Abstract
Purpose
Knowledge, attitudes, and patient preferences about genetic testing and subsequent risk management for cancer prevention among average risk populations are understudied, especially among Hispanics. This study was to assess these items by conducting an in-person survey in this understudied population.
Methods
We conducted in-person surveys using a self-administered, structured questionnaire among young women in 2017. Survey questions were adapted from other validated surveys. This study had 677 participants in the final analyses. Data were collected in 2017 and analyzed in 2018 and 2019.
Results
Participants had little knowledge about genes or breast cancer risk, but most felt that genetic testing for cancer prevention is “a good idea” (87.0%), “a reassuring idea” (84.0%), and that “everyone should get the test” (87.7%). Most (64.0%) of these women would pay up to $25 for the test, 29.3% would pay $25-$500, and <10% would pay more than $500 for the test. When asked about a hypothetical scenario of high breast cancer risk, 34.2% Hispanics and 24.5% non-Hispanics would choose chemoprevention. Women would be less like to choose risk reduction procedures, such as mastectomy (19.6% among Hispanics and 15.1% among non-Hispanics) and salpingo-oophorectomy (11.8% among Hispanics and 10.7% among non-Hispanics).
Conclusions
In this low-income, mostly Hispanic population, knowledge about genetic testing and cancer risk is poor, but most have positive opinions about genetic testing for cancer prevention. However, their strong preference for chemoprevention and lesser preference for prophylactic surgeries in a hypothetical scenario underscore the importance of genetic counseling and education.
Keywords: genetic testing, breast cancer, cancer risk, Hispanic, risk management
Introduction
The cancer burden in the United States is high. It is estimated that 1,735,350 new cases of invasive cancer will be diagnosed in the US in 2018.1 Many cancers tend to aggregate in families2, and genetic factors and inheritance contribute a significant portion to some cancers’ development, especially breast cancer, colorectal cancer, and prostate cancer.3 Genetic testing can detect altered cancer susceptibility genes, which put some people at increased risk for developing cancer.4 After receiving genetic testing, mutation carriers can reduce their cancer risk via risk-reducing surgeries, chemoprevention, and intensive screening.5 More and more cancer-driving gene mutations have been identified during last two decades, and the use of cancer genetic testing is increasing.6
Currently, genetic testing is only recommended for cancer prevention among individuals with a high probability for oncogenic mutations based on their family history and ancestry.7 Numerous guidelines and algorithms have been developed to provide quantitative approaches for identification of high-risk individuals for risk assessment and testing. 8–10 Currently, most carriers will not receive genetic testing until they are already diagnosed with cancer.11–14 Only about 5.5% of mutation carriers without cancer have been identified. However, next-generation sequencing technologies are dramatically reducing costs for genetic testing and sequencing.15 Lower costs will make multigene testing more accessible, which may render population-level testing feasible and potentially cost-effective. However, population-based screening is still currently limited by the cost of testing, concerns regarding privacy, and freedom of choice. It is important to know whether this type of test is acceptable to patients and how it is viewed among different populations.16
The purpose of this study was to examine sociodemographic factors associated with knowledge of and attitudes toward genetic testing of altered cancer susceptibility genes among young women in a clinical sample. This study chose breast cancer prevention because breast cancer is the second most prevalent cancer besides skin cancers and the second leading cause of cancer death among US women.17 Moreover, BRCA1/2 genes have been extensively studied and tested.14,18 Early detection of harmful BRCA mutations could potentially be used for the prevention of breast cancer.
Methods
From May 26 to July 21, 2017, this study conducted a self-administered survey using a structured questionnaire among adult women 18–65 years old who attended any of five reproductive health clinics in Southeast Texas. All five clinics are administered by a single academic institution, The University of Texas Medical Branch. Women attending these clinics are primarily from low-income families, with about 80% having an annual family income < $30,000. Clinic personnel invited women upon check-in to participate in the survey administered in the waiting room before they saw their providers. Women were informed that participation in the survey was completely voluntary. Study personnel approached those who expressed their willingness to participate, gave them a brief verbal description of the research and goals, and asked if they would agree to complete an anonymous survey that took approximately 15 minutes to complete. They obtained oral consent from the participants and allowed participants to choose either a paper questionnaire or an electronic version. Participants were given a small gift valued ≤$5 upon completion of the questionnaire as reimbursement for their time and effort. This study ensured participants only completed the survey once, while maintaining their anonymity: study personnel maintained a cumulative database containing the clinic numbers of all women who had previously been approached across all clinics and did not invite these women to participate again. The Institutional Review Board approved this study, including a waiver of written consent.
Survey questions were adapted from the National Health Interview Survey,19 the survey on cost sharing and hereditary cancer risk,20 and the prenatal screening survey.21,22 For this study, this study focused on questions about participants’ demographics, knowledge of breast cancer risk and genetic testing, attitudes toward genetic testing for cancer prevention, and willingness to pay for genetic testing. This study assessed the internal consistency reliability of the knowledge index and attitude scale using Cronbach’s alpha. Questions about knowledge of genes and cancer risk and questions about attitude towards genetic testing both had acceptable internal consistency (standardized Cronbach’s alpha 0.73 and 0.81, respectively). This study reported results according to individual question items rather than the overall scale scores. The questionnaire was available in both English and Spanish. The Spanish surveys were direct translation from the English survey by experienced translators and were back translated into English by another experienced translator for consistency checkup. Study protocol, and survey questionnaires for both pregnant women and non-pregnant women were presented as the Supplemental materials.
Statistical Analysis
Data were collected in 2017 and analyzed in 2018 and 2019. This study used SAS software version 9.4 (SAS Institute; Carey, NC) for all statistical analyses. Descriptive analyses included chi-square and Fisher’s exact (when applicable) tests for categorical variables and t tests for continuous variables. This study used multivariate logistic regression models to assess factors associated with binomial outcomes, such as attitudes toward genetic testing for cancer prevention. Age, race/ethnicity, country of birth, education level, and marital status were included in multivariable models. Respondents with missing data were excluded from multivariable models. Pregnant and non-pregnant women were examined separately in sensitivity analyses.
Results
Study population
This study received 795 survey responses from the five reproductive health clinics with patient populations mainly composed of young women. After excluding women with incomplete responses to questions about genetic testing, those < 18 years or > 65 years, and those with a history of breast cancer, this study retained 677 women for these analyses. Among those, 77.3% were Hispanics. The mean age of the sample was 28.1 years in the Hispanic group and 29.4 years in the non-Hispanic group. Among Hispanics, 25.2% had a college degree or above, 30.7% did not finish high school, 41.5% were currently married, 34.0% only read and spoke Spanish, 37.1% spoke only Spanish at home, and 27.3% were born in the US (Table 1). Among non-Hispanics, 63.6% had a college degree or above, 10.4% did not finish high school, 32.5% were currently married, 3.4% only read and spoke a native language other than English, 3.9% spoke only a non-English language at home, and 68.2% were born in the US.
Table 1.
Characteristics of the participants (N = 677)
| Prevalence % (95% CI) | ||
|---|---|---|
| Hispanic (n = 523) | Non-Hispanic (n = 154) | |
| Pregnant | 46.8 (42.6–51.1) | 47.4 (39.5–55.3) |
| College | 25.2 (21.4–29.0) | 63.6 (56.0–71.3) |
| Did not finish high school | 30.7 (26.7–34.7) | 10.4 (5.6–15.2) |
| Age < 30 years old | 61.0 (56.8–65.2) | 55.8 (48.0–63.7) |
| Currently married | 41.5 (37.3–45.7) | 32.5 (25.1–39.9) |
| Single, never married | 21.2 (17.7–24.7) | 28.6 (21.4–35.7) |
| Only read and speak native language | 34.0 (30.3–37.8) | 3.4 (0.7–6.0) |
| Speak only native language at home | 37.1 (33.3–41.0) | 3.9 (1.1–6.8) |
| Born in the US | 27.3 (23.5–31.2) | 68.2 (60.8–75.6) |
Boldface indicates statistical significance (p<0.05).
Knowledge about genes and cancer risk
Overall, knowledge about genes and cancer risk was low (Table 2). For the statement, “a parent can pass a cancer gene to their child that increases their child’s chance of getting cancer,” only 48.3% of Hispanics and 52.0% of non-Hispanics chose the correct answer “True.” For the statement, “very few women have a cancer gene that increases their chance of getting breast cancer,” 24.2% of Hispanics and 22.3% of non-Hispanics chose the correct answer. Hispanics were less likely to choose the correct answer than non-Hispanics, for statements regarding unhealthy environment (23.6% vs. 35.4%) or diet (27.6% vs. 41.1%) and breast cancer risk.
Table 2.
Knowledge about genes and cancer risk (N = 677)
| Prevalence of correct answers % (95% CI) | ||
|---|---|---|
| Hispanic (n = 523) | Non-Hispanic (n = 154) | |
| A parent can pass a cancer gene to their child that increases their child's chance of getting cancer (True) | 48.3 (43.9–52.7) | 52.0 (44.0–60.1) |
| A person who does not have a cancer gene can still get cancer (True) | 60.4 (56.1–64.7) | 67.8 (60.3–75.3) |
| Very few women have a cancer gene that increases their chance of getting breast cancer (True) | 24.2 (20.4–28.0) | 22.3 (15.6–29.0) |
| A person with a family member who had cancer has a higher chance of getting cancer (True) | 56.4 (52.0–60.8) | 45.9 (37.8–54.0) |
| Cancer at younger ages (< 50) is LESS likely due to a person having a cancer gene (False) | 20.0 (16.5–23.6) | 21.5 (14.8–28.3) |
| Mammograms often do not detect cancer until after it spreads to other parts of the body (False) | 36.1 (31.9–40.4) | 42.1 (34.0–50.1) |
| Having the breasts removed will prevent breast cancer (True) | 16.3 (13.0–19.5) | 11.0 (5.9–16.1) |
| An unhealthy environment may increase a person's chance of getting breast cancer (True) | 23.6 (19.8–27.3) | 35.4 (27.6–43.1) |
| An unhealthy diet may increase a person's chance of getting breast cancer (True) | 27.6 (23.6–31.6) | 41.1 (33.1–49.1) |
Boldface indicates statistical significance(p<0.05).
Attitude towards genetic testing for cancer prevention
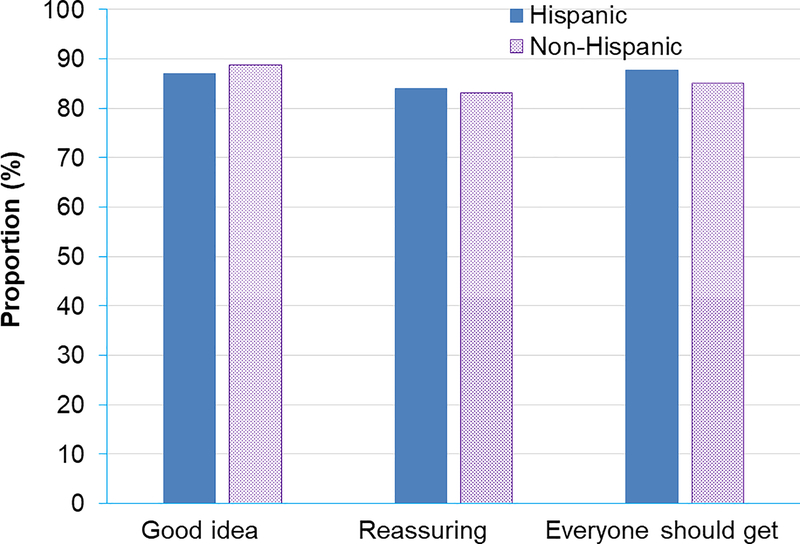
Both Hispanics and non-Hispanics had highly positive attitudes towards genetic testing for cancer prevention. Among Hispanics, 87.0% thought it was a good idea, 84.0% thought it was a reassuring idea, and 87.7% agreed that everyone should get genetic testing for cancer prevention (Figure 1). Among non-Hispanics, 88.8% thought it was a good idea, 83.2% thought it was a reassuring idea, and 85.0% agreed that everyone should get genetic testing for cancer prevention. Multivariate logistic regression models revealed that attitude towards genetic testing for cancer prevention did not differ by age, race / ethnicity, country of birth, or marital status. However, women who did not finish high school were significantly less likely to think that “genetic testing for cancer prevention is a good idea” (adjusted odds ratio 0.53, 95% confidence interval 0.30–0.91).
Figure 1.
Attitudes towards genetic testing for breast cancer prevention.
Willingness to pay for genetic testing
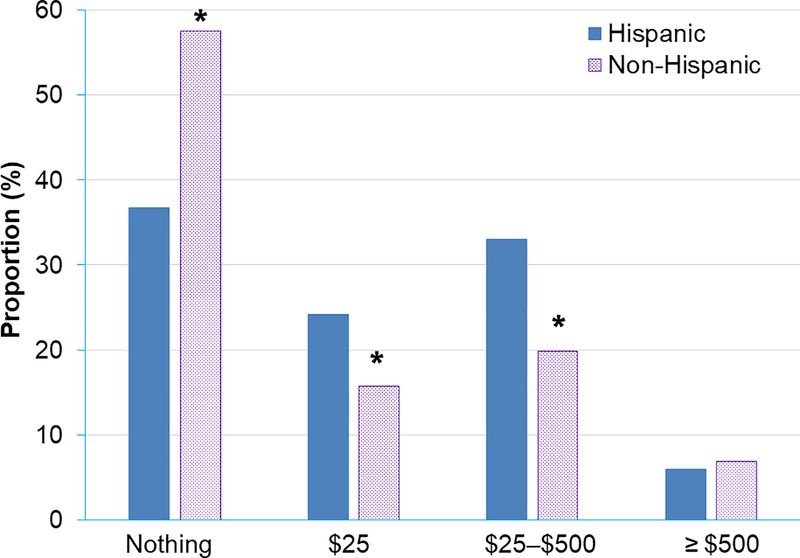
Most (64.0%) women would pay up to $25 for the test, 29.3% would pay $25-$500, and < 10% would pay more than $500 for the test (Figure 2). A higher proportion of non-Hispanics compared to Hispanics reported that they were only willing to pay nothing for genetic testing (57.5% vs 36.8%, P < .001), respectively. Further, a lower proportion of non-Hispanics were willing to pay $25 (15.8% vs 24.2%, P = .03) or $25–$500 (19.9% vs 33.0%, P = .002) compared to Hispanics, respectively.
Figure 2.
Willingness to pay for genetic testing for cancer prevention.
Preference of risk management plans
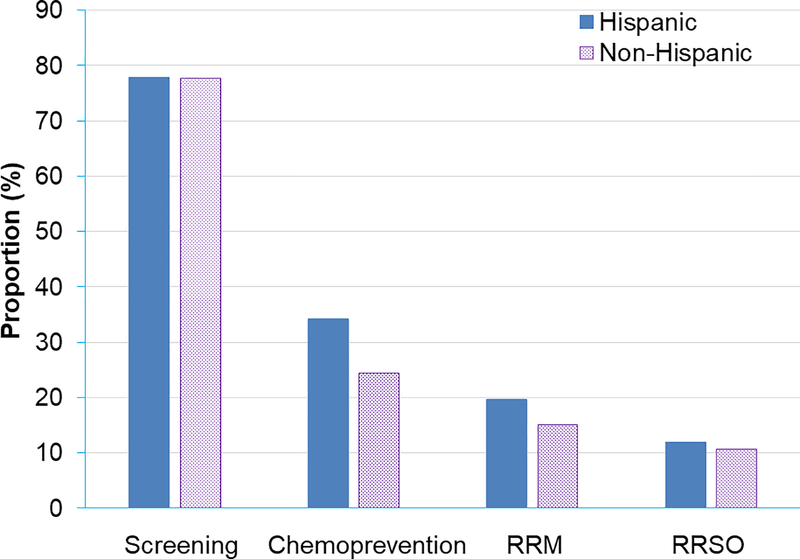
This study presented women with a hypothetical scenario of high genetic risk for breast cancer and asked them to choose among several risk management options described. This study did not provide details about the risks and benefits of those options. They could choose more than one option ranging from early and intensive screening with mammogram or MRI, chemoprevention with Tamoxifen / Raloxifene, risk-reducing mastectomy (RRM), and risk-reducing salpingo-oophorectomy (RRSO). Overall, 77.8% of Hispanics and 77.6% of non-Hispanics said they would choose screening (Figure 3). However, 34.2% of Hispanics and 24.5% of non-Hispanics said they would choose chemoprevention. Very few women responded that they would choose the highly-effectively risk reduction procedures, such as RRM (19.6% of Hispanics and 15.1% of non-Hispanics) and RRSO (11.8% of Hispanics and 10.7% of non-Hispanics). Multivariable logistic regression models revealed that being born in the US was associated with the choice of intensive screening (adjusted odds ratio 1.76, 95% confidence interval 1.10–2.82), not being born in the US and not finishing high school were associated with the choice of chemoprevention (adjusted odds ratio 2.02, 95% confidence interval 1.32–3.08, and 1.63, 95% confidence interval 1.09–2.43, respectively), and not being born in the US was strongly associated with the choice of RRM (adjusted odds ratio 1.80, 95% confidence interval 1.07–3.02). There was no significant difference in the choice of risk management options between Hispanics and non-Hispanics.
Figure 3.
Preference for risk-management plans.
This study performed a sensitivity analysis to examine differences in outcomes between pregnant women and non-pregnant women. The results were similar between those two groups compared to results observed among the total sample (results not shown).
Discussion
This study conducted self-administered surveys in a clinical sample of women to assess knowledge, attitudes, and patient preferences about genetic testing and subsequent risk management for cancer prevention. Although their knowledge levels were low, these women seemed accepting of genetic testing for cancer prevention. However, this high approval rate needs to be interpreted with caution as high acceptance does not necessarily lead to high uptake.23 The preference of chemoprevention over risk-reducing surgeries in this sample indicated that these women may not be familiar with the risks and benefits of different risk management options. This study underscores the importance of increased education about genetic testing in general and particularly for women who may be at increased risk. Additionally, it is important to increase the availability of genetic counseling services as genetic screening becomes more widely available.
Genetic testing can be used for disease prevention if susceptible individuals are adequately identified before disease occurs. For example, since 2005, the US Preventive Services Task Force (USPSTF), the National Comprehensive Cancer Network (NCCN), and other professional organizations have recommended genetic testing for women whose family histories or ethnic backgrounds are associated with increased risks for BRCA mutations.9,10 USPSTF predicted that ~2% of the general population would meet these “high-risk” criteria,9 but studies using detailed family histories found that actually 6%–12.4% of women met USPSTF’s “high-risk” criteria.24,25 Current clinical criteria and practice guidelines26,27 for BRCA testing are based mainly on personal and family history of breast / ovarian cancer and Ashkenazi Jewish ancestry, but these testing criteria identify only a small portion of high-risk mutation carriers.28 Early detection of mutation carriers is pivotal for the success of cancer prevention programs,11 but, in the US, the majority of BRCA mutation carriers have not been identified.16 Population-wide screening for BRCA mutations has been proposed,29 especially as the cost for genetic testing and sequencing is rapidly decreasing. However, debate about the appropriateness, access to follow-up services and privacy concerns remain. Nevertheless, it is important to increase knowledge and improve attitudes about genetic testing so that when available and if appropriate, there will be improved uptake of these tests.
In this sample, this study found these women generally had low knowledge of genes and cancer risk but an extremely high level of approval for genetic testing. This is in agreement with a systematic review of 39 studies from the US and 2 from Australia which assessed knowledge and attitudes towards genetic counselling / testing for cancer risk prediction in ethnic minority groups. They reported low awareness and knowledge of genetic testing for cancer susceptibility but generally positive attitudes towards genetic testing among ethnic minority groups including African Americans, Asian Americans, and Hispanics.30 Thus, acceptance of genetic testing is positive among the general population.
High acceptance does not necessarily lead to high uptake.23 Cost and lack of insurance may inhibit access to genetic services by the underserved, as this survey indicated more than half were unwilling to pay more than $25 for the test. Other barriers to genetic testing may potentially play a role in a lower uptake in the US as well.31–33 Fears of genetic information misuse is an inevitable concern.34 Another barrier to testing uptake is the psychological burden when a potentially life-threatening mutation is identified. Anxiety about the future is an inevitable consequence, and making decisions about life-saving, but life-altering, surgical prophylaxis is highly stressful. Moreover, the lack of effective ways to communicate risk estimates to patients after pretest genetic counseling also contributes to low uptake of genetic testing.35 The interplay of these barriers and concerns impedes uptake of genetic testing, even when relatives are notified that they should be tested due to an identified mutation carrier in their immediate family.35,36 Nonetheless, fundamental culture changes would be necessary to foster a nondiscriminatory approach to people with pathogenic genetic mutations, as this information could be misused to determine insurability or employment if policy is not adequate to guide the use of this information.34 A balanced view of genetic information is needed to protect patients and their families from negative social consequences and encourage them to undergo needed testing.37 To avoid increasing health disparities, infrastructure for test delivery and delivery modes must be improved to increase the accessibility of genetic testing and related counseling to the general public.
The participants’ stated preference for chemoprevention over risk-reducing surgeries is not the usual course of action when pathogenic mutations are detected. Among identified mutation carriers without prior cancer diagnosis, chemoprevention (Tamoxifen, Raloxifene) is rarely chosen.38 About 30%–50% choose RRM, and 60%–75% choose RRSO. The remaining 25%–30% choose surveillance only by MRI / mammogram. While this method is reasonable, the drop-off in surveillance behavior is significant over time, and very few patients continue regular screenings 5 years after genetic testing.38 Chemoprevention halves the risk of invasive breast cancer.39 In contrast, RRM, reduces breast cancer risk by over 90%.40 Ovarian cancer risk can be reduced by 80% by RRSO, which also reduces breast cancer risk and mortality by > 50%.26 It is safe to conclude that most participants in this study would have chosen risk reduction strategies differently, if they had been adequately informed of the risks and benefits of each of the treatment options through thorough genetic counseling. High-quality decision making requires high-quality information support, which the survey respondents did not have when responding. Thus, any conclusions about their selection of possible risk reduction options are not true choices under real-world conditions. However, it highlights the importance of genetic counseling in this population. Management and treatment of mutation carriers are complex clinically,41 and patients’ decisions about risk reduction strategies differ based on personal preferences, including future child-bearing plans. Psychological and ethical risks of genetic testing are another concern. The high preference for chemoprevention and low preference for prophylactic surgeries in a hypothetical scenario among women in this study underscore the importance of genetic counseling before and after genetic testing to maximize the benefits of the test for disease prevention while ensuring understanding and consideration of patient values and desires. Future research to better understand how cultural factors may play a role in management decision-making is warranted.
The strengths of this study include its large sample size, unique sample of low-income young women, and high proportion of Hispanic women. However, this study has some limitations. This study used a convenience sample of women attending reproductive health clinics in Southeast Texas, so the findings may not be applicable to the general US population or other age groups. In addition, intention may not necessarily lead to action, and a high level of approval of genetic testing for cancer prevention among these women does not guarantee uptake, even if tests are available to them. Furthermore, this sample contains very few women with a family history of cancer, and this study excluded 2 women with breast cancer diagnoses. Women with a personal or family history of breast cancer may have a higher level of knowledge of genetic testing and cancer.
In conclusion, knowledge about genetic testing and cancer risk was poor in this low-income population. However, most of them expressed positive opinions about genetic testing for cancer prevention. Genetic counseling prior to and after the testing is vital to the success of cancer prevention, considering these women’s relatively high preference for chemoprevention and low preference for prophylactic surgeries in a hypothetical scenario of a positive test result. This study underscores the importance of increased education about genetic testing in general and particularly for women who may be at increased risk. Additionally, it is important to increase the availability of genetic counseling services as genetic screening becomes more widely available.
Supplementary Material
Acknowledgments
Funding: Dr. Guo is currently supported by the National Cancer Institute of the National Institutes of Health under Award Number K07CA222343. Dr. Guo was and Dr. Fuchs is currently supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program–BIRCWH; Berenson, PI) from the Office of Research on Women’s Health (ORWH) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH). Drs. Cofie and Brown were postdoctoral fellows supported by an institutional training grant (National Research Service Award T32HD055163, Berenson, PI) from the NICHD at the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Role of the Funding Source: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflicts of interest/Competing interests
The authors report no conflicts of interest.
Declarations
Ethics approval (include appropriate approvals or waivers)
The Institutional Review Board at The University of Texas Medical Branch approved this study, including a waiver of written consent.
Consent to participate
Oral consent from the participants was obtained.
Availability of data and material
Data and material are available upon request to the corresponding author, Dr. Fangjian Guo (faguo@utmb.edu).
Code availability (software application or custom code)
Code is available upon request to the corresponding author, Dr. Fangjian Guo (faguo@utmb.edu).
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Pomerantz MM, Freedman ML. The Genetics of Cancer Risk. Cancer J. 2011;17(6):416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. [DOI] [PubMed] [Google Scholar]
- 4.Weitzel JN, Blazer KR, MacDonald DJ, Culver JO, Offit K. Genetics, genomics, and cancer risk assessment: State of the Art and Future Directions in the Era of Personalized Medicine. CA Cancer J Clin. 2011;61(5):327–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robson M, Offit K. Clinical practice. Management of an inherited predisposition to breast cancer. N Engl J Med. 2007;357(2):154–162. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, & risk management, April, 2007. Cancer. 2008;113(10):2627–2637. [DOI] [PubMed] [Google Scholar]
- 7.Nelson HD, Pappas M, Zakher B, Mitchell JP, Okinaka-Hu L, Fu R. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2014;160(4):255–266. [DOI] [PubMed] [Google Scholar]
- 8.Antoniou AC, Hardy R, Walker L, et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet. 2008;45(7):425–431. [DOI] [PubMed] [Google Scholar]
- 9.Nelson HD, Huffman LH, Fu R, Harris EL, Force USPST. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(5):362–379. [DOI] [PubMed] [Google Scholar]
- 10.FitzGerald MG, MacDonald DJ, Krainer M, et al. Germ-line BRCA1 mutations in Jewish and non-Jewish women with early-onset breast cancer. N Engl J Med. 1996;334(3):143–149. [DOI] [PubMed] [Google Scholar]
- 11.Levy-Lahad E, Lahad A, King MC. Precision medicine meets public health: population screening for BRCA1 and BRCA2. J Natl Cancer Inst. 2015;107(1):420. [DOI] [PubMed] [Google Scholar]
- 12.Metcalfe KA, Eisen A, Lerner-Ellis J, Narod SA. Is it time to offer BRCA1 and BRCA2 testing to all Jewish women? Curr Oncol. 2015;22(4):e233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp Z, Breast Unit RMNHSFT, London, United Kingdom, Cancer Genetics Unit RMNHSFT, London, United Kingdom, et al. Evaluation of Cancer-Based Criteria for Use in Mainstream BRCA1 and BRCA2 Genetic Testing in Patients With Breast Cancer. JAMA Network Open. 2020;2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Color Test $249. https://getcolor.com/kit/how-it-works. Accessed September 9, 2016.
- 16.Drohan B, Roche CA, Cusack JC, Hughes KS. Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol. 2012;19(6):1732–1737. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society. Breast Cancer Facts & Figures 2015–2016. Atlanta: American Cancer Society, Inc. 2015. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-046381.pdf. Accessed on June 30, 2016. In. [Google Scholar]
- 18.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98(23):1694–1706. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. Survey Description, National Health Interview Survey, 2013. Hyattsville, Maryland. 2014.
- 20.Matro JM, Ruth KJ, Wong YN, et al. Cost sharing and hereditary cancer risk: predictors of willingness-to-pay for genetic testing. J Genet Couns. 2014;23(6):1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Berg M, Timmermans DR, Ten Kate LP, van Vugt JM, van der Wal G. Are pregnant women making informed choices about prenatal screening? Genet Med. 2005;7(5):332–338. [DOI] [PubMed] [Google Scholar]
- 22.Kooij L, Tymstra T, Berg P. The attitude of women toward current and future possibilities of diagnostic testing in maternal blood using fetal DNA. Prenat Diagn. 2009;29(2):164–168. [DOI] [PubMed] [Google Scholar]
- 23.Galbraith KV, Lechuga J, Jenerette CM, Moore LA, Palmer MH, Hamilton JB. Parental acceptance and uptake of the HPV vaccine among African-Americans and Latinos in the United States: A literature review. Soc Sci Med. 2016;159:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palomaki GE, McClain MR, Steinort K, Sifri R, LoPresti L, Haddow JE. Screen-positive rates and agreement among six family history screening protocols for breast/ovarian cancer in a population-based cohort of 21- to 55-year-old women. Genet Med. 2006;8(3):161–168. [DOI] [PubMed] [Google Scholar]
- 25.McClain MR, Palomaki GE, Hampel H, Westman JA, Haddow JE. Screen positive rates among six family history screening protocols for breast/ovarian cancer in four cohorts of women. Fam Cancer. 2008;7(4):341–345. [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. Fort Washington, PA: National Comprehensive Cancer Network, 2017. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed October 5, 2017. In. [Google Scholar]
- 27.Hampel H, Bennett RL, Buchanan A, Pearlman R, Wiesner GL, Guideline Development Group AeCoMGaGPPaGCaNSoGCPGC. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17(1):70–87. [DOI] [PubMed] [Google Scholar]
- 28.Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer. 2000;83(10):1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111(39):14205–14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hann KEJ, Freeman M, Fraser L, et al. Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Public Health. 2017;17(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinney AY, Croyle RT, Dudley WN, Bailey CA, Pelias MK, Neuhausen SL. Knowledge, attitudes, and interest in breast-ovarian cancer gene testing: a survey of a large African-American kindred with a BRCA1 mutation. Prev Med. 2001;33(6):543–551. [DOI] [PubMed] [Google Scholar]
- 32.Sankar P, Wolpe PR, Jones NL, Cho M. How do women decide? Accepting or declining BRCA1/2 testing in a nationwide clinical sample in the United States. Community Genet. 2006;9(2):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters N, Rose A, Armstrong K. The association between race and attitudes about predictive genetic testing. Cancer Epidemiol Biomarkers Prev. 2004;13(3):361–365. [PubMed] [Google Scholar]
- 34.Hudson KL. Prohibiting genetic discrimination. N Engl J Med. 2007;356(20):2021–2023. [DOI] [PubMed] [Google Scholar]
- 35.Finlay E, Stopfer JE, Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landsbergen K, Verhaak C, Kraaimaat F, Hoogerbrugge N. Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer. 2005;4(2):115–119. [DOI] [PubMed] [Google Scholar]
- 37.Surbone A. Social and ethical implications of BRCA testing. Ann Oncol. 2011;22 Suppl 1:i60–66. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MD, Isaacs C, Graves KD, et al. Long-term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer. 2012;118(2):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93(21):1633–1637. [DOI] [PubMed] [Google Scholar]
- 41.Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K, Oncology ASoC. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893–901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.