Abstract
Objectives:
The aim was to develop a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for the detection of hepatitis C virus (HCV) in a single closed tube.
Methods:
Plasma samples were collected from 200 HCV-infected patients. HCV-RNA was detected by one-step RT-LAMP processed at 65 °C for 60 min. The amplified products were detected by hydroxynaphthol blue (HNB)-dependent visual method and gel electrophoresis. Specificity was tested against other viruses. Sensitivity was determined using serial dilutions of extracted RNA.
Results:
The RT-LAMP assay detected 97.5% of HCV-RNA genotype 1, 91.1% of genotype 3, and 100% of genotype 6. The color change was evidenced with the naked eye. The assay demonstrated a clinical sensitivity of 95.5% and specificity of 100%, as well as no cross-reactivity with other viruses (i.e., hepatitis B virus, HIV). The limit of detection was as low as 10 ng per reaction for HCV genotypes 1a and 6, while it was 100 ng for genotype 3a. The assay showed a 100% detection threshold at a viral load of 5.00 log10 IU/mL in the clinical samples tested.
Conclusions:
This study demonstrated the use of an RT-LAMP assay for the detection of HCV in a simple, rapid, and cost-effective manner, which will be useful in resource-limited settings to allow the identification of individuals in need of HCV treatment.
Keywords: Hepatitis C virus, Loop-mediated isothermal amplification, Reverse transcription, Hydroxynaphthol blue
Introduction
Hepatitis C virus (HCV) is a leading cause of cirrhosis and liver cancer. An estimated 71 million people are chronically infected with HCV worldwide (Asselah et al., 2018). HCV is classified into six major genotypes designated as genotypes 1–6 and these genotypes present variations in global distribution, transmission, and disease progression (Kuiken and Simmonds, 2009; Messina et al., 2015). Among HCV genotypes characterized in Thailand, subtype 3a is predominant (36.4%), followed by 6 (20.9%), 1a (19.9%), 1b (12.6%), 3b (9.7%), and 2a (0.5%) (Wasitthankasem et al., 2015).
Although more than 95% of patients with an HCV infection can now be cured with the use of direct-acting antiviral agents (DAA), most HCV-infected individuals are unaware of their infection. Anti-HCV antibody detection alone is insufficient for the diagnosis of HCV infection, as the presence of anti-HCV may reflect past infection with spontaneous clearance and not active disease. Thus, testing for HCV-RNA is still critical to confirm ongoing HCV replication and therefore make a clinical decision to treat. This approach is of great importance in resource-limited settings, since its simplicity and low-cost can help during large-scale diagnostic testing and for monitoring patients afterwards.
Nucleic acid-based detection techniques are the most reliable methods for the diagnosis of HCV infection. Many molecular diagnostic assays have been developed, including reverse transcription PCR (RT-PCR) and real-time RT-PCR (Daniel et al., 2008; Gonzalez-Perez et al., 2003; Nakatani et al., 2010). However, these require expensive laboratory facilities, specialized equipment, and well-trained personnel, and are time-consuming, which limits their application for on-site HCV diagnosis, especially in remote areas and resource-limited settings (Parida, 2008). For this reason, there is still a need to develop simple, cost-effective, and rapid diagnostic tools to identify chronic HCV infection. Indeed, the National Institutes of Health of the United States have identified the need for rapid tests for HCV as a high priority (NIH, 2020).
Loop-mediated isothermal amplification (LAMP) is a nucleic acid-based amplification method that amplifies target sequences with high specificity and efficiency in a short period of time under isothermal conditions (Notomi et al., 2000). This technique combines at least four specific LAMP primers and Bacillus stearothermophilus (Bst) DNA polymerase, which can amplify the target DNA and replace the synthesized DNA strand in the meantime, therefore allowing the specific LAMP primers to complete the reaction with high efficacy and precision. Reverse transcription LAMP (RT-LAMP) is similar to LAMP, but the template used is RNA instead of DNA. The equipment needed for the LAMP reaction is a regular laboratory water bath or heat block that furnishes a constant temperature without an expensive thermocycler machine (Bentaleb et al., 2016; Zhang et al., 2019), and the results from LAMP can be observed immediately by visual observation, through turbidity or dye staining. This testing approach would therefore be ideal in terms of cost-effectiveness and easy applicability in urban and rural areas.
Currently, several LAMP-based assays have been designed for the detection of various pathogens including HCV, but they detect a limited number of genotypes and a small number of clinical specimens have been used for assay validation (Kargar et al., 2012; Nyan and Swinson, 2016; Wang et al., 2011; Yang et al., 2011; Zhao et al., 2017). In this study, we introduce an RT-LAMP assay for the detection of HCV in Thailand, in which the reaction color is observed in a single closed tube. In addition, this assay was evaluated using clinical samples.
Materials and methods
Clinical specimens
Plasma samples were retrieved from 200 HCV-infected individuals who had undergone routine HCV viral load testing and HCV genotyping in the Faculty of Associated Medical Sciences, Chiang Mai University, Thailand. All samples were negative for hepatitis B virus (HBV), while five were positive for human immunodeficiency virus (HIV). These samples were kept at −70 °C. The HCV viral load was initially confirmed using a real-time RT-PCR assay (COBAS AmpliPrep/COBAS TaqMan HCV Test; Roche Molecular Systems, Pleasanton, CA, USA). The HCV genotypes were identified by in-house direct sequencing of a core region of the HCV genome. There were 81 HCV genotype 1, 79 HCV genotype 3, one HCV genotype 4, and 39 HCV genotype 6.
As ‘other-virus’ controls, plasma samples of 20 individuals infected with HIV and 20 infected with HBV who were undergoing routine HIV and HBV viral load testing in the Faculty of Associated Medical Sciences, Chiang Mai University were also collected to test the specificity of the RT-LAMP for the detection of HCV. In addition, 30 plasma samples were retrieved from healthy donors to serve as a negative control. These specimens were confirmed not to contain infectious agents according to the regulations for blood safety for humans of the Blood Bank Section, Maharaj Nakorn Chiangmai Hospital.
Ethical approval for the use of these specimens was obtained from the Research Institute for Health Sciences Human Experimentation Committee (RIHES HEC), Chiang Mai University (Study code: 14/62).
Design of the RT-LAMP primers
Based on the study of Nyan and Swinson (2016), the DN1 primer sets were initially designed from the highly conserved region in the 5′-non-coding region (NCR) of HCV genotype 6a. This primer set was aligned with HCV genotypes predominantly circulating in Thailand, in particular genotype 1, 3, and 6 sequences, which were obtained from the HCV sequence database (https://hcv.lanl.gov/content/index). It was found that some positions of these primers did not match perfectly, thus this primer set was modified to be more specific to the HCV circulating in Thailand. The set consisted of the following: forward inner primer (FIP), reverse inner primer (RIP), loop forward primer (LF), loop reverse primer (LR), forward outer primer (F3), and reverse outer primer (R3) (Table 1). The oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA, USA).
Table 1.
Oligonucleotide sequences of the primer set targeted to HCV 5′-NCR (modified from the study by Nyan and Swinson (2016)).
| Primer | Nucleotide sequences (5′–3′) | Length |
|---|---|---|
| DN1M-F3 | CGGGAGAGCCATAGTGGT | 18 |
| DN1M-R3 | WGGAWGTGTGCTCATGATGCACG | 23 |
| DN1M-FIP | TGAGCGGGTTTDATCCAAGATTTTTGCGGAACCGGTGAGTAC | 42 |
| DN1M-RIP | CCGCRAGACYGCTAGCCGAGTTTTACCCTATCAGGCAGTACCAC | 44 |
| DN1M-LF | TCGTCCYGGCRATTCCGG | 18 |
| DN1M-LR | TAGTGTTGGGTCGCGAAAG | 19 |
HCV, hepatitis C virus; NCR, non-coding region.
RT-LAMP reaction and product detection
Viral RNA was extracted from plasma specimens using the NucleoSpin RNA Virus kit (Macherey-Nagel, Düren, Germany), according to the manufacturer’s protocol.
Following optimization of the assay, the RT-LAMP reaction was performed in a total reaction volume of 25 μl with the reaction mixture composed of 1.6 μM each of primers FIP and RIP, 0.2 μM each of primers F3 and R3, 0.4 μM each of primers LF and LR, 1.4 mM deoxynucleotide (dNTP) mix, 8 mM MgSO4, 8 U Bst 2.0 WarmStart DNA Polymerase (New England Biolabs, MA, USA), 7.5 U WarmStart RTx Reverse Transcriptase (New England Biolabs, MA, USA), 120 μM hydroxynaphthol blue-HNB (Honeywell, Charlotte, North Carolina, USA), and 5 μl of RNA template.
The amplification reaction was performed at 65 ° C for 60 min and inactivated at 80 °C for 10 min. For confirmation, 5 μl RT-LAMP products were electrophoresed in a 2% agarose gel, stained with RedSafe (iNtRON Biotechnology, Gyeonggi-do, Korea), and visualized under a UV transilluminator. The products of a positive RT-LAMP appeared on the stained gel as a typical ladder pattern, with many bands of different sizes.
Based on the color change of the reaction mixture induced by the pre-added HNB, the RT-LAMP result could also be visualized with the naked eye, without the need for electrophoresis or a UV transilluminator. The principle of this process is as follows: HNB is a metal ion-binding indicator dye that is known to bind the Mg2+ ion and change its color depending on the pH and Mg2+ concentration present in the reaction mixture. The color of HNB is purple at a Mg2+ concentration of 6 mM or higher, but as the DNA synthesis progresses, the concentration of Mg2+ ion decreases due to its binding to pyrophosphate; when it reaches below 6 mM, the color of HNB changes to sky blue (Goto et al., 2009). The samples that turned sky blue were considered as positive, while those that turned purple were considered as negative.
Specificity and sensitivity testing
To test the specificity of this RT-LAMP assay for the detection of HCV-RNA, the RNA and DNA of other related viruses, including HBV and HIV obtained from corresponding patients, were used as a template to test for cross-reactivity of the developed assay. The amplified products were visualized with the naked eye and by gel electrophoresis.
The limit of detection (LOD) of the RT-LAMP assay was evaluated by testing serial dilutions of extracted HCV-RNA genotypes 1, 3, and 6 ranging from 100 to 0.01 ng/μl, and 1 μl of each serial dilution was used as a template in the developed RT-LAMP assay. Each reaction was performed five times per genotype.
Assessment of the feasibility of using the developed RT-LAMP assay in the clinical setting
To assess the feasibility of using the developed RT-LAMP assay in the clinical setting, the results of RT-LAMP were compared to those of real-time PCR for all 270 clinical specimens: 200 HCV, 20 HIV, 20 HBV, and 30 healthy control samples. The binary variable agreement was used to analyze the results.
Results
Optimization of the RT-LAMP reaction
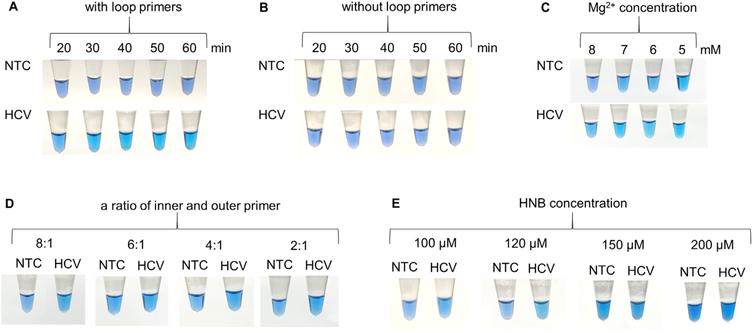
To optimize the RT-LAMP reaction, the procedures were conducted under different conditions. The amplification time at 65 °C was assessed at 10-min intervals, from 20 to 60 min, and then inactivated at 80 °C for 10 min. A sky blue color was observed with the naked eye in samples with a positive result for HCV-RNA at 30, 40, 50, and 60 min after amplification, with an increase in intensity (Figure 1A). However, an optimal RT-LAMP reaction time of 60 min was chosen to potentially increase the sensitivity in cases with a low HCV viral load.
Figure 1.
Optimization of the RT-LAMP reaction for the detection of HCV. Visual detection of RT-LAMP products by pre-adding HNB based on different conditions: (A) incubation time, (B) effect of loop primers, (C) Mg2+ concentration, (D) ratio between the inner and outer primers, and (E) HNB concentration. HCV = hepatitis C virus; NTC = no template control. In Figure 1C, the NTC reaction tubes turned sky blue due to a reduction in Mg2+ concentration, even though the amplification process had not yet been started. Figure 1D shows that an increase in inner primers may result in a non-specific amplification, reducing the Mg2+ concentration in the reaction mixture and then presenting as sky blue, even though in NTC tubes.
In some certain situations, the LAMP reaction can be performed with only four primers to amplify six distinct regions on the target gene. However, an additional pair of loop primers can increase the sensitivity of the LAMP reaction to amplify eight distinct regions. To make the tested RT-LAMP reaction simpler, the two loop primers were excluded, and the RT-LAMP reaction with four primers was tested using HCV-RNA with the amplification time at 65 o C and at 10-min intervals. The results showed that the RT-LAMP reaction with four primers failed to produce any visible color change (Figure 1B). Thus, in this RT-LAMP reaction, adding loop primers is necessary to increase the amplification efficiency.
The effects of the Mg2+ concentration, ratio between the inner and outer primers, and the HNB concentration on the RT-LAMP reaction are also shown in Figure 1. At an Mg2+ concentration of 8 mM, the tube with HCV turned sky blue, whereas the tube with no template remained purple. When the Mg2+ concentration was decreased from 7 mM to 5 mM, all tested samples turned sky blue, even with no template controls (Figure 1C). Based on different ratios of inner and outer primers at 8:1, 6:1, 4:1, and 2:1 in the RT-LAMP, the negative color response (purple) was most obvious at the ratio of 8:1 (Figure 1D). At different HNB concentrations of 100, 120, 150, and 200 μM, a sky blue color (positive) and purple color (negative) were clearly observed with the naked eye at both 100 μM and 120 μM (Figure 1E).
Put together, the optimized RT-LAMP reaction consisted of 60 min of incubation time with loop primers, 8 mM Mg2+, a ratio of inner to outer primers of 8:1, and 120 μM HNB.
Specificity of the RT-LAMP reaction
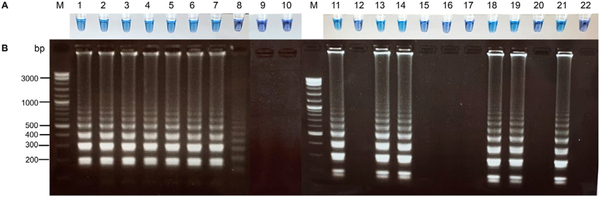
The specificity of the RT-LAMP assay for the detection of HCV was investigated in the presence of specific and non-specific viral nucleic acid templates (HCV, HIV, and HBV). A color change from purple to sky blue in the reaction mixture induced by pre-added HNB was only observed in tubes containing specific HCV genomes (Figure 2). Consistent with the visual results, the product of positive HCV-RNA appeared on the stained gel as a typical ladder pattern with bands of different sizes, while no bands were detected in either the non-specific viral genomes or negative controls.
Figure 2.
Specificity testing of the RT-LAMP assay for the detection of HCV. (A) Visual detection of RT-LAMP products by pre-adding HNB. A color change from purple to sky blue was only observed in tubes containing specific HCV genomes, whereas negative samples were purple. (B) Electrophoresis pattern of the RT-LAMP products in the same order as in panel A. The results showed the presence of typical ladder-like banding patterns in HCV samples, but the absence of a banding pattern in HIV samples, HBV samples, and negative controls. M = 1 kb marker; 1 = HCV 1b; 2 = HCV 3a; 3 = HCV 6; 4 = HCV 6; 5 = HCV 6; 6 = HCV 1a; 7 = PC; 8 = NTC; 9, 10 = healthy blood donors; 11 = HCV 1b; 12 = HBV; 13 = HCV 1a; 14 = HCV 1b; 15 = HIV; 16 = HCV 1a (viral load = 3163 IU/mL); 17 = HBV; 18 = HCV 1b; 19 = HCV 6; 20 = HIV; 21 = PC; 22 = NTC. (HCV, hepatitis C virus; HIV, human immunodeficiency virus; HBV, hepatitis B virus; PC, positive control; NTC, no template control).
Sensitivity of the RT-LAMP reaction
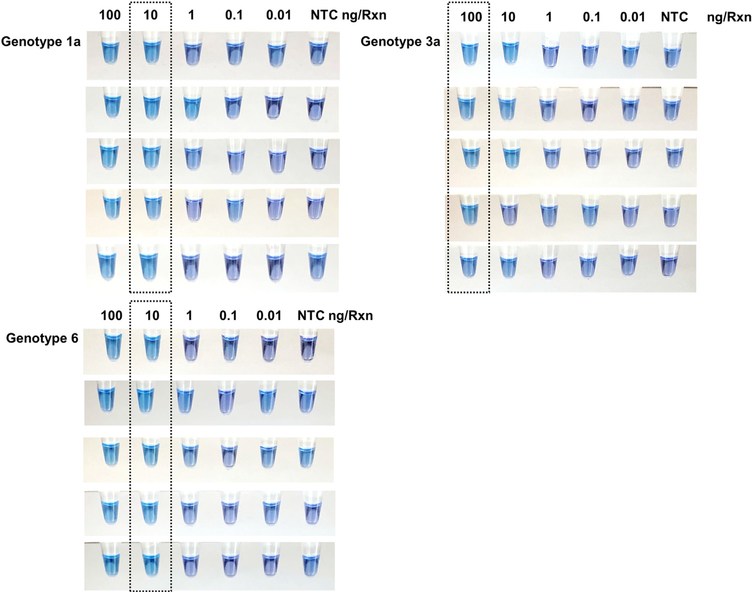
The sensitivity of the RT-LAMP reaction was tested by 10-fold serial dilution of extracted HCV-RNA. The RT-LAMP products resulted in an obvious color change from purple to sky blue in reaction tubes containing 100 ng/μl and 10 ng/μl (Figure 3). The results showed that the detection limit of the RT-LAMP method (100% detection) was about 10 ng/μl for HCV genotypes 1a and 6, whereas it was 100 ng/μl for HCV genotype 3a.
Figure 3.
Sensitivity testing of the RT-LAMP assay for the detection of HCV. Visual detection of RT-LAMP products by pre-adding HNB. Sensitivity was evaluated using serial dilutions of HCV-RNA (genotypes 1a, 3a, and 6) ranging from 100 to 0.01 ng/μl. The assay detected down to 10 ng per reaction of HCV-RNA for genotypes 1a and 6, and 100 ng for HCV genotype 3a, which resulted in an obvious color change from purple to sky blue in the reaction tubes (dashed box). (NTC, no template control).
Clinical evaluation of RT-LAMP for the detection of HCV
All 270 plasma specimens from HCV-, HIV-, and HBV-infected individuals and healthy blood donors were initially tested using a real-time PCR and later by RT-LAMP. RT-LAMP was positive in 173/196 samples and negative in 68/70 samples (Table 2). Four samples positive by real-time RT-PCR yielded inconclusive results by RT-LAMP. The clinical sensitivity of RT-LAMP for the detection of HCV was 88.3% and specificity was 97.1%. In the first-round RT-LAMP analysis, 25 samples gave discordant results between RT-LAMP and real-time RT-PCR, while four samples yielded inconclusive results by RT-LAMP, which required retesting for resolution. After R T-LAMP retesting, 20 of these 29 samples were conclusive (i.e., two samples became negative and 18 samples became positive), while nine samples still gave discordant results. Among these nine samples, six were genotype 3a, two were genotype 1a, and one was genotype 3. The HCV viral loads of these nine samples ranged from 3.48 to 4.72 log10 IU/mL (3062 to 52 955 IU/mL) based on real-time PCR. Thus, in this second investigation, RT-LAMP was positive in 191/200 and negative in all 70 negative samples (Table 2). The clinical sensitivity of RT-LAMP for the detection of HCV was 95.5%. There was no cross-reactivity between RT-LAMP reactions of HCV; the specificity was 100%.
Table 2.
Results of the RT-LAMP assay for all clinical specimens.
| Methods |
RT-LAMP assay (I) |
RT-LAMP assay (II) |
Total | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Inconclusivea | Positive | Negative | |||
| Real-time PCR assay | Positive | 173 (88.3%) | 23 (11.7%) | 4 | 191 (95.5%) | 9 (4.5%) | 200 |
| Negative | 2 (2.9%) | 68 (97.1%) | – | 0 | 70 (100%) | 70b | |
RT-LAMP, reverse transcription loop-mediated isothermal amplification assay. (I) the first interpretation; (II) the second interpretation.
Inconclusive refers to a color in a reaction tube indicating a weakly to moderately positive result, but a banding pattern appearing on the gel not specific for HCV amplification, or vice versa. After RT-LAMP retesting, three samples became positive, while one became negative.
These samples included 20 HIV-infected patient samples, 20 HBV-infected patient samples, and 30 healthy donor samples.
Based on HCV viral load, the RT-LAMP assay showed a better detection threshold at a viral load of 5.00 log10 IU/mL where the rate of detection was 100% (Table 3).
Table 3.
Results of the RT-LAMP assay for the detection of HCV on a panel of known HCV viral loads.
| HCV viral load (IU/mL) | All |
Genotype 1 |
Genotype 3 |
Genotype 6 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | N | n | % | N | n | % | |
| 7.01–8.00 log10 | 23 | 23 | 100 | 10 | 10 | 100 | 7 | 7 | 100 | 6 | 6 | 100 |
| 6.01–7.00 log10 | 96 | 96 | 100 | 39 | 39 | 100 | 33 | 33 | 100 | 24 | 24 | 100 |
| 5.01–6.00 log10 | 47 | 47 | 100 | 18 | 18 | 100 | 21 | 21 | 100 | 7 | 7 | 100 |
| 4.01–5.00 log10 | 28 | 23 | 82.1 | 12 | 10 | 83.3 | 14 | 11 | 78.6 | 2 | 2 | 100 |
| 3.01–4.00 log10 | 6 | 2 | 33.3 | 2 | 2 | 100 | 4 | 0 | 0 | |||
| Total | 200 | 191 | 95.5 | 81 | 79 | 97.5 | 79 | 72 | 91.1 | 39 | 39 | 100 |
HCV, hepatitis C virus; RT-LAMP, reverse transcription loop-mediated isothermal amplification assay.
Discussion
The great majority of HCV-infected individuals are asymptomatic or unaware of their infection. Therefore, early detection of HCV infection is crucial for immediate initiation of antiviral treatment and the control the disease progression. In this study, we developed an RT-LAMP assay for the simple, rapid, and cost-effective detection of HCV-RNA in clinical samples. Compared to RT-PCR or real-time RT-PCR, the RT-LAMP is simpler to perform, and no thermal cycler is needed. The major advantages of the RT-LAMP are its high specificity (since it uses six primers recognizing eight distinct regions on the target sequence), high sensitivity, and rapidity under isothermal conditions (2 h including the extraction step compared to 3–4 h for the real-time RT-PCR assay). The reverse transcription and DNA amplification can be performed in single tube in the RT-LAMP assay. In addition, an attractive property of the RT-LAMP assay is that results of RT-LAMP products can be observed immediately with the naked eye. The cost of RT-LAMP for a single reaction is also significantly cheaper than RT-PCR and real-time RT-PCR (Zhao et al., 2017).
In this study, HNB was pre-added in the RT-LAMP reaction in a closed tube to avoid a carry-over contamination in the post amplification process. HNB allows visual detection with the naked eye without equipment or a UV lamp, and we confirmed that the results of visual detection were equal to those of gel electrophoresis. Of note, the pre-addition of HNB in the RT-LAMP reaction did not inhibit amplification efficiency (Goto et al., 2009). Therefore, in the absence of other intercalating dyes and a turbidimeter, HNB would be ideal for use in resource-limited settings, as it is low cost and the results can be judged easily by the color change.
In this study, the LAMP primers used in the study by Nyan and Swinson (2016) were modified to specifically target all of the HCV genotypes predominantly circulating in Thailand, especially HCV genotypes 1a, 1b, 3a, 3b, and 6. It was demonstrated that the loop primers play a critical role in amplification using this primer set. Other studies have also reported that adding loop primers can increase the sensitivity of the LAMP reaction (Parida et al., 2004; Yang et al., 2011).
In the first-round RT-LAMP analysis, 29 discordant samples were retested for resolution. After RT-LAMP retesting, 20 of the 29 discordant samples were conclusive. For the two of 20 that became negative, possible explanations include technical or handling errors. For those 18 that became positive, the most likely explanations may be related to the viral load in the samples being near the limit of detection of the RT-LAMP, or random events associated with primers, target RNA, and the enzyme interaction leading to amplification in the retesting. Testing in duplicate may help to improve this issue.
In brief, this RT-LAMP method was able to detect the intended genotypes of HCV, showing a 100% detection rate for HCV genotype 6, 97.5% for HCV genotype 1, and 91.1% for HCV genotype 3. However, nine HCV samples did not yield positive results with this RT-LAMP, which may be due to a low viral load in these samples or degradation of viral RNA. In this study, a diagnostic sensitivity of 95.5% was thus revealed. It was demonstrated that this RT-LAMP assay was able to detect 100% of 39 samples with HCV genotype 6, which is comparable to the results of the study by Wang et al., who evaluated nine samples using a different primer set (Wang et al., 2011). Our assay also revealed appropriate specificity for HCV, as no false-positive results were found when testing with other viruses.
To determine the detection limit of the test, HCV-RNA serial dilutions were tested by RT-LAMP. However, the serial dilution of HCV-RNA in this study differed from that in a previous study (Nyan and Swinson, 2016) regarding the measurement unit, making comparison more difficult. In the study by Nyan and Swinson, the HCV-RNA serial dilution ranged from 105 to 0.1 IU per reaction, with a detection limit of 100 IU per reaction (100% detection rate), whereas in our study, it ranged from 100 to 0.01 ng per reaction, with a detection limit of 10–100 ng per reaction depending on the genotype. This difference may also be explained by the utilization of the DN3 primer set in the study of Nyan and Swinson, whereas a modified DN1 primer set was used and applied in our study. Other factors including the dilution method, extraction method, LAMP reaction, or even product detection could have influenced the difference.
Based on HCV viral load, the assay showed a 100% detection threshold at a viral load of 5.00 log10 IU/mL for the clinical samples tested. This appears to be a rather high threshold level; however, this method may still be useful for the screening or diagnosis of acute HCV-infected individuals, since the HCV viremia is generally higher than 104 IU/mL during acute infection (Glynn et al., 2005), or in chronic HCV-infected individuals with a high viral load. In addition, to ensure the result accuracy, this test should be performed in duplicate or in consecutive order and also interpreted with other laboratory results such as liver function tests.
Although RT-LAMP assays for the detection of HCV have been evaluated in previous studies, most of them used gel electrophoresis or added dye at the end of the reaction, such as SYBR Green I or GelGreen dye, for the detection of the RT-LAMP products (Esfahani et al., 2010; Kargar et al., 2012; Lakshmi et al., 2016; Nyan and Swinson, 2016; Wang et al., 2011; Yang et al., 2011). These methods have several limitations, such as the generation of hazardous waste, high contamination due to opening the reaction tubes, and the need for a UV lamp to interpret the results. In addition, previous studies have needed to add calcein/Mn2+ to visualize the HCV products, but have been performed in a limited number of HCV genotypes (Zhao et al., 2017). HNB has been used to visualize HCV products from an RT-LAMP assay previously, but that study evaluated only 83 samples of HCV genotypes 1–4 in sub-Saharan Africa (Odari, 2017).
This study had some limitations. First, HCV genotypes other than HCV-1, 3, and 6 were not investigated due to the unavailability of samples in our setting. However, in the Southeast Asia region, these are the three predominant genotypes, found in up to 80% of all HCV-infected patients (Messina et al., 2015; Wasitthankasem et al., 2015). Second, the distribution of HCV viral load among all samples validated in this study did not balance; most of the samples were obtained from untreated patients who had a high HCV viral load.
In conclusion, this study evaluated an RT-LAMP assay for the detection of the three predominant genotypes of HCV in Thailand, by observing the reaction color in a single closed tube. It is believed that RT-LAMP will be a useful tool to screen for and diagnose chronic HCV infection in a simple, rapid, and cost-effective manner, especially under conditions where sophisticated and expensive equipment are not available.
Acknowledgements
The research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under grant number #D43TW009345awarded to the Northern Pacific Global Health Fellows Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported in part by Chiang Mai University, Thailand. The authors would like to express their gratitude to Nipapan Leetrakul, Blood Bank Section, Maharaj Nakorn Chiangmai Hospital, Chiangmai, Thailand.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Declaration of Competing Interest
The authors report no declarations of interest.
Ethical approval
Use of these specimens was ethically approved by the Research Institute for Health Sciences Human Experimentation Committee (RIHES HEC), Chiang Mai University (Study code: 14/62).
References
- Asselah T, Marcellin P, Schinazi RF. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure?. Liver Int 2018;38 Suppl 1:7–13, doi: 10.1111/liv.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentaleb EM, Abid M, El Messaoudi MD, Lakssir B, Ressami EM, Amzazi S, et al. Development and evaluation of an in-house single step loop-mediated isothermal amplification (SS-LAMP) assay for the detection of Mycobacterium tuberculosis complex in sputum samples from Moroccan patients. BMC Infect Dis 2016;16:517, doi: 10.1186/s12879-016-1864-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel HD, Grant PR, Garson JA, Tedder RS, Chandy GM, Abraham P. Quantitation of hepatitis C virus using an in-house real-time reverse transcriptase polymerase chain reaction in plasma samples. Diagn Microbiol Infect Dis 2008;61:415–20, doi: 10.1016/j.diagmicrobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Esfahani SN, Shahhosseiny MH, Yaghmai P, Praivar K, Moslemi E, Amini HK. Rapid and simple detection of Hepatitis C virus by reverse transcriptase -loop-mediated isothermal amplification method. Afr J Microbiol Res 2010;4:2580–6. [Google Scholar]
- Glynn SA, Wright DJ, Kleinman SH, Hirschkorn D, Tu Y, Heldebrant C, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion 2005;45:994–1002, doi: 10.1111/j.1537-2995.2005.04390.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez I, Vina-Rodriguez A, Cayarga AA, Rosa IG, Gonzalez YJ. Design of an antisense reverse-transcriptase-polymerase chain reaction primer efficient for all hepatitis C virus genotypes: comparison of its performance vs a commercial primer. Anal Biochem 2003;315:281–4. [DOI] [PubMed] [Google Scholar]
- Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 2009;46:167–72, doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- Kargar M, Askari A, Doosti A, Ghorbani-Dalini S. Loop-mediated isothermal amplification assay for rapid detection of hepatitis C virus. Indian J Virol 2012;23:18–23, doi: 10.1007/s13337-012-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken C, Simmonds P. Nomenclature and numbering of the hepatitis C virus. Methods Mol Biol 2009;510:33–53, doi: 10.1007/978-1-59745-394-3_4. [DOI] [PubMed] [Google Scholar]
- Lakshmi V, Neeraja M, Lavanya V, Priyanka E, Sharma S, Dash P, et al. Application of real time loop mediated isothermal amplification assay on dried blood spots in the detection Of HCV RNA among high risk patients. J Emerg Dis Virol 2016;2, doi: 10.16966/2473-1846.111. [DOI] [Google Scholar]
- Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015;61:77–87, doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani SM, Santos CA, Riediger IN, Krieger MA, Duarte CA, Lacerda MA, et al. Development of hepatitis C virus genotyping by real-time PCR based on the NS5B region. PLoS One 2010;5:e10150, doi: 10.1371/journal.pone.0010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. Request Funding for Rapid Point-of-Care Hepatitis C Virus Diagnostics. Available from:. 2020. https://www.niaid.nih.gov/grants-contracts/hepatitis-c-virus-diagnostics.
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 2000;28:E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyan DC, Swinson KL. A method for rapid detection and genotype identification of hepatitis C virus 1–36 by one-step reverse transcription loop-mediated isothermal amplification. Int J Infect Dis 2016;43:30–6, doi: 10.1016/j.ijid.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Odari EO. Evaluation of a reverse transcriptase (Rt) loop mediated isothermal amplification assay for detection of hepatitis C Genotypes 1–4 viruses under limited logistical conditions. J Human Virol Retrovirol 2017;5, doi: 10.15406/jhvrv.2017.05.00145. [DOI] [Google Scholar]
- Parida M, Posadas G, Inoue S, Hasebe F, Morita K. Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol 2004;42:257–63, doi: 10.1128/jcm.42.1.257-263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida MM. Rapid and real-time detection technologies for emerging viruses of biomedical importance. J Biosci 2008;33:617–28, doi: 10.1007/s12038-008-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QQ, Zhang J, Hu JS, Chen HT, Du L, Wu LQ, et al. Rapid detection of hepatitis C virus RNA by a reverse transcription loop-mediated isothermal amplification assay. FEMS Immunol Med Microbiol 2011;63:144–7, doi: 10.1111/j.1574-1695X.2011.00828.x. [DOI] [PubMed] [Google Scholar]
- Wasitthankasem R, Vongpunsawad S, Siripon N, Suya C, Chulothok P, Chaiear K, et al. Genotypic distribution of hepatitis C virus in Thailand and Southeast Asia. PLoS One 2015;10:e0126764, doi: 10.1371/journal.pone.0126764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Fang MX, Li J, Lou GQ, Lu HJ, Wu NP. Detection of hepatitis C virus by an improved loop-mediated isothermal amplification assay. Arch Virol 2011;156:1387–96, doi: 10.1007/s00705-011-1001-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cao J, Zhu M, Xu M, Shi F. Loop-mediated isothermal amplification-lateral-flow dipstick (LAMP-LFD) to detect Mycoplasma ovipneumoniae. World J Microbiol Biotechnol 2019;35:31, doi: 10.1007/s11274-019-2601-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Liu J, Sun D. Detection of HCV genotypes 1b and 2a by a reverse transcription loop-mediated isothermal amplification assay. J Med Virol 2017;89:1048–54, doi: 10.1002/jmv.24747. [DOI] [PubMed] [Google Scholar]