Abstract
The treatment of venous thromboembolism (VTE) in patients with cancer is challenging because these patients have increased risks of both recurrent VTE and major bleeding, along with patient‐specific and cancer‐related factors that influence the approach to treatment. Historically, anticoagulant therapy with low‐molecular‐weight heparin (LMWH), given for both initial and long‐term treatment, has been the preferred approach recommended by practice guidelines. Most recently, the National Comprehensive Cancer Network (NCCN) guidelines indicate that the direct oral anticoagulants (DOACs) apixaban, edoxaban, or rivaroxaban are preferred for patients without gastric or gastroesophageal lesions. DOACs have been associated with an increased risk of major bleeding in patients with gastrointestinal and possibly genitourinary cancers, and DOACs should either not be used (especially in those with intact intraluminal tumors) or be used with caution in patients with these cancers. Fatal or life‐threatening bleeding occurs with similar frequency with DOACs or LMWH, and most major bleeding with DOACs can be managed with transfusion and standard measures. The patient's willingness and ability to comply with LMWH injections, and their treatment preference, should also be considered. Patients with cancer who have VTE should be treated with anticoagulation for a minimum of 6 months. Anticoagulation should be continued indefinitely while cancer is active or under treatment or if there are persistent risk factors for recurrent VTE. This article summarizes the evidence from clinical trials of LMWH and DOACs that underpins the NCCN guideline recommendations, addresses several controversies and caveats regarding anticoagulant treatment, and offers evidence‐based, practical suggestions on patient selection for treatment with DOACs.
Implications for Practice
Several randomized trials support the addition of direct oral anticoagulants (DOACs) to the therapeutic armamentarium for cancer‐associated venous thromboembolism (VTE). These agents come with unique risks and patient‐ and cancer‐specific variables that must be evaluated during the course of a patient's cancer care. This narrative review discusses findings from clinical trials of low‐molecular‐weight heparin and DOACs for the treatment of cancer‐associated VTE, evidence that supports the recent National Comprehensive Cancer Network guideline recommendations. A personalized approach to treatment is proposed that addresses patient selection for treatment with DOACs, factors that influence efficacy and safety, controversies and caveats, and suggestions for their resolution in clinical practice.
Keywords: Cancer‐associated thrombosis, Venous thromboembolism, Malignancy, Treatment
Short abstract
The management of venous thromboembolism (VTE) in patients with cancer poses challenges for the treating clinician beyond the elevated risks of recurrence and major bleeding. This article summarizes the evidence from clinical trials of low‐molecular‐weight heparin and direct oral anticoagulants that underpins NCCN guidelines, addresses controversies regarding anticoagulant treatment, and offers suggestions for patient selection for treatment.
Introduction
Patients with cancer are at significantly higher risk of developing venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), than the general population [1]. The management of VTE in patients with cancer poses challenges for the treating clinician beyond the elevated risks of recurrence and major bleeding. Patients with cancer also have a higher case fatality of recurrence compared with patients with VTE who do not have cancer [2, 3]. Patients with cancer may undergo procedures requiring temporary cessation of anticoagulation, have acute complications requiring intermittent hospitalization, suffer from nausea and vomiting limiting oral intake, develop thrombocytopenia from chemotherapy, and proceed through various cancer‐related therapies with differing—and sometimes unpredictable—effects on the metabolism of other drugs. The recent approval of several direct oral anticoagulants (DOACs) broadens the arsenal of therapies previously limited to parenteral agents and vitamin K antagonists (VKAs), providing clinicians with an opportunity to individualize the treatment of cancer‐associated VTE throughout the course of care of their patients with cancer.
Materials and Methods
The studies that formed the basis for the acceptance of low‐molecular‐weight heparin (LMWH) as the “gold standard” for the treatment of cancer‐associated VTE prior to the introduction of the DOACs are summarized and critiqued. The four randomized clinical trials that support the safety and efficacy of three different DOACs compared with dalteparin are reviewed in more detail. The patient‐ and cancer‐related factors that appear to impact safety in these studies are identified. Taken together with real‐world data and common clinical challenges, these factors are incorporated into a clinical decision‐making algorithm for choosing an anticoagulation strategy for patients with cancer‐associated VTE. We conclude by addressing specific clinical controversies where data are lacking to provide expert suggestions on optimal management.
Results
Synopsis of Current Evidence
LMWH as the “Gold Standard” Therapy
In 2003, the results of the multinational CLOT (Randomized Comparison of Low‐Molecular‐Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) trial made LMWH the gold standard for the treatment of acute DVT and PE in patients with cancer [4]. In this study, patients randomized to receive 6 months of therapy with the LMWH, dalteparin, had a reduced risk of recurrent VTE (9%) compared with those who received initial treatment (5–7 days) with dalteparin followed by the VKA warfarin (17%). There was no statistically significant difference in their risk of bleeding or overall mortality.
Four additional randomized controlled clinical trials comparing LMWH products other than dalteparin with warfarin for treatment of cancer‐associated VTE have been published (Table 1) [5, 6, 7, 8]. As in CLOT, there was no difference in rates of major bleeding or death between treatment arms in any of these studies. Only one other study, LITE (Long‐Term Innovations in Treatment Program), demonstrated a statistically significant reduction in VTE recurrence in favor of tinzaparin (at 3 months) [5].
Table 1.
Cancer‐associated venous thromboembolism: Low‐molecular‐weight heparin trials
| Variable | Trial | ||||
|---|---|---|---|---|---|
| CLOT [4] | Meyer et al. [8] | ONCENOX [7] | LITE [5] | CATCH [6] | |
| LMWH |
Dalteparin (200 IU/kg per day × 30 days, then 150 IU/kg per day) |
Enoxaparin (1.5 mg/kg once daily) |
Enoxaparin 1.0 mg/kg b.i.d. × 5 days, then 1.0 mg/kg per day (group 1a) Enoxaparin 1.5 mg/kg daily (group 1b) |
Tinzaparin 175 IU/kg daily Treatment duration 3 months |
Tinzaparin 175 IU/kg daily |
| Comparator | Warfarin | Warfarin | Warfarin | Warfarin | Warfarin |
| n | 672 | 146 | 102 | 200 | 900 |
| Follow‐up (months) | 6 | 3 | 6 | 3, 12 | 6 |
| Tumor type, % | NP | ||||
| Breast | 16.7 | 13.1 | NP | NP | 9.3 |
| Colorectal | 16.1 | 15.1 (digestive tract) | NP | NP | 13.2 |
| Lung | 13.4 | 11.0 | NP | NP | 11.6 |
| GU | 12.8 | 16.4 | NP | NP | 10.4 |
| GYN | 10.1 | 11.0 | NP | NP | 22.6 |
| Pancreas | 4.3 | NP | NP | NP | NP |
| Brain | 4.0 | NP | NP | NP | NP |
| Hem | 10.4 | 11.0 | NP | 6.5 | 10.4 |
| METS | 67.7 | 52.7 | 58.4 | 41.5 | 54.7 |
| Qualifying thrombus, % | |||||
| DVT | 69.2 | 30.1 | 83.2 | 93 | 56.8 |
| PE ± DVT | 31.4 | 69.9 | 29.7 | 21 | 43.2 |
| Efficacy outcomes, % | |||||
| VTE |
Dalteparin, 8.0 Warfarin, 15.8 |
Enoxaparin, 3.5 Warfarin, 5.1 |
Enoxaparin 1 mg/kg daily, 3.4 Enoxaparin 1.5 mg/kg daily, 3.1 Warfarin, 6.7 |
3 months: Tinzaparin, 6 Warfarin, 10 12 months: Tinzaparin, 7 Warfarin, 16 |
Tinzaparin, 6.9 Warfarin, 10.0 |
| DVT |
Dalteparin, 4.2 Warfarin, 11.0 |
NP |
Enoxaparin 1 mg/kg, 3.4 Enoxaparin 1.5 mg/kg, 3.1 Warfarin, 6.7 |
NP |
Tinzaparin, 2.7 Warfarin, 5.3 |
| Nonfatal PE |
Dalteparin, 2.4 Warfarin, 2.7 |
NP |
Enoxaparin 1 mg/kg, 0 Enoxaparin 1.5 mg/kg, 0 Warfarin, 0 |
NP |
Tinzaparin, 0.7 Warfarin, 0.4 |
| Fatal PE |
Dalteparin, 1.5 Warfarin, 2.1 |
NP |
Enoxaparin 1 mg/kg, 0 Enoxaparin 1.5 mg/kg, 0 Warfarin, 0 |
Tinzaparin, 0 Warfarin, 3 |
Tinzaparin, 3.8 Warfarin, 3.8 |
| Safety outcomes, % | |||||
| Major bleeding |
Dalteparin, 6% Warfarin, 4% |
Enoxaparin, 7.0% Warfarin, 6.0% |
Enoxaparin 1 mg/kg, 6.5% Enoxaparin 1.5 mg/kg 11.1% Warfarin, 2.9% |
Tinzaparin, 7% Warfarin, 7% |
Tinzaparin, 2.7% Warfarin, 2.4% |
| Any bleeding |
Dalteparin, 14% Warfarin, 19% |
NP | NP |
Tinzaparin, 27% Warfarin, 24% |
Tinzaparin, 25.4% Warfarin, 24.4% |
| Death |
Dalteparin, 39% Warfarin, 41% |
Enoxaparin, 11.3% Warfarin, 22.7% |
Enoxaparin 1 mg/kg, 22.6% Enoxaparin 1.5 mg/kg, 41.7% Warfarin, 32.4% |
3 months: Tinzaparin, 20% Warfarin, 19% 12 months: Tinzaparin, 47% Warfarin, 47% |
Tinzaparin, 34.7% Warfarin, 32.2% |
| Reference | N Engl J Med 2003;349:146–153 | Arch Intern Med 2002;162:1729–1735 | Clin Appl Thromb Hemost 2006;12:389–396 | Am J Med 2006;119:1062–1072 | JAMA 2015;314:677–686 |
Abbreviations: DVT, deep vein thrombosis; GU, genitourinary; GYN, gynecologic; Hem, hematologic; LMWH, low‐molecular‐weight heparin; METS, metastatic cancer; NP, not published; PE, pulmonary embolism; VTE, venous thromboembolism.
Panel 1: DOAC recommendations.
Patients with cancer for whom DOAC is the preferred initial therapy for VTE
Ambulatory patients with cancer with an intact upper gastrointestinal tract that can take oral medications
Hospitalized patients with cancer for whom surgical intervention is not planned
DOACs not recommended for patients with
Creatinine clearance <30 mL/min
Luminal gastrointestinal lesion
Luminal genitourinary lesion
Recent (<3 months) history of peptic ulcer disease or other bleeding lesion
Anticancer therapies that significantly affect P‐glycoprotein, CYP3A4, or CYP2J2 pathways
Severe hepatic impairment with coagulopathy
Surgery or invasive procedure imminent
One hypothesis as to why these other studies did not corroborate the improved efficacy of LMWH seen in CLOT is that each LMWH has a unique composition with the potential for heterogeneity in clinical effects. CLOT was the only trial that used dalteparin, which could account for its superior performance. However, both the LITE and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) studies compared tinzaparin with warfarin. Unlike LITE, CATCH failed to demonstrate a statistically significant efficacy benefit for tinzaparin over warfarin (6‐month cumulative VTE recurrence risk of 7.2% vs. 10.5%; hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.41–1.03; p = .07) [6]. The authors suggested that the inadvertent inclusion of a healthier cancer population in CATCH (54.7% with metastatic disease vs. 67.7% in CLOT and 23% with an Eastern Cooperative Oncology Group [ECOG] score of 2 vs. 36% in CLOT) and/or a higher prevalence of active cancer treatment in CLOT (78% vs. 53% in CATCH) was the reason for the lower overall recurrent thrombosis rate and lack of detectable benefit with LMWH. Indeed, the CLOT trial included the largest percentage of patients with metastatic disease across these trials. The ONCENOX study was not far behind with 58.4% of patients presenting with metastatic disease, yet it failed to show a benefit for either of two doses of enoxaparin over warfarin in prevention of recurrent VTE [7].
Thus, although LMWH was established as the gold standard for treatment after publication of the CLOT study, the results of individual randomized clinical trials have not consistently shown an efficacy benefit over warfarin. Given the difficulty in maintaining therapeutic warfarin levels in the setting of active cancer treatment and the suggestion of superior efficacy of LMWH with similar safety in meta‐analyses of pooled studies [9, 10], LMWH as a class became the recommended first‐line treatment for VTE in patients with cancer who have adequate renal function in all major treatment guidelines [11, 12, 13, 14]. However, real‐world data suggest that physicians continued to prescribe warfarin despite the guidelines and that patient compliance with LMWH is low, with only 37% still on therapy at 6 months (vs. 61% of patients prescribed oral agents) [15]. These concerns, coupled with the inconsistent results from individual trials, left an open door for the introduction of new agents for patients with cancer who have VTE.
Clinical Trial Evidence for DOACs
Several trials have assessed DOACs for the treatment of VTE in patients with cancer [16, 17, 18, 19] (Table 2). The Hokusai VTE Cancer Trial Investigators compared a strategy of LMWH for 5 days followed by oral edoxaban 60 mg daily with dalteparin (200 IU/kg per day every 30 days, then 150 IU/kg daily) for at least 6 months and up to 12 months for the treatment of acute symptomatic or incidentally detected proximal leg DVT or PE [16]. Patients had to have active cancer or a diagnosis of cancer within the prior 2 years. Of the 1,050 patients enrolled, 53% had metastatic disease, and 98% had active cancer. The median duration of antithrombotic treatment was 211 days for the edoxaban arm and 184 days for the dalteparin arm. The primary composite outcome included recurrent VTE or major bleeding, which occurred in 12.8% of the edoxaban‐treated patients and 13.5% of the dalteparin‐treated patients (p = .006 for noninferiority; HR, 0.97; 95% CI, 0.70–1.36). Recurrent VTE occurred in 41 patients (7.9%) in the edoxaban group and in 59 patients (11.3%) in the dalteparin group (difference in risk, −3.4 percentage points; 95% CI, −7.0–0.2). Major bleeding occurred in 36 patients (6.9%) in the edoxaban group and in 21 patients (4.0%) in the dalteparin group (difference in risk, 2.9 percentage points; 95% CI, 0.1–5.6). In a subgroup analysis of patients with gastrointestinal (GI) malignancy, major bleeding rates favored dalteparin therapy (edoxaban 13.2% vs. dalteparin 2.4%; p = .0169). Bleeding rates were similar in the patients with non‐GI cancer. There were no fatal bleeding events in the edoxaban arm and one fatal bleed in the dalteparin arm. All‐cause mortality rates were similar for both arms (39.5% vs. 36.6%).
Table 2.
Cancer‐associated thrombosis treatment trials with direct oral anticoagulants
| Variable | Hokusai VTE Cancer | SELECT‐D | ADAM VTE | Caravaggio |
|---|---|---|---|---|
| Number of participants | 1,050 (1,046) | 406 | 300 (287) | 1,170 (1,155) |
| Study drug, n | Edoxaban, 522 | Rivaroxaban, 203 | Apixaban, 145 | Apixaban, 576 |
| Comparator drug, n | Dalteparin, 524 | Dalteparin, 203 | Dalteparin, 142 | Dalteparin, 579 |
| DVT only, n (%) | ||||
| Total | 389 (32.7) | 110 (27.1) | 106 (36.9) | 517 (44.7) |
| Study arm | 194 (37.2) edoxaban | 53 (26.1) rivaroxaban | 54 (36.7) apixaban | 272 (47.2) apixaban |
| Comparator arm | 195 (37.2) dalteparin | 57 (28.1) dalteparin | 52 (35.1) dalteparin | 245 (42.3) dalteparin |
| PE with or without DVT, n (%) | ||||
| Total | 657 (62.8) | 295 (72.6) | 156 (54.3) | 638 (55.2) |
| Study arm | 328 (62.8) edoxaban | 150 (73.9) rivaroxaban | 81 (55.9) apixaban | 304 (52.8) apixaban |
| Comparator arm | 329 (62.8) dalteparin | 145 (71.4) dalteparin | 75 (52.8) dalteparin | 334 (57.7) dalteparin |
| Incidental thrombosis, n (%) | ||||
| Total | 340 (32.5) | 213 (52.5) | Not reported in manuscript | 230 (19.9) |
| Study arm | 167 (32.0) edoxaban | 108 (53.2) rivaroxaban | 116 (20.1) apixaban | |
| Comparator arm | 173 (33.0) dalteparin | 105 (51.7) dalteparin | 114 (19.7) dalteparin | |
| Metastatic disease, n (%) | Or recurrent locally advanced | |||
| Total | 554 (53.0) | 236 (58.1) | 193 (67.2) | 785 (68.0) |
| Study arm | 274 (52.5) edoxaban | 118 (58.1) rivaroxaban | 96 (65.3) apixaban | 389 (67.5) apixaban |
| Comparator arm | 280 (53.4) dalteparin | 118 (58.1) dalteparin | 97 (66.0) dalteparin | 396 (68.4) dalteparin |
| VTE recurrence, n (%) | ||||
| Total | 100 (9.6) | 36 (8.9) | 10 (3.5) | 78 (6.8) |
| Study arm | 41 (7.9) edoxaban | 8 (3.9) rivaroxaban | 1 (0.7) apixaban | 32 (5.6) apixaban |
| Comparator arm | 59 (11.3) dalteparin | 18 (8.9) dalteparin | 9 (6.3) dalteparin | 46 (7.9) dalteparin |
| Major bleeding, n (%) | ||||
| Total | 57 (5.4) | 17 (4.2) | 2 (0.7) | 45 (3.9) |
| Study arm | 36 (6.9) edoxaban | 11 (5.4) rivaroxaban | 0 apixaban | 22 (3.8) apixaban |
| Comparator arm | 21 (4.0) dalteparin | 6 (3.0) dalteparin | 2 (1.4) dalteparin | 23 (4.0) dalteparin |
| CRNMB, n (%) | ||||
| Total | 134 (12.8) | 32 (7.9) | 16 (5.6) | 87 (7.5) |
| Study arm | 76 (14.6) edoxaban | 25 (12.3) rivaroxaban | 9 (6.2) apixaban | 52 (9.0) apixaban |
| Comparator arm | 58 (11.1) dalteparin | 7 (3.4) dalteparin | 7 (4.2) dalteparin | 35 (6.0) dalteparin |
| Deaths (any cause), n (%) | ||||
| Total | 399 (38.1) | 104 (25.6) | 38 (13.2) | 288 (24.9) |
| Study arm | 206 (39.5) edoxaban | 48 (23.6) rivaroxaban | 23 (16) apixaban | 135 (23.4) apixaban |
| Comparator arm | 193 (36.6) dalteparin | 56 (27.6) dalteparin | 15 (11) dalteparin | 153 (26.4) dalteparin |
Abbreviations: CRNMB, clinically relevant nonmajor bleeding; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Panel 2: Patients with cancer for whom low‐molecular‐weight heparin is the preferred initial therapy for venous thromboembolism.
Ambulatory patients who have gastrointestinal malignancies with luminal lesions
Ambulatory patients who have genitourinary malignancies with luminal lesions
Ambulatory patients who cannot take oral medications or lack an intact upper gastrointestinal tract
Ambulatory or hospitalized patients whose anticancer therapy has significant drug interactions with DOACs
Ambulatory or hospitalized patients who have thrombocytopenia >50,000 cells/L; dose reduction recommended for platelets 25,000–50,000 cells/L
Hospitalized patients for whom surgical intervention is planned
The SELECT‐D trial compared rivaroxaban (15 mg twice daily for 3 weeks, then 20 mg once daily for a total of 6 months) with dalteparin (200 IU/kg daily during month 1, then 150 IU/kg daily for months 2–6) for treatment of acute symptomatic leg DVT or symptomatic or incidental PE in patients with active cancer [17]. To qualify for the trial, patients had to have an ECOG performance score ≤2. The primary outcome was VTE recurrence. Secondary outcomes were major and clinically relevant nonmajor bleeding. During the recruitment period, 406 patients were randomized. Of these, 70% were actively receiving cancer therapy, and 58% had metastatic disease. Nearly half of qualifying thrombi (48%) were symptomatic, and 52% were found incidentally. VTE recurrence rates at 6 months were 4% for the rivaroxaban arm and 11% for the dalteparin arm (HR, 0.43; 95% CI, 0.19 to 0.99). There were two patients with symptomatic PE and one fatal PE in each treatment arm. The primary tumor site influenced VTE recurrence rates. Patients with stomach or pancreas cancer had more than a fivefold greater risk of recurrence (HR, 5.55; 95% CI, 1.97–15.66), whereas those with lung, lymphoma, gynecologic, or bladder cancer had more than a twofold increased risk (HR, 2.69; 95% CI, 1.11–6.53). Patients presenting with symptomatic VTE as a qualifying event also had more than twofold increased risk of recurrence compared with those with an incidental PE (HR, 2.78; 95% CI, 1.20–6.41). Major bleeding rates were 6% for rivaroxaban and 4% for dalteparin (HR, 1.83; 95% CI, 0.68 to 4.96). Most major bleeding events involved the GI tract. Patients with upper GI cancer were more likely to experience a major bleed with rivaroxaban. Clinically relevant nonmajor bleeding was also more frequent with rivaroxaban (13% vs. 4% HR, 3.76; 95% CI, 1.63 to 8.69) and primarily involved GI or genitourinary (GU) sources. There were no intracranial bleeds. Overall survival at 6 months was similar for both arms (rivaroxaban 75% vs. dalteparin 70%).
The ADAM VTE trial randomized 300 patients with cancer and VTE to receive either apixaban (10 mg twice daily for 7 days followed by 5 mg twice daily) for 6 months or subcutaneous dalteparin (200 IU/kg for 1 month followed by 150 IU/kg once daily) [18]. The primary outcome was major bleeding. Major bleeding up to 6 months occurred in none assigned to apixaban and 1.4% assigned to dalteparin (p = .138; HR and 95% CI not estimable because of no bleeding events in the apixaban arm). The major bleeding events in the dalteparin arm included one retroperitoneal and one intracranial bleed. Secondary outcomes included VTE recurrence and a composite of major plus clinically relevant nonmajor bleeding. Recurrent VTE occurred in 0.7% in the apixaban group and 6.3% in the dalteparin group (HR, 0.099; 95% CI, 0.013–0.780; p = .0281).
The Caravaggio trial compared apixaban with dalteparin for 6 months for the treatment of cancer‐associated VTE [19]. Enrollment criteria included confirmed acute proximal leg DVT or segmental PE in the setting of active cancer. Primary brain tumors, intracerebral metastasis, and acute leukemia were excluded. Of the 1,155 recruited patients, 97% had active cancer. The primary efficacy outcome, recurrent VTE, occurred in 5.6% of apixaban‐treated patients compared with 7.9% of dalteparin‐treated patients (HR, 0.63; 95% CI, 0.37–1.07; p < .001 for noninferiority and p = .09 for superiority). Rates for recurrent DVT were similar (2.3% vs. 2.6%), with borderline lower recurrent PE rates in the apixaban arm (3.3% vs. 5.5%). The primary safety outcome, International Society on Thrombosis and Haemostasis (ISTH) major bleeding, was similar for both treatment groups (3.8% vs. 4.0%), including GI bleeding, in the overall trial population (1.9% vs. 1.7%). The rates of major bleeding in the subgroup with GI cancer at study entry were not provided. Mortality rates were similar (23.4% vs. 26.4%) and primarily related to cancer progression. Of note, the event‐free survival composite (recurrent VTE, major bleeding, or death) favored apixaban therapy (73.3% vs. 68.6%; HR, 1.36; 95% CI, 1.05–1.76). In subgroup analysis, there was a statistically significant interaction between treatment and age; the HRs for both recurrent VTE and major bleeding favored apixaban in patients <65 years of age but favored dalteparin in patients >75 years of age [19].
Which Patients Should Receive a DOAC?
Based on these randomized clinical trials, contemporary guidelines, including the most recent National Comprehensive Cancer Network (NCCN) guidelines, cite the DOACs as preferred or acceptable options for VTE treatment in many, but not all, patients with cancer [14, 20] (Panel 1). In a meta‐analysis, DOACs were associated with lower VTE recurrence but higher bleeding rates compared with dalteparin [21]. In particular, GI bleeds were higher with edoxaban, and both GI and GU bleeds were higher with rivaroxaban. Bleeding rates were very low with both apixaban and dalteparin in the ADAM VTE trial, which suggests that the patient population may be less representative of most patients with cancer. In the Caravaggio trial, apixaban was equivalent to dalteparin in the rate of major bleeding, and a subgroup analysis suggests that patients younger than 65 years may have experienced significantly lower rates of bleeding with apixaban than older patients or patients treated with dalteparin.
Other authors have proposed reasonable algorithms for deciding when to initiate a DOAC versus a LMWH [22]. The following describes in detail when to avoid or alter dosing of DOACs, consistent with the most recent NCCN guidelines (Panel 2) [14].
Which Patients Should Not Receive a DOAC?
GI and GU cancers have shown increased risk of major bleeding with DOACs. DOACs should either not be used (especially in those with intact intraluminal tumors) or be used with caution in patients with these cancers. About 75% of the major bleeding events occur in patients with unresected tumors. Fatal or life‐threatening bleeding occurs with similar frequency to LMWH, and most major bleeds with the DOACs can be managed with transfusion and standard measures. The patient's willingness and ability to comply with LMWH injections, and their treatment preference, should also be considered.
Rivaroxaban should not be used in patients with creatinine clearance (CrCl) <30 cc/minute or end‐stage renal disease (ESRD). The apixaban trials excluded patients with serum creatinine >2.5 mg/dL or CrCl <25 cc/minute. Edoxaban dose should be reduced to 30 mg daily for patients with CrCl 30 to 50 cc/minute, or a body weight of ≤60 kg [16]. Edoxaban should be avoided in patients with CrCl <30 cc/minute or ESRD.
Avoid use of rivaroxaban in patients on strong dual CYP3A4 and concomitant P‐glycoprotein inhibitor and those on strong dual CYP3A4 and concomitant P‐glycoprotein inducer. Patients on apixaban and strong dual CYP3A4 and concomitant P‐glycoprotein inhibitor should have apixaban decreased by 50%. Apixaban should be avoided in those patients on strong dual CYP3A4 and concomitant P‐glycoprotein inducer. Edoxaban dose should be reduced to 30 mg once daily in patients who were receiving concomitant treatment with potent P‐glycoprotein inhibitors. Edoxaban should be avoided in patients on any concomitant administration of P‐glycoprotein inducer.
DOACs are not recommended for patients with Child‐Turcotte‐Pugh class B or C cirrhosis.
Patients should not be on DOACs if they have active bleeding, a spinal puncture, or neuroaxial anesthesia.
There is limited evidence with DOAC use in patients with cancer who experience chemotherapy‐induced thrombocytopenia. Care should be taken in patients with an expected decrease in platelet count.
Patients with recent (<1 month) brain surgery (major surgery) or metastatic brain lesions with melanoma, renal cell carcinoma, or thyroid cancer should not receive DOACs. Data are limited for all anticoagulants in the setting of brain metastases.
What Is the Recommended Approach to Initiating Anticoagulation in a Patient with Cancer‐Associated VTE?
Upon diagnosis of VTE, we suggest a two‐step assessment (Fig. 1) of patient‐ and cancer‐related variables to quickly enable the clinician to choose between the three available classes of anticoagulation (DOACs, LMWH, oral VKAs).
Figure 1.
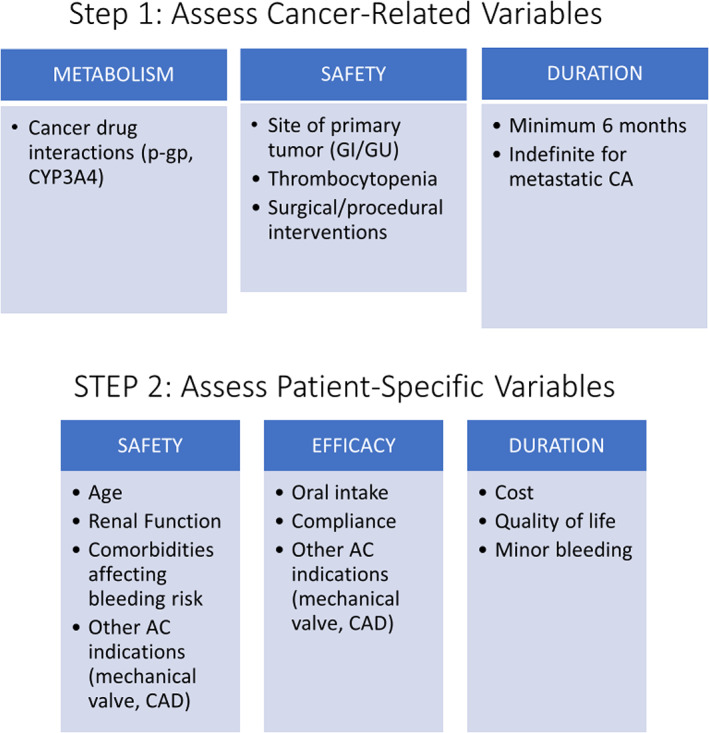
Two‐step approach to choosing between three available classes of anticoagulants (direct oral anticoagulant, low‐molecular‐weight heparin, or oral vitamin K antagonist).Abbreviations: AC, anticoagulation; CA, cancer; CAD, coronary artery disease; GI, gastrointestinal; GU, genitourinary; p‐gp, P‐glycoprotein.
Panel 3: Patients with cancer for whom warfarin is the preferred therapy for venous thromboembolism.
Patients who have a contraindication to DOACs and cannot or will not use LMWH
Patients who have end‐stage renal disease nearing or on hemodialysis
Step 1. Assess patient‐specific variables that may affect safety, efficacy, and/or duration of treatment for cancer‐associated thrombosis:
For patients with ESRD, use unfractionated heparin followed by warfarin.
For patients with impaired absorption because of poor oral intake, surgically absent stomach or intestines, or significantly impaired absorption for any reason, use LMWH.
For patients with other indications for warfarin, such as mechanical heart valves, continue warfarin.
For patients requiring antiplatelet therapy of higher intensity than baby aspirin, consult cardiology to determine whether this can be reduced; if not, consider LMWH.
For patients requiring treatment with drugs metabolized by P‐glycoprotein or CYP3A4 for other conditions, review drug interactions prior to choosing a DOAC.
For patients with compliance challenges, consider once‐daily dosing of rivaroxaban or edoxaban, rather than twice daily dosing of apixaban. However, edoxaban requires initial treatment with LMWH for 5 days, which may be a drawback for some patients.
Patients who cannot or do not want to use DOACs or LMWH should use warfarin (Panel 3).
Step 2. Assess cancer‐related variables that may affect metabolism, safety, or duration of anticoagulation. These include the following:
Primary tumor type and area(s) of involvement: in GI malignancies, especially when a luminal lesion is present, use LMWH; also consider use of LMWH in GU cancers when a luminal lesion is present.
Current cancer therapy: if potential drug‐drug interactions between cancer therapy and DOACs identified, use LMWH; if cancer therapy has caused thrombocytopenia or is expected to cause thrombocytopenia with platelets <50,000/mcL, use dose‐adjusted LMWH and/or platelet transfusion per recommendations of the Scientific and Standardization Committee of the ISTH [23].
Central lines: if thrombosis is central line‐related but the line is needed for administration of chemotherapy and is still functioning, leave central line in place and initiate therapy with DOAC if other contraindications are not present.
Controversies and Caveats
How Long Should Patients Receive Anticoagulant Treatment?
Patients with cancer and VTE should be treated with anticoagulation for a minimum of 6 months. This differs slightly from the recent NCCN guidelines [14], which recommend a minimum duration of 3 months, because all of the clinical trials of LMWH or DOACs evaluated treatment for 6 months.
Patients with cancer and VTE should continue anticoagulation beyond 6 months if the malignancy is still present, unless there are contraindications to anticoagulation (i.e., there is some detectable solid tumor or hematologic malignancy is not in remission).
Patients with cancer who have been diagnosed with VTE and treated with anticoagulation for 6 months may discontinue anticoagulation if they have no evidence of disease or are in complete remission.
-
If cancer is metastatic and/or cancer therapy is ongoing beyond 6 months of treatment for VTE, consider continuing anticoagulation.
Consider prophylactic dosing of DOAC if both bleeding risk and risk of recurrent VTE are relatively low.
Consider prophylactic dose of LMWH if bleeding risk is moderate or high and risk of recurrent VTE is relatively low.
Consider full‐dose DOAC if bleeding risk is low and risk of recurrent VTE is moderate or high.
Consider full‐dose LMWH if bleeding risk is high and risk of recurrent VTE is moderate or high.
What Is the Explanation for LMWH Being Less Effective Than DOACs?
A meta‐analysis of randomized trials suggests a lower risk of recurrent VTE at the expense of a higher risk of bleeding than LMWH in patients with cancer‐associated VTE [21]. Although this might suggest that DOACs are simply more potent anticoagulants, two important variables may account for the difference in efficacy. First, the dose of dalteparin was reduced in the CLOT study after the first month of treatment based on expert consensus rather than evidence. Second, as demonstrated in real‐world data, compliance with LMWH likely decreases after the first couple of months [15].
What Is the Explanation for the Higher Bleeding Risk with DOACs in Patients with Cancer‐Associated VTE?
In most clinical trials of DOACs, major bleeding was cancer‐related and predominantly gastrointestinal. Increased GI bleeding events were also seen in other patient populations. For example, in atrial fibrillation (AF) stroke prevention trials, all DOACs appear to be associated with less intracranial hemorrhage than warfarin but more GI bleeding [24]. Elevated levels of active drug in the lumen of the GI tract may contribute to this risk. A substudy of the Caravaggio trial demonstrated that bleeding was highest in apixaban‐treated patients over the age of 75 years [19]. Similarly, a meta‐analysis of clinical trials using rivaroxaban or dabigatran for AF risk of major bleeding was highest in patients ≥75 years of age [25]. Although careful patient selection may result in comparable safety with LMWH, it is not clear that DOACs are inherently superior anticoagulants.
Are the DOACs Interchangeable in Terms of Efficacy and Safety?
All DOAC trials demonstrated at least noninferiority to LMWH for the combined endpoints of safety and efficacy [16, 17, 18, 19]. In an observational study at the Mayo Clinic, there was no significant difference in the rate of recurrence or major bleeding among patients treated with rivaroxaban, apixaban, or enoxaparin. Treatment choice was based on shared decision making between the patient and provider [26]. However, rivaroxaban was associated with a higher rate of clinically relevant nonmajor bleeding and lower mortality. The DOACs have not been compared against each other, and therefore, it is reasonable to consider them appropriate as a class—with the exception of dabigatran, which has not been specifically studied in a randomized trial in patients with cancer‐associated VTE.
What Should Be Done for Patients with Cancer When There Is Clot Progression or Recurrence While on Treatment for VTE?
There are no published data to answer this question, but the authors agree that in patients who experience clot progression while taking a DOAC, it is reasonable to switch to a treatment regimen of LMWH, such as dalteparin 200 IU once daily for one month, followed by 150 IU once daily thereafter, or enoxaparin 1 mg/kg every 12 hours. For patients with thrombosis progression while receiving LMWH, one option is to increase the dose by 20% as published by Carrier et al. [27]. It is also reasonable, given the possibility of noncompliance, to switch to a DOAC in this setting.
How Should Patients Receiving Anticoagulant Treatment for VTE Be Followed?
There are no published data to answer this particular question, but patients with cancer should be followed carefully for thrombus resolution, bleeding, and changes in renal or liver function early in the treatment of cancer‐associated thrombosis. We recommend following patients at least every 4 to 6 weeks initially. The frequency can be reduced to every 12 weeks once clot‐related signs and symptoms have resolved and cancer‐related interventions are less frequent or stable.
Conclusion
The treatment of cancer‐associated thrombosis presents several challenges, including increased risks of bleeding and recurrent VTE. In this review, we have assessed the available data on anticoagulant options in patients with cancer and VTE and have provided evidence‐based suggestions to guide management and shared decision making. The DOACs provide an improved therapeutic option for many, but not all, patients with cancer‐associated VTE. Assessment of patient‐ and cancer‐related variables, and patient preference are key to choosing an anticoagulant regimen that optimizes risks and benefits for the individual patient.
Author Contributions
Conception/design: Casey O'Connell, Carmen P. Escalante, Samuel Z. Goldhaber, Robert McBane, Jean M. Connors, Gary E. Raskob
Manuscript writing: Casey O'Connell, Carmen P. Escalante, Samuel Z. Goldhaber, Robert McBane, Jean M. Connors, Gary E. Raskob
Final approval of manuscript: Casey O'Connell, Carmen P. Escalante, Samuel Z. Goldhaber, Robert McBane, Jean M. Connors, Gary E. Raskob
Disclosures
Jean M. Connors: Bristol‐Myers Squibb, Pfizer, Portola, Abbott, Takeda (H), Abbott (C/A), CSL Behring (RF—institution). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This work was funded through an educational grant from Bristol‐Myers Squibb–Pfizer Alliance to the North American Thrombosis Forum. The funder of this work had no role in the design, preparation, or writing of the report. We thank Aviva Schwartz, Kathryn Mikkelsen, and the North American Thrombosis Forum (Boston, MA) for their invaluable comments and support during this work.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Cohen AT, Katholing A, Rietbrock S et al. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population‐based cohort study. Thromb Haemost 2017;117:57–65. [DOI] [PubMed] [Google Scholar]
- 2. Prandoni P, Lensing AW, Piccioli A et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484–3488. [DOI] [PubMed] [Google Scholar]
- 3. Abdulla A, Davis WM, Ratnaweera N et al. A meta‐analysis of case fatality rates of recurrent venous thromboembolism and major bleeding in patients with cancer. Thromb Haemost 2020;120:702–713. [DOI] [PubMed] [Google Scholar]
- 4. Lee AY, Levine MN, Baker RI et al.; Randomized Comparison of Low‐Molecular‐Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–153. [DOI] [PubMed] [Google Scholar]
- 5. Hull RD, Pineo GF, Brant RF et al.; LITE Trial Investigators. Long‐term low‐molecular‐weight heparin versus usual care in proximal‐vein thrombosis patients with cancer. Am J Med 2006;119:1062–1072. [DOI] [PubMed] [Google Scholar]
- 6. Lee AY, Kamphuisen PW, Meyer G et al.; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: A randomized clinical trial. JAMA 2015;314:677–686. [DOI] [PubMed] [Google Scholar]
- 7. Deitcher SR, Kessler CM, Merli G et al.; ONCENOX Investigators. Secondary prevention of venous thromboembolic events in patients with active cancer: Enoxaparin alone versus initial enoxaparin followed by warfarin for a 180‐day period. Clin Appl Thromb Hemost 2006;12:389–396. [DOI] [PubMed] [Google Scholar]
- 8. Meyer G, Marjanovic Z, Valcke J et al. Comparison of low‐molecular‐weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch Intern Med 2002;162:1729–1735. [DOI] [PubMed] [Google Scholar]
- 9. Carrier M, Cameron C, Delluc A et al. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer‐associated thrombosis: A systematic review and meta‐analysis. Thromb Res 2014;134:1214–1219. [DOI] [PubMed] [Google Scholar]
- 10. Kahale LA, Hakoum MB, Tsolakian IG et al. Anticoagulation for the long‐term treatment of venous thromboembolism in people with cancer. Cochrane Database Syst Rev 2018;(6):CD006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kearon C, Akl EA, Ornelas J et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–352. [DOI] [PubMed] [Google Scholar]
- 12. Farge D, Debourdeau P, Beckers M et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost 2013;11:56–70. [DOI] [PubMed] [Google Scholar]
- 13. Mandala M, Falanga A, Roila F. Management of venous thromboembolism in cancer patients: ESMO clinical recommendations. Ann Oncol 2008;19:ii126–27. [DOI] [PubMed] [Google Scholar]
- 14. National Comprehensive Cancer Network . NCCN guideline on cancer‐associated venous thromboembolic disease. Version 1. 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed October 11, 2020.
- 15. Khorana AA, McCrae KR, Milentijevic D et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer‐associated thrombosis. Res Pract Thromb Haemost 2017;1:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raskob GE, van Es N, Verhamme P et al.; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med 2018;378:615–624. [DOI] [PubMed] [Google Scholar]
- 17. Young AM, Marshall A, Thirlwall J et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT‐D). J Clin Oncol 2018;36:2017–2023. [DOI] [PubMed] [Google Scholar]
- 18. McBane RD, Wysokinski WE, Le‐Rademacher JG et al. Apixaban and dalteparin in active malignancy‐associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost 2020;18:411–421. [DOI] [PubMed] [Google Scholar]
- 19. Agnelli G, Becattini C, Meyer G et al.; Caravaggio Investigators. Apixaban for the treatment of venous thromboembolism associated with cancer. New Engl J Med 2020;382:1599–1607. [DOI] [PubMed] [Google Scholar]
- 20. Khorana AA, Noble S, Lee AYY et al. Role of direct oral anticoagulants in the treatment of cancer‐associated venous thromboembolism: Guidance from the SSC of the ISTH. J Thromb Haemost 2018;16:1891–1894. [DOI] [PubMed] [Google Scholar]
- 21. Li A, Garcia DA, Lyman GH et al. Direct oral anticoagulant (DOAC) versus low‐molecular weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta‐analysis. Thromb Res 2019;173:158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bannow BT, Lee A, Khorana AA et al. Management of cancer‐associated thrombosis in patients with thrombocytopenia: Guidance from the SSC of the ISTH. J Thomb Haemost 2018;16:1246–1249. [DOI] [PubMed] [Google Scholar]
- 23. Ay C, Beyer‐Westendorf J, Pabinger I. Treatment of cancer‐associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol 2019;30:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abraham N, Horsley‐Silva J. Gastrointestinal bleeding secondary to the new anticoagulants. Curr Opinion Gastroenterol 2016;32:474–480. [DOI] [PubMed] [Google Scholar]
- 25. Lin L, Lim WS, Zhou HJ et al. Clinical and safety outcomes of oral antithrombotics for stroke prevention in atrial fibrillation: A systematic review and network meta‐analysis. J Am Med Dir Assoc 2015;16:1103. [DOI] [PubMed] [Google Scholar]
- 26. Wysokinski WE, Houghton DE, Casanegra AI et al. Comparison of apixaban to rivaroxaban and enoxaparin in acute cancer‐associated venous thromboembolism. Am J Hematol 2019;94:1185–1192. [DOI] [PubMed] [Google Scholar]
- 27. Carrier M, Le Gal G, Cho R et al. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost 2009;7:760–765. [DOI] [PubMed] [Google Scholar]


