Abstract
Following intensive efforts since their discovery little more than 10 years ago, cell replacement therapy using induced pluripotent stem (iPS) cells is now becoming reality. However, there remain several obstacles in the translation of basic research to clinical application, obstacles known as the “Valley of Death”. With regards to regenerative medicine using iPS cells for Parkinson's disease, we have developed a method for the 1) efficient induction of dopaminergic neurons from human iPS cells and 2) sorting dopaminergic progenitor cells using a floor plate marker, CORIN. The grafted CORIN+ cells survived well and functioned as midbrain dopaminergic neurons in the Parkinson's disease model rats and monkeys, and showed minimal risk of tumor formation. Based on these results, we performed a pre-clinical study using a clinical-grade iPS cell line and finally started a clinical trial to treat Parkinson's disease patients in August 2018. Here, I discuss the key issues to crossing the Valley of Death: scientific rationale, pre-clinical study, and clinical trial.
Keywords: Parkinson's disease, Induced pluripotent stem cell, Dopaminergic neuron, Transplantation, Translational research, Clinical trial
1. Crossing the Valley of Death
More than 20 and 10 years have passed since the first reports of human embryonic stem (ES) and induced pluripotent stem (iPS) cells, respectively. Currently in the field of regenerative medicine, clinical trials using pluripotent stem cells are conducted mainly for retinal diseases and spinal cord injury. One reason for the limited range of diseases is the many hurdles of translating basic research to clinical application, including a sophisticated cell facility, the high financial cost, and human resources. Accordingly, these obstacles are called the “Valley of Death”. To cross this valley, good scientific rationale, pre-clinical studies and clinical trials are indispensable. We began basic research pursuing a cell-based therapy for Parkinson's disease (PD) 20 years ago. Finally, in August 2018, we started a clinical trial. In this short paper, I highlight our efforts leading to crossing the Valley of Death.
2. Scientific rationale
2.1. Fetal cell transplantation for PD
PD is a neurodegenerative disease in which dopaminergic (DA) neurons that project from the midbrain substantia nigra to the striatum progressively die. As a result, patients mainly exhibit motor dysfunctions such as bradykinesia (slowed movement), rigidity and tremors. To replace the lost DA neurons, the transplantation of fetal midbrain, which contains DA neurons, has been attempted since 1987. A number of clinical cases have proved that this treatment can improve the symptoms of the patients [1,2]. Moreover, positive effects for over 10 years have been reported [[3], [4], [5]]. Now, a new clinical trial is ongoing in Europe seeking to optimize the treatment protocols of this strategy [6]. However, there are several issues regarding fetal-based treatment, including ethical concerns using fetal tissue, difficulty in obtaining a sufficient amount of fetal brain tissue, and contamination of serotonergic neurons, which may cause dyskinesia (involuntary movement). Although the efficacy of fetal cell transplantation has been demonstrated, it is not yet admitted as a standard treatment. In this situation, ES/iPS cells are expected as alternative cell sources for DA neurons.
2.2. Induction of neural differentiation from iPS cells
The differentiation of pluripotent stem cells into various somatic cells is mainly controlled by bone morphogenic protein (BMP), transforming growth factor (TGF)/Activin/Nodal and Wnt signals [7]. In order to induce neural cells, it is important to inhibit both BMP and TGF/Activin/Nodal signals. Because this inhibits the SMAD1/5/8 and SMAD2/3 intracellular pathways, this method is called dual SMAD inhibition [8].
For the treatment of PD, we need to induce midbrain DA neurons, which originate from the mesencephalic floor plate, from ES/iPS cells [9]. For this purpose, in addition to the above-mentioned dual SMAD inhibition, we induce the ventral midbrain by moderately activating Wnt signals and ventralizing with Sonic hedgehog (Shh) [[10], [11], [12]].
2.3. In vivo studies using animal models
Given that midbrain DA neurons can be induced from human ES/iPS cells, their safety and efficacy should be verified using animal PD models. These models are generated by administrating 6-hydroxydopamine (6-OHDA) into rats or 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) into monkeys. When human ES cell-derived DA neurons were grafted into the striatum of these rat [11,13] and monkey [14] models, the cells extended neuronal fibers and improved the behavior of the animals. Moreover, the effect in the rat models was identical to that of human fetal midbrain [15]. These results suggest that ES/iPS cell-derived DA neurons can be used to treat PD.
Regarding safety, the biggest concern when using ES/iPS cells is the risk of tumor formation that is caused by proliferating cells such as undifferentiated ES/iPS cells and neural stem cells [14,[16], [17], [18]]. Another concern, like in the case of fetal cell transplantation, is graft-induced dyskinesia (involuntary movement), caused by contaminating serotonergic neurons [19,20]. Due to the nature of pluripotent stem cells, cultured cells during differentiation cannot be homogeneous. In order to avoid the risks of heterogeneous cell populations, including the aforementioned contaminating cells, the removal of unwanted cells by sorting midbrain DA neurons with antibodies against Corin [21], a marker for floor plate, and Alcam [22], a marker for vascular endothelial cells in the central nervous system, is reported. This cell sorting procedure not only enhances the safety and efficacy by improving the quality of the final cell products, but also contributes to quality control, and is considered to be an important step for clinical application.
We have established a protocol for producing DA progenitor cells from human iPS cells by sorting Corin + cells for the treatment of PD [21]. The DA progenitor cells survived in the striatum of rat PD models without any tumor formation and improved the abnormal behavior of the animals. Furthermore, we conducted an in vivo study using cynomolgus monkey models as a simulation of a clinical trial [23]. In that study, iPS cell lines were established from healthy individuals and PD patients, and DA progenitor cells were prepared using the above protocol. A total of about five million cells were transplanted into the bilateral putamen, and the monkeys were followed up for two years. The neurological status was evaluated by the behavioral score in seven items and automatic quantification of spontaneous movements by video recording. The transplantation of both healthy individual- (n = 4) and patient- (n = 4) derived cells resulted in significant behavioral improvement. Dopamine synthesis by the transplanted cells was confirmed by a 18F-DOPA PET study, and histological analysis of the brain slices revealed that more than 100,000 DA neurons survived per animal. Importantly, there was no tumor formation or abnormal findings in the animals.
These results indicated that human iPS cell-derived DA progenitor cells can function as DA neurons without side effects in rodent and monkey PD models.
3. Pre-clinical study using a clinical grade cell line
As a next step towards clinical application, we performed a pre-clinical study to confirm the safety and efficacy of a clinical grade human iPS cell line (QHJI-01) [24]. This cell line was established at our institute from a healthy individual who has most common human leukocyte antigen (HLA) haplotype in the Japanese population [25]. Here I provide the essence of the study.
3.1. Quality control of iPS cells
iPS cells have the morphological characteristics of pluripotent stem cells and express specific markers (TRA-1-60, TRA-2-49/6E, SSEA-4), both of which are used to evaluate their quality. When considering clinical application, it is equally important to conduct sterility, mycoplasma, and endotoxin assays in accordance with Japanese Pharmacopoeia standards. In addition, an analysis of the karyotype, plasmid survival, genomic and epigenomic abnormalities, and virus contamination of the iPS cells is required.
There is still debate about the interpretation of the genome analysis results, in part because iPS cells are a new cell type and not all genetic mutations cause malignant phenotypes. Currently, there is a website called “Catalogue of somatic mutations in cancer (COSMIC)”, which is updated daily [26]. In Japan, a list of genes known to be involved in carcinogenesis was published in 2013 by the Japanese government [27]. We confirmed that no abnormalities in the genes listed in COSMIC or the Japanese government in the clinical-grade iPS cell line and the derived final products.
3.2. Quality control of the final product
The final product of the differentiated iPS cells is DA progenitor cells, which we define as Foxa2+Tuj1+ cells and set the threshold 80% of the final cell population for transplantation. The rest of the cells are midbrain glial cells that support DA neurons. For safety, we confirmed by flow cytometry that no iPS cells (OCT3/4, TRA-2-49/6E-positive) or early neural stem cells (SOX1, PAX6-positive) are in the final population. In addition, as mentioned above with clinical-grade iPS cells, sterility, mycoplasma and endotoxin tests were performed. Also in the case of iPS cells, a final analysis was performed to confirm that there are no plasmid or genomic/epigenomic abnormalities.
In this pre-clinical study, these evaluations were repeated more than 20 times. Six cell preparations were also subjected to the following in vivo evaluation.
3.3. In vivo study
Even if the in vitro studies reveal no problems with the cells, it is necessary to investigate cell dynamics in the brain, since cell transplantation is the final goal. In particular, the verification of tumorigenicity, toxicity, and biodistribution for safety is essential.
The tumorigenicity test was performed using immunodeficient mice (NOG mice): 40 males and 40 females for cell transplantation, and 25 males and 25 females for controls (medium only). DA progenitor cells (2 × 105) were transplanted into the striatum and observed for 52 weeks. Histological analysis showed no signs of graft overgrowth or cell proliferation, and no malignant findings. In addition, no graft-related toxicity or distant metastasis to other organs was observed.
The efficacy of the clinical-grade cells was confirmed using 6-OHDA-lesioned rats. DA progenitor cells (4 × 105) were transplanted into the striatum of the injured side, and behavioral analysis was performed for 20 weeks. Abnormal rotational movement was improved, and subsequent histological analysis revealed good survival of the DA neurons.
4. Clinical trial
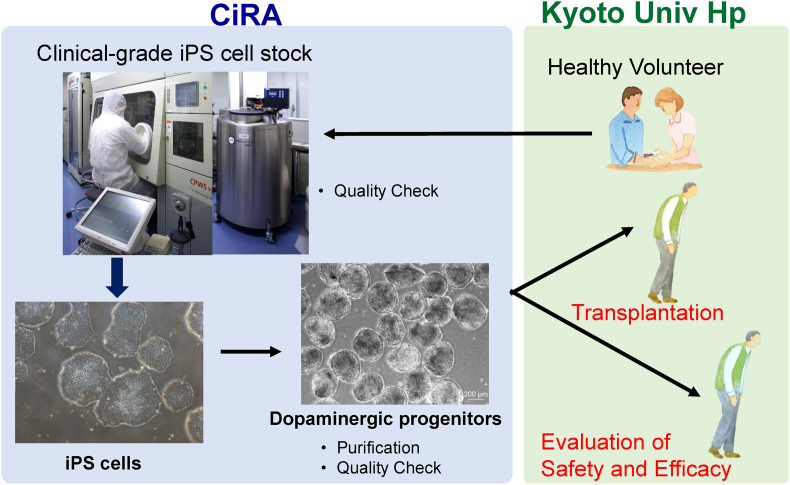
There are two tracks of clinical application in Japan. One is clinical research, which is based on the Act on the Safety of Regenerative Medicine. The other is clinical trial, which is based on the Revised Pharmaceutical Affairs Act. We chose the latter. Since a clinical trial is aimed at approval and the establishment of a new cell-based therapy, cooperation with companies, regulatory agencies and hospitals is essential. To that end, we have started collaboration with several groups from the early stages of the development. After these efforts, our clinical trial started with the approval of the Pharmaceuticals and Medical Devices Agency (PMDA) and the institutional review board <UMIN000033564> [28] <JMA-IIA00384)> [29] (Fig. 1).
Fig. 1.
Outline of our clinical trial. Clinical-grade iPS cell line has been established at CiRA, from which DA progenitor cells are induced. These cells are transplanted into PD patients at Kyoto University Hospital, then the safety and efficacy of the cells will be evaluated.
4.1. Outline of clinical trial plan
The purpose of the clinical trial is “evaluation of safety and efficacy in transplantation of human iPS cell-derived DA progenitor cells into the putamen of PD patients”. Recruitment of the patients started in August 2018, and the first surgery was performed in October 2018 at Kyoto University Hospital. This is a single arm, non-randomized and open phase I/II study. The target sample size is seven cases. The key inclusion criteria are shown in Table 1. The observation period is two years after surgery.
Table 1.
Design and key inclusion criteria of our clinical trial.
| <Design> | <Inclusion criteria> |
|---|---|
| Phase I/II study | PD patients |
| Single institute (Kyoto university hospital) | Uncontrollable by medical treatment |
| Non-randomized, Open | 50 to 69 y.o. |
| No control group (pre vs. post) | suffering from PD more than 5 years |
| 7 patients | Hoehn & Yahr scale: Stage III-V (off), I-III (on) |
| Responsive for l-dopa (>30%) |
4.2. Surgical technique
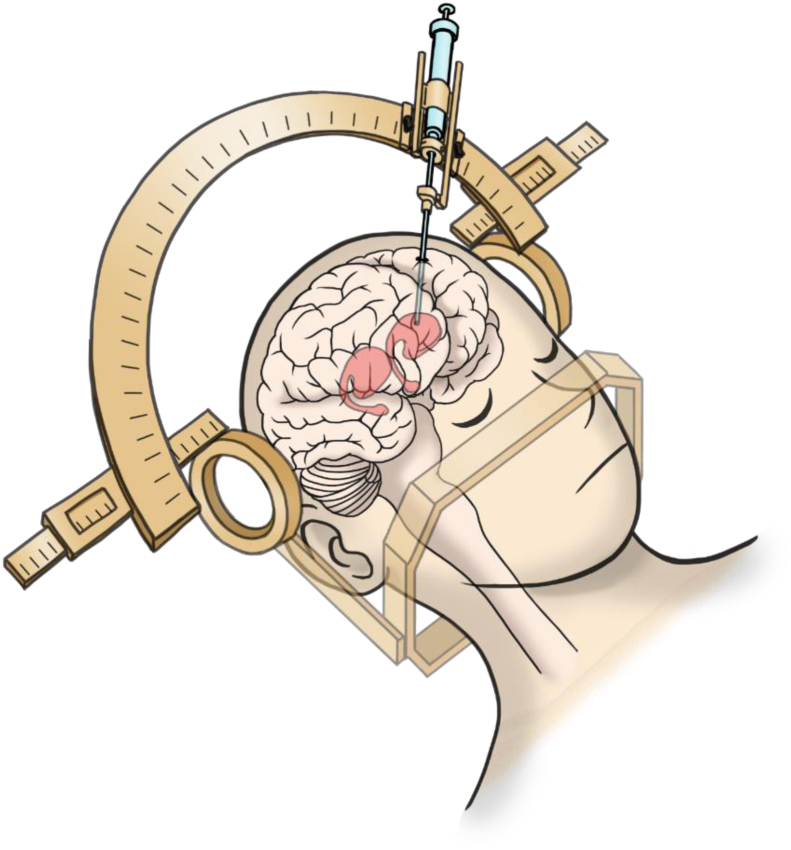
Under general anesthesia, approximately five million cells are stereotaxically transplanted through burr holes to the bilateral putamen (Fig. 2). We are using three tracts per side and inject approximately 200,000 cells at four points each (12 points total per side). The cells are injected as spheres with a diameter of about 400 μm. For this cell injection, we have developed a new needle that can be attached to a stereotaxic device.
Fig. 2.
Surgical procedure. The cells are transplanted into bilateral putamen through burr holes by using a stereotaxic device.
4.3. Immunosuppression
As mentioned above, we are using an iPS cell line established from a healthy person who has the most common HLA type in Japan as a homozygote: HLA A (24:02), B (52:01), C (12:02), DRB1 (15:02), DQB1 (06:01), DPB1 (09:01), which matches 17% of the Japanese population [30]. In a study using cynomolgus monkeys, it was confirmed that the immune response is reduced, and the survival of DA neurons is increased by HLA-matched transplantation [31]. In this clinical trial, HLA is not necessarily matched, and it is possible that not all HLA including minor antigens match. Therefore, an immunosuppressant (tacrolimus) will be administered for one year after the surgery.
4.4. Primary and secondary endpoints
Primary endpoints are related to safety: 1) the incidence and severity of adverse events and 2) the presence or absence of graft overgrowth in the brain at 24 months after transplantation. Graft size is assessed by MRI. Secondary endpoints relate to efficacy such as 1) MDS-UPDRS, 2) daily average on-time and off-time, 3) Hoehn & Yahr severity, 4) PDQ-39 score, and 5) EQ-5D-5L. As a more objective surrogate marker, we are employing a PET study to evaluate the function as DA neurons (DOPA) and the proliferation (FLT) of the grafted cells, and also the immune response by the host brain (GE-180).
5. Future outlook
Clinical trials of cell transplantation for PD using human ES cells are currently underway in Australia <NCT02452723> [32] and China <NCT03119636 (ChineseASZQ-003)> [33], and other trials are about to start [34]. Recently, a single case, not a clinical trial, of autologous transplantation of iPS cell-derived DA neurons was reported, in which the grafted cells survived for two years without any adverse effect [35]. Because animal experiments cannot recapitulate the outcome of human treatment perfectly, reverse translation based on the outcomes of these clinical trials will be important for the better treatment for PD.
In PD, it is estimated that only about 1/3 of the DA neuron population remains in symptomatic patients. At present, cell transplantation has been attempted for patients in the advanced stage, when the number of DA neurons is even lower. If safety and efficacy are confirmed in this clinical trial, the expectation is that treatment in the early stage will become an option. This shift will change the purpose of the treatment from “improving severe symptoms” to “delaying the advanced stage” of PD.
Cell replacement therapy does not improve the pathology of PD. Considering reports of the accumulation of α-synuclein in transplanted fetal cells [36], it is possible that transplanted pluripotent stem cell-derived cells will also be affected by the pathological brain environment. Cell transplantation can be expected to improve symptoms by increasing the amount of DA neurons, but the progression of the disease cannot be stopped. Conversely, a disease modifying therapy may be able to slow or stop the progression of the disease, but it cannot improve the symptoms. When these two are combined in the future, a complete treatment for PD will be achieved.
Declaration of Competing Interest
JT receives a grant for a collaborative research by Sumitomo Dainippon Pharma.
Acknowledgements
I thank Drs. A. Morizane, D. Doi, T. Kikuchi and all the lab members and collaborators for the previous and current work. I also thank Dr. Peter Karagiannis (CiRA) for critical reading of the manuscript, and Mrs. Misaki Ouchida for illustration. Our project has been supported by the following grants: the Highway Project for Realization of Regenerative Medicine from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Research Center Network for Realization of Regenerative Medicine from the Japan Agency for Medical Research and Development (AMED), and the Research Project for Practical Applications of Regenerative Medicine from AMED.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Barker R.A., Drouin-Ouellet J., Parmar M. Cell-based therapies for Parkinson disease—past insights and future potential. Nat Rev Neurol. 2015;11:492–503. doi: 10.1038/nrneurol.2015.123. [DOI] [PubMed] [Google Scholar]
- 2.Barker R.A., Barrett J., Mason S.L., Björklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 2013;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- 3.Kefalopoulou Z., Politis M., Piccini P., Mencacci N., Bhatia K., Jahanshahi M. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71:83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallett P.J., Cooper O., Sadi D., Robertson H., Mendez I., Isacson O. Long-term health of dopaminergic neuron transplants in Parkinson's disease patients. Cell Rep. 2014;7:1755–1761. doi: 10.1016/j.celrep.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W., Englund E., Widner H., Mattsson B., van Westen D., Lätt J. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc Natl Acad Sci U S A. 2016;113:6544–6549. doi: 10.1073/pnas.1605245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker R.A., TRANEURO consortium Designing stem-cell-based dopamine cell replacement trials for Parkinson's disease. Nat Med. 2019;25:1045–1053. doi: 10.1038/s41591-019-0507-2. [DOI] [PubMed] [Google Scholar]
- 7.Murry C.E., Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono Y., Nakatani T., Sakamoto Y., Mizuhara E., Minaki Y., Kumai M. Differences in neurogenic potential in floor plate cells along an antero-posterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 10.Kirkeby A., Grealish S., Wolf D., Nelander J., Wood J., Lundblad M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi J., Liu Y., Liu H., Chen H., Emborg M.E., Zhang S.C. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cell. 2012;30:1655–1663. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinbeck J.A., Choi S.J., Mrejeru A., Ganat Y., Deisseroth K., Sulzer D. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nat Biotechnol. 2015;33:204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doi D., Morizane A., Kikuchi T., Onoe H., Hayashi T., Kawasaki T. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson's disease. Stem Cell. 2012;30:935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- 15.Grealish S., Diguet E., Kirkeby A., Mattsson B., Heuer A., Bramoulle Y. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell. 2014;15:653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brederlau A., Correia A.S., Anisimov S.V., Elmi M., Paul G., Roybon L. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cell. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- 17.Elkabetz Y., Panagiotakos G., Al Shamy G., Socci N.D., Tabar V., Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsukawa M., Nakajima Y., Fukumoto A., Doi D., Takahashi J. Fail-safe therapy by gamma-ray irradiation against tumor formation by human induced pluripotent stem cell-derived neural progenitors. Stem Cell Dev. 2016;25:815–825. doi: 10.1089/scd.2015.0394. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson T., Carta M., Winkler C., Björklund A., Kirik D. Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesias in a rat model of Parkinson's disease. J Neurosci. 2007;27:8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Politis M., Wu K., Loane C., Quinn N.P., Brooks D.J., Rehncrona S. Serotonergic neurons mediate dyskinesia side effects in Parkinson's patients with neural transplants. Sci Transl Med. 2010;2:38ra46. doi: 10.1126/scitranslmed.3000976. [DOI] [PubMed] [Google Scholar]
- 21.Doi D., Samata B., Katsukawa M., Kikuchi T., Morizane A., Ono Y. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014;2:337–350. doi: 10.1016/j.stemcr.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bye C.R., Jönsson M.E., Björklund A., Parish C.L., Thompson L.H. Transcriptome analysis reveals transmembrane targets on transplantable midbrain dopamine progenitors. Proc Natl Acad Sci USA. 2015;112:1946–1955. doi: 10.1073/pnas.1501989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kikuchi T., Morizane A., Doi D., Magotani H., Onoe H., Hayashi T. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature. 2017;548:592–596. doi: 10.1038/nature23664. [DOI] [PubMed] [Google Scholar]
- 24.Doi D., Magotani H., Kikuchi T., Ikeda M., Hiramatsu S., Yoshida K. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson's disease. Nat Commun. 2020 doi: 10.1038/s41467-020-17165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umekage M., Sato Y., Takasu N. Overview: an iPS cell stock at CiRA. Inflamm Regen. 2019;39:17. doi: 10.1186/s41232-019-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.https://cancer.sanger.ac.uk/census
- 27.https://www.pmda.go.jp/files/000155938.pdf
- 28.https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000038278
- 29.https://dbcentre3.jmacct.med.or.jp/JMACTR/App/JMACTRE02_04/JMACTRE02_04.aspx?kbn=3&seqno=8542
- 30.Okita K., Matsumura Y., Sato Y., Okada A., Morizane A., Okamoto S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 31.Morizane A., Kikuchi T., Hayashi T., Mizuma H., Takara S., Doi H. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 2017;8:385. doi: 10.1038/s41467-017-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.https://clinicaltrials.gov/ct2/show/NCT02452723?term=stem+cell&cond=Parkinson+Disease&rank=13
- 33.https://clinicaltrials.gov/ct2/show/NCT03119636?term=stem+cell&cond=Parkinson+Disease&rank=14
- 34.Barker R.A., Parmar M., Studer L., Takahashi J. Human trials of stem cell-derived dopamine neurons for Parkinson's disease: dawn of a new era. Cell Stem Cell. 2017;21:569–573. doi: 10.1016/j.stem.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Schweitzer J.S., Song B., Herrington T.M., Park T.-Y., Lee N., Ko S. Personalized iPSC-derived dopamine progenitor cells for Parkinson's disease. N Engl J Med. 2020;382:1926–1932. doi: 10.1056/NEJMoa1915872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olanow C.W., Brundin P. Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder? Mov Disord. 2013;28:31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]