Abstract
The SARS-CoV-2 global pandemic has seen rapid spread, disease morbidities and death associated with substantive social, economic and societal impacts. Treatments rely on re-purposed antivirals and immune modulatory agents focusing on attenuating the acute respiratory distress syndrome. No curative therapies exist. Vaccines remain the best hope for disease control and the principal global effort to end the pandemic. Herein, we summarize those developments with a focus on the role played by nanocarrier delivery.
Keywords: SARS-CoV-2, COVID-19 vaccine, Nanovaccine, mRNA vaccine
Graphical abstract
1. Overview: pathways toward an effective COVID-19 vaccine
In late 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first emerged and on March 11, 2020 it was declared a pandemic [1]. Clinical outcomes ranged from asymptomatic infection to severe acute respiratory distress syndrome (ARDS) and death. The World Health Organization (WHO) named the resultant disease complex coronavirus disease 2019 (COVID-19) [2,3]. COVID-19 has negatively impacted the global socioeconomic well-being of the world's population. Global lack of health care, infrastructure, and preparedness has intensified the pandemic's impact [4].
Viral detection, mobilization and control of person-to-person spread served as the primary means for containment. Induction of effective host antiviral immunity against SARS-CoV-2 comes secondary to infection [5,6]. An uncontrolled innate immune response is the signature of virus-induced pro-inflammatory responses for an ARDS. Alveolar macrophage inflammation disrupts cell and tissue homeostasis leading to end-organ lung disease [7,8]. In the absence of a vaccine, virus-induction of adaptive humoral immune responses can attenuate disease progression [9].
A vaccine can elicit protective antiviral responses against SARS-CoV-2. Short of containment it is the most effective means to prevent infection in susceptible people [10]. To this end, there are more than 137 vaccine candidates in development and 23 in Phase 2 or 3 trials [11,12]. One promising candidate BNT162b2 is already approved while several others are soon to be approved for prevention in the United States of America (USA) [13,14]. However, how long an induced immune response remains effective is in question. Final outcomes will depend, in part, on the continuance of a neutralizing antibody response, the limitations seen on viral mutations and the long-term induction of antiviral memory T cell responses [15,16]. The long term efficacy of a SARS-CoV-2 vaccine requires limited boosting and divergent geographic, co-morbid and age-associated population efficacy [17]. Efficacy is defined as long-term prevention of viral infection and in halting transmission in broad populations [18].
2. SARS-CoV-2 pharmacological agents
Currently, there are no potent effective antiviral therapies available for SARS-CoV-2 [19]. Nonetheless, the United States Food and Drug Administration (US FDA) approvals have sped drug repurposing [20]. Medications now availalbe were developed to treat other viral infections [21,22]. However, newer drugs or drug formulations remain under development. Ongoing efforts are in producing drugs that not only combat viral infection but modulate antiviral immunity [15,23]. Pharmacological agents target parts of the SARS-CoV-2 life cycle [24,25]. In silico viral gene profiles have uncovered prospective medicines that inhibit the viral protease, the hemagglutinin esterase (HE), the spike (S)/envelop (E) proteins, and RNA-dependent polymerases [26]. These and drug candidates that target the angiotensin-converting enzyme 2 (ACE2) receptor are major candidates for COVID-19 treatment.
-
i.
Broad-spectrum antiviral drugs: Hydroxychloroquine (HCQ) is an anti-malarial drug used successfully to treat systemic lupus erythematosus and rheumatoid disease [27]. It has anti-inflammatory activitiese with known control of interleukin 6, 17, and 22 (IL-6, IL-17, and IL-22) cytokines [28,29]. While HCQ received initial global attention based on symptom similarities between malaria and COVID-19, its use for COVI-19 remains controversial [30]. Its “putative” mechanisms of action include alkalization of intracellular pH, inhibition of lysosomal activity of antigen-presenting cells (APCs), and effects on cathepsins, mitogen-activated protein kinases, and autophagosomal functions which results in structural damage to SARS-CoV-2 S proteins [[31], [32], [33], [34], [35], [36], [37], [38]]. HCQ can also attenuate cytokine production and autophagy [39,40]. However, based on variable clinical outcomes and toxicities, the US FDA revoked emergency-use authorization (EUA) for COVID-19 treatments on June 15th, 2020 [41,42].
-
ii.
Protease inhibitors: Lopinavir/ritonavir and other human immunodeficiency virus type one (HIV) proteases were approved by the US FDA for HIV-1 infection. Promising results as an inhibitor of the 3-chymotrypsin-like protease of SARS-CoV-2 were reported [43]. However, use of the drugs for COVID-19 are limited [44]. Darunavir is an inhibitor of HIV-1 protease and cobicistat is a potent inhibitor of cytochrome P450 3A. All, regrettably, are limited against SARS-CoV-2 [45].
-
iii.
Nucleoside analogs and inhibitors: Remdesivir: Remdesivir (GS-5734), a broad-spectrum antiviral drug, acts on the viral RNA-dependent RNA polymerases (RdRp) to suppress viral replication. It has activity against coronaviruses and was shown to be effective during the Ebola virus 2014–2016 outbreak and in some SARS-CoV-2 studies [46,47]. At the outset of the pandemic, remdesivir was used for COVID-19 treatments in late stage disease. Early successful reports were reinforced by a more extensive double-blind placebo-controlled study demonstrating shortened times on ventilator assistance precluding improvements in disease mortalities [48,49]. Soon remdesivir received a EUA by the US FDA for COVID-19 that included all age groups independent of disease severity [48,50]. However, on November 9, 2020, a follow on study concluded no clinical benefit for remdesivir in hospitalized patients [51]. Ribavirin: Ribavirin is a guanine nucleoside analog that prevents viral replication by acting on the viral RdRp. To date, there are no published reports on therapeutic efficacy for SARS-CoV-2 with only mild therapeutic benefit in MERS-CoV infections when used with interferon alpha (IFN-α) [52]. Favipiravir (T-705): Favipiravir is known to undergo intracellular activation to favipiravir ribofuranosyl-5-triphosphate (RTP); a purine nucleotide that inhibits viral replication by control of RNA polymerase. Favipiravir has antiviral activity against a spectrum of RNA viruses such as influenza H1N1 and Ebola [53]. Laboratory studies demonstrate antiviral activity against SARS-CoV-2 [54]. In June 2020, the Drug Controller General of India (DGCI) approved the drug during ongoing clinical trials [55,56]. Other drug candidates under development for SARS-CoV-2 include umifenovir (arbidol), an antiviral drug that interferes with the spike protein-ACE2 interactions. The drug acts by inhibiting cell membrane-SARS-CoV-2 envelope fusion [57].
-
iv.
Immunotherapeutics: Impaired immunity with compromised lung functions and pro-inflammatory cytokine levels are COVID-19 features [5,58]. Adaptive immune dysfunctions include lymphopenia and activation, granulocyte and monocyte dysfunction, and elevated immunoglobulin G (IgG) and total antibody levels [6,59]. These are present in blood and convalescent plasma of infected people [22,60,61]. Control of inflammation is achieved by immune modulation [22,62,63]. Convalescent plasma: Convalescent plasma from recovered COVID-19 patients contains antibodies that can neutralize viral infection [64]. However, adverse events have been reported that include fever, allergic reactions, transfusion-related lung injury, life-threatening bronchospasm and circulatory overload. These are present in patients with cardiorespiratory disorders [65]. A cocktail of monoclonal antibodies was used successfully for USA President Donald J. Trump which now received an EUA [66]. Mesenchymal Stem Cells (MSC): MSC-based therapy is being developed for treatment of pneumonia [67]. Transplantation of MSCs possesses self-renewal and anti-inflammatory properties resulting in pulmonary epithelial cell repair and defense against a cytokine storm and promotion of alveolar fluid clearance [68]. Regulatory T cells (Treg): The inflammatory processes seen during ARDS-asssociated COVID-19 may be linked to Treg dysfunction. Therefore, Treg therapy may serve to improve oxygenation and attenuate pro-inflammatory cytokines [69,70]. A novel allogeneic cell therapy (CK0802) developed by Cellenkos Inc. consists of Tregs administered to overcome immune dysfunction through resolving chronic inflammation in COVID-19 patients [71]. Such treatments serve to halt respiratory deterioration. IL-6 inhibitor: A key mediator in the COVID-19 cytokine storm is IL-6 [72], a driver of inflammatory responses. Targeting the IL-6/IL-6 receptor (IL-6R) signalling can halt inflammatory activities [73]. Tocilizumab and sarilumab, are humanized anti-IL-6R antibodies that inhibit IL-6 signalling [[74], [75], [76]]. Both are in Phase 3 clinical trials for COVID-19 [12]. Janus kinase (JAK) Inhibitors: Inhibition of the JAK signalling pathway together with IL-6 can ameliorate abnormal cytokine levels [77]. Baricitinib, fedratinib, and ruxolitinib are JAK inhibitors used for rheumatoid arthritis and myelofibrosis. COVID-19 patients treated with baricitinib in combination with lopinavir, ritonavir or remdesivir demonstrated reductions in viral and inflammatory activities [78,79]. Dihydroorotate dehydrogenase (DHODH) inhibitors: While DHODH inhibitors can suppress viral replication and modulate cytokines they have a low therapeutic index [80]. Others: NK cell-based immunotherapy is less defined for COVID-19 [60,81]. PEGylated IFNα-2α and 2β, can stimulate innate antiviral responses for SARS-CoV-2 [19]. Clinical trial involving combination therapy of PEGylated IFN-α with ribavirin has been reported (ChiCTR2000029387). Camostat mesylate inhibits host transmembrane proteases restricting viral host cell entry [20]. The anti-vascular endothelial factor bevacizumab (used in cancer), the monoclonal antibody eculizumab (used in autoimmune conditions), and a sphingosine-1-phosphate receptor modulator fingolimod which sequesters lymphocytes are under study [20,58,82].
3. Herd immunity
Whether the elimination of COVID-19 is possible through herd immunity and vaccines is uncertain [83,84]. Previously, two human influenza viruses (H1N1 and H2N2) disappeared after pandemics explained by herd immunity [85]. The prior pandemics led to global immunity through the aid of highly-effective vaccination. These led to viral elimination. Two pathogenic avian influenza viruses also disappeared through herd immunity and vaccination. However, human person-to-person passage led to viral mutation and an epidemic [86,87]. These events support the idea that SARS-CoV-2 elimination can occur through herd immunity [88] defined as the point at which the proportion of infection in susceptible individuals falls below the threshold needed for viral transmission [89]. When herd immunity begins to take effect, susceptible individuals benefit from indirect protection of infection [83]. For SARS-CoV-2, herd immunity would be determined by: (i) the percentage of the population immune to infection (herd immunity threshold (HIT); (ii) the length and effectiveness of the SARS-CoV-2 immune response; and (iii) the stability of the virus epitopes to mutation [90]. The HIT depends upon the basic reproduction number (R0), the average number of people spawned by the one infected person in fully succeptible mixed populations. The HIT is calculated using formula 1-1/R0 and different mathematical models predicted HIT for COVID-19 closer to 60 to 70% suggesting very high population need to be immune to the infection to achieve herd immunity [84,91]. As of December 10, 2020, 69 million people have been infected and 48 million people have recovered worldwide from the SARS-CoV-2 infection representing 0.90% and 0.62% of the world's population [92]. Therefore, it is far below a significant percentage of the world's population that could become immune to SARS-CoV-2 to confer herd immunity [88]. The Ferguson report estimated that with R0 2.4 the healthcare systems in the USA and the United Kingdom (UK) will incur more deaths then expected. The report suggested strict government interventions to prevent rapid viral spread which were soon implemented in different countries [93]. Sweden experienced rapid spread of COVID-19 without herd immunity and ten times greater number of deaths than neighboring Norway who implemented strict preventive measures. However, in a part of Sweden, Stockholm County, by April 11, 2020, the net hospital admissions decreased significantly when 17% of the population was infected, whether this was due to herd immunity is not clear [94]. To evaluate societal costs in achieving global SARS-CoV-2 herd immunity, the overall infection fatality rate (IFR) need be considered. Understandably, IFR will be lower than the reported case fatality rate (CFR) due to the numbers of asymptomatic individuals. If one combines infection fatality data with numbers of individuals needed to reach HIT, estimations of the expected number of deaths can be determined [83]. Achieving herd immunity remain theoretical [88,95].
Effective antiviral immunity rests in its durability. SARS-CoV immunity can decline over time in levels of IgG, IgM, and IgA neutralizing antibodies [96]. While SARS-CoV antigen-specific memory B-cells decline, memory antiviral T cell responses require sustenance [97]. T cell responses persist >10 years in those who recovered from MERS-CoV and SARS-CoV infections. Whether these are protective is not known [59]. It was demonstrated by computer modeling that immunity against SARS-CoV-2 could be transient. If operative, there is a likelihood that, like influenza A and B viruses, SARS-CoV-2 may lead to biennial or annual outbreaks. Therefore, a need exists to determine the extent and duration of immunity against SARS-CoV-2 [98]. This need is further complicated because the antibody response that is mounted against SARS-CoV-2 is not always accompanied by viral clearance [99]. Antibody levels vary based on age [100], re-infection rates [101], and length of illness. Cases with SARS-CoV-2 re-infection have been reported and virus strains with different genome sequences in a single person [102]. Re-infection is distinguished from relapse and prolonged viral shedding [103]. Although the number of re-infection is limited compared to new infections, re-infection is linked to how long-term neutralizing antibody titers are sustained [104]. Emergence of mutant strains of SARS-CoV-2 and viral epitope stability affect re-infections [105]. COVID-19 has a lower fatality rate than SARS-CoV and MERS-CoV infections but higher than flu (~3% versus 0.1%) [106]. However, since SARS-CoV-2 is more contagious than the flu, and has spread rapidly across the globe, the 1.5 million deaths from COVID-19 in the past 10 months has already exceeded the total number of deaths from the last five flu seasons [92,107,108]. Viral infections with low fatality rates enable the host to mount an immune response, that create selection pressure for the emergence of mutant virus strains [109]. Considering re-infection, limited immunity, virus mutations and need for herd immunity underlies the importance of vaccination.
4. Viral vaccines
Bacteria and viruses are pathogens that commonly elicit disease [110]. For a successful pathogen to survive and grow, it must have ability to: (i) inhabit the host; (ii) avoid or circumvent host immune responses; (iii) grow by affecting host biological machineries to its benefit; and (iv) transmit itself from one host to another [110,111]. Viral infections possess nucleic acids surrounded by a protein shield. Viruses can also be classified based on various criterias including genetic material, number of nucleic acid strands, envelope, shape, and structure [111,112]. Some viruses contain an envelop whereas some do not. For example, adenoviruses and adeno-associated viruses (AAVs) are non-enveloped viruses, whereas herpesvirus and coronavirus are enveloped. The viral envelope-membrane, when present, consists of lipid and glycoproteins and it is acquired during detachment of virus from the host cell [113]. Upon exposure, body's immune system mount a specific response enabling the host to be protected upon re-exposure [110,113]. Vaccines harness the body's immune responses providing protective immune responses against infection. Vaccines are broadly available against tuberculosis, diphtheria, tetanus, pertussis, Haemophilus Influenzae Type b, Haemophilus Influenzae Type b, cholera, typhoid, Streptococcus pneumoniae and viral infections that include influenza, hepatitis, diphtheria, measles, mumps, and polio. Efforts are still under development for chikangunya, dengue, malaria, cytomegalovirus and leishmaniasis [114].
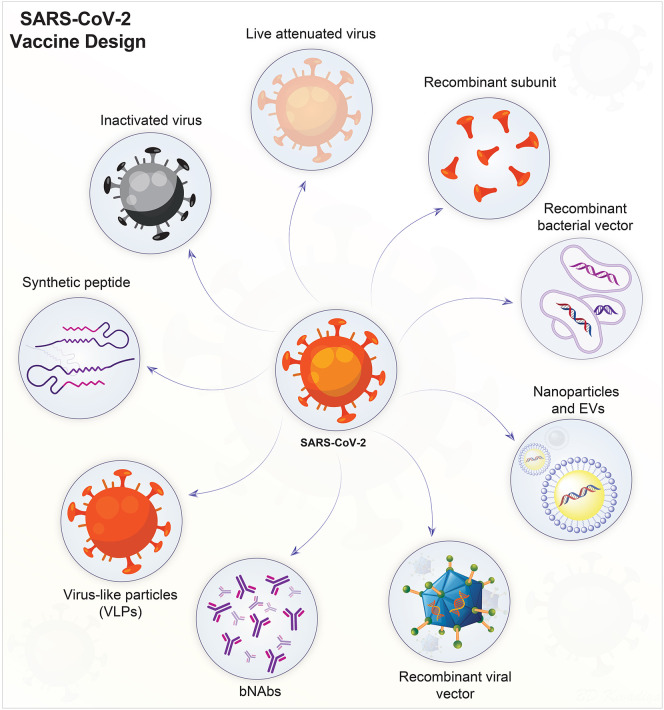
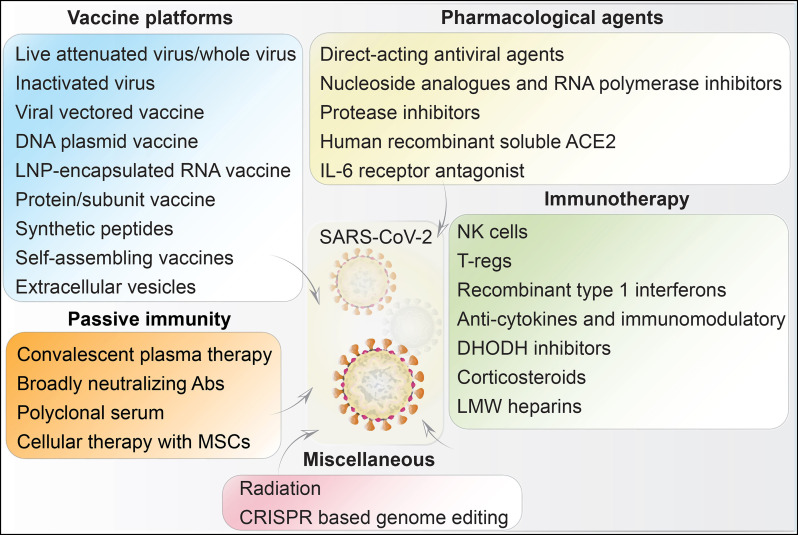
SARS-CoV-2 vaccine development had now achieved US FDA approval [12]. What is now available is able to induce long-lasting viral neutralizing antibodies that prevent viral attachment (through a ACE2 receptor) to epithelial cells in the mucosal layers and type II pneumocytes [16,115]. Vaccine should also induce sustained humoral and cellular immune responses which would generate long-lasting memory T cell responses. Further features of an effective vaccine include ease of administration (single dose, mucosal), storage, production, and scale-up [15,116]. The spike (S), nucleocapsid (N), membrane (M), and envelope (E) glycoproteins are known immunogenic proteins for SARS-CoV-2. The spike S protein is the major target for vaccine development, as it is involved in the viral entry via ACE2 receptors [115,117]. Several COVID-19 vaccine candidates have or are being developed. Viral vaccines against SARS-CoV-2 include: (i) live and attenuated; (iii) inactivated; (iv) nucleic acids; (v) viral vectors (self- and non-replicating); and (vi) protein and subunits; (Fig. 1 ). These can be propagated in animals, chicken embryos, and cell cultures and tissues [118,119]. Currently vaccines are approved for marketing in different countries to control more than 30 infectious diseases [120]. Vaccines are monovalent, effective against a single antigen or a pathogen (Rotarix) and polyvalent/multivalent, effective against strains of the same pathogen (RotaTeq) [116,121].
Fig. 1.
SARS-CoV-2 vaccine designs. Live-attenuated viruses are produced by serial passage in relevant tissue culture systems. Virus inactivation is produced by radiation, heat, or chemical treatments. Both live-attenuated and inactivated viruses are capable of inducing protective antiviral immune responses. Viral vectors are employed to deliver specific antigens through the genome of another virus. DNA vaccines, carried by recombinant bacterial vectors, are generated in relevant microorganisms or in cell cultures. When injected into a host they provide relevant virus-specific protein synthesis needed to generate an immune response. Recombinant subunits are antigenic determinants of SARS-CoV-2, obtained by recombinant DNA technology. VLPs contain no genetic materials but resemble the SARS-CoV-2 virus by virtue of specific surface antigenic proteins. Broadly neutralizing antibodies (bNAbs) are capable of binding to multiple conserved sites on viral spike proteins obtained from different viral strains, and thereby prevent virus neutralization escape. They may also function to attenuate virus evolution. Synthetic peptides can be designed to inhibit the receptor-binding domain (RBD) on the spike protein that is crucial for SARS-CoV-2 to gain host cell entry. Nanoparticles and extracellular vesicles (EVs) are the emerging technologies for the degevelopment of safer vaccines against SARS-CoV-2. Nanoparticles are decorated with antigenic molecules, while EVs serve as natural carrier of viral proteins, wherein both inducing antiviral immune responses.
4.1. Live attenuated
These use live viruses to elicit protective immune responses. India and China use a live virus vaccine to boost small pox immunization. They expose people to material obtained from patients infected with a mild virus form [121,122]. Live vaccine raises the possibility of viral virulence reversal [123]. Therefore, live virus vaccines need be developed by altering the viral genome and selecting non-pathogenic mutant strains incapable of causing disease [124]. Live virus vaccines can also be produced by viral attenuation. These are viruses weakened in their pathogenicity but can elicit antiviral immune responses without causing disease. One of the most widely used strategy is serial passages of virus in chick embryo fibroblasts and vero cells. This is a well accepted practice for the vaccine development [125,126].
As the virus replicates through each passage, it loses virulence [119]. Attenuated viral vaccines against measles, mumps, and chickenpox were developed [127]. Another means used to generate live attenuated vaccines involves deletion or viral gene mutation essential for viral growth. These defective viruses cannot replicate in a human host [128] but can induce immune responses [129]. The first replication-defective live viral vaccine was developed against herpes simplex virus-1 (HSV-1) [130,131]. Replication-defective HSV-2 missing the genes essential for viral DNA synthesis was produced [132]. Another approach is to grow virus at reduced temperatures. Attenuated viruses can also be developed by inserting viral proteins into an attenuated cold-adapted virus [133]. Live attenuated virus induce no or limited infection [134]. However, these strains show an ability to induce herd immunity by shedding of viral particles and by host to host transmission. Live-attenuated vaccines are capable of eliciting illness in the immunosuppressed individuals [123]. To overcome this limitation, codon deoptimization is used. Codon deoptimization vaccines are completely safe due to substitution of several nucleotides from the virus coding sequence [135,136]. Recently, live attenuated vaccine was produced against Ebola and found to be >97% effective in 90,000 individuals [137]. Currently, Griffith University in collaboration with Indian Immunologicals Ltd. developed codon deoptimized live attenuated vaccine for SARS-CoV-2. The vaccine can induce long lasting protective immunity after single dose without cross reactivity to MERS-CoV and SARS-CoV [138]. Existing vaccine manufacturing facilities are capable to develop live attenuated vaccine for large scale production [139].
4.2. Inactivated viruses
These viruses are inactivated by radiation, heat, and chemicals such as binary ethyleneimine and formalin. This leads to an inability to cause illness. However, inactivated viruses maintain the ability to induce host immunity that recognize and destroy pathogens. This vaccine type contain viral particles from inactivated virus and hence do not develop pathogenicity [116,140]. The inactivated vaccine developed against poliovirus is an example, where virus is inactivated by formaldehyde treatment. Due to inactivated vaccine development, no new polio infection cases have been reported since 1999 and as a result disease eradication was declared in 2015 [141,142]. Another examples of an inactivated vaccine are those for typhoid, rabies, influenza and hepatitis A [120,143]. Such vaccines have set regulatory requirments for short-term protection as they, as a group, generate a weaker immune responses and require more frequent boosters for long-term immunity than other vaccine candidates. On balance, they are relatively stable and do not require refrigeration and can be freeze-dried and easily transported. Inactivated vaccines are boosted with adjuvants, such as saponins, alum, immune complexes and liposomes [124,144]. Currently, nine SARS-CoV-2 vaccines are being developed using this technology. Sinovac Biotech is developing inactivated vaccine PiCoVacc which is now known as CoronaVac against SARS-CoV-2 and this candidate induces broad neutralizing antibodies against ten different viral strains in multiple species that include primates. After three immunization of CoronaVac (6 μg per dose), complete protection was observed in macaques challenged with SARS-CoV-2 [145]. CoronaVac has already been tested in the human Phase 1/2 trials. 142 healthy volunteers were enrolled in Phase 1 while 600 healthy volunteers were enrolled in Phase 2 studies. CoronaVac was found safe and well tolerated in all studies. The neutralizing antibody titer rate was above 90%, confirming a robust protective immune response against SARS-CoV-2 [146]. Currently, Sinovac Biotech is studying CoronaVac in Brazil in collaboration with Instituto Butantan in a Phase 3 clinical trial where 90,000 healthy participants are or soon to be enrolled [147]. Another inactivated vaccine Covaxin is developed by the Indian Pharmaceutical company Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV). Preliminary results of a Phase 1/2 clinical trial showed that Covaxin is safe and effective. Currently, a Phase 3 trial with Covaxin is ongoing with 26,000 participants [148]. Other inactivated vaccine candidates are being developed by Beijing Institute of Biological Products in collaboration with Sinopharm and was found safe and able to generate a high antiviral antibody titer among participants as observed in a Phase 1/2 clinical trial [149]. Currently, a Phase 3 trial in the United Arab Emirates (UAE) in 15,000 participants is ongoing [147].
4.3. Nucleic acid – DNA
DNA vaccines are a harmless complement to conventional live- and inactivated-virus vaccines [120]. DNA vaccines are generally safe and stable compared to conventional vaccines because the vectors used are non-replicating and express only the antigen of interest. Therefore, unlike viral vector vaccines, they are not able to revert to the disease-causing form. DNA vaccines also lack vector induced immunity which allow their use with other vaccines in the same individual [150]. In short time intervals, DNA vaccines are produced in bulk quantities. Additionally, such types of vaccines can provoke both humoral and cell-mediated immune responses but at lower levels when compared to conventional vaccines. Maintaining high-level protein expression is challenging with this platform (Fig. 2 ) [151]. Under the transcriptional control of the CMV/R promoter, a West Nile virus DNA vaccine was shown to be safe and well tolerated [152]. A DNA vaccine against Rift Valley fever virus (RVFV) [153] and Chikungunya virus (CHIKV) elicited strong immune responses that protected mice and non-human primates after viral challenge [154]. Recently, the EBOV-GP DNA vaccine was shown to induce long-term immune responses and neutralizing antibodies in nonhuman primates [155]. A HIV-1 DNA vaccine DNA-4, which encodes the Pol(rt), gp140, Nef, and Gag proteins of HIV-1, was found to be safe, well-tolerated and capable to induce robust immune responses [156]. DNA vaccines from the envelope (E), ectodomain (domains I, II, and III), and the non-structural 1 (NS1) protein of dengue virus serotype 2 (DENV2) protected against lethal DENV2 challenges [157]. A DNA vaccine encoding the SARS-CoV S-protein induced T cell and neutralizing antibody responses in animals [158]. Despite the encouraging preclinical outcomes, no DNA vaccines yet are approved for human use based on their regulatory uncertainty. A vaccine candidate developed in this platform must have to go through vigorous regulatory process that could delay approval. Currently, three DNA vaccine candidates are under investigation for COVID-19. Inovio Pharmaceuticals with the International Vaccine Institute and Korea National Institute of Health are developing the SARS-CoV-2 vaccine INO-4800, using a DNA-based platform. In Phase 1 clinical trial with 40 healthy voluenteers, 94% participants showed protective immune responses six weeks after two doses (1 and 2 mg) of INO-4800. After the early encouraging results, Inovio is now recruiting healthy participants for a Phase 2/3 trial [159]. The India-based pharmaceutical company Zydus Cadila is investigating a plasmid DNA vaccine candidate ZyCov-D for SARS-CoV-2. After seeing protective immmune responses in mice, rats, guinea pigs and rabbits, investigators are recruiting healthy voluenteers for a Phase 1/2 trial [160]. Genexine Inc. is developing DNA vaccine GX-19 for COVID-19 with the support of the Korean Government. Currently, recruitment of 120 healthy volunteers is under progress to initiate a Phase 1 clinical trial [161].
Fig. 2.
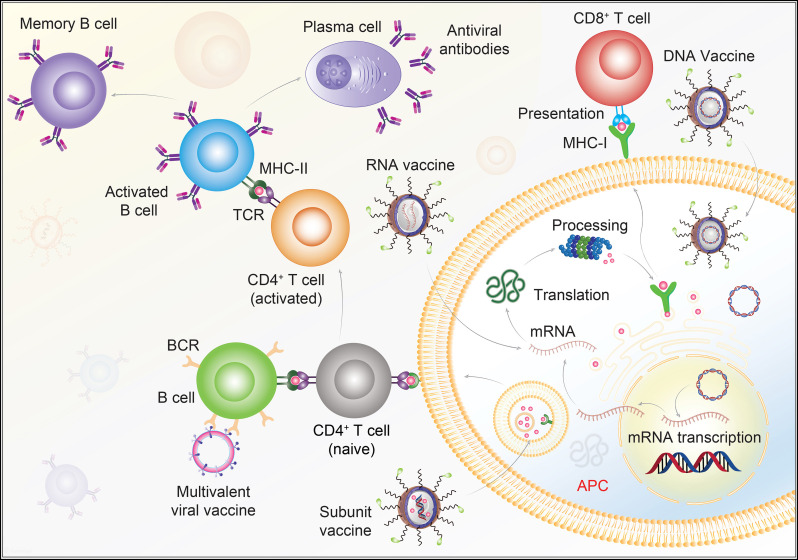
Schematics for vaccine-induced antiviral immunity. DNA vaccines carry viral genes, which are released inside the target APCs. The inserted genes are transcribed, then translated to antigenic viral proteins that are either presented through the APC to CD8 T cells through MHC-I TCR interactions. Alternatively, the viral protein of interest is presented to CD4 T cells by MHC-II TCR interactions. Cytotoxic CD8 T cells kill infected cells and B cells make antibodies by CD4 T cell dependent activation. These released antibodies are directed to target specific viral antigens. RNA vaccines incorporate mRNA into target APCs and undergo parallel pathway events once the immunogenic proteins are synthesized. Subunit vaccine consists of the antigenic determinants of the viral pathogen, which enters the target cells and subsequently releases the specific viral subunits. The subunits are engulfed into endosomes, which when fused with the membranes, present the viral antigens to the CD4 T cells for both T- and B- cell mediated antiviral immunity. Multivalent viral vaccines are also designed to display multiple antigens in order to enhance immunogenicity. Abbreviations; Antigen presenting cells, APCs; class I major histocompatibility complex, MHC-I; class II major histocompatibility complex, MHC-II, T-cell receptor,TCR; B-cell receptor, BCR. The illustration is prepared in-house and schematic ideas and technical details were followed as presented in previous published report [17].
4.4. Viral vectors
Vector-based vaccines are kind of live attenuated vaccines which use available viral vectors with known safety profiles. Vectors deliver specific gene that encodes for a specific antigen. When viral vectors are injected, a gene of interest transcribes a specific antigenic protein, and thereby elicits immunity. Vectors do not require adjuvants and are capable of eliminating virus-infected cells by inducing a cytotoxic T cell (CTL) responses [[162], [163], [164]]. Existing viral vectros can be divided into two categories: replicating (replication-competent) and non-replicating (replication-deficient). The replicating viral vector produces infectious viruses capable of infecting target cells to produce viral antigens. The most widely used replicating vectors include the measles and the vesicular stomatitis virus, both are single-stranded, and antisense RNA viruses able to deliver heterologous antigens that can induce both cellular and humoral immune responses [165]. The major advantage of the measles virus vector (MVV) is that it elicits life-long cellular and humoral immunity. Due to helical structure of measles virus, vectors prepared from it can tolerate insertion of genes over a size of 6Kb and vectors are stable even after multiple passages [166].
The recombinant versions of replicating viral vectors have better safety profile and superior infectivity over their native form. Recombinant MVV can elicite broad neutralizing antibodies against multiple conserved epitopes of viral spike proteins of divergent strains and therefore precluding virus escape [167,168]. Recombinant MVV is also known for more specific targeted antigen delivery to the macrophages and can be used as multivalent vector for HIV-1, West Nile virus, and others [169,170]. In contrast, non-replicating viral vectors include adenovirus, adeno-associated virus (AAV), herpes virus, and alpha virus. Originally, they were developed from simian adenoviruses and some other pathogens such as Ebola virus, RVFV, and Zika virus [171]. Adenovirus-based viral vectors are most commonly used. The E1A and E1B genomic regions of adenovirus are replaced by genes encoding target viral antigens [172]. Adenoviral vectors can express gene inserts of size over 8Kb, and hence can be used to generate multivalent vaccines [173]. Adenoviral vector Ad5 can provoke CD8 T cells and strong antiviral responses and provide ease of large scale production [174,175]. Other adenovirus-based vectors (Ad26 and Ad35) are candidates for HIV-1 vaccine due for their abilities to infect functional memory T cells [176,177]. The key advantages of non-replicating adenovirus vectors include ease of genetic alterations, stability, safety, higher growth rate, strong immune response, and thermostability [178].
In spite of structural similarities, AAV display improved stability over adenoviruses. The protein coverings of a virus (capsid) and specific activating protein assembly facilitate their targeted delivery potential [179,180]. The recombinant AAV vectors (rAAV) are superior over native AVV where the Rep and Cap genes of AAV are replaced with the genes encoded for target antigens to elicit neutralizing antibodies [181]. AAV-based vectors are used as a tool in gene therapy for the treatment of various disorders like muscular atrophy, inherited blindness, and haemophilia [182]. Despite advantages, viral vectors have several limitations. Vector induced immunity is common with this technology that reduces the efficacy of the vaccine. Host genome integtation is another key limitation with viral vector leading to the risk of tumorigenesis. Also certain viral vectors like AAV have low titer production which become limiting step in bulk vaccine production. However, due to highly specific delivery and long lasting immune response after single administration, viral vector platform is still well accepted and in the past this technology has shown successful eradiation of smallpox [162]. Technological developments have increased host immune responses as well as the large-scale production of viral vectors that ease the regulatory requirements of this technology [116].
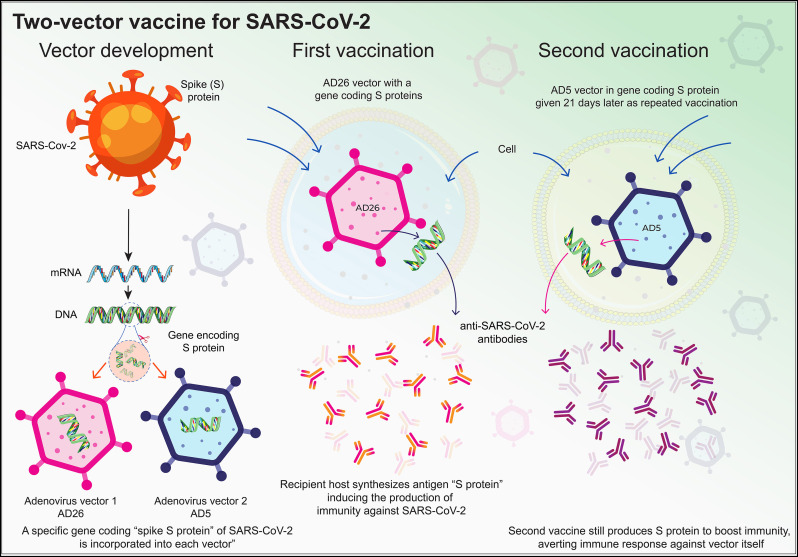
Currently, many COVID-19 vaccines are under development with this technology. AD26 associated vaccine against SARS-CoV-2 showed complete protection in rhesus macaques after infection [183]. Thereafter, a series of AD26 vectors encoding different SARS-CoV-2 spike protein epitopes were developed with encouraging outcomes [184,185]. Recently, Russia approved a COVID-19 vaccine Sputnik V exhibiting two different adenovirus vectors (rAd26 and rAd5), both carrying the gene for SARS-CoV-2 S glycoprotein (rAd26-S and rAd5-S) (Fig. 3 ).
Fig. 3.
Two-vector viral vaccines. Developed by Gamaleya Center in Russia, two-vector vaccine, as the name suggests, uses two different vectors (Ad5 and Ad26). Vector development involves the use of S-protein mRNA to generate the complementary DNA, followed by insertion of this S-protein encoding DNA into adenoviral vectors, Ad26 and Ad5. Ad26 vector encoding S-protein was administered as first vaccination followed by a booster dose of Ad5 vector encoding the same S-protein 21 days later. Inside the recipient, these vectors generate the S-proteins, which upon entering the circulation induces protective immunity. The illustration is prepared in-house and schematic ideas and technical details were followed as presented in previous published report [186,187].
The premature decision was made after the encouraging results of Sputnik V in Phase 1 and Phase 2 clinical trials, with just 38 participants in each study [186]. In Phase 1 study, both rAd5-S and rAd26-S vaccines were found safe and well tolerated for up to 28 days. In Phase 2 study, rAd26 was administered on day 1 followed by a booster dose of rAd5 on day 21 where 100% of the participants showed seroconversion and high SARS-CoV-2 antibody titer than COVID-19 convalescent plasma. Both antigen-specific CD4 and CD8 T cells were observed in all the participants [186]. However, large-scale Phase 2 clinical trial results are needed for US FDA approval and to vaccinate the general population with Sputnik V [187]. Other COVID-19 vaccines developed using this technology include Ad5-nCoV, AZD1222, aAPC, and LV-SMENP-DC. Ad5-nCoV, developed by CanSino Biologics Inc., is the first vaccine to reach Phase 2 clinical trial. In Phase 1 clinical trial, Ad5-nCoV was found well tolerated and immunogenic in 108 participants 28 days post vaccination [188]. In Phase 2 study, single immunization of Ad5-nCoV induced significant SARS-CoV-2 neutralizing antibodies and immunogenic response in all 508 participants [189]. Ad5-nCoV has already been approved for the military use in the China and currently it is under Phase 3 clinical trial investigation in russia [190]. AZD1222, formerly known as ChAdOx1-S is being developed by the University of Oxford with AstraZeneca. Currently, Phase 2/3 clinical trila is ongoing but the interim results of Phase 2 study showed better tolerance of AZD1222 in older adults compared to the young adults with equivalent immunogenicity across different age groups 28 days after single booster immunization. To note, when tested in the animlas, no complete protection against SARS-CoV-2 infection was observed [191]. AZD1222 is the first vaccine candidate being investigated in the Phase 3 clinical trial. Previously, Phase 3 study of AZD1222 was put on hold after suspected serious adverse reactions in the UK trial participants which is now resumed after recommendations of the independent safety review committee and the United Kingdom Medicines and Healthcare Regulatory Agency [192]. AstraZeneca has agreement with Europe's Inclusive Vaccines Alliance to supply 400 million doses of AZD1222 by the end of 2020 [193]. Vaccines aAPC and LV-SMENP-DC are made by Shenzhen Geno-Immune Medical Institute which are currently in Phase 1/2 clinical trial (NCT04276896).
4.5. Protein and subunits
These vaccines utilize viral proteins or protein fragments and able to elicit strong humoral and cellular CD4 and CD8 T cell antiviral responses (Fig. 2). Currently, many vaccines are under development which are based on the protein subunit technology. Such vaccines devoid of using infectious virus and instead use purified specific antigenic component, therefore eliminate isssues of virus attenuation/inactivation or virulence reversal to offer better safety profile compared to the other viral vaccines [194]. However, like other viral vaccines, protein subunit vaccines have limitations, major one is limited efficacy. In this technology, the isolated antigenic fragment of the pathogen likely denatured that conjugate with untargeted antibodies leading to the diminished efficacy [195]. Hovever, recombinant DNA technologies have successfully overcome such limitations as evidenced by the approval of first recombinant protein subunit vaccine against hepatitis B which has now controlled the disease to near elimination [196]. It has also open the way for regulatory relaxations for the vaccines developed by this technology [197]. Furhther advancements made in this vaccine technology make them more suitable for the immunocompromised individuals. For the production of recombinant proteins, E.coli [[198], [199], [200]] and yeasts are used [200,201].
Several protein subunit vaccines are marketed against diseases including influenza, HPV, and hepatitis B [202]. To improve immunogenic action of such vaccines, diverse formulations of vaccines with potent adjuvants have been employed [144]. The subunit vaccine for hepatitis B is an example of a single antigen-containing subunit vaccines, whereas influenza vaccine is an example of a subunit vaccine that contains two antigens (haemagglutinin and neuraminidase) [124,197,198,203]. Large scale production of purified antigenic protein is also challenging which can be improved by various expression systems including plant-based [204]. Envelope glycoproteins E1 and E2 of Chikungunya virus (CHIKV) were expressed in insect cells as well as in bacterial expression systems to develop subunit vaccines effective against CHIKV [205]. The subunit vaccines developed from the capsid protein (CP) of astrovirus, protected mink litters against astrovirus infection better than the whole CP [206]. Recombinant subunit vaccines against herpes zoster showed significant reduction in the risk of post therapeutic neuralgia [207,208]. Currently, several vaccine candidates have been developed using this technology against COVID-19 focused on the SARS-CoV-2 S protein or specific domain within the S protein such as receptor binding domain (RBD). The University of Queensland in collaboration with GSK and Dynavax is developing stabilized pre-fusion recombinant protein subunit vaccine against SARS-CoV-2 using its Molecular Clamp technology that lock the SARS-CoV-2 specific protein in the three-dimentional shape with ability to develop humoral immune response against appropriate viral epitopes [12]. Novavax has developed a protein subunit vaccine, NVX-CoV2373 by combining its nanoparticle technology. The recombinant pre-fusion SARS-CoV-2 S protein is expressed in the Baculovirus system and uses Matrix-M adjuvant to enhance the protective immune response against SARS-CoV-2 S protein. Novavax is planning to initate Phase 1 clinical trial after encouraging observations in the animal studies where NVX-CoV2373 induced high levels of neutralizing antibodies against SARS-CoV-2 S protein [209]. MigVax Ltd. has developed oral subunit vaccine technology against poultry coronaviruses and now it is developing COVID-19 vaccine with this technology [210].
5. Cell-based
Deployment of CTL is a promising vaccine approach for different pathologies. T cell's unique T cell receptors (TCRs) recognize and activate against viral-specific antigens which drive immune responses [211,212]. Adoptive transfer of virus epitope-specific T cells showed protective immune responses against various viral infections including adenovirus [213], Epstein-Barr virus (EBV) [214], human cytomegalovirus (CMV), etc [215]. Antigen-targeted TCRs have limitations of cross-reactivity and induction of autoimmunity [216]. To overcome such limations, recently combination immunotherapy has been applied for the treatment of various cancers and chronic viral infections where antigen targeted cells are delivered along with other immunemodulators [217]. During viral infection, activated T cells express the programmed death (PD-1) and other inhibitory receptors that make T cells dysfunctional. To overcome such problems, antigen-targeted T cells should be administered alongside the anti-PD1 antibody, otherwise inhibitory receptors are genetically ablated to better control the viral infection [[218], [219], [220], [221], [222]]. Another cell-based vaccine approach uses whole cells or cell lysates as a source of antigen or as a platform to deliver antigens. Virus infected cells are irradiated or healthy cells are transfected with a virus to provoke humoral and cellular immune responses where endogenous dendritic cells (DCs) play a vital role in the development of neutralizing antibodies [223]. DCs are potential candidate for the cell-based vaccine. DCs play an intermediate role between the innate and adptive immunity. DCs sense foreign antigens, processs and represent them to the T cells to contribute protective immune responses [211]. Like T cells, DCs can be manipulated ex vivo to confirm antigen specificity. DCs can be modified by different technologies such as loading of DCs with the immunogenic viral protein, transfection with viral protein, or loading with viral mRNA, each strategy induce vaccine of different potency. DC-based vaccines can also be incorporated with the immunomodulatory agents or their gene to improve therapeutic outcomes [224,225]. DC-based vaccine PROVENGE (sipuleucel-T), loaded with a prostatic acid phosphatase (PAP)-granulocyte-macrophage colony-stimulating factor (GM-CSF) fusion protein has proven effective in patients with prostate cancer [226] and became the first US FDA approved DC-based vaccine for prostate cancer [227]. Because of such favourable immunotherapeutic activities, DCs have been targeted for the development of vaccines against a range of infectious diseases. However, cell-based vaccine development is complex, labor-intensive, and expensive. Large scale production is challenging compared to the traditional viral vaccines to fulfill the global demand in case of COVID-19. FLUCELVAX is the only cell-based vaccine approved by the US FDA for the influenza infection. The FLUCELVAX produces better protection in the people above 65 years of age compared to the traditional flue vaccines. Effective cell banking ensures adequate supply of cells to scale-up FLUCELVAX in case of emergency [228]. University of Manitoba is developing DC-based vaccine for COVID-19 which is currently under preclinical development [229].
6. Adjuvants
COVID-19 containment can only be achieved using effective vaccine strategy. Different technologies used for COVID-19 vaccine development have their own limitations. The inactivated and subunit vaccines often exhibit low immunogenicity therefore frequent booster dose administration is required. Also the effectiveness of the nucleic acid vaccines is questionable due to lack of any licensed vaccines for the human use [230,231]. The globally spread COVID-19 has affected a large population of all the ages due to lack of appropriate prophylactic measures. To fullfill the overwhelming demand in COVID-19 pandemic, a vaccine with high immuniogenicity, faster onset of action, low toxicity, and minimal dose injection is primary requiremet. All outcomes can be achieved by incorporating suitable adjuvants. [232]. A formulation or an immunostimulatory reagent which is designed to improve the performance of a vaccine is adjuvant. Adjuvants enhances antigen-specific protective immune responses and targeted antigen delivery of a vaccine and therefore reduce vaccine dose and associated toxicities [233,234]. An ideal adjuvant should offer heterologous antibody response, potential to kill or neutralize diverse pathogens, and effective T cell responses [235,236]. Moreover, adjuvants should be cheap and widely available with good biodegradable and biocompatible properties [237]. Strategies developed specifically for viral vaccine adjuvants are broadly classified based on formulation and composition which include Toll-like receptor (TLR) agonists, aluminium formulations, and emulsions, all have been tested preclinically in the coronavirus vaccines as discussed below.
6.1. Toll-like receptor agonists
APCs recognize viral antigenic peptide which serve as pathogen-associated molecular patterns (PAMPs) through various pattern recognition receptors (PRRs). Toll like receptors (TLRs) of different subtypes are known as PRRs. Activation of TLR lead to induction of robust humoral and cellular immune responses against invading pathogen. Therefore, use of small molecules that activate TCRs on the APCs are frequently incorporated in the vaccine to improve immune responses [238]. Due to ability to directly interact with the APCs and B cells, TLR agonists amplify the innate and adaptove immune responses [239]. Few examples of the TLR agonists are monophosphoryl lipid A (MPL, a derivative of Salmonella Minnesota R959) (TLR4), lipopolysaccharide (LPS) (TLR4), bacterial DNA (TLR7/8), and cysteine-phosphate-guanine (CpG) oligonucleotide (TLR9) which serve as PAMPs [240]. MPL is effective in eliciting both humoral and cell-based immunogenicity and capable of inducing Th1 and Th2 type responses, has been approved in the human vaccines for hepatitis (Fendrix®), HPV (Cervarix™), and malaria (Mosquirix) [241]. SARS-CoV RBD subunit vaccine (S318-510) elicited immune responses were potentiated by incorporating adjuvant alum inducing Th2 cells mediated immune responses. Addition of CpG oligonucleotide in the alum adjuvanted vaccine added Th1 responses and offered dual immune arm activation [242]. Similar dual immune induction was observed with the MERS-CoV N terminal domain (NTD) vaccine upon incorporation of CpG oligonucleotide with aluminium [243]. UV-inactivated SARS-CoV vaccine has reported to induce systemic proinflammation leading to eosinophil infiltration in the animal lungs. Such limitations of inactivated vaccines can be successfully overcome by addition of TLR agnist LPS [244].
6.2. Aluminium
Aluminium hydroxide or alum is the most commonly used adjuvant in the vaccine formulations. Alum adjuvant has been used in the licensed vaccines for the human use against diphtheria, tetanus, pertussis, and hepatitis [245]. Insoluble aluminium salts were the first generation of vaccine adjuvants founded on the discovery of an alum-precipitated diphtheria toxoid suspension. They show improved immunogenicity compared to soluble toxoid [246,247]. The performance of aluminium adjuvant, in part, depends on the composit concentration and absorption of the antigen on a preformed aluminium gel [248]. Alum adjuvants form depot at the injection site followed by slow release of the target antigen to enable its prolonged interactions with the APCs. Alum adjuvants also facilitate antigen uptake of the APCs to activate T cell responses [249]. A SARS-CoV viral vaccine sequencialy inactivated by formaldehyde and UV induced high antibody titer against S protein and neutralizing antibodies in the mice whereas the protective effects were improved by the adjuvant aluminium hydroxide [250]. A SARS-CoV inactivated vaccine induced dose dependent neutralizing antibody responses in the mice and protected them from the viral infection. Such protective effects of vaccine were further improved upon addition of alum adjuvant [251]. Alum in combination with CpG oligonucleotide had demonstrated enhanced humoral and cellular responses in the mice immunized with either inactived or S-based or RBD-based MERS-CoV vaccines [252,253].
6.3. Emulsions
A decade after the discovery of alum-based formulations, emulsion adjuvants were developed by Freund [254]. Emulsions are a dispersion of two or more immiscible liquids composed of oil, emulsifiers, and excipients [255]. Emulsions are broadly categorized into two types: water-in-oil and oil-in-water. Both emulsion adjuvants are similar in physical structure, dimensions, follow an extended time of presence at the injection site and prolonged antigen release. Emulsion adjuvants have been demonstrated to activate APCs and improve their antigen uptake. They also promote APCs migration to the target tissue to induce robust CD4 and CD8 T cell responses [256]. MF59 is an oil-in-water emulsion adjuvant, consists squalene and Tween 80 and Span 85 surfactants. In 1997, the Fluad® vaccine adjuvanted with MF59 was first approved for seasonal flu in Europe. Other marketed influenza vaccines consist MF59 as an adjuvant are Focetria® and Celtura® [257]. MF59 when used with the cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) has improved the antibody responses offered by different DNA vaccines compared to the unadjuvanted DNA vaccines [258,259]. SARS-CoV inactivated vaccine induced S protein specific antibodies and Th2 biased immune responsese were potentiated by the adjuvant MF59 leading to improved viral protection of mice [260]. A SARS-CoV inactivated vaccine induced neutralizing antibody and T cell responses were boosted by the MF59 but not CD8 T cell responses [261]. A MERS-CoV RBD subunit vaccine (S377-588-Fc, S377-588 protein fused with Fc of human IgG) induced detectable neutralizing antibodies and T cell responses in the mice. When vaccine efficacy assessed in the presence of different emulsion adjuvants, Freund's adjuvant, aluminium, montanide ISA51, MF59, and monophosphoryl lipid A; MF59 was found as the most potent adjuvant to substantially improve immune responses [262]. W805EC is an oil-in-water nanoemulsion which is formulated without squalene can substantially improve the protective spectrum of influenza vaccine [263]. Hemagglutinin influenza vaccine with glucopyranosyl lipid A (GLA) oil-in-water emulsion showed improved immune responses in mice [264]. AF03 is a squalene-based emulsion adjuvant present in the marketed influenza vaccine Humenza® [265]. AS01 and AS03 are oil-in-water emulsions developed by GlaxoSmithKline. AS01 consists of MPL and a saponin-based molecule (QS-21) while AS03 consists of α-tocopherol, squalene, and polysorbate 80. The influenza vaccine Pandemrix® is adjuvanted with AS03 to induce antigen-specific immune responses [266]. An inactivated SARS-CoV whole virus vaccine induced high neutralizing antibody titer in the mice after two dose administration. However, vaccine adjuvanted with either AS01 or AS03 showed early and robust antiviral responses where AS01 performed slightly better compared to the AS03. Additionally adjuvanted vaccine offered complete protection of hamsters against wild-type SARS-CoV infection [267]. Montanide series emulsions (ISA-51, −206, −720, etc) are metabolizable squalene-based water-in-oil emulsions. Such adjuvants eliminate the cytotoxic effects of Freund's adjuvant due to their biodegradability [268,269]. A SARS-CoV DNA vaccine expressing recombinant NTD induced antigen-specific CD8 T cell responses and neutralizing antibodies in mice. Co-administration of adjuvants montanide and CpG further improved vaccine performance [270]. MERS-CoV RBD vaccine induced high neutralizing antibody titer and protcted mice against viral challenge when adjuvated with montanide ISA-51 [271].
Few COVID-19 vaccine studies have discussed the adjuvant used in their formulation. NVX-CoV2373 is a recombinant (SARS-CoV-2) S protein nanoparticle vaccine uses Matrix-M, a saponin-based adjuvant, in the formulation. Matrix-M improved the recruitment of APCs at the site of injection and thereby increase T cell activation in the nearby lymph nodes [272]. A COVID-19 vaccine developed by the molecular modeling approaches, COVAX-19 is formulated with the Advax adjuvant which is a novel microcrystalline polysaccharide particle engineered from the delta inulin, potentiated the immunostimulatory potential of co-delivered antigen recombinant SARS-CoV-2 S protein [273]. In BNT162b1, an mRNA COVID-19 vaccine, mRNA itself serve as an adjuvant and therefore allow synchronized delivery of antigen and adjuvant [274]. The lipid nanocarriers used in the formulation of vaccines can also serve as adjuvant. The dicetyl phosphate when incorporated into the nonovaccine against diphtheria toxoid improved the protective immune responses by serving as an adjuvant [275]. Overall, the adjuvants with desired properties are critical for effective vaccine delivery.
7. Nanovaccines
Nanoparticles share size distribution with the viruses and therefore like viruses, nanoparticles can enter the virus targeted cells. Nanoparticles can be loaded with the antigen in the form of nucleic acids (DNA and mRNA) or the protein subunits and therefore allow targeted expression or direct delivey of viral antigen. Recently nanoparticles have gained significant attention for the development of effective vaccines against various pathogens including SARS-CoV-2. For effective vaccine responses both innate and adptive immune system should be activated synchronizely which has now successfully achieved with the help of innovative nanovaccine formulations such as solid-lipid nanoparticles, liposomes, polymeric nanoparticles, protein nanoparticles, and virus-like particles (VLP) [17,276]. Adjuvants pay a vital role in the nanovaccine mediated desired immune responses by assisting antigen delivery to the target APCs and reduce off-target side effects. Targeted antigen delivery reduces the dose of vaccine required to elicite effective immune response which is very essential consideration for the development of SARS-CoV-2 vaccines to achieve global demand [276,277,278]. Following approaches are used for antigens and adjuvants codelivery, (i) direct conjugation of antigen and adjuvant, (ii) encapsulation within or decorated onto the nanoparticle, and (iii) use of delivery vehicle as adjuvant. Nanovaccine formulations can enhance antigen stability by maintaining native confirmation and protect them from proteolytic degradation [279]. In the promising mRNA based COVID-19 nanovaccine BNT162b, the trimerized confirmation of SARS-CoV-2 RBD and pre-fused confirmation of S protein are maintained and secreted from the nanoformulation to allow induction of broadly neutralizing antibodies [280]. Nanovaccines further offer advantage of sustained release of target antigen due to their enhanced lipophilicity [281]. In addition to the unique physicochemical characteristics, nanoparticles allow flexibility of surface engineering by incorporating cell-targeting peptides, proteins or polymers on their surface (Fig. 4 ) [282,283]. Delivery of the antigen to the lymphatic system is the frontmost requirement for vaccine efficacy. Nanovaccines have ability to cross the intestinal space and reach the lymphatic tissue. Intranasal delivery of nanovaccine can easilty target the lymph nodes nearby the lung which would be advantageous for fighting against COVID-19 [283,284]. Such properties make nanovaccines a versatile delivery vehicle for the treatment of COVID-19.
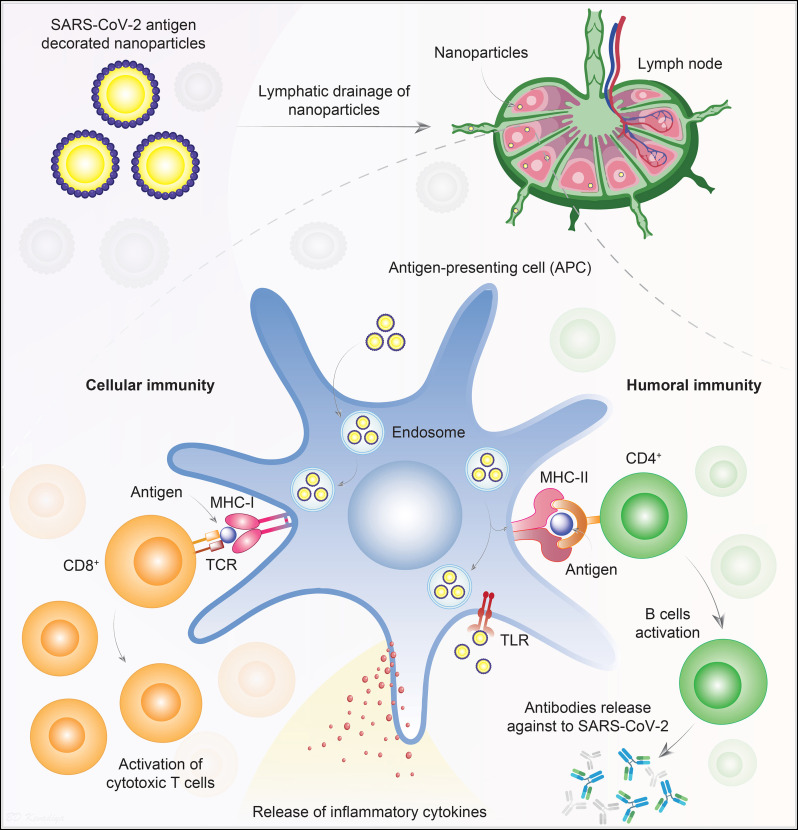
Fig. 4.
Targeted delivery of SARS-CoV-2 antigens. Nanoparticles are decorated on the surface to present SARS-CoV-2 antigens to efficiently enter APCs. Lymphatic drainage of the nanoparticles brings them in close proximity to the immune cells, particularly the APCs. Nanoparticles stimulate the APCs in different ways. APCs engulf the nanoparticles into endosomes and then presents the NP's surface engineered antigen to CD8 T lymphocytes via membrane-bound MHC-I and TCR interactions. Also, nanoparticles are ligands for the TLRs, which activates the APCs and induce secretion of pro-inflammatory cytokines. Following the interaction between MHC-I and TCR, in the presence of co-stimulatory molecules and cytokines, the activated CD8 T cells kill the infected cells by inducing cytotoxicity. Nanoparticles surface engineered antigens can also be presented to helper CD4 T cells via MHC-II. Subsequently, CD4 T cells activate the B cells to produce protective antibodies against the SARS-CoV-2 antigen. Abbreviations; Antigen presenting cells, APCs; class I major histocompatibility complex, MHC-I; class II major histocompatibility complex, MHC-II; T-cell receptor,TCR; Toll-like receptor, TLR.
7.1. mRNA nanovaccine
The mRNA-based nanovaccines have benefits over other technologies which include short development time, simple manufacturing and purification processes regardless of the antigen, and most importantly, mimic natural infection to promote potent cellular and humoral immunity by eliciting CD4 and CD8 T cell responses (Fig. 2) [285,286]. Multiple mRNAs can be combined into a single vaccine to deliver mRNA transcripts of interest into the host cell cytosol, which allows the encoding of one or more antigen(s). Such favourable properties designate mRNA vaccine as a forefront runner for the development against COVID-19 [21]. To note, no commercial vaccine is available till date developed with this technology which mandate strict regulatory requirements to undergo the candidate before approval for the general public.
Mainly two types of mRNA constructs have been evaluated: self-amplifying mRNA and non-replicating mRNA [286]. Both types of mRNAs are synthetically produced, encoding target antigens' 5′ and 3′ untranslated region, cap structure, and open-reading frame, through the use of a cell-free enzymatic transcription reaction. Self-amplifying mRNA vaccines possess genetically-engineered replication machinery that is obtained from the positive-stranded mRNA viruses [286]. Delivery of the intact mRNA vaccine from the injection site to the target cell cytosol for the initiation of protein translation is as important as manufacturing of the mRNA construct. mRNA is labile and prone to degradation primarily from nuclease activity within the cells; hence, efficient protection of the mRNA is critical during administration [285]. Lipid nanoparticles could serve as safe and compatible intracellular delivery platform for the development of successful mRNA vaccine. Lipid nanoparticles offer (i) sustained mRNA confirmation (iii) protection of mRNA cargos against nuclease degradation and; (ii) efficient cellular uptake for targeted mRNA delivery [17,276]. In the past, lipid nanocarrier system has delivered RNA for the therapeutic applications. In 2018, the first lipid nanoparticle formulated siRNA product Onpattro® was approved by the US FDA for the treatment of polyneuropathy caused by hereditary transthyretin-mediated amyloidosis, which established the standard for the clinical safety of lipid nanoparticle-based siRNA formulations [287]. Lipid nanoparticles are generally composed of ionizable lipids, cholesterol, PEGylated lipids, and phospholipids to deliver an mRNA construct. The ionizable cationic lipids create a lipid bilayer shell that allow mRNA encapsulation within an aqueous core for endosomal escape [288]. New-generation cationic lipids and lipoids are developed that maintain neutral or mild cationic charge at physiological pH to reduce nonspecific lipid-protein interactions [289]. Cholesterol provides stability to the lipid bilayer membrane and facilitates cellular transfection. PEGylated lipids serve to sterically stabilize the nanoparticles and reduce nonspecific protein bindings. Decoration of the outer surface with targerting moieties and encapsulation of multiple antigens for tailor-made immunization are additional advantages with lipid nanoparticles [290]. Several companies have used nanovaccine technology for the development of SARS-CoV-2 vaccines. Moderna, an mRNA-based biotechnology company, initiated the first mRNA nanovaccine using their patented nanovaccine technology (WO2017070626 and WO2018115527). Moderna first developed an mRNA vaccines that encodes the MERS-CoV antigens: (i) S or its fragment (S1); (ii) E, (iii) membrane (M); or (iii) nucleocapsid (N) protein. These vaccines are effective in inducing antigen-specific immune responses. Moderna encapsulated the mRNA mixture into the cationic lipid nanoparticles and intradermally injected the vaccine into mice, which lead to the encoding MERS-CoV S proteins' translation in vivo for the subsequent induction of humoral immune responses. The MERS-CoV mRNA nanovaccine encoding the full-length S protein reduced more than 90% of the viral load in the lungs and induced a significant amount of neutralizing antibody against MERS-CoV in the New Zealand white rabbits [291]. Research on MERS-CoV vaccines led to funding from CEPI (Coalition for Epidemic Preparedness Innovations) to manufacture an mRNA nanovaccine against SARS-CoV-2 (mRNA-1273). This led to the first mRNA nanovaccine to enter clinical trial (NCT04283461) for SARS-CoV-2. In mRNA-1273 vaccine, two proline amino acids were substituted at 986 and 987 positions in the S2 cleavage site to maintain stability of the prefused S mRNA [291]. The findings from the Moderna SARS-CoV-2 vaccine trial raised optimism. In the preliminary Phase 1 clinical trial, Moderna investigated a dose-escalation study of mRNA-1273 in 45 healthy adults (18 to 55 years of age), who were vaccinated at two time points, 28 days apart, with three different doses (25 μg, 100 μg or 250 μg). Commensurately, antibody responses were highest with the higher dose after the first vaccination and titers were increased after the second vaccination. Serum-neutralizing activity was detected using pseudotyped lentivirus reporter single-round-of-infection neutralization assay (PsVNA) and live wild-type SARS-CoV-2 plaque-reduction neutralization testing (PRNT) assay. No participant had measurable neutralizing antibody responses before vaccination however all participants showed higher antibody responses after second vaccination in both PsVNA and PRNT assays. mRNA-1273 stimulated Th1-biased CD4 T cell responses in all participants. From the lower dose groups (50 μg and 100 μg), all the participants showed mild or moderate side effects. However, one or more adverse events were reported in few participants from the 250 μg dose group. Similar adverse events were presented in clinical trials with high dose of avian influenza mRNA vaccine (influenza A/H10N8 and influenza A/H7N9) manufactured by Moderna's lipid nanoparticle technology [292]. The Phase 1 clinical trial was expanded to included 40 older adults of 56 to 70 years or ≥ 71 years of age who received two doses of mRNA-1273 vaccine (50 μg and 100 μg). In this study, 100 μg dose induced higher antigen-binding and neutralizing antibodies in all the older participants while the associated side effects were mainly mild or moderate [293]. In Phase 2 clinical trial, Moderna is demonstrating safety and immunogenicity of mRNA-1273 in 600 healthy participants across all age groups (above 18 years) where participants will receive either 50 μg or 100 μg dose twice at 28 days interval with follow-up for 12 months after the second vaccination (NCT04405076). Moderna has already initiated a Phase 3 clinical trial in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) in 30,000 young adult participants to test mRNA-1273 at 100 μg dosage. The primary endpoint of this study is prevention of symptomatic COVID-19 disease while the secondary endpoints include prevention of severe COVID-19 disease and infection by SARS-CoV-2 (NCT04470427) [292]. On November 15, 2020, the interim results of mRNA-1273 Phase 3 clinical trial were released that comprised 95 cases of symptomatic COVID-19 where 90 cases belongs to the placebo group and 5 cases from the vaccinated group. The vaccine was found safe and well-tolerated and showed 94.5% efficacy in studied candidates with statistical significance [294]. Based upon the promising interim results of Phase 3 trial, Moderna received the US FDA EUA of mRNA-1273 for COVID-19 treatment [295].
BioNTech and Pfizer have jointly developed BNT162(b1,b2,b3) vaccines for COVID-19 (NCT04368728). BNT162b1 is a nucleoside-modified mRNA vaccine that encodes trimerized SARS-CoV-2 receptor-binding domain (RBD) which is a key target for viral neutralizing antibodies. BNT162b2 is mRNA vaccine that encodes a membrane-anchored SARS-CoV-2 full-length S protein stabilized in the prefusion confirmation. Nucleotide modifications in the RBD and S pretein sequences increase RNA stability from enzymatic degradation and protect RNA confirmations in the native form to improve immunogenicity. Both vaccines are encapsulated in lipid nanoparticles for more efficient mRNA delivery into the target cells after intramuscular injection. The Phase 1/2 clinical trial results of BNT162b1 were available in August 2020 from 45 healthy adults of 18–55 years of age. Dose ascalation study was performed using 10 μg, 30 μg or 100 μg of BNT162b1. 10 μg and 30 μg doses were given twice at 21 days interval while 100 μg dose was given once due to increased risk of reactogenicity. Dose dependant RBD-binding IgG levels and SARS-CoV-2 neutralizing titers in the sera were detected at 21 days after the first dose that substantially increased after the second dose suggested robust immunogenicity in all participants. Geometric mean neutralizing titer levels were 1.8 to 2.8 fold higher than of COVID-19 convalescent human sera [296,297]. The extended results of the BNT162b1 Phase 1/2 clinical trial showed higer titers of broadly neutralizing antibodies in all participants with concurrent induction of RBD-specific CD4 and CD8 T cells. Surprisingly, all the participants showed Th1 biased SARS-CoV-2 immune responses after BNT162b1 immunization [298]. In a separate Phase 1 clinical trial, BNT162b2 was assessed with BNT162b1 in 195 healthy young (18 to 55 years of age) and older adults (65 to 85 years of age) at four dose levels (10 μg, 20 μg, 30 μg, and 100 μg), each injected twice 21 days apart except the highest dose. Both, BNT162b1 and BNT162b2 elicited identical dose dependant SARS-CoV-2 nutralizing antibody titers higher than the COVID-19 convalescent serum. However, BNT162b2 was associated with lower incidences and severity of adverse reactions in both young and older adults compared to the BNT162b1. Therefore, BNT162b2 was selected for the advancement in the Phase 2/3 clinical safety and efficacy assessment trials [280]. Recently, BioNTech and Pfizer concluded the Phase 3 clinical trial results with BNT162b2. The study enrolled more than 43,000 young and older adult participants in approximately 150 clinical sites across different corners of the world. The results indicated 95% efficacy of the BNT162b2 vaccine in the study participant with or without prior SARS-CoV-2 infection including adults over 65 years of age without any serious adverse reactions [299]. After the positive Phase 3 clinical trial conclusion, BNT162b2 vaccine recently received emergency approval from UK's healthcare regulator the Medicines and Healthcare Products Regulatory Agency (MHRA) for the treatment of COVID-19 [300]. BNT162b2 has also been approved for marketing in the Bahrain, Canada and USA for the COVID-19 treatment [14,301].
Acuitas Therapeutics (Vancouver) and Imperial College (London) developed a self-amplifying RNA (saRNA) lipid nanoparticles encapsulated with the pre-fusion stabilized SARS-CoV-2 S protein. They characterized both the humoral and cellular immune response, as well as the neutralization capacity of a pseudo-typed SARS-CoV-2. The mice were immunized with two injections, one month apart, at doses ranging from 0.01 μg to 10 μg. After 6 weeks, robust SARS-CoV-2 S protein specific IgG antibodies were seen in animals in a dose-dependent manner. Even at the lowest dose, saRNA nanovaccine induced higher neutralizing antibody teters in mice compared to the recovered COVID-19 patients or from other reported subunit vaccines for the MERS-CoV, SARS-CoV, and SARS-CoV-2. Higher viral neutralization, cellular responses, and antibody titers were demonstrated with saRNA nanovaccine in comparision to the electroporated plasmid DNA, the positive control, suggesting success of lipid nanoformulation for saRNA delivery [302].
Vaccines must be stored in a narrow temperature range to maintain their stability and efficacy. Cold shipment chain for the vaccine, consumes almost 80% of the total vaccine development cost which limit vaccine access in the countries with low and emerging economies [303]. To overcone these challenges, a thermostable mRNA nanovaccine was developed by the Suzhou Abogen Biosciences in collaboration with Walvax Biotechnology and People's Liberation Army Academy of Military Sciences in China for COVID-19. The vaccine candidate ARCoV was developed by encapsulating an mRNA encoding the RBD of SARS-CoV-2 S glycoprotein in the lipid nanoparticles using a preformed vesicle method [304,305]. After intramuscular injection in mice, ARCoV (30 μg) readily biodistributed in the upper abdomen 6 h post injection. At the injection site, expression of target SARS-CoV-2 RBD was colocalized with CD11b-positive monocytes, CD163-positive macrophages, and CD103-positive dendritic cells with no local inflammation, suggesting vaccine ability to recruit key APCs. ARCoV induced robust SARS-CoV-2 RBD specific IgG and neutralizing antibodies and antigen-specific T cell responses which were further elevated by ARCoV booster administration. Similar protective immune responses were also observed in non-human primates (cynomolgus monkeys) after ARCoV immunization [306,307]. ARCoV was found stable at different temperatures including 4 °C, 25 °C, and 37 °C upto a week confirming its thermostability [308].
An alphavirus-derived replicating RNA vaccine encoding the SARS-CoV-2 S protein, repRNA-CoV2S, was developed using lipid inorganic nanoparticles to enhance vaccine stability, immunogenicity, and targeted delivery. After a single or multiple intramuscular injections, repRNA-CoV2S induced robust SARS-CoV-2 S protein IgG antibodies and antigen-specific T cell responses in rodents and monkeys. Additionally, the neutralizing antibody responses were comparable to the COVID-19 convalescent plasma. The repRNA-CoV2S also induced robust immune responses in aged mice suggesting its potential for the elderly population. repRNA-CoV2S should be further evaluated in clinical studies to confirm its immunogenicity in humans [309].
7.2. Protein and subunit nanovaccine
Novavax Inc. is developing multiple nanoparticle vaccines for the COVID-19 treatment [310]. A promising candidate, recombinant SARS-CoV-2 full length S protein nanoparticle vaccine NVX-CoV2373 was generated by incorporating mutations at the S1 and S2 cleavage site to protect from proteolytic degradation and at the heptad repeat 1 site to maintain pre-fusion confugarations. NVX-CoV2373 was evaluated in Phase 1/2 clinical trials at two different dosages (5 μg and 25 μg) in 131 healthy participants, for each dose two intramuscular injections were administered 21 days apart. NVX-CoV2373 was found to be safe and elicited strong immune responses, with SARS-CoV-2 S protein specific IgG and neutralizing antibodies that exceeded the levels of COVID-19 convalescent serum. The immunogenicity was improved by the sponin-based Matrix-M1 adjuvant, which induced Th1 biased immune responses [272]. A Phase 3 clinical trial of NVX-CoV2373 in combination with influenza nanoparticles vaccine NanoFlu™, initiated in the UK after enrolling 10,000 elderly participants from 18 to 84 years of age. The study has now extended to include 30,000 participants from different parts of the world to reach better conclusions [311]. Novavax Inc. is collaborating with Takeda Pharmaceutical to establish the infrastructure and scale-up manufacturing with the aim of developing 250 million doses of the NVX-CoV2373 vaccine to fulfill global demand [312].
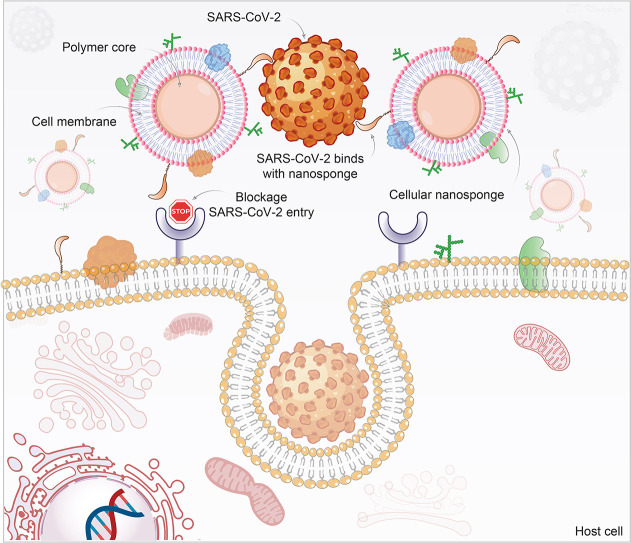
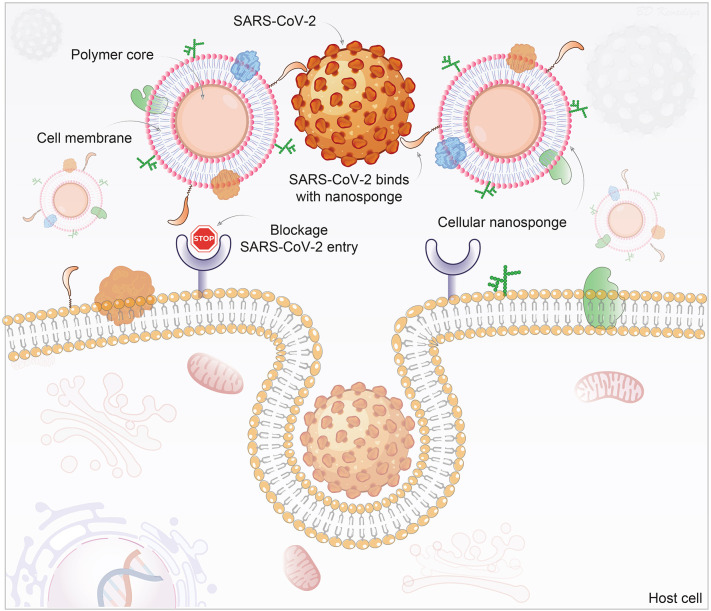
During the past months, researchers have realized challenges in the development of therapeutics and preventive measures against SARS-CoV-2 mutations [313]. To overcome virus mutations associated complications, a novel cellular nanosponge concept was introduced as an active cellular entry-level neutralizing agent against SARS-CoV-2 [314]. In this technology, nanoparticle cores were synthesized (<100 nm size) using biodegradable polymer, poly(D,L-lactic-co-glycolic acid) (PLGA), followed by their coating with the plasma membrane derived from either human lung epithelial type II cells or human macrophages. Developed nanosponges (~100 nm size) display the same protein RBD required by SARS-CoV-2 for cellular entry. Both epithelial-nanosponge and macrophage-nanosponge showed SARS-CoV-2 neutralization and protected Vero E6 cells against infection in a concentration-dependent manner. As long as the SARS-CoV-2 target remains the identified host cell, the nanosponge can neutralize the infection, thereby such platform offers (i) broad-acting countermeasures against SARS-CoV-2 mutations and (ii) protection against other emerging coronavirus species (Fig. 5 ). Both nanosponges were found safe upon intratracheal administration (300 μg) which is very important to treat respiratory diseases including COVID-19. Overall, the nanosponge platform offers significant benefits over other therapies to fight against the rapidly mutating SARS-CoV-2 [314].
Fig. 5.
Schematic of nanoparticles used as a decoy to the SARS-CoV-2 virus. Polymeric nanoparticle cores are wrapped with cell membranes derived from SARS-CoV-2 target cells, human lung epithelial type II cells, or macrophages. The inheritance of the surface antigenic profiles of the target cells allows the nanosponges to act as decoys to the circulating viruses and be independent of the status of mutation and strain. They serve to prevent virus entry to the host's natural target cells. The illustration is prepared in-house and schematic ideas and technical details were followed as presented in previously published report [314].
A recombinant SARS-CoV-2 S1 subunit nanovaccine with two different adjuvants, amphiphilic adjuvant monophosphoryl lipid A (MPLA) and CpG oligiodeoxynucleotides (ODN) is developed. In mice, it induced robust humoral immune responses and strong CD4 and CD8 T cell activation compared to the free S1 subunit vaccine with traditional alum adjuvant.
Further studies are required to assess safety and clinical translatability of this nanovaccine for the COVID-19 treatment [315]. University of Washington School of Medicine researchers are developing a novel nanovaccine platfrom where viral antigen(s) is fused on the surface of self-assembling de novo designed nanoparticles that allow unprecedented molecular configuration control of the resulting vaccine [316]. Researchers tailored self-assembling nanoparticles by presenting the ectodomains of influenza, HIV, and RSV viral glycoprotein trimers with the aim to induce better protective immune responses compared to direct antigen delivery [316]. Administration of HIV Env trimer-expressing nanoparticles in rhesus macaques showed accumulation of nanoparticles in the B cell follicles of the draining lymph nodes within two days after immunization, leading to enhanced protective immune responses [317]. As SARS-CoV-2 also exhibits trimeric S protein-like influenza, HIV, and RSV, researchers are developing a nanovaccine against COVID-19 using this technology [316].
8. Virus like particles (VLPs)
From various nanovaccine strategies, VLP is one of the preferred option with several advantages. VLPs are structurally identical to the virus particles, but lack viral genome. Therefore, VLPs are termed as non-replicating, and non-pathogenic viral capsid protein based vaccine [[318], [319], [320]]. VLPs can be either enveloped or non-enveloped. Enveloped VLPs contain viral proteins on their outer lipid membrane formed through budding from respective host cells, whereas non-enveloped VLPs lack lipid layers and are composed of one or more viral structural proteins [321]. VLPs can be decorated on their surface to display target antigen using recombinant fusion protein expression or chemical cross-linking with bifunctional linkers [322,323]. VLPs also allow encapsulation of target antigenic gene or peptide fragment for the immune cell presentation and activation. VLPs can be loaded with single or multiple capsid proteins and can be formulated in different diameters ranging from 20 to 200 nm [324,325]. VLPs hold all the conformational epitopes to the native virus to allow repetitive antigen presentation. This multi-antigen nanoassembly allow recognization of VLPs by APCs through TLRs and PRRs. Upon recognition, APCs can transport VLPs to the target tissue and difficult to reach lymph nodes where VLPs can directly bind with the B cells and with the T cells through the route of the MHC-I and MHC-II, leading to the induction of strong humoral and cellular immune responses without need of adjuvants. However, adjuvants are frequenty employed in the VLP vaccines to improve immunogenicity in the older adults [[324], [325], [326], [327]]. VLPs engineered with multiple viral antigens can provoke the immune response against multiple viral strains [328]. Recently, VLPs are developed using plug-and-play technologies such as AviTag [329] and SpyCatcher/SpyTag [330]. These technologies allow the conjugation of antigens through either reaction of biotin-streptavidin or the irreversible formation of an isopeptide. VLPs are potential vaccine candidates against diseases like malaria, dengue, and influenza [331,332]. Additionally, the recombinant VLP based nanovaccines are already commercially available to protect against hepatitis B, hepatitis E, HPV, and porcine circovirus type-2 (PCV2) [333]. Such commercialy available VLP vaccines have already set a platfrom for the future vaccine candidates developed with this technology and relaxations in their regulatory requirments are expected. VLP vaccines generally carry small size viral epitopes that accommodate within the particle and therefore loading of larget epitopes such as HIV and influenza haemagglutinin are always challenging [334].
In the past, VLP vaccines were produced from the human serum. The antigen expressing VLPs were isolated from the asymptomatic individuals with chronic viral infection. Although, the isolated VLPs were inactivated and purified, their safety is always uncertain [335]. Therefore, the eukaryotic expression systems are employed for the safe VLPs production. Recombivax HB® and Engerix-B® are recombinant VLP vaccines against HBV produced by Merck and GlaxoSmithKline, respectively. These VLP vaccines are produced from the Saccharomyces cerevisiae system that stably express hepatitis B virus surface antigen (HBsAg). The vaccines are safe and consist 20 nm size VLPs with octahedral symmetrical structure of the antigen [336]. The HPV prophylactic VLP vaccine Gardasil® is synthesized by expressing HPV L1 capsid protein in Saccharomyces cerevisiae (yeast). The HPV L1 protein self-assembles into immunogenic non-infectious VLPs with the 40–60 nm size, and can induce similar neutralizing antibody responses as the native virions [337]. Porcilis PCV® is also a safe and effective prophylactic VLP vaccine against PCV associated diseases, and is formed by the ORF2 capsid protein of PCV2 using the baculovirus expression system [338,339]. The major disadvantages of the VLP vaccines produced from the a eukaryotic expression systems are that they offer low VLPs yield and expensive to scale-up and therefore such vaccine produce only partial immunity. As an alternative, mammalian expression systems are used for cost effective and large scale VLP vaccine development with the goal to incude better immune responses [322,323]. A VLP vaccine Sci-B-Vac®, exhibiting three HBV surface antigens S, pre--S1, and pre--S2, is produced from the mammalian Chinese hamster ovary (CHO) cells produces higher antibody titers against HBV compared to the vaccines generated from the eukaryotic expression systems [340]. A hepatitis E virus (HEV) VLP vaccine Hecolin® primarily consists of hepatitis E capsid protein that self assembles into 20–30 nm particles is prepared using a cost effective recombinant Escherichia coli expression system [341]. A recombinant HPV vaccine Cecolin® is recently approved for marketing in China is also developed from Escherichia coli expression system [342]. Novavax is currently developing a VLP nanovaccine tINV against influenza H3N2 virus. A recombinant hemagglutinin (HA) trivalent nanoparticle influenza vaccine (tNIV) adjuvanted with Matrix-M, is produced using the Sf9 insect cells and recombinant baculovirus platformt. In Phase 1/2 clinical trial with 330 adults (greater than 60 years of age), tNIV induced robust neutralizing antibody response against three different influenza H3N2 virus strains [343].
Several VLP nanovaccines have been tested preclinically against betacoronaviruses. MERS-CoV VLP vaccine was prepared by assembeling viral S protein on the surface of nanoparticles (100-200 nm) secreted from the BM5 insect cells that co-express viral E and N proteins using mechanical extrusion. The resulting VLPs express all the viral components, S, E and N proteins, and able to bind human dipeptidyl peptidase 4 (DPP4) which is the target receptor for MERS-CoV host entry [344]. In another study, a chimeric VLP nanovaccine was produced by co-expressing MERS-CoV RBD and canine parvovirus (CPV) VP2 structural proteins on the surface of self-assembling spherical virus particles (~25 nm) produced from recombinant baculoviruses expression system. The VLPs have structural morphology with CPV virions and their immunization in mice produced RBD-specifc neutralizing antibodes and elicited Th1 and Th2 mediated immune responses against MERS-CoV infection [345]. A recombinant SARS-CoV VLP nanovaccine containing both the SARS-CoV S and influenza M1 proteins produced using the baculovirus Sf6 insect cell expression system was shown to elicit an immune response in mice with a reduction in lung virus titers to below detectable levels when administered intramuscularly or intranasally [346]. A VLP vaccine co-expressing the SARS-CoV S protein along with E, M, and N proteins of the mouse hepatitis virus produced from the mammalian CHO cells was found to protect mice from the SARS-CoV challenge by triggering the release of neutralizing antibodies that are responsible for viral suppression in the lungs [347]. Several VLP vaccines are currently under development as a potential treatment of COVID-19. Medicago Inc. (Quebec, Canada) announced Phase 1 clinical trial of a recombinant coronavirus like particle COVID-19 vaccine (CoVLP) (NCT04450004). The company uses VLPs that are derived from an innovative plant-based production platform. They use plants as mini-factories, which produce proteins that self-assemble into the VLPs. The Phase 1 clinical study is intended to assess the safety, tolerability, and immunogenicity of CoVLP in 180 healthy adults of 18–55 years of age. CoVLP vaccine will be administered at three dose levels (3.75 μg, 7.5 μg, and 15 μg), unadjuvanted or adjuvanted with either CpG 1018 or AS03 [348]. In preclinical testing, CoVLP vaccine induced high SARS-CoV-2 nutralizing antibody titers after a single adjuvanted dose [349,350]. Fudan University and Shanghai Jiao Tong University in china are developing COVID-19 VLP vaccine (RQ3013-VLP) that encodes mRNA of three SARS-CoV-2 structural proteins, S, M and E using the HEK293A cell platform. A single intramuscular dose of RQ3013-VLP in mice delivered 6 μg of S, 2.5 μg of M, and 1.5 μg of E mRNAs which induced higher S-protein specific binding antibodies and neutralizing antibodies and stronger CD4 and CD8 immune responses compared to the RQ3012-Spike vaccine which encodes SARS-CoV-2 S protein mRNA into the lipid nanoparticles. These results suggest VLP technology as a better platform for mRNA vaccine development compared to the traditional lipid nanoparticle formulation [351]. Although, the VLPs display repetative structures of the pathogen-derived epitopes, viral epitope sequences may not be sufficient immunogenic to elicit strong immune responses. Therefore, to increase the viral epitope density on the VLP surface, self-assembling protomers are employed during VLP production. University of Briston in collaboration with Imophoron Ltd. has developed ADDomer VLP platform which generates adenovirus-derived multimeric protein-based self-assembling VLPs to display multiple immunogenic epitopes from pathogens. First ADDomer VLP vaccine was developed against Chikungunya infectious disease and now researchers are developing COVID-19 vaccine using this technology [352,353].
9. Current SARS-CoV-2 vaccines
A year has passed since the WHO declared a global pandemic for SARS-CoV-2. The pandemic has changed a normal way of life on an international scale. This is true in both developing and developed countries and sped by the lack of preparedness. The healthcare ramifications will persist beyond the period of infection. Clinical research into treating patients infected with COVID-19 and limiting the transmission of the disease is of immediate importance. Host immune response to SARS-CoV-2 determines the pathogenesis and subsequent clinical implications of the disease, and understanding such immune effects will be critical to intervention. Before a proficient vaccine or a targeted drug is approved for use, the management of host SARS-CoV-2 immune response serves as a only viable clinical option. One of the primary aspects of such a plan is to manage the inflammatory response of the infection, especially in regards to the release of proinflammatory cytokines. [20,354]. Currently, different antiviral agents and immunomodulatory therapies are used for COVID-19-associated complications but with limited efficacy. To induce passive immunity, convalescent plasma, neutralizing antibodies, and MSCs are employed in clinics. Radiation and gene-editing technologies are under development [5,22]. In order to achieve herd immunity and eliminate COVID-19, development of successful vaccines against SARS-CoV-2 using various technologies is highly desirable and certainly worth intensive consideration (Fig. 6 ). Due to viral mutation frequencies and successive infection waves, multiple doses of SARS-CoV-2 vaccine may be necessary for prevention. Theoretically, the next two years could be vital for the elimination of SARS-CoV-2 through vaccination, as SARS-CoV-2 presumably would not have diversified into distinct lineages within this period. Thus, one vaccine strain or antigen can possibly match all lineages of SARS-CoV-2 that circulate worldwide within this timeline. Simultaneously, establishment of the natural herd immunity over the time would aid in eliminating COVID-19 disease [355].
Fig. 6.
Treatments for COVID-19. Currently, repurposed and recently approved antiviral agents are used to suppress COVID-19 disease complications that include the signs and symptoms of ARDS. A number of immunotherapies are being used and are currently being tested in randomized clinical trials of COVID-19. Vaccines are the principal challenge for SARS-CoV-2 to achieve herd immunity and eliminate SARS-CoV-2 infection and its consequences. Passive immunity can be achieved by convalescent plasma or neutralizing antibodies from recovered COVID-19 patients. Additionally, radiation and CRISPR based genome editing technologies are under development for the SARS-CoV-2 elimination.
The use of a vaccine in at-risk populations is regarded as the safest option, though despite there being a plethora of vaccines in clinical development Phases, no vaccine has yet to achieve full US FDA approval [12]. Such a vaccine would protect at-risk individuals as well as curb the transmission of disease.
Beyond the challenges of vaccine development and delivery, each vaccine requires rigorous testing to not only prove its' efficacy, but also to demonstrate its safety across mixed population. The WHO's criteria for vaccine prospects in Phase 2b/3 trials includes the vaccine's safety profile, potential for efficacy, stability, implementation, and availability. The specific criteria presented also revealed limitations and strengths of the different vaccines [10,12]. The global nature of the pandemic and the disease's transmission are pushing such criteria to a more rigorous standard including, if at all possible, a single booster dose vaccination. The dosage will be critical as it will need to be potent enough to provide immune system priming, and thus control any viral infection. Yet the vaccine's dosage must accommodate the global population with minimal-to-no deleterious effects. Finally, the stability of the vaccine must be robust to handle different environments and temperatures without losing efficacy. Different trials are ongoing using a broad array of vaccines, each with their respective strengths and weaknesses [15,16].
Currently, approximately 120 candidates are under development using different technologies, some of which have not been used as a licensed-vaccine before. The approaches being used include the use of nucleic acids (DNA or RNA), inactivated or live attenuated virus, viral vectors, and recombinant proteins or VLPs [16,116]. Different vaccine candidates being developed using said technologies are summarized in Table 1 . Challenges to develop an effective vaccine consist of technical barriers, such as the choice of proteins or domains that would provoke more protective antibodies, prior exposure to other viruses which impairs immunogenicity in the viral vector vaccine, need for an adjuvant, effectiveness in elderly patients, feasibility of large-scale production, regulation (e.g., ensuring safety and effectiveness), and legal barriers and issues (e.g., technology transfer and licensing agreements) including the potential duration of immunity as well as the number of vaccine doses needed to confer immunity. Other approaches for prevention are also emerging, which include hyperimmune globulin, monoclonal antibodies, and convalescent plasma. If they prove to be effective, these approaches could be used in high-risk individuals, including health care workers and older adults [10,118]. Regeneron's anti-SARS-CoV-2 antibody cocktail has already received EUA from the US FDA [66].
Table 1.
| # | Vector | Organizations | Formulation candidate | Trial phase |
|---|---|---|---|---|
| 1 | Viral | Johnson & Johnson, Janssen Pharmaceutical Companies + Beth Israel Deaconess Medical Center (Harvard Medical School), Emergent BioSolutions, Catalent |
Ad26 (alone or with MVA boost) |
Phase 1 (NCT04509947) Phase 1–2 (NCT04436276) Phase 2 (NCT04535453) Phase 3 (NCT04505722) Phase 3 (NCT04614948) Name: AdVac®Ad26.COV2-S |
| Geovax Labs and BravoVax |
MVA encoded VLP |
Pre-Clinical Name: GV-MVA-VLP™ |
||
| Jenner Institute (University of Oxford) + Cobra Biologics + Oxford Biomedica + Merck KGaA + Halix BV + Pall Corporation + SGS + India's Serum Institute + AstraZeneca + Catalent Biologics and CSL Limited [18] |
AZD 1222 |
Phase 1/2 (NCT04324606, not yet recruiting) Phase 1–2 (UK) (2020–001072-15) Phase 1–2 (South Africa) (PACTR202006922165132) Phase 1–2 (Japan) (NCT04568031, Active, not recruiting) Phase 2b-3 (UK) (2020–001228-32) Phase 3 (Brazil) ISRCTN89951424 Phase 3 (USA) NCT04516746 Phase 3 (India) CTRI/2020/08/027170 |
||
| Tonix Pharmaceuticals and Southern Research |
Horsepox vector expressing S protein |
Pre-Clinical Name: TNX-1800 |
||
| Altimmune + University of Alabama + DynPort Vaccine Company |
Adenovirus based NasoVAX expressing SARS2-CoV spike protein |
Pre-Clinical Name: AdCOVID™ and T-COVID™ |
||
| Greffex |
Ad5 S (GREVAX™ platform) |
Pre-Clinical Name: GreVac™ |
||
| Vaxart + emergent BioSolutions + KindredBio |
VAAST Oral Vaccine platform |
Phase 1 NCT04563702 |
||
| Gamaleya Research Institute |
Adeno-based – sputnik V |
Phase 1 (NCT04436471, NCT04437875) Phase 2 NCT04587219 Phase 2–3 NCT04640233 (Not yet recruiting) Phase 3 (Belarus) NCT04564716 (Not yet recruiting) Phase 3 (Russia) NCT04530396 Phase 3 (Vnezuela) NCT04642339 (Not yet recruiting) |
||
| CanSino Biologics + Beijing Institute of BioTech + Canadian Center for Vaccinology at Dalhousie University + Precision NanoSystems + Petrovax [188,189] |
Adenovirus Type 5 Vector |
Phase 1 (ChiCTR2000030906) Phase 2 (ChiCTR2000031781) Phase 1 NCT04568811 Phase 2 NCT04566770 Phase 3 NCT04526990 Phase 3 NCT04540419 |
||
| Zydus Cadila Healthcare Ltd. |
DNA plasmid vaccine + Adjuvant |
Phase 1–2 (CTRI/2020/07/026352) |
||
| Institut Pasteur + Themis + University of Pittsburgh + Merck |
Measles Vector |
Phase 1 (NCT04497298) |
||
| ReiThera + Leukocare + Univercells |
Replication defective Simian Adenovirus (GRAd) encoding SARSCoV-2 S |
Phase 1 NCT04528641 |
||
| Centro Nacional Biotecnologia (CNB-CSIC), Spain |
MVA expressing structural proteins |
Pre-Clinical |
||
| University of Manitoba |
Dendritic cellbased vaccine |
Pre-Clinical |
||
| Bharat Biotech + Thomas Jefferson University |
Recombinant deactivated rabies virus |
Pre-Clinical |
||
| BiOCAD and IEM | Live viral vectored vaccine based on attenuated influenza virus backbone (intranasal) | Pre-Clinical | ||
| 2 |
DNA |
Inovio Pharmaceuticals + Beijing Advaccine Biotechnology + Ology Bioservices + VGXI + Richter-Helm + Thermo Fisher Scientific |
INO-4800 DNA with electroporation |
Phase 1 (USA) (NCT04336410) Phase 1–2 (South Korea) (NCT04447781) Phase 2–3 (USA) NCT04642638 |
| Osaka University + AnGes + Takara Bio + Cytiva + Brickell Biotech |
DNA Plasmid |
Phase 1–2 (NCT04463472) JapicCTI-205,328 Title: AG0301-COVID19 Phase 1–2 NCT04527081 |
||
| Applied DNA Sciences + Takis Biotech + Evvivax |
Linear DNA |
Pre-Clinical |
||
| Zydus Cadila |
DNA plasmid vaccine |
Phase 1–2 (CTRI/2020/07/026352) |
||
| Genexine Inc. + Binex + GenNBio + International Vaccine Institute + Korea Advanced Institute of Science and Technology + Pohang University of Science and Technology + PT Kalbe Pharma |
GX-19 DNA |
Phase 1/2a (NCT04445389) |
||
| Symvivo + Nucleus Network |
DNA BacTRL-Spike (oral) |
Phase 1 (NCT04334980) |
||
| CureVac |
mRNA |
Phase 1 (NCT04449276) Phase 2 (NCT04515147) |
||
|
3 |
RNA |
Moderna + NIAID + Lonza + Catalent Inc. + BIOQUAL [292] |
mRNA-1273 LNPencapsulated mRNA |
Phase 1 (NCT04283461) Phase 2 (NCT04405076) Phase 3 (NCT04470427) |
| BioNTech + Pfizer + Fosun Pharma + University of Rochester Medical Center (URMC) + Rochester Regional Health (RRH) [357] |
mRna BNT162b2 |
Phase 1–2 (UTRN U1111-1249-4220) Phase 1–2 (Germany) NCT04537949 EudraCT Number (Germany) (2020–001038-36) Phase 2–3 (USA) (NCT04368728) Phase 1–2 (ChiCTR2000034825) Phase 1 (Japan) NCT04588480 |
||
| Tongji University and Chinese Center for Disease Control and Prevention + Stemirna Therapeutics |
mRNA |
Pre-Clinical |
||
| Imperial College London |
saRNA |
Phase 1 (ISRCTN17072692) |
||
| Codagenix + Serum Institute of India |
Codon deoptimized live attenuated |
Phase 1 NCT04619628 |
||
| Arcturus Therapeutics + Duke-NUS |
mRNA Lunar-Cov19 |
Phase 1–2 (NCT04480957) |
||
| People's Liberation Army Academy of Military Sciences + Walvax Biotech |
mRNA |
Phase 1 (ChiCTR2000034112) |
||
| Sanofi Pasteur + Translate Bio |
mRNA |
Pre-Clinical |
||
|
4 |
Live |
German Center for Infection Research (DZIF) + CanVirex AG |
Measles Virus (S, N targets) |
Pre-Clinical |
| German Center for Infection Research (DZIF) + Ludwig-Maximilians University of Munich + IDT Biologika GmbH + Universitätsklinikum Hamburg-Eppendorf + Philipps University Marburg Medical Center |
MVA-S-encoded |
Phase 1 NCT04569383 |
||
| Novavax + Emergent BioSolutions + FUJIFILM Diosynth Biotechnologies + Serum Institute of India |
NVX-CoV2373 Recombinant glycopreotein |
Phase 1 (NCT04368988) Phase 2 (NCT04533399) Phase 3 (UK) (2020–004123-16) Phase 3 (USA/Mexico) NCT04611802 |
||
| 5 | Protein | Clover Biopharmaceuticals + GSK + Dynavax |
Native like Trimeric subunit Spike Protein |
Phase 1 (NCT04405908) |
| Baylor College of Medicine + Texa Children's Hospital + PATH |
S1 or RBD protein |
Pre-Clinical |
||
| Fudan University + Shanghai JiaoTong University + RNACure Biopharma |
LNP encapsulated mRNA cocktail encoding RBD |
Pre-Clinical |
||
| VIDO-InterVac + University of Saskatchewan, Canada |
Adjuvanted microsphere peptide |
Pre-Clinical |
||
| University of Queensland, Australia + Dynavax + GSK + Viroclinics Xplore + CSL Limited |
Molecular clamp stabilized Spike protein |
Phase 1 (ACTRN12620000674932) Phase 1 (ISRCTN51232965) |
||
| Vaxart + Emergent BioSolutions + KindredBio |
VAAST Oral Vaccine platform |
Phase 1 (NCT04563702) |
||
| Generex/ EpiVax | Ii-Key peptide | Pre-Clinical | ||
| ExpreS2ion |
Drosophila S2 insect cell expression system VLPs |
Pre-Clinical |
||
| Vaxil Bio |
Peptide |
Pre-Clinical |
||
| Sanofi Pasteur + GSK |
S protein (baculovirus production) |
Phase 1–2 (NCT04537208) |
||
| iBio/CC-Pharming |
Subunit protein, plant produced |
Pre-Clinical |
||
| Saiba + University of Bern |
RBD displayed on virus-like particles |
Pre-Clinical |
||
| Covaxx + university of Nebraska Medical Center (UNMC) + DASA |
Multitope Peptide-Based Vaccine (MVP) |
Phase 1 (NCT04545749) |
||
|
6 |
VLP |
Imophoron + Bristol University |
VLP ADDomerTM multiepitope display |
Pre-Clinical |
| Fundan University + Shanghai JiaoTong University + RNACure Biopharma |
LNP encapsulated mRNA cocktail encoding VLP |
Pre-Clinical |
||
| Medicago Inc. |
Plant-derived VLP |
Phase 1 (NCT04450004) Phase 2–3 (NCT04636697) |
||
|
7 |
Inactivated or Attenuated |
Beijing Institute of BioProducts (Sinopharm) + Chinese Center for Disease Control and Prevention |
Inactivated |
Phase 1–2 (ChiCTR2000032459) Phase 3 (ChiCTR2000034780) |
| Wuhan Institute of Biological Products/Sinopharm [140] |
Inactivated |
Phase 1–2 (ChiCTR2000031809) Phase 3 (ChiCTR2000034780) Phase 3 (NCT04560881) |
||
| Institute of Medical Biology + Chinese Academy of Medical Sciences |
Inactivated |
Phase 1 (NCT04412538) Phase 1–2 (NCT04470609) |
||
| Sinovac + Instituto Butatan |
Inactivated + alum CoronaVac |
Phase 1–2 (Jiangsu) (NCT04352608) Phase 1–2 (Hebei) (NCT04383574) Phase 1–2 (Hebei children) (NCT04551547) Phase 3 (Brazil) (NCT04456595) Phase 3 (Turkey) (NCT04582344) Phase 3 (Indonesia) (669/UN6.KEP/EC/2020) Phase 3 (China) (NCT04617483) |
||
| 8 | Uncatorgized | Shenzhen GenoImmune Medical Institute |
Pathogen-specific aAPC |
Phase 1 (NCT04299724) |
| Shenzhen GenoImmune Medical Institute | LV-SMENP-DC | Phase 1 (NCT04276896) | ||
According to the WHO [12], more than thirty vaccine candidates are in Phase 2/3 clinical trials, two are approved in UK, USA, UAE, and Canada, as of December 15, 2020. From different clinical trial candidates, Sinovac, Wuhan Institute, and Beijing Institute are using the inactivated virus as their platforms. Moderna and Pfizer/BioNTech are using mRNA-based platforms, while Oxford University is using its proprietary nonreplicating chimpanzee adenovirus platform. CanSino Biologics Inc. is using a nonreplicating adenovirus type 5 vector platform, while Anhui Zhifel Longcom Biopharmaceutical is using a protein subunit platform. All of them intend to use intramuscular injection as the route of administration. Codiak BioSciences Inc. collaborated with Ragon Institute is investigating the exosome-based vaccine. With all the COVID-19 vaccine candidates, induction of robust neutralizing antibodies and antigen-specific T cell responses in the lung and other mucosal surfaces are expected for complete elimination of SARS-CoV-2 [356].
10. Summary
Mass immunization through vaccination stands as the highest priority for control of the SARS-CoV-2 pandemic. Significant efforts are underway to complete this task and by the time of this publication, this goal will be on its ways toward a successful conclusion. Understandably, critical needs will remain and include how effective the chosen vaccine in the longer term and how to preclude adverse reactions. What underlies the immunological profiles for protective immunity and how long-lasting that immunity will be. Nonetheless, until these questions are addressed and a vaccine comes available parallel immune-based and pharmacological therapeutic options will be required to move forward with due diligence. While a many divergent vaccines and therapies are in the offering, full implementation is dependent on carefully-crafted clinical trial results and delivery. The bar to clear approvals for this task is high. Obstacles include product safety, efficacy, and robust sustained antiviral immune responses. The results of the ongoing vaccine efforts designed to eliminate SARS-CoV-2 infection will prove invaluable.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
Acknowledgements
We thank Doug Meigs, University of Nebraska Medical Center, for critical review of the manuscript. This work was supported by the National Institutes of Health R01 MH121402-01A1; R01 MH121402P01; R01 AG043540, R01 AG043530, P01 DA028555, P30 MH062261, R01 MH115860, R01 NS034249, R01 NS036126, the Carol Swartz Emerging Neuroscience Fund and the Margaret R. Larson Professorship.
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [Google Scholar]
- 4.Lange S.J., Ritchey M.D., Goodman A.B., Dias T., Twentyman E., et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions — United States. MMWR Morb. Mortal. Wkly Rep. 2020;69:795–800. doi: 10.15585/mmwr.mm6925e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machhi J., Herskovitz J., Senan A.M., Dutta D., Nath B., et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. NeuroImmune Pharmacol. 2020;15:359–386. doi: 10.1007/s11481-020-09944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X., Wang T., Cai D., Hu Z., Chen J., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., Wang J., Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golonka R.M., Saha P., Yeoh B.S., Chattopadhyay S., Gewirtz A.T., Joe B., Vijay-Kumar M. Harnessing innate immunity to eliminate SARS-CoV-2 and ameliorate COVID-19 disease. Physiol. Genomics. 2020;52:217–221. doi: 10.1152/physiolgenomics.00033.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. (e1039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and immunotherapeutics. Signal. Transduct. Target Ther. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://covidvax.org/
- 12.World Health Organization . 2020. Draft landscape of COVID-19 candidate vaccines.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Google Scholar]
- 13.McKinsey & Company On Pins and Needles: Will COVID-19 Vaccines ‘Save the World’? 2020. https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/on-pins-and-needles-will-covid-19-vaccines-save-the-world
- 14.U.S. Food and Drug Administration FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
- 15.Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- 16.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 17.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski J.K., Steinmetz N.F. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 18.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV), Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 20.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 21.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owji H., Negahdaripour M., Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020;88:106924. doi: 10.1016/j.intimp.2020.106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrugia G., Plutowski R.W. Innovation lessons from the COVID-19 pandemic. Mayo Clin. Proc. 2020;95:1574–1577. doi: 10.1016/j.mayocp.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 25.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theerawatanasirikul S., Kuo C.J., Phetcharat N., Lekcharoensuk P. In silico and in vitro analysis of small molecules and natural compounds targeting the 3CL protease of feline infectious peritonitis virus. Antivir. Res. 2020;174:104697. doi: 10.1016/j.antiviral.2019.104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen M.S. Hydroxychloroquine for the prevention of Covid-19 - searching for evidence. N. Engl. J. Med. 2020;383:585–586. doi: 10.1056/NEJMe2020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khuroo M.S. Chloroquine and hydroxychloroquine in coronavirus disease 2019 (COVID-19). Facts, fiction and the hype: a critical appraisal. Int. J. Antimicrob. Agents. 2020;56:106101. doi: 10.1016/j.ijantimicag.2020.106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yazdany J., Kim A.H.J. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann. Intern. Med. 2020;172:754–755. doi: 10.7326/M20-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotteau V., Teyton L., Peleraux A., Nilsson T., Karlsson L., Schmid S.L., Quaranta V., Peterson P.A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 32.Wu S.F., Chang C.B., Hsu J.M., Lu M.C., Lai N.S., Li C., Tung C.H. Hydroxychloroquine inhibits CD154 expression in CD4(+) T lymphocytes of systemic lupus erythematosus through NFAT, but not STAT5, signalling. Arthritis Res. Ther. 2017;19:183. doi: 10.1186/s13075-017-1393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Borne B.E., Dijkmans B.A., de Rooij H.H., le Cessie S., Verweij C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 34.Kuznik A., Bencina M., Svajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 35.Ewald S.E., Lee B.L., Lau L., Wickliffe K.E., Shi G.P., Chapman H.A., Barton G.M. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hacker H., Mischak H., Miethke T., Liptay S., Schmid R., Sparwasser T., Heeg K., Lipford G.B., Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vollmer J., Tluk S., Schmitz C., Hamm S., Jurk M., Forsbach A., Akira S., Kelly K.M., Reeves W.H., Bauer S., Krieg A.M. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An J., Woodward J.J., Sasaki T., Minie M., Elkon K.B. Cutting edge: antimalarial drugs inhibit IFN-beta production through blockade of cyclic GMP-AMP synthase-DNA interaction. J. Immunol. 2015;194:4089–4093. doi: 10.4049/jimmunol.1402793. [DOI] [PubMed] [Google Scholar]
- 39.Cook K.L., Warri A., Soto-Pantoja D.R., Clarke P.A., Cruz M.I., Zwart A., Clarke R. Hydroxychloroquine inhibits autophagy to potentiate antiestrogen responsiveness in ER+ breast cancer. Clin. Cancer Res. 2014;20:3222–3232. doi: 10.1158/1078-0432.CCR-13-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mocholi E., Dowling S.D., Botbol Y., Gruber R.C., Ray A.K., Vastert S., Shafit-Zagardo B., Coffer P.J., Macian F. Autophagy is a tolerance-avoidance mechanism that modulates TCR-mediated signalling and cell metabolism to prevent induction of T cell anergy. Cell Rep. 2018;24:1136–1150. doi: 10.1016/j.celrep.2018.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Food and Drug Administration Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
- 42.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao B., Wang Y., Wen D., Liu W., Wang J., et al. A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao T.T., Qian J.D., Zhu W.Y., Wang Y., Wang G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020;92:556–563. doi: 10.1002/jmv.25729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Buyck C., Van Loock M., Woodfall B., Ciesek S. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. J. Med. Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 47.Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med. Infect. Dis. 2020;34:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., et al. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y., Zhang D., Du G., Du R., Zhao J., et al. Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Self W.H., Semler M.W., Leither L.M., Casey J.D., Angus D.C., et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324:2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shannon A., Selisko B., Le N.T., Huchting J., Touret F., Piorkowski G., Fattorini V., Ferron F., Decroly E., Meier C., Coutard B., Peersen O., Canard B. Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat. Commun. 2020;11:4682. doi: 10.1038/s41467-020-18463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020;76:370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Ju L., Zhang J., Wang X. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 58.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rokni M., Ghasemi V., Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: Comparison with SARS and MERS. Rev. Med. Virol. 2020;30 doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Market M., Angka L., Martel A.B., Bastin D., Olanubi O., Tennakoon G., Boucher D.M., Ng J., Ardolino M., Auer R.C. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front. Immunol. 2020;11:1512. doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suvakov S., Richards C., Nikolic V., Simic T., McGrath K., Krasnodembskaya A., McClements L. Emerging therapeutic potential of mesenchymal stem/stromal cells in preeclampsia. Curr. Hypertens. Rep. 2020;22:37. doi: 10.1007/s11906-020-1034-8. [DOI] [PubMed] [Google Scholar]
- 63.Bari E., Ferrarotti I., Saracino L., Perteghella S., Torre M.L., Corsico A.G. Mesenchymal stromal cell secretome for severe COVID-19 infections: premises for the therapeutic use. Cells. 2020;9 doi: 10.3390/cells9040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu S.T.H., Lin H.M., Baine I., Wajnberg A., Gumprecht J.P., et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat. Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 65.Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;323:1561–1562. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 66.BioPharma-Reporter Regeneron's COVID-19 Antibody Cocktail Receives EUA from FDA. 2020. https://www.biopharma-reporter.com/Article/2020/11/23/Regeneron-s-COVID-19-antibody-cocktail-receives-EUA-from-FDA
- 67.Soo Y.O., Cheng Y., Wong R., Hui D.S., Lee C.K., et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin. Microbiol. Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J., Hu C., Chen L., Tang L., Zhu Y., Xu X., Chen L., Gao H., Lu X., Yu L., Dai X., Xiang C., Li L. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering (Beijing) 2020;6:1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gladstone D.E., Kim B.S., Mooney K., Karaba A.H., D'Alessio F.R. Regulatory T cells for treating patients with COVID-19 and acute respiratory distress syndrome: two case reports. Ann. Intern. Med. 2020;173:852–853. doi: 10.7326/L20-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abbasi J. Regulatory T cells tested in patients with COVID-19 ARDS. JAMA. 2020;324:539. doi: 10.1001/jama.2020.13765. [DOI] [PubMed] [Google Scholar]
- 71.PR Newswire, Cellenkos Inc Focuses on the Development of Cell-Therapy for Treatment of COVID-19 Mediated Acute Respiratory Distress Syndrome (CoV-ARDS) 2020. https://www.prnewswire.com/news-releases/cellenkos-inc-focuses-on-the-development-of-cell-therapy-for-treatment-of-covid-19-mediated-acute-respiratory-distress-syndrome-cov-ards-301025278.html
- 72.Yip M.S., Leung H.L., Li P.H., Cheung C.Y., Dutry I., Li D., Daeron M., Bruzzone R., Peiris J.S., Jaume M. Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS. Hong Kong Med. J. 2016;22:25–31. [PubMed] [Google Scholar]
- 73.Salome B., Magen A. Dysregulation of lung myeloid cells in COVID-19. Nat. Rev. Immunol. 2020;20:277. doi: 10.1038/s41577-020-0303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burmester G.R., Rigby W.F., van Vollenhoven R.F., Kay J., Rubbert-Roth A., Kelman A., Dimonaco S., Mitchell N. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomized controlled trial. Ann. Rheum. Dis. 2016;75:1081–1091. doi: 10.1136/annrheumdis-2015-207628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yokota S., Itoh Y., Morio T., Origasa H., Sumitomo N., Tomobe M., Tanaka K., Minota S. Tocilizumab in systemic juvenile idiopathic arthritis in a real-world clinical setting: results from 1 year of postmarketing surveillance follow-up of 417 patients in Japan. Ann. Rheum. Dis. 2016;75:1654–1660. doi: 10.1136/annrheumdis-2015-207818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan F., Fabbri L., Stewart I., Robinson K., Smyth A.R., Jenkins G. A systematic review of Anakinra, Tocilizumab, Sarilumab and Siltuximab for coronavirus-related infections. medRxiv. 2020 doi: 10.1101/2020.04.23.20076612. [DOI] [Google Scholar]
- 77.Luo W., Li Y.X., Jiang L.J., Chen Q., Wang T., Ye D.W. Targeting JAK-STAT signalling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 2020;41:531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schett G., Sticherling M., Neurath M.F. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immunol. 2020;20:271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kleo Pharmaceuticals Kleo Pharmaceuticals Enters into Research Collaboration with Green Cross LabCell (GCLC) To Rapidly Develop COVID-19-Targeting Allogeneic NK Cell Combination Therapy. 2020. https://www.kleopharmaceuticals.com/kleo-pharmaceuticals-enters-into-research-collaboration-with-green-cross-labcell-gclc-to-rapidly-develop-covid-19-targeting-allogeneic-nk-cell-combination-therapy/
- 82.Korsukewitz C., Reddel S.W., Bar-Or A., Wiendl H. Neurological immunotherapy in the era of COVID-19 - looking for consensus in the literature. Nat. Rev. Neurol. 2020;16:493–505. doi: 10.1038/s41582-020-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwok K.O., Lai F., Wei W.I., Wong S.Y.S., Tang J.W.T. Herd immunity - estimating the level required to halt the COVID-19 epidemics in affected countries. J. Inf. Secur. 2020;80:e32–e33. doi: 10.1016/j.jinf.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palese P., Wang T.T. Why do influenza virus subtypes die out? A hypothesis. mBio. 2011;2 doi: 10.1128/mBio.00150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu S., Zhuang Q., Wang S., Jiang W., Jin J., Peng C., Hou G., Li J., Yu J., Yu X., Liu H., Sun S., Yuan L., Chen J. Control of avian influenza in China: Strategies and lessons. Transbound. Emerg. Dis. 2020;67:1463–1471. doi: 10.1111/tbed.13515. [DOI] [PubMed] [Google Scholar]
- 87.Zeng X., Tian G., Shi J., Deng G., Li C., Chen H. Vaccination of poultry successfully eliminated human infection with H7N9 virus in China. Sci. China Life Sci. 2018;61:1465–1473. doi: 10.1007/s11427-018-9420-1. [DOI] [PubMed] [Google Scholar]
- 88.Aschwanden C. The false promise of herd immunity for COVID-19. Nature. 2020;587:26–28. doi: 10.1038/d41586-020-02948-4. [DOI] [PubMed] [Google Scholar]
- 89.Anderson R.M., May R.M. Vaccination and herd immunity to infectious diseases. Nature. 1985;318:323–329. doi: 10.1038/318323a0. [DOI] [PubMed] [Google Scholar]
- 90.Mallory M.L., Lindesmith L.C., Baric R.S. Vaccination-induced herd immunity: Successes and challenges. J. Allergy Clin. Immunol. 2018;142:64–66. doi: 10.1016/j.jaci.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Syal K. COVID-19: Herd immunity and convalescent plasma transfer therapy. J. Med. Virol. 2020;92:1380–1382. doi: 10.1002/jmv.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferguson N., Laydon D., Nedjati Gilani G., Imai N., Ainslie K., et al. Report 9: Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. Imperical College London. 2020:1–20. [Google Scholar]
- 94.Kavaliunas A., Ocaya P., Mumper J., Lindfeldt I., Kyhlstedt M. Swedish policy analysis for Covid-19. Health Policy Technol. 2020;9:598–612. doi: 10.1016/j.hlpt.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rubin R. Difficult to determine herd immunity threshold for COVID-19. JAMA. 2020;324:732. doi: 10.1001/jama.2020.14778. [DOI] [PubMed] [Google Scholar]
- 96.Mo H., Zeng G., Ren X., Li H., Ke C., Tan Y., Cai C., Lai K., Chen R., Chan-Yeung M., Zhong N. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirology. 2006;11:49–53. doi: 10.1111/j.1440-1843.2006.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang F., Quan Y., Xin Z.T., Wrammert J., Ma M.J., Lv H., Wang T.B., Yang H., Richardus J.H., Liu W., Cao W.C. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J. Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 98.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao J., Yuan Q., Wang H., Liu W., Liao X., et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu F., Wang A., Liu M., Wang Q., Chen J., et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 101.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moore J.L., Ganapathiraju P.V., Kurtz C.P., Wainscoat B. A 63-year-old woman with a history of non-hodgkin lymphoma with persistent SARS-CoV-2 infection who was seronegative and treated with convalescent plasma. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Madan M., Kunal S. COVID-19 reinfection or relapse: an intriguing dilemma. Clin. Rheumatol. 2020;39:3189. doi: 10.1007/s10067-020-05427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seow J., Graham C., Merrick B., Acors S., Pickering S., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang M., Li M., Ren R., Li L., Chen E.Q., Li W., Ying B. International expansion of a novel SARS-CoV-2 mutant. J. Virol. 2020;94 doi: 10.1128/JVI.00567-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.World Health Organization Q&A: Influenza and COVID-19 - Similarities and Differences. 2020. https://www.who.int/westernpacific/news/q-a-detail/q-a-similarities-and-differences-covid-19-and-influenza
- 107.Centers for Disease Control & Prevention Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States — 2019–2020 Influenza Season. 2020. https://www.cdc.gov/flu/about/burden/2019-2020.html
- 108.CNN More People Have Died from Covid-19 than in the Past 5 Flu Seasons Combined. And Coronavirus is Much More Contagious. 2020. https://www.cnn.com/2020/10/06/health/flu-covid-19-deaths-comparison-trnd/index.html
- 109.Biswas A., Bhattacharjee U., Chakrabarti A.K., Tewari D.N., Banu H., Dutta S. Emergence of novel coronavirus and COVID-19: whether to stay or die out? Crit. Rev. Microbiol. 2020;46:182–193. doi: 10.1080/1040841X.2020.1739001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Janeway C.A., Jr., Travers P., Walport M. Infectious Agents and How They Cause Disease. 5th ed. Garland Science; New York: 2001. Immunobiology: the immune system in health and disease; pp. 455–465. [Google Scholar]
- 111.Alberts B., Johnson A., Lewis J. 4th ed. Garland Science; New York: 2002. Molecular Biology of the Cell. Pathogens and Infection; pp. 1263–1268. [Google Scholar]
- 112.Louten J. In: Essential Human Virology. Louten J., editor. Academic Press; Boston: 2016. Chapter 2 - virus structure and classification; pp. 19–29. [Google Scholar]
- 113.Afrough B., Dowall S., Hewson R. Emerging viruses and current strategies for vaccine intervention. Clin. Exp. Immunol. 2019;196:157–166. doi: 10.1111/cei.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fauci A.S., Marston H.D. PUBLIC HEALTH. Toward an HIV vaccine: a scientific journey. Science. 2015;349:386–387. doi: 10.1126/science.aac6300. [DOI] [PubMed] [Google Scholar]
- 115.Peeples L., Feature News. Avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proc. Natl. Acad. Sci. U. S. A. 2020;117:8218–8221. doi: 10.1073/pnas.2005456117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thanh Le T., Andreadakis Z., Kumar A., Gomez Roman R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 117.Pandey S.C., Pande V., Sati D., Upreti S., Samant M. Vaccination strategies to combat novel corona virus SARS-CoV-2. Life Sci. 2020;256:117956. doi: 10.1016/j.lfs.2020.117956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buckland B.C. The process development challenge for a new vaccine. Nat. Med. 2005;11:S16–S19. doi: 10.1038/nm1218. [DOI] [PubMed] [Google Scholar]
- 119.Hajj Hussein I., Chams N., Chams S., El Sayegh S., Badran R., Raad M., Gerges-Geagea A., Leone A., Jurjus A. Vaccines through centuries: major cornerstones of global health. Front. Public Health. 2015;3:269. doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Delany I., Rappuoli R., De Gregorio E. Vaccines for the 21st century. EMBO Mol. Med. 2014;6:708–720. doi: 10.1002/emmm.201403876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levine M.M., Woodrow G.C., Kaper J.B., Cobon G.S., editors. New Generation Vaccines. 2nd ed. Marcel Dekker; New York: 1997. [Google Scholar]
- 122.Salisbury D., Ramsay M., Noakes K., editors. Immunization Against Infectious Disease. Green Book; 2006. [Google Scholar]
- 123.Jang Y.H., Seong B.L. Principles underlying rational design of live attenuated influenza vaccines. Clin. Exp. Vaccine Res. 2012;1:35–49. doi: 10.7774/cevr.2012.1.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee N.H., Lee J.A., Park S.Y., Song C.S., Choi I.S., Lee J.B. A review of vaccine development and research for industry animals in Korea. Clin. Exp. Vaccine Res. 2012;1:18–34. doi: 10.7774/cevr.2012.1.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hanley K.A. The double-edged sword: How evolution can make or break a live-attenuated virus vaccine. Evolution. 2011;4:635–643. doi: 10.1007/s12052-011-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Minor P.D., vaccines Live attenuated. Historical successes and current challenges. Virology. 2015;479–480:379–392. doi: 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 127.Clem A.S. Fundamentals of vaccine immunology. J. Global Infect. Dis. 2011;3:73–78. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dudek T., Knipe D.M. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344:230–239. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 129.Loudon P.T., Blakeley D.M., Boursnell M.E., Day D.A., Duncan I.A., Lowden R.C., McLean C.S., Martin G., Miller J.C., Shaw M.L. Preclinical safety testing of DISC-hGMCSF to support phase I clinical trials in cancer patients. J. Gene Med. 2001;3:458–467. doi: 10.1002/jgm.206. [DOI] [PubMed] [Google Scholar]
- 130.Nguyen L.H., Knipe D.M., Finberg R.W. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J. Virol. 1992;66:7067–7072. doi: 10.1128/jvi.66.12.7067-7072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morrison L.A., Knipe D.M. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology. 1996;220:402–413. doi: 10.1006/viro.1996.0328. [DOI] [PubMed] [Google Scholar]
- 132.Da Costa X., Kramer M.F., Zhu J., Brockman M.A., Knipe D.M. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J. Virol. 2000;74:7963–7971. doi: 10.1128/jvi.74.17.7963-7971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gomez Lorenzo M.M., Fenton M.J. Immunobiology of influenza vaccines. Chest. 2013;143:502–510. doi: 10.1378/chest.12-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pulendran B., Ahmed R. Immunological mechanisms of vaccination. Nat. Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Le Nouen C., Collins P.L., Buchholz U.J. Attenuation of human respiratory viruses by synonymous genome recoding. Front. Immunol. 2019;10:1250. doi: 10.3389/fimmu.2019.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meng J., Lee S., Hotard A.L., Moore M.L. Refining the balance of attenuation and immunogenicity of respiratory syncytial virus by targeted codon deoptimization of virulence genes. mBio. 2014;5 doi: 10.1128/mBio.01704-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.First Ebola vaccine approvedNat. Biotechnol. 2020;38:6. doi: 10.1038/s41587-019-0385-7. [DOI] [PubMed] [Google Scholar]
- 138.Pharmaceutical Technology Indian Immunologicals, Griffith University to Develop Covid-19 Vaccine. 2020. https://www.pharmaceutical-technology.com/news/indian-immunologicals-covid-19-vaccine/
- 139.Ulmer J.B., Valley U., Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat. Biotechnol. 2006;24:1377–1383. doi: 10.1038/nbt1261. [DOI] [PubMed] [Google Scholar]
- 140.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Salk J.E., Krech U., Youngner J.S., Bennett B.L., Lewis L.J., Bazeley P.L. Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. Am. J. Public Health Nations Health. 1954;44:563–570. doi: 10.2105/ajph.44.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morales M., Tangermann R.H., Wassilak S.G. Progress toward polio eradication - worldwide, 2015–2016. MMWR Morb. Mortal. Wkly Rep. 2016;65:470–473. doi: 10.15585/mmwr.mm6518a4. [DOI] [PubMed] [Google Scholar]
- 143.Cunningham A.L., Garcon N., Leo O., Friedland L.R., Strugnell R., Laupeze B., Doherty M., Stern P. Vaccine development: from concept to early clinical testing. Vaccine. 2016;34:6655–6664. doi: 10.1016/j.vaccine.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 144.Vogel F.R. Improving vaccine performance with adjuvants. Clin. Infect. Dis. 2000;30(Suppl. 3):S266–S270. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 145.Gao Q., Bao L., Mao H., Wang L., Xu K., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.SINOVAC Sinovac Announces Positive Preliminary Results of Phase I/II Clinical Trials for Inactivated Vaccine Candidate Against COVID-19. 2020. http://www.sinovac.com/?optionid=754&auto_id=904
- 147.ContagionLive The COVID-19 Live Vaccine Tracker. 2020. https://www.contagionlive.com/view/the-covid19-live-vaccine-tracker
- 148.Wire T. COVID-19: Bharat Biotech's Covaxin Expected To Be 60% Effective, Company Says. 2020. https://science.thewire.in/health/covid-19-bharat-biotechs-covaxin-expected-to-be-60-effective-company-says/
- 149.Isakova-Sivak I., Rudenko L. Lancet Infect Dis. 2020. A promising inactivated whole-virion SARS-CoV-2 vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu M.A., Wahren B., Karlsson Hedestam G.B. DNA vaccines: recent developments and future possibilities. Hum. Gene Ther. 2006;17:1051–1061. doi: 10.1089/hum.2006.17.1051. [DOI] [PubMed] [Google Scholar]
- 151.Restifo N.P., Ying H., Hwang L., Leitner W.W. The promise of nucleic acid vaccines. Gene Ther. 2000;7:89–92. doi: 10.1038/sj.gt.3301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ledgerwood J.E., Pierson T.C., Hubka S.A., Desai N., Rucker S., et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J. Infect. Dis. 2011;203:1396–1404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boshra H., Lorenzo G., Rodriguez F., Brun A. A DNA vaccine encoding ubiquitinated Rift Valley fever virus nucleoprotein provides consistent immunity and protects IFNAR(−/−) mice upon lethal virus challenge. Vaccine. 2011;29:4469–4475. doi: 10.1016/j.vaccine.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 154.Mallilankaraman K., Shedlock D.J., Bao H., Kawalekar O.U., Fagone P., et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Patel A., Reuschel E.L., Kraynyak K.A., Racine T., Park D.H., et al. Protective efficacy and long-term immunogenicity in cynomolgus macaques by Ebola virus glycoprotein synthetic DNA vaccines. J. Infect. Dis. 2019;219:544–555. doi: 10.1093/infdis/jiy537. [DOI] [PubMed] [Google Scholar]
- 156.Akulova E., Murashev B., Verevochkin S., Masharsky A., Al-Shekhadat R., et al. The increase of the magnitude of spontaneous viral blips in some participants of phase II clinical trial of therapeutic optimized HIV DNA vaccine candidate. Vaccines (Basel) 2019;7 doi: 10.3390/vaccines7030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pinto P.B.A., Assis M.L., Vallochi A.L., Pacheco A.R., Lima L.M., et al. T cell responses induced by DNA vaccines based on the DENV2 E and NS1 proteins in mice: importance in protection and immunodominant epitope identification. Front. Immunol. 2019;10:1522. doi: 10.3389/fimmu.2019.01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.PipelineReview . 2020. INOVIO announces initiation of Phase 2 segment of Phase 2/3 clinical trial for COVID-19 DNA vaccine candidate INO-4800.https://pipelinereview.com/index.php/2020111776561/Vaccines/INOVIO-announces-initiation-of-Phase-2-segment-of-Phase-2/3-clinical-trial-for-COVID-19-DNA-vaccine-candidate-INO-4800.html [Google Scholar]
- 160.BusinessLine Zydus Cadila Gets DCGI Nod for Human Trials of Covid-19 Vaccine. 2020. https://www.thehindubusinessline.com/companies/zydus-cadila-gets-dcgi-nod-for-human-trials-of-covid-19-vaccine/article31977182.ece
- 161.BioWorld South Korea's Genexine begins phase I/IIa trials for COVID-19 vaccine. 2020. https://www.bioworld.com/articles/435995-south-koreas-genexine-begins-phase-iiia-trials-for-covid-19-vaccine
- 162.Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel) 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Small J.C., Ertl H.C. Viruses - from pathogens to vaccine carriers. Curr. Opin. Virol. 2011;1:241–245. doi: 10.1016/j.coviro.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rollier C.S., Reyes-Sandoval A., Cottingham M.G., Ewer K., Hill A.V. Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol. 2011;23:377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 165.Lundstrom K. Self-replicating RNA viruses for RNA therapeutics. Molecules. 2018;23 doi: 10.3390/molecules23123310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zuniga A., Wang Z., Liniger M., Hangartner L., Caballero M., Pavlovic J., Wild P., Viret J.F., Glueck R., Billeter M.A., Naim H.Y. Attenuated measles virus as a vaccine vector. Vaccine. 2007;25:2974–2983. doi: 10.1016/j.vaccine.2007.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lorin C., Delebecque F., Labrousse V., Da Silva L., Lemonnier F., Brahic M., Tangy F. A recombinant live attenuated measles vaccine vector primes effective HLA-A0201-restricted cytotoxic T lymphocytes and broadly neutralizing antibodies against HIV-1 conserved epitopes. Vaccine. 2005;23:4463–4472. doi: 10.1016/j.vaccine.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 168.Lorin C., Mollet L., Delebecque F., Combredet C., Hurtrel B., Charneau P., Brahic M., Tangy F. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J. Virol. 2004;78:146–157. doi: 10.1128/JVI.78.1.146-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Baldo A., Galanis E., Tangy F., Herman P. Biosafety considerations for attenuated measles virus vectors used in virotherapy and vaccination. Hum. Vaccin Immunother. 2016;12:1102–1116. doi: 10.1080/21645515.2015.1122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brandler S., Tangy F. Recombinant vector derived from live attenuated measles virus: potential for flavivirus vaccines. Comp. Immunol. Microbiol. Infect. Dis. 2008;31:271–291. doi: 10.1016/j.cimid.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 171.Dicks M.D., Spencer A.J., Edwards N.J., Wadell G., Bojang K., Gilbert S.C., Hill A.V., Cottingham M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wold W.S., Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lauer K.B., Borrow R., Blanchard T.J. Multivalent and multipathogen viral vector vaccines. Clin. Vaccine Immunol. 2017;24 doi: 10.1128/CVI.00298-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Humphreys I.R., Sebastian S. Novel viral vectors in infectious diseases. Immunology. 2018;153:1–9. doi: 10.1111/imm.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tatsis N., Ertl H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tan W.G., Jin H.T., West E.E., Penaloza-MacMaster P., Wieland A., et al. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J. Virol. 2013;87:1359–1372. doi: 10.1128/JVI.02055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Baden L.R., Karita E., Mutua G., Bekker L.G., Gray G., et al. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: a randomized trial. Ann. Intern. Med. 2016;164:313–322. doi: 10.7326/M15-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Coughlan L. Factors which contribute to the immunogenicity of non-replicating adenoviral vectored vaccines. Front. Immunol. 2020;11:909. doi: 10.3389/fimmu.2020.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Balakrishnan B., Jayandharan G.R. Basic biology of adeno-associated virus (AAV) vectors used in gene therapy. Curr. Gene Ther. 2014;14:86–100. doi: 10.2174/1566523214666140302193709. [DOI] [PubMed] [Google Scholar]
- 180.Ogden P.J., Kelsic E.D., Sinai S., Church G.M. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science. 2019;366:1139–1143. doi: 10.1126/science.aaw2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Johnson P.R., Schnepp B.C., Connell M.J., Rohne D., Robinson S., Krivulka G.R., Lord C.I., Zinn R., Montefiori D.C., Letvin N.L., Clark K.R. Novel adeno-associated virus vector vaccine restricts replication of simian immunodeficiency virus in macaques. J. Virol. 2005;79:955–965. doi: 10.1128/JVI.79.2.955-965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li C., Samulski R.J. Engineering adeno-associated virus vectors for gene therapy, Nat. Rev. Gen. Therm. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 183.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomized phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Burki T.K. The Russian vaccine for COVID-19. Lancet Respir. Med. 2020;8:e85–e86. doi: 10.1016/S2213-2600(20)30402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomized, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.News18 CanSino's Covid-19 Vaccine Candidate Approved for Military Use in China. 2020. https://www.news18.com/news/world/cansinos-covid-19-vaccine-candidate-approved-for-military-use-in-china-2692251.html
- 191.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomized, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.AstraZeneca AstraZeneca to supply Europe with up to 400 million doses of Oxford University's vaccine at no profit. https://www.astrazeneca.com/media-centre/articles/2020/astrazeneca-to-supply-europe-with-up-to-400-million-doses-of-oxford-universitys-potential-covid-19-vaccine.html
- 194.Liljeqvist S., Stahl S. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 1999;73:1–33. doi: 10.1016/s0168-1656(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 195.Vartak A., Sucheck S.J. Recent advances in subunit vaccine carriers. Vaccines (Basel) 2016;4 doi: 10.3390/vaccines4020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Degos F. Protein subunit vaccines: example of vaccination against hepatitis B virus. Rev. Prat. 1995;45:1488–1491. [PubMed] [Google Scholar]
- 197.Huzair F., Sturdy S. Biotechnology and the transformation of vaccine innovation: the case of the hepatitis B vaccines 1968–2000. Stud. Hist. Phil. Biol. Biomed. Sci. 2017;64:11–21. doi: 10.1016/j.shpsc.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tripathi N.K. Production and purification of recombinant proteins from Escherichia coli. ChemBioEng Rev. 2016;3:116–133. [Google Scholar]
- 199.Baeshen M.N., Al-Hejin A.M., Bora R.S., Ahmed M.M., Ramadan H.A., Saini K.S., Baeshen N.A., Redwan E.M. Production of biopharmaceuticals in E. coli: current scenario and future perspectives. J. Microbiol. Biotechnol. 2015;25:953–962. doi: 10.4014/jmb.1412.12079. [DOI] [PubMed] [Google Scholar]
- 200.Berlec A., Strukelj B. Current state and recent advances in biopharmaceutical production in Escherichia coli, yeasts and mammalian cells. J. Ind. Microbiol. Biotechnol. 2013;40:257–274. doi: 10.1007/s10295-013-1235-0. [DOI] [PubMed] [Google Scholar]
- 201.Porro D., Gasser B., Fossati T., Maurer M., Branduardi P., Sauer M., Mattanovich D. Production of recombinant proteins and metabolites in yeasts: when are these systems better than bacterial production systems? Appl. Microbiol. Biotechnol. 2011;89:939–948. doi: 10.1007/s00253-010-3019-z. [DOI] [PubMed] [Google Scholar]
- 202.Arvin A.M., Greenberg H.B. New viral vaccines. Virology. 2006;344:240–249. doi: 10.1016/j.virol.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 203.Baxter D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. (Lond.) 2007;57:552–556. doi: 10.1093/occmed/kqm110. [DOI] [PubMed] [Google Scholar]
- 204.Tison E., Marpeau L., Pigne A., Tessier F., Barrat J. Treatment of acute non-chlamydial salpingitis. Study of the efficacy and tolerance of a single-therapy antibiotic: augmentin. J. Gynecol. Obstet. Biol. Reprod. (Paris) 1988;17:513–519. [PubMed] [Google Scholar]
- 205.Gao S., Song S., Zhang L. Recent progress in vaccine development against chikungunya virus. Front. Microbiol. 2019;10:2881. doi: 10.3389/fmicb.2019.02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bidokhti M.R.M., Ullman K., Hammer A.S., Jensen T.H., Chriel M., Byrareddy S.N., Baule C. Immunogenicity and efficacy evaluation of subunit astrovirus vaccines. Vaccines (Basel) 2019;7 doi: 10.3390/vaccines7030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yancey K.B. Commentary regarding: efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. H Lal, AL Cunningham, O Godeaux et al., N. Engl. J. Med. 372:2087–2096, 2015. Dermatol. Ther. 2016;29:300–301. doi: 10.1111/dth.12330. [DOI] [PubMed] [Google Scholar]
- 208.Cunningham A.L., Lal H., Kovac M., Chlibek R., Hwang S.J., et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 209.Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C., Smith G.E., Frieman M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.MigVax . 2020. MigVax is developing an oral COVID-19 sub-unit vaccine.https://www.migvax.com/ [Google Scholar]
- 211.Machhi J., Kevadiya B.D., Muhammad I.K., Herskovitz J., Olson K.E., Mosley R.L., Gendelman H.E. Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders. Mol. Neurodegener. 2020;15:32. doi: 10.1186/s13024-020-00375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen Z., John Wherry E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Qian C., Campidelli A., Wang Y., Cai H., Venard V., et al. Curative or pre-emptive adenovirus-specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: a successful phase I/II multicenter clinical trial. J. Hematol. Oncol. 2017;10:102. doi: 10.1186/s13045-017-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Icheva V., Kayser S., Wolff D., Tuve S., Kyzirakos C., et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J. Clin. Oncol. 2013;31:39–48. doi: 10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- 215.Feuchtinger T., Opherk K., Bethge W.A., Topp M.S., Schuster F.R., et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 216.Soon C.F., Zhang S., Suneetha P.V., Antunes D.A., Manns M.P., Raha S., Schultze-Florey C., Prinz I., Wedemeyer H., Sallberg Chen M., Cornberg M., Hepatitis E. Virus (HEV)-specific T cell receptor cross-recognition: implications for immunotherapy. Front. Immunol. 2019;10:2076. doi: 10.3389/fimmu.2019.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.David P., Drabczyk-Pluta M., Pastille E., Knuschke T., Werner T., et al. Combination immunotherapy with anti-PD-L1 antibody and depletion of regulatory T cells during acute viral infections results in improved virus control but lethal immunopathology. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Attanasio J., Wherry E.J. Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity. 2016;44:1052–1068. doi: 10.1016/j.immuni.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zelinskyy G., Myers L., Dietze K.K., Gibbert K., Roggendorf M., et al. Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute friend retrovirus infection but are highly cytotoxic and control virus replication. J. Immunol. 2011;187:3730–3737. doi: 10.4049/jimmunol.1101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Urbani S., Amadei B., Tola D., Pedrazzi G., Sacchelli L., Cavallo M.C., Orlandini A., Missale G., Ferrari C. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J. Hepatol. 2008;48:548–558. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 221.Akhmetzyanova I., Drabczyk M., Neff C.P., Gibbert K., Dietze K.K., Werner T., Liu J., Chen L., Lang K.S., Palmer B.E., Dittmer U., Zelinskyy G. PD-L1 expression on retrovirus-infected cells mediates immune escape from CD8+ T cell killing. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stadtmauer E.A., Fraietta J.A., Davis M.M., Cohen A.D., Weber K.L., et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367 doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhou Y., Zhang Y., Yao Z., Moorman J.P., Jia Z. Dendritic cell-based immunity and vaccination against hepatitis C virus infection. Immunology. 2012;136:385–396. doi: 10.1111/j.1365-2567.2012.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cintolo J.A., Datta J., Mathew S.J., Czerniecki B.J. Dendritic cell-based vaccines: barriers and opportunities. Future Oncol. 2012;8:1273–1299. doi: 10.2217/fon.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Golchin A. Cell-based therapy for severe COVID-19 patients: clinical trials and cost-utility. Stem Cell Rev. Rep. 2020 doi: 10.1007/s12015-020-10046-1. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Le D.T., Pardoll D.M., Jaffee E.M. Cellular vaccine approaches. Cancer J. 2010;16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Anassi E., Ndefo U.A. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P T. 2011;36:197–202. [PMC free article] [PubMed] [Google Scholar]
- 228.Centers for Drug Control anf Prevention Cell-Based Flu Vaccines. 2020. https://www.cdc.gov/flu/prevent/cell-based.htm
- 229.University of Manitoba A treatment for COVID-19 will be found, reassures UM virologist. 2020. https://news.umanitoba.ca/a-treatment-for-covid-19-will-be-found-reassures-um-virologist/
- 230.Liang Z., Zhu H., Wang X., Jing B., Li Z., Xia X., Sun H., Yang Y., Zhang W., Shi L., Zeng H., Sun B. Adjuvants for coronavirus vaccines. Front. Immunol. 2020;11:589833. doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Gupta T., Gupta S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int. Immunopharmacol. 2020;86:106717. doi: 10.1016/j.intimp.2020.106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nalwa H.S. A special issue on reviews in nanomedicine, drug delivery and vaccine development. J. Biomed. Nanotechnol. 2014;10:1635–1640. doi: 10.1166/jbn.2014.2033. [DOI] [PubMed] [Google Scholar]
- 233.Pashine A., Valiante N.M., Ulmer J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005;11:S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 234.Caetano L.A., Almeida A.J., Goncalves L.M. Approaches to tuberculosis mucosal vaccine development using nanoparticles and microparticles: a review. J. Biomed. Nanotechnol. 2014;10:2295–2316. [PubMed] [Google Scholar]
- 235.Petrovsky N., Aguilar J.C. Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 236.Singh M. D. O'Hagan, Advances in vaccine adjuvants. Nat. Biotechnol. 1999;17:1075–1081. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 237.Bowen W.S., Svrivastava A.K., Batra L., Barsoumian H., Shirwan H. Current challenges for cancer vaccine adjuvant development. Expert Rev. Vaccines. 2018;17:207–215. doi: 10.1080/14760584.2018.1434000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schijns V.E., Degen W.G. Vaccine immunopotentiators of the future. Clin. Pharmacol. Ther. 2007;82:750–755. doi: 10.1038/sj.clpt.6100394. [DOI] [PubMed] [Google Scholar]
- 239.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 240.Singh M., Kazzaz J., Ugozzoli M., Baudner B., Pizza M., et al. MF59 oil-in-water emulsion in combination with a synthetic TLR4 agonist (E6020) is a potent adjuvant for a combination Meningococcus vaccine. Hum. Vaccin Immunother. 2012;8:486–490. doi: 10.4161/hv.19229. [DOI] [PubMed] [Google Scholar]
- 241.Kazzaz J., Singh M., Ugozzoli M., Chesko J., Soenawan E. D.T. O'Hagan, Encapsulation of the immune potentiators MPL and RC529 in PLG microparticles enhances their potency. J. Control. Release. 2006;110:566–573. doi: 10.1016/j.jconrel.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 242.Zakhartchouk A.N., Sharon C., Satkunarajah M., Auperin T., Viswanathan S., et al. Immunogenicity of a receptor-binding domain of SARS coronavirus spike protein in mice: implications for a subunit vaccine. Vaccine. 2007;25:136–143. doi: 10.1016/j.vaccine.2006.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jiaming L., Yanfeng Y., Yao D., Yawei H., Linlin B., et al. The recombinant N-terminal domain of spike proteins is a potential vaccine against Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Vaccine. 2017;35:10–18. doi: 10.1016/j.vaccine.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Iwata-Yoshikawa N., Uda A., Suzuki T., Tsunetsugu-Yokota Y., Sato Y., et al. Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine. J. Virol. 2014;88:8597–8614. doi: 10.1128/JVI.00983-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tomljenovic L., Shaw C.A. Aluminium vaccine adjuvants: are they safe? Curr. Med. Chem. 2011;18:2630–2637. doi: 10.2174/092986711795933740. [DOI] [PubMed] [Google Scholar]
- 246.Oleszycka E., Lavelle E.C. Immunomodulatory properties of the vaccine adjuvant alum. Curr. Opin. Immunol. 2014;28:1–5. doi: 10.1016/j.coi.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 247.Gupta R.K. Aluminium compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998;32:155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 248.Brito L.A., Malyala P. D.T. O'Hagan, Vaccine adjuvant formulations: a pharmaceutical perspective. Semin. Immunol. 2013;25:130–145. doi: 10.1016/j.smim.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 249.Hogenesch H. Mechanism of immunopotentiation and safety of aluminium adjuvants. Front. Immunol. 2012;3:406. doi: 10.3389/fimmu.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Spruth M., Kistner O., Savidis-Dacho H., Hitter E., Crowe B., et al. A double-inactivated whole virus candidate SARS coronavirus vaccine stimulates neutralizing and protective antibody responses. Vaccine. 2006;24:652–661. doi: 10.1016/j.vaccine.2005.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tang L., Zhu Q., Qin E., Yu M., Ding Z., et al. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 2004;23:391–394. doi: 10.1089/104454904323145272. [DOI] [PubMed] [Google Scholar]
- 252.Deng Y., Lan J., Bao L., Huang B., Ye F., et al. Enhanced protection in mice induced by immunization with inactivated whole viruses compare to spike protein of middle east respiratory syndrome coronavirus. Emerg. Microbes Infect. 2018;7:60. doi: 10.1038/s41426-018-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lan J., Deng Y., Chen H., Lu G., Wang W., et al. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Stills H.F., Jr. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J. 2005;46:280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 255.Jansen T., Hofmans M.P., Theelen M.J., Manders F., Schijns V.E. Structure- and oil type-based efficacy of emulsion adjuvants. Vaccine. 2006;24:5400–5405. doi: 10.1016/j.vaccine.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 256.Morel S., Didierlaurent A., Bourguignon P., Delhaye S., Baras B., et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 257.Vesikari T., Groth N., Karvonen A., Borkowski A., Pellegrini M. MF59-adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second year seasonal vaccination. Vaccine. 2009;27:6291–6295. doi: 10.1016/j.vaccine.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 258.Ott G., Barchfeld G.L., Van Nest G. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine. 1995;13:1557–1562. doi: 10.1016/0264-410x(95)00089-j. [DOI] [PubMed] [Google Scholar]
- 259.Ott G. The adjuvant MF59: the 1998 perspective, clinical performance and mechanism of action. Res. Immunol. 1998;149:25–27. [Google Scholar]
- 260.Stadler K., Roberts A., Becker S., Vogel L., Eickmann M., Kolesnikova L., Klenk H.D., Murphy B., Rappuoli R., Abrignani S., Subbarao K. SARS vaccine protective in mice. Emerg. Infect. Dis. 2005;11:1312–1314. doi: 10.3201/eid1108.041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kong W.P., Xu L., Stadler K., Ulmer J.B., Abrignani S., Rappuoli R., Nabel G.J. Modulation of the immune response to the severe acute respiratory syndrome spike glycoprotein by gene-based and inactivated virus immunization. J. Virol. 2005;79:13915–13923. doi: 10.1128/JVI.79.22.13915-13923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang N., Channappanavar R., Ma C., Wang L., Tang J., et al. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell. Mol. Immunol. 2016;13:180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Das S.C., Hatta M., Wilker P.R., Myc A., Hamouda T., Neumann G., Baker J.R., Jr., Kawaoka Y. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine. 2012;30:6871–6877. doi: 10.1016/j.vaccine.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Treanor J.J., Essink B., Hull S., Reed S., Izikson R., et al. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE + GLA) adjuvant. Vaccine. 2013;31:5760–5765. doi: 10.1016/j.vaccine.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 265.Klucker M.F., Dalencon F., Probeck P., Haensler J. AF03, an alternative squalene emulsion-based vaccine adjuvant prepared by a phase inversion temperature method. J. Pharm. Sci. 2012;101:4490–4500. doi: 10.1002/jps.23311. [DOI] [PubMed] [Google Scholar]
- 266.Garcon N., Vaughn D.W., Didierlaurent A.M. Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines. 2012;11:349–366. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- 267.Roberts A., Lamirande E.W., Vogel L., Baras B., Goossens G., et al. Immunogenicity and protective efficacy in mice and hamsters of a beta-propiolactone inactivated whole virus SARS-CoV vaccine. Viral Immunol. 2010;23:509–519. doi: 10.1089/vim.2010.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Geale P.F., Sheehy P.A., Giles C., Thomson P.C., Wynn P.C. Efficacy of two adjuvant systems to promote humoral immunity to the pre-proghrelin peptide obestatin in pigs: consequences for the growth of piglets to weaning. Anim. Prod. Sci. 2020;60:356–362. [Google Scholar]
- 269.Nordly P., Madsen H.B., Nielsen H.M., Foged C. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opi.n Drug Deliv. 2009;6:657–672. doi: 10.1517/17425240903018863. [DOI] [PubMed] [Google Scholar]
- 270.Azizi A., Aucoin S., Tadesse H., Frost R., Ghorbani M., Soare C., Naas T., Diaz-Mitoma F. A combined nucleocapsid vaccine induces vigorous SARS-CD8+ T-cell immune responses. Genet. Vaccines Ther. 2005;3:7. doi: 10.1186/1479-0556-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Du L., Zhao G., Kou Z., Ma C., Sun S., Poon V.K., Lu L., Wang L., Debnath A.K., Zheng B.J., Zhou Y., Jiang S. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J. Virol. 2013;87:9939–9942. doi: 10.1128/JVI.01048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Keech C., Albert G., Cho I., Robertson A., Reed P., et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Vaxine COVID-19. 2020. https://vaxine.net/covid-19/
- 274.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 275.Perrie Y., Crofts F., Devitt A., Griffiths H.R., Kastner E., Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Adv. Drug Deliv. Rev. 2016;99:85–96. doi: 10.1016/j.addr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 276.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14:12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 277.Vijayan V., Mohapatra A., Uthaman S., Park I.K. Recent advances in nanovaccines using biomimetic immunomodulatory materials. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Joshi V.B., Geary S.M., Salem A.K. Biodegradable particles as vaccine delivery systems: size matters. AAPS J. 2013;15:85–94. doi: 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bishop C.J., Kozielski K.L., Green J.J. Exploring the role of polymer structure on intracellular nucleic acid delivery via polymeric nanoparticles. J. Control. Release. 2015;219:488–499. doi: 10.1016/j.jconrel.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Krishnamachari Y., Geary S.M., Lemke C.D., Salem A.K. Nanoparticle delivery systems in cancer vaccines. Pharm. Res. 2011;28:215–236. doi: 10.1007/s11095-010-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhou X., Jiang X., Qu M., Aninwene G.E., 2nd V., Jucaud J.J., Moon Z. Gu, Sun W., Khademhosseini A. Engineering antiviral vaccines. ACS Nano. 2020;14:12370–12389. doi: 10.1021/acsnano.0c06109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Itani R., Tobaiqy M., Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10:5932–5942. doi: 10.7150/thno.46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wu Y., Wei W., Zhou M., Wang Y., Wu J., Ma G., Su Z. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials. 2012;33:2351–2360. doi: 10.1016/j.biomaterials.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 285.Jackson N.A.C., Kester K.E., Casimiro D., Gurunathan S., DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Iavarone C., O’Hagan T., Yu D., Delahaye N.F., Ulmer J.B. Mechanism of action of mRNA-based vaccines. Expert Rev. Vaccines. 2017;16:871–881. doi: 10.1080/14760584.2017.1355245. [DOI] [PubMed] [Google Scholar]
- 287.Barba A.A., Bochicchio S., Dalmoro A., Lamberti G. Lipid delivery systems for nucleic-acid-based-drugs: from production to clinical applications. Pharmaceutics. 2019;11 doi: 10.3390/pharmaceutics11080360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Li W., Szoka F.C., Jr. Lipid-based nanoparticles for nucleic acid delivery. Pharm. Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 289.Sahay G., Querbes W., Alabi C., Eltoukhy A., Sarkar S., et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 2013;31:653–658. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Moderna Moderna Ships mRNA Vaccine Against Novel Coronavirus (mRNA-1273) for Phase 1 Study. 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-ships-mrna-vaccine-against-novel-coronavirus-mrna-1273
- 292.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.National Institute of Health Promising Interim Results from Clinical Trial of NIH-Moderna COVID-19 Vaccine. 2020. https://www.nih.gov/news-events/news-releases/promising-interim-results-clinical-trial-nih-moderna-covid-19-vaccine
- 295.Moderna Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization. 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study/
- 296.GlobeNewsWire . 2020. BioNTech and Pfizer announce regulatory approval from German authority Paul-Ehrlich-Institut to commence first clinical trial of COVID-19 vaccine candidates.https://www.globenewswire.com/news-release/2020/04/22/2019785/0/en/BioNTech-and-Pfizer-announce-regulatory-approval-from-German-authority-Paul-Ehrlich-Institut-to-commence-first-clinical-trial-of-COVID-19-vaccine-candidates.html [Google Scholar]
- 297.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 years of age: interim report. medRxiv. 2020 doi: 10.1101/2020.06.30.20142570. [DOI] [Google Scholar]
- 298.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 299.Pfizer Pfizer and Biontech Conclude Phase 3 Study of Covid-19 Vaccine Candidate, Meeting all Primary Efficacy Endpoints. 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
- 300.Pharmaceutical Technology UK authorises world's first Covid-19 vaccine, Pfizer/BioNTech's BNT162b2. 2020. https://www.pharmaceutical-technology.com/features/pfizer-covid-19-vaccine-approved-uk/
- 301.Pharmaceutical Business Review Canada has approved Pfizer and BioNTech's mRNA vaccine BNT162b2 for the prevention of Covid-19. https://www.pharmaceutical-business-review.com/news/canada-approves-bnt162b2-covid-19/
- 302.McKay P.F., Hu K., Blakney A.K., Samnuan K., Brown J.C., et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020;11:3523. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jocelyn K. Temperature concerns could slow the rollout of new coronavirus vaccines. Science. 2020 [Google Scholar]
- 304.Maurer N., Wong K.F., Stark H., Louie L., McIntosh D., Wong T., Scherrer P., Semple S.C., Cullis P.R. Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys. J. 2001;80:2310–2326. doi: 10.1016/S0006-3495(01)76202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 306.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., et al. SARS-CoV-2 infection in children. N. Engl. J. Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhang N.N., Li X.F., Deng Y.Q., Zhao H., Huang Y.J., et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–1283. doi: 10.1016/j.cell.2020.07.024. (e1216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Erasmus J.H., Khandhar A.P., O'Connor M.A., Walls A.C., Hemann E.A., et al. An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 2020:12. doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Drug Development and Delivery Novavax Advances Development of Novel COVID-19 Vaccine. 2020. https://drug-dev.com/novavax-advances-development-of-novel-covid-19-vaccine/
- 311.Novavax Novavax Initiates Phase 3 Efficacy Trial of COVID-19 Vaccine in the United Kingdom. 2020. https://ir.novavax.com/news-releases/news-release-details/novavax-initiates-phase-3-efficacy-trial-covid-19-vaccine-united
- 312.Takeda Novavax and Takeda Announce Collaboration for Novavax’ COVID-19 Vaccine Candidate in Japan. 2020. https://www.takeda.com/newsroom/newsreleases/2020/novavax-and-takeda-announce-collaboration-for-novavax-covid-19-vaccine-candidate-in-japan/
- 313.Cyranoski D. Profile of a killer: the complex biology powering the coronavirus pandemic. Nature. 2020;581:22–26. doi: 10.1038/d41586-020-01315-7. [DOI] [PubMed] [Google Scholar]
- 314.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H., Fang R.H., Gao W., Griffiths A., Zhang L. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20:5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lixin L., Zhijia L., Haolin C., Hong L., Qiang G., Feng C., Guangxia G., Yongming C. 2020. A Translatable Subunit Nanovaccine for COVID-19. [Google Scholar]
- 316.U.o.W. Institute for Protein Design De novo Nanoparticles as Vaccine Scaffolds. 2020. https://www.ipd.uw.edu/2020/08/de-novo-nanoparticles-vaccine-scaffolds/
- 317.Martin J.T., Cottrell C.A., Antanasijevic A., Carnathan D.G., Cossette B.J., et al. Targeting HIV Env immunogens to B cell follicles in nonhuman primates through immune complex or protein nanoparticle formulations. NPJ Vaccines. 2020;5:72. doi: 10.1038/s41541-020-00223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Roldao A., Mellado M.C., Castilho L.R., Carrondo M.J., Alves P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 319.Buonaguro L., Tornesello M.L., Buonaguro F.M. Virus-like particles as particulate vaccines. Curr. HIV Res. 2010;8:299–309. doi: 10.2174/157016210791208659. [DOI] [PubMed] [Google Scholar]
- 320.Purcell A.W., McCluskey J., Rossjohn J. More than one reason to rethink the use of peptides in vaccine design, Nat. Rev. Drug Discov. 2007;6:404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 321.Mateu M.G. Virus engineering: functionalization and stabilization. Protein Eng. Des. Sel. 2011;24:53–63. doi: 10.1093/protein/gzq069. [DOI] [PubMed] [Google Scholar]
- 322.Zepeda-Cervantes J., Ramirez-Jarquin J.O., Vaca L. Interaction between virus-like particles (VLPs) and pattern recognition receptors (PRRs) from dendritic cells (DCs): toward better engineering of VLPs. Front. Immunol. 2020;11:1100. doi: 10.3389/fimmu.2020.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Qian C., Liu X., Xu Q., Wang Z., Chen J., Li T., Zheng Q., Yu H., Gu Y., Li S., Xia N. Recent progress on the versatility of virus-like particles. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kushnir N., Streatfield S.J., Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chung Y.H., Cai H., Steinmetz N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020;156:214–235. doi: 10.1016/j.addr.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mathieu C., Rioux G., Dumas M.C., Leclerc D. Induction of innate immunity in lungs with virus-like nanoparticles leads to protection against influenza and Streptococcus pneumoniae challenge. Nanomedicine. 2013;9:839–848. doi: 10.1016/j.nano.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 327.Zeltins A. Construction and characterization of virus-like particles: a review. Mol. Biotechnol. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Young K.R., McBurney S.P., Karkhanis L.U., Ross T.M. Virus-like particles: designing an effective AIDS vaccine. Methods. 2006;40:98–117. doi: 10.1016/j.ymeth.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 329.Thrane S., Janitzek C.M., Agerbaek M.O., Ditlev S.B., Resende M., Nielsen M.A., Theander T.G., Salanti A., Sander A.F. A novel virus-like particle based vaccine platform displaying the placental malaria antigen VAR2CSA. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Brune K.D., Leneghan D.B., Brian I.J., Ishizuka A.S., Bachmann M.F., Draper S.J., Biswas S., Howarth M. Plug-and-Display: decoration of Virus-Like Particles via isopeptide bonds for modular immunization. Sci. Rep. 2016;6:19234. doi: 10.1038/srep19234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Chua A.J., Vituret C., Tan M.L., Gonzalez G., Boulanger P., Ng M.L., Hong S.S. A novel platform for virus-like particle-display of flaviviral envelope domain III: induction of Dengue and West Nile virus neutralizing antibodies. Virol. J. 2013;10:129. doi: 10.1186/1743-422X-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Pitoiset F., Vazquez T., Bellier B. Enveloped virus-like particle platforms: vaccines of the future? Expert Rev. Vaccines. 2015;14:913–915. doi: 10.1586/14760584.2015.1046440. [DOI] [PubMed] [Google Scholar]
- 333.Jain N.K., Sahni N., Kumru O.S., Joshi S.B., Volkin D.B., Russell Middaugh C. Formulation and stabilization of recombinant protein based virus-like particle vaccines. Adv. Drug Deliv. Rev. 2015;93:42–55. doi: 10.1016/j.addr.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 334.Grgacic E.V., Anderson D.A. Virus-like particles: passport to immune recognition. Methods. 2006;40:60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Gerety R.J., Tabor E. Newly licensed hepatitis B vaccine. Known safety and unknown risks. JAMA. 1983;249:745–746. [PubMed] [Google Scholar]
- 336.Lacson E., Teng M., Ong J., Vienneau L., Ofsthun N., Lazarus J.M. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodial. Int. 2005;9:367–375. doi: 10.1111/j.1492-7535.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 337.McNeil C. Who invented the VLP cervical cancer vaccines? J. Natl. Cancer Inst. 2006;98:433. doi: 10.1093/jnci/djj144. [DOI] [PubMed] [Google Scholar]
- 338.Martelli P., Ferrari L., Morganti M., De Angelis E., Bonilauri P., Guazzetti S., Caleffi A., Borghetti P. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 2011;149:339–351. doi: 10.1016/j.vetmic.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 339.Charlton Hume H.K., Vidigal J., Carrondo M.J.T., Middelberg A.P.J., Roldao A., Lua L.H.L. Synthetic biology for bioengineering virus-like particle vaccines. Biotechnol. Bioeng. 2019;116:919–935. doi: 10.1002/bit.26890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Shouval D., Roggendorf H., Roggendorf M. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S vaccine. Med. Microbiol. Immunol. 2015;204:57–68. doi: 10.1007/s00430-014-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhao Q., Li S., Yu H., Xia N., Modis Y. Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol. 2013;31:654–663. doi: 10.1016/j.tibtech.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 342.Qiao Y.L., Wu T., Li R.C., Hu Y.M., Wei L.H., et al. Efficacy, Safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J. Natl. Cancer Inst. 2020;112:145–153. doi: 10.1093/jnci/djz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Shinde V., Fries L., Wu Y., Agrawal S., Cho I., et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N. Engl. J. Med. 2018;378:2346–2348. doi: 10.1056/NEJMc1803554. [DOI] [PubMed] [Google Scholar]
- 344.Kato T., Takami Y., Kumar Deo V., Park E.Y. Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J. Biotechnol. 2019;306:177–184. doi: 10.1016/j.jbiotec.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wang C., Zheng X., Gai W., Wong G., Wang H., et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antivir. Res. 2017;140:55–61. doi: 10.1016/j.antiviral.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Liu Y.V., Massare M.J., Barnard D.L., Kort T., Nathan M., Wang L., Smith G. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29:6606–6613. doi: 10.1016/j.vaccine.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Lokugamage K.G., Yoshikawa-Iwata N., Ito N., Watts D.M., Wyde P.R., Wang N., Newman P., Kent Tseng C.T., Peters C.J., Makino S. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine. 2008;26:797–808. doi: 10.1016/j.vaccine.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Dynavax Dynavax Announces First Participants Dosed in Phase 1 Clinical Trial Evaluating Medicago's COVID-19 Vaccine Candidate with Dynavax's CpG 1018 Adjuvant. 2020. https://investors.dynavax.com/news-releases/news-release-details/dynavax-announces-first-participants-dosed-phase-1-clinical-0
- 349.Review E.P. Plant-based COVID-19 Vaccine Enters Phase I Trials. 2020. https://www.europeanpharmaceuticalreview.com/news/124092/plant-based-covid-19-vaccine-enters-phase-i-trials/
- 350.Pillet S., Couillard J., Trepanier S., Poulin J.F., Yassine-Diab B., Guy B., Ward B.J., Landry N. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate-Two randomized Phase II clinical trials in 18 to 49 and >/=50 years old adults. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lu J., Lu G., Tan S., Xia J., Xiong H., Yu X., Qi Q., Yu X., Li L., Yu H., Xia N., Zhang T., Xu Y., Lin J. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020;30:936–939. doi: 10.1038/s41422-020-00392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Vragniau C., Bufton J.C., Garzoni F., Stermann E., Rabi F., et al. Synthetic self-assembling ADDomer platform for highly efficient vaccination by genetically encoded multiepitope display. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aaw2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.University of Bristol New Vaccine Platform Used to Develop COVID-19 Candidates. 2020. http://www.bristol.ac.uk/news/2020/april/covid-19-vaccine-platform.html
- 354.Kumar S., Zhi K., Mukherji A., Gerth K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses. 2020;12 doi: 10.3390/v12050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Zhou X., Li Y., Li T., Zhang W. Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin. Microbiol. Infect. 2020;26:957–959. doi: 10.1016/j.cmi.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Codiak Codiak BioSciences Collaborates with Ragon Institute to Evaluate the exoVACC™ Vaccine Platform in SARS-CoV-2 and HIV. 2020. https://www.codiakbio.com/news/press-releases/codiak-biosciences-collaborates-with-ragon-institute-to-evaluate-the-exovacc-vaccine-platform-in-sars-cov-2-and-hiv
- 357.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., et al. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]