Summary
We describe an outbreak of carbapenem-resistant Acinetobacter baumannii (CRAB) in a COVID-19 dedicated hospital. The suspected mechanism of transfer was an environmental source that persisted despite evacuation and terminal cleaning of the entire hospital, and transmitted through healthcare workers' hands or equipment. This outbreak demonstrates that practices to prevent the spread of multidrug-resistant organisms must not be neglected during the COVID-19 pandemic.
Keywords: Carbapenem-resistant Acinetobacter baumannii (CRAB), Outbreak investigation, COVID-19 pandemic, Infection control, Screening, Environmental sampling
Introduction
Carbapenem-resistant Acinetobacter baumannii (CRAB) is a common multidrug-resistant organism (MDRO) responsible for nosocomial infections, including hospital-acquired pneumonia, bloodstream infections, urinary-tract infections and wound infections, and is associated with a high case fatality rate [1,2]. CRAB contaminates the hospital environment and is able to survive for prolonged periods on dry surfaces [1,3]. It is resistant to common disinfectants, leading to outbreaks that are hard to contain and that affect the most vulnerable and critically ill patients [1]. Strict adherence to infection control practices are vital to interrupt transmission of CRAB in hospitals [4].
In this study, we describe a monoclonal outbreak of CRAB in a dedicated hospital for the treatment of COVID-19 patients and the challenges of maintaining routine infection control practices in the pandemic setting.
Materials and methods
Setting
Hasharon Hospital is a 240-bed academic hospital in central Israel. Before the COVID-19 pandemic, CRAB was endemic in Hasharon (CRAB clinical infection incidence: 81/100,000 patient-days in 2019, unpublished data, National Center for Infection Control, Ministry of Health, Israel).
In late February 2020, with rising COVID-19 incidence in the country, the Israeli Ministry of Health designated Hasharon as a dedicated COVID-19 inpatient facility with 3 medical wards (B, C, and D, each including a step-up unit for patients requiring respiratory support) and one ICU. The ICU was a converted anesthesia recovery unit (multi-patient room) renovated to accommodate up to 20 ventilated COVID-19 patients. All current patients were discharged or transferred and major infrastructure changes were implemented to ensure separation between patient and provider areas. Following evacuation, all hospital rooms were terminally cleaned and disinfected using bleach and adjunctive ultraviolet light (UVL) (UVDI-360 Room Sanitizer, UltraViolet Devices Inc., California, USA). All staff were trained in correct use of personal protective equipment (PPE) and other measures to prevent SARS-CoV-2 transmission.
On March 10th, 2020 the hospital reopened. The first COVID-19 patients were admitted to ward D. Before the hospital was evacuated, CRAB patients had been cohorted in one of the multi-patient rooms in this ward. After reopening, this room served as a step-up unit for stable COVID-19 patients needing respiratory support.
Screening and microbiological methods
Screening of high-risk patients for CRAB carriage has been conducted at Hasharon since April 2019. Screening cultures were performed upon admission for patients from long-term care facilities or who were hospitalized in the ICU in the past 3 months, as well as upon admission and weekly for mechanically ventilated patients. This practice continued in the COVID-19 wards. Screening cultures were obtained from buccal mucosa and the rectum using swabs, and skin was sampled using pre-moistened sponges (Polywipe, Medical Wire & Equipment, Wiltshire, England) [5]. Tracheal aspirates were collected from ventilated patients. Specimens were inoculated directly and after overnight enrichment in tryptic soy broth onto CHROMagar MDR Acinetobacter plates (Hylabs, Rehovot, Israel), and incubated overnight at 37ºC. Suspicious colonies were isolated and identified to the Acinetobacter baumannii-calcoaceticus complex level using the Microflex MALDI Biotyper (Bruker Daltonics, Bremen, Germany). Meropenem resistance was confirmed by disc diffusion. Clinical samples were obtained for culture as clinically indicated. Identification of clinical isolates was by MALDI-TOF as above, and carbapenem resistance was determined by VITEK-2 (bioMérieux, Marcy l'Etoile, France).
As part of the CRAB outbreak investigation, environmental samples were obtained from the outbreak wards using sterile pre-moistened nylon-flocked swabs (FLOQSwab; Copan Italia S.P.A., Brescia, Italy). Samples were processed as for screening cultures above.
Molecular typing
All CRAB isolates recovered from clinical and environmental samples were sent to the National Laboratory for Antibiotic Resistance and Investigation of Outbreaks (National Institute for Infection Control and Antibiotic Resistance, Ministry of Health, Israel) for investigation. CRAB identification was confirmed by polymerase chain reaction (PCR) for OXA-51 and gyrB genes. Sequencing of the blaOXA-51-like gene was used to assign isolates to international clonal lineages (IC) [6]. Fourier-transform infrared spectroscopy (FT-IR; IR Biotyper, Bruker Daltonics, Bremen, Germany) was used to determine clonal relatedness. Samples were prepared according to the manufacturer's instructions. Spectra were analyzed using OPUS 7.5 software (Bruker Daltonics, Bremen, Germany). Hierarchical cluster analysis (HCA) was generated by the OPUS 7.5 software using the Pearson correlation coefficient option. Identification of carbapenemases was performed by PCR for the detection of blaOXA-23-like, blaOXA-24-like and blaOXA-58-like genes [7].
Results
Outbreak description and epidemiologic investigation
The first CRAB case was detected on March 27th, 2020, two weeks after reopening the hospital for COVID-19 patients. This patient and the four subsequent patients involved in the CRAB outbreak are described in Table I. No known CRAB patients were hospitalized after reopening of the hospital and prior to identification of CRAB in the index patient.
Table I.
Cases in an outbreak of carbapenem-resistant A. baumannii (CRAB) in a dedicated COVID-19 hospital
| Case | Patient description∗ | Hospital admission date | Unit on admission | Date of mechanical ventillation | Date of ICU admission | Date of first positive CRAB culture | Unit in which positive culture was taken | Unit in which CRAB was acquired | Type and site of positive CRAB culture | CRAB clinical infection | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 year-old male, with HTN, DM, and HL; arrived from home | March 24, 2020 | Ward D | March 25, 2020 | March 27, 2020 | March 27, 2020 | On admission to ICU | Ward D step-up unit | Surveillance - skin | No | Died |
| 2 | 82 year-old male, with HTN, DM, HL, IHD and TIA; arrived from home | March 13, 2020 | Ward D | March 24, 2020 | NA | March 29, 2020 | Ward D step-up unit | Ward D step-up unit | Surveillance - sputum and femoral catheter tip | No | Discharged |
| 3 | 81 year-old male, with PAF, HL, CKD and gout; arrived from home | March 20, 2020 | Ward D | March 20, 2020 | NA | March 29, 2020 | Ward D step-up unit | Ward D step-up unit | Surveillance - rectum and femoral catheter tip | Yes, central line-associated bloodstream infection | Died |
| 4 | 46 year-old male, with morbid obesity and DM; arrived from home | March 24, 2020 | Ward D | March 25, 2020 | March 26, 2020 (bed adjacent to patient 1) | March 30, 2020 | ICU | ICU | Surveillance - skin | No | Discharged |
| 5 | 63 year-old male, with history of cured LY; arrived from home | April 2, 2020 | ICU | April 2, 2020 | April 2, 2020 | April 16, 2020 | ICU | ICU | Clinical - sputum | Yes, ventilator-associated pneumonia | Discharged |
HTN - hypertension; DM - diabetes mellitus; HL - hyperlipidemia; IHD - ischemic heart disease; TIA - transient ischemic attack; CKD - chronic kidney disease; PAF - paroxysmal atrial fibrillation; LY – lymphoma.
Following detection of the first four patients, CRAB screening of all patient in ward D and the ICU uncovered no additional cases. Environmental sampling was performed in ward D and the ICU. In ward D, 56 environmental samples were obtained from all rooms in the ward and in the step-up unit. Seven of them were positive for CRAB, all from the step-up unit: 3 from the step-up unit's medication room; 3 from the resuscitation cart used for intubation procedures; and one from a faucet. Questioning of the cleaning staff revealed that the medication room was not part of the enhanced terminal cleaning performed before the hospital was re-opened to accept COVID-19 patients. In the ICU, 60 environmental samples were obtained and all were negative.
Microbiological investigation
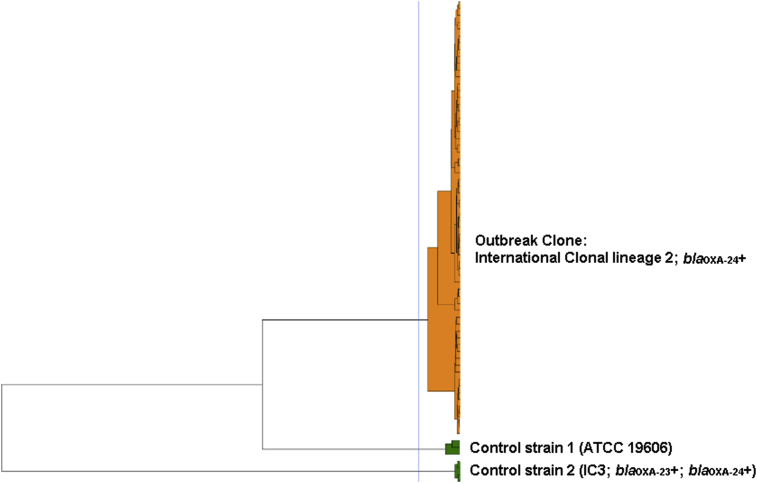
All isolates were confirmed in the central laboratory as CRAB with meropenem MIC >16ug/ml. All isolates belonged to international clonal lineage 2 (blaOXA-66 allele) and harbored the blaOXA-24-like carbapenemase. In FT-IR analysis, all clinical and environmental isolates clustered together (Figure 1).
Figure 1.
Fourier-transform infrared spectroscopy (FT-IR) bio-typing analysis of carbapenem-resistant Acinetobacter baumannii (CRAB) clinical and environmental specimens (n=24) from the Hasharon outbreak. The blue line represents the automatic cut-off value (0.042) generated by the manufacturer's software. A. baumannii ATCC 19606 and a CRAB isolate belonging to international clonal lineage 3 (IC3) and harboring blaOXA-23 and blaOXA-24 genes were added as control strains to increase discriminatory power. Clusters composed of two or more isolates are shown in orange and singletons in green.
Outbreak control measures
The following measures were taken to prevent further transmission: (1) Ward D was closed to new admissions. Terminal cleaning was performed using bleach and UVL. Repeat environmental samples were obtained after this round of cleaning and all were negative, at which point the ward was reopened. The medication room, suspected as the source of the outbreak, was closed permanently. (2) Hand hygiene awareness was strengthened and monitoring was performed. (3) CRAB patients were cohorted in the step-up unit in ward D and in a separate area within the ICU. Staff members who cared for CRAB-positive patients were not allowed to care for any CRAB –negative patient without first leaving the cohort area and doffing all PPE. (4) As part of contact precautions for CRAB patients, staff wore disposable gowns over the COVID-19 PPE coveralls.
These measures succeeded in controlling the outbreak and no further CRAB cases were detected in ward D. However a fifth patient was diagnosed in the ICU 2 weeks after the initial cluster and implementation of infection control measures. This patient overlapped with patient 4 in the ICU. After patient 5 was identified, repeat environmental sampling in the ICU was negative and no further cases were detected.
Discussion
The first three patients acquired CRAB in a multi-patient step-up unit in ward D, which served to cohort CRAB patients before the hospital was closed and repurposed as a COVID-19 facility. We presume that an environmental reservoir persisted in the medication room, which was not properly terminally cleaned. Environmental sampling was not performed prior to reopening. The fourth and fifth patients acquired CRAB in the ICU, likely due to cross transmission from other patients.
Risk factors for the acquisition of CRAB include endotracheal intubation and carbapenem use [8,9]. All patients in our outbreak were mechanically ventilated, but none were exposed to carbapenems before acquisition of CRAB. CRAB acquisition has been described after an average hospital stay of 42 days [10]. In contrast, patients in our outbreak had an unusually short length of stay before CRAB acquisition (median 9 days), possibly due to severe pneumonitis in these patients, immunosuppression due to COVID-19 lymphopenia and treatments (such as corticosteroids and tocilizumab) or a combination of these.
This outbreak demonstrates the speed of re-emergence of CRAB when an environmental source has not been controlled. Environmental contamination has been described as key to the dissemination of CRAB. Healthcare workers' hands can serve as vehicles for transmission either from contaminated surfaces to patients or from a colonized patient to other patients [1]. In a hospital endemic for CRAB, terminal cleaning should be accompanied by environmental sampling to make sure no reservoir has been left.
One might expect that the heightened infection control measures put in place to prevent COVID-19 transmission in hospitals would have a beneficial side effect of reducing transmission of MDROs. Our report demonstrates that MDRO outbreaks can occur in COVID-19 wards. In fact, an emphasis on preventing transmission of COVID-19 may lead to lapses in routine infection control activities, thus facilitating MDRO transmission. For example, unorthodox work practices used when treating COVID-19 patients, such as double gloving, may cause confusion about when to perform hand hygiene. Second, contact precautions are designed to minimize provider risk according to COVID-19 status, not MDRO carriage status. Third, shortages in PPE may force facilities to suspend the use of single-use gowns for contact precautions for MDRO carriers. Fourth, long patient stays in multi-patient COVID-19 rooms may create fewer opportunities for terminal cleaning. Fifth, staff education that emphasizes minimizing provider risk and correct use of PPE may cause confusion regarding what constitutes a clean environment for the patients [11]. Finally, co-infection with MDROs may not be suspected in the setting of a pandemic, and hospitals may have suspended their pre-pandemic protocols for MDRO surveillance.
In summary, this CRAB outbreak stresses the importance of continuing routine infection control practices despite the changes in healthcare delivery brought about by the COVID-19 pandemic. These include strict adherence to hand hygiene, including monitoring and feedback, screening for CRAB and contact precautions for CRAB carriers, and meticulous environmental cleaning and disinfection.
Author contributions
Tamar Gottesman: Conceptualization, Investigation, Writing - Original Draft.
Rina Fedorowsky: Investigation.
Rebecca Yerushalmi: Investigation.
Jonathan Lellouche: Methodology, Investigation.
Amir Nutman: Conceptualization, Methodology, Investigation, Writing - Review & Editing.
Acknowledgements
We thank Dr Elizabeth Temkin for her helpful comments on this manuscript.
Conflict of interest statement
All authors report no conflicts of interest relevant to this article.
Financial support
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong D., Nielsen T.B., Bonomo R.A., Pantapalangkoor P., Luna B., Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin Microbiol Rev. 2017;30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nutman A., Lerner A., Schwartz D., Carmeli Y. Evaluation of carriage and environmental contamination by carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect. 2016;22 doi: 10.1016/j.cmi.2016.08.020. 949.e5-949.e7. [DOI] [PubMed] [Google Scholar]
- 4.Tacconelli E., Cataldo M.A., Dancer S.J., De Angelis G., Falcone M., Frank U. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20:1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 5.Nutman A., Temkin E., Lellouche J., Ben David D., Schwartz D., Carmeli Y. Detecting carbapenem-resistant Acinetobacter baumannii (CRAB) carriage: Which body site should be cultured? Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pournaras S., Gogou V., Giannouli M., Dimitroulia E., Dafopoulou K., Tsakris A. Single-locus-sequence-based typing of blaOXA-51-like genes for rapid assignment of Acinetobacter baumannii clinical isolates to international clonal lineages. J Clin Microbiol. 2014;52:1653–1657. doi: 10.1128/JCM.03565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans B.A., Amyes S.G.B. OXA β-Lactamases. Clin Microbiol Rev. 2014;27 doi: 10.1128/CMR.00117-13. 241 LP-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Wang Q., Zhao C., Chen H., Li H., Wang H. Prospective multi-center evaluation on risk factors, clinical characteristics and outcomes due to carbapenem resistance in Acinetobacter baumannii complex bacteraemia: experience from the Chinese Antimicrobial Resistance Surveillance of Nosocomial Infections (CARES) Network. J Med Microbiol. 2020 doi: 10.1099/jmm.0.001222. [DOI] [PubMed] [Google Scholar]
- 9.Sheng W.H., Liao C.H., Lauderdale T.L., Ko W.C., Chen Y.S., Liu J.W. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis. 2010;14 doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]
- 10.Playford E.G., Craig J.C., Iredell J.R. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: risk factors for acquisition, infection and their consequences. J Hosp Infect. 2007;65:204–211. doi: 10.1016/j.jhin.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Gon G., Dancer S., Dreibelbis R., Graham W.J., Kilpatrick C. Reducing hand recontamination of health workers during COVID-19. Infect Control Hosp Epidemiol. 2020;41:870–871. doi: 10.1017/ice.2020.111. [DOI] [PMC free article] [PubMed] [Google Scholar]