Abstract
Stroke is the leading cause of death and the main cause of disability in surviving patients. The detrimental interaction between immune cells, glial cells, and matrix components in stroke pathology results in persistent inflammation that progresses to fibrosis. A substantial effort is being directed towards understanding the exact neuroinflammatory events that take place as a result of stroke. The initiation of a potent cytokine response, along with immune cell activation and infiltration in the ischemic core, has massive acute deleterious effects, generally exacerbated by comorbid inflammatory conditions. There is secondary neuroinflammation that promotes further injury, resulting in cell death, but conversely plays a beneficial role, by promoting recovery. This highlights the need for a better understanding of the neuroinflammatory and fibrotic processes, as well as the need to identify new mechanisms and potential modulators. In this review, we summarize several aspects of stroke-induced inflammation, fibrosis, and include a discussion of cytokine inhibitors/inducers, immune cells, and fibro-inflammation signaling inhibitors in order to identify new pharmacological means of intervention.
Keywords: Cytokines, Fibrosis, Immune cell infiltration, Neuroinflammation, Perlecan, Stroke
1. Introduction
Stroke, a sudden interruption of blood supply to the brain or blood vessel in the brain, causes neurological deficits (Ojaghihaghighi et al., 2017). Stroke is the 3rd leading cause of death, and every year 15 million people are affected in the world (Jayaraj et al., 2019). In the United States, it is the 5th leading cause, and about 140,000 people die each year (https://www.cdc.gov/). The stroke risk varies with gender and age (Roy-O’Reilly and McCullough, 2014). Mortality rates are similar for both men and women below 45 years of age, but women aged 45–74 years are at less risk of stroke mortality than men of the same age (Reeves et al., 2008). Different types of stroke include ischemic stroke (clots), which accounts for 87% of total stroke cases, hemorrhagic stroke (bleeds), transient ischemic attack (TIA, also known as mini-strokes), cryptogenic (of unknown cause) stroke, and brain stem stroke (lack of body function) (https://www.stroke.org/). Ischemic stroke is further classified into two subgroups, thrombotic strokes and embolic strokes. Thrombotic strokes are caused by blood clots that develop in the brain blood vessels, whereas embolic strokes are caused by blood clots arising from the body via the bloodstream. Inflammation, initiated by blood supply to the brain is interrupted and is known to play a dual role in a stroke pathology and having both detrimental and beneficial effects (Iadecola and Anrather, 2011). The immune system consists of a wide variety of immune cells, which are important for brain development and function and contribute to several neurological diseases including stroke (Iadecola and Anrather, 2011). The circulating immune cells, such as neutrophils, lymphocytes, macrophages, and endothelial cells, are actively involved in the inflammatory process (Perera et al., 2006). Whereas microglia, astrocytes, and neurons are active contributors to inflammation in brain ischemia. Previous studies suggest that components of the immune system are involved in all stages of stroke-induced inflammation (Iadecola and Anrather, 2011; Urra et al., 2009). The inflammatory and immune responses to stroke results from activation of the innate and adaptive immune system, which may enhance tissue injury and also promote healing in stroke (McCombe and Read, 2008). In this review, we aim to summarize currently known information about how inflammation and, fibrosis underlies stroke pathology, and a better understanding immunology of stroke could be used to guide future research and intervention strategies.
2. Inflammatory response to ischemic stroke
Ischemic stroke causes decreased blood flow in the brain, loss of cellular integrity, and subsequent cellular damage resulting in inflammation (Rock et al., 2010). The onset of ischemic stroke leads to activation of stress signals via tissue hypoxia, glutamate excitation, oxidonitrosative stress, which causes activation of glial cells in the brain. There are two mechanisms of action in the immune system, adaptive immunity and innate immunity (Nakamura and Shichita, 2019). Microglial cells are resident myeloid cells derived from yolk-sac progenitors and are a key component of the innate immune response system (Gomez et al., 2015). At the same time, in response to the stroke injury, other bone marrow derived monocytes are recruited to the damaged tissue and exhibit morphology similar to that of microglia. Danger associated molecular patterns (DAMPS) such as high-mobility group box 1 protein (HMGB-1) derived from dying neurons (Agalave and Svensson, 2015), and further activates innate microglia and other immune cells, via mediating toll like receptors (TLR), which in turn produce many proinflammatory cytokines, chemokines, and matrix metalloproteinases (MMPs). MMP 9 is upregulated by HMGB1 via TLR4 and its induced cytokines as TNFα or IL-1β mediating cellular death after ischemic stroke (Qiu et al., 2010). Time-dependent inhibition of MMP-9 improves stroke outcomes via the degradation of DAMPS (Cauwe et al., 2009). Previous studies have demonstrated local and systematic inflammatory responses after stroke in humans (Zaremba and Losy, 2001; Pedersen et al., 2004). The studies with human brains by histology during autopsy showed that neutrophil recruitment starts from the first day and drastically increased within 2 to 3 days after stroke whereas macrophage infiltration begins after three days and persistent for several years in infarct regions (Chuaqui and Tapia, 1993; Mena et al., 2004).
3. Immune cells
Leukocytes, mainly neutrophils invade first to infiltrate the ischemic region and decreases rapidly with time (Jin et al., 2010). In humans, the circulating neutrophils constitute 50–70%, compared 75–90% in rodents (Mestas and Hughes, 2004). Infiltrating neutrophils contributes to inflammation and progression to injury by generation of pro-inflammatory signals, such as inducible nitric oxide synthase (iNOS), matrix metalloproteinases (MMPs), toll like receptor 2 (TLR2), antigen presenting proteins, chemokines and, immunoglobulins which are differentially expressed in mice and humans (Mestas and Hughes, 2004; Iadecola and Anrather, 2011). The use of antileukocyte strategies with antiadhesion molecule such as beta2-integrins are proven to be more effective in transient middle cerebral occlusion (tMCAO) model but not in permanent middle cerebral artery occlusion (pMCAO) (Yilmaz and Granger, 2008). Immune cells such as T lymphocytes, especially CD8+ T and CD4+ T cells, influx into the brain within 3 hr and 24 hr respectively, and are responsible for the damaging effect of this acute phase of stroke (Gill and Veltkamp, 2016; Rogove et al., 2002). Cytokines are key mediators in the inflammatory response following stroke (Doll et al., 2014). While the recent study reported the deleterious effect of interferon-γ in inflammation after stroke (Seifert et al., 2014). Increasing evidence suggests that immune cells are not only involved in the critical event of neuroinflammation as key contributors to stroke pathogenesis but are also important players in the maintenance of central nervous system (CNS) homeostasis (Greenwood et al., 2011). Inhibition of aberrant infiltration of immune cells and pro-inflammatory cytokine is vital to counter the deleterious effects of inflammation in stroke. Immune cells inhibitors/inducers for stroke therapy listed in table1.
Table 1.
Immune cell inhibitors/inducers for stroke therapy.
| Immune cells | Immune cell inhibitors/inducers | Study organism | Experimental study | Biological effects | Reference |
|---|---|---|---|---|---|
| Microglia | Pexidartinib (PLX3397. 290 mg in 1 kg chow) | CS7BL/6 J and Cx3CrlGFP/ + mice | fMCAO (60 mm) | ↑ in Cx3CrlGTP/+, − (no change) in BBB injury and ↑ in brain injury with ↑ in TNFα. IL-6. MCP-1, KC (CXCL1), 1L-4 | (Szalay et al., 2016) |
| Macrophages | Anti-CCR2 monoclonal antibody MC-21 (IP) | Male C57BL/6 J and B6SJL (CD45.1), CX3CR1-EGFP (CD45.2). (β-actin-GFP + C57BL/C mice | fMCAO | ↑ in MDMs. TGFβ, CD163. Yml, | (Wattananit et al., 2016) |
| DHA sodium salt (10 mg/kg, IP) | CS7BL/6 mice (both male and female. 8–12 wk) | tMCAO (60 min) | ↓ infiltration of peripheral macrophages (CD11b + CD45highLy6G −). neutrophils (CD11b + CD4ShighLy6G +). B lymphocytes (CD19 + CD3−), T lymphocytes (CD3 + CD19−). and ↑ in CD206 + Ibal +, 1L-10. Arginase-1. TGFβ | (Cai et al., 2018) | |
| T cells (Treg) | Monoclonal antibody CD28SA (300 μg. IP) | Male C57BL/6 J mice (8–12wk) | pMCAO. fMCAO (60 min) | ↑ in IL-10, Treg cells and CD45. and ↓ CD11b +, MUCH on macrophages/dendritic cells. | (Na et al., 2015) |
| B cells | Rituximab (Micromedex, 100 μg IP) | Transgenic human CD20+ expressing (hCD20 + /−) mice | tMCAO | ↓ infarct volumes and didnť alter neurogenesis | (Ortega et al., 2020) |
| Dendritic cells | Transgenic GFP + overexpress sTNFR1 cells (2 × 106. IV) | Adult male SD | tMCAO | ↓ TNF-α and infarct volumes | (Works et al., 2013) |
Table Abbreviations, ↑: Increase; ↓: Decrease; −: No change; fMCAO: Filamentous Middle Cerebral Artery Occlusion; BBB: Blood Brain Barrier; TNFα: Tumor Necrosis Factor Alpha; IL: Interleukin; MCP: Mast Cell Protease; MDMs: Monocyte-derived macrophages; TGF-β: Transforming Growth Factor Beta; CD:Cluster Of Differentiation; tMCAO: Transient Middle Cerebral Artery Occlusion; Treg: Infiltrating Regulatory T Cells; IP: Intraperitoneally; pMCAO: Permanent Middle Cerebral Artery Occlusion: MHC: major histocompatibility complex; GFP: Green Fluorescent Protein; sTNFR1: Soluble Tumor Necrosis Factor Receptor-1; IV: Intravenously; wk.: Week: SD: Sprague-Dawley; hCD-20: Human Anti-CD20: mo:Months; CXCL: C-X-C Motif Chemokine Ligand.
3.1. Neutrophils
Neutrophils are the first and critical cells to invade into injured tissue after stroke, and the severity of pathogenesis depends on their influx in the ischemic region (Chen et al., 2016). After invading the CNS, in the diseased state, neutrophils transform into two different phenotypes, namely N1 phenotype (proinflammatory property) and N2 phenotype (anti-inflammatory property) (Easton, 2013; Kolaczkowska and Kubes, 2013; Segel et al., 2011). Studies have shown the increased expression of N2 markers such as chitinase-like protein (Chil3 protein or YM1) YM1/Chil3, and Arginase 1+ proteins after stroke, whereas, N1 neutrophil markers like YM1− were lesser in the ischemic brain following experimental stroke induced by middle cerebral artery occlusion (MCAO) in TLR4 knockout (KO) mice (Garcia-Culebras et al., 2019). The shift in N2 polarization by peroxisome proliferator-activated receptor gamma (PPARγ) activation with the agonist rosiglitazone is an essential event of inflammation that participates in neuroprotection after stroke in pMCAO mice model (Cuartero et al., 2013). Recent evidence indicates that neutrophil accumulation is downregulated with the administration of all-trans retinoic acid (atRA) in the MCAO mouse model (Cai et al., 2019). With the decrease in neutrophil accumulation, they transform toward the N2 phenotype in stroke lesions and reduce infarct volume. The atRA treatment suppressed STAT1 signaling through enhancing the expression of suppressor of cytokine signaling-1 protein (SOCS1) (Cai, Wang, 2019). Neutrophil migration into the stroke lesion can be determined by myosin1f levels in the brain after stroke and further study demonstrated that myosin1f KO mice subjected to MCAO had smaller infarcts than wildtype controls (Wang et al., 2019a). Given that neutrophil-mediated proinflammatory cytokines such as interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), interferon-gamma (IFN-γ), and IL-6 are increased in ischemic stroke (Kostulas et al., 1999), targeting them is essential for therapeutic approaches to limiting inflammation after stroke (Doll et al., 2014; Jickling et al., 2015). Blockade of neutrophils by a recombinant neutrophil inhibitory factor (rNIF) such as UK-279, 276, which selective binding to CD11b integrin have not been effective in clinical trials (Del 2004). Similarly, the use of rNIF against neutrophil β2 Integrin CD18 was failed to improve long-term outcomes after stroke (Smith et al., 2015).
3.2. Microglia
Microglia are the resident macrophages of the brain and possess an ability to maintain constant population at a homeostatic level in the CNS (Bruttger et al., 2015), whilst playing a key role in the immuno-surveillance of the CNS and maintaining a homeostatic environment by eliminating cellular debris (see (Thurgur and Pinteaux, 2019) for review). Microglial activation is considered to be the first step in the initiation of inflammation after stroke and is considered to be detrimental (Block et al., 2007). However, a recent study demonstrated that selective elimination of microglia leads to increased infarct volume and neuronal death with calcium overload, which is reversed by microglial repopulation, and the same study further found that microglia perform a critical role in the clearing of damaged neurons and promoting neuronal survival in the injured mice brain (Szalay et al., 2016). Microglial cells express macrophage like markers under normal physiological conditions, although several candidate markers like CD45 and CD39 can be used to differentiate microglia from the peripheral macrophage population (Dudvarski et al., 2016). Furthermore, several studies have identified markers such as Iba1high, CD206− CD45low, CD163− CD11b+, MHCII+, F480+, Cx3cr1high, Ly6C− being specifically expressed by activated microglia in the brain (Ginhoux et al., 2010; Kierdorf et al., 2013). Activation of microglia varies with the acute and delayed phase after injury and remains active from weeks to months after acute injury (Kabba et al., 2018).
Previously, microglia were referred to as M1/M2-like phenotype (activated macrophage 1 (M1) and alternatively activated macrophage 2 (M2)). However, several studies have recently demonstrated that microglial phenotypes are complex and cannot be classified strictly into just these two different classical phenotypes (Chiu et al., 2013; Geissmann et al., 2010; Kan et al., 2015). Regardless, the morphological and phenotypic changes of the microglia after stroke are often accompanied by increased expression of IL-6, TNF-α, MMPs, as well as chemokines CCL2, CX3CL1, MIP-1, and free radicals (Kabba et al., 2018). A cell based therapy using preconditioned microglia by oxygen glucose deprivation (OGD) induces microglia to acquire an M2-like phenotype leading to secretion of neurotrophic factors vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), as well as MMP-9, rendering microglial modulation as a therapeutic strategy for ischemic stroke in rats (Kanazawa et al., 2017). One mechanism by which ischemic preconditioning triggers neuroprotection is via induction of low-grade inflammation. In line with this, our group previously reported that intra-arterial administration of low dose IL-1α reduces microglial activation, improves functional outcomes and triggers neurogenesis and angiogenesis after experimental stroke, demonstrating that low levels of IL-1α regulate microglial activation and has likely beneficial effects on neuroinflammation following stroke (Salmeron et al., 2019). A human study reported the intravenously administration of minocyclin with 10 mg/kg alone and in combination with tissue plasminogen activator is safe and was effective in multiple preclinical stroke models by reduction of microglial activation, reduced NO production, and inhibition of MMP activity in stroke pateints (Fagan et al., 2010).
3.3. Macrophages
Macrophages are one of the blood-borne monocytes that infiltrate the brain parenchyma after stroke from perivascular spaces. They perform a critical role in neuroinflammation after ischemic stroke. They migrate through endothelial cells of the blood-brain barrier (BBB) to the stroke lesion under the action of cytokines, chemokines, and cell adhesion molecules (Jian et al., 2019). Macrophages are characterized as expressing different markers such as CD206+, CD163+, CD45high, CD11b+, MHCIIhigh, Ly6Clow, F480+, Cx3cr1low, Iba1low under diseased state (Goldmann et al., 2016; Zeisel et al., 2015; Faraco et al., 2017; Faraco et al., 2016) and are commonly referred to as non-parenchymal macrophages, to differentiate them from microglia. According to Ritzel and colleagues, more recently activated, BrdU-positive (a marker of cell division) macrophages rather than microglia were observed in the ischemic brain of mice, and these macrophages exhibited increased IL-1β production (Ritzel et al., 2015). The anti-inflammatory role of macrophages is characterized by expression of several cytokines including TGF-β, IL-4, IL-10, and IL-13 following stroke (Hu et al., 2015; Jian et al., 2019). A study revealed that half of the monocyte derived macrophages/mononuclear cells accumulate in the stroke-injured hemisphere (Wattananit et al., 2016). Another half of spontaneously recruited monocytes migrate to the injured site and contribute to long-term behavioral recovery via expression of TGF-β, Ym1, and CD163 in mice after MCAO (Wattananit et al., 2016).
3.4. Lymphocytes
Lymphocytes are essential subtypes of white blood cells in immune systems and include T cells and B cells. They are mainly involved in both pathogenesis and protective mechanisms in ischemic stroke (Liesz et al., 2015).
3.4.1. T lymphocytes (T cells)
T cells have multiple roles in ischemic stroke and cause inflammation after entering infarcted tissue by the release of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1β, IL-17, and IL-21 (Pawluk et al., 2020; Rayasam et al., 2018). One study suggests that CD8 T cell recruitment to the CNS is increased in aged mice after MCAO via the release of TNF-α, IFN-γ, and monocyte chemoattractant protein 1 (MCP-1/CCL2) (Ritzel et al., 2016). CD4+ T cells invade into infarct tissue via release of proinflammatory cytokines such as IL-1, IFN-γ, and IL-17 (Arumugam et al., 2005). Fas ligand (FasL) belongs to the TNF family of cytokines and plays a critical role in stroke pathology. FasL mutation reduces stroke injury by attenuating CD4+ T cells and nuclear factor-kappa B (NF-κB)-mediated M1 microglia polarization in mice model of MCAO (Zhao et al., 2018). PDPK1 is downstream of FasL signaling and inhibition of PDPK1, by treatment with its inhibitor BX-912, reduces cytotoxicity of CD8+ T cells after stroke in mice (Fan et al., 2020). Anti-CD4 depletion antibody administered to mice after stroke resulted in an improved behavioral score, and levels of IFN-γ -inducible protein (IP-10) were elevated in serum and brain of male mice following MCAO (Harris et al., 2020). CD3+CD4−CD8− T cells (double-negative T cell; DNT) were upregulated in peripheral blood after stroke. Targeting infiltrating DNTs with microglial FasL/PTPN2/TNF-α mediated signaling is a promising therapeutic approach in ischemic stroke (Meng et al., 2019). Gamma delta T cells (γδT) cells increases ischemic damage by releasing IL-17 (Gelderblom et al., 2012b). Th1 cells aggravate brain injury by secreting IL-2, IL-12, and IFN- γ, whereas Th2 cells are neuroprotective by production of anti-inflammatory cytokines include IL-4, IL-5, IL-10, and IL-13 (Dolati et al., 2018; Filiano et al., 2017). Infiltrating regulatory T cells (Treg cells) are one of the most important T cells sub-population known to regulate adaptive immunity, and have beneficial role in ischemic stroke (Liesz et al., 2013). A recent study characterized sirtuin2 expression in infiltrating Treg cells after acute stroke in mouse tMCAO, demonstrating increased expression and decreased anti-inflammatory activity in infiltrating Treg cells. Furthermore, the use of the hypoxia-inducible factor 1-alpha (HIF-1α) inhibitor PX-478 abolished microglia mediated Sirt2 expression (Shu et al., 2019).
3.4.2. B lymphocytes (B cells)
B cells play critical roles in host defense against pathogens including antibody production, and modulation of T cell responses (Selvaraj et al., 2016). They are categorized into B1, B2, and regulatory B cells (Breg cells) based on pathogenic and regulatory function. Breg cells consist of seven different subsets including two immature and five mature cell types. Several studies confirmed a role of B cells in synthesizing cerebrospinal fluid–specific immunoglobulin such as IgG, IgM, and IgA after stroke (Pruss et al., 2012; Rostrom and Link, 1981) and secrete IL-10 (Duddy et al., 2007). B cells infiltrate into infarcted tissue in a delayed manner (Doyle et al., 2015), however depletion of B cells affects neither stroke-induced brain injury nor behavioral function (Schuhmann et al., 2017). Berchtold and collaborators (Daniel et al., 2019) reported that B-cell-deficient mice (μMT KO) did not affect stroke volume in mice after MCAO (Ren et al., 2011), a finding that is in line with a previous study using Rag1 KO mice that reported no effect on stroke following tMCAO (Yilmaz et al., 2006). However, another study showed that B-lymphocytes infiltrate the infarcted tissue within weeks after stroke (Doyle et al., 2015). In this process, B cells undergo isotype switching, leading to IgA and IgG antibodies being expressed and this resulted in dementia in mice that underwent the distal MCAO (dMCAO) surgery (Doyle et al., 2015). B cells have an anti-inflammatory role as regulatory B (Breg) cells via the expression of IL-10 and were shown to strongly reduce the infarct volume in ischemic mice (Seifert et al., 2018). This observation demonstrated that the transfer of enriched Breg cell populations might be of important therapeutic value in stroke patients (Seifert et al., 2018). A study evaluated the effect of fingolimod, and it was administered orally (0.5 mg per day for 3 consecutive days) in 22 patients by comparing 11 control patients. The results showed that 11 fingolimod recipients had lower circulating lymphocyte and better neurological outcomes (Fu et al., 2014).
3.5. Dendritic cells
Dendritic cells/antigen-presenting cells (APCs) are immune cells responsible for the initiation of the adaptive immune response, and a recent study demonstrated that these cells are also found in the brain parenchyma after stroke (Kostulas et al., 2002). Conventional type 2 dendritic cells, IRF4+/CD172a+ infiltrate into ischemic brain while expressing IL-23 (Gelderblom et al., 2018). IL-23 receptor KO mice have been reported to be protected against ischemic stroke, albeit with defective IL-17 levels (Gelderblom et al., 2018). The study showed increased expression of dendritic cell-associated C-type lectin-1 (dectin-1) and spleen tyrosine kinase (Syk), which triggers neuroinflammation in a mouse model of cerebral focal ischemia. The dectin-1 antagonist laminarin-1 and Syk inhibitor piceatannol decrease TNF-α as well as inducible nitric oxide synthase (iNOS) expression, resulting in smaller infarct volume, and improved neurological score in a mouse model of cerebral focal ischemia (Ye et al., 2020). Hence, dectin-1/Syk mediated signaling is an important therapeutic target after ischemic stroke (Ye et al., 2020). Fisetin, a flavonoid administered before and after experimental cerebral ischemia, reduced the number of CD11c+ cells in the brain in a mouse tMCAO model (Gelderblom et al., 2012a). This study also suggests that fisetin mediated suppression of NFκB activation and JNK/Jun phosphorylation is neuroprotective in cerebral ischemia as well (Gelderblom et al., 2012a).
4. Pro and anti-inflammatory cytokines role in neuroinflammation after stroke
Cytokines are immunoregulatory molecules released by immune cells in systemic circulation as well as in the CNS in response to various stimuli in order to re-establish homeostasis. An anti-inflammatory response opposes pro-inflammatory cytokine signals, and an imbalance between them leads to localized tissue and organ damage in stroke. Cytokine inhibitors/inducers for stroke therapy are listed in table2.
Table 2.
Cytokine inhibitors/inducers for stroke therapy.
| Cytokine | Cytokine Inhibitor/inducer | Study organism | Experimental study | Biological effects | Reference |
|---|---|---|---|---|---|
| IL-1α | IL-la (50 pg/μL. LA) | Male C57BL/6 mice | CCA/M CAO (60 min) followed by reperfuston for 7 days | IL-lα reduced infarct volumes via ↓ IL-1β,1L-6, or CXCL-1 levels and ↑ expression of PECAM. ICAM-1. and VEGFR2 | (Salmeron et al., 2019) |
| IL-1Ra | IL-IRa overexpressing mice | CS7BL/6-Tg (UBC-GFP)30Scha/J (Stock No. 004353) (GFP-TG) breeding pairs | tMCAO (40 min) | ↑ IL-IRa and ↓ expression of IL-1β produced by microglia mediating MAPK signaling in the ischemic cortex | (Clausen et al., 2016) |
| IL-1β | Monoclonal anti-IL-lβ antibody (10 μg/g IV) | JunD siRNA (siJunD)-treated mice with CS7BL/6 J WT (wild type) background mice | tMCAO (45 min) | ↓ in infarct volume. – (no change) in 1L-6, TNF-α. and 4-hydroxynonenal levels | (Diaz-Canestro et al., 2019) |
| IL-4 | IL-4 KO | CS7BL/6 J WT, | tMCAO (60 min) | ↓ in smi32/MBP ratio | (Zhang et al., 2019) |
| IL-10 | Recombinant IL-10 | SD rats (8 wk) | tMCAO (90 min) | ↓ in TNF-α. IL-1β. 1L-6 | (Nakajima et al., 2017) |
| ICV IL-10 | CS7BL/6 J (10–12 wk) | pMCAO | ↓ in PD-L1. CXCL9 RE | (Liesz et al., 2014) | |
| IL-13 | Recombinant IL-13 | Male (4-mo) BALB/cOlaHsd mice | MCAO | − (no change) in GFAP. Ibal. ↓ in C.D45+, ↑ in Ibal +/Argl + | (Kolosowska et al., 2019) |
| IL-33 | Recombinant IL-33 | Male C57BL/6 mice (8–10 wk) | CCA/MCA (30 min) | ↓ in IFN-γ + T cells, ↑ in Foxp3+ T cells. IL-4, IL-10. TGF-β in spleen tissues | (Xiao et al., 2019) |
Table Abbreviations, ↑: Increase; ↓: Decrease; −: No change; IL-lα: Interleukin 1 alpha; CCA/MCAO: Central Carotid Artery/ Middle Cerebral Artery Occlusion; Intracerebroventricular; I A: Intra Arterial; IP, Intraperitoneally; IV: Intravenously; min: Minutes; hr Hours; mo: Month; wk.: Week; Yr: year, IL: Interleukin; CXCL: C-X-C Motif Chemokine Ligand; PECAM-1: Platelet/endothelial cell adhesion molecule-1; ICAM-1: Intercellular Adhesion Molecule 1; VEGFR-2: Vascular endothelial growth factor receptor 2; IL-IRa: Interleukin-1 Receptor Antagonist; MAPK: Mitogen-activated protein kinase; TNFα: Tumor Necrosis Factor Alpha; WT: Wild Type; GFP: Green Fluorescent Protein; SD: Sprague-Dawley: KO: Knock Out; tMCAO: Transient Middle Cerebral Artery Occlusion: IL-1β: Interleukin 1 Beta: siRNA: Small Interfering RNA; SMI32: a marker of demyelinated axons; MBP: major myelin protein; ICV: Intracerebroventricular. pMCAO: Permanent Middle Cerebral Artery Occlusion; GFAP: Glial fibrillary acidic protein; Interferon gamma; FOXP3:Forkhcad Box P3; TGF-β: Transforming Growth Factor Beta.
4.1. Pro-inflammatory cytokines
4.1.1. Interleukin-1 (IL-1)
IL-1 is a master regulator of inflammation and immunity and plays a pivotal role in most, if not all, inflammatory disease. The role of IL-1 in neuroinflammation induced by stroke and its associated risk factors that are linked with raised systemic inflammatory profile has been long established (Sobowale et al., 2016). Hence, current therapies aimed at targeting IL-1 actions in stroke are currently underway. The IL-1 family comprises 11 cytokines and a large family of IL-1 related receptors. These networks of cytokines regulate innate immune cells and play a key role in inflammation after stroke (Dinarello, 2011). IL-1β is the main released isoform of the IL-1 family, expressed primarily by immune cells, as a pro-IL-1β released extracellularly via an NLR family pyrin domain containing 3 (NLRP3)/caspase-1 dependent mechanism (Weber et al., 2010). The role of IL-1β in stroke has been long established with early studies demonstrating that IL-1β is expressed in the brain after experimental stroke (Liu et al., 1993; Wiessner et al., 1993). Several published studies demonstrated that pharmacological inhibition of IL-1β actions by using the IL-1 receptor antagonist (IL-1Ra) confer neuroprotection after experimental stroke (Garcia et al., 1995, Relton and Rothwell, 1992). Interestingly, studies using IL-1 KO mice showed that genetic deletion of IL-1β failed to affect ischemic brain injury, whereas deletion of IL-1β and IL-1α (second main isoform that is intracellularly stored) induces a significant reduction in brain damage (Boutin et al., 2001), hence both IL-1α and IL-1β play an important compensatory effect in stroke. Further study found that both centrally- and peripherally-derived IL-1α and IL-1β contribute to stroke pathogenesis (Denes et al., 2013). IL-1α is also expressed by microglia after stroke, although expression precedes that of IL-1β expression (Luheshi et al., 2011), suggesting that both isoforms may exert specific non-overlapping actions in stroke, In light of this, our recent study found that IL-1α, but not IL-1β, induces brain cells to generate the laminin-like globular domain 3 (LG3) neuroprotective protein fragment of the extracellular matrix component perlecan, a prominent heparan sulfate proteoglycan extracellular matrix (ECM) component of the BBB (Saini et al., 2011), whereas IL-1α is a key inducer of angiogenesis and neurogenesis after stroke (Salmeron et al., 2019). Therefore, alternative strategies aimed at selectively targeting IL-1α or IL-1β might prove more effective than complete IL-1 blockade by IL-1ra. In a randomized phase, II study reported that of intravenous (IV) administration of IL-1ra in patients with acute stroke showed reduced cerebral inflammation and greater reduction in National Institutes of Health Stroke Scale at three months (Emsley et al., 2005).
4.1.2. Interleukin-6 (IL-6)
IL-6 is a pro-inflammatory cytokine and is a critical biomarker used to predict stroke associated infection in elderly patients (Kwan et al., 2013). Studies proved that significantly higher serum IL-6 levels correlate with infarct volume and cerebral perfusion deficits in stroke-affected patients than healthy controls (Hotter et al., 2019; Jenny et al., 2019; Saroj et al., 2018). Il6 gene polymorphisms also have a significant association with increased risk of ischemic stroke (Zhou et al., 2019). Recently, studies using pigs show IL-6 was increased after stroke and further elevated by wild type tissue-type plasminogen activator (tPA). Indeed, co-administration of LMT-28 with wild type tPA blocked JNK and endothelin 1 mediated increase of IL-6, reducing cerebrovascular autoregulation impairment, which in turn, would lead to improved outcomes of tPA treatment after stroke in pigs (Armstead et al., 2019). Although IL-6 has proinflammatory properties, it also has beneficial potential; IV administration of IL-6 to stroked mice results in reduced infarct volume and improved functional outcomes in IL-6 KO mice (Gronhoj et al., 2017).
4.1.3. Interleukin-18 (IL-18)
IL-18 is a cytokine of the IL-1 family and proinflammatory cytokine that plays a critical role in neuroinflammation and stroke (Kandikattu et al., 2019). A recent study demonstrated that serum IL-18 levels were higher in stroke patients, is increased with the severity of the stroke, and can be used as a diagnostic marker for stroke (Hao et al., 2019). Il18 (137G/C and 607C/A) gene polymorphisms are associated with an increased risk of ischemic stroke (Zhou et al., 2019). IL-18 level was reported to be increased in ischemic mice brain that showed depression-like behaviors, and the blockage of endogenous IL-18 by IL-18 binding protein rescued depressive phenotypes in spatial restraint-stressed mice. IL-18-mediated depressive behaviors are regulated by the interaction between the IL-18 receptor and NKCC1. NKCC1 antagonist bumetanide showed a therapeutic effect for post-stroke depression in IL-18-induced depressive mice (Wu et al., 2020). However, a study from our group showed that genetic deletion of IL-18 in mice had no effect on brain injury after stroke (Wheeler et al., 2003), indicating that IL-18 may be involved in mediating the depression occurring due to stroke, more than other aspects of stroke pathogenesis.
4.2. Anti-inflammatory cytokines
4.2.1. Interleukin-4 (IL-4)
IL-4 triggers a pleiotropic phenotype in both microglia and macrophages and is involved in diverse immune responses of M2 microglia polarization (Francos et al., 2016). Results from a previous study showed that IL-4 induces PPAR-γ mediated activation of M2 polarization. Protein expression of translocator protein (TSPO) antagonist PK11195 treatment modulates IL-4 expression that subsequently promotes increased expression of CD206, Arg-1, YM-1, and FIZZ-1 under hypoxic ischemia (Zhou et al., 2020). Intraperitoneal administration of dimethyloxalylglycine, a HIF-1α activator, reduced the infarct size by promoting IL-4 and IL-10 levels (Yang et al., 2018). Elevated HIF-1α activity had a synergistic effect with limb remote ischemic preconditioning (RIPC) on reducing infarction volume after stroke in rats by inhibiting prolyl hydroxylase (PHD) enzyme inactivation (Yang et al., 2018). In accordance with this notion, mice that lacked IL-4 were associated with worse neurological score outcome, along with increased immune cell infiltration and Th1/Th2 ratio in the infarct region (Xiong et al., 2011). Together, these findings indicate that IL-4 signaling has a beneficial role of reducing inflammation in the ischemic core.
4.2.2. Interleukin-10 (IL-10)
IL-10 mediated neuroprotection is well studied in brain injury. As an anti-inflammatory cytokine, it promotes neuronal cell survival via various signaling pathways such as suppression of tumorigenicity 2 (ST2)/IL-33 (Liu et al., 2020; Mills, 2001). Previous study using IL-10 KO mice showed that increased infarct volume and neurologic deficits were observed in ischemic mice (Perez et al., 2013). IL-10 deficiency in mice was reported to promote the expression of CTLA-4, a T-cell inhibitory molecule in the ischemic tissue (Perez et al., 2013). A recent study explained that transplanted mesenchymal stem cell (MSC)/IL-10 intravenously injected through the catheter at 0 or 3 hr after ischemia-reperfusion significantly reduced infarct volume and enhanced motor functional recovery at 72 hr and 7 days after MCAO in rats as an acute phase of ischemic stroke (Nakajima et al., 2017). A recent meta-analysis revealed Il10 gene polymorphism (1082A/G) to be associated with ischemic stroke (Liu et al., 2017). In another study focused on different promoter regions of IL-10, functional polymorphisms at −1082 promoter region of IL-10 was found to be rare in the Chinese Han population compared to American/European people, and it was concluded that it might be a protective factor for ischemic stroke (Tong et al., 2018).
4.2.3. Interleukin-13 (IL-13)
IL-13 is a key anti-inflammatory cytokine secreted by activated T cells. Transplanting IL13-expressing MSCs leads to a M2 microglia phenotypic switch with a significant increase of Arg-1 and decreased expression of major histocompatibility complex II (MHC-II) in a mouse model of MCAO (Hamzei et al., 2018). A recent study revealed that peripherally administered IL-13 after pMCAO in mouse significantly reduced the infarct volume, increased levels of Arg1 and Ym1, and improved neurologic deficit functions (Kolosowska et al., 2019). A previous study has shown TREM2 as neuroprotective in the ischemic penumbra of a mouse model of MCAO. TREM2 expression also increased in IL-4/IL-13-treated microglia under OGD (Zhai et al., 2017).
4.2.4. Interleukin-33 (IL-33)
IL-33 is a cytokine of the IL-1 family and a crucial mediator of the immune response (Chen et al., 2018; Liew et al., 2016). IL-33 is neuroprotective after ischemic stroke via regulating the inflammatory response (Luo et al., 2015). A recent study demonstrates the role of IL-33/suppression of tumorigenicity 2 signaling in microglial activation and neuroprotection after stroke in mice (Yang et al., 2017). The activated microglia, in turn, release IL-10 which helps in neuronal survival under in vitro OGD conditions (Yang et al., 2017). Previous findings demonstrate that the administration of recombinant mouse IL-33 protein to mice before MCAO helps to attenuate brain damage and CNS inflammation (Luo et al., 2015). The neuroprotective effect of IL-33 might be associated with inhibition of neuroinflammation via turning on the T helper 1 and 2 (Th1/Th2) response while suppressing Th17 immune response (Luo et al., 2015). IL-33 acts as a neuroprotectant via mediating regulatory T cell (Tregs) response in experimental ischemic stroke (Xiao et al., 2019). However, another study showed that IL-33 treatment increased the number of Tregs in the MCAO model and suggested that IL-33-ST2 signaling-mediated neuroprotection in stroke (Liu et al., 2020).
5. Mechanisms of neuroinflammation and the inflammatory pathway
Fig. 1 depicts a schematic of stroke-induced neuroinflammation. Stroke induces activation of stress signals, hypoxia, and oxidonitrosative stress that leads to activation of NLRP3 and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling, infiltration of immune cells and cytokines, and neuroinflammation. Several reports indicate that these signals are activated via mitochondrial damage (Gong et al., 2018; Kandikattu et al., 2017a), lysosomal degradation (Aftabizadeh et al., 2019), reactive oxygen species (ROS) induction (Xu et al., 2018), Ca2+ release mediated ER stress (Guo et al., 2018a; Kandikattu et al., 2020; Nakka et al., 2010), and autophagy (Kandikattu et al., 2017b; Wang et al., 2019b). Understanding the stress inducers that activate inflammatory pathways and the development of drugs that inhibit inflammation is key in the treatment of stroke. Other pharmacological therapies with anti-inflammatory activity for stroke therapy are listed in table 3.
Fig.1.
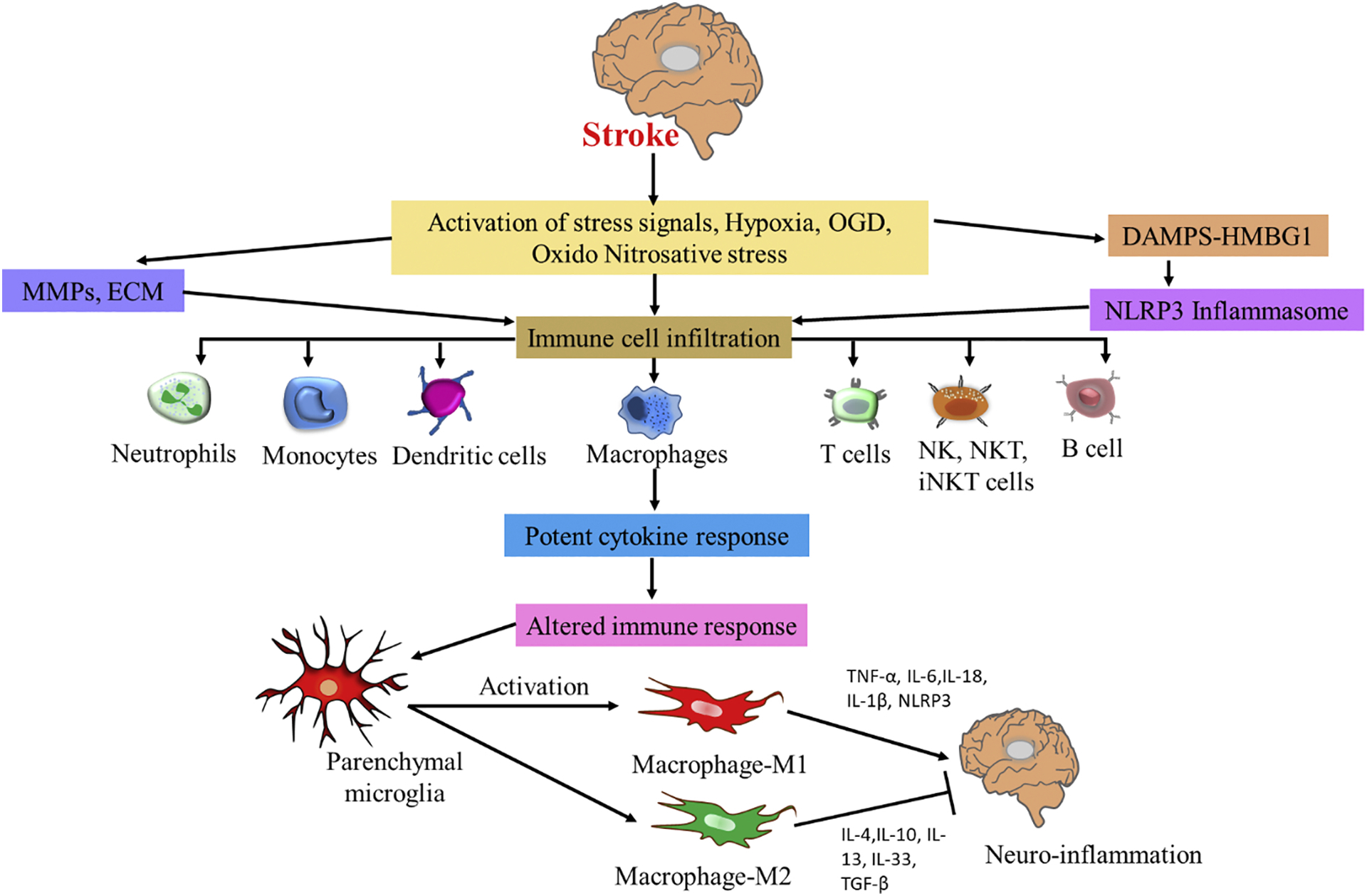
Schematic of stroke-induced neuroinflammation. Stroke induces activation of stress signals, hypoxia, OGD, oxido-nitrosative stress, DAMPS such as high-mobility group box 1 protein (HMGB-1) derived from dying neurons, and it further activates innate microglia and other immune cells, via mediating toll like receptors (TLR), which in turn produce many pro-inflammatory cytokines, and matrix metalloproteinases (MMPs), which leads to activation of NLRP3 signaling and infiltration of immune cells and potent cytokine response and neuroinflammation. Following stroke, activated parenchymal microglial cells transforms to M1 and M2 macrophage polarization. M1 macrophages release proinflammatory cytokines whereas M2 macrophages release anti-inflammatory cytokines and inhibit neuroinflammation.
Table 3.
Other pharmacological therapies with anti-inflammatory activity for stroke therapy.
| Cell types | inhibitors | Study organism | Experimental study | Biological effects | Reference |
|---|---|---|---|---|---|
| Microglia | .Melatonin (20 mg/kg. IP) | Male C57BL/6 J mice (8–10 wk) | dMCAO | STAT3 mediated pathway with ↓ In pSTAT3. CD11b. CD86, iNOS, TNF-α. 1L-6. and IL-1β and ↑ in CD206. Arg-1. YM1/2, TGF-β. IL-10 | (Liu et al., 2019) |
| Berberine hydrochloride. BP1108 (50 mg/kg/day) | Male C57BL/6 mice | tMCAO (45 min) | AMPK mediated signaling with ↓ in IL-1β. CD32. TNF-α. and iNOS, p-AMPK and ↑ in CD206. Arg-1. IL-10, Ym1/2 | (Zhu et al., 2019) | |
| α-lipoid acid (50 mg/kg) | Male SD | MCAO (30 min) | ↓ in lba-1-TNF-α-specifk cells | (Wu et al., 2016) | |
| Neurons | Fluoxetine (40 mg/kg. IP) | C57B1V6 J mice (3 mo.) | t.MCAO (1 h)/24hR. Drug administered 1 h and 12 h after tMCAO | Fluoxetine was inhibiting IL-10. Bax. and p53 and ↑anti-apoptotic protein Bcl-2 level. | (Shan et al., 2016) |
| JNK inhibitor SP 600125 and the ET-1 antagonist BQ 123 | Yorkshire pigs (1.1–1.6 kg; 2–7 days old) | Photothrombotic stroke | ↓ in 1L-6 levels | (Armstead et al., 2019) | |
| Neutrophils | rtPA (10 mg/kg. femoral vein) and NLRP3 shRNA | SD rats | Thromboembolic focal cerebral ischemia model | PPARY/SIRT6/Fox03a mediated pathway with ↑ in PPARγ. SIRT6 and ↓ in p-FoxO3a. 1L-6, IL-1β. TNF-α; inhibited the microglia activation and neutrophils infiltration | (Guo et al., 2018b) |
Table Abbreviations, ↑: Increase; ↓: Decrease: −: No change; IL-lα: Interleukin 1 alpha: Intracerebroventricular. IA: Intra Arterial; IP. Intraperitoncally; IV: Intravenously; min: Minutes; hr. Hours; mo: Month: wk.: Week; Yr: year; 1L Interleukin; dMCAO: Distal middle cerebral artery occlusion; STAT3: Signal transducer and activator of transcription 3; pSTAT3: Phospho- signal transducer and activator of transcription 3; iNOS: Inducible nitric oxide synthase; CD: Cluster Of Differentiation; IL-1β: Interleukin 1 Beta; TNF: tumor necrosis factor, TGF-β: Transforming Growth Factor Beta; p-AMPK: Phospho-AMPK;PPARs: Peroxisome proliferator- activated receptors; SIRT6: Sirtuin 6; FOXO: Forkhcad box class O; Arg1: Arginase-1;; tMCAO: Transient Middle Cerebral Artery Occlusion; JNK: c-Jun N-terminal kinase; ET-1: Endothelin 1; ET-1: Endothelin 1; PPARγ: Peroxisome Proliferator-Activated Receptor Gamma: NLRP3:NLR Family Pyrin Domain Containing 3; FOXP3:Forkhcad Box P3: Sirt6:Sirtuin 6; Ibal: Ionized calcium binding adaptor molecule 1.
5.1. DAMPs and NLRP3 Inflammasome pathway
Damaged-associated molecular patterns (DAMPs) are released from injured tissue after stroke (Umahara et al., 2018). DAMPS are a large family of factors which constitute pathogen-associated molecular patterns (PAMPS) and alarmins (Tang et al., 2012). Alarmins are further divided into protein alarmins such as HMGB1 or heat shock proteins (HSPs), and non-protein alarmins such as adenosine triphosphate (ATP) (Gulke et al., 2018; Lucchese et al., 2019). HMGB1 is a cytokine-like nuclear protein and mediator of neuroinflammation and pathogenesis after ischemic stroke (Ye et al., 2019). HMGB1 is released into the bloodstream and induces inflammation via its receptors TLR2, TLR4, and receptor for the advanced glycation end product (RAGE) (Ye et al., 2019). HMGB1 is highly expressed in blood under both acute phase and for about 2 weeks after stroke in rats (Kim et al., 2006). Previous experiments have demonstrated that lipopolysaccharides administration induced the release of HMGB1 in a rat model of MCAO (Kim et al., 2018). In this study, blocking HMGB1 function by treatment with HPep1 reduces infarct volume and is considered as a therapeutic target for preventing lipopolysaccharides induced post-stroke infection (Kim et al., 2018).
NLRP3 inflammasomes are known as one of the novel inflammatory pathways discovered in ischemic stroke. They are responsible for mediating cellular damage and death after stroke (Abulafia et al., 2009). Under stroke-induced stimuli, NLRP3 assembles by an apoptosis-associated speck-like protein containing a caspase (ASC), and caspase-1 leading to release and maturation of IL-1β. NLRP3 inflammasomes are first expressed in microglial cells, followed by microvascular endothelial cells and neurons under oxygen-glucose deprivation/reoxygenation (OGD/R) (Gong et al., 2018). Mitochondrial dysfunction leads to NLRP3 activation in microglia in vitro. Hence, the mitochondrial protector diazoxide could inhibit NLRP3 mediated inflammation after stroke in the rat model of tMCAO (Gong et al., 2018). NLRP3-inflammasome inhibitor, MCC950 treatment, reduced infarction via decreased expression of TNF-α, Poly (ADP-ribose) polymerase (PARP), Caspase-3, and IκBα levels and showed protection in mouse model of tMCAO (Ismael et al., 2018). However, contradicting results from another study suggest, through the use of NLRP3 KO mice or targeting NLRP3 with a pharmacological inhibitor, MCC950 showed that NLRP3 is not a critical mediator in ischemic brain damage (Lemarchand et al., 2019).
6. Fibrosis
Inflammation is a prime driver for the induction of fibrosis in various diseases, including stroke. Immune cell infiltration and cytokine response in stroke induce profibrotic proteins like TGF-β, collagens, MMPs accumulation, ECM deposition, and epithelial to mesenchymal transmission in infarct regions of ischemic stroke.
6.1. Extracellular matrix deposition in ischemic stroke
ECM proteins are rapidly increased in tissue and cells under pathological stress conditions. The ECM consists of a group of proteins that bind to cell surface receptors and regulates many genes involved in cellular behavior. ECM proteins have both harmful as well as protective roles in stroke (Kawakita et al., 2019). In the stroke environment, the composition of ECM proteins is altered, which is implicated in BBB disruption that causes brain damage (Baeten and Akassoglou, 2011).
6.1.1. ECM proteins of the BBB in stroke
ECM proteins participate in the multicellular activity and support systems for many cells when in complex with integrins (heterodimeric receptors containing a transmembrane α and β protein subunit), (Edwards and Bix, 2019). ECM proteins, mainly composed of proteoglycans, glycoproteins, and collagens, are primarily present in the basement membrane (BM) of brain microvessels between endothelial cells (Summers et al., 2013) and astrocyte end-feet (Baeten and Akassoglou, 2011). ECM proteins, fibronectin, laminins and collagen type I and type IV play a critical role in fibrosis and are induced in the infarct region after stroke (Baeten and Akassoglou, 2011; Summers et al., 2013). Platelet-derived growth factor receptor β (PDGFRβ) is an essential molecule for pericyte proliferation and survival and is a mediator of fibrosis induced in stroke. Research from our group demonstrated that a protein portion of perlecan (an ECM proteoglycan) called domain V (DV) plays a crucial role in modulating PDGF responses in angiogenesis (Bix et al., 2007; Bix and Iozzo, 2008). Administration of SU11652, an inhibitor of PDGFRβ, reduces fibrosis through decreased desmin and α-smooth muscle actin (α-SMA) levels in vascular cells (Makihara et al., 2015). However, further studies reported that fibrotic scar development after stroke does not primarily occur by PDGFRß+ pericytes, and that it is not a contributor to the fibrotic ECM in pMCAO mice (Roth et al., 2020). Previous studies showed that the fibronectin-splicing variant containing extra domain A (Fn-EDA) was elevated in the plasma of diabetes mellitus and hypercholesterolemia comorbid patients (Dhanesha et al., 2015). Further studies showed smaller infarcts and lesser expression of phospho-NF-κB p65, IL-1β, and TNF-α levels in the MCAO model of apolipoprotein E-deficient mice expressing Fn-EDA (Fn-EDA+:Apoe KO mice) (Dhanesha et al., 2015). Still further studies investigated the role of plasma versus endothelial Fn-EDA in stroke exacerbation in the comorbid condition of hyperlipidemia. These observations suggest that plasma Fn-EDA KO:Apoe KO mice displayed improved stroke outcomes compared with endothelial Fn-EDA KO (Fn-EDAfl/flTie2Cre) mice, and endothelial-specific KO mice did not contribute to stroke outcome. Hence it suggests that plasma Fn-EDA exacerbates stroke outcome by promoting post-ischemic secondary thrombosis. Therefore, targeting plasma Fn-EDA may help to reduce brain damage after reperfusion (Dhanesha et al., 2019).
6.1.2. ECM receptors of the BBB in stroke
Ubiquitously expressed cellular ECM receptors are primarily dystroglycan and integrins, (Edwards and Bix, 2019). Amongst many different types of integrins, endothelial cells express fibronectin receptor α5β1, α4β1 and αvβ3 integrins (Guell and Bix, 2014; Roberts et al., 2017). We have demonstrated that α5 integrin, (an obligate pair to the β1 integrin subunit) endothelial cell-specific knockout mice (α5-EC-KO) were resistant to ischemic infarction after tMCAO in mice. We further demonstrated that α5 integrin destabilizes the BBB via decreased expression of claudin-5 after stroke, suggesting that this integrin could be a therapeutic target for stroke (Guell and Bix, 2014; Roberts et al., 2017). This was further confirmed by post-stroke administration of the α5β1 inhibitor, ATN-161, which significantly reduced the expression of α5β1 in the infarct region, stabilized the BBB, and increased collagen IV expression, whilst further reducing CXCL12, MMP-9, IL-1β, and CD45 + cells in the brain. These results suggest that ATN-161 is a promising novel stroke therapeutic (Edwards et al., 2019).
α4β1 integrin, also known as VLA-4 or CD49d/CD29, is primarily localized in lymphocytes, monocytes, and macrophages. Some researchers in both rodent and preclinical studies reported that inhibition of α4 showed reduced infarct volumes and improved functional deficits (Becker, 2002; Llovera et al., 2015) in stroke patients. A recent study by using hydrogels precisely controlled α3/α5β1 integrin binding and promoted endothelial sprout clumping under in vitro (Li et al., 2017). Further, these hydrogels (containing nV and fragments) were injected directly into the stroke cavity promoted non-tortuous blood vessel formation and non-leaky blood vessels by 10 days after stroke. Hence precisely controlled integrin activation from a biomaterial can be used to direct therapeutic vessel regeneration and reduce VEGF-induced vascular permeability in vivo (Li et al., 2017). In preclinical studies targeting αMβ2, by rNIF (UK279276) and humanized Hu23F2G (Leukarrest) were failure to target αMβ2 integrin might be due to not increase of αMβ2 expression in human stroke patients compared to rodents (Caimi et al., 2001). α6β4 integrin is expressed on both astrocytes and endothelial cells (Milner and Campbell, 2006). The expression was decreases within 2–4 hrs after MCAO and increases from day 4 to day 14 (Wagner et al., 1997). A recent study on myeloid-specific integrin α9β1 KO mice improved stroke outcome by inhibiting post-ischemia/reperfusion inflammation and reduced fibrin, platelet thrombi, neutrophil, NETosis, and decreased phospho-NF-κB, tumor necrosis factor-α, and IL-1β levels in tMCAO mice brain. (Dhanesha et al., 2020).
6.1.3. Role of perlecan in BBB after stroke
ECM proteins generated and proteolytically processed with BBB disruption such as perlecan, play important roles in pathology after stroke (Lee et al., 2011). Research from our group demonstrated that one such proteolytic protein component of perlecan, DV, plays a crucial role in modulating PDGF responses in angiogenesis (Bix et al., 2007; Bix and Iozzo, 2008). Perlecan DV, a C-terminal perlecan fragment that can be generated by an unknown protease(s) in vivo after experimental stroke (Lee et al., 2011), can be further processed into the protein fragment LG3 via proteases cathepsins B and L (Bix et al. 2004; Gonzales et al. 2005; Cailhier et al. 2008 and Saini and Bix 2012), and BMP-1 (Gonzalez et al., 2005). LG3 has been shown to be present in the blood, cerebrospinal fluid and the urine of human patients with end-stage kidney disease, thus demonstrating that it is a proteolytic fragment in vivo, especially in humans (Adkins et al., 2002; Cartier et al., 2004; Pieper et al., 2004). Increased levels of LG3 were observed in primary fetal cortical neurons (FCN) under OGD/reperfusion condition, which was not blocked by the cathepsin L specific inhibitor, Z-FY-CHO. However, the cathepsin B inhibitor CA074 could inhibit LG3 production under OGD (Saini et al., 2011). Furthermore, IL-1α treatment increases LG3 levels via cathepsin L and B mediated expression in FCN (Saini and Bix, 2012), and promotes angiogenesis in brain endothelial cell cultures, and induces angiogenesis derived VEGF and CXCL1 expression and therefore, IL-1α is neuroprotective against stroke (Salmeron et al., 2016; Salmeron et al., 2019). Increased expression of DV and LG3 in the ischemic core of both mice and rats after stroke was shown to be beneficial (Bix, 2013; Saini and Bix, 2012). In seeming agreement with the importance of endogenous DV after stroke, perlecan hypomorph mice that express 10% of total protein (Pln−/− mice) showed larger ischemic stroke lesions following stroke (Lee et al., 2011; Yanagihara et al., 1984). Further, perlecan DV expression is reported to be upregulated in the brains of stroke patients (Trout et al., 2020). Studies using perlecan deficient mice showed significantly fewer NPCs at the subventricular zone (SVZ) following MCAO and leads to larger infarcts and decreases in neurogenesis (Trout et al., 2020). Administration of DV to mice 7 days after stroke enhanced neurogenesis and improved neurological deficit function with less infarct volume (Trout et al., 2020). Collectively, these studies suggest that perlecan DV could be a novel therapeutic for ischemic stroke (Bix, 2013; Marcelo and Bix, 2014; Parham et al., 2014; Trout et al., 2020).
6.1.4. Role of other basement membrane components in the BBB after stroke
Laminin is a heterotrimeric protein that occurs in 15 different isoforms (including α, β and γ subunits). The increased expression of laminin in both endothelial cells and astrocytes of ischemic penumbra are observed within 24 hrs after rat MCAO (Kang and Yao, 2020). Endothelial laminin-10 is essential for BBB integrity after in vitro OGD by regulating occludin and ZO-1 expression and localization to the extracellular cell wall, decreasing paracellular resistance through the endothelial cells (Kangwantas et al., 2016). Type IV collagen are a major component of all basement membranes (Mao et al., 2015). A recent study with small stroke patient populations revealed the variation in COL4A1 and COL4A2 genes causes of weakness of the basement vascular membranes causes perinatal arterial ischemic stroke (Kocak et al., 2020). However, collagen IV-deficient mice (null allele of the Col4a1/2 locus in mice) die at E10.5– E11.5 due to vascular bleeding in the heart and arteries (Poschl et al., 2004). The proteolytic fragment of type IV collagen, tumstatin are generated by MMP-9 proteolysis and are known to suppress angiogenesis via αVβ3 integrin (Hamano et al., 2003). Studies on collagen IV expression need more investigation and improvement before any determination of collagen IV’s impact on stroke severity, or poststroke recovery can be made (Edwards and Bix, 2019).
6.2. Matrix metalloproteinases in ischemic stroke
MMPs are a large family of proteolytic enzymes with crucial roles in ECM remodeling and BBB disruption (Chang et al., 2016). In turn, they affect leukocyte infiltration and subsequent inflammation, in addition to cerebral edema. Neutrophils express different types of MMPs at the injury site after ischemic stroke (Jickling et al., 2015). A number of studies have highlighted the increase in levels of MMPs 1, 2, 3, 8, 9, 10 and 13 in response to stroke (Chelluboina et al., 2015a; Chelluboina et al., 2015b; Cuadrado et al., 2009; Hafez et al., 2016; Han et al., 2016; Hirono et al., 2018; Ma et al., 2016; Orbe et al., 2011; Roncal et al., 2017). In addition, recent meta-analyses have revealed Mmp gene polymorphisms that are implicated in predispositions to ischemic stroke (Wang et al., 2018; Zhang et al., 2018). Although most of the MMPs act as a pro-inflammatory factor, MMP-9 has a vital role in neuronal proliferation and apoptosis (Morancho et al., 2010; Vandooren et al., 2014). Time-dependent inhibition of MMP-9 improves stroke outcomes via the degradation of DAMPS (Cauwe et al., 2009). Indeed, the timing of inhibition of MMPs is thought to be critical in terms of therapeutic effect, because MMPs have been shown to play different roles based on timing relative to stroke onset. In the early stages after stroke, MMPs have been reported to contribute to the injury process (Asahi et al., 2001; Lee and Lo, 2004; Lo et al., 2003), while later on, they contribute to the repair and recovery process (Zhao et al., 2006). Previous results demonstrated that MMP-12 upregulation leads to increased expression of other proteases such as MMP-9 and MMP-2 that could contribute to disruption of tight junction proteins in ischemic tissue after MCAO (Chelluboina et al., 2015a).
Finally, the activity of MMPs is tightly regulated via interactions with Tissue Inhibitors of Metalloproteinases (TIMPs) (Cuadrado et al., 2009; Hirono et al., 2018) and ADAMs (a disintegrin and metalloproteinases) (Montaner et al., 2019), as well as the interactions with other MMPs. Recent studies have shown that MMP-12 KO in rats and genetic deletion of MMP-12 in mice causes major alterations in the expression of other MMPs and that nature of this alteration is different in rats from mice (Chelluboina et al., 2015b; Nalamolu et al., 2018). TIMP-1 has been shown to inhibit MMP-9 and is implicated in post-stroke BBB preservation (Fujimoto et al., 2008). Therefore, it is possible to target MMPs directly, as well as indirectly via TIMP activity modulation for therapeutic intervention.
7. Conclusion and future therapeutic perspective
Stroke is a cerebrovascular disease that affects millions of people every year. Immuno-inflammatory mechanisms leading to ischemic damage are still not fully understood, but accumulating evidence suggests that inflammation is a key element in stroke pathogenesis. Activation of immune cells releases many inflammatory cytokines and critical inflammatory mediators that are not only harmful but also exhibits beneficial effect during inflammation, and fibrosis after stroke. How the temporal expression patterns and polarization into different immune cell subsets affects their function after stroke should be taken into special consideration. The role of DAMPS and NLRP3 signals and recognition of extracellular matrix proteins and the intracellular molecular switches could be promising therapeutic approaches for post-stroke inflammation and progression to fibrosis.
Highlights:
Stroke is a disorder of blood vessels accompanied with the detrimental interaction between glia, neurons, vascular cells, and matrix components.
Illustrates immune cell infiltration, cytokines response in stroke
Discusses fibrosis ECM deposition, MMPs in stroke
Elucidates the neuroprotective role of perlecan domain V in stroke via its angiogenesis property
Summarizes cytokine inhibitors/inducers, immune cell, and inflammation signaling inhibitors, and other pharmacological therapies for stroke.
Funding:
NIH R01NS089515 to G.J.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors have declared that no conflict of interest exists.
References
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD, 2009. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab 29(3), 534–544. https://doi.10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL and Pounds JG, 2002. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics. 1(12), 947–955. https://doi.10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- Aftabizadeh M, Tatarek-Nossol M, Andreetto E, El Bounkari O, Kipp M, Beyer C, Kapurniotu A, 2019. Blocking Inflammasome Activation Caused by beta-Amyloid Peptide (Abeta) and Islet Amyloid Polypeptide (IAPP) through an IAPP Mimic. ACS Chem. Neurosci 10(8), 3703–3717. https://doi.10.1021/acschemneuro.9b00260. [DOI] [PubMed] [Google Scholar]
- Agalave NM, Svensson CI, 2015. Extracellular high-mobility group box 1 protein (HMGB1) as a mediator of persistent pain. Mol. Med 20, 569–578.https://doi.10.2119/molmed.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Hekierski H, Pastor P, Yarovoi S, Higazi AA, Cines DB, 2019. Release of IL-6 After Stroke Contributes to Impaired Cerebral Autoregulation and Hippocampal Neuronal Necrosis Through NMDA Receptor Activation and Upregulation of ET-1 and JNK. Transl. Stroke Res 10(1), 104–111. https://doi.10.1007/s12975-018-0617-z. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Granger DN, Mattson MP, 2005. Stroke and T-cells. Neuromolecular Med. 7(3), 229–242. https://doi.10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Lo EH, 2001. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci 21(19), 7724–7732. https://www.ncbi.nlm.nih.gov/pubmed/11567062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten KM, Akassoglou K, 2011. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol 71(11), 1018–1039. https://doi.10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, 2002. Anti-leukocyte antibodies: leukarrest (Hu23F2G) and enlimomab (R6.5) in acute stroke. Curr. Med. Res. Opin 18(2), 18–22. https://doi.10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- Bix GJ, 2013. Perlecan domain V therapy for stroke: a beacon of hope? ACS Chem. Neurosci 4(3), 370–374. https://doi.10.1021/cn300197y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Höök M, Reed CC, 2004. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin. J Cell Biol. 166(1):97–109. https://doi.10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Iozzo RA, Woodall B, Burrows M, McQuillan A, Campbell S, Iozzo RV, 2007. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the alpha2beta1-integrin receptor. Blood 109(9), 3745–3748. https://doi.10.1182/blood-2006-08-039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Iozzo RV, 2008. Novel interactions of perlecan: unraveling perlecan’s role in angiogenesis. Microsc. Res. Tech 71(5), 339–348. https://doi.10.1002/jemt.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS, 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci 8(1), 57–69. https://doi.10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ, 2001. Role of IL-1alpha and IL-1beta in ischemic brain damage. J. Neurosci 21(15), 5528–5534. https://www.ncbi.nlm.nih.gov/pubmed/11466424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, Waisman A, 2015. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity 43(1), 92–106. https://doi.10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Cai W, Liu S, Hu M, Sun X, Qiu W, Zheng S, Lu Z, 2018. Post-stroke DHA Treatment Protects Against Acute Ischemic Brain Injury by Skewing Macrophage Polarity Toward the M2 Phenotype. Transl. Stroke Res 9(6), 669–680. https://doi.10.1007/s12975-018-0662-7. [DOI] [PubMed] [Google Scholar]
- Cai W, Wang J, Hu M, Chen X, Lu Z, Bellanti JA, Zheng SG, 2019. All trans-retinoic acid protects against acute ischemic stroke by modulating neutrophil functions through STAT1 signaling. J. Neuroinflammation, 16(1), 175. https://doi.10.1186/s12974-019-1557-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhier JF, Sirois I, Laplante P, Lepage S, Raymond MA, Bras-sard N, Prat A, Iozzo RV, Pshezhetsky AV, Hebert MJ, 2008.Caspase-3 activation triggers extracellular cathepsin L release andendorepellin proteolysis. J. Biol. Chem 283, 27220–27229. https://doi.10.1074/jbc.M801164200. [DOI] [PubMed] [Google Scholar]
- Caimi G, Canino B, Ferrara F, Montana M, Musso M, Porretto F, et al. , 2001. Granulocyte integrins before and after activation in acute ischaemic stroke. J. Neurol. Sci 186, 23–26. https://doi.10.1016/s0022-510x(01)00495-6. [DOI] [PubMed] [Google Scholar]
- Cartier L, Garcia L, Kettlun AM, Castaneda P, Collados L, Vá squez F, Giraudon P, Belin MF, Valenzuela MA, 2004. Extracellular matrix protein expression in cerebrospinal fluid from patients with tropical spastic paraparesis associated with HTLV-I and Creutzfeldt-Jakob disease. cand J Clin Lab Invest. 64(2), 101–107. doi:https://10.1080/00365510410004308. [DOI] [PubMed] [Google Scholar]
- Cauwe B, Martens E, Proost P, Opdenakker G, 2009. Multidimensional degradomics identifies systemic autoantigens and intracellular matrix proteins as novel gelatinase B/MMP-9 substrates. Integr. Biol (Camb) 1(5–6), 404–426. https://doi.10.1039/b904701h. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Stanfill A, Pourmotabbed T, 2016. The Role of Matrix Metalloproteinase Polymorphisms in Ischemic Stroke. Int. J. Mol. Sci 17(8). https://doi.10.3390/ijms17081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK, 2015. Matrix Metalloproteinase-12 Induces Blood-Brain Barrier Damage After Focal Cerebral Ischemia. Stroke 46(12), 3523–3531. https://doi.10.1161/STROKEAHA.115.011031. [DOI] [PubMed] [Google Scholar]
- Chelluboina B, Warhekar A, Dillard M, Klopfenstein JD, Pinson DM, Wang DZ, Veeravalli KK, 2015. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci. Rep 5, 9504. doi:https://10.1038/srep09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang X, Zhang C, Wang W, Chen R, Jiao H, Cui L, 2016. Anti-Inflammation of Natural Components from Medicinal Plants at Low Concentrations in Brain via Inhibiting Neutrophil Infiltration after Stroke. Mediators Inflamm. 9537901. https://doi.10.1155/2016/9537901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Tsai TH, Yang JL, Li LC, 2018. Therapeutic Strategies for Targeting IL-33/ST2 Signalling for the Treatment of Inflammatory Diseases. Cell Physiol. Biochem 49(1), 349–358. https://doi.10.1159/000492885. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP, Maniatis T, 2013. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 4(2), 385–401. https://doi.10.1016/j.celrep.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuaqui R, Tapia J, 1993. Histologic assessment of the age of recent brain infarcts in man. J. Neuropathol Exp. Neurol 52(5), pp.481–489. https://doi.10.1097/00005072-199309000-00006. [DOI] [PubMed] [Google Scholar]
- Clausen BH, Lambertsen KL, Dagnaes-Hansen F, Babcock AA, von Linstow CU, Meldgaard M, Finsen B, 2016. Cell therapy centered on IL-1Ra is neuroprotective in experimental stroke. Acta Neuropathol. 131(5), 775–791. https://doi.10.1007/s00401-016-1541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado E, Rosell A, Penalba A, Slevin M, Alvarez-Sabin J, Ortega-Aznar A, Montaner J, 2009. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J. Proteome Res 8(6), 3191–3197. https://doi.10.1021/pr801012x. [DOI] [PubMed] [Google Scholar]
- Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Moro MA, 2013. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist rosiglitazone. Stroke 44(12), 3498–3508. https://doi.10.1161/STROKEAHA.113.002470 [DOI] [PubMed] [Google Scholar]
- Daniel Berchtold LW, Christian Meisel Dr. Andreas Meisel., 2019. Friend or foe? – B cells in stroke Neuroforum, 25(3). doi: 10.1515/nf-2018-0031. [DOI] [Google Scholar]
- Del Zoppo GJ, 2004. Lessons from stroke trials using anti-inflammatory approaches that have failed. In Neuroinflammation in Stroke (pp. 155–184). Springer, Berlin, Heidelberg. https://doi.10.1007/978-3-662-05426-0_9. [DOI] [PubMed] [Google Scholar]
- Denes A, Wilkinson F, Bigger B, Chu M, Rothwell NJ, Allan SM, 2013. Central and haematopoietic interleukin-1 both contribute to ischaemic brain injury in mice. Dis. Model Mech 6(4), 1043–1048. https://doi.10.1242/dmm.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanesha N, Ahmad A, Prakash P, Doddapattar P, Lentz SR, Chauhan AK, 2015. Genetic Ablation of Extra Domain A of Fibronectin in Hypercholesterolemic Mice Improves Stroke Outcome by Reducing Thrombo-Inflammation. Circulation 132(23), 2237–2247. https://doi.10.1161/CIRCULATIONAHA.115.016540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanesha N, Chorawala MR, Jain M, Bhalla A, Thedens D, Nayak M, Chauhan AK, 2019. Fn-EDA (Fibronectin Containing Extra Domain A) in the Plasma, but Not Endothelial Cells, Exacerbates Stroke Outcome by Promoting Thrombo-Inflammation. Stroke 50(5), 1201–1209. https://doi.10.1161/STROKEAHA.118.023697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanesha N, Jain M, Tripathi A, Doddapattar P, Chorawala M, Bathla G, Nayak MK, Ghatge M, Lentz SR, Kon S and Chauhan AK, 2020. Targeting Myeloid-Specific Integrin α9β1 Improves hort and Long-Term Stroke Outcomes in Murine Models With Preexisting Comorbidities by Limiting Thrombosis And Inflammation. Circulation Research. 10.1161/CIRCRESAHA.120.316659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Canestro C, Reiner MF, Bonetti NR, Liberale L, Merlini M, Wust P, Camici GG, 2019. AP-1 (Activated Protein-1) Transcription Factor JunD Regulates Ischemia/Reperfusion Brain Damage via IL-1beta (Interleukin-1beta). Stroke 50(2), 469–477. https://doi.10.1161/STROKEAHA.118.023739. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117(14), 3720–3732. https://doi.10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolati S, Ahmadi M, Khalili M, Taheraghdam AA, Siahmansouri H, Babaloo Z, Yousefi M, 2018. Correction to: Peripheral Th17/Treg imbalance in elderly patients with ischemic stroke. Neurol. Sci 39(4), 655. https://doi.10.1007/s10072-018-3265-x. [DOI] [PubMed] [Google Scholar]
- Doll DN, Barr TL, Simpkins JW, 2014. Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis. 5(5), 294–306. https://doi.10.14336/AD.2014.0500294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Quach LN, Sole M, Axtell RC, Nguyen TV, Soler-Llavina GJ, Buckwalter MS, 2015. B-lymphocyte-mediated delayed cognitive impairment following stroke. J. Neurosci 35(5), 2133–2145. https://doi.10.1523/JNEUROSCI.4098-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Bar-Or A, 2007. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol 178(10), 6092–6099. https://doi.10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, & Schmidt MHH, 2016. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol 131(3), 347–363. https://doi.10.1007/s00401-015-1524-y. [DOI] [PubMed] [Google Scholar]
- Easton AS, 2013. Neutrophils and stroke - can neutrophils mitigate disease in the central nervous system? Int. Immunopharmacol 17(4), 1218–1225. https://doi.10.1016/j.intimp.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Edwards DN, Bix GJ, 2019. Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am. J. Physiol. Cell. Physiol 316(2), C252–C263. https://doi.10.1152/ajpcell.00151.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DN, Salmeron K, Lukins DE, Trout AL, Fraser JF, Bix GJ, 2019. Integrin alpha5beta1 inhibition by ATN-161 reduces neuroinflammation and is neuroprotective in ischemic stroke. J. Cereb. Blood Flow Metab 271678X19880161. https://doi.10.1177/0271678X19880161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley HCA, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ and Tyrrell PJ, 2005. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 76(10), pp.1366–1372. https://doi.10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC, 2010. Minocycline to improve neurologic outcome in stroke (MINOS) a dose-finding study. Stroke, 41(10), 2283–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Zhang CJ, Zhu L, Chen J, Zhang Z, Liu P, Xu Y, 2020. FasL-PDPK1 Pathway Promotes the Cytotoxicity of CD8(+) T Cells During Ischemic Stroke. Transl. Stroke Res https://doi.10.1007/s12975-019-00749-0. [DOI] [PubMed] [Google Scholar]
- Faraco G, Park L, Anrather J, Iadecola C, 2017. Brain perivascular macrophages: characterization and functional roles in health and disease. J. Mol. Med (Berl) 95(11), 1143–1152. https://doi.10.1007/s00109-017-1573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Iadecola C, 2016. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin. Invest 126(12), 4674–4689. https://doi.10.1172/JCI86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, Kipnis J, 2017. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat. Rev. Neurosci 18(6), 375–384. https://doi.10.1038/nrn.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, Lopez-Vales R, 2016. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia 64(12), 2079–2092. https://doi.10.1002/glia.23041. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, Han W, Xue R, Liu Q, Hao J, Yu C, 2014. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A 111(51), 18315–18320. https://doi.10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Takagi Y, Aoki T, Hayase M, Marumo T, Gomi M, Nozaki K, 2008. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J. Cereb. Blood Flow Metab 28(10), 1674–1685.https://doi.10.1038/jcbfm.2008.59 [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Relton JK, 1995. Interleukin-1 receptor antagonist decreases the number of necrotic neurons in rats with middle cerebral artery occlusion. Am. J. Pathol 147(5), 1477–1486. https://www.ncbi.nlm.nih.gov/pubmed/7485410. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Culebras A, Duran-Laforet V, Pena-Martinez C, Moraga A, Ballesteros I, Cuartero MI, Lizasoain I, 2019. Role of TLR4 (Toll-Like Receptor 4) in N1/N2 Neutrophil Programming After Stroke. Stroke 50(10), 2922–2932. https://doi.10.1161/STROKEAHA.119.025085 [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, & Randolph GJ, 2010. Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol 10(6), 453–460. https://doi.10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Gallizioli M, Ludewig P, Thom V, Arunachalam P, Rissiek B, Magnus T, 2018. IL-23 (Interleukin-23)-Producing Conventional Dendritic Cells Control the Detrimental IL-17 (Interleukin-17) Response in Stroke. Stroke 49(1), 155–164. https://doi.10.1161/STROKEAHA.117.019101. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Lewerenz J, Birkenmayer G, Orozco D, Ludewig P, Magnus T, 2012. The flavonoid fisetin attenuates postischemic immune cell infiltration, activation and infarct size after transient cerebral middle artery occlusion in mice. J. Cereb. Blood Flow Metab 32(5), 835–843. https://doi.10.1038/jcbfm.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Magnus T, 2012. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 120(18), 3793–3802. https://doi.10.1182/blood-2012-02-412726. [DOI] [PubMed] [Google Scholar]
- Gill D, Veltkamp R, 2016. Dynamics of T cell responses after stroke. Curr. Opin. Pharmacol 26, 26–32. https://doi.10.1016/j.coph.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Merad M, 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005), 841–845. https://doi.10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Prinz M, 2016. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol 17(7), 797–805. https://doi.10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Rodewald HR, 2015. Tissue-resident macrophages originate from yolk-sac-derived erythromyeloid progenitors. Nature 518(7540), 547–551. https://doi.10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Pan J, Shen Q, Li M, Peng Y, 2018. Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J. Neuroinflammation 15(1), 242. https://doi.10.1186/s12974-018-1282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B,Greenspan DS, Iozzo RV, 2005. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem 280, 7080–7087. https://doi.10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B, 2011. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol 37(1), 24–39. https://doi.10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- Gronhoj MH, Clausen BH, Fenger CD, Lambertsen KL, Finsen B, 2017. Beneficial potential of intravenously administered IL-6 in improving outcome after murine experimental stroke. Brain Behav. Immun 65, 296–311. https://doi.10.1016/j.bbi.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Guell K, Bix GJ, 2014. Brain endothelial cell specific integrins and ischemic stroke. Expert Rev. Neurother 14(11), 1287–1292. https://doi.10.1586/14737175.2014.964210. [DOI] [PubMed] [Google Scholar]
- Gulke E, Gelderblom M, Magnus T, 2018. Danger signals in stroke and their role on microglia activation after ischemia. Ther. Adv. Neurol. Disord 11, 1756286418774254. https://doi.10.1177/1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wang X, Zhao Y, Yang Q, Ding H, Dong Q, Cui M, 2018. Ketogenic Diet Improves Brain Ischemic Tolerance and Inhibits NLRP3 Inflammasome Activation by Preventing Drp1-Mediated Mitochondrial Fission and Endoplasmic Reticulum Stress. Front. Mol. Neurosci 11, 86. https://doi.10.3389/fnmol.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Yu S, Chen X, Zheng P, Hu T, Duan Z, Liu X, 2018. Suppression of NLRP3 attenuates hemorrhagic transformation after delayed rtPA treatment in thromboembolic stroke rats: Involvement of neutrophil recruitment. Brain Res. Bull 137, 229–240. https://doi.10.1016/j.brainresbull.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Hafez S, Abdelsaid M, El-Shafey S, Johnson MH, Fagan SC, Ergul A (2016). Matrix Metalloprotease 3 Exacerbates Hemorrhagic Transformation and Worsens Functional Outcomes in Hyperglycemic Stroke. Stroke 47(3), 843–851. https://doi.10.1161/STROKEAHA.115.011258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R, 2003. Physiological levels of tumstatin, a fragment of collagen IV α3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via αVβ3 integrin. Cancer cell, 3(6), 589–601. 10.1016/S1535-6108(03)00133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei Taj S, Le Blon D, Hoornaert C, Daans J, Quarta A, Praet J, Hoehn M, 2018. Targeted intracerebral delivery of the anti-inflammatory cytokine IL13 promotes alternative activation of both microglia and macrophages after stroke. J. Neuroinflammation 15(1), 174. https://doi.10.1186/s12974-018-1212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JE, Lee EJ, Moon E, Ryu JH, Choi JW, Kim HS, 2016. Matrix Metalloproteinase-8 is a Novel Pathogenetic Factor in Focal Cerebral Ischemia. Mol. Neurobiol 53(1), 231–239. https://doi.10.1007/s12035-014-8996-y. [DOI] [PubMed] [Google Scholar]
- Hao Y, Ding J, Hong R, Bai S, Wang Z, Mo C, Guan Y, 2019. Increased interleukin-18 level contributes to the development and severity of ischemic stroke. Aging (Albany NY) 11(18), 7457–7472. https://doi.10.18632/aging.102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NM, Roy-O’Reilly M, Ritzel RM, Holmes A, Sansing LH, O’Keefe LM, Chauhan A, 2020. Depletion of CD4 T cells provides therapeutic benefits in aged mice after ischemic stroke. Exp. Neurol 326, 113202. https://doi.10.1016/j.expneurol.2020.113202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono J, Sanaki H, Kitada K, Sada H, Suzuki A, Lie LK, Hasegawa H, 2018. Expression of tissue inhibitor of metalloproteinases and matrix metalloproteinases in the ischemic brain of photothrombosis model mice. Neuroreport. 29(3), 174–180. https://doi.10.1097/WNR.0000000000000946. [DOI] [PubMed] [Google Scholar]
- Hotter B, Hoffmann S, Ulm L, Meisel C, Fiebach JB, Meisel A, 2019. IL-6 Plasma Levels Correlate With Cerebral Perfusion Deficits and Infarct Sizes in Stroke Patients Without Associated Infections. Front. Neurol 10, 83. https://doi.10.3389/fneur.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015). Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol 11(1), 56–64. https://doi.10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J, 2011. The immunology of stroke: from mechanisms to translation. Nat. Med 17(7), 796–808. https://doi.10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismael S, Zhao L, Nasoohi S, Ishrat T, 2018. Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep, 8(1), 5971. https://www.ncbi.nlm.nih.gov/pubmed/29654318. doi: 10.1038/s41598-018-24350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA, 2019. Neuroinflammation: friend and foe for ischemic stroke. J. Neuroinflammation 16(1), 142. https://doi.10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny NS, Callas PW, Judd SE, McClure LA, Kissela B, Zakai NA, Cushman M, 2019. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology 92(20), e2375–e2384. https://doi.10.1212/WNL.0000000000007416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Z, Liu R, Zhu X, Smerin D, Zhong Y, Gu L, Xiong X, 2019. The Involvement and Therapy Target of Immune Cells After Ischemic Stroke. Front. Immunol 10, 2167. https://doi.10.3389/fimmu.2019.02167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR, 2015. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J. Cereb. Blood Flow Metab 35(6), 888–901. https://doi.10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G, 2010. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc Biol 87(5), pp.779–789. doi: https://10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Pang T, 2018. Microglia: Housekeeper of the Central Nervous System. Cell Mol. Neurobiol 38(1), 53–71. https://doi.10.1007/s10571-017-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan MJ, Lee JE, Wilson JG, Everhart AL, Brown CM, Hoofnagle AN, Colton CA, 2015. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J. Neurosci 35(15), 5969–5982. https://doi.10.1523/JNEUROSCI.4668-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa M, Miura M, Toriyabe M, Koyama M, Hatakeyama M, Ishikawa M, Shimohata T, 2017. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci. Rep 7, 42582. https://doi.10.1038/srep42582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandikattu HK, Deep SN, Razack S, Amruta N, Prasad D, Khanum F, 2017. Hypoxia induced cognitive impairment modulating activity of Cyperus rotundus. Physiology & behavior 175, 56–65. [DOI] [PubMed] [Google Scholar]
- Kandikattu HK, Rachitha P, Jayashree G, Krupashree K, Sukhith M, Majid A, Khanum F, 2017. Anti-inflammatory and anti-oxidant effects of Cardamom (Elettaria repens (Sonn.) Baill) and its phytochemical analysis by 4D GCXGC TOF-MS. Biomedicine & Pharmacotherapy 91, 191–201. [DOI] [PubMed] [Google Scholar]
- Kandikattu HK, Venkateshaiah SU, Mishra A, 2019. Synergy of interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine & growth factor reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandikattu H, Venkateshaiah S, Mishra A, 2020. Chronic Pancreatitis and the Development of Pancreatic Cancer. Endocrine, Metabolic & Immune Disorders Drug Targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Yao Y, 2020. Basement membrane changes in ischemic stroke. Stroke, 51(4), pp.1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangwantas K, Pinteaux E, Penny J, 2016. The extracellular matrix protein laminin-10 promotes blood–brain barrier repair after hypoxia and inflammation in vitro. J. neuroinflammation, 13(1), p.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita F, Kanamaru H, Asada R, Suzuki H, 2019. Potential roles of matricellular proteins in stroke. Exp. Neurol 322, 113057. https://doi.10.1016/j.expneurol.2019.113057. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Prinz M, 2013. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci 16(3), 273–280. https://doi.10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kim ID, Lee H, Kim SW, Lee HK, Choi J, Han PL, Lee JK, 2018. Alarmin HMGB1 induces systemic and brain inflammatory exacerbation in post-stroke infection rat model. Cell Death Dis. 9(4), 426. https://doi.10.1038/s41419-018-0438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee JK, 2006. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci 26(24), 6413–6421. https://doi.10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocak O, Carman KBCB, Yarar C, Kodera H, Saitsu H, Matsumoto N, 2020. COL4A1 and COL4A2 Mutations Analyses with Perinatal Arterial İschemic troke. Trop. Health Med. Research, 2(1), 18–25. [Google Scholar]
- Kolaczkowska E, Kubes P, 2013. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol 13(3), 159–175. doi:https://10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kolosowska N, Keuters MH, Wojciechowski S, Keksa-Goldsteine V, Laine M, Malm T, Dhungana H, 2019. Peripheral Administration of IL-13 Induces Anti-inflammatory Microglial/Macrophage Responses and Provides Neuroprotection in Ischemic Stroke. Neurotherapeutics 16(4), 1304–1319. https://doi.10.1007/s13311-019-00761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H, 2002. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke 33(4), 1129–1134. https://doi.10.1161/hs0402.105379. [DOI] [PubMed] [Google Scholar]
- Kostulas N, Pelidou SH, Kivisakk P, Kostulas V, Link H, 1999. Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke 30(10), 2174–2179. https://doi.10.1161/01.str.30.10.2174. [DOI] [PubMed] [Google Scholar]
- Kwan J, Horsfield G, Bryant T, Gawne-Cain M, Durward G, Byrne CD, Englyst NA, 2013. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp. Gerontol 48(9), 960–965. https://doi.10.1016/j.exger.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, Bix GJ, 2011. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J. Clin. Invest 121(8), 3005–3023. https://doi.10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Lo EH, 2004. Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J. Cereb. Blood Flow Metab 24(7), 720–727. https://doi.10.1097/01.WCB.0000122747.72175.47. [DOI] [PubMed] [Google Scholar]
- Lemarchand E, Barrington J, Chenery A, Haley M, Coutts G, Allen JE, Brough D, 2019. Extent of Ischemic Brain Injury After Thrombotic Stroke Is Independent of the NLRP3 (NACHT, LRR and PYD Domains-Containing Protein 3) Inflammasome. Stroke 50(5), 1232–1239. https://doi.10.1161/STROKEAHA.118.023620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Nih LR, Bachman H, Fei P, Li Y, Nam E, Dimatteo R, Carmichael ST, Barker TH and Segura T, 2017. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat Mater. 16(9), 953–961. https://doi.10.1038/nmat4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Bauer A, Hoheisel JD, Veltkamp R, 2014. Intracerebral interleukin-10 injection modulates post-ischemic neuroinflammation: an experimental microarray study. Neurosci. Lett 579, 18–23. https://doi.10.1016/j.neulet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Liesz A, Hu X, Kleinschnitz C, Offner H, 2015. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke 46(5), 1422–1430. https://doi.10.1161/STROKEAHA.114.008608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Na SY, Hammerling GJ, Garbi N, Karcher S, Veltkamp R, 2013. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J. Neurosci 33(44), 17350–17362. https://doi.10.1523/JNEUROSCI.4901-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Girard JP, Turnquist HR, 2016. Interleukin-33 in health and disease. Nat. Rev. Immunol 16(11), 676–689.doi:https://10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- Liu T, McDonnell PC, Young PR, White RF, Siren AL, Hallenbeck JM, Feurestein GZ, 1993. Interleukin-1 beta mRNA expression in ischemic rat cortex. Stroke 24(11), 1746–1750; discussion 1750–1741. https://doi.10.1161/01.str.24.11.1746. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu R, Pei L, Si P, Wang C, Tian X, Song B, 2020. Regulatory T cell is critical for interleukin-33-mediated neuroprotection against stroke. Exp. Neurol 328, 113233. https://doi.10.1016/j.expneurol.2020.113233. [DOI] [PubMed] [Google Scholar]
- Liu X, Li Q, Zhu R, He Z, 2017. Association of IL-10–1082A/G Polymorphism with Ischemic Stroke: Evidence from a Case-Control Study to an Updated Meta-Analysis. Genet. Test Mol. Biomarkers 21(6), 341–350. https://doi.10.1089/gtmb.2016.0409. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Ran YY, Qie SY, Gong WJ, Gao FH, Ding ZT, Xi JN, 2019. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci. Ther 25(12), 1353–1362. https://doi.10.1111/cns.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovera G, Hofmann K, Roth S, Salas-Perdomo A, Ferrer-Ferrer M, Perego C, et al. (2015). Results of a preclinical randomized controlled multicenter trial (pRCT): anti-CD49d treatment for acute brain ischemia. Sci. Transl. Med 7:299ra121. https://doi.10.1126/scitranslmed.aaa9853. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA, 2003. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci 4(5), 399–415. https://doi.10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lucchese G, Floel A, Stahl B, 2019. Cross-Reactivity as a Mechanism Linking Infections to Stroke. Front. Neurol 10, 469. https://doi.10.3389/fneur.2019.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi NM, Kovacs KJ, Lopez-Castejon G, Brough D, Denes A, 2011. Interleukin-1alpha expression precedes IL-1beta after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J. Neuroinflammation 8, 186. https://doi.10.1186/1742-2094-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhou Y, Xiao W, Liang Z, Dai J, Weng X, Wu X, 2015. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 1597, 86–94. https://doi.10.1016/j.brainres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Ma F, Martinez-San Segundo P, Barcelo V, Morancho A, Gabriel-Salazar M, Giralt D, Rosell A, 2016. Matrix metalloproteinase-13 participates in neuroprotection and neurorepair after cerebral ischemia in mice. Neurobiol. Dis 91, 236–246. https://doi.10.1016/j.nbd.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Makihara N, Arimura K, Ago T, Tachibana M, Nishimura A, Nakamura K, Kitazono T, 2015. Involvement of platelet-derived growth factor receptor beta in fibrosis through extracellular matrix protein production after ischemic stroke. Exp. Neurol 264, 127–134. https://doi.10.1016/j.expneurol.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Mao M, Alavi MV, Labelle-Dumais C, Gould DB, 2015. Type IV collagens and basement membrane diseases: cell biology and pathogenic mechanisms. In Current topics in membranes (Vol. 76, pp. 61–116). Academic Press. https://doi.10.1016/bs.ctm.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Marcelo A, Bix G, 2014. Investigating the role of perlecan domain V in post-ischemic cerebral angiogenesis. Methods Mol. Biol 1135, 331–341. https://doi.10.1007/978-1-4939-0320-7_27. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Read SJ, 2008. Immune and inflammatory responses to stroke: good or bad?. Int J Stroke. 3(4), pp.254–265. doi: https://10.1111/j.1747-4949.2008.00222.x. [DOI] [PubMed] [Google Scholar]
- Mena H, Cadavid D and Rushing EJ, 2004. Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol. 108(6), 524–530. https://doi.10.1007/s00401-004-0918-z. [DOI] [PubMed] [Google Scholar]
- Meng H, Zhao H, Cao X, Hao J, Zhang H, Liu Y, Xu Y, 2019. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc. Natl. Acad. Sci. U S A 116(12), 5558–5563. https://doi.10.1073/pnas.1814394116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J and Hughes CC, 2004. Of mice and not men: differences between mouse and human immunology. J Immunol. 172(5), 2731–2738. https://doi.10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Mills CD, 2001. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit. Rev. Immunol 21(5), 399–425. https://www.ncbi.nlm.nih.gov/pubmed/11942557. [PubMed] [Google Scholar]
- Milner R and Campbell IL, 2006. Increased expression of the β4 and α5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-α in the central nervous system. Molecular and Cellular Neuroscience, 33(4), 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J, Ramiro L, Simats A, Hernandez-Guillamon M, Delgado P, Bustamante A, Rosell A, 2019. Matrix metalloproteinases and ADAMs in stroke. Cell Mol. Life Sci 76(16), 3117–3140. https://doi.10.1007/s00018-019-03175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morancho A, Rosell A, Garcia-Bonilla L, Montaner J, 2010. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann. N Y Acad. Sci 1207, 123–133. https://doi.10.1111/j.1749-6632.2010.05734.x. [DOI] [PubMed] [Google Scholar]
- Na SY, Mracsko E, Liesz A, Hunig T, Veltkamp R, 2015. Amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice. Stroke 46(1), 212–220. https://doi.10.1161/STROKEAHA.114.007756. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A, Okada T, 2017. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol. Ther. Methods Clin. Dev 6, 102–111. https://doi.10.1016/j.omtm.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Shichita T, 2019. Cellular and molecular mechanisms of sterile inflammation in ischaemic stroke. J. Biochem 165(6), 459–464. https://doi.10.1093/jb/mvz017. [DOI] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Raghubir R, 2010. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 17(2), 189–202. https://doi.10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- Nalamolu KR, Chelluboina B, Magruder IB, Fru DN, Mohandass A, Venkatesh I, Veeravalli KK, 2018. Post-stroke mRNA expression profile of MMPs: effect of genetic deletion of MMP-12. Stroke Vasc. Neurol 3(3), 153–159. https://doi.10.1136/svn-2018-000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaghihaghighi S, Vahdati SS, Mikaeilpour A, Ramouz A, 2017. Comparison of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World J. Emerg. Med 8(1), 34–38. https://doi.10.5847/wjem.j.1920-8642.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbe J, Barrenetxe J, Rodriguez JA, Vivien D, Orset C, Parks WC, Paramo JA, 2011. Matrix metalloproteinase-10 effectively reduces infarct size in experimental stroke by enhancing fibrinolysis via a thrombin-activatable fibrinolysis inhibitor-mediated mechanism. Circulation 124(25), 2909–2919. https://doi.10.1161/CIRCULATIONAHA.111.047100. [DOI] [PubMed] [Google Scholar]
- Ortega SB, Torres VO, Latchney SE, Whoolery CW, Noorbhai IZ, Poinsatte K, Stowe AM, 2020. B cells migrate into remote brain areas and support neurogenesis and functional recovery after focal stroke in mice. Proc. Natl. Acad. Sci. U S A 117(9), 4983–4993. https://doi.10.1073/pnas.1913292117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham C, Auckland L, Rachwal J, Clarke D, Bix G, 2014. Perlecan domain V inhibits amyloid-beta induced brain endothelial cell toxicity and restores angiogenic function. J. Alzheimers Dis 38(2), 415–423. https://doi.10.3233/JAD-130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluk H, Wozniak A, Grzesk G, Kolodziejska R, Kozakiewicz M, Kopkowska E, Kozera G, 2020. The Role of Selected Pro-Inflammatory Cytokines in Pathogenesis of Ischemic Stroke. Clin. Interv. Aging, 15, 469–484. https://doi.10.2147/CIA.S233909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen ED, Waje-Andreassen U, Vedeler CA, Aamodt G, Mollnes TE, 2004. ystemic complement activation following human acute ischaemic stroke. Clin ExpImmunol. 137(1), 117–122. https://doi.10.1111/j.1365-2249.2004.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera MN, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, Donnan GA, 2006. Inflammation following stroke. J. Clin. Neurosci 13(1), 1–8. 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Perez-de Puig I, Miro F, Salas-Perdomo A, Bonfill-Teixidor E, Ferrer-Ferrer M, Marquez-Kisinousky L, Planas AM, 2013. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J. Cereb. Blood Flow Metab 33(12), 1955–1966. https://doi.10.1038/jcbfm.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R, Gatlin CL, McGrath AM, Makusky AJ, Mondal M, Seonarain M, Field E, Schatz CR, Estock MA, Ahmed N Anderson NG, 2004. Characterization of the human urinary proteome: a method for high‐resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1400 distinct protein spots. Proteomics, 4(4), pp.1159–1174. https://10.1002/pmic.200300661. [DOI] [PubMed] [Google Scholar]
- Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U, 2004. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development, 131(7), pp.1619–1628. [DOI] [PubMed] [Google Scholar]
- Pruss H, Iggena D, Baldinger T, Prinz V, Meisel A, Endres M, Schwab JM, 2012. Evidence of intrathecal immunoglobulin synthesis in stroke: a cohort study. Arch Neurol 69(6), 714–717. https://doi.10.1001/archneurol.2011.3252. [DOI] [PubMed] [Google Scholar]
- Qiu J, Xu J, Zheng Y, Wei Y, Zhu X, Lo EH, Sims JR, 2010. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke 41(9), 2077–2082. https://doi.10.1161/STROKEAHA.110.590463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayasam A, Hsu M, Kijak JA, Kissel L, Hernandez G, Sandor M, Fabry Z, 2018. Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology, 154(3), 363–376. https://doi.10.1111/imm.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Lisabeth L, 2008. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 7(10), 915–926. https://doi.10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton JK, Rothwell NJ, 1992. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res. Bull 29(2), 243–246. https://doi.10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H, 2011. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J. Neurosci 31(23), 8556–8563. https://doi.10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard SA, Sackey M, Su Z and Xu H, 2017. Pivotal neuroinflammatory and therapeutic role of high mobility group box 1 in ischemic stroke. Biosci Rep. 37(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Crapser J, Patel AR, Verma R, Grenier JM, Chauhan A, McCullough LD, 2016. Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury. J. Immunol 196(8), 3318–3330. https://doi.10.4049/jimmunol.1502021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD, 2015. Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflammation 12, 106. https://doi.10.1186/s12974-015-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J, de Hoog L, Bix GJ, 2017. Mice deficient in endothelial alpha5 integrin are profoundly resistant to experimental ischemic stroke. J. Cereb. Blood Flow Metab 37(1), 85–96. https://doi.10.1177/0271678X15616979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H, 2010. The sterile inflammatory response. Annu. Rev. Immunol 28, 321–342. https://doi.10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogove AD, Lu W, Tsirka SE, 2002. Microglial activation and recruitment, but not proliferation, suffice to mediate neurodegeneration. Cell Death Differ. 9(8), 801–806. https://doi.10.1038/sj.cdd.4401041. [DOI] [PubMed] [Google Scholar]
- Roncal C, Martinez de Lizarrondo S, Salicio A, Chevilley A, Rodriguez JA, Rosell A, Orbe J, 2017. New thrombolytic strategy providing neuroprotection in experimental ischemic stroke: MMP10 alone or in combination with tissue-type plasminogen activator. Cardiovasc Res 113(10), 1219–1229. https://doi.10.1093/cvr/cvx069. [DOI] [PubMed] [Google Scholar]
- Rostrom B, Link B, 1981. Oligoclonal immunoglobulins in cerebrospinal fluid in acute cerebrovascular disease. Neurology 31(5), 590–596. https://doi.10.1212/wnl.31.5.590. [DOI] [PubMed] [Google Scholar]
- Roth M, Enstrom A, Aghabeick C, Carlsson R, Genove G, Paul G, 2020. Parenchymal pericytes are not the major contributor of extracellular matrix in the fibrotic scar after stroke in male mice. J. Neurosci. Res 98(5), 826–842. https://doi.10.1002/jnr.24557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-O’Reilly M, & McCullough LD (2014). Sex differences in stroke: the contribution of coagulation. Exp. Neurol 259, 16–27. https://doi.10.1016/j.expneurol.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini MG, Bix GJ, 2012. Oxygen-glucose deprivation (OGD) and interleukin-1 (IL-1) differentially modulate cathepsin B/L mediated generation of neuroprotective perlecan LG3 by neurons. Brain Res. 1438, 65–74. https://doi.10.1016/j.brainres.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini MG, Pinteaux E, Lee B, Bix GJ, 2011. Oxygen-glucose deprivation and interleukin-1alpha trigger the release of perlecan LG3 by cells of neurovascular unit. J. Neurochem 119(4), 760–771. https://doi.10.1111/j.1471-4159.2011.07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini MG, Bix GJ, 2012., Oxygen–glucose deprivation (OGD) and interleukin-1 (IL-1) differentially modulate cathepsin B/L mediated generation of neuroprotective perlecan LG3 by neurons. Brain res. 1438, 65–74. https://doi.10.1016/j.brainres.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron KE, Maniskas ME, Edwards DN, Wong R, Rajkovic I, Trout A, Bix GJ, 2019. Interleukin 1 alpha administration is neuroprotective and neuro-restorative following experimental ischemic stroke. J. Neuroinflammation, 16(1), 222. https://doi.10.1186/s12974-019-1599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron K, Aihara T, Redondo-Castro E, Pinteaux E, Bix G, 2016. IL-1alpha induces angiogenesis in brain endothelial cells in vitro: implications for brain angiogenesis after acute injury. J. Neurochem 136(3), 573–580. https://doi.10.1111/jnc.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroj Choudhary DC, Mishra TK, Agarwal Sarita. (2018). Temporal profile of serum levelsof il-6 in acute ischemic stroke and its relationship with stroke severity and outcome in Indian population. Int. J. Intg. Med. Sci 5(1). [Google Scholar]
- Schuhmann MK, Langhauser F, Kraft P, Kleinschnitz C, 2017. B cells do not have a major pathophysiologic role in acute ischemic stroke in mice. J. Neuroinflammation 14(1), 112. https://doi.10.1186/s12974-017-0890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel GB, Halterman MW, Lichtman MA, 2011. The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol 89(3), 359–372. https://doi.10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Vandenbark AA, Offner H, 2018. Regulatory B cells in experimental stroke. Immunology 154(2), 169–177. https://doi.10.1111/imm.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Collier LA, Chapman CB, Benkovic SA, Willing AE, Pennypacker KR, 2014. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J. Neuroimmune Pharmacol 9(5), pp.679–689. https://doi.10.1007/s11481-014-9560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj UM, Poinsatte K, Torres V, Ortega SB, Stowe AM, 2016. Heterogeneity of B Cell Functions in Stroke-Related Risk, Prevention, Injury, and Repair. Neurotherapeutics 13(4), 729–747. https://doi.10.1007/s13311-016-0460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan H, Bian Y, Shu Z, Zhang L, Zhu J, Ding J, Hu G, 2016. Fluoxetine protects against IL-1beta-induced neuronal apoptosis via downregulation of p53. Neuropharmacology 107, 68–78. https://doi.10.1016/j.neuropharm.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Shu L, Xu CQ, Yan ZY, Yan Y, Jiang SZ, Wang YR, 2019. Post-Stroke Microglia Induce Sirtuin2 Expression to Suppress the Anti-inflammatory Function of Infiltrating Regulatory T Cells. Inflammation, 42(6), 1968–1979. https://doi.10.1007/s10753-019-01057-3. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Denes A, Tyrrell PJ, Di Napoli M, 2015. Phase II anti-inflammatory and immune-modulating drugs for acute ischaemic stroke. Expert Opin. Investig. Drugs 24(5), pp.623–643. https://doi.10.1517/13543784.2015.1020110. [DOI] [PubMed] [Google Scholar]
- Sobowale OA, Parry-Jones AR, Smith CJ, Tyrrell PJ, Rothwell NJ, Allan SM, 2016. Interleukin-1 in Stroke: From Bench to Bedside. Stroke 47(8), 2160–2167. https://doi.10.1161/STROKEAHA.115.010001. [DOI] [PubMed] [Google Scholar]
- Summers L, Kangwantas K, Rodriguez-Grande B, Denes A, Penny J, Kielty C, Pinteaux E, 2013. Activation of brain endothelial cells by interleukin-1 is regulated by the extracellular matrix after acute brain injury. Mol. Cell Neurosci 57, 93–103. https://doi.10.1016/j.mcn.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, Denes A, 2016. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun 7, 11499. https://doi.10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT, 2012. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev 249(1), 158–175. https://doi.10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurgur H, Pinteaux E, 2019. Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions After Central Nervous System Disorders. Neuroscience 405, 55–67. https://doi.10.1016/j.neuroscience.2018.06.046. [DOI] [PubMed] [Google Scholar]
- Tong Y, Jiang S, Cai L, Guan X, Hou S, Wang Z, Liu J, 2018. Identification of Functional Genetic Polymorphisms at IL-10 Promoter Region and their Association with Risk of Ischemic Stroke in Chinese Han Population. J. Nutr. Health Aging 22(7), 779–784. https://doi.10.1007/s12603-018-1012-x. [DOI] [PubMed] [Google Scholar]
- Trout AL, Kahle MP, Roberts JM, Marcelo A, de Hoog L, Boychuk JA, Bix GJ, 2020. Perlecan Domain-V Enhances Neurogenic Brain Repair After Stroke in Mice. Transl. Stroke Res https://doi.10.1007/s12975-020-00800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umahara T, Uchihara T, Hirokawa K, Hirao K, Shimizu S, Hashimoto T, Hanyu H, 2018. Time-dependent and lesion-dependent HMGB1-selective localization in brains of patients with cerebrovascular diseases. Histol. Histopathol 33(2), 215–222. https://doi.10.14670/HH-11-914. [DOI] [PubMed] [Google Scholar]
- Urra X, Cervera Á, Villamor N, Planas AM, Chamorro A, 2009. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience, 158(3), 1174–1183. https://doi.10.1016/j.neuroscience.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Vandooren J, Van Damme J, Opdenakker G, 2014. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog. Brain Res 214, 193–206. https://doi.10.1016/B978-0-444-63486-3.00009-8. [DOI] [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ, 1997. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin α6β4 during focal cerebral ischemia/reperfusion. Stroke, 28(4), pp.858–865. https://doi.10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Wang B, Wang Y, Zhao L, 2018. MMP-9 gene rs3918242 polymorphism increases risk of stroke: A meta-analysis. J. Cell Biochem 119(12), 9801–9808. https://doi.10.1002/jcb.27299. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin H, Wang W, Wang F, Zhao H, 2019. Myosin1f-mediated neutrophil migration contributes to acute neuroinflammation and brain injury after stroke in mice. J. Neuroinflammation 16(1), 77. https://doi.10.1186/s12974-019-1465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Meng C, Zhang J, Wu J, Zhao J, 2019. Inhibition of GSK-3beta alleviates cerebral ischemia/reperfusion injury in rats by suppressing NLRP3 inflammasome activation through autophagy. Int. Immunopharmacol 68, 234–241. https://doi.10.1016/j.intimp.2018.12.042. [DOI] [PubMed] [Google Scholar]
- Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, Kokaia Z, 2016. Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J. Neurosci 36(15), 4182–4195. https://doi.10.1523/JNEUROSCI.4317-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M, 2010. Interleukin-1beta (IL-1beta) processing pathway. Sci. Signal 3(105), cm2. https://doi.10.1126/scisignal.3105cm2. [DOI] [PubMed] [Google Scholar]
- Wheeler RD, Boutin H, Touzani O, Luheshi GN, Takeda K, & Rothwell NJ (2003). No role for interleukin-18 in acute murine stroke-induced brain injury. J Cereb Blood Flow Metab, 23(5), 531–535. https://doi.10.1097/01.WCB.0000059587.71206.BA [DOI] [PubMed] [Google Scholar]
- Wiessner C, Gehrmann J, Lindholm D, Topper R, Kreutzberg GW, Hossmann KA, 1993. Expression of transforming growth factor-beta 1 and interleukin-1 beta mRNA in rat brain following transient forebrain ischemia. Acta Neuropathol. 86(5), 439–446. https://doi.10.1007/BF00228578. [DOI] [PubMed] [Google Scholar]
- Works MG, Koenig JB, Sapolsky RM, 2013. Soluble TNF receptor 1-secreting ex vivo-derived dendritic cells reduce injury after stroke. J. Cereb. Blood Flow. Metab 33(9), 1376–1385. https://doi.10.1038/jcbfm.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Zhang G, Zhao C, Yang Y, Miao Z, Xu X, 2020. Interleukin-18 from neurons and microglia mediates depressive behaviors in mice with post-stroke depression. Brain Behav. Immun https://doi.10.1016/j.bbi.2020.04.004. [DOI] [PubMed] [Google Scholar]
- Wu MH, Huang CC, Chio CC, Tsai KJ, Chang CP, Lin NK, Lin MT, 2016. Inhibition of Peripheral TNF-alpha and Downregulation of Microglial Activation by Alpha-Lipoic Acid and Etanercept Protect Rat Brain Against Ischemic Stroke. Mol. Neurobiol 53(7), 4961–4971. https://doi.10.1007/s12035-015-9418-5. [DOI] [PubMed] [Google Scholar]
- Xiao W, Guo S, Chen L, Luo Y, 2019. The role of Interleukin-33 in the modulation of splenic T-cell immune responses after experimental ischemic stroke. J. Neuroimmunol 333, 576970. https://doi.10.1016/j.jneuroim.2019.576970. [DOI] [PubMed] [Google Scholar]
- Xiong X, Barreto GE, Xu L, Ouyang YB, Xie X, Giffard RG, 2011. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke 42(7), 2026–2032. https://doi.10.1161/STROKEAHA.110.593772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhang L, Ye X, Hao Q, Zhang T, Cui G, Yu M, 2018. Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflammasome activation in BV2 cells after cerebral ischemia reperfusion. Inflamm. Res 67(1), 57–65. https://doi.10.1007/s00011-017-1095-6. [DOI] [PubMed] [Google Scholar]
- Yanagihara R, Garruto RM, Gajdusek DC, Tomita A, Uchikawa T, Konagaya Y, Gibbs CJ Jr.,1984. Calcium and vitamin D metabolism in Guamanian Chamorros with amyotrophic lateral sclerosis and parkinsonism-dementia. Ann. Neurol 15(1), 42–48. https://doi.10.1002/ana.410150108. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu C, Du X, Liu M, Ji X, Du H, Zhao H, 2018. Hypoxia Inducible Factor 1alpha Plays a Key Role in Remote Ischemic Preconditioning Against Stroke by Modulating Inflammatory Responses in Rats. J. Am. Heart Assoc 7(5). https://doi.10.1161/JAHA.117.007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu H, Zhang H, Ye Q, Wang J, Yang B, Hu X, 2017. ST2/IL-33-Dependent Microglial Response Limits Acute Ischemic Brain Injury. J. Neurosci 37(18), 4692–4704. https://doi.10.1523/JNEUROSCI.3233-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XC, Hao Q, Ma WJ, Zhao QC, Wang WW, Yin HH, Cui GY, 2020. Dectin-1/Syk signaling triggers neuroinflammation after ischemic stroke in mice. J. Neuroinflammation 17(1), 17. https://doi.10.1186/s12974-019-1693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Zeng Z, Jin T, Zhang H, Xiong X, Gu L, 2019. The Role of High Mobility Group Box 1 in Ischemic Stroke. Front. Cell Neurosci 13, 127. https://doi.10.3389/fncel.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G and Granger DN, 2008. Cell adhesion molecules and ischemic stroke. Neurol Res. 30(8), pp.783–793. https://doi.10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN, 2006. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113(17), 2105–2112. https://doi.10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zaremba J, Losy J, 2001. The levels of TNF-alpha in cerebrospinal fluid and serum do not correlate with the counts of the white blood cells in acute phase of ischaemic stroke. Folia Morphol (Warsz). 60(2), 91–97. [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Linnarsson S, 2015. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347(6226), 1138–1142. https://doi.10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Li F, Chen X, Jia J, Sun S, Zhou D, Wang Q, 2017. Triggering Receptor Expressed on Myeloid Cells 2, a Novel Regulator of Immunocyte Phenotypes, Confers Neuroprotection by Relieving Neuroinflammation. Anesthesiology 127(1), 98–110. https://doi.10.1097/ALN.0000000000001628. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li W, Guo Y, Li D, Liu Y, Xu S, 2018. MMP Gene Polymorphisms, MMP-1 −1607 1G/2G, −519 A/G, and MMP-12 −82 A/G, and Ischemic Stroke: A Meta-Analysis. J. Stroke Cerebrovasc. Dis 27(1), 140–152. https://doi.10.1016/j.jstrokecerebrovasdis.2017.08.021. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhu W, Xu F, Dai X, Shi L, Cai W, Hu X, 2019. The interleukin-4/PPARgamma signaling axis promotes oligodendrocyte differentiation and remyelination after brain injury. PLoS Biol. 17(6), e3000330. https://doi.10.1371/journal.pbio.3000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Lo EH, 2006. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med 12(4), 441–445. https://doi.10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wan L, Chen Y, Zhang H, Xu Y, Qiu S, 2018. FasL incapacitation alleviates CD4(+) T cells-induced brain injury through remodeling of microglia polarization in mouse ischemic stroke. J. Neuroimmunol 318, 36–44. https://doi.10.1016/j.jneuroim.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Zhou D, Ji L, Chen Y, 2020. TSPO Modulates IL-4-Induced Microglia/Macrophage M2 Polarization via PPAR-gamma Pathway. J. Mol. Neurosci 70(4), 542–549. https://doi.10.1007/s12031-019-01454-1. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhu X, Wang J, Cheng Y, Ma A, Pan X, 2019. Association between interleukin-18 (137G/C and 607C/A) gene polymorphisms and risk of ischemic stroke: a meta-analysis. Neuroreport 30(2), 89–94. https://doi.10.1097/WNR.0000000000001165. [DOI] [PubMed] [Google Scholar]
- Zhu J, Cao D, Guo C, Liu M, Tao Y, Zhou J, Li Y, 2019. Berberine Facilitates Angiogenesis Against Ischemic Stroke Through Modulating Microglial Polarization via AMPK Signaling. Cell Mol. Neurobiol 39(6), 751–768. https://doi.10.1007/s10571-019-00675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


