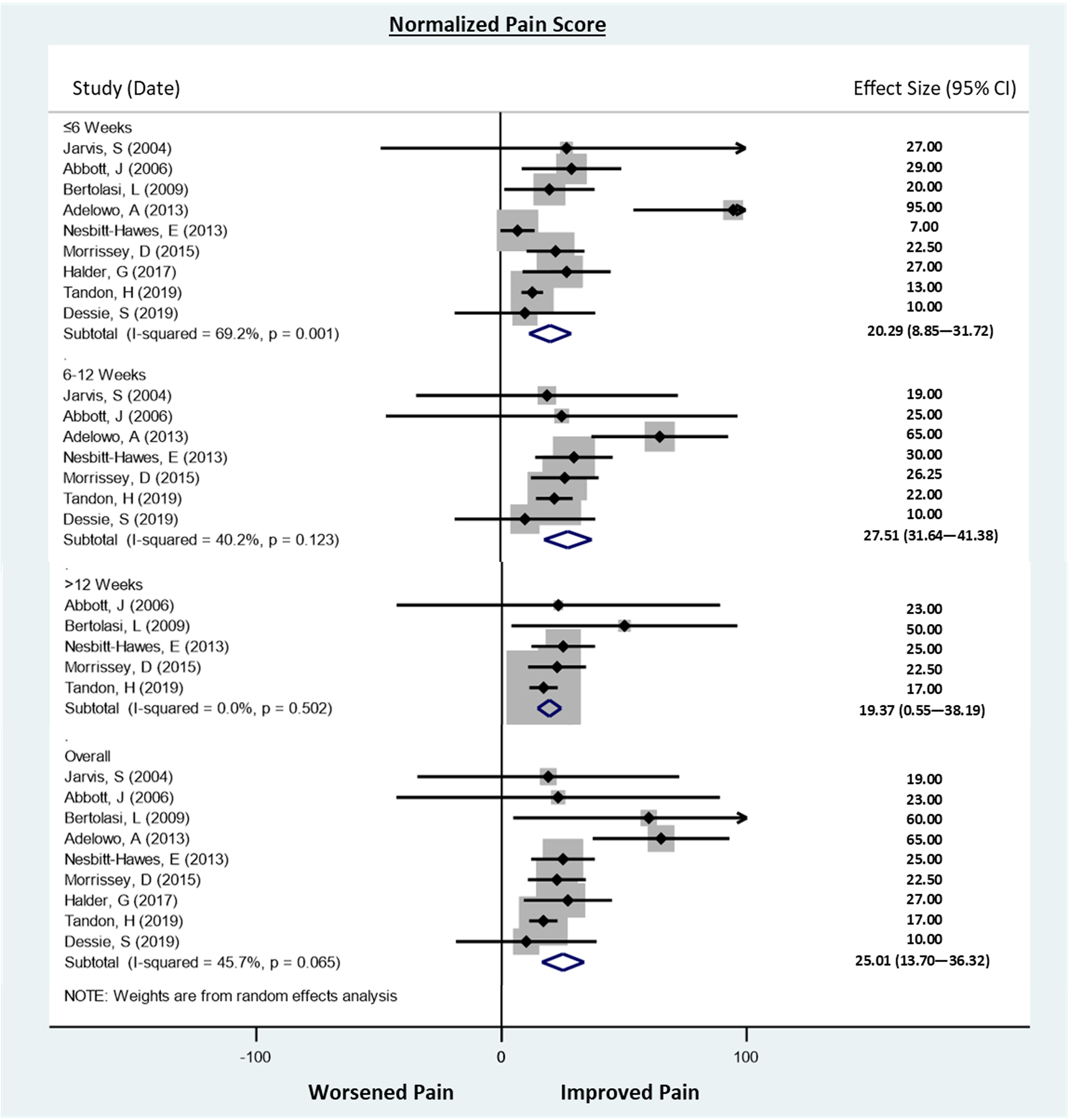
Pain outcomes were converted to a 100-point pain scale for those studies using scales other than the VAS. Scores were measured at median 2 weeks, 4 weeks and mean 6 weeks in the ≤6 weeks analysis. In the >6 to ≤12 weeks analysis, values were collected at median 8 weeks, and 12 weeks. In the >12 week analysis, values were collected at 24 weeks, 26 weeks and 120 weeks. P-trend for change in pain score with time p=0.232.
Figure 3. Funnel plot for assessment of publication bias in studies that evaluated the effectiveness of botulinum toxin injections to the pelvic floor for treatment of pelvic floor myofascial pain in female patients.
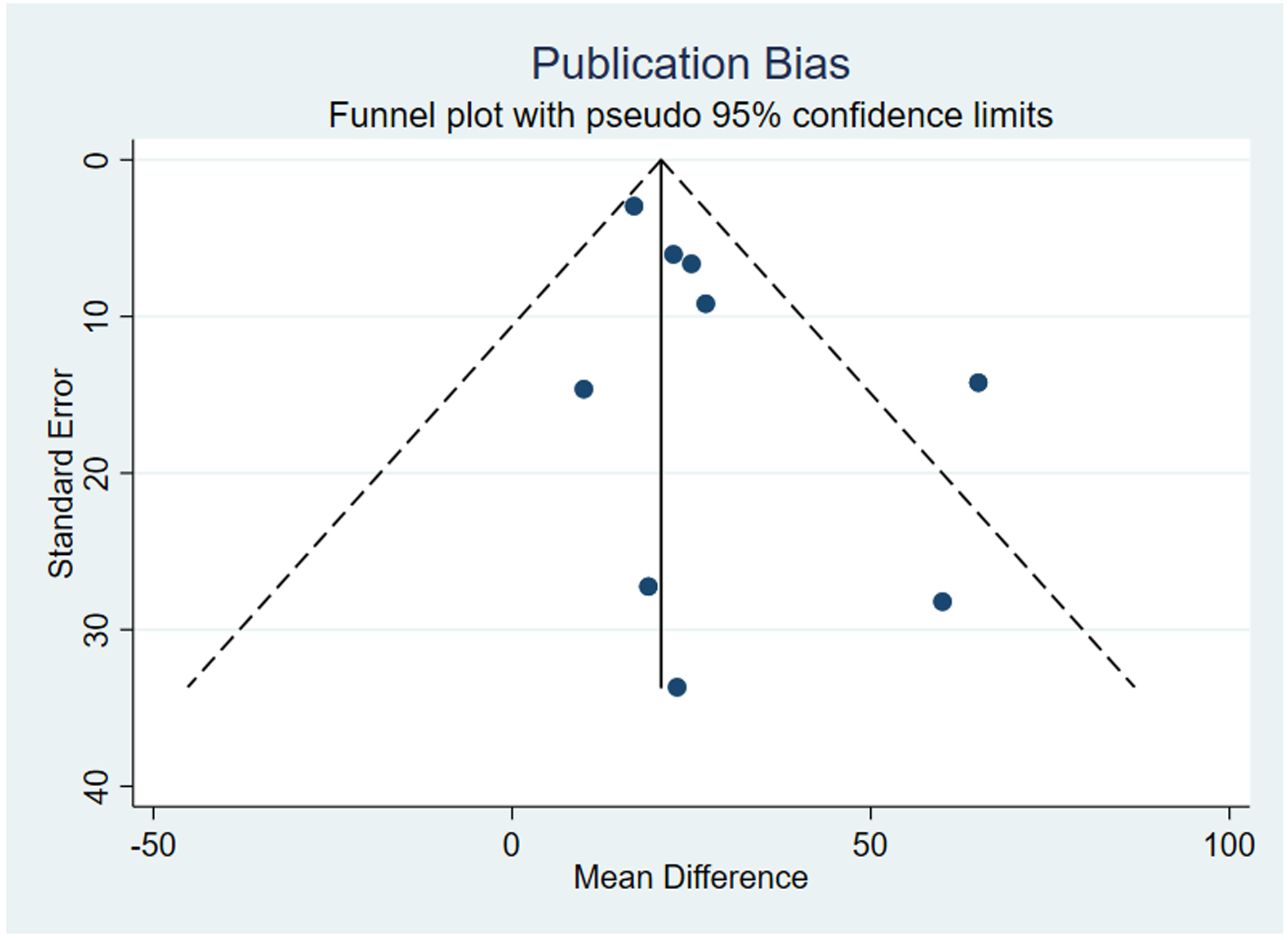
Minimal publication bias was noted among included studies.
To investigate whether our findings for pain varied by characteristics of injection and study methodology, we performed several additional stratified analyses. Each of these analyses used data collected at ≤6 weeks to allow for inclusion of all studies. When the data were stratified by total dose of botulinum toxin, there was a 33.4-point reduction in pain for <100 IU of botulinum toxin (95% CI −5.81 – 72.6) compared to a statistically significant 24.9-point reduction for ≥100 IU (95% CI 11.2 – 38.5; p=0.461 comparing the two doses; Table 3). When change in pain scores was stratified by use of EMG for identification of injection site, there was a 29.5-point reduction in pain scores in those that did not use EMG to guide injection site (95% CI 4.05–54.9) compared to a 20.7-point reduction among studies that did use EMG (95% CI −11.0 – 52.4), which was not significantly different (p=0.543). In analyses stratified by concomitant therapy, a non-significant 29.4-point reduction in pain scores was observed in studies evaluating botulinum toxin injections alone (95% CI −6.73 – 65.5) compared to a significant 23.9-point reduction in studies that evaluated botulinum toxin injections together with other therapies (95% CI 12.8 – 35.0; p=0.741 for the comparison of studies with and without concomitant therapies). Similar results were observed in sensitivity analyses including one additional unpublished RCT, except that use of botulinum toxin injections alone (without concomitant treatment) was associated with a statistically significant, albeit smaller, pain reduction [26.9 points vs 29.4 [Supplemental Table]).
Table 3. Meta-analysis of studies that evaluated the effectiveness of botulinum toxin injections to the pelvic floor for treatment of pelvic floor myofascial pain in female patients stratified by treatment differences and including secondary outcomes.
. Methods of analysis and reporting secondary outcomes varied among studies and did not permit meta-analysis for all studies that reported on secondary outcomes. VAS, visual analogue scale; IU, international units; EMG, electromyography. Data included from only published studies (excludes Bartley et al).
| Factor | Stratum | Studies | Point Estimate | 95% CI | p-value |
|---|---|---|---|---|---|
| VAS reduction (0–100) | |||||
| Botox Dose | <100 IU | 3[10, 14, 15] | 33.4 | −5.81 – 72.6 | 0.066 |
| ≥100 IU | 6[11, 16–20] | 24.9 | 11.2 – 38.5 | 0.007 | |
| Method of Detection | Digital palpation | 6[10, 11, 14, 16, 18, 19] | 29.5 | 4.05 – 54.9 | 0.033 |
| EMG ± digital palpation | 3[15, 17, 20] | 20.7 | −11.0 – 52.4 | 0.083 | |
| Concomitant Therapy | No concomitant therapy | 5[10, 14, 16, 18, 20] | 29.4 | −6.73 – 65.5 | 0.079 |
| Any concomitant therapy | 4[11, 15, 17, 19] | 23.9 | 12.8 – 35.0 | 0.014 | |
| Dysmenorrhea | <4 weeks | 4[10, 14–16] | 1.66 | −33.1 – 36.5 | 0.804 |
| 4–11 weeks | 3[10, 14, 16] | 8.40 | −5.69 – 22.5 | 0.095 | |
| ≥12 weeks | 3[10, 15, 16] | 3.29 | −30.8 – 37.4 | 0.680 | |
| Dyspareunia | <4 weeks | 4[10, 14–16] | 22.3 | −20.5 – 65.1 | 0.151 |
| 4–11 weeks | 4[10, 14, 16, 17] | 34.8 | 6.64 – 62.9 | 0.031 | |
| ≥12 weeks | 4[10, 15–17] | 24.7 | 17.9 – 31.6 | 0.006 | |
| Dyschezia | <4 weeks | 3[10, 14, 16] | 13.1 | −6.81 – 33.1 | 0.082 |
| 4–11 weeks | 3[10, 14, 16] | 11.9 | 0.56 – 23.3 | 0.047 | |
| ≥12 weeks | 2[10, 16] | 12.8 | −15.5 – 41.0 | 0.110 | |
| SF-12 improvement | |||||
| SF-12 Mental | <4 weeks | 3[10, 15, 17] | 2.41 | −7.62 – 12.4 | 0.204 |
| 4–11 weeks | 2[10, 17] | 5.80 | 4.93 – 6.66 | 0.008 | |
| ≥12 weeks | 3[10, 15, 17] | 5.29 | −40.4 – 51.0 | 0.382 | |
| SF-12 Physical | <4 weeks | 3[10, 15, 17] | 2.73 | 0.64 – 4.82 | 0.035 |
| 4–11 weeks | 2[10, 17] | 3.18 | −2.21 – 8.57 | 0.085 | |
| ≥12 weeks | 3[10, 15, 17] | 5.73 | −4.50 – 16.0 | 0.091 | |
| Change in pressure (mmHg) | |||||
| Manometry – maximum contracting pressures | <4 weeks | 3[10, 16, 17] | −14.7 | −28.6 – −0.85 | 0.045 |
| 4–11 weeks | 3[10, 16, 17] | −10.1 | −19.7 – −0.57 | 0.046 | |
| ≥12 weeks | 3[10, 16, 17] | −6.29 | −15.2 – 27.8 | 0.325 | |
| Manometry – resting pressures | <4 weeks | 4[10, 14, 16, 17] | −15.3 | −23.0 – −7.67 | 0.008 |
| 4–11 weeks | 4[10, 14, 16, 17] | −12.0 | −17.2 – −6.81 | 0.007 | |
| ≥12 weeks | 3[10, 16, 17] | −9.28 | −12.8 – −5.73 | 0.008 |
To explore the effect of botulinum toxin independent of a possible placebo effect, we next investigated the difference in changes in pain scores between patients receiving botulinum injection vs comparator in the two published and one unpublished RCT. This analysis demonstrated a significantly greater reduction in pain in the botulinum toxin arm compared to the comparator (Figure 4). When analyzed by time point, this difference was significant at ≤6 weeks [standardized mean difference (SMD) 0.37, 95% CI 0.03–0.70] but not at 7–12 weeks (SMD 0.21, 95% CI −0.12–0.55). Differences were not explored past 12 weeks because Dessie et al did not measure outcomes past this time point.
Figure 4. Meta-analysis of change in pain score after therapy in studies that evaluated botulinum toxin vs a comparator.
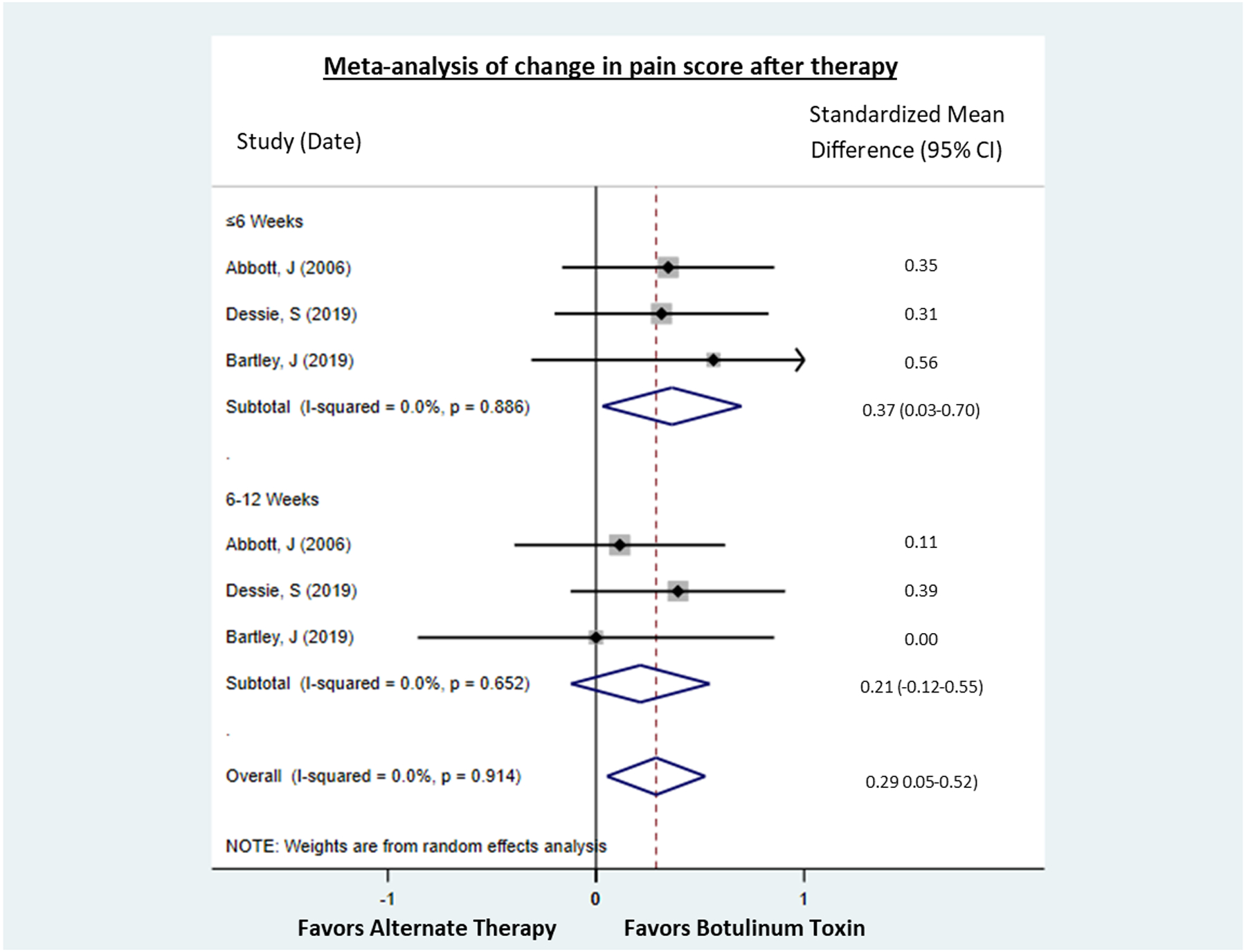
Two studies compared botuinum toxin to a placebo and one, unpublished RCT, compared botulinum toxin to an active treatment (Kenalog). Differences were not explored past 12 weeks because Dessie et al did not measure outcomes past this time point. Treatment with botulinum toxin was associated with a greater reduction in pain score at ≤6 weeks and overall.
Secondary Outcomes
Secondary outcomes assessed among the studies included dysmenorrhea (4/9, 44%),[18, 22–24] dyspareunia (6/9, 67%),[18, 22–25, 27] bladder symptoms (3/9, 33%),[18, 22, 23] bowel symptoms/dyschezia (5/9, 56%),[18, 22–24, 27] quality of life (7/9, 78%),[18, 19, 22, 23, 25, 28] sexual activity (3/10, 30%),[18, 23, 25] pelvic floor manometry (5/9, 56%),[18, 22–25] and pelvic floor EMG (1/9, 11%).[23] The unpublished RCT measured quality of life. Methods of assessment, timing, and reporting of the secondary outcomes varied between the studies. Homogeneity in reporting allowed for meta-analysis of changes in VAS scores in dysmenorrhea, dyspareunia, and dyschezia; physical and mental SF-12 quality of life scores; and final manometry scores at rest and with maximum contraction.
Pelvic floor botulinum toxin injection was associated with a minimal, non-significant reduction in VAS scores for dysmenorrhea at all times points (Table 3), which appears to have been driven by an increase in dysmenorrhea in 1 study.[23] All other studies observed decreases ranging from 7–27 points. Dyspareunia was improved at all time points, with significant reductions observed at 4–11 weeks (34.8-point reduction, 95% CI 6.64 – 62.9) and ≥12 weeks (24.7-point reduction, 95% CI 17.9 – 31.6). Dyschezia was also improved at all time points, with a significant reduction observed at 4–11 weeks (11.9-point reduction, 95% CI 0.56 – 23.3).
SF-12 mental and physical quality of life scores improved after pelvic floor botulinum toxin injection, with significant improvements observed at 4–11 weeks for SF-12 mental component scores (5.80-point improvement, 95% CI 4.93 – 6.66) and at <4 weeks for SF-12 physical component scores (2.73-points improvement, 95% CI 0.64 – 4.82).
Maximum contracting pressures as measured on pelvic floor manometry significantly decreased at 4 weeks (14.7 mmH2O reduction, 95% CI, −28.6 to −0.85) and 4–11 weeks (10.1 mmH2O reduction, 95% CI, −19.7 to −0.57). Maximum contracting pressures were not significantly decreased past 12 weeks. The reduction in maximum contracting pressures significantly decreased over time (p=0.04). Resting manometry pressures were significantly decreased at all time points [15.3 mmH2O reduction at 4 weeks (95% CI, −23.0 to −7.67), 12.0 mmH2O reduction at 4–11 weeks (95% CI, −17.2 to −6.81), and 9.28 mmH2O reduction at ≥12 weeks (95% CI, −12.8 to −5.73). There was not a significant change in resting pressures over time (p=0.09). Similar results were observed for all secondary outcomes when data from one additional, unpublished RCT was included.
Adverse events were reported in all studies. The frequency of these complications within each study, however, was not consistently reported. The most commonly reported adverse events included constipation,[19, 25–28] urinary incontinence,[18, 19, 23, 25] fecal incontinence,[18–20, 25, 26] and cold or flu-like symptoms.[18, 22, 24] Urinary retention[19, 20, 26, 27] and gastrointestinal side effects[18, 22] were also reported in multiple studies. Other adverse events included urinary tract infection,[19, 20, 27] bleeding complications,[18] pelvic/back pain,[18, 20, 28] and vulvar irritation.[24] No studies reported infectious complications aside from urinary tract infections.
Discussion
This systematic review and meta-analysis was conducted to determine the effectiveness of botulinum toxin injection on pelvic floor myofascial pain through a comprehensive synthesis of the available evidence, which included data from 2 small RCTs, 4 prospective cohort studies, 1 retrospective cohort study, and 2 case series with more than 10 participants. This is the first study to assimilate the data on this novel treatment strategy for pelvic floor myofascial pain. Our comprehensive search strategy completed by a medical librarian and rigorous attention to article screening and data extraction by two independent reviewers lend confidence to the findings from this analysis.
Although the overall number of available studies was limited, we found a significant decrease in pain scores associated with pelvic floor botulinum toxin use. When compared to either placebo or active control, reduction in pain scores was greater with botulinum toxin. Reductions in pain scores were observed despite significant variability in dose, method for identification of injection sites, number of injection sites, and muscles injected, and at all time points (≤6, 7–12, and >12 weeks), suggesting a sustained influence of injection. While many aspects of pelvic floor botulinum toxin injections have not been standardized, this work supports this therapy as a promising treatment for refractory pelvic floor myofascial pain. Larger, well-powered RCTs should now be conducted to optimize the injection protocol.
In addition to statistical significance, clinical significance is also important for evaluating the effectiveness of therapies. A minimal clinically important difference of 30 points on the VAS has previously been identified as representing significant pain reduction[29], which is similar in magnitude to our observed maximum decrease of 27.5 points on a normalized 100-point scale at 7–12 weeks post-injection. Thus, our findings suggest that use of botulinum toxin injections to the pelvic floor may result in both a statistically and clinically significant reduction in pain scores. This is especially promising for patients with pelvic floor myofascial pain refractory to traditional therapies like pelvic floor PT.
In addition to reductions in pain, our findings suggest that botulinum toxin injections to the pelvic floor also lead to improvements in secondary outcomes related to pain and quality of life. While reported in only a small subset of included studies, we observed significant or suggestive improvement in dyspareunia, dyschezia, and SF-12 mental and physical component scores at most time points investigated. As expected, manometry resting and maximum contracting pressures significantly decreased after botulinum toxin injection. The greatest reduction in pressure was noted at 4 weeks with deterioration in effect over time. This decrease in muscle tone at rest and with contraction may have contributed to the improvements noted in dyspareunia. Although not investigated in the current studies, it is also possible that decreased resting and contraction pressure could negatively affect other symptoms and outcomes, such as urinary and fecal symptoms, which in turn could negatively impact quality of life. This possibility should be explored in future studies including women with both pelvic floor myofascial pain and incontinence.
For this systematic review and meta-analysis, we restricted inclusion to studies that provided a quantitative assessment of pain pre- and post-treatment. Our conclusions are limited by the wide variability in botulinum toxin dose, location, and quantitative assessment of pain, and the small number of studies with small sample sizes, which made it difficult to estimate the precision of our pooled values with confidence. However, the strong statistical significance of our main pooled estimate and the consistency of the direction of findings from almost all individual studies lend support to our conclusions. Additionally, only 2 published studies included a randomized comparison group (placebo control). The inclusion of data from the RCT by Bartley et al, while not published, permitted us to perform a meta-analysis of the effect of botulinum toxin vs a comparator, which suggested that botulinum toxin is superior to placebo or active control in the treatment of pelvic floor myofascial pain. This finding should be interpreted with caution, however, given the low number of studies available for inclusion, the inclusion of data from a non-published source, and the significant clinical heterogeneity between studies. Nevertheless, these findings support further exploration. Finally, based on our inclusion criteria, we may have excluded studies that only addressed other possibly relevant outcomes including dyspareunia, dysmenorrhea, sexual function, and bowel and bladder symptoms but did not provide an assessment of pain. However, we believe it is unlikely that any significantly relevant studies were excluded because the indication for botulinum toxin use would likely be pain.
Data on the use of botulinum toxin for treatment of myofascial pain in the pelvic floor continue to emerge. In addition to determining the efficacy of this promising therapy, future studies should investigate the optimal method for identifying injection sites (i.e., palpation, EMG, ultrasound), botulinum toxin dose, number of injection sites, dose per site, and ideal follow-up time for assessment of greatest efficacy. Given the apparent improvement in secondary outcomes, future studies should assess these outcomes as well. Additionally, data on the utility of single vs repeat injections and the efficacy of botulinum toxin compared to other management options needs to be further evaluated.
Overall, this comprehensive synthesis of the available literature provides evidence to support a role for botulinum toxin injections for treatment of pelvic floor myofascial pain and associated pelvic floor symptoms. Our findings suggest both a clinically and statistically significant improvement in pain scores and secondary outcomes after botulinum toxin injection to the pelvic floor muscles. Future RCTs should determine the efficacy of this treatment, and clarify the optimal dose, method for identifying injection sites (i.e. palpation, EMG, ultrasound), number of injection sites, dose per site, and duration of effect to support application for FDA approval and use of this currently off-label therapy in clinical practice.
Supplementary Material
Supplemental Figure. Meta-analysis of pooled results from published studies that evaluated the effectiveness of botulinum toxin injections to the pelvic floor for treatment of pelvic floor myofascial pain in female patients. Pain outcomes were converted to a 100-point pain scale for those studies using scales other than the VAS. Scores were measured at median 2 weeks, 4 weeks and mean ≤6 weeks in the 6 weeks analysis. In the >6 to ≤12 weeks analysis, values were collected at median 8 weeks, and 12 weeks. In the >12 week analysis, values were collected at 24 weeks, 26 weeks and 120 weeks. P-trend for change in pain score with time p=0.931.
Acknowledgments:
Dr. Meister was supported by an NIH Reproductive Epidemiology Training Grant (T32HD055172-08) and a Clinical and Translational Science Award held at Washington University in St. Louis (UL1 TR002345) during this study. The authors would like to acknowledge Kim Lipsey, Medical Librarian at the Becker Medical Library at Washington University in St. Louis, for assistance with the systematic literature search. This study was presented as a poster at the 45th Annual Scientific Meeting of the Society of Gynecologic Surgeons in Tucson, Arizona on April 1, 2019.
Footnotes
FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: None
Contributor Information
Melanie R. MEISTER, Kansas City, Kansas; Department of Obstetrics & Gynecology, Division of Female Pelvic Medicine and Reconstructive Surgery, University of Kansas School of Medicine.
Allison BRUBAKER, St. Louis, Missouri; Department of Obstetrics & Gynecology, Washington University in St. Louis.
Siobhan SUTCLIFFE, St. Louis, Missouri; Department of Surgery, Division of Public Health Sciences, Washington University in St. Louis.
Jerry L. LOWDER, St. Louis, Missouri; Department of Obstetrics & Gynecology, Division of Female Pelvic Medicine & Reconstructive Surgery, Washington University in St. Louis.
References
- 1.Bo K, Frawley H, Haylen B, et al. , An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. International Urogynecoly Journal, 2017. 28(2): p. 191–213. [DOI] [PubMed] [Google Scholar]
- 2.Spitznagle T and Robinson C, Myofascial pelvic pain. Obstet Gynecol Clin N Am, 2014. 41. [DOI] [PubMed] [Google Scholar]
- 3.Moldwin R and Fariello J, Myofascial trigger points of the pelvic floor: associations with urological pain syndromes and treatment stategies including injection therapy. Curr Urol Rep, 2013. 14: p. 409–417. [DOI] [PubMed] [Google Scholar]
- 4.Adams K, Gregory W, Osmundsen B, et al. , Levator myalgia: why bother? Int Urogynecol J, 2013. 24: p. 1687–1693. [DOI] [PubMed] [Google Scholar]
- 5.Pastore E and Katzman W, Recognizing myofascial pelvic pain in the female patient with chronic pelvic pain. J Obstet Gynecol Neonatal Nurs, 2012. 41(5): p. 680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald C, Neville C, Mallinson T, et al. , Pelvic floor muscle examination in female chronic pelvic pain. J Reprod Med, 2011. 56. [PubMed] [Google Scholar]
- 7.Peters K, Carrico D, Kalinowski S, et al. , Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology, 2007. 70(1): p. 16–18. [DOI] [PubMed] [Google Scholar]
- 8.Meister M, Sutcliffe S, Badu A, et al. , Pelvic floor myofascial pain severity and pelvic floor disorder symptom bother: is there a correlation? Am J Obstet Gynecol, 2019. 221(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff B, Joyce C, Brincat C, et al. , Pelvic floor myofascial pain in patients with symptoms of urinary tract infection. Int J Gynaecol Obstet, 2019. 145(2): p. 205–211. [DOI] [PubMed] [Google Scholar]
- 10.Dixon A, Fitzgerald C, and Brincat C, Severity and bother of prolapse symptoms in women with pelvic floor myofascial pain. Int Urogynecol J, 2019. 30(11): p. 1829–1834. [DOI] [PubMed] [Google Scholar]
- 11.Bassaly R, Tidwell N, Bertolino S, et al. , Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J, 2011. 22(4): p. 413–418. [DOI] [PubMed] [Google Scholar]
- 12.Sahrmann S, Diagnosis and treatment of movement impairment syndromes. 2001: Mosby. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borg-Stein J and Iaccarino M, Myofascial pain syndrome treatments. Phys Med Rehabil Clin N Am, 2014. 25(20): p. 357–374. [DOI] [PubMed] [Google Scholar]
- 14.Brown C, Glazer H, Vogt V, et al. , Subjective and objective outcomes of Botulinum toxin type A treatment in vestibulodynia: Pilot Data. J Reprod Med, 2006. 51: p. 635–641. [PubMed] [Google Scholar]
- 15.Kwanchuay P, Petchnumsin T, Yiemsiri P, et al. , Efficacy and safety of single Botulinum Toxin Type A (Botox) injection for relief of upper trapezius myofascial trigger point: a randomized, double-blind, placebo-controlled study. J Med Assoc Thai, 2015. 98(12): p. 1231–1236. [PubMed] [Google Scholar]
- 16.Tanner-Smith E and Tipton E, Robust variance estimation with dependent effect sizes: practical considerations including a software tutorial in Stata and spss. Res Synth Methods, 2014. 5(1): p. 13–30. [DOI] [PubMed] [Google Scholar]
- 17.Kontopantelis E, Springate D, and Reeves D, A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One, 2013. 8(7): p. e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott J, Jarvis S, Lyons S, et al. , Botulinum toxin type A for chronic pain and pelvic floor spasm in women: A randomized controlled trial. Obstet Gynecol, 2006. 103(4): p. 915–923. [DOI] [PubMed] [Google Scholar]
- 19.Dessie S, von Bargen E, Hacker M, et al. , A randomized, double-blind, placebo-controlled trial of onabotulinumtoxinA trigger point injections for myofascial pelvic pain. Am J Obstet Gynecol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartley J, Onabotulinumtoxin A Versus Kenalog for Chronic Pelvic Pain. 2019.
- 21.Harris P, Taylor R, Thielke R, et al. , Research electronic data capture (RedCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 2009. 42(2): p. 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis S, Abbott J, Lenart M, et al. , Pilot study of botulinum toxin type A in the treatment of chronic pelvic pain associated with spasm of the levator ani muscles. Australian and New Zealand J Obstet Gynecol, 2004. 44: p. 46–50. [DOI] [PubMed] [Google Scholar]
- 23.Bertolasi L, Frasson E, Cappelletti J, et al. , Botulinum neurotoxin type A injections for vaginismus secondary to vulvar vestibulitis syndrome. Obstet Gynecol, 2009. 114(5): p. 1008–1016. [DOI] [PubMed] [Google Scholar]
- 24.Nesbitt-Hawes E, Won H, Jarvis S, et al. , Improvement in pelvic pain with botulinum toxin type a - single vs repeat injections. Toxicon, 2013. 63(83–87): p. 83. [DOI] [PubMed] [Google Scholar]
- 25.Morrissey D, El-Khawand D, Ginzburg N, et al. , Botulinum toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg, 2015. 21: p. 277–282. [DOI] [PubMed] [Google Scholar]
- 26.Adelowo A, Hacker M, Shapiro A, et al. , Botulinum toxin type A (BOTOX) for refractory myofascial pelvic pain. Female Pelvic Med Reconstr Surg, 2013. 19: p. 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halder G, Scott L, Wyman A, et al. , Botox combined with myofascial release physical therapy as a treatment for myofascial pelvic pain. Investig Clin Urol, 2017. 58: p. 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tandon H, Stratton P, Sinaii N, et al. , Botulinum toxin for chronic pelvic pain in women with endometriosis: a cohort study of a pain-focused treatment. Reg Anesth Pain Med, 2019. 44: p. 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Hobden E, Stiell I, et al. , Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med, 2003. 10(10): p. 1128–1130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Meta-analysis of pooled results from published studies that evaluated the effectiveness of botulinum toxin injections to the pelvic floor for treatment of pelvic floor myofascial pain in female patients. Pain outcomes were converted to a 100-point pain scale for those studies using scales other than the VAS. Scores were measured at median 2 weeks, 4 weeks and mean ≤6 weeks in the 6 weeks analysis. In the >6 to ≤12 weeks analysis, values were collected at median 8 weeks, and 12 weeks. In the >12 week analysis, values were collected at 24 weeks, 26 weeks and 120 weeks. P-trend for change in pain score with time p=0.931.


