Abstract
Background
Numerous clinical trials and observational studies have investigated various pharmacological agents as potential treatment for Coronavirus Disease 2019 (COVID-19), but the results are heterogeneous and sometimes even contradictory to one another, making it difficult for clinicians to determine which treatments are truly effective.
Methods and findings
We carried out a systematic review and network meta-analysis (NMA) to systematically evaluate the comparative efficacy and safety of pharmacological interventions and the level of evidence behind each treatment regimen in different clinical settings. Both published and unpublished randomized controlled trials (RCTs) and confounding-adjusted observational studies which met our predefined eligibility criteria were collected. We included studies investigating the effect of pharmacological management of patients hospitalized for COVID-19 management. Mild patients who do not require hospitalization or have self-limiting disease courses were not eligible for our NMA. A total of 110 studies (40 RCTs and 70 observational studies) were included. PubMed, Google Scholar, MEDLINE, the Cochrane Library, medRxiv, SSRN, WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov were searched from the beginning of 2020 to August 24, 2020. Studies from Asia (41 countries, 37.2%), Europe (28 countries, 25.4%), North America (24 countries, 21.8%), South America (5 countries, 4.5%), and Middle East (6 countries, 5.4%), and additional 6 multinational studies (5.4%) were included in our analyses. The outcomes of interest were mortality, progression to severe disease (severe pneumonia, admission to intensive care unit (ICU), and/or mechanical ventilation), viral clearance rate, QT prolongation, fatal cardiac complications, and noncardiac serious adverse events. Based on RCTs, the risk of progression to severe course and mortality was significantly reduced with corticosteroids (odds ratio (OR) 0.23, 95% confidence interval (CI) 0.06 to 0.86, p = 0.032, and OR 0.78, 95% CI 0.66 to 0.91, p = 0.002, respectively) and remdesivir (OR 0.29, 95% CI 0.17 to 0.50, p < 0.001, and OR 0.62, 95% CI 0.39 to 0.98, p = 0.041, respectively) compared to standard care for moderate to severe COVID-19 patients in non-ICU; corticosteroids were also shown to reduce mortality rate (OR 0.54, 95% CI 0.40 to 0.73, p < 0.001) for critically ill patients in ICU. In analyses including observational studies, interferon-alpha (OR 0.05, 95% CI 0.01 to 0.39, p = 0.004), itolizumab (OR 0.10, 95% CI 0.01 to 0.92, p = 0.042), sofosbuvir plus daclatasvir (OR 0.26, 95% CI 0.07 to 0.88, p = 0.030), anakinra (OR 0.30, 95% CI 0.11 to 0.82, p = 0.019), tocilizumab (OR 0.43, 95% CI 0.30 to 0.60, p < 0.001), and convalescent plasma (OR 0.48, 95% CI 0.24 to 0.96, p = 0.038) were associated with reduced mortality rate in non-ICU setting, while high-dose intravenous immunoglobulin (IVIG) (OR 0.13, 95% CI 0.03 to 0.49, p = 0.003), ivermectin (OR 0.15, 95% CI 0.04 to 0.57, p = 0.005), and tocilizumab (OR 0.62, 95% CI 0.42 to 0.90, p = 0.012) were associated with reduced mortality rate in critically ill patients. Convalescent plasma was the only treatment option that was associated with improved viral clearance rate at 2 weeks compared to standard care (OR 11.39, 95% CI 3.91 to 33.18, p < 0.001). The combination of hydroxychloroquine and azithromycin was shown to be associated with increased QT prolongation incidence (OR 2.01, 95% CI 1.26 to 3.20, p = 0.003) and fatal cardiac complications in cardiac-impaired populations (OR 2.23, 95% CI 1.24 to 4.00, p = 0.007). No drug was significantly associated with increased noncardiac serious adverse events compared to standard care. The quality of evidence of collective outcomes were estimated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework. The major limitation of the present study is the overall low level of evidence that reduces the certainty of recommendations. Besides, the risk of bias (RoB) measured by RoB2 and ROBINS-I framework for individual studies was generally low to moderate. The outcomes deducted from observational studies could not infer causality and can only imply associations. The study protocol is publicly available on PROSPERO (CRD42020186527).
Conclusions
In this NMA, we found that anti-inflammatory agents (corticosteroids, tocilizumab, anakinra, and IVIG), convalescent plasma, and remdesivir were associated with improved outcomes of hospitalized COVID-19 patients. Hydroxychloroquine did not provide clinical benefits while posing cardiac safety risks when combined with azithromycin, especially in the vulnerable population. Only 29% of current evidence on pharmacological management of COVID-19 is supported by moderate or high certainty and can be translated to practice and policy; the remaining 71% are of low or very low certainty and warrant further studies to establish firm conclusions.
In this meta-analysis, Min Seo Kim and colleagues synthesise results from randomized trials and observational studies on COVID-19 treatments.
Author summary
Why was the study done?
Numerous clinical trials and observational studies have investigated various pharmacological agents as potential treatments for Coronavirus Disease 2019 (COVID-19), but systematic synthesis of this large body of information is not readily available.
Results from these studies are heterogeneous and sometimes even contradictory to one another, making it difficult for clinicians to determine which treatments are truly effective.
Level of evidence behind these drugs are diverse and must be classified into categories to effectively inform policy and practice.
What did the researchers do and find?
In randomized controlled trials (RCTs), remdesivir and corticosteroid were shown to reduce COVID-19 aggravation and mortality rates.
In the whole dataset, including data from RCTs and observational studies, anti-inflammatory agents (corticosteroid, tocilizumab, anakinra, and IVIG), convalescent plasma, and remdesivir were associated with improved clinical outcomes of COVID-19.
Hydroxychloroquine provides no benefit in mitigating COVID-19 disease course while posing safety risks, especially to vulnerable populations.
What do these findings mean?
These findings could help prioritize further research on drugs of possible benefit.
Only 29% of current evidence on pharmacological management of COVID-19 is based on moderate/high evidence certainty and can be reflected in practice and policy; remaining 71% are of low or very low evidence certainty and warrant further studies to establish firm conclusions.
Introduction
Numerous Coronavirus Disease 2019 (COVID-19) clinical trials and observational studies are underway, and over 47 pharmacological agents and regimens have been investigated as potential treatments of COVID-19. However, despite all such research efforts, conclusive consensus on treatment is still lacking. To the contrary, the increasing volume of information on the pharmacologic management of COVID-19 patients has led to a wider divergence in the management of COVID patients across institutions worldwide. Although studies supporting these various treatment regimens have been published, they utilize different designs, investigating different medications under various settings; as a result, the evidence on pharmacologic treatment of COVID-19 is scattered and heterogeneous. A network meta-analysis (NMA) enables a single coherent ranking of such numerous interventions, and it can thus aid decision-makers who must choose among an array of treatment options [1].
We conducted an NMA with selective predefined eligibility criteria for both published and unpublished data and investigated 47 treatment regimens for comparative efficacy and safety. We incorporated 110 studies (40 randomized controlled trials (RCTs) and 70 confounder-adjusted observational studies). The level of certainty behind the evidence for each outcome was also evaluated to assist the decision-making of clinicians and policy makers.
Methods
Search strategy and selection criteria
We searched PubMed, Google Scholar, MEDLINE, the Cochrane Library, medRxiv, SSRN, WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov for RCTs and observational studies that evaluated treatment responses to pharmacological management in COVID-19 patients, from the beginning of 2020 to August 24, 2020. Reference lists of review articles were also reviewed to search for additional articles that may not have been retrieved by the prespecified searching strategy. We had no restriction on language, but all included studies were written in English. This study was reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [2] (S1 PRISMA Checklist). The study protocol is publicly available on PROSPERO (CRD42020186527) and medRxiv [3]. Since this review did not involve any individual patient data, ethical approval was not required.
Participants, interventions, comparisons, outcomes, and study design (PICOS) of all included studies are described in S1 Table. We included studies investigating the effect of pharmacological management of patients hospitalized for COVID-19 management. Mild patients who do not require hospitalization or have self-limiting disease courses were not eligible for our network meta-analysis (NMA). The outcomes of interest were mortality, progression to severe disease (severe pneumonia, admission to intensive care unit (ICU), and/or mechanical ventilation), viral clearance rate, QT prolongation, fatal cardiac complications, and noncardiac serious adverse events.
We contacted principal investigators of unpublished studies identified in trial registries and regulatory submissions to obtain unpublished data. Inclusion of unpublished data in NMAs is not uncommon [4–11] and reduces risk of selection and publication bias while increasing the density of study data. This is especially beneficial in the study of COVID-19 as all data were generated relatively recently, and the bulk of the relevant data are still in the unpublished stages. Preprints have been used in meta-analysis relatively frequently for the urgent topic of COVID-19 [9–12], and American Gastroenterological Association (AGA) recently published a management guideline for the gastrointestinal manifestation of COVID-19 patients based on the result of meta-analysis incorporating preprints [11]. We contacted authors of included preprints from medRxiv and SSRN, and any change in the results was updated.
We included both RCTs and baseline-adjusted observational studies; the rationale is that inclusion of real-world data from nonrandomized studies has the potential to improve precision of findings from RCTs if appropriately integrated [13] and that the volume of information provided by these studies is necessary to assess adverse events of low to moderate incidence [14–16]. As observational studies are more vulnerable to bias, we included only the studies that accounted for relevant confounding variables by showing that baseline characteristics were similar (p > 0.05 for baseline characteristics) or through methods such as propensity score matching (PSM), subgroup analyses, or regression model adjustment.
Following studies were excluded: studies without a proper control group; studies of children or adolescents (<18 years) as they may have a different disease course [17–19]; observational studies with significant differences in baseline characteristics between groups and did not perform adequate adjustments; and studies investigating the effect of medication initiated prior to the diagnosis of COVID-19 (e.g., ACEi/ARB for hypertensive patients).
Data extraction and quality assessment
The study search and data extraction were independently conducted by 3 authors (MS Kim, MH An, and WJ Kim). Manuscript and supplementary materials of the included studies were reviewed for relevant information which was extracted according to a prespecified protocol. Any discrepancy or ambiguity in this process was resolved by discussion. Authors of certain included studies were contacted in case of missing or unclear information. Nonrandomized studies were qualitatively assessed using the Newcastle-Ottawa Scale (NOS) [20], and RCTs were assessed with the Jadad scale [21]. All studies were assessed for risk of bias (RoB) using the Risk of Bias 2 (RoB2) tool for randomized studies and Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool for nonrandomized studies [22]. The quality of evidence of collective outcomes were estimated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework [23]. A comparison-adjusted funnel plot with Egger test was constructed to assess for publication bias [24].
Control groups consisted of patients who received standard care or placebo. Patients who received hydroxychloroquine or corticosteroids were subdivided according to the dosage they received. For hydroxychloroquine, most studies reported 400 mg hydroxychloroquine daily for maintenance, and this was considered the standard prescription; patients who received daily maintenance dosage of over 600 mg (>600 mg/day) hydroxychloroquine were classified into a separate high-dose hydroxychloroquine group. For corticosteroids, average daily dosage of 40 mg methylprednisolone (or equivalent) was regarded as the standard dosage, while 1 to 2 mg/kg/day methylprednisolone (or equivalent) was regarded as high dose. A total of 1 mg methylprednisolone was considered equivalent to 0.1875 mg dexamethasone and 5 mg hydrocortisone.
A critically ill patient was defined as a patient who received invasive mechanical ventilation or needed intensive care in the ICU before or soon after beginning the treatment of interest, while moderate-severe patients were defined as patients hospitalized in a non-ICU setting at admission. The mortality rate of patients included in our mortality analyses were 13.1% for moderate-severe (non-ICU) patients and 40.5% for critically ill (ICU) patients on average.
Data synthesis and statistical analysis
We conducted a random-effects NMA within a frequentist framework using STATA (Stata Corp, College Station, Texas, United States of America, version 15.0) and R (version 3.6.0) software [25]. Direct and indirect (and mixed) comparison were accomplished through the self-programmed routines of STATA [24,26] and the netmeta package of R [27]. Further details of the methodology for NMA are described elsewhere [28,29]. The effect estimation was in odds ratios (OR) for dichotomous variables and mean difference (MD) for continuous variables, both with 95% confidential intervals (CI). When median (interquartile range) was presented for continuous variables of interest, it was converted to mean (standard deviation) by calculation [30,31]. A 2-sided p-value of less than 0.05 was regarded as statistically significant.
Statistical heterogeneity was estimated using restricted maximum likelihood method [32] and expressed with Higgins I2 statistics and the Cochran Q test [33]. The net heat plot was constructed to visualize the inconsistency matrix and detect specific comparisons which introduced large inconsistencies [34]. The rank of effect estimation for each treatment was investigated using the surface under the cumulative rank curve (SUCRA) of P rank score of R [35].
Prespecified subgroup and sensitivity analyses were performed to determine whether the results were affected by the patient severity, treatment protocol, and study design. The primary outcomes were separately analyzed for moderate to severe patients (non-ICU at admission) and critically ill patients (ICU) as these patients may respond differently to treatments. Sensitivity analyses were conducted by restricting the analyses to only RCTs, only published studies, and excluding studies with high/serious RoB.
Results
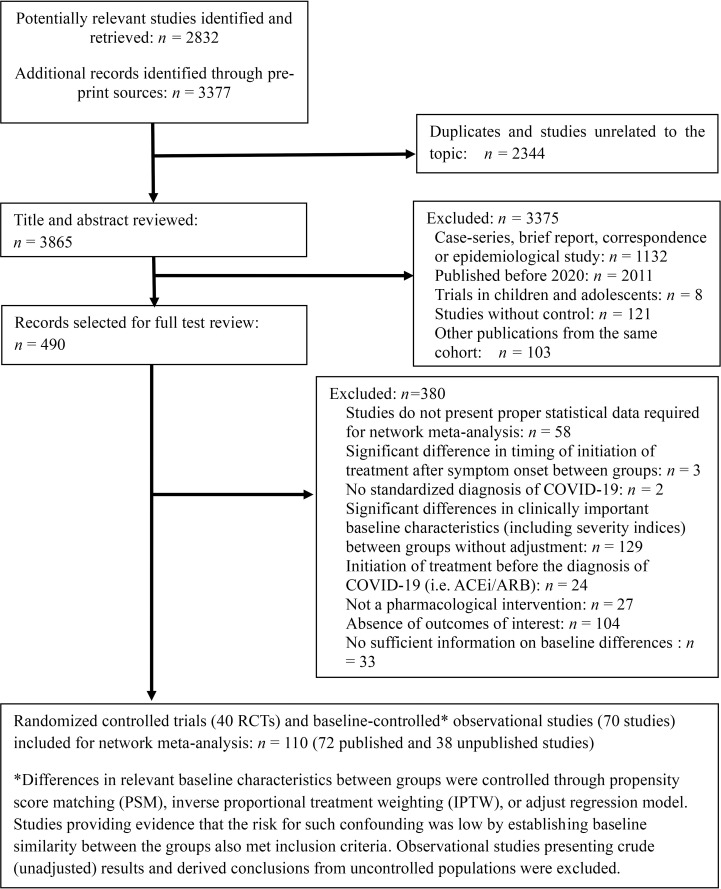
The initial search identified 6,209 articles. These studies were assessed for inclusion using the prespecified inclusion and exclusion criteria described in methods. Title and abstract of 3,865 articles were assessed, and 490 studies were found suitable for full-text review. After excluding 380 studies, 40 RCTs and 70 observational studies were finally included in our NMA (Fig 1). A total of 49,569 COVID-19 patients were included. Background characteristics and reference list of included studies are presented in S1 Table. The RoB in included studies were generally low to moderate (S2 Table).
Fig 1. PRISMA diagram showing selection of articles for pairwise and network meta-analysis.
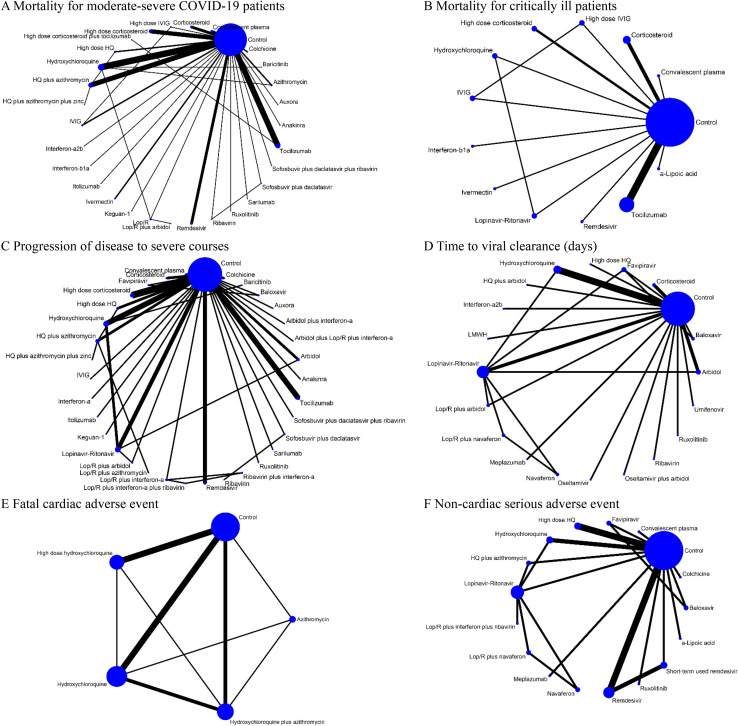
For both pairwise meta-analysis and NMA, the primary outcomes presented no evidence of heterogeneity (S3 Table), except for mortality rate of critically ill patients (I2 = 62.0%) and viral clearance rate (I2 = 83.8%). Inconsistency, which represents discordance of direct and indirect comparisons, was also evaluated for outcomes, but none were subject to global inconsistency. We visualized the network of comparisons as shown in Fig 2. In the network, each regimen is represented by a unique node, meaning different nodes were designated for different dosages of the same drug. Lines indicate direct head-to-head comparison of agents, and the thickness of line corresponds to the number of trials in the comparison. Size of the node corresponds to the number of studies behind the intervention. Detailed information of studies included in the analysis for cardiac adverse events are presented in Table 1, and the certainty of evidence (GRADE) for each outcome is summarized in Table 2.
Fig 2.
Network of eligible comparisons for primary outcomes (A) Mortality for moderate-severe COVID-19 patients (non-ICU at admission). (B) Mortality for critically ill patients (ICU). (C) Progression of disease to severe courses (i.e., progression to severe pneumonia, admission to ICU, and/or mechanical ventilation). (D) Time to viral clearance (days). (E) Fatal cardiac adverse events (torsades de pointes, cardiac arrest, and severe ventricular arrhythmia). (F) Noncardiac serious adverse events. Lines indicate direct comparison of agents, and the thickness of line corresponds to the number of trials in the comparison. Size of node corresponds to the number of studies that involve the intervention. HQ, hydroxychloroquine; ICU, intensive care unit; Lop/R, lopinavir-ritonavir.
Table 1. Studies included for analysis of QT prolongation and fatal cardiac complications after taking hydroxychloroquine alone or hydroxychloroquine with azithromycin.
| Study | Baseline characteristics | Assessed fatal cardiac complications | Incidence (%) |
|---|---|---|---|
| Studies including relatively high portion of patients with poor cardiac function (>10% of patients have CAD/CHD*) | |||
| Rosenberg et al. [65] | Proportion of patients with cardiovascular comorbidities was high at baseline (approximately 30%), and proportions of cardiovascular comorbidities were significantly different between groups in crude analysis. However, adjustments were made for sex, age category (<65 vs ≥65 years), diabetes, any chronic lung disease, cardiovascular disease, abnormal chest imaging, respiration rate >22/min, O2 saturation <90%, elevated creatinine, and AST >40 U/L. Adjusted odds ratios for prolonged QT interval were presented. Obesity was significantly higher in pharmacologic treatment groups (hydroxychloroquine, hydroxychloroquine, alone, and azithromycin alone) compared to control (neither drug). Obesity was not adjusted for. Median age 63 Coronary heart disease (12.7%) |
Cardiac arrest | Hydroxychloroquine+Azithromycin: 15.5% Hydroxychloroquine alone: 13.6% Azithromycin alone: 6.1% Neither drugs: 7.1% |
| Mercuro et al. [66] | Baseline QTc interval was longer in the hydroxychloroquine group compared to hydroxychloroquine plus azithromycin group. Therefore, we used the change in QTc intervals (ΔQTc) in each group for analysis. Mean age 60.1 Coronary heart disease (11.1%) and atrial fibrillation (13.3%) |
Torsades de pointes | Hydroxychloroquine+Azithromycin: .8% Hydroxychloroquine: 0% |
| Ramireddy et al. [67] | Baseline QTc intervals were significantly different between groups. Therefore, we used change in QTc intervals (posttreatment QTc-baseline QTc, ΔQTc) of each group for analysis. Mean age 62.3, mean BMI 27.8 Heart failure (20%) |
Syncope, torades de pointes, or other lethal arrhythmias | Azithromycin alone: 0% Hydroxychloroquine+Azithromycin: 0% |
| Saleh et al. [68] | Sex, structural heart disease, cirrhosis, other medications known to cause QT prolongation were comparable between hydroxychloroquine and hydroxychloroquine plus azithromycin groups. Mean age 58.5, mean BMI 28.2 Mean ejection fraction (61.9%), coronary artery disease (11.4%) |
Monomorphic ventricular arrhythmia | Hydroxychloroquine: 2.4% Hydroxychloroquine+Azithromycin: 5.0% |
| Bessiere et al. [69] | ICU setting. Median age 68, median BMI 28 Structural heart disease (20%) |
Severe ventricular arrhythmia including torades de pointes | Hydroxychloroquine: 0% Hydroxychloroquine+Azithromycin: 0% |
| Borba et al. [70] | Randomized controlled trial. Generally, baseline characteristics were well controlled between groups, but preexisting heart disease was more frequent in the high dosage group (p-value not provided). Mean age 51.1 Heart disease (17.9%) in high-dose hydroxychloroquine group |
Ventricular tachycardia | High-dose hydroxychloroquine: 4.8% Standard-dose hydroxychloroquine: 0% |
| Horby et al. [71] | Randomized controlled trial Mean age 65.3 Heart disease (26% but perhaps including hypertension) |
Ventricular tachycardia or fibrillation | High-dose hydroxychloroquine: 0.9% No hydroxychloroquine: 0.7% |
| Studies including relatively low portion of patients with poor cardiac function (<10% of patients have CAD/CHD*) | |||
| Mahevas et al. [72] | Baseline characteristics were generally balanced and controlled (i.e., COPD/asthma, HF, CVD, DM, CKD, LC, immunosuppression). Median age 60 Chronic heart failure (4%) |
Severe arrhythmia | Hydroxychloroquine: 1.1% (first-degree atrioventricular block) No hydroxychloroquine: 1.0% (left bundle branch block) |
| Tang et al. [73] | Randomized controlled trial. Mild to moderate patients with low rate of cardiovascular comorbidity presence. Mean age 46.1, BMI 23.5 Hypertension was presented in 6% of patients, and the prevalence of structural heart disease is expected to be less than 6% |
Severe arrhythmia | Hydroxychloroquine: 0% No hydroxychloroquine: 0% |
| An et al. [74] | Patients in the conservative treatment group had milder baseline features than patients in the hydroxychloroquine plus azithromycin group. Mean age 42.2 and mean BMI 23 Very low prevalence of cardiovascular comorbidities, perhaps due to young age. |
Cardiac arrest | Hydroxychloroquine+Azithromycin: 0% No hydroxychloroquine: 0% |
| Boulware et al. [75] | Randomized controlled trial. Mild patient group. Only the adverse event-related data from this study were used in this network meta-analysis, as this study investigates the effect of hydroxychloroquine as a prophylactic measure which is not the focus of our efficacy meta-analysis. Median age 41 Cardiovascular disease (0.7%, not include hypertension) |
Cardiac arrhythmia | High-dose hydroxychloroquine: 0% No hydroxychloroquine: 0% |
| Skipper et al. [76] | Randomized controlled trial. Mild patient group. Only the adverse event-related data from this study were used in this network meta-analysis, as this study investigates the effect of early hydroxychloroquine intake in nonhospitalized COVID-19 or probable COVID-19 patients with high-risk exposure which is not the focus of our efficacy analysis. Median age 40 Cardiovascular disease (1.2%, not include hypertension) |
Cardiac arrhythmia | High-dose hydroxychloroquine: 0% No hydroxychloroquine (placebo): 0% |
| Mitja et al. [77] | Randomized controlled trial. Mild patient group. Only the adverse event-related data from this study were used in this network meta-analysis, as this study investigates the effect of early hydroxychloroquine intake in nonhospitalized COVID-19 which is not the focus of our efficacy analysis. Median age 41.6 Cardiovascular disease (9.6% in control group and 14.7% in HQ group; however, note that this prevalence includes hypertension and therefore rate of structural/functional cardiac comorbidities such as CAD and CHD may be lower) |
Cardiac arrhythmia | Hydroxychloroquine: 0% No hydroxychloroquine: 0% |
| Cavalcanti et al. [78] | Randomized controlled trial. Mild-to-moderate patient group. Median age 50.3 Heart failure (1.5%) |
Cardiac arrhythmia/ventricular tachycardia | Hydroxychloroquine plus azithromycin: 1.3% High-dose hydroxychloroquine: 1.5% Azithromycin alone: 0% Control (neither medication): 0.6% |
| Mitja et al. [79] | Randomized controlled trial. Mild patient group (postexposure group). Only the adverse event-related data from this study were used in this network meta-analysis, as this study investigates the effect of prophylactic hydroxychloroquine intake in postexposure patients which is not the focus of our efficacy analysis. Median age 48.6 Cardiovascular disease (14.9% in control group and 11.6% in HQ group; however, note that the prevalence includes hypertension and therefore the prevalence of structural/functional cardiac comorbidities such as CAD and CHD may be lower) |
Cardiac arrhythmia | Hydroxychloroquine: 0% No hydroxychloroquine: 0% |
| Lofgren et al. [80] | Randomized controlled trial. Mild patient group (post- and pre-exposure groups). Only the adverse event-related data from this study were used in this network meta-analysis, as this study investigates the effect of prophylactic hydroxychloroquine intake which is not the focus of our efficacy analysis. Median age 40 Mostly healthworkers |
Ventricular tachycardia | Hydroxychloroquine (daily intake): 0% Placebo: 0% |
*CAD/CHD, coronary artery disease/congestive heart disease.
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus Disease 2019; CVD, cardiovascular disease; DM, diabetes mellitus; HF, heart failure; ICU, intensive care unit; LC, liver cirrhosis.
Table 2. Certainty of evidence evaluated with GRADE framework.
| Comparisons (vs. Control) | Study No. † | Effect size (95% CI), p-value | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | GRADE |
|---|---|---|---|---|---|---|---|---|---|
| Mortality in moderate to severe patients (non-ICU at admission), odds ratio | |||||||||
| High-dose corticosteroid plus tocilizumab | 1 | 0.04 (0.01, 0.17), p < 0.001 | Observational study | Downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Interferon-a2b | 1 | 0.05 (0.01, 0.39), p = 0.004 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Baricitinib | 1 | 0.05 (0.00, 1.04), p = 0.053 | Observational study | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Itolizumab | 1 | 0.10 (0.01, 0.92), p = 0.042 | Observational study | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Sofosbuvir plus daclatasvir plus ribavirin | 1 | 0.13 (0.01, 2.64), p = 0.184 | RCT | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Ruxolitinib | 1 | 0.13 (0.01, 2.74), p = 0.190 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Interferon-b1a | 1 | 0.13 (0.01, 2.96), p = 0.201 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Sofosbuvir plus daclatasvir | 3 | 0.26 (0.07, 0.88), p = 0.030 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Anakinra | 1 | 0.30 (0.11, 0.82), p = 0.019 | Observational study | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| High-dose corticosteroid | 7 | 0.38 (0.24, 0.63), p < 0.001 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| Tocilizumab | 9 | 0.43 (0.30, 0.60), p < 0.001 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Sarilumab | 1 | 0.35 (0.06, 2.10), p = 0.251 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Keguan-1 | 1 | 0.31 (0.01, 8.11), p = 0.482 | RCT | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Colchicine | 2 | 0.39 (0.07, 2.33), p = 0.302 | RCT | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | Moderate |
| High-dose IVIG | 1 | 0.37 (0.02, 7.17), p = 0.511 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low‡ |
| Convalescent plasma | 3 | 0.48 (0.24, 0.96), p = 0.038 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Remdesivir | 4 | 0.52 (0.34, 0.80), p = 0.003 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Auxora | 1 | 0.47 (0.05, 4.19), p = 0.499 | RCT | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| HQ plus azithromycin plus zinc | 1 | 0.56 (0.28, 1.10), p = 0.092 | Observational study | No downgrade | Downgrade* | Downgrade | No downgrade | No downgrade | Very Low |
| LOP/R plus arbidol | 1 | 0.58 (0.03, 10.79), p = 0.715 | Observational study | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| LOP/R | 3 | 0.66 (0.33, 1.35), p = 0.255 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Azithromycin | 2 | 0.70 (0.46, 1.09), p = 0.114 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Ivermectin | 2 | 0.76 (0.25, 2.33), p = 0.631 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Ribavirin | 2 | 0.86 (0.33, 2.24), p = 0.758 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| IVIG | 2 | 1.00 (0.25, 3.96), p = 1.000 | RCT | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | High‡ |
| HQ | 11 | 0.93 (0.77, 1.13), p = 0.465 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| Corticosteroid | 4 | 1.00 (0.74, 1.33), p = 1.000 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| High-dose HQ | 2 | 1.15 (0.77, 1.71), p = 0.490 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High‡ |
| HQ plus azithromycin | 8 | 1.13 (0.89, 1.44), p = 0.323 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| Mortality in critically ill patients (ICU), odds ratio | |||||||||
| High-dose IVIG | 1 | 0.13 (0.03, 0.49), p = 0.003 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low‡ |
| Ivermectin | 1 | 0.15 (0.04, 0.57), p = 0.005 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| a-Lipoic acid | 1 | 0.17 (0.02, 1.61), p = 0.122 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| HQ | 1 | 0.45 (0.12, 1.70), p = 0.239 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low‡ |
| Interferon-b1a | 1 | 0.47 (0.11, 1.94), p = 0.297 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Tocilizumab | 6 | 0.62 (0.42, 0.90), p = 0.012 | Observational study | No downgrade | Downgrade | No downgrade | No downgrade | No downgrade | Very Low |
| Convalescent plasma | 1 | 0.72 (0.19, 2.72), p = 0.628 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| IVIG | 1 | 0.73 (0.22, 2.45), p = 0.610 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low‡ |
| High-dose corticosteroid | 2 | 0.74 (0.38, 1.46), p = 0.385 | RCT | No downgrade | Downgrade | No downgrade | No downgrade | No downgrade | High‡ |
| LOP/R | 1 | 0.78 (0.19, 3.27), p = 0.734 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Remdesivir | 1 | 0.92 (0.38, 2.25), p = 0.855 | RCT | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Moderate |
| Corticosteroid | 3 | 0.91 (0.50, 1.68), p = 0.763 | Observational study | No downgrade | Downgrade | No downgrade | No downgrade | No downgrade | Low‡ |
| Progression to severe course (progress to severe pneumonia or admission to ICU), odds ratio | |||||||||
| Baricitinib | 1 | 0.02 (0.00, 0.32), p = 0.006 | Observational study | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| High-dose corticosteroid | 5 | 0.11 (0.06, 0.19), p < 0.001 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| Sofosbuvir plus daclatasvir plus ribavirin | 1 | 0.09 (0.00, 1.85), p = 0.119 | RCT | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Ruxolitinib | 1 | 0.09 (0.00, 1.91), p = 0.122 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Auxora | 1 | 0.17 (0.03, 1.07), p = 0.059 | RCT | Downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Low |
| Anakinra | 1 | 0.22 (0.09, 0.56), p = 0.002 | Observational study | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| IVIG | 1 | 0.20 (0.03, 1.22), p = 0.081 | RCT | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | High‡ |
| Keguan-1 | 1 | 0.18 (0.01, 3.90), p = 0.275 | RCT | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Remdesivir | 3 | 0.28 (0.16, 0.50), p < 0.001 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Itolizumab | 1 | 0.26 (0.07, 1.03), p = 0.055 | Observational study | Downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| Arbidol plus interferon-a | 1 | 0.23 (0.01, 5.34), p = 0.360 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Colchicine | 2 | 0.33 (0.06, 1.89), p = 0.213 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Tocilizumab | 4 | 0.39 (0.24, 0.62), p = 0.001 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Sofosbuvir plus daclatasvir | 3 | 0.37 (0.09, 1.62), p = 0.187 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Ribavirin plus interferon-a | 1 | 0.33 (0.02, 6.41), p = 0.464 | RCT | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Arbidol | 2 | 0.49 (0.15, 1.64), p = 0.247 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Corticosteroid | 2 | 0.51 (0.35, 0.76), p < 0.001 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| Convalescent plasma | 2 | 0.53 (0.28, 0.98), p = 0.043 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| LOP/R plus interferon-a | 2 | 0.62 (0.12, 3.26), p = 0.572 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| LOP/R plus interferon-a plus ribavirin | 1 | 0.70 (0.05, 9.71), p = 0.790 | RCT | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Sarilumab | 1 | 0.82 (0.23, 2.90), p = 0.758 | Observational study | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| Arbidol plus LOP/R plus interferon-a | 1 | 0.85 (0.16, 4.59), p = 0.850 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| HQ | 6 | 0.92 (0.56, 1.51), p = 0.742 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| HQ plus azithromycin plus zinc | 1 | 0.96 (0.42, 2.18), p = 0.922 | Observational study | No downgrade | Downgrade* | Downgrade | No downgrade | No downgrade | Very Low |
| Interferon-a | 1 | 1.33 (0.15, 11.95), p = 0.799 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| LOP/R | 5 | 1.08 (0.53, 2.21), p = 0.833 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| High-dose HQ | 2 | 1.12 (0.82, 1.53), p = 0.476 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High‡ |
| LOP/R plus arbidol | 2 | 1.60 (0.28, 9.11), p = 0.596 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Ribavirin | 1 | 1.67 (0.25, 11.01), p = 0.594 | Observational study | No downgrade | Downgrade* | Downgrade | No downgrade | No downgrade | Very Low |
| Baloxavir | 1 | 3.31 (0.12, 91.51), p = 0.480 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| HQ plus azithromycin | 3 | 1.76 (0.90, 3.44), p = 0.098 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| LOP/R plus azithromycin | 1 | 4.78 (0.45, 51.11), p = 0.196 | Observational study | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Favipiravir | 1 | 6.97 (0.29, 168.34), p = 0.232 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Viral clearance rate, odds ratio | |||||||||
| LOP/R plus arbidol | 1 | 15.83 (0.60, 417.8), p = 0.098 | Observational study | Downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Convalescent plasma | 1 | 11.39 (0.89, 145.7), p = 0.061 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Meplazumab | 1 | 8.67 (0.48, 156.43), p = 0.143 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| LOP/R plus navaferon | 1 | 6.27 (0.28, 138.55), p = 0.245 | RCT | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Navaferon | 1 | 3.51 (0.16, 76.55), p = 0.425 | RCT | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| LOP/R | 5 | 2.88 (0.50, 16.69), p = 0.238 | Observational study | No downgrade | Downgrade | No downgrade | No downgrade | No downgrade | Very Low |
| Baloxavir | 1 | 2.11 (0.14, 32.18), p = 0.591 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Arbidol | 1 | 1.69 (0.16, 18.46), p = 0.667 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Favipiravir | 2 | 1.61 (0.21, 12.32), p = 0.647 | RCT | No downgrade | Downgrade | No downgrade | No downgrade | No downgrade | Moderate |
| HQ | 5 | 1.34 (0.36, 5.08), p = 0.667 | RCT | No downgrade | Downgrade | No downgrade | No downgrade | No downgrade | High‡ |
| Umifenovir | 1 | 0.79 (0.06, 9.88), p = 0.855 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Darunavir plus cobicistat | 1 | 0.71 (0.05, 10.84), p = 0.806 | RCT | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Corticosteroid plus IVIG | 1 | 0.63 (0.05, 7.67), p = 0.717 | Observational study | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low‡ |
| High-dose HQ | 1 | 0.58 (0.05, 6.46), p = 0.658 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Moderate‡ |
| Time to viral clearance (days), mean difference | |||||||||
| Meplazumab | 1 | −10.00 (−16.79, −3.21), p = 0.045 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| HQ | 5 | −4.01 (−5.28, −2.73), p = 0.011 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| Favipiravir | 2 | −3.83 (−6.77, −0.89), p = 0.009 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Interferon-a2b | 1 | −3.00 (−8.17, 2.17), p = 0.615 | Observational study | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| Corticosteroid | 2 | −2.12 (−5.19, 0.95), p = 0.464 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Moderate‡ |
| Ribavirin | 1 | −1.30 (−3.41, 0.81), p = 0.521 | Observational study | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| LOP/R plus navaferon | 1 | −0.59 (−2.82, 1.64), p = 0.453 | RCT | No downgrade | Downgrade* | Downgrade | No downgrade | No downgrade | Low |
| Oseltamivir | 1 | 0.15 (−2.21, 2.51), p = 0.948 | Observational study | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| LMWH | 1 | 0.28 (−8.99, 9.56), p = 0.974 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Navaferon | 1 | 0.31 (−1.90, 2.52), p = 0.927 | RCT | No downgrade | Downgrade* | Downgrade | No downgrade | No downgrade | Low |
| Ruxolitinib | 1 | 0.72 (−5.34, 6.77), p = 0.911 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| HQ plus arbidol | 1 | 1.26 (−9.46, 11.98), p = 0.687 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Umifenovir | 1 | 0.93 (−2.64, 4.50), p = 0.817 | Observational study | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| High-dose HQ | 1 | 1.00 (−2.28, 4.28), p = 0.839 | RCT | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | High‡ |
| Arbidol | 2 | 0.96 (−0.85, 2.76), p = 0.489 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| LOP/R | 6 | 1.31 (−0.27, 2.89), p = 0.137 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Baloxavir | 1 | 7.32 (−10.20, 24.84), p = 0.735 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Oseltamivir plus arbidol | 1 | 4.57 (−0.21, 9.36), p = 0.079 | Observational study | No downgrade | Downgrade* | No downgrade | No downgrade | No downgrade | Very Low |
| LOP/R plus arbidol | 2 | 4.12 (0.79, 7.45), p = 0.032 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| ΔQTc interval from baseline (msec) of HQ plus AZ and AZ alone compared to ΔQTc of control (HQ alone), mean difference | |||||||||
| HQ plus azithromycin (vs. HQ) |
20.97 (12.60, 28.98), p < 0.001 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low | |
| Azithromycin (vs. HQ) |
4.09 (−15.76, 23.94), p = 0.522 | Observational study | Downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low | |
| Proportion of patients experiencing QTc prolongation (>500 ms or delta >60 ms), odds ratio | |||||||||
| High-dose HQ | 3 | 2.24 (1.04, 4.82), p = 0.039 | RCT | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | Moderate |
| HQ plus azithromycin | 7 | 2.01 (1.26, 3.20), p = 0.003 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| HQ | 7 | 1.39 (0.90, 2.17), p = 0.147 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Azithromycin | 3 | 1.09 (0.63, 1.87), p = 0.754 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Fatal cardiac complication after HQ (TdP, cardiac arrest, and severe ventricular arrhythmia)—overall study, odds ratio | |||||||||
| HQ plus azithromycin | 7 | 2.10 (1.23, 3.60), p = 0.007 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| HQ | 9 | 1.53 (0.88, 2.66), p = 0.132 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| High-dose HQ | 6 | 1.52 (0.73, 3.17), p = 0.264 | RCT | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | Moderate |
| Azithromycin | 2 | 0.55 (0.26, 1.18), p = 0.125 | Observational study | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | Very Low |
| Fatal cardiac complication after HQ (TdP, cardiac arrest, and severe ventricular arrhythmia)—studies with CAD/CHD <10% at baseline | |||||||||
| High-dose HQ | 4 | 1.34 (0.38, 4.71), p = 0.648 | RCT | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | Moderate |
| HQ plus azithromycin | 3 | 1.25 (0.27, 5.74), p = 0.774 | RCT | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | Moderate |
| HQ | 4 | 1.05 (0.26, 4.25), p = 0.946 | RCT | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | Moderate |
| Fatal cardiac complication after HQ (TdP, cardiac arrest, and severe ventricular arrhythmia)—studies with CAD/CHD >10% at baseline | |||||||||
| HQ plus azithromycin | 5 | 2.23 (1.24, 4.00), p = 0.007 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| HQ | 5 | 1.65 (0.90, 3.02), p = 0.104 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| High-dose HQ | 2 | 1.42 (0.54, 3.73), p = 0.477 | RCT | No downgrade | No downgrade | No downgrade | Downgrade | No downgrade | Moderate |
| Azithromycin | 2 | 0.59 (0.27, 1.30), p = 0.191 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Noncardiac serious adverse events, odds ratio | |||||||||
| Ruxolitinib | 1 | 0.09 (0.00, 1.89), p = 0.121 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| LOP/R plus interferon plus ribavirin | 1 | 0.09 (0.00, 2.43), p = 0.152 | RCT | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Short-term used remdesivir | 2 | 0.40 (0.26, 0.62), p < 0.001 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| LOP/R | 5 | 0.58 (0.31, 1.10), p = 0.095 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| HQ | 3 | 0.61 (0.26, 1.44), p = 0.259 | Observational study | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | Low |
| Navaferon | 1 | 0.58 (0.03, 10.51), p = 0.713 | RCT | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| LOP/R plus navaferon | 1 | 0.58 (0.03, 10.51), p = 0.713 | RCT | No downgrade | Downgrade* | Downgrade | Downgrade | No downgrade | Very Low |
| Meplazumab | 1 | 0.63 (0.04, 11.16), p = 0.753 | Observational study | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| Remdesivir | 4 | 0.71 (0.55, 0.90), p = 0.005 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Baloxavir | 1 | 1.00 (0.06, 16.76), p = 1.000 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Favipiravir | 1 | 1.00 (0.06, 16.76), p = 1.000 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| a-Lipoic acid | 1 | 1.00 (0.06, 16.76), p = 1.000 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| HQ plus azithromycin | 1 | 1.05 (0.14, 7.93), p = 0.962 | Observational study | Downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Very Low |
| High-dose HQ | 3 | 2.15 (0.98, 4.72), p = 0.056 | RCT | No downgrade | No downgrade | No downgrade | No downgrade | No downgrade | High |
| Colchicine | 1 | 4.72 (0.22, 100.72), p = 0.320 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
| Convalescent plasma | 1 | 5.10 (0.24, 108.86), p = 0.297 | RCT | No downgrade | Downgrade* | No downgrade | Downgrade | No downgrade | Low |
CAD, coronary artery disease; CHD, congestive heart disease; CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HQ, hydroxychloroquine; ICU, intensive care unit; IVIG, intravenous immunoglobulin; LMWH, low molecular weight heparin; LOP/R, lopinavir-ritonavir; RCT, randomized controlled trial.
* Downgraded by one when unable to evaluate inconsistency/heterogeneity due to lack of sufficient data (a single study).
‡ Upgrade by one for dose-response gradient.
† Number of studies investigated on each intervention.
Rationale
Study design: If randomized trials form the evidence base, the quality rating starts at “high.” If observational studies form the evidence, base the quality rating starts at “low.”
Risk of bias: Downgraded for failure to conceal random allocation or blind participants in randomized controlled trials or failure to adequately control for confounding in observational studies.
Inconsistency: Downgraded if heterogeneity represented by I2 statistics or global inconsistency (Q statistic to assess consistency under the assumption of a full design-by-treatment interaction random effects model) was high.
Indirectness. Downgraded when assumption of transitivity is challenged, or the result is solely derived from indirect comparisons.
Imprecision: Downgraded when confidential interval (CI) is relatively too large compared to other active drugs.
Publication bias: Downgraded when substantial asymmetry is observed in funnel plot or p < 0.05 in Egger test.
GRADE Definition (suggested by Puhan et al. in “A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis”)
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate, i.e., the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited, i.e., the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate, i.e., the true effect is likely to be substantially different from the estimate of effect.
Mortality in non-ICU settings
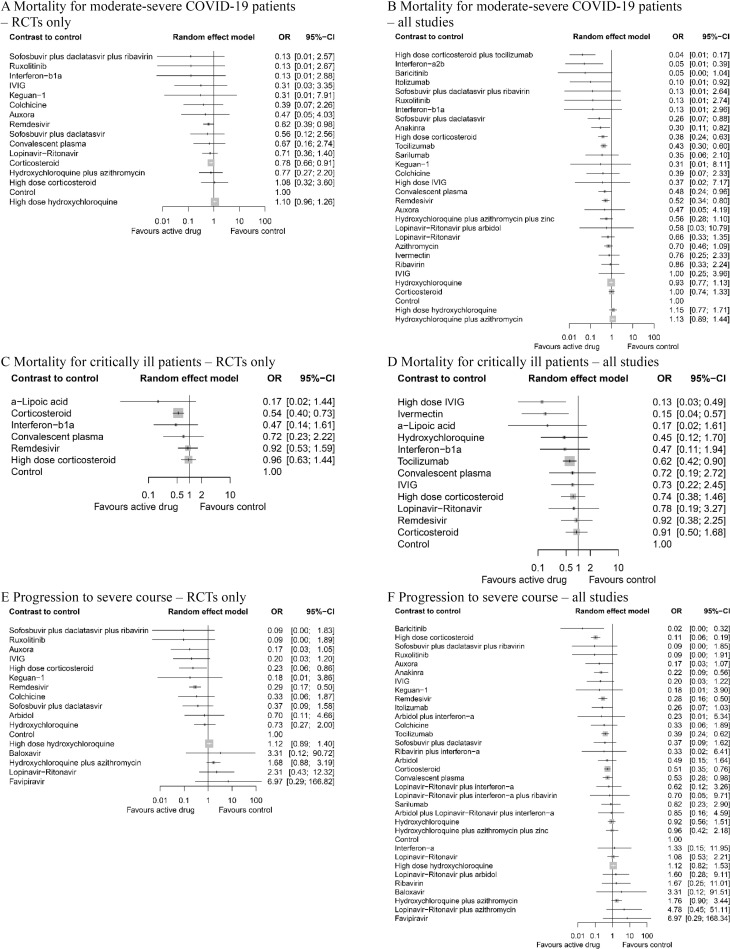
Remdesivir (OR 0.62, 95% CI 0.39 to 0.98, p = 0.041) and corticosteroids (OR 0.78, 95% CI 0.66 to 0.91, p = 0.002) significantly reduced the mortality in moderate-to-severe patients in analysis of RCTs (Fig 3A). High-dose corticosteroid plus tocilizumab (OR 0.04, 95% CI 0.01 to 0.17, p < 0.001, very low (GRADE)), interferon-a2b (OR 0.05, 95% CI 0.01 to 0.39, p = 0.004, very low), itolizumab (OR 0.10, 95% CI 0.01 to 0.92, p = 0.042, very low), sofosbuvir plus daclatasvir (OR 0.26, 95% CI 0.07 to 0.88, p = 0.030, high), anakinra (OR 0.30, 95% CI 0.11 to 0.82, p = 0.019, very low), high-dose corticosteroid (OR 0.38, 95% CI 0.24 to 0.63, p < 0.001, moderate), tocilizumab (OR 0.43, 95% CI 0.30 to 0.60, p < 0.001, low), convalescent plasma (OR 0.48, 95% CI 0.24 to 0.96, p = 0.038, low), and remdesivir (OR 0.52, 95% CI 0.34 to 0.80, p = 0.003, high) were associated with reduced mortality in moderate-to-severe patients hospitalized in a non-ICU setting compared to the control group (Fig 3B).
Fig 3. Network meta-analysis of pharmacological interventions compared with control (standard care) for efficacy outcomes.
Mortality for moderate-severe patients (non-ICU at admission) from (A) RCTs and (B) all studies. Mortality for critically ill patients (ICU) from (C) RCTs and (D) all studies. Progression to severe course (i.e., progression to severe pneumonia, admission to ICU, and/or mechanical ventilation) from (E) RCTs and (F) all studies. Effect estimates are presented in OR with 95% CI. Pharmacological agents are ranked by SUCRA value. CI, confidence interval; ICU, intensive care unit; OR, odds ratio; RCT, randomized controlled trial; SUCRA, surface under the cumulative ranking curve.
Mortality in ICU settings
In critically ill patients hospitalized in the ICU, corticosteroid (OR 0.54, 95% CI 0.40 to 0.73, p < 0.001) was the only agent showing effect in analysis with RCTs (Fig 3C). High-dose IVIG (OR 0.13, 95% CI 0.03 to 0.49, p = 0.003, low), ivermectin (OR 0.15, 95% CI 0.04 to 0.57, p = 0.005, very low), and tocilizumab (OR 0.62, 95% CI 0.42 to 0.90, p = 0.012, very low) were associated with lower mortality (Fig 3D).
Progression to severe disease
High-dose corticosteroid (OR 0.23, 95% CI 0.06 to 0.86, p = 0.032) and remdesivir (OR 0.29, 95% CI 0.17 to 0.50, p < 0.001) were shown to be effective in the sensitivity analysis using only RCTs (Fig 3E). Baricitinib (OR 0.02, 95% CI 0.00 to 0.32, p = 0.006, very low), high-dose corticosteroid (OR 0.11, 95% CI 0.06 to 0.19, p < 0.001, moderate), anakinra (OR 0.22, 95% CI 0.09 to 0.56, p = 0.002, very low), remdesivir (OR 0.28, 95% CI 0.16 to 0.50, p < 0.001, high), tocilizumab (OR 0.39, 95% CI 0.24 to 0.62, p < 0.001, low), corticosteroid (OR 0.51, 95% CI 0.35 to 0.76, p < 0.001, moderate), and convalescent plasma (OR 0.53, 95% CI 0.28 to 0.98, p = 0.043, low) was associated with lower rate of progression to severe disease (Fig 3F).
Viral clearance rate (negative conversion rate within 14 days)
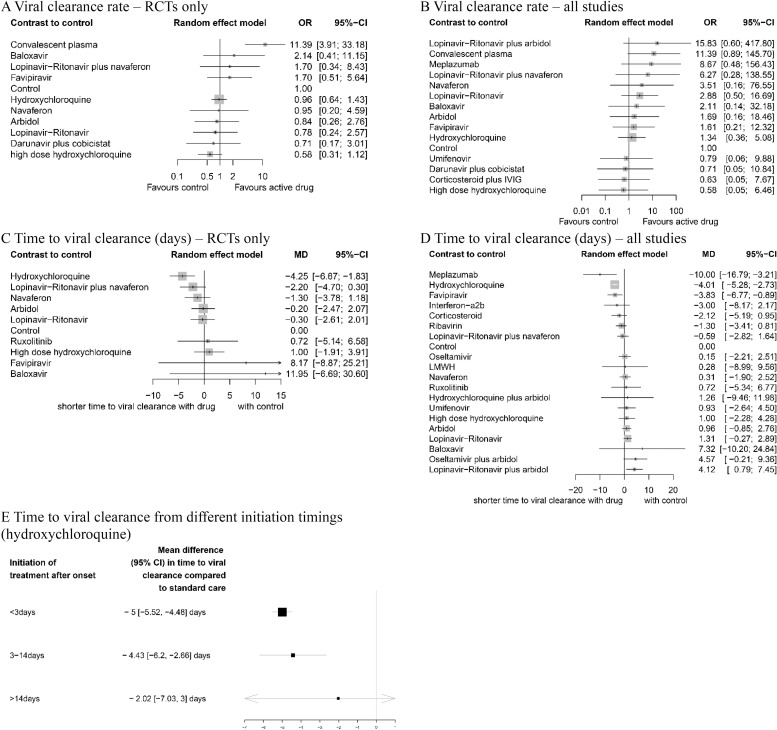
In RCTs, the use of convalescent plasma (OR 11.39, 95% CI 3.91 to 33.18, p < 0.001, low) showed significantly higher viral clearance rate compared to standard supportive therapy (Fig 4A). No active drug was associated with improved viral clearance rate within 14 days in mixed studies (Fig 4B).
Fig 4. Network meta-analysis of pharmacological interventions compared with control (standard care) for viral clearance.
Viral clearance rate (proportion of patients converted to PCR-negative status) from (A) RCTs and (B) all studies. Time to viral clearance (days) from (C) RCTs and (D) all studies. (E) Time to viral clearance from different hydroxychloroquine treatment initiation timings after symptom onset. Effect estimates are presented in OR for viral clearance rate and MD for time to viral clearance, with 95% CI. Pharmacological agents are ranked by SUCRA value. CI, confidence interval; MD, mean difference; OR, odds ratio; RCT, randomized controlled trial; SUCRA, surface under the cumulative ranking curve.
Time to viral clearance (days)
In RCTs, hydroxychloroquine was associated with the reduced time to viral clearance (Fig 4C). Meplazumab (MD −10.00, 95% CI −16.79 to −3.21, p = 0.045, very low), hydroxychloroquine (MD −4.01, 95% CI −5.28 to −2.73, p = 0.011, moderate), and favipiravir (MD −3.83, 95% CI −6.77 to −0.89, p = 0.009, high) were associated with shorter time to viral shedding compared with standard care (Fig 4D).
Time to treatment initiation from symptom onset
The effect of the timing of hydroxychloroquine treatment initiation after the symptom onset (Fig 4E) was assessed. Treatment initiated after 14 days (MD −2.02, 95% CI −7.03 to 3.00) from symptom onset did not reduce the time to viral clearance compared to standard care.
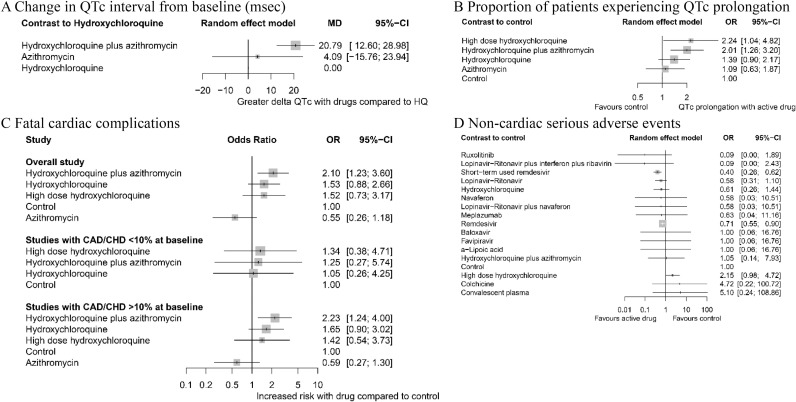
QTc prolongation
Compared to hydroxychloroquine monotherapy, the prolongation of QTc interval after treatment initiation was statistically significantly longer in the hydroxychloroquine plus azithromycin group (MD 20.79 ms, 95% CI 12.60 to 28.98, low) (Fig 5A). The proportion of patients experiencing QTc prolongation (defined by QTc interval >500 ms or ΔQTc >60 ms) was also significantly higher in the hydroxychloroquine plus azithromycin group and high-dose hydroxychloroquine group compared to the control group (OR 2.01, 95% CI 1.26 to 3.20, p = 0.003, low and OR 2.24, 95% CI 1.04 to 4.82, p = 0.039, moderate, respectively) (Fig 5B).
Fig 5. Network meta-analysis of safety of different pharmacological interventions.
(A) Change in QTc interval (ΔQTc) from baseline (msec). (B) Proportion of patients experiencing QTc prolongation (>500 ms or ΔQTc >60 ms). (C) Fatal cardiac complication after hydroxychloroquine administration (torsades de pointes, cardiac arrest, and severe ventricular arrhythmia). (D) Noncardiac serious adverse events. Effect estimates are presented in OR and MD with 95% CI. Pharmacological agents are ranked by SUCRA value. CAD, coronary artery disease; CHD, congestive heart disease; CI, confidence interval; HQ, hydroxychloroquine; MD, mean difference; OR, odds ratio; SUCRA, surface under the cumulative ranking curve.
Fatal cardiac complications (torsades de pointes, cardiac arrest, or severe ventricular arrhythmia)
The associations between fatal cardiac complications and hydroxychloroquine, azithromycin, or hydroxychloroquine plus azithromycin therapy were analyzed (Fig 5C). Overall, treatment with hydroxychloroquine plus azithromycin showed a significant association (OR 2.10, 95% CI 1.23 to 3.60, p = 0.007, low) while others did not. We further subdivided the included studies based on prevalence of coronary artery disease (CAD) and congestive heart disease (CHD) at baseline. In studies in which >10% of the baseline population had CAD/CHD, the risk of fatal cardiac complication was statistically significantly higher in patients receiving hydroxychloroquine plus azithromycin. In studies in which <10% of the baseline population had CAD/CHD, no notable difference in incidence of fatal heart complication was observed in any treatment group.
Noncardiac serious adverse events
No agent or regimen was associated with noncardiac severe adverse events (Fig 5D). To the contrary, there was a protective effect, i.e., decreased rate of adverse events with both short-term (5 days regimen) and standard (10 days regimen) remdesivir compared to standard care. High-dose hydroxychloroquine (>600 mg/day) was more prone to risk of severe adverse events (i.e., nausea, vomiting, and diarrhea that required discontinuation of the treatment) compared to standard care, but this difference was not statistically significant.
Subgroup and sensitivity analysis
The results of our subgroup and sensitivity analysis are reported in S4 Table. The assessments of other specific complications such as nausea/vomiting, diarrhea, hypoalbuminemia, anemia, leukopenia, lymphopenia, elevated AST/ALT, elevated CK, and increase total bilirubin are also presented in S4 Table.
Discussion
To our knowledge, this is the first comprehensive NMA of pharmacological treatment for COVID-19. Our conclusions provide possible insights on the use of individualized treatment strategies based on clinical setting and severity. For moderate and severe patients hospitalized in non-ICU settings, corticosteroids, tocilizumab, anakinra, remdesivir, and convalescent plasma were associated with reduced risk of progression to severe pneumonia, admission to ICU, and/or mechanical ventilation. Among these agents, corticosteroids and remdesivir further showed survival benefit compared to standard care. For ICU-based critically ill patients, corticosteroids reduced mortality from RCT evidence; high-dose IVIG, ivermectin, and tocilizumab may be associated with reduced mortality when including observational data. Hydroxychloroquine, a topic of much debate, was not shown to reduce mortality rate or prevent progression to severe disease in our analysis.
We analyzed 47 active pharmacologic agents and their combinations in a large-scale analysis incorporating 49,569 COVID-19 patients. Our study included unpublished data to integrate recent investigations and avoid selection and publication bias, as done in previous studies [4–7]. We did not limit our inclusions to RCTs and incorporated observational studies as we deemed that, in this analysis, the inclusion of real-world evidence from nonrandomized studies has the potential to add validity to certain findings [13], provide additional information regarding low-to-moderate incidence adverse events [14–16], and improve the density of the network [14]. Many previous NMAs included observational studies with this rationale [14–16,36,37], but inclusion of observational studies to an NMA requires careful integration to avoid biases from these observational studies pervading the meta-analysis [38]; as such, we exclusively included cohort studies that adjusted for confounders through methods such as PSM, subgroup analyses, and/or regression modeling or established similarity in the baseline characteristics (p > 0.05) of the groups being compared so that such adjustments are not necessary or irrelevant.
Hydroxychloroquine was not shown to reduce mortality rate or progression to severe disease. Such accumulated empirical results on hydroxychloroquine is supported by a recent in vitro study that revealed chloroquine does not prevent SARS Coronavirus 2 (SARS-CoV-2) entry into human lung cells and the subsequent spread through pulmonary tissue [39]
The potential cardiotoxicity of hydroxychloroquine and azithromycin is a widely shared concern in treating COVID-19 with these medications. According to our quantitative synthesis, incidence of QT prolongation was significantly higher in the patients who received hydroxychloroquine plus azithromycin compared to those who received standard care (Fig 5B). In addition, this combination of hydroxychloroquine and azithromycin was also associated with increased rate of fatal cardiac complications such as torsades de pointes, cardiac arrest, and severe ventricular arrhythmia in the cardiac-impaired population with a pooled incidence of 12.27%; in comparison, the pooled fatal cardiac complications rate in healthy populations with preserved cardiac function was about 0.01%. It should also be noted that noncardiac serious adverse events (nausea, vomiting, and/or diarrhea requiring discontinuation of the medication) were more frequent in high-dose (>600 mg/day) hydroxychloroquine monotherapy compared to standard care (Fig 5D), but the difference was not statistically significant. Strict monitoring should be implemented in all patients receiving hydroxychloroquine with or without azithromycin to maintain a tolerable safety margin.
Corticosteroids, tocilizumab (monoclonal IL-6 receptor antibody), anakinra (IL-1 receptor antagonist), and IVIG were associated with significantly reduced mortality in COVID-19 patients (Fig 3A–3D). Corticosteroids have been widely used in managing inflammation [40–42]. Other 3 drugs are also known anti-inflammatory agents that have been conventionally used in hyperimmune or autoimmune conditions; tocilizumab and anakinra have been used for the management of severe rheumatoid arthritis [43,44] and juvenile idiopathic arthritis [45–47]; IVIG was used for management of Kawasaki disease [18,48], inflammatory muscle diseases [49,50], and sepsis [51]. As there is accumulating evidence for an hyperimmune response characterized by the release of pro-inflammatory cytokines in severe and deceased COVID-19 patients [52–56], suppression of the inflammatory response and potential cytokine storm with immune-modulatory therapies was proposed as a potential therapeutic target; the results of this NMA support the efficacy of these treatments. Effectiveness of anti-inflammatory agents (corticosteroids, tocilizumab, anakinra, and IVIG) and ineffectiveness of antiviral agents, except for remdesivir, in hospitalized COVID-19 patients suggest that the management of hyperreactivity of the host immune response is advantageous over targeting viral replication itself.
Although corticosteroids and tocilizumab were highlighted as potential therapeutic agents against COVID-19 here, there are safety concerns regarding superinfection and other side effects related to these agents. Somers and colleagues reported that COVID-19 patients who received tocilizumab experienced significantly more superinfection compared to standard care, but they also stated that increase in superinfection did not significantly impact fatality [57]. This may indicate that the vulnerability to infection induced by tocilizumab may be manageable, but caution is still warranted to avoid bacterial infection [58]. Long-term use of corticosteroids may also entail side effects as reported in the past [59]; however, this safety concern is not expected to be of huge concern as most regimens for COVID-19 do not last more than 10 days [60–63] and short-term use was sufficient. Indeed, the recent RECOVERY trial showed that oral or intravenous dexamethasone (at a dose of 6 mg once daily) for up to 10 days reduces 28-day mortality [63]. These results regarding corticosteroids may have the farthest-reaching effect during this global pandemic, as they are available worldwide at relatively low cost.
Limitations
Our study has several limitations. First, some of the results were derived from a single study (i.e., Ruxolitinib) or studies with high RoB. To account for such weakness in evidence, we assessed the certainty of evidence for each outcome using the GRADE framework as summarized in Table 2. Second, for certain treatment agents, many articles have been published among which only one or few have been included in our analysis (e.g., convalescent plasma). This is because we prospectively collected studies that adhered to predefined inclusion criteria, and studies that did not adequately account for confounding or those prone to significant bias were filtered out. The excluded studies are listed and described in S5 Table with reasons for exclusion. Third, we included observational studies and unpublished data. While such inclusions may introduce biases into the final analysis, we judged the benefits overweigh the risks for reasons we mentioned in methods. Furthermore, we attempted to minimize biases by exclusively including observational studies that accounted for potential confounders and further conducted sensitivity analyses in which the same analysis was performed using only RCTs. Lastly, some of the results derived from this NMA lacks the support of pairwise meta-analysis. However, the methodological power of NMA is credible as empirical evidence supported that NMAs were 20% more likely to provide stronger evidence against the null hypothesis than conventional pairwise meta-analyses [64]. Accordingly, our NMA can offer meaningful implications for guiding management of COVID-19 until future studies build up stronger evidence.
The gradient of evidence levels analyzed in this review may assist the decision-making of clinicians and policymakers. Although numerous studies reported consistent results on beneficial effect of anti-inflammatory agents, the certainty of evidence for these agents are either low or very low because conclusions on tocilizumab, anakinra, and IVIG are based on observational studies. RCTs on these anti-inflammatory agents are required to confirm these findings and increase the level of evidence.
Conclusions
Corticosteroids and remdesivir may effectively improve clinical outcomes of COVID-19 as shown in RCTs; there is evidence of associations for other agents from the observational data that tocilizumab, anakinra, itolizumab, IVIG, and convalescent plasma may also provide clinical benefits. Hydroxychloroquine was not associated with improved clinical outcomes for COVID-19, while posing both cardiac and noncardiac safety risks and warrant appropriate patient selection. Only 29% of current evidence on pharmacological management of COVID-19 is on moderate/high evidence certainty and can therefore be confidently incorporated into practice and policy.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Abbreviations
- AGA
American Gastroenterological Association
- CAD
coronary artery disease
- CHD
congestive heart disease
- CI
confidence interval
- COVID-19
Coronavirus Disease 2019
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICU
intensive care unit
- IVIG
intravenous immunoglobulin
- MD
mean difference
- NMA
network meta-analysis
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- PICOS
participants, interventions, comparisons, outcomes, and study design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSM
propensity score matching
- RCT
randomized controlled trial
- RoB
risk of bias
- RoB2
Risk of Bias 2
- ROBINS-I
Risk of Bias in Nonrandomized Studies of Interventions
- SARS-CoV-2
SARS Coronavirus 2
- SUCRA
surface under the cumulative ranking curve
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korean government (MSIP) (NRF-2015R1A5A2009656), received by TH. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? The Lancet. 2015;386(9994):628–30. 10.1016/S0140-6736(15)61478-7 [DOI] [PubMed] [Google Scholar]
- 2.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 3.Kim MS, Kim WJ, An MH. Comparative Efficacy and Safety of Pharmacological Managements for Hospitalized COVID-19 Patients: Protocol for Systematic Review and Trade-Off Network Meta-Analysis. medRxiv. 2020;[preprint]:2020.05.18.20103697. 10.1101/2020.05.18.20103697 [DOI] [Google Scholar]
- 4.Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. The Lancet. 2019;394(10202):939–51. 10.1016/S0140-6736(19)31135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slee A, Nazareth I, Bondaronek P, Liu Y, Cheng Z, Freemantle N. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. The Lancet. 2019;393(10173):768–777. 10.1016/S0140-6736(18)31793-8 [DOI] [PubMed] [Google Scholar]
- 6.Eyding D, Lelgemann M, Grouven U, Härter M, Kromp M, Kaiser T, et al. Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ. 2010;341:c4737 10.1136/bmj.c4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinatongthai P, Kongwatcharapong J, Foo CY, Phrommintikul A, Nathisuwan S, Thakkinstian A, et al. Comparative efficacy and safety of reperfusion therapy with fibrinolytic agents in patients with ST-segment elevation myocardial infarction: a systematic review and network meta-analysis. The Lancet. 2017;390(10096):747–59. 10.1016/S0140-6736(17)31441-1 [DOI] [PubMed] [Google Scholar]
- 8.Langhorne P, Ramachandra S, Collaboration SUT. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev. 2020;(4). 10.1002/14651858.CD000197.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynants L, Van Calster B, Bonten MM, Collins GS, Debray TP, De Vos M, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J-W, Han T-W, Woodward M, Anderson CS, Zhou H, Chen Y-D, et al. The impact of 2019 novel coronavirus on heart injury: A systemic review and Meta-analysis. Prog Cardiovasc Dis. 2020;63(4):518–524. 10.1016/j.pcad.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, et al. AGA Institute Rapid Review of the GI and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020;159(1):320–334.e27. 10.1053/j.gastro.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viner RM, Russell SJ, Croker H, Packer J, Ward J, Stansfield C, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. The Lancet Child & Adolescent Health. 2020;4(5):397–404. 10.1016/S2352-4642(20)30095-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efthimiou O, Mavridis D, Debray TP, Samara M, Belger M, Siontis GC, et al. Combining randomized and non-randomized evidence in network meta-analysis. Stat Med. 2017;36(8):1210–26. 10.1002/sim.7223 [DOI] [PubMed] [Google Scholar]
- 14.Marconi E, Bettiol A, Ambrosio G, Perduca V, Vannacci A, Troiani S, et al. Efficacy and safety of pharmacological treatments for Patent Ductus Arteriosus closure: a systematic review and network meta-analysis of clinical trials and observational studies. Pharmacol Res. 2019:104418 10.1016/j.phrs.2019.104418 [DOI] [PubMed] [Google Scholar]
- 15.Tricco AC, Zarin W, Cardoso R, Veroniki A-A, Khan PA, Nincic V, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. BMJ. 2018;363:k4029 10.1136/bmj.k4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng C, Wei J, Persson MS, Sarmanova A, Doherty M, Xie D, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52(10):642–50. 10.1136/bjsports-2017-098043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Gu J, Chen Q, Deng N, Li J, Huang L, et al. Clinical and epidemiological characteristics of pediatric SARS-CoV-2 infections in China: A multicenter case series. PLoS Med. 2020;17(6):e1003130 10.1371/journal.pmed.1003130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369 10.1136/bmj.m2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383(4):334–46. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. 2012. Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [cited 2020 June 31]. [Google Scholar]
- 21.Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20(5):448–52. 10.1016/s0197-2456(99)00026-4 [DOI] [PubMed] [Google Scholar]
- 22.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions BMJ. 2016;355:i4919 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 24.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. Plos ONE. 2013;8(10):e76654 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, Niu Y, Wu J, Gu H, Zhang C. Software and package applicating for network meta-analysis: A usage-based comparative study. J Evid Based Med. 2018;11(3):176–83. 10.1111/jebm.12264 [DOI] [PubMed] [Google Scholar]
- 26.Shim S, Yoon B-H, Shin I-S, Bae J-M. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39 10.4178/epih.e2017047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. Plos ONE. 2014;9(12):e115065 10.1371/journal.pone.0115065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MS, Rhim HC, Park A, Kim H, Han K-M, Patkar AA, et al. Comparative efficacy and acceptability of pharmacological interventions for the treatment and prevention of delirium: A systematic review and network meta-analysis. J Psychiatr Res. 2020;125:164–176. 10.1016/j.jpsychires.2020.03.012 [DOI] [PubMed] [Google Scholar]
- 29.Rhim HC, Kim MS, Choi S, Tenforde AS. Comparative Efficacy and Tolerability of Nonsurgical Therapies for the Treatment of Midportion Achilles Tendinopathy: A Systematic Review With Network Meta-analysis. Orthop J Sports Med. 2020;8(7):2325967120930567 10.1177/2325967120930567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21(16):2313–24. 10.1002/sim.1201 [DOI] [PubMed] [Google Scholar]
- 33.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 34.Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol. 2013;13(1):35 10.1186/1471-2288-13-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanters S, Socias ME, Paton NI, Vitoria M, Doherty M, Ayers D, et al. Comparative efficacy and safety of second-line antiretroviral therapy for treatment of HIV/AIDS: a systematic review and network meta-analysis. The Lancet HIV. 2017;4(10):e433–e41. 10.1016/S2352-3018(17)30109-1 [DOI] [PubMed] [Google Scholar]
- 37.Datta S, Shah L, Gilman RH, Evans CA. Comparison of sputum collection methods for tuberculosis diagnosis: a systematic review and pairwise and network meta-analysis. Lancet Glob Health. 2017;5(8):e760–e71. 10.1016/S2214-109X(17)30201-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cameron C, Fireman B, Hutton B, Clifford T, Coyle D, Wells G, et al. Network meta-analysis incorporating randomized controlled trials and non-randomized comparative cohort studies for assessing the safety and effectiveness of medical treatments: challenges and opportunities. Syst Rev. 2015;4(1):147 10.1186/s13643-015-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann M, Mösbauer K, Hofmann-Winkler H, Kaul A, Kleine-Weber H, Krüger N, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020. Epub 2020/07/23. 10.1021/acschembio.0c00553 . [DOI] [PubMed] [Google Scholar]
- 40.Huebner KD, Shrive NG, Frank CB. Dexamethasone inhibits inflammation and cartilage damage in a new model of post-traumatic osteoarthritis. J Orthop Res. 2014;32(4):566–72. 10.1002/jor.22568 [DOI] [PubMed] [Google Scholar]
- 41.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120(2–3):69–75. 10.1016/j.jsbmb.2010.03.058 [DOI] [PubMed] [Google Scholar]
- 42.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–23. 10.1056/NEJMra050541 [DOI] [PubMed] [Google Scholar]
- 43.Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol. 2011;38(1):10–20. 10.3899/jrheum.100717 [DOI] [PubMed] [Google Scholar]
- 44.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. The Lancet. 2008;371(9617):987–97. 10.1016/S0140-6736(08)60453-5 [DOI] [PubMed] [Google Scholar]
- 45.Sahraoui A, Kloster-Jensen K, Ueland T, Korsgren O, Foss A, Scholz H. Anakinra and tocilizumab enhance survival and function of human islets during culture: implications for clinical islet transplantation. Cell Transplant. 2014;23(10):1199–211. Epub 2013/05/03. 10.3727/096368913X667529 . [DOI] [PubMed] [Google Scholar]
- 46.Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, Van Rossum MA, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63(2):545–55. 10.1002/art.30128 [DOI] [PubMed] [Google Scholar]
- 47.Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. The Lancet. 2008;371(9617):998–1006. 10.1016/S0140-6736(08)60454-7 [DOI] [PubMed] [Google Scholar]
- 48.Oates-Whitehead RM, Baumer JH, Haines L, Love S, Maconochie IK, Gupta A, et al. Intravenous immunoglobulin for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2003;(4). 10.1002/14651858.CD004000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes RA, Swan AV, van Doorn PA. Cochrane Review: Intravenous immunoglobulin for Guillain-Barré syndrome. Evidence-Based Child Health: A Cochrane Review Journal. 2011;6(4):1176–231. 10.1002/14651858.CD008630.pub2 [DOI] [PubMed] [Google Scholar]
- 50.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33. 10.1146/annurev.immunol.26.021607.090232 [DOI] [PubMed] [Google Scholar]
- 51.Alejandria MM, Lansang MAD, Dans LF, Mantaring JB III. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev. 2013;(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan F, Fabbri L, Stewart I, Robinson K, Smyth AR, Jenkins G. A systematic review of Anakinra, Tocilizumab, Sarilumab and Siltuximab for coronavirus-related infections. medRxiv. 2020;[preprint]:2020.04.23.20076612. 10.1101/2020.04.23.20076612 [DOI] [Google Scholar]
- 53.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. Epub 2020/03/13. 10.1093/cid/ciaa248 PubMed Central PMCID: PMC7108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;395(10229):1033 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–70. 10.1038/s41577-020-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. medRxiv. 2020;[preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossotti R, Travi G, Ughi N, Corradin M, Baiguera C, Fumagalli R, et al. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis. J Infect. 2020;81(4):e11–e17. 10.1016/j.jinf.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–65. 10.1517/14740338.2016.1140743 [DOI] [PubMed] [Google Scholar]
- 60.Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With COVID-19 (Metcovid): A Randomised, Double-Blind, Phase IIb, Placebo-Controlled Trial. Clin Infect Dis. 2020;ciaa1177 10.1093/cid/ciaa1177 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corral L, Bahamonde A, delas Revillas FA, Gomez-Barquero J, Abadia-Otero J, Garcia-Ibarbia C, et al. GLUCOCOVID: A controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. MedRxiv. 2020;[preprint]. [Google Scholar]
- 62.Ma Q, Qi D, Deng X, Yuan G, Tian W, Cui Y, et al. Corticosteroid therapy for patients with severe novel Coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24(15):8194–201. 10.26355/eurrev_202008_22508 [DOI] [PubMed] [Google Scholar]
- 63.Group RC. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020;NEJMoa2021436. 10.1056/NEJMoa2021436 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikolakopoulou A, Mavridis D, Furukawa TA, Cipriani A, Tricco AC, Straus SE, et al. Living network meta-analysis compared with pairwise meta-analysis in comparative effectiveness research: empirical study. BMJ. 2018;360:k585 Epub 2018/03/02. 10.1136/bmj.k585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020. 10.1001/jama.2020.8630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1036–1041. 10.1001/jamacardio.2020.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, et al. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J Am Heart Assoc. 2020;9(12):e017144 10.1161/JAHA.120.017144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saleh M, Gabriels J, Chang D, Kim BS, Mansoor A, Mahmood E, et al. The Effect of Chloroquine, Hydroxychloroquine and Azithromycin on the Corrected QT Interval in Patients with SARS-CoV-2 Infection. Circulation: Arrhythmia and Electrophysiology. 0(0). 10.1161/CIRCEP.120.008662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, et al. Assessment of QT Intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5(9):1067–1069. 10.1001/jamacardio.2020.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(4):e208857–e. 10.1001/jamanetworkopen.2020.8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020;[preprint]:2020.07.15.20151852. 10.1101/2020.07.15.20151852 [DOI] [Google Scholar]
- 72.Mahévas M, Tran V-T, Roumier M, Chabrol A, Paule R, Guillaud C, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369 10.1136/bmj.m1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849 10.1136/bmj.m1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.An MH, Kim MS, Park Y, Kim B-O, Kang SH, Kimn WJ, et al. Treatment Response to Hydroxychloroquine and Antibiotics for mild to moderate COVID-19: a retrospective cohort study from South Korea. medRxiv. 2020;[preprint]:2020.07.04.20146548. 10.1101/2020.07.04.20146548 [DOI] [Google Scholar]
- 75.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020. Epub 2020/06/04. 10.1056/NEJMoa2016638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173(8):623–631. 10.7326/M20-4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitjà O, Corbacho-Monné M, Ubals M, Tebe C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis. 2020. 10.1093/cid/ciaa1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitja O, Ubals M, Corbacho M, Alemany A, Suner C, Tebe C, et al. A Cluster-Randomized Trial of Hydroxychloroquine as Prevention of Covid-19 Transmission and Disease. medRxiv. 2020;[preprint]:2020.07.20.20157651. 10.1101/2020.07.20.20157651 [DOI] [Google Scholar]
- 80.Lofgren SM, Nicol MR, Bangdiwala AS, Pastick KA, Okafor EC, Skipper CP, et al. Safety of Hydroxychloroquine among Outpatient Clinical Trial Participants for COVID-19. medRxiv. 2020;[preprint]:2020.07.16.20155531. 10.1101/2020.07.16.20155531 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.