Abstract
Background
The COVID-19 pandemic that emerged in 2019 has imposed huge consequences, including economic losses and threats to human health, which are still affecting many aspects throughout the world.
Scope and approach
This review provides an overview of SARS-CoV-2 infection, the cause of COVID-19, and explores its impact on the food supply system and food safety. This review examines the potential risk of transmission through food and environmental surfaces before discussing an effective inactivation strategy to control the COVID-19 pandemic in the aspect of food safety. This article also suggests effective food safety management post-COVID-19.
Key findings and conclusions
Respiratory viruses including SARS-CoV-2 are responsible for huge impacts on the global economy and human health. Although food and water are not currently considered priority transmission routes of SARS-CoV-2, infection through contaminated food and environmental surfaces where the virus can persist for several days cannot be ignored, particularly when the surrounding environment is unhygienic. This approach could help determine the exact transmission route of SARS-CoV-2 and prepare for the post-COVID-19 era in the food safety sector.
Keywords: COVID-19, SARS-CoV-2, Transmission route, Food supply chain, Food safety, Inactivation, Post-COVID-19 era
1. Introduction
Huge global attention has been brought to bear on COVID-19, a disease that emerged in the Wuhan province of China and rapidly spread throughout the entire country and then to nearly 50 others all over the world, resulting in the declaration of a pandemic by the World Health Organization (WHO) on March 11, 2020 (WHO, 2020a). The WHO identified a novel group 2B betacoronavirus that causes viral pneumonia and subsequently announced the standard nomenclature for the disease as COVID-19 on February 11, 2020 (WHO, 2020a). At the same time, this novel coronavirus was named SARS-CoV-2 by the International Committee on Taxonomy of Viruses (ICTV) (Gorbalenya et al., 2020; Hui et al., 2020). This virus is highly homologous to two other coronaviruses that have emerged over the past two decades: SARS-CoV that causes Severe Acute Respiratory Syndrome (SARS) and MERS-CoV that causes Middle East Respiratory Syndrome (MERS), both of which resulted in high mortality and morbidity. Although the mortality rate of COVID-19 is lower than SARS, its transmission rate is higher, which could be explained by mutation and enhanced genetic recombination at the S-protein in the receptor-binding domain (RBD) of SARS-CoV-2 (Shereen, Khan, Kazmi, Bashir, & Siddique, 2020).
At the time of this review, the COVID-19 pandemic has resulted in approximately 28 million confirmed cases in more than 218 countries, resulting in over 919,000 deaths and the lockdown of a third of the world's population (CoronaBoard, 2020). Still, COVID-19 continues to spread and thousands of new cases occur every day worldwide due to the lack of specific antiviral treatments for this virus (the data on the COVID-19 reported cases globally are presented in Fig. 1 ). In addition, each country is facing adverse impacts on their economies due to the COVID-19 infection, with marketing problems throughout food supply chains being one of the worst-hit areas.
Fig. 1.
Worldwide COVID-19 outbreak.
At this point in time, the exact origin of COVID-19 is unknown. However, several studies have suggested that bats are the native host of SARS-CoV-2 as this new virus is 96% homologous with SARS-CoV according to a phylogenic comparison (Zhou et al., 2020). However, an intermediate host that could have helped this virus cross the species barrier to infect humans has not yet been identified. It is known that this virus is easily transmissible by human-to-human contact through respiratory droplets, which is the main transmission route of infection, as well as contaminated fomites, which has recently been found to be significant, while food surfaces can also be a carrier for the virus (Mullis, Saif, Zhang, Zhang, & Azevedo, 2012). The WHO and Centers for Disease Control and Prevention (CDC) have declared that there is no evidence of transmission and direct contamination of SARS-CoV-2 via food and water (CDC, 2020), but the possibility of spreading the virus by consuming food served on contaminated surfaces, during packaging in a contaminated room, or by transmission during the handling or sharing of food with an infected person cannot be ignored (Galanakis, 2020). Indeed, it has been reported that physical contact and shared food during a conference resulted in a few people catching COVID-19 in Singapore in January 2020 (Pung et al., 2020), which suggests that food may also be a potential medium for infection by SARS-CoV-2. Therefore, it is essential to understand the exact transmission route including potential infection through food with scientific evidence to search for new approaches to control the rapid spread of the virus and to update international guidelines during the pandemic situation. In this review, an overview of COVID-19, including the epidemiology and transmission of SARS-CoV-2 is presented, and explore potential transmission routes through the environment and food, and then discusses proper strategies to inactivate SARS-CoV-2. This paper also reviews the impact of the COVID-19 pandemic crisis on the food supply system before discussing how to prepare for the post-COVID-19 era in the aspect terms of food safety.
2. Epidemiology and transmission of SARS-CoV-2
Coronaviruses are a subfamily of the Coronaviridae (enveloped viruses with a single-stranded genome of RNA (i.e., are positive-sense)) belonging to the order Nidovirales with crown-like spikes on their outer surface of the virus (De Wilde, Snijder, Kikkert, & van Hemert, 2017). SARS-CoV and MERS-CoV, which emerged in 2002 and 2014, respectively, are betacoronaviruses that caused serious worldwide epidemics. Based on the full-length genome sequences, samples collected from the original site of the COVID-19 outbreak (the Huanan Seafood Market in Wuhan) tested positive for a new type of betacoronavirus with more than 99.98% nucleotide sequence identity (Zhou et al., 2020). Although the exact origin of SARS-CoV-2 has not yet been identified, there is a close phylogenetic relationship of the virus with SARS-CoV from Chinese horseshoe bats (family: Rhinolophidae) as they have 96% homology in their nucleotide sequences at the whole-genome level (Wan, Shang, Graham, Baric, & Li, 2020; Zhou et al., 2020). Although an intermediate animal host for SARS-CoV-2 has not yet been identified, it has been reported that pangolin is a potential candidate as the new virus shares 99% genetic homology with the pangolin SARS-CoV (Lam et al., 2020). Therefore, researchers are still trying to discover other potential animal hosts of SARS-CoV-2, which is important for discovering how the virus crossed the species barrier to infect humans and for setting a control strategy to prevent the spread of COVID-19.
An initial investigation has shown that some patients infected with SARS-CoV-2 who developed pneumonia in China visited the seafood market where live animals were being sold and may have used infected animals or birds as a food source. However, further investigations revealed that some individuals who contracted the disease had not visited the seafood market, indicating the person-to-person spread of the virus via coughing and sneezing that released invisible respiratory droplets and/or aerosols that were then inhaled through the nose and mouth (Wang, Tang, & Wei, 2020).
Although fundamental knowledge regarding the role of surfaces and food in COVID-19 infection is limited, recent studies have suggested the potential transmission of the SARS-CoV-2 virus from environmental surfaces, including food, water, and other commonly touched fomites (Garraturo et al., 2020; Kampf, Todt, Pfaender, & Steinmann, 2020). Surfaces can be contaminated with various viruses via direct contact with droplets or fluids from infected individuals, after which the survival of a particular virus can be highly variable from a few hours to many days depending on the virus strain, type of surface, temperature, and relative humidity (Kampf et al., 2020). Besides, other potential transmission routes of SARS-CoV-2, such as fecal-oral transmission, have been suggested. More recently, gastrointestinal symptoms and asymptomatic infections among young children have been reported (Chan et al., 2020). Another study indicated that SARS-CoV-2 can be detected in urine and stool samples from laboratory-confirmed COVID-19 patients (Wu et al., 2020), suggesting the possibility of transmission through food and/or water. Therefore, great precautions should be taken when monitoring fecal-oral transmission and more in-depth research is required to verify this hypothesis. This is particularly important to verify the potential transmission risk from food or water and to consequently manage this pandemic.
The symptoms of COVID-19 infection are generally similar to influenza, e.g. fever and coughing (Li et al., 2020), while other symptoms include headaches, breathing difficulties (dyspnea), sputum production, and hemoptysis (Carlos, de la Cruz, Cao, Pasnick, & Jamil, 2020; Wang, Tang, & Wei, 2020). In more severe cases, death can occur due to massive alveolar damage and progressive respiratory failure; the fatality rate was 2% when confirmed cases reached 66,567 globally (Chan et al., 2020). The estimated average incubation period of SARS-CoV-2 is approximately 3–7 days based on the report of the first cases in Wuhan (Li et al., 2020). It is important to adjust the quarantine time based on the accurate incubation period to prevent virus transmission by asymptomatic people. In addition, people with a high risk of contracting COVID-19 are those who are in close contact with the infected or sub-clinically asymptomatic infected individuals. In particular, older (≥60 years) and immunocompromised people are more vulnerable to infection by SARS-CoV-2 than children due to the associated overwhelming inflammation (Carraturo et al., 2020), which suggests that the state of individuals’ immune system could affect their susceptibility to infection and death. Understanding the infectious dose including the number of particles required to cause a detectable infection in humans and animals is critical to control COVID-19; however, this information is not yet conclusively known for SARS-CoV-2. However, Basu (2020) quantified the infectious dose in humans for COVID-19 at 300 particles based on the nasopharyngeal transmission trends and inhalation of droplets, although a single virion could potentially establish an infection in highly susceptible individuals.
3. Impact of the COVID-19 pandemic crisis on the food supply system
The food system is comprehensive, multifaceted, highly interconnected, and has the potential to address food security, safety, nutrition/quality, and manufacturing allocation (Abbaspourrad, Padilla-Zakour, Wiedmann, Moraru, & Goddard, 2017; Clancy, 2017). Food products often require ingredients for multi-element formulations that are not regionally available; the lack of such ingredients can lead to significant challenges for food producers. Thus, it is conceivable that such a system has been created on multiple scales, from industrial to regional, state, country, and global levels (Bhunnoo, 2019). However, the production capacity to meet the global demand for food is drawing close attention (De Lima, Fioriolli, Padula, & Pumi, 2018). The Food and Agriculture Organization (FAO, 2020a) stated that COVID-19 is affecting agriculture in two crucial ways, namely in terms of the supply of and demand for food, which are directly related to food security, which is therefore at risk. A food supply chain is a link that connects farm systems to consumers’ tables via processes related to production, packaging, distribution, and storage (Chen, Brahma, Mackay, Cao, & Aliakbarian, 2020). During the COVID-19 pandemic, all categories of the food supply chain, including fresh vegetables, fruit, bakery items, perishable goods, and food grains, have been extremely compromised (Ivanov & Dolgui, 2020). Food safety is one of the four pillars of the food system that has been badly affected by the COVID-19 pandemic (Galanakis, 2020).
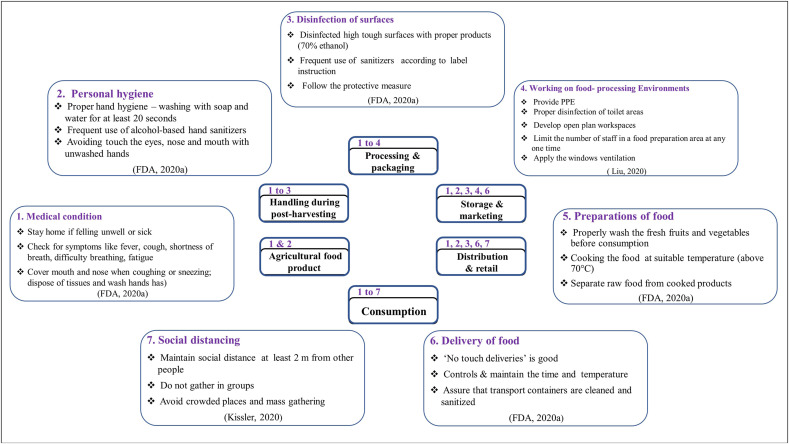
Fig. 2 summarizes the proposed safety measures for the food sector during the pandemic at each stage of the food chain from farms to consumers. Workers are grouped by treatment conditions, personal hygiene, surface disinfection, work environment cleanliness, preparation and delivery of food, and social distancing. Despite all the safety standards implemented in all parts of the food chain, the last stage (consumption) needs the most safety considerations at the consumer level, as this is clearly the main source of infection. Likewise, it is of utmost importance that the food sector ensures that the food on consumers’ plates is safe and poses no risk to consumer health at any stage of the process (even at the moment of delivery). Besides, there are preventive measures (e.g., during food preparation) that are largely implemented before consumption. For instance, at the beginning of the crisis, many restaurants, cafeterias, and health authorities in Central Europe stopped serving rare steak and meat as a general precaution against viruses and pathogens even though the foodborne transmission of SARS-CoV-2 is unsupported by scientific evidence (Euractiv, 2020). Moreover, some companies in the USA (such as those carrying out meat processing) have entirely ceased production during the COVID-19 pandemic (Reiley, 2020).
Fig. 2.
Safety guideline during COVID-19 pandemic for the food sector at each step of the food chain.
Food demand refers to consumers’ eagerness and ability to pay for specific goods and services within a given period of time (Gottheil, 2013). Food demand has decreased slightly nowadays because of uncertainty and the declining purchasing capacity of people. Moreover, these long-term pandemic conditions could create a worsening situation because of the lack of income and job losses (FAO, 2020b). Indeed, the growing demand for food and beverages online is increasing daily due to the COVID-19 pandemic (FAO, 2020a). A shortage of food items is inevitable under such strict lockdown conditions, during which most logistics activities have stopped. Narayanan et al. (2020) mentioned that ordering food items from online-based companies such as Zomato and Swiggy has been prohibited because of the threat of infection.
Food insecurity is growing due to the economic crisis caused by the COVID-19 pandemic, and the number of people facing food insecurity worldwide could double by the end of 2020 (World Food Program, 2020). Both developing and developed countries are facing the same situation due to increased food insecurity during the COVID-19 pandemic, while vulnerable and low-income population groups are more severely affected (Fitzpatrick, Harris, & Drawve, 2020). Government authorities must play a vital role in supporting access to healthy food (FAO, 2020c). Food security requires that everyone has unbounded access to food that allows them to meet their basic needs (Rosales & Mercado, 2020). Failure to act swiftly implies an impending food crisis, which will have the greatest impact on the most vulnerable population groups. Management should keep global food supply chains running and reduce the impact of the pandemic on food systems; social programs could mitigate the effects of short-term crises. Currently, about 820 million are among the most vulnerable group who experience chronic hunger and do not consume enough caloric energy to lead a normal life (FAO, 2020a). This group of people cannot afford interruptions in their livelihoods or limited access to food, as created by a situation like the COVID-19 pandemic and the consequences could be serious with the spread of the virus in countries where such people live with health systems of limited capacity.
A second vulnerable group, small farmers, could be prevented from working on their land and accessing markets to sell their products or buy seeds and other essential inputs. The third vulnerable group is children from low-income families who are mainly nourished by food provided by social programs; suspending these programs due to the pandemic puts their food security and nutrition at risk, and consequently limits their capacity to cope with diseases (FAO, 2020d). Thus, each country must direct its actions to maintain social food programs while taking necessary precautions to avoid transmitting the virus.
4. Persistence of SARS-CoV-2 in various environments
4.1. Food and food-contact surfaces
It is known that the first cases of COVID-19 were associated with the Huanan Seafood Market (Li et al., 2020), where live wild animals such as bats, snakes, and marmots, as well as animal organs, are sold, which suggests the zoonotic transmission of SARS-CoV-2. Although the WHO has indicated that food is not a transmission route for COVID-19 (WHO, 2020b), many authorities including the US Food and Drug Administration Agency (FDA) (2020), and the European Food Safety Authority (2020) ((EFSA 2020)) continue to gather information related to the potential persistence of the virus on food and track the exact intermediate host for this virus.
Meat from beef, poultry, pork, and wild animals are known to be abundant in heparin sulfate, which is required for SARS-CoV-2 to interact with host tissue epithelia (Mycroft-West et al., 2020). This virus’ persistence in the environment and on food-contact surfaces such as plastic, wood, rubber, and stainless steel means that it can survive for several days, so meat tissue surfaces could be a potential or even critical transmission route for COVID-19 infection (Van Doremalen et al., 2020). Studies on the persistence of coronaviruses in food are extremely rare. Van Doremalen, Bushmaker, Karesh, and Munster (2014) investigated the survival of MERS-CoV in dromedary camel milk and found that the virus spiked in all samples stored at 22 °C with a great loss of infectivity when stored at 4 °C; the virus in the dromedary camel milk survived at 4 °C for 72 h, while the infectivity was lost at 22 °C after 48 h. Mullis et al. (2012) described the stability of bovine coronavirus on refrigerated romaine lettuce leaves to examine the potential foodborne transmission of the virus and found that they were detectable for at least 14 days, with the virus becoming more stable at lower temperature and relative humidity, suggesting that contaminated vegetables could be a potential route for the transmission of zoonotic coronaviruses to humans. Similar findings were reported for human coronavirus (HuCoV) 229E (Yépiz-Gómez, Gerba, & Bright, 2013) on lettuce leaves stored at 4 °C; the virus particles decreased by 0.2 log10 after two days and became inactive after four days. These studies are particularly important because of reporting the potential zoonotic transmission via fresh produce to which heat treatment cannot be applied to inactivate viruses and demonstrated that coronaviruses can survive on fresh produce for several days at the usual refrigeration storage temperature in the average consumer household. More recently, Dai et al. (2020) reported the prolonged survival of SAS-CoV-2 in salmon at low temperatures; SARS-CoV-2 remained viable in salmon at 4 °C for eight days and survived for 2 day at 25 °C, confirming that the infectivity of SARS-CoV-2 is associated with temperature and insinuating the potential risk of infection from fish or seafood that are mostly stored and transported while refrigerated. This also indicates that SARS-CoV-2 can survive for longer than MERS-CoV (Van Doremalen et al., 2014) in food stored at 4 °C, although it should be noted that this was with a different food item.
Very limited published scientific papers have reported on the length of time SARS-CoV-2 can remain viable on food or food-contact surfaces. At this stage, the transmission of the virus via food has not been evidenced but ensuring proper and constant personal hygiene including handwashing and safe waste-management practices could be the best way to prevent the human-to-human transmission of SARS-CoV-2. In addition, various food items including meat, poultry, and seafood that are stored at low temperatures need to be inspected to ensure food safety against SARS-CoV-2.
4.2. Environmental surfaces
The significance of transmission of SARS-CoV-2 via contaminated surfaces has been recently suggested. It is known that many viruses, including SARS-CoV and MERS-CoV, can survive on different biological and non-biological surfaces such as plastic, metal, wood, and stainless steel, for hours or even months. Obviously, the surfaces of a variety of foodstuffs can also be a vehicle for viral transmission if they are exposed to unhygienic conditions (Mullis et al., 2012).
COVID-19 is highly contagious as it is transmitted by human-to-human contact; one main mechanism for transmitting this virus is self-inoculation of the mucous membranes in the nose, eyes, and/or mouth from contaminated dry surfaces (Otter et al., 2016:; Dowell et al., 2004). During the illness, viruses can shed in large numbers in human body secretions including blood, saliva, nasal fluid, urine, and feces. Subsequently, an infected person may touch inanimate surfaces or objects, and infectious virus particles can be transferred to the facial mucosa of uninfected individuals. Coronavirus persistence on different environmental surfaces based on the current literature is summarized in Table 1 .
Table 1.
Persistence of coronaviruses on different types of inanimate surfaces.
| Type of surface | Virus | Inoculum | Temperature (°C) | Persistence | Log reduction | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Steel | SARS-CoV-2 | 103.7 | NG | 3–4days | 3.2 | Van Doremalen et al., 2020 | ||
| SARS-CoV-2 | 105.8 | 22 °C | 7days | 5.8 | Alex Chin et al. (2020) | |||
| HCoV 229E | 10³ | 21 °C | >5days | 2 | Warnes, Little, and Keevil (2015) | |||
| Copper | SARS-CoV-2 | 103.2 | NG | 4–8h | 1.7 | Van Doremalen et al., 2020 | ||
| Glass | SARS-CoV Strain P9 |
106 | RT | 4days | 6 | Duan et al. (2003) | ||
| HCoV 229E | 103 | 21 °C | >5days | 2.5 | Warnes et al. (2015) | |||
| SARS-CoV-2 | 105.8 | 22 °C | 4days | 5.8 | Alex Chin et al. (2020) | |||
| Aluminum | HCoV 229E | 5.5 × 10⁵ | 21 °C | <8h | 3 | Sizun, Yu, and Talbot (2000) | ||
| HCoV OC43 | 5.15 × 10⁵ | 21 °C | <2h | 3 | ||||
| Wood | SARS-CoV Strain P9 |
10⁶ | RT | 4days | 6 | Duan et al. (2003) | ||
| SARS-CoV-2 | 105.6 | 22 °C | 1–2days | 5.6 | Alex Chin et al. (2020) | |||
| Latex | HCoV 229E | 5.5 × 105 | 21 °C | 3–6h | 3 | Sizun et al. (2000) | ||
| HCoV OC43 | 5.15 × 105 | 21 °C | <1h | 3 | ||||
| Paper | SARS-CoV strain GVU6109 | 104 | RT | <5min | ∼1.7 | Lai, Cheng, and Lim (2005) | ||
| SARS-CoV strain GVU6109 | 105 | RT | 3h | ∼2.7 | ||||
| SARS-CoV strain GVU6109 | 106 | RT | 24h | ∼3.7 | ||||
| SARS-CoV Strain P9 |
106 | RT | 4–5days | 6 | Duan et al. (2003) | |||
| SARS-CoV-2 | 104.8 | 22 °C | 3h | 4.8 | Alex Chin et al. (2020) | |||
| Tissue paper | SARS-CoV-2 | 107.8 | 22 °C | 30min | 5.5 | |||
| Banknote | SARS-CoV-2 | 107.8 | 22 °C | 2d | 6 | |||
| Cardboard | SARS-CoV-2 | 102.5 | NG | 24h | 2 | Van Doremalen et al., 2020 | ||
| Cotton | SARS-CoV strain GVU6109 | 104 | RT | 5min | ∼1.7 | Lai et al. (2005) | ||
| SARS-CoV strain GVU6109 | 105 | RT | 1h | ∼2.7 | ||||
| SARS-CoV strain GVU6109 | 106 | RT | 24h | ∼3.7 | ||||
| Silicon rubber | HCoV 229E | 103 | 21 °C | 3days | 3 | Warnes et al. (2015) | ||
| Ceramic | HCoV 229E | 103 | 21 °C | >5days | 2 | |||
| Teflon | HCoV 229E | 103 | 21 °C | >5days | 2.5 | |||
| PVC | HCoV 229E | 103 | 21 °C | >5days | 2 | |||
| Plastic | SARS-CoV Strain HKU39849 |
107 | 22–25 °C | 5–28days | 5 | Chan et al. (2011) | ||
| SARS-CoV Strain P9 |
106 | RT | 4days | 6 | Duan et al. (2003) | |||
| SARS-CoV strain FFM-1 | 107 | RT | 6–9days | ∼5 | Rabenau et al. (2005) | |||
| HCoV 229E | 107 | RT | 2–6days | ∼5 | ||||
| SARS-CoV-2 | 103.7 | NG | 3–4days | 3.2 | Van Doremalen et al., 2020 | |||
| SARS-CoV-2 | 105.8 | 22 °C | 7days | 5.8 | Alex Chin et al. (2020) | |||
| Metal | SARS-CoV Strain P9 |
106 | RT | 5days | NG | Duan et al. (2003) | ||
| Brass | 95–100% Cu | HCoV 229E | 103 | 21 °C | 10min | 3 | Warnes et al. (2015) | |
| 85% Cu | HCoV 229E | 103 | 21 °C | 50min | 3 | |||
| 60% Cu | HCoV 229E | 103 | 21 °C | 2h | 2.5 | |||
| Copper nickel | 90% Cu | HCoV 229E | 103 | 21 °C | 20min | 3 | ||
| 79% Cu | HCoV 229E | 103 | 21 °C | 30min | 3 | |||
| 70% Cu | HCoV 229E | 103 | 21 °C | 4h | 3 | |||
| Zinc | HCoV 229E | 103 | 21 °C | 2h | 0.5 | Warnes et al. (2015) | ||
| Cloth | SARS-CoV-2 | 107.8 | 22 °C | 1d | 4.8 | Alex Chin et al. (2020) | ||
| Surgical Mask | Outer layer | SARS-CoV-2 | 107.8 | 22 °C | 7d | 5.8 | ||
| Inner layer | SARS-CoV-2 | 107.8 | 22 °C | 4d | 5.8 | |||
| Cotton gauze sponges | HCoV 229E | 5.5 × 105 | 21 °C | 6h | 3 | Sizun et al. (2000) | ||
| HCoV OC43 | 5.15 × 105 | 21 °C | <1h | 3 | ||||
| HCoV: Human coronavirus, NG: Not Given, RT: Room Temperature (20–25 °C) | ||||||||
Van Doremalen et al. (2020) analyzed the persistence of SARS-CoV-2 in aerosols and surfaces, including plastic, stainless steel, copper, and cardboard, and compared the results with SARS-CoV, the most closely related human coronavirus. SARS-CoV-2 persisted in aerosols for 3 h with a reduction in the infectious particle titer from 103.5 to 102.7 TCID50 (the median tissue culture infectious dose) per liter of air; this was similar to SARS-CoV, with a reduction from 104.3 to 103.5 TCID50 per liter. SARS-CoV-2 could survive for 72 and 48 h after application to plastic and stainless steel, respectively, but no viable virus titer was measured on copper and cardboard at 4 h and 24 h after application, respectively. The stability kinetics of SARS-CoV were similar to SARS-CoV-2 under the experimental circumstances, indicating that other factors such as the viral load in the upper respiratory tract and potential inoculum shed are associated with the epidemiologic characteristics of these viruses.
Alex et al. (2020) investigated the stability of SARS-CoV-2 on different surfaces. No infectious virus could be recovered from printing or tissue paper at 3 h post-exposure and from wood and cloth at two days post-exposure. However, SARS-CoV-2 was more stable on other fomites; it survived for four days on glass and banknotes and seven days on stainless steel and plastic. The authors also found that a significant virus titer (2.79 ± 0.46 log TCID50/ml) still survived on the outer layer of a surgical mask seven days after inoculation, suggesting extra precaution when wearing or disposing of them. Kampf et al. (2020) analyzed the persistence of HuCoVs on different types of inanimate surface and revealed various infectious periods from 2 h up to nine days. The authors also found that certain environmental conditions such as temperature and humidity could influence the viability of the viruses; higher temperatures (30 or 40 °C) decreased the survival duration of the coronaviruses on inanimate surfaces whereas the viruses remained viable for up to 9 day at 4 °C. All of this information from the literature clearly indicates that frequent contact with fomites and other objects is a potential source of viral transmission.
5. Inactivation of and control measures for the SARS-CoV-2 virus in the food system
The food industry is facing huge uncertainties regarding the presence of SARS-CoV-2 in food production and distribution (Oliveira, Abranches, & Lana, 2020). Currently, there are no approved specific antiviral drugs, cures, or vaccines for SARS-CoV-2 and so prevention relies on personal hygiene, including adequate disinfection of environments and food-contact surfaces (Yang, 2020) and social distancing (Makroo, Majid, Siddiqi, Greiner, & Dar, 2020). Therefore, this chapter will discuss the inactivation methods by analyzing the current literature.
5.1. SARS-CoV-2 inactivation via chemical disinfectants
Inactivation of coronaviruses including SARS-CoV-2 has been studied widely and the use of biocidal surfaces could be effective at reducing the spread of viruses. Since the start of the COVID-19 pandemic, significant efforts have been made to remove SARS-CoV-2 from environmental surfaces but very limited data are available on removing the virus from food surfaces. At this point, disinfection is likely the best practice in community and household settings to reduce the spread of COVID-19 infection. A wide variety of disinfectants are available that are generally cost-effective, easy to use, and have a range of uses on commonly touched surfaces. Coronaviruses including SARs-CoV-2 are known to be susceptible to and easily inactivated by certain biocidal agents such as chlorine and its derivate and ethanol (Quevedo-León et al., 2020). Zuber and Brüssow (2020) indicated that surface disinfection to inactivate human and animal coronaviruses can be achieved with 62–71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 min. Table 2 reports various chemical disinfectants against coronaviruses.
Table 2.
Virucidal efficacy of disinfectants against coronaviruses.
| Disinfectant | Type of assay | Concentration | Exposure time | Virus | Reduction of viral infectivity | Reference |
|---|---|---|---|---|---|---|
| Ethanol | suspension test | 80% | 30s | SARS-CoV strain FFM-1 | ≥4.25 | Rabenau et al. (2005) |
| 85% | 30s | SARS-CoV strain FFM-1 | ≥5.5 | |||
| 95% | 30s | SARS-CoV strain FFM-1 | ≥5.5 | |||
| 70% | 10min | MHV-2 | >4.20 | Saknimit, Inatsuki, Sugiyama, and Yagami (1988) | ||
| 70% | 10min | MHV-N | >3.91 | |||
| 70% | 10min | CCV strain I-71 | >3.28 | |||
| 78% | 30s | SARS-CoV strain FFM-1 | ≥5.01 | Rabenau et al. (2005) | ||
| 20% | 30s | SARS-CoV-2 | 1.08 | Yin, Ling, Hong, and Yan (2020) | ||
| 20% | 1min | SARS-CoV-2 | 1.33 | |||
| 20% | 3min | SARS-CoV-2 | 1.75 | |||
| 20% | 5min | SARS-CoV-2 | 1.92 | |||
| 30% | 30s | SARS-CoV-2 | 4.42 | |||
| 30% | 1min, 3min, 5min | SARS-CoV-2 | ≥4.75 | |||
| 40, 50, 60, 75% | 30s, 1min, 3min, 5min | SARS-CoV-2 | ≥4.75 | |||
| QCT with stainless steel | 70% | 1min | HCoV 229E | ≥3.0 | Satter et al. (1989) | |
| 62% | 1min | MHV | 2.66 | Hulkower, Casanova, Rutala, Weber, and Sobsey (2011) | ||
| 70% | 1min | MHV | 3.92 | |||
| 71% | 1min | MHV | 1.98 | |||
| 62% | 1min | TGEV | 4.04 | |||
| 70% | 1min | TGEV | 3.19 | |||
| 71% | 1min | TGEV | 3.51 | |||
| Sodium Hypochlorite | suspension test | 0.001% | 10min | MHV-2 | 0.57 | Saknimit et al. (1988) |
| 0.001% | 10min | MHV-N | 0.26 | |||
| 0.001% | 10min | CCV strain I-71 | 0.90 | |||
| 0.01% | 10min | MHV-2 | 2.82 | |||
| 0.01% | 10min | MHV-N | 2.26 | |||
| 0.01% | 10min | CCV strain I-71 | 1.05 | |||
| 0.21% | 30s | MHV-1 | ≥4.0 | Dellanno, Vega, and Boesenberg (2009) | ||
| QCT with stainless steel | 0.06% | 1min | MHV | 0.62 | Hulkower et al. (2011) | |
| 0.06% | 1min | TGEV | 0.35 | |||
| 0.01% | 1min | HCoV 229E | <3.0 | Satter et al. (1989) | ||
| 0.1% | 1min | HCoV 229E | >3.0 | |||
| 0.5% | 1min | HCoV 229E | >3.0 | |||
| Hydrogen Peroxide | suspension test | 0.5% | 1min | HCoV 229E | >4.0 | Omidbakhsh and Sattar (2006) |
| MHV: Murine coronavirus, CCV: Canine coronavirus, HCoV: Human coronavirus, TGEV: A coronavirus which infects pigs | ||||||
Kampf et al. (2020) confirmed the effective inactivation of SARS-CoV-2 on various surfaces and found that the virus is more resistant on smooth surfaces than hard ones; 62–71% ethanol reduced SARS-CoV-2 infectivity by 2.0–4.0 log10 within 1 min, while 0.1–0.5% sodium hypochlorite or 2% glutardialdehyde was also effective, with >3.0 log10 reduction in the viral titer within 1 min. Alex et al. (2020) also evaluated the virucidal effects of various disinfectants against SARS-CoV-2 under different environmental conditions; no infectious virus particles were detected after 5 min treatment with household bleach, ethanol (70%), povidone-iodine, chloroxylenol (0.05%), chlorhexidine (0.05%), or benzalkonium chloride (0.1%). The exception was hand soap, which required 15 min to completely inactivate SARS-CoV-2. Yoshimoto et al. (2020) evaluated the SARS-CoV-2 inactivation effect of acetic acid and vinegar for food safety, which were 4% and 6%, respectively, and effectively reduced the viral load by over 4 log10 after 5 min treatment. The WHO (2020c) recommends the cleaning and disinfection of environmental surfaces with hospital-level disinfectants to reduce the COVID-19 infection. They indicate that bleach (e.g., dilution 1:100 of 5% sodium hypochlorite to a final concentration of 0.05%) or 0.5% hydrogen peroxide are effective. Earlier, they recommended a concentration of 70% ethanol for the inactivation of viruses on small surfaces (WHO, 2014); however, these chemical agents are not always safe for environments and human health, and so verification of the inactivation efficacy for each chemical disinfectant is necessary for safe application, particularly if it involves food and water for human consumption. Moreover, it is essential to search for proper disinfectants that can be used directly on food surfaces and to establish appropriate doses and methods to reduce the risk of SARS-CoV-2 infection.
5.2. Heat inactivation
Many studies have shown that the persistence of coronaviruses is influenced by various environmental conditions, particularly temperature and relative humidity, which can thus be used as public intervention measures. Heat inactivation could be considered and successfully applied for food safety if the kinetics of inactivation and diminished virus infectivity are understood (Steardo, Steardo Jr, Zorec, & Verkhratsky, 2020). Increased temperature has been associated with a reduction in coronavirus titer and decreased relative humidity can decrease their infectivity (Aboubakr, Sharafeldin, & Goyal, 2020). The viability of SARS-CoV is degraded and rapidly lost at high temperatures and high relative humidity (Chan et al., 2011). In addition, MERS-CoV was removed from dromedary camel, cow, and goat milk spiked with the virus after heat treatment at 63 °C for 20 min (Van Doremalen et al., 2014). Similarly, SARS-CoV in protein-containing solutions lost its infectivity after heat treatment at 60 °C for 30 min (Rabenau, Cinatl, et al., 2005; Rabenau, Kampf, Cinatl, & Doerr, 2005). More recently, Pastorino, Touret, Gilles, de Lamballerie, and Charrel (2020) evaluated the effect of three heat inactivation protocols (56 °C for 30 min, 60 °C for 60 min, and 92 °C for 15 min) on SARS-CoV-2 using infected cell culture supernatant, virus-spiked human sera, and nasopharyngeal samples. They observed a 4 log10 TCID50 reduction regardless of the protocol and the type of sample. However, samples containing viral loads >6 log10TCID50 still remained infectious after 56 °C at 30 min and 60 °C at 60 min, although viral loads <10 TCID50 did not. Thus, they suggested taking precautions when handling food contaminated with a high viral load. Alex Chin et al. (2020) inhibited SARS-CoV-2 by heat treatment for 5 min at 70 °C. Similarly, SARS-CoV-2 in both human sera and sputum samples was inactivated within 30 and 15 min at 56 and 65 °C, respectively (Wang, Lien, Liu, & Selveraj, 2020). Henwood (2020) reported that treatment at 56–67 °C for 60–90 min is sufficient to inactivate SARS-CoV-2. These results indicate that food is probably safe from SARS-CoV-2 when cooked at the general cooking temperature (70 °C).
Although SARS-CoV-2 is unstable to heat, there may still be limitations in using heat treatment for inactivating SARS-CoV-2. Due to the very limited knowledge about the relationship between SARS-COV-2 and foodstuffs, many studies on inactivating the virus have relied on the previous data of studies on other coronaviruses. Moreover, heat inactivation cannot be applied to many fresh food products. Freezing is another conventional method for preserving various foodstuffs, although transmitting the virus from frozen food remains possible. Therefore, finding an effective alternate means of reducing the risk of viral transmission through fresh foods is an attractive target for future research.
5.3. Ultraviolet (UV) treatment
The WHO (2020d) issued guidance on infection, prevention, and control (IPC) strategies regarding the prevention of droplets, contact, and airborne transmission, along with support treatment for COVID-19 and strategies to extend the lifespan of medical equipment and to disinfect fomites. These strategies include using UV light-based innovations, robot-controlled UV surface disinfection in hospital rooms, and microbial inactivation in food safety applications.
Viruses are known to be especially vulnerable to UV at wavelengths near 253.7 nm (the UVC range) as the maximum absorption wavelength of DNA molecules is around 260 nm (Quevedo-León et al., 2020). At present, scant data are available on the inactivation ability of UV against coronaviruses (Table 3 ). Darnell, Subbarao, Feinstone, and Taylor (2004) inactivated SARS-CoV with UV exposure at 254 nm, showing partial inactivation at 1 min with increasing efficiency up to 6 min, which was indicated by a 400-fold decrease in the virus: the greater inactivation by UV was due to the greater intensity of the UVC light and close proximity to the light source. SARS-CoV and SARS-CoV-2 are structurally similar, thus it is assumed that SARS-CoV and SARS-CoV-2 will show similar UV inactivation efficacy, even though viral sensitivity to UV can vary widely.
Table 3.
The effect of UV on coronavirus.
| Virus | Waves | Intensity of UV | Notes | Reference | |
|---|---|---|---|---|---|
| SARS-CoV-2 | MOI 0.05 | 254 nm | 3.7 mJ/cm2 | SARS-CoV-2 replication was completely inactivated at UV-C dose of 3.7 mJ/cm2 after 6 days | Bianco et al. (2020) |
| MOI 5 | 3.7 mJ/cm2 | The UV-C dose of 3.7 mJ/cm2 was effective in a reduction of viral replication (3 log reduction after 24 h). | |||
| MOI 1000 | 16.9 mJ/cm2 | Viral replication was totally inactivated at a dose 16.9 mJ/cm2. | |||
| SARS-CoV | BSC's UV lamp |
134 μW/cm2 | After 15min UV exposure, the titer of virus went down to 1.8 × 102 TCID50/mL (initial titer was 3.8 × 107 TCID50/mL). But, the virus was not completely inactivated (18.8 TCID50/mL), even after 60min of irradiation. |
Kariwa, Fujii, and Takashima (2006) | |
| SARS-CoV strain P9 | 260 nm | >90 μW/cm2 | After 15 min UV exposure, the CPE of SARS-CoV was reduced from 51 to 75% to less than 25% and dropped to an undetectable level after 60 min irradiation. | Duan et al. (2003) | |
| SARS-CoV | 365 nm (UV-A) | 2133 μW/cm2 | For more than 15min, UV-A exposure didn't have significant effects on virus inactivation. | Darnell., 2004 | |
| 254 nm (UV-C) | 4016 μW/cm2 | UV-C exposure to virus showed increasing efficiency up to 6min (resulting in a 400-fold decrease in infectious virus). And there was no additional inactivation from 6 to 1 min. |
|||
| MOI 0.05: Low-level concentration observed in closed environments (e.g. hospital rooms) MOI 5: Intermediate-level concentration corresponds to the average concentration found in the sputum of COVID-19 infected patients MOI 1000: High-level concentration corresponds to that observed in terminally diseased COVID-19 patients BSC's UV lamp: Biosafety Cabinet's UV lamp (typically 254 nm) | |||||
The majority of UV-based inactivation studies have been conducted on target viruses suspended in water, thus this approach may be suitable for water-based food and environmental samples. It is known that inactivation doses are generally higher in water than on solid surfaces and various factors such as the type and structure of the surface as well as the relative humidity of the air and the temperature can influence the UV dose to inactivate viruses (International Ultraviolet Association, 2020). Recently, Bianco et al. (2020) evaluated the veridical effects of UVC irradiation on SARS-CoV-2 in water for different exposure doses and virus concentrations (1,000, 5, and 0.5 MOI (multiplicity of infection)); for UVC treatment at 254 nm; a dose of 3.7 mJ cm2 reduced SARS-CoV-2 by 3 log10 in water and a dose of 16.9 mJ cm2 completely inactivated all virus particles. Inagaki, Saito, Sugiyama, Okabayashi, and Fujimoto (2020) decontaminated SARS-CoV-2 using deep UV light-emitting diodes (DUV-LEDs) with a dose of roughly 38 mJ cm2 at 280 nm, showing a reduction of 3 log10. These results are extremely important for developing efficient UV-based methods for reducing the spread of COVID-19. Further UV-based inactivation studies are required to establish important details regarding exposure time and dose for quantification as well as robust validation before the large-scale application of germicidal UV-based methods.
6. Roles of food ingredients in the immune system
The COVID-19 pandemic is related to other well-known outbreaks over the past 20 years, including SARS and MERS, which are lower respiratory diseases with similar clinical representations in the early stages of infection (fever and cough) and result in significant mortality among vulnerable individuals (those who do not have a strong immune system, those who smoke, and the elderly) (Das, 2020). The consumption of vitamin-rich and functional foods can improve the immune system to help suppress viruses (Gibson et al., 2012). Vitamin C, which enhances the immune system and is essential for the growth and repair of body tissues, is known to play a protective role (Li et al., 2020). In addition, vitamin A contains several fat-soluble compounds (including retinol, retinoic acid, and β-carotene) that play a major role in immune function and are known to reduce infection susceptibility (Huang, Liu, Qi, Brand, & Zheng, 2018). For example, isotretinoin controls the downregulation of angiotensin-converting enzyme 2 (ACE2), which is a cellular protein required for the entry of SARS-COV-2 (Sinha, Cheng, Aldape, Schiff, & Ruppin, 2020). Besides, Vitamins D and E can increase resistance to COVID-19 (Im, Kim, & Min, 2016). Bioactive lipids such as arachidonic acid and other unsaturated fatty acids can enhance resistance to and recovery from SARS-CoV-2. Natural polyphenols including hesperidin and rutin have demonstrated efficacy as COVID-19 main protease (Mpro) inhibitors, which are considered a major target for therapeutic drugs (Adem, Eyupoglu, Sarfraz, Rasul, & Ali, 2020). It has also been shown that herbal and Chinese medicines aid in the treatment of viral diseases. For example, ginseng root is useful in the treatment of respiratory viral diseases (Im et al., 2016; Kolodziej, 2011). Astragalus membranaceus is commonly used to treat colds and upper respiratory infections (Luo et al., 2020), while Pelargonium sidoides is an effective herbal remedy for the prevention of respiratory viruses (Kolodziej, 2011). Chinese herbal formulas have been used to treat H1N1 and SARS influenza in high-risk populations, suggesting that they could provide an alternative approach to COVID-19 treatment and prevention (Luo et al., 2020). Some bioactive foods discovered in Chinese medicine (e.g., plant-derived phenolic compounds, flavonoids from litchi seeds, and quercetin) are known to inhibit SARS 3-chymotrypsin-like protease (3CLpro) enzymatic activity. This enzyme is essential for SARS-CoV replication and could become a treatment agent against SARS-CoV-2 and a supporting care agent for COVID-19 patients (Yang, Islam, Wang, Li, & Chen, 2020). Dietary supplements containing vitamins, bioactive lipids, flavonoids, and herbs could be a useful tool against COVID-19 by aiding the human immune system. Nevertheless, there is still no strong evidence as of August 7, 2020 that such bioactive ingredients will sufficiently boost the immune function to prevent or cure COVID-19. Moreover, in the new era of the COVID-19 pandemic, the search is on for potential drugs to enhance the immune system in the future.
7. Future prospects: Preparation for post-COVID-19 food systems
The COVID-19 pandemic has broad implications for international ties, economic growth, and sustainable agriculture. Overall, identifying the key challenges for agriculture and food policies after COVID-19 is necessary to choose adequate equipment and rewards, to maintain appropriate relationships among various policy fields, and to weigh up the value of different social goals. The effect of COVID-19 on agriculture and food system output and the principal difficulties during the post-COVID-19 recovery period will involve food and trading markets, food safety, and food practices.
7.1. Challenges for food markets and trade
The interest in short supply chains and national food safety will grow following the COVID-19 pandemic. Countries and companies are trying to diversify their manufacturing locations to minimize costs and some supply chains have fully changed to provide sufficient medical equipment during emergencies.
Critical items can be supplied locally, including medical supplies, medical equipment, and essential food products. There are major implications for the cost of food as countries may refrain from their competitive use for the large-scale production of certain foodstuffs while using interregional and international trade to ensure access to a broader range of commodities.
Another important challenge is sustaining affordable food prices for poorer consumers who mainly rely on access to food markets resulting from free and transparent trade flows. It is necessary to ensure food safety quality and to develop their internal spatial configuration for regional wet markets, which would support poor and vulnerable customers through the provision of healthy foods; this would largely depend on indirect welfare systems.
7.2. Challenges to food security and safety
Food safety is a scientific discipline describing how food is handled, prepared, and stored in ways that prevent foodborne diseases. It requires a variety of protocols to prevent unnecessary health risks. Effective food control systems are essential for protecting consumer health and safety. The new global food trade framework puts significant responsibilities on both importing and exporting countries to improve their food safety systems and to adopt and execute food safety policies based on risk (M&M Technologies, 2012). Meanwhile, existing trade laws require the implementation of defined food safety requirements, so encouraging greater commercial integration will require a stronger emphasis on safety and wellness. It should be concluded that food safety impacts both intensive and extensive margins in domestic and international trade, while manufacturers may prefer informal markets if they find the formal criteria of food protection too onerous. Therefore, food safety has trade implications. Public food safety concerns regarding the post-COVID-19 era are massive and expertise in the tools to ameliorate them is scarce. The COVID-19 crisis is beginning to focus authorities' attention on the major food security and health issues, including food security risks and ways of reducing them, as well as better communicating with both the general public and the private food sector. The major challenge for the recovery package will be to close the policy–implementation gap, thereby making food safety and hygienic practices the new benchmark from farm to fork.
The COVID-19 response among all stakeholders, including governments, the agri-food industry, regulators, and consumers, has the potential to change food safety, and fostering such demand depends partly on future research into food safety costs, performance evaluations, and risk communication (Roy, 2020). Although the information on proving a link between SARS-CoV-2 transmission and food is limited, the potential role of food as a carrier of viruses is being researched intensively by many authorities such as the EFSA and the US FDA to control the spread of COVID-19 worldwide.
7.3. Limitations and future research suggestions for food safety in the post-COVID-19 era
The COVID-19 pandemic caused by SARS-CoV-2 has brought about changes in every sector in the world as well as in different aspects of our daily lives. Within the COVID-19 pandemic and the forthcoming post-lockdown period, the food industry should strictly follow Food Safety Management Systems (FSMS) based on the Hazard Analysis Critical Control Point (HACCP) principles at every stage of food processing, manufacturing, packaging, and marketing process to prevent food contamination. Although coronaviruses cannot multiply in food (they need an animal or human host), more research is required to manage risks when handling raw food such as meat, fish, shrimp, and eggs. In addition, intensive research should be conducted to discover safer food packaging materials and ways of avoiding cross-contamination.
The post-pandemic phase may result in major reviews of food systems with special emphasis on resilience. Up until the pandemic crisis, the discourse within the food research community was dominated by the design and manufacture of healthy and safe foods. The main issues are relevant to the sustainability, circular economy, energy and water efficiency, and climate-friendly practices of products and processes. One of the greatest challenges in crisis planning is developing food systems that are sufficiently resilient to continue functioning. The potential policy tools for cities and local governments to strengthen the resilience of city food systems are summarized in Table 4 . Hecht, Biehl, Barnett, and Neff (2019) identified 10 factors that contribute to organization-level resilience in food supply chains: formal emergency planning, staff training, staff attendance, redundancy of food supply, food suppliers’ infrastructure, location, service providers, insurance, and post-event learning. Implementation of any changes toward a more resilient future for food safety requires that key stakeholders, including industry, policymakers, governments, and consumers, all have an active role.
Table 4.
Potential policy tools for cities and local governments to strengthen the resilience of food systems during and post-pandemic phase.
| 1. FAO published a document with five specific recommendations that countries should measure to avoid vital crises in food supply chains, including: - (I) “Expand and improve emergency food assistance and social protection programs”, - (II) “give smallholder farmers support to both enhance their productivity and market the food they produce, also through e-commerce channels”, - (III) “keep the food value chain alive by focusing on key logistics bottlenecks”, - (IV) “address trade and tax policies to keep the global trade open”, - (V) “manage the macroeconomic ramifications.” Cullen (2020) |
2. FAO has recommended a series of actions to assure the sustainability of agri-food companies during the crisis, including: - to practice the awareness of companies close to normal prices, - to protect the food market in the long term, - development of strategic management and partnerships with companies in the food sector, - service providers from local companies and chambers of commerce, among other measures. (FAO, 2020a) |
| 3. FAO has advised countries to develop logistical strategies to reduce the loss and waste of agricultural food products (mainly perishable food products), due to barriers of transportation routes, restrictions on transportation and social distance, in order to ensure sufficient supply food for all, mostly for the vulnerable. FAO (2020b) |
4. Some city authorities and governments have been taken few policy during COVID-19: - Some city authorities (e.g. Wuhan, New York) have taken steps to stop the increasing of food insecurity during COVID-19 by developing plan to identify vulnerable populations and provide food to them. - Some city authorities provide support civil society organizations to co-ordinate their work in delivering urgent relief food for vulnerable people (e.g. Toronto). - Some city authorities provide food vouchers to vulnerable people to access a healthy diet (e.g. Seattle). - Some city develops online maps to support citizens find available food services and food relief (e.g. Milan) C40 Cities (2020) |
| 5. The government has expanded support to non-governmental groups addressing the challenge of food insecurity. - For example, food banks, are presently challenged to maintain and expand their capacity to address food insecurity. - Food banks are particularly important to the most food insecure households. (Tarasuk, St-Germain, & Loopostra, 2019) |
6. COVID-19 offers government the opportunity to review their ‘current resilient toolkit’ for food systems and to establish new plan or policy. Few policy or plan that might be considered as part of a future food system resilience toolkit, based on the lessons of COVID-19 pandemic, including: - To identify the vulnerable populations and make a data collection list. - To develop the real-time data collection to assess the impact on food security - To develop a plan to address ongoing food insecurity after the initial shock - To develop a government food resilience policy and program that aims to bolster the food system resilience - To ensure ongoing population surveys to understand the distribution of food insecurity - To assure emergency relief for most vulnerable populations - To support civil society organizations to co-ordinate their work in delivering urgent relief food for vulnerable people - To aid city and local markets to keep open during shocks like COVID-19 - To help community gardens - To promote online platforms that directly support farmer livelihoods - To protect peri-urban farmland through land use planning policy - To establish a connection between food system, industry, civil society, and government works in rural areas |
Agri-food systems may take advantage of opportunities to make changes such as making more use of locally produced food; this would remove dependence on long-distance transportation and distribution by third parties with a major carbon footprint. Shifts in the paradigm for safe food practices should be reinforced by promoting safety habits that were developed and acquired during the pandemic. The food science and technology community will be positioned to strategically plan and contribute to the recovery of the food sector in collaboration with other allied disciplines and stakeholders. The role of food scientists and technologists in shaping government policies and in decision-making strategies must be considered to ensure the food supply chain's readiness to respond to any future pandemic.
As the COVID-19 hazard could be long-lasting, there is no alternative to developing an accurate and fast detection method for SARS-CoV-2 in food surfaces and the surrounding environment to ensure food safety. The COVID-19 pandemic has brought new challenges for researchers to ensure food security by detecting SARS-CoV-2 in environments where food is manufactured, packaged, and distributed. Moving to the post-COVID-19 era, the food industry and food supply will increasingly depend on the development of relevant bioanalytical tools alongside rules and regulations including good hygiene practices, cleaning, sanitation, and maintaining social distance among workers in the food industry, all of which should be followed strictly.
8. Conclusions
The COVID-19 pandemic crisis has started a new epoch in the food industry. At time of writing, the whole world is fighting SARS-CoV-2 to reduce the outbreak. Understanding the epidemiology of COVID-19 and investigating the exact transmission route of the virus is not simple in a pandemic. Up to now, there has been no confirmed evidence concerning the transmission of SARS-CoV-2 through the ingestion of contaminated food and water. However, questions remain unanswered due to the stability of SARS-CoV-2 under a variety of environmental conditions and its persistence on commonly touched surfaces, including food-contact surfaces. Therefore, evaluating the potential impact of the virus on food safety is an extremely important issue for governments, the food industry, and consumers worldwide. Some effective strategies such as heating, chemical disinfectants, and UV to inactivate the virus are covered herein. However, further studies, including adequate strategies for inactivating SARS-CoV-2 through international guidelines, are required to ensure food safety and to control the COVID-19 pandemic.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This Research was supported by a grant (20162MFDS039) from Ministry of Food and Drug Safety in 2020.
References
- Abbaspourrad A., Padilla-Zakour O., Wiedmann M., Moraru C.I., Goddard J.M. Taking a systems approach to clean label challenges. Food Technology. 2017;71(11):32–43. [Google Scholar]
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transboundary and Emerging Diseases. 2020;133(1510):6. doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adem S., Eyupoglu V., Sarfraz I., Rasul A., Ali M. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: An in silico strategy unveils a hope against CORONA. Preprints. 2020 doi: 10.20944/preprints202003.0333.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex Chin J.C., Perera M., Hui K., Yen H.L., Chan M., Poon L. Stability of SARS-CoV-2 in different environmental conditions. 2020. medRxiv. [DOI] [PMC free article] [PubMed]
- Basu S. Close-range exposure to a COVID-19 carrier: Transmission trends in the respiratory tract and estimation of infectious dose. medRxiv. 2020 doi: 10.1101/2020.07.27.20162362. 2020. [DOI] [Google Scholar]
- Bhunnoo R. The need for a food-systems approach to policy making. The Lancet. 2019;393:1097–1098. doi: 10.1016/S0140-6736(18)32754-5. 10176. [DOI] [PubMed] [Google Scholar]
- Bianco A., Biasin M., Pareschi G., Cavalleri A., Cavatorta C., Fenizia F., et al. SSRN; 2020. UV-C irradiation is highly effective in inactivating and inhibiting SARS-CoV-2 replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C40 Cities . 2020. COVID-19 and food: FAQs. Updated 14 May. C40 Cities. [Google Scholar]
- Carlos W.G., de la Cruz C.S., Cao B., Pasnick S., Jamil S. COVID-19 disease due to SARS-CoV-2 (novel coronavirus) American Journal of Respiratory and Critical Care Medicine. 2020;201(4):7–8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- Carraturo F., Del Giudice C., Morelli M., Cerullo V., Libralato G., Galdiero E., et al. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environmental Pollution. 2020;265 doi: 10.1016/j.envpol.2020.115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html/
- Chan K.H., Peiris J.M., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Advances in Virology. 2011;2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. The Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Brahma S., Mackay J., Cao C., Aliakbarian B. The role of smart packaging system in food supply chain. Journal of Food Science. 2020;85(3):517–525. doi: 10.1111/1750-3841.15046. [DOI] [PubMed] [Google Scholar]
- Clancy K. Digging deeper: Transdisciplinary and systems approaches to food security. Journal of Agriculture, Food Systems, and Community Development. 2017;7(4):13–16. doi: 10.5304/jafscd.2017.074.012. [DOI] [Google Scholar]
- CoronaBoard COVID 19 dashboard. 2020. http://coronaboard.com/global/ko/
- Cullen M.T. COVID-19 and the risk to food supply chains. How to respond? 2020. http://www.fao.org/2019-ncov/analysis/en/
- Dai M., Li H., Yan N., Huang J., Zhao L., Xu, et al. Long-term survival of salmon-attached SARS-CoV-2 at 4°C as a potential source of transmission in seafood markets. 2020. bioRxiv preprint. [DOI]
- Darnell M.E.R., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. Journal of Virological Methods. 2004;121(1):85–91. doi: 10.1016/j.viromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Archives of Medical Research. 2020;51(3):282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lima D.P., Fioriolli J.C., Padula A.D., Pumi G. The impact of Chinese imports of soybean on port infrastructure in Brazil: A study based on the concept of the “Bullwhip effect”. Journal of Commodity Markets. 2018;9:55–76. doi: 10.1016/j.jcomm.2017.11.001. [DOI] [Google Scholar]
- De Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. In: Roles of host gene and non-cording RNA expression in virus infection: Current topics in microbiology and immunology. Tripp R.A., Tompkins S.M., editors. Springer; Cham: 2017. Host factors in coronavirus replication; pp. 1–42. [DOI] [Google Scholar]
- Dellanno C., Vega Q., Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. American Journal of Infection Control. 2009;37(8):649–652. doi: 10.1016/j.ajic.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell S.F., Simmerman J.M., Erdman D.D., Wu J.S.J., Chaovavanich A., Javadi M., et al. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clinical Infectious Diseases. 2004;39(5):652–657. doi: 10.1086/422652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.M., Zhao X.S., Wen R.F., Huang J.j., Pi G.H., Zhang S.X., et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomedical and Environmental Sciences. 2003;16:246–255. [PubMed] [Google Scholar]
- EFSA Coronaviruses: No evidence that food is a source or transmission route. 2020. https://www.efsa.europa.eu/en/news/coronavirus-no-evidence-food-source-or-transmission-route/
- Euractiv No evidence of COVID-19 transmission through food, says EFSA. 2020. https://www.euractiv.com/section/coronavirus/news/no-evidence-of-covid-19-transmission-through-food-says-efsa/
- European Food Safety Authority Coronaviruses: No evidence that food is a source or transmission route. 2020. https://www.efsa.europa.eu/en/news/coronavirus-no-evidence-food-source-or-transmission-route/
- Fitzpatrick K.M., Harris C., Drawve G. Assessing food insecurity in the United States during COVID-19 pandemic. Montana. 2020;34:3. [Google Scholar]
- Food and Agriculture Organization Q&A: COVID-19 pandemic–impact on food and agriculture. 2020. http://www.fao.org/2019-ncov/q-and-a/impact-on-food-and-agriculture/en/
- Food and Agriculture Organization Director-General urges G20 to ensure that food value chains are not disrupted during COVID-19 pandemic. 2020. http://www.fao.org/news/story/en/item/1268254/icode/
- Food and Agriculture Organization Urban food systems and COVID-19: The role of cities and local governments in responding to the emergency. 2020. [DOI]
- Food and Agriculture Organization FAO warns about the impact of COVID-19 on school feeding in Latin America and the Caribbean. 2020. http://www.fao.org/americas/noticias/ver/es/c/1267028/
- Galanakis C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods. 2020;9(4):523. doi: 10.3390/foods9040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraturo F., del Guidice C., Morelli M., Cerullo V., Libralato G., Galdiero E., et al. Persistence of SARs-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environmental Pollution. 2020;265 doi: 10.1016/j.envpol.2020.115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Edgar J.D., Neville C.E., Gilchrist S.E., McKinley M.C., Patterson C.C., et al. Effect of fruit and vegetable consumption on immune function in older people: A randomized controlled trial. American Journal of Clinical Nutrition. 2012;96(6):1429–1436. doi: 10.3945/ajcn.112.039057. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., Groot R.J.D., Drosten C., Gulyaeva A.A., et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses-a statement of the coronavirus study group. 2020. bioRxiv. [DOI]
- Gottheil F.M. 7th ed. Cengage; Boston: 2013. Principles of Microeconomics. [Google Scholar]
- Hecht A.A., Biehl E., Barnett D.J., Neff R.A. Urban food supply chain resilience for crises threatening food security: A qualitative study. Journal of the Academy of Nutrition and Dietetics. 2019;119(2):211–224. doi: 10.1016/j.jand.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Henwood A.F. Coronavirus disinfection in histopathology. Journal of Histotechnology. 2020;43(2):102–104. doi: 10.1080/01478885.2020.1734718. [DOI] [PubMed] [Google Scholar]
- Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of vitamin A in the immune system. Journal of Clinical Medicine. 2018;7(9):258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Azhar E.I., Madani T.A., Nitoumi F., Kock R., Dar O., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower R.L., Casanova L.M., Rutala W.A., Weber D.J., Sobsey M.D. Inactivation of surrogate coronaviruses on hard surfaces by health care germicides. American Journal of Infection Control. 2011;39(5):401–407. doi: 10.1016/j.ajic.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K., Kim J., Min H. Ginseng, the natural effectual antiviral: Protective effects of Korean red ginseng against viral infection. Journal of Ginseng Research. 2016;40(4):309–314. doi: 10.1016/j.jgr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H., Saito A., Sugiyama H., Okabayashi T., Fujimoto S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerging Microbes & Infections. 2020;9(1):1744–1747. doi: 10.1080/22221751.2020.1796529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Ultraviolet Association SARS-CoV-2 UV dose-response behavior. 2020. https://iuva.org/resources/covid-19/SARS%20CoV2%20Dose%20Response%20White%20Paper.pdf
- Ivanov D., Dolgui A. Viability of intertwined supply networks: Extending the supply chain resilience angles towards survivability. A position paper motivated by COVID-19 outbreak. International Journal of Production Research. 2020;58(10):2904–2915. doi: 10.1080/00207543.2020.1750727. [DOI] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariwa H., Fujii N., Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212:119–123. doi: 10.1159/000089211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej H. Antimicrobial, antiviral and immunomodulatory activity studies of Pelargonium sidoides (EPs® 7630) in the context of health promotion. Pharmaceuticals. 2011;4(10):1295–1314. doi: 10.3390/ph4101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.Y., Cheng P.K., Lim W.W. Survival of severe acute respiratory syndrome coronavirus. Clinical Infectious Diseases. 2005;41(7):e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T.Y., Jia N., Zhang Y.W., Shum M.H., Jiang J.F., Zhu H.C., et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England Journal of Medicine. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chinese Journal of Integrative Medicine. 2020;26(4):243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M&M Technologies Food safety and food security. 2012. https://www.foodsafetymagazine.com/signature-series/food-safety-and-food-security/
- Makroo H., Majid D., Siddiqi M.A., Greiner R., Dar B.N. COVID-19 pandemic and its implication on food system. Preprints. 2020 doi: 10.20944/preprints202008.0321.v1. [DOI] [Google Scholar]
- Mullis L., Saif L.J., Zhang Y., Zhang X., Azevedo M.S. Stability of bovine coronavirus on lettuce surfaces under household refrigeration conditions. Food Microbiology. 2012;30(1):180–186. doi: 10.1016/j.fm.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycroft-West C., Su D., Elli S., Li Y., Guimond S., Miller G., et al. The 2019 coronavirus (SARS-CoV-2) surface protein (spike) S1 receptor binding domain undergoes conformational change upon heparin binding. 2020. bioRxiv. [DOI]
- Narayanan L., Pandit M., Basu S., Karmakar A., Bidhan V., Kumar H., et al. Impact of lockdown due to COVID-19 outbreak: Lifestyle changes and public health concerns in India. Preprints. 2020 doi: 10.20944/preprints202006.0129.v1. [DOI] [Google Scholar]
- Oliveira T.C., Abranches M.V., Lana R.M. Food security in Brazil in the context of the SARS-CoV-2 pandemic. Cadernos de Saúde Pública. 2020;36(4) doi: 10.1590/0102-311X00055220. [DOI] [PubMed] [Google Scholar]
- Omidbakhsh N., Sattar S.A. Broad-spectrum microbicidal activity, toxicologic assessment, and materials compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectant. American Journal of Infection Control. 2006;34(5):251–257. doi: 10.1016/j.ajic.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. Journal of Hospital Infection. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Heat inactivation of different types of SARS-CoV-2 samples: What protocols for biosafety, molecular detection and serological diagnostics? Viruses. 2020;12(7):735. doi: 10.3390/v12070735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pung R., Chiew C.J., Young B.E., Chin S., Chen M.I., Clapham H.E., et al. Investigation of three clusters of COVID-19 in Singapore: Implications for surveillance and response measures. The Lancet. 2020;395(1029):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo-León R., Bastías-Montes J.M., Espinoza-Tellez T., Ronceros B., Balic I., Muñoz O. Inactivation of coronaviruses in food industry: The use of inorganic and organic disinfectants, ozone, and UV radiation. Scientia Agropecuaria. 2020;11(2):257–266. doi: 10.17268/sci.agropecu.2020.02.14. [DOI] [Google Scholar]
- Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Medical Microbiology and Immunology. 2005;194(1–2):1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H.F., Kampf G., Cinatl J., Doerr H.W. Efficacy of various disinfectants against SARS coronavirus. Journal of Hospital Infection. 2005;61(2):107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley L. Meat processing plants are closing due to covid-19 outbreaks. Beef shortfalls may follow. 2020. https://www.washingtonpost.com/business/2020/04/16/meat-processing-plants-are-closing-due-covid-19-outbreaks-beef-shortfalls-may-follow/
- Rosales G., Mercado W. Effect of changes in food prices on quinoa consumption and rural food security in Peru. Scientia Agropecuaria. 2020;11(1):83–93. doi: 10.17268/sci.agropecu.2020.01.10. [DOI] [Google Scholar]
- Roy D. World food safety day 2020: COVID-19 offers an opportunity for India's food systems to deliver on safety and health. 2020. https://www.ifpri.org/blog/world-food-safety-day-2020-covid-19-offers-opportunity-indias-food-systems-deliver-safety-and/
- Saknimit M., Inatsuki I., Sugiyama Y., Yagami K.I. Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Experimental Animals. 1988;37(3):341–345. doi: 10.1538/expanim1978.37.3_341. [DOI] [PubMed] [Google Scholar]
- Sattar S.A., Springthorpe V.S., Karim Y., Loro P. Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiology and Infection. 1989;102(3):493–505. doi: 10.1017/S0950268800030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Cheng K., Aldape K., Schiff E., Ruppin E. Systematic cell line-based identification of drugs modifying ACE2 expression. Preprints. 2020 doi: 10.20944/preprints202003.0446.v1. [DOI] [Google Scholar]
- Sizun J., Yu M.W.N., Talbot P.J. Survival of human coronaviruses 229E and OC43 in suspension and after drying on surfaces: A possible source of hospital-acquired infections. Journal of Hospital Infection. 2000;46(1):55–60. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steardo L., Steardo L., Jr., Zorec R., Verkhratsky A. Neuroinfection may attribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiologica. 2020;229(3) doi: 10.1111/alph.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasuk V., St-Germain A.A.F., Loopostra R. The relationship between food banks and food security: Insights from Canada. VOLUNTAS. International Journal of Voluntary and Nonprofit organizations. 2019 doi: 10.1007/s11266-019-00092-w. [DOI] [Google Scholar]
- US Food and Drug Administration Agency Food safety and the coronavirus disease 2019 (COVID-19) 2020. https://www.fda.gov/food/food-safety-during-emergencies/food-safety-and-coronavirus-disease-2019-covid-19/
- Van Doremalen N., Bushmaker T., Karesh W.B., Munster V.J. Stability of Middle East respiratory syndrome coronavirus in milk. Emerging Infectious Diseases. 2014;20(7):1263–1264. doi: 10.3201/eid2007.140500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Lien C., Liu S., Selveraj P. Effective heat inactivation of SARS-CoV-2. 2020. medRxiv. [DOI]
- Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. Journal of Medical Virology. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. Journal of Virology. 2020;94(7):94e00127. doi: 10.1128/JVl.00127-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes S.L., Little Z.R., Keevil C.W. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6(6) doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Food Programme COVID-19 will double number of people facing food crises unless swift action is taken. 2020. https://www.wfp.org/news/covid-19-will-double-number-people-facing-food-crises-unless-swift-action-taken/
- World Health Organization . Infection prevention and control of epidemic and pandemic Prone Acute respiratory infections in health care. WHO Press; Geneva: 2014. Annex, G. Use of disinfectants: Alcohol and bleach; pp. 65–66. [PubMed] [Google Scholar]
- World Health Organization Coronavirus disease (COVID-19) pandemic. 2020. http://www.who.int/emergencies/diseases/novel-coronavirus-2019/
- World Health Organization COVID-19 and food safety: Guidance for food businesses. 2020. http://www.who.int/publications-detail/covid-19-and-food-safety-guidance-for-ffod-businesses
- World Health Organization Cleaning and disinfection of environmental surfaces in the context of COVID-19: Interim guidance. 2020. https://www.who.int/publications/i/item/cleaning-and-disinfection-of-environmental-surfaces-inthe-context-of-covid-19
- World Health Organization Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected. 2020. https://www.who.int/publications/i/item/10665-331495/
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged persistence of SARS-CoV-2 viral RNA in faecal samples. The Lancet Gastroenterology & Hepatology. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Does hand hygiene reduce SARS-CoV-2 transmission? Graefe’s Archive for Clinical and Experimental Ophthalmology. 2020;258:1133–1134. doi: 10.1007/s00417-020-04652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. International Journal of Biological Sciences. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yépiz-Gómez M.S., Gerba C.P., Bright K.R. Survival of respiratory viruses on fresh produce. Food and Environmental Virology. 2013;5(3):150–156. doi: 10.1007/s12560-013-9114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Ling W., Hong S., Yan X. Vitro inactivation of SARS-CoV-2 by commonly used disinfection products and methods. 2020. Preprints. [DOI] [PMC free article] [PubMed]
- Yoshimoto J., Ono C., Tsuchiya Y., Kabuto S., Kishi M., Matsuura Y. Virucidal effect of acetic acid and vinegar on SARS-CoV-2. 2020. Preprints. [DOI]
- Zhou P., Yang X., Wang X., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber S., Brüssow H. COVID-19: Challenges for virologists in the food industry. Microbial Biotechnology. 2020:1–13. doi: 10.1111/1751-7915.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]