Introduction
The myeloid zinc finger (MZF) protein family encompasses different transcription factors (TFs) including the myeloid zinc finger protein 1 (MZF-1), also known as zinc finger protein 42 (ZNF42) (Hromas et al., 1991). Assessing the role of MZF-1 in the granulocyte colony-stimulating factor (G-CSF)-induced differentiation of neutrophil in mice, Murai et al. (1997) unexpectedly isolated a novel MZF cDNA form that they named MZF-2. They suggested that MZF-1 and MZF-2 are produced from a single gene by using two alternative transcription initiation sites (Murai et al., 1997). The newly MZF-2 isolated was predicted to be longer than MZF-1. In this initial report by Murai et al. (1997) the human and the murine MZF-2 (hMZF-2 and mMZF-2, respectively), were predicted to have a 75.3% identity between their amino acids (aa) sequences. The hMZF-2 and mMZF-2 proteins contain 13 zinc finger motifs each, which are identical to those reported in the MZF-1 protein (Morris et al., 1994; Murai et al., 1997). It was also proposed that both hMZF-1 and hMZF-2 most likely recognize and bind to the same consensus sequences (5′-AGTGGGA-3′ and 5′-CGGGGAGGGGGAA-3′) (Murai et al., 1997). In a complementary study, the same authors investigated only the mMZF-2 form and evaluated its transcriptional regulatory ability in myeloid cells (Murai et al., 1998). In this review, we question the actual existence of hMZF-2 as a transcription factor involved in hTERT expression and regulation.
hMZF-2 and hTERT Gene
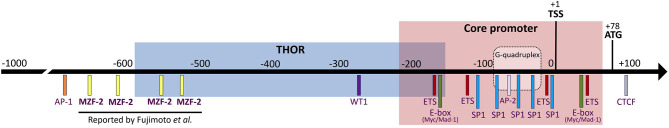
According to the above reports, the hMZF-2 protein was supposed to bind to the distal region of the recently identified telomerase reverse transcriptase (TERT) hypermethylated oncogenic region (THOR) (Figure 1). THOR epigenetic modifications were shown to be a crucial regulator of the hTERT gene re-expression in solid tumors and leukemia (Lee et al., 2019) (Figure 1). Indeed, hTERT expression, a limiting factor of the telomerase activity (TA), is elevated in 85 to 90% of human cancers, thus promoting survival, proliferation, and invasion capacities of tumor cells (Ramlee et al., 2016). hTERT can be regulated through the binding of TFs (either repressors or activators) to its promoter region. MZF-2 was classified among the suppressors of the hTERT gene in human and canine (Long et al., 2005; Kyo et al., 2008). Due to the lack of appropriate and validated hMZF-2 antibodies, no chromatin immunoprecipitation experiments were done, and therefore, the binding of MZF-2 to the hTERT promoter was reported only as a result of indirect in vitro experiments. So far, Fujimoto et al. (2000) predicted that hMZF-2 can bind to 4 sites, all of them being located on the hTERT promoter at positions −514, −543, −619, and −687 (Figure 1). Since this initial report, these four binding sites were presented in several figures of book chapters or review articles on telomerase regulation, including recently published ones (Ducrest et al., 2002; Pericuesta et al., 2006; Jafri et al., 2016; Lewis and Tollefsbol, 2016; ElHajj et al., 2017; Heidenreich and Kumar, 2017; Eitsuka et al., 2018; Srinivas et al., 2020), without any additional report that stated unambiguously the existence of hMZF-2 while the presence of other regulators of the hTERT gene, located further upstream of the transcription start site (TSS), were clearly reported to influence the hTERT expression, such as the activator protein 1 (AP-1), vitamin D (3) receptor (VDR), signal transducer and activator of transcription 3 (STAT3), and nuclear factor κB (NF-κB) (Ramlee et al., 2016).
Figure 1.
The promoter region of hTERT including the core promoter and the TERT hypermethylated oncogenic region (THOR). The transcription start site (TSS, +1) and the translation start site (start codon ATG, +78) are indicated in addition to the binding sites for the “elusive” hMZF-2 (myeloid zinc finger-2) as predicted by Fujimoto et al. (2000) as well as other common transcription factors, such as CTCF (CCCTC-binding factor), ETS (E26 transformation-specific or E-twenty-six), E-box (enhancer-box, where Myc/Mad-family can bind), SP1 (specificity protein 1), AP-2 (activator protein-2), WT1 (Wilms' tumor 1), and AP-1 (activator protein-1). The location of the G-quadruplex structure that can be adopted by the hTERT promoter is also represented.
hMZF-2 in the Databases
Blasting the forward and reverse primers (CCGGAGATGGGTCACAGTCC and TTGCTGAACACCTTGCCAC) used by Fujimoto et al. to amplify MZF-2 transcripts (Fujimoto et al., 2000), we obtained very significant alignments with MZF-1 and its mRNA variants. Such findings can be explained by the hypothesis that MZF-2 is transcribed from the same gene as MZF-1 (Murai et al., 1997). Moreover, the human form hMZF-2 sequence is still absent in the genomic and proteomic databases, while the murine form remains to be validated. In the UCSC Genome Browser on Human (genome.ucsc.edu), the OMIM (omim.org), the NCBI (ncbi.nlm.nih.gov/gene), and the Ensembl (ensembl.org) databases, only MZF-1 exists. In the GeneCards database (genecards.org), a search for “MZF-2” directs to the MZF-1 gene and to the biological region LOC110806263 which refers to the TERT 5′ regulatory region on the hTERT promoter and citing the paper by Fujimoto et al. (2000). In the proteomic database UniProt (uniprot.org), information concerning MZF-2 in mouse (Mus musculus) is available under the label “experimental evidence at transcript level,” but no information is indicated for the human MZF-2 form.
Discussion
In a recent review article published in 2020, Brix et al. (2020) regrouped information on MZF-1 and its role in regulating cancer invasion. They also discussed MZF-1 transcript variants. They stated that the first MZF-1 isoform isolated and characterized was believed to be the full-length MZF-1 (485 aa) until the identification of the long isoforms (734 aa), named MZF-2a in mouse and MZF1B/C in human (Brix et al., 2020). Brix et al. defined hMZF-2 as the largest form of hMZF-1, or “full-length hMZF-1” (Brix et al., 2020). However, the 734 aa full-length hMZF1 (MZF1B/1C) differs in length from the 775 aa hMZF-2 predicted initially by (Murai et al., 1997; Peterson and Morris, 2000) (Supplementary Figure 1). As for the structural domains in MZF, the SCAN domain that mediates interactions between members of a mammalian subfamily of zinc-finger transcription factors is shared between MZF-1 and mMZF-2 (uniport.org), while this information is not available for hMZF-2.
Herein, we summarize the available information regarding MZF-2 published as original research articles (Murai et al., 1997, 1998; Fujimoto et al., 2000) and those published in review articles (Ducrest et al., 2002; Pericuesta et al., 2006; Jafri et al., 2016; Lewis and Tollefsbol, 2016; ElHajj et al., 2017; Heidenreich and Kumar, 2017; Eitsuka et al., 2018; Srinivas et al., 2020). All the published reports, as well as the search in genomic databases, lead us to be doubtful about the real existence of the human form hMZF-2. From these reports, it is not clearly demonstrated whether hMZF-2 is another isoform of hMZF-1. Twenty-three years after its discovery, data concerning hMZF-2 genomic or proteomic sequences are still unpublished. No antibody against the hMZF-2 protein is available. If it is true that hMZF-2 refers to the full-length hMZF-1 as mentioned by Brix DM et al. in 2020, why is this information lacking in the genomic databases? Most of the hMZF-2 original research articles were published before the availability of a reference genome. However, we aimed to highlight the lack of biological evidence that confirm the existence of hMZF-2, functionally differentiate hMZF-2 from hMZF-1, and unequivocally state its ability to regulate the hTERT gene. Therefore, we urgently suggest that the four theoretical hMZF-2-binding sites on the hTERT promoter should be no longer assigned to this “elusive” transcription factor until further clear experimental evidence is reported (Figure 1). Indeed, the precise identification of the TFs' binding sites on the promoter of the oncogene hTERT would refine insights into the epigenetic regulation of hTERT activity in cancer.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The work of AC and EC was supported by grants from the French National Institute of Health and Medical Research (INSERM), the French Society of Dermatology (SFD), Hubert Curien Partnership (PHC-CEDRE), and ERASMUS+. The work of ES-B team was supported by grants from INSERM, the National Center for Scientific Research (CNRS), and the Ligue Nationale Contre le Cancer (Comité Ile-de France).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.581115/full#supplementary-material
Pairwise alignment comparison between the human MZF-1 and the mouse MZF-2 proteins showing that the N-terminal region is missing in the full length MZF-1.
References
- Brix D. M., Bundgaard Clemmensen K. K., Kallunki T. (2020). Zinc finger transcription factor MZF1-A specific regulator of cancer invasion. Cells 9:223. 10.3390/cells9010223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducrest A.-L., Szutorisz H., Lingner J., Nabholz M. (2002). Regulation of the human telomerase reverse transcriptase gene. Oncogene 21, 541–552. 10.1038/sj.onc.1205081 [DOI] [PubMed] [Google Scholar]
- Eitsuka T., Nakagawa K., Kato S., Ito J., Otoki Y., Takasu S., et al. (2018). Modulation of telomerase activity in cancer cells by dietary compounds: a review. Int. J. Mol. Sci. 19:478. 10.3390/ijms19020478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElHajj J., Garsuault D., Bouyer C., Nguyen E., Hilal G., Ségal-Bendirdjian E. (2017). “Telomeres and telomerase in neuroblastoma,” in Neuroblastoma—Current State and Recent Updates. Intechopen. Available online at: https://www.intechopen.com/books/neuroblastoma-current-state-and-recent-updates/telomeres-and-telomerase-in-neuroblastoma
- Fujimoto K., Kyo S., Takakura M., Kanaya T., Kitagawa Y., Itoh H., et al. (2000). Identification and characterization of negative regulatory elements of the human telomerase catalytic subunit (hTERT) gene promoter: possible role of MZF-2 in transcriptional repression of hTERT. Nucleic Acids Res. 28, 2557–2562. 10.1093/nar/28.13.2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich B., Kumar R. (2017). TERT promoter mutations in telomere biology. Mutat. Res. 771, 15–31. 10.1016/j.mrrev.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Hromas R., Collins S. J., Hickstein D., Raskind W., Deaven L. L., O'Hara P., et al. (1991). A retinoic acid-responsive human zinc finger gene, MZF-1, preferentially expressed in myeloid cells. J. Biol. Chem. 266, 14183–14187. [PubMed] [Google Scholar]
- Jafri M. A., Ansari S. A., Alqahtani M. H., Shay J. W. (2016). Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 8:69. 10.1186/s13073-016-0324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyo S., Takakura M., Fujiwara T., Inoue M. (2008). Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 99, 1528–1538. 10.1111/j.1349-7006.2008.00878.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. D., Leão R., Komosa M., Gallo M., Zhang C. H., Lipman T., et al. (2019). DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J. Clin. Invest. 129, 223–229. 10.1172/JCI121303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. A., Tollefsbol T. O. (2016). Regulation of the telomerase reverse transcriptase subunit through epigenetic mechanisms. Front. Genet. 7:83. 10.3389/fgene.2016.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., Argyle D. J., Gault E. A., Campbell S., Nasir L. (2005). The canine telomerase catalytic subunit (dogTERT): characterisation of the gene promoter and identification of proximal core sequences necessary for specific transcriptional activity in canine telomerase positive cell lines. Gene 358, 111–120. 10.1016/j.gene.2005.05.030 [DOI] [PubMed] [Google Scholar]
- Morris J. F., Hromas R., Rauscher F. J. (1994). Characterization of the DNA-binding properties of the myeloid zinc finger protein MZF1: two independent DNA-binding domains recognize two DNA consensus sequences with a common G-rich core. Mol. Cell. Biol. 14, 1786–1795. 10.1128/MCB.14.3.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai K., Murakami H., Nagata S. (1997). A novel form of the myeloid-specific zinc finger protein (MZF-2). Genes Cells Devoted Mol Cell Mech. 2, 581–591. 10.1046/j.1365-2443.1997.1430341.x [DOI] [PubMed] [Google Scholar]
- Murai K., Murakami H., Nagata S. (1998). Myeloid-specific transcriptional activation by murine myeloid zinc-finger protein 2. Proc. Natl. Acad. Sci. U.S.A. 95, 3461–3466. 10.1073/pnas.95.7.3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericuesta E., Ramírez M. A., Villa-Diaz A., Relaño-Gines A., Torres J. M., Nieto M., et al. (2006). The proximal promoter region of mTert is sufficient to regulate telomerase activity in ES cells and transgenic animals. Reprod Biol Endocrinol. 4:5. 10.1186/1477-7827-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M., Morris J. (2000). Human myeloid zinc finger gene MZF produces multiple transcripts and encodes a SCAN box protein. Gene 254, 105–118. 10.1016/S0378-1119(00)00281-X [DOI] [PubMed] [Google Scholar]
- Ramlee M. K., Wang J., Toh W. X., Li S. (2016). Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes 7:50. 10.3390/genes7080050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas N., Rachakonda S., Kumar R. (2020). Telomeres and telomere length: a general overview. Cancers 12:558. 10.3390/cancers12030558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pairwise alignment comparison between the human MZF-1 and the mouse MZF-2 proteins showing that the N-terminal region is missing in the full length MZF-1.