Abstract
Lessons Learned
Panitumumab monotherapy showed favorable efficacy and feasibility in the treatment of frail or elderly patients with RAS wild‐type unresectable colorectal cancer.
It is especially effective for left‐sided tumors; therefore, panitumumab as first‐line treatment could be an additional therapeutic option for frail elderly patients, particularly in those who are unsuitable for upfront oxaliplatin‐based or irinotecan‐based combination regimens.
Background
First‐line panitumumab monotherapy is expected to be well tolerated and improve survival in patients ineligible for intensive chemotherapy. However, its safety and efficacy in chemotherapy‐naïve frail or elderly patients with unresectable RAS wild‐type (WT) colorectal cancer (CRC) have not been studied. The aim of this phase II trial was to evaluate the efficacy and safety of panitumumab as first‐line treatment.
Methods
We conducted a multicenter phase II study on patients aged ≥76 years or ≥65 years considered unsuitable for intensive chemotherapy. Panitumumab 6 mg/kg of intravenous infusion was administered every 2 weeks. The primary endpoint was disease control rate (DCR). Secondary endpoints included progression‐free survival (PFS), overall survival (OS), response rate (RR), time to treatment failure (TTF), and incidence of grade 3 or 4 toxicities.
Results
Thirty‐six patients (median age: 81 [range, 67–88] years) were enrolled between February 2017 and August 2018. Two patients were excluded from the analysis of efficacy: one from lack of image examination at baseline and the other from lack of a measurable lesion. Thirty‐three (91.6%) patients had a performance status (PS) of 0 or 1, whereas two (5.6%) patients and one (2.8%) patient had a PS of 2 and 3, respectively. Twenty‐eight patients (77.8%) had left‐sided CRC, whereas eight (22.2%) had right‐sided CRC. The RR was 50.0% (95% confidence interval [CI], 32.4–67.6), including three patients (8.8%) who had complete responses. A total of 26.5% had stable diseases, resulting in a DCR of 76.5% (90% CI, 61.5–87.7). The RR of patients with left‐ and right‐sided tumors was 65.4% (95% CI, 44.3–82.8) and 0.0% (95% CI, 0.0–36.9), respectively. Major grade 3 or 4 nonhematologic toxicities were rash (n = 6, 16.7%), hypomagnesemia (n = 4, 11.1%), fatigue (n = 3, 8.3%), paronychia (n = 2, 5.6%), and hyponatremia (n = 2, 5.6%). The only grade 3 hematologic toxicity was neutropenia (n = 1, 2.8%).
Conclusion
Panitumumab monotherapy showed favorable efficacy and feasibility in frail or elderly patients with RAS WT unresectable CRC. Survival analysis including OS, PFS, and TTF is currently in progress.
Keywords: Colorectal cancer, Panitumumab, Elderly patients, RAS wild‐type, OGSG1602
Discussion
The present study is the first phase II study on the efficacy of first‐line panitumumab in frail elderly patients with RAS WT CRC. Our results showed that the primary endpoint, DCR, was improved (76.5%, p < .001; 90% CI, 61.5–87.7), including three cases (8.8%) of CR (Fig. 1). Furthermore, the RR and DCR of the patients with left‐sided tumors were 65% and 80%, respectively, whereas those of the patients with right‐sided tumors were 0% and 62.5%, respectively (Fig. 2). First‐line panitumumab treatment seems to be a viable therapeutic option in frail elderly patients, particularly in those who exhibit left‐sided tumors and/or are unsuitable for upfront oxaliplatin‐based or irinotecan‐based combination regimens.
Figure 1.
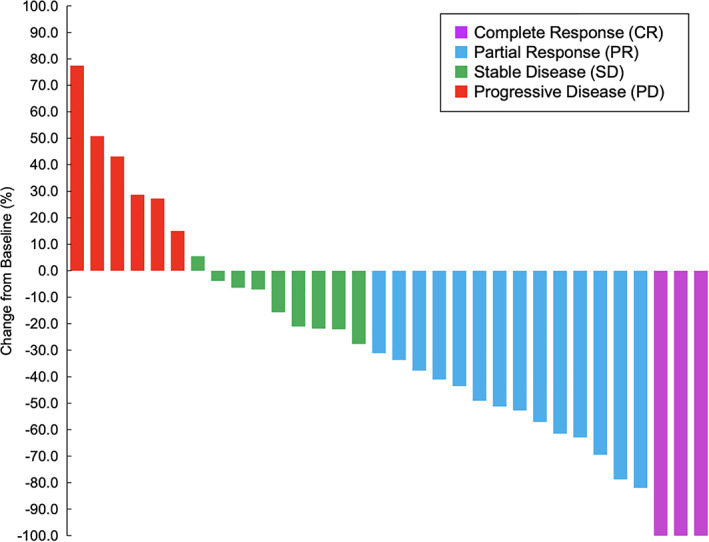
Waterfall plot (n = 32).
Figure 2.
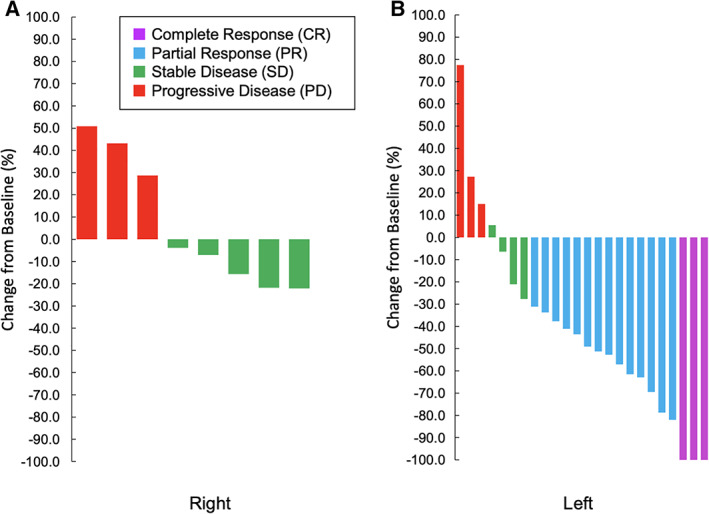
Waterfall plot by tumor location (right, n = 8; left, n = 24). Right: cecum colon, ascending colon, transverse colon. Left: descending colon, sigmoid colon, rectum.
Trial Information
| Disease | Colorectal cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | None |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Disease control rate |
| Secondary Endpoints | Overall response rate, progression‐free survival, overall survival, safety, time to treatment failure |
| Additional Details of Endpoints or Study Design | |
| The aim of the Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG) 1602 phase II study was to assess the efficacy of panitumumab as a first‐line treatment for patients with RAS WT unresectable CRC and who were ineligible for intensive chemotherapy. Therefore, the primary endpoint was set as the DCR, defined as the proportion of the best overall response from either complete response (CR), partial response (PR), or stable disease (SD), according to RECIST 1.1. The DCR was also assessed by an independent review committee. Disease reassessments were performed by means of contrast‐enhanced computed tomography every 8 weeks. Secondary endpoints were as follows: OS, defined as the time from enrollment to death from any cause; PFS, defined as the time from enrollment to disease progression or death from any cause; RR, defined as a proportion of best overall response of CR or PR; TTF, defined as the time from enrollment to discontinuation of treatment for any reason, including disease progression, treatment toxicity, or death; and the incidence of grade 3/4 toxicities according to CTCAE ver. 4.0. The null hypothesis was that DCR was 45%, and the alternative hypothesis was that DCR was >70%; this was assessed using an exact p value of .05 and a power of 0.90 based on the Clopper‐Pearson method. Thus, the sample size was 33. The total sample size was set to 36 to account for deviations. All statistical analyses were conducted at the OGSG Data Center. The Data and Safety Monitoring Committee (DSMC) of the OGSG independently reviewed the efficacy and safety data obtained from the present study. Protocol compliance, safety, and on‐schedule study progress were monitored by the DSMC. The OGSG Protocol Review Committee approved this study protocol on October 18, 2016. Approval was obtained from the Institutional Review Board before starting patient accrual at each institution. This trial was registered at the University hospital Medical Information Network (UMIN) Clinical Trials Registry as UMIN000024528 on December 1, 2016. The study was conducted according to the guidelines of the Declaration of Helsinki and the International Conference on Harmonization E6 Good Clinical Practice. The present study was supported by OGSG and funded by Takeda Pharmaceutical Company Limited. The ethical committee or institutional review committee at each site approved the protocol before the initiation of the study. All patients were required to sign a written informed consent form. |
|
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Generic/Working Name | New drug |
| Trade Name | Panitumumab |
| Company Name | Takeda Pharmaceutical Company Limited. |
| Drug Type | Antibody |
| Drug Class | EGFR |
| Dose | 6 mg/kg |
| Route | IV |
| Schedule of Administration |
|
| Panitumumab 6 mg/kg of intravenous infusion was administered every 2 weeks. Patients received treatment until the appearance of progressive disease, unacceptable toxicities, patient withdrawal, physician decision, or planned conversion surgery with the intention of curative resection. The use of minocycline, skin moisturizer, and sunscreen were recommended as prevention for skin toxicities. After the second cycle, the treatment protocol was started if skin toxicities (acne, dry skin, nail changes) were grade ≤ 2 and hypomagnesemia was grade ≤ 1 on day one of the cycle or the day before the scheduled date. If treatment could not be started within 28 days, the patients were withdrawn from the study. If patients exhibited grade 3 skin toxicities or hypomagnesemia, the dose was reduced (−1 level: 4.8 mg/kg, −2 level: 3.6 mg/kg). |
|
Patient Characteristics
| Number of Patients, Male | 20 (55.6%) |
| Number of Patients, Female | 16 (44.4%) |
| Stage | Twenty patients (55.6%) had stage IV disease, whereas 16 patients (44.4%) had recurrent disease. |
| Age | Median (range): 81 (67–88) years |
| Number of Prior Systemic Therapies | Median: 0 |
| Performance Status: ECOG |
0 — 18(50.0%) 1 — 15(41.6%) 2 — 2(5.6%) 3 — 1(2.8%) Unknown — |
| Other | A total of 36 patients were enrolled between February 2017 and August 2018. Thirty patients (83.3%) were aged ≥76 years, and six patients (16.7%) were aged <76. Regarding tumor location, 28 patients (77.8%) had left‐sided CRC, whereas 8 patients (22.2%) had right‐sided CRC. Notable medical histories were as follows: 16 patients (44.4%) had hypertension, 6 patients (16.7%) had diabetes, 5 patients (13.9%) had stroke, and 3 patients (8.3%) had ischemic heart disease. |
| Cancer Types or Histologic Subtypes | Tubular adenocarcinoma, 34 (94.4%); poorly differentiated adenocarcinoma, 2 (5.6%) |
Primary Assessment Method
| Title | Clinical response by tumor location (Light side colon) |
| Number of Patients Evaluated for Efficacy | 26 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 3 (11.5%) |
| Response Assessment PR | n = 14 (53.8%) |
| Response Assessment SD | n = 4 (15.4%) |
| Response Assessment PD | n = 3 (11.5%) |
| Response Assessment OTHER | n = 2 (7.7%) |
| Outcome Notes | The RR of the patients with left‐sided tumors (tumors located in the descending colon, sigmoid colon, or rectum) was 65.4% (95% CI, 44.3–82.8; Fig. 2.). |
| Title | Clinical response by tumor side (Right side colon) |
| Number of Patients Evaluated for Efficacy | 8 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 5 (62.5%) |
| Response Assessment PD | n = 3 (37.5%) |
| Response Assessment OTHER | n = 0 (0%) |
| Outcome Notes | The RR of the patients with right‐sided tumors (tumors located in the cecum, ascending colon, or transverse colon) was 0.0% (95% CI, 0.0–36.9; Fig. 2). |
| Title | Disease control rate, response rate |
| Number of Patients Screened | 36 |
| Number of Patients Enrolled | 36 |
| Number of Patients Evaluable for Toxicity | 36 |
| Number of Patients Evaluated for Efficacy | 34 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 3 (8.8%) |
| Response Assessment PR | n = 14 (41.2%) |
| Response Assessment SD | n = 9 (26.5%) |
| Response Assessment PD | n = 6 (17.6%) |
| Response Assessment OTHER | n = 2 (5.9%) |
| (Median) Duration Assessments PFS | 5.8 months, CI: 5.3–10.0 |
| (Median) Duration Assessments Duration of Treatment | 4.2 months |
| Outcome Notes |
Thirty‐four patients were included in the analysis of efficacy; 2 of 36 patients were excluded: one from lack of image examination at baseline and the other from lack of a measurable lesion. The RR was 50.0% (95% CI, 32.4–67.6), including three cases (8.8%) of CR, and SD was 26.5%, yielding 76.5% of DCR (p < .001, 90% CI, 61.5–87.7; Fig. 1). The median number of panitumumab dose administered was 8 (range, 1–16). Eleven patients (30.6%) had doses reduced by one level, and four patients (11.1%) had doses reduced by two levels. Sixteen patients (44.4%) discontinued study treatment because of progressive disease. Two patients (5.6%) discontinued treatment and received surveillance after CR, two patients (5.6%) underwent conversion therapy, and one patient (2.8%) received palliative radiation therapy for distance metastasis after tumor shrinkage. Five patients (13.9) discontinued treatment because toxicities; one experienced paronychia grade 3, stomatitis grade 1, and fatigue grade 1; one experienced rash grade 2, fatigue grade 2, stomatitis grade 1, and hypomagnesemia grade 2; one experienced sarcopenia; one experienced hypomagnesemia grade 2 and one experienced infusion reaction grade 3. Five patients (13.9%) were unable to start the next cycle within four weeks; two because of hypomagnesemia; two because of rash; and one because of both hypomagnesemia and rash. Three patients (8.3%) discontinued treatment because of other reasons. Two patients (5.6%) have continued treatment until this analysis. |
Secondary Assessment Method
| Title | Progression‐free survival, overall survival, time to treatment failure |
| Number of Patients Enrolled | 36 |
| Number of Patients Evaluated for Efficacy | 34 |
| (Median) Duration Assessments PFS | 5.8 months, CI: 5.3–10.0 |
| (Median) Duration Assessments Duration of Treatment | 4.2 months |
| Outcome Notes |
Kaplan‐Meier curves for PFS and OS of the 34 patients are displayed in Figures 3 and 4, respectively; however, the data of PFS and OS are preliminary and immature at the data cut‐off date (January 10, 2019). The median PFS of the 34 patients was 5.8 months (95% CI, 5.3–10.0; Fig. 3). The median PFS of patients with left‐sided tumors was 5.8 months (95% CI, 5.4–15.7), whereas that of patients with right‐sided tumors was 4.9 months (95% CI, 1.9–not available). The 6‐ and 12‐month OS rates for the 34 patients were 84.9% (95% CI, 73.5–98.0) and 69.9% (95% CI, 54.9–88.9), respectively (Fig. 4). The 6‐ and 12‐month OS rates for those with left‐sided tumors were 87.8% (95% CI, 75.7–100) and 78.5% (95% CI, 63.4–97.3), respectively, whereas for those with right‐sided tumors they were 75.0% (95% CI, 50.3–100) and 37.5% (95% CI, 13.0–100), respectively. The median TTF was 4.2 months (95% CI, 3.1–5.8). |
| Title | Treatment delivery |
| Outcome Notes |
The median number of panitumumab doses administered was 8 (range, 1–16). Eleven patients (30.6%) had doses reduced by one level, and four patients (11.1%) had doses reduced by two levels. Sixteen patients (44.4%) discontinued study treatment because of progressive disease. Two patients (5.6%) discontinued treatment and received surveillance after CR, two patients (5.6%) underwent conversion therapy, and one patient (2.8%) received palliative radiation therapy for distance metastasis after tumor shrinkage. Five patients (13.9) discontinued treatment because of toxicities; one experienced paronychia grade 3, stomatitis grade 1, and fatigue grade 1; one experienced rash grade 2, fatigue grade 2, stomatitis grade 1, and hypomagnesemia grade 2; one experienced sarcopenia; one experienced hypomagnesemia grade 2 and one experienced infusion reaction grade 3. Five patients (13.9%) were unable to start the next cycle within four weeks; two because of hypomagnesemia; two because of rash; and one because of both hypomagnesemia and rash. Three patients (8.3%) discontinued treatment because of other reasons. Two patients (5.6%) have continued treatment until this analysis. |
Figure 3.
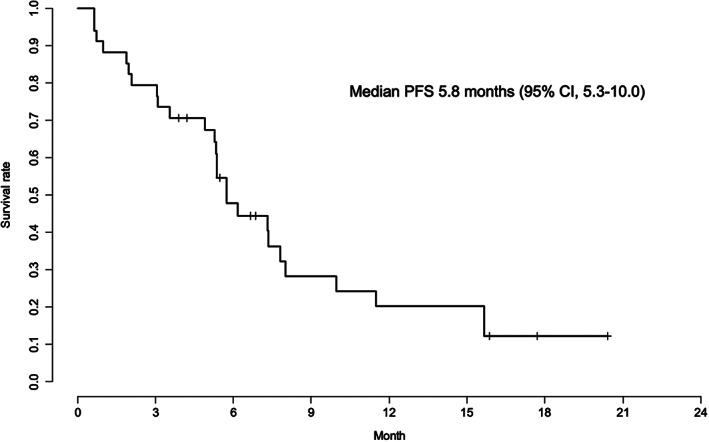
Progression‐free survival (n = 34). Abbreviations: CI, confidence interval; PFS, progression‐free survival.
Figure 4.
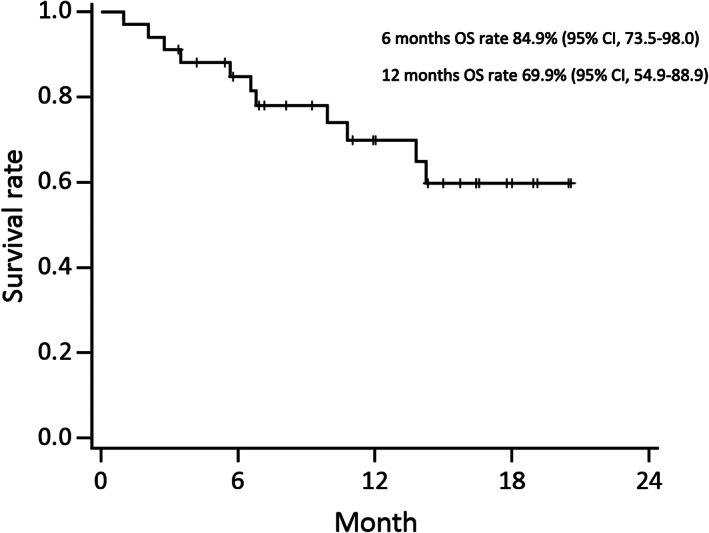
Overall survival (n = 34). Abbreviations: CI, confidence interval; OS, overall survival.
Adverse Events
All Cycles
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
|---|---|---|---|---|---|---|---|
| Hypoalbuminemia | 61% | 19% | 17% | 3% | 0% | 0% | 39% |
| Nail infection | 77% | 14% | 3% | 6% | 0% | 0% | 23% |
| Fatigue | 64% | 22% | 6% | 8% | 0% | 0% | 36% |
| Anorexia | 75% | 11% | 11% | 3% | 0% | 0% | 25% |
| Mucositis oral | 75% | 19% | 3% | 3% | 0% | 0% | 25% |
| Aspartate aminotransferase increased | 86% | 14% | 0% | 0% | 0% | 0% | 14% |
| Alanine aminotransferase increased | 86% | 11% | 3% | 0% | 0% | 0% | 14% |
| Hypokalemia | 86% | 11% | 3% | 0% | 0% | 0% | 14% |
| Hyperkalemia | 86% | 11% | 0% | 0% | 3% | 0% | 14% |
| Creatinine increased | 89% | 11% | 0% | 0% | 0% | 0% | 11% |
| Nausea | 88% | 6% | 3% | 3% | 0% | 0% | 12% |
| Diarrhea | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Hyponatremia | 94% | 0% | 0% | 6% | 0% | 0% | 6% |
| Dysgeusia | 97% | 0% | 3% | 0% | 0% | 0% | 3% |
| White blood cell decreased | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Neutrophil count decreased | 94% | 3% | 0% | 3% | 0% | 0% | 6% |
| Anemia | 80% | 14% | 6% | 0% | 0% | 0% | 20% |
| Hypomagnesemia | 39% | 25% | 25% | 11% | 0% | 0% | 61% |
| Hypocalcemia | 61% | 28% | 11% | 0% | 0% | 0% | 39% |
| Rash acneiform | 24% | 28% | 31% | 17% | 0% | 0% | 76% |
Adverse Events Legend All patients were evaluable for safety. The observed major grade 3 or 4 nonhematologic toxicities were rash (n = 6, 17%), hypomagnesemia (n = 4, 11%), fatigue (n = 3, 8%), paronychia (n = 3, 6%), and hyponatremia (n = 3, 6%), whereas the only grade 3 hematologic toxicity was neutropenia (n = 1, 3%). There was no adverse event‐related death.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Advanced colorectal cancer (CRC) is the second most common cause of cancer‐related death worldwide after lung cancer [1]. Although the development of new cytotoxic drugs has increased the median survival time of patients with metastatic colorectal cancer (mCRC) from 8 to approximately 30 months over the past 2 decades [2, 3, 4, 5], frail elderly patients are often excluded from randomized trials or represent a minority of enrolled patients [6], despite the high prevalence of mCRC in this population [7, 8]. More than 60% of patients who are newly diagnosed with cancer are aged 65 years or older, which makes this the most common population seen in most oncology practices [9]. Risks associated with old age should be taken into account when treatment options are considered, as elderly patients are more likely to present with age‐related decline in organ function and comorbidities at diagnosis [10, 11]. Therefore, several trials targeting frail or elderly mCRC patients were undertaken [12, 13]. For frail elderly patients, for whom oxaliplatin‐ or irinotecan‐based doublet therapy would be inappropriate, less intensive regimens, such as capecitabine plus bevacizumab or reduced‐dose oxaliplatin plus 5‐fluorouracil (5‐FU), were more optimal [8, 12, 13].
Panitumumab and cetuximab are epidermal growth factor receptor (EGFR)‐inhibiting monoclonal antibodies. The results of a recent prospective trial in frail, older patients who were not candidates for cytotoxic chemotherapy suggest that the use of panitumumab as a first‐line therapy would be well tolerated and could improve survival even in patients with RAS wild‐type (WT) mCRC who are not considered eligible for intensive chemotherapy [14, 15]. In a phase II trial in Spain, Sastre et al. reported that panitumumab first‐line therapy in frail elderly patients (≥70‐year, performance status [PS] 0–2) with KRAS WT mCRC had a median overall survival (OS) and progression‐free survival (PFS) of 7.1 months (95% confidence interval [CI], 5.0–12.3) and 4.3 months (95% CI, 2.8–6.4), respectively [14]. Pietrantonio et al. also reported a prospective study on the efficacy and safety of panitumumab in frail elderly patients with WT RAS and WT BRAF [15]. Including 75% of patients who received panitumumab as second‐line therapy, the median PFS and OS rates were 6.4 months (95% CI, 4.9–8) and 14.3 months (95% CI, 10.9–17.7), respectively [15]. However, the efficacy of panitumumab as a first‐line therapy has not been investigated extensively in patients with WT RAS who are ineligible for intensive chemotherapy. Thus, we conducted a phase II trial on the efficacy of panitumumab in frail elderly patients with WT RAS aged ≥76 years or ≥ 65 years who are ineligible for intensive chemotherapy. Here we report the results of the primary endpoint disease control rate (DCR).
Our results showed that the primary endpoint, DCR, was improved (76.5%, p < .001, 90% CI, 61.5–87.7), including three cases (8.8%) of CR. Furthermore, the response rate (RR) and DCR of the patients with left‐sided tumors were 65% and 80%, respectively, whereas that of the patients with right‐sided tumors were 0% and 62.5%, respectively. First‐line panitumumab treatment seems to be a viable therapeutic option in frail elderly patients, particularly in those who exhibit left‐sided tumors and/or are unsuitable for upfront oxaliplatin‐based or irinotecan‐based combination regimens.
The FOCUS2 trial, which reported on 5‐FU–based treatment, showed that the RR and DCR of reduced‐dose oxaliplatin plus 5‐FU were 38% and 71%, respectively [13]. However, the AVEX trial showed that the RR and DCR of capecitabine plus bevacizumab treatment were 19% and 74%, respectively [12]. Although the DCR of left‐sided tumors in this trial was comparable with previous reports of a 5‐FU based regimen, the RR tended to be better [12, 13]. Regarding the previous reports on panitumumab, Sastre et al. showed that the RR was 9.1% and DCR was 63.6% for patients with KRAS WT [14]. In addition, Pietrantonio et al. showed that the RR was 32.5% and DCR was 72.5% for patients with RAS WT, 75% of whom received panitumumab as a second‐line treatment [15]. Compared with these two reports, our results were favorable; this may be because our trial included RAS WT and chemo‐naive patients. Furthermore, our trial included many more patients with left‐sided tumors than those in previous studies [14, 15], as the day of enrollment of the first participant was February 9, 2017, which dated after the first report in American Society of Clinical Oncology 2016, which revealed that left‐sided colon cancer was favorable for anti‐EGFR monoclonal antibodies [16]. As a result, our trial showed higher RR and DCR values than those reported in previous trials [12, 13, 14, 15].
Regarding the safety profile, older age does not seem to negatively increase serious toxicities, despite the higher‐risk status of this population. Grade 3 rash, a well‐described panitumumab‐associated adverse event, was seen in 16.2% and hypomagnesemia was 11.1% of the patients, and no adverse event (AE)‐related deaths occurred. However, five patients discontinued treatment because of toxicities; panitumumab‐related toxicities including rash, hypomagnesemia, stomatitis, and diarrhea were comparable with previous studies [14, 15]. Furthermore, five patients were unable to start the next cycle because of prolonged toxicities; this trial allowed for only 28 days to start the next trial, which might be too short to recover from panitumumab‐related toxicities. In contrast, of the patients treated with capecitabine plus bevacizumab in the AVEX trial, 60% experienced grade 3 or worse adverse events, 30% experienced serious adverse events, and 7% experienced AEs‐related death, primarily because of cardiac disorder [12]. So, the careful patient selection is required to use bevacizumab for elderly patients [17]. Indeed, 44.4% of patients had a medical history of hypertension, diabetes, stroke, and/or ischemic heart disease in our trial. In the FOCUS‐2 trial, reduced 5‐FU plus oxaliptalin and capecitabine plus oxaliplatn resulted in 33% and 43% occurrence rates of grade 3 or worse adverse events, respectively; these included diarrhea, neurosensory toxicity, nausea, vomiting, and neutropenia [13]. Compared with panitumumab, the more severe side effects of a cytotoxic regimen could significantly affect a patient's quality of life and compliance with treatment. Overall, in view of this treatment's improved efficacy and lower toxicity compared with conventional chemotherapeutic agents, the use of panitumumab monotherapy in frail elderly patients appears to be promising; however, panitumumab can cause dermatologic toxicities.
The use of panitumumab as first‐line treatment for right‐sided tumors requires more careful consideration. In our results, DCR and RR were 62.5% and 0% respectively; however, several reports of first‐line clinical trials assessing tumors in this location revealed that cytotoxic chemotherapy plus anti‐EGFR antibody therapy showed some tumor response, although the benefit for long‐term survival was poor [18, 19, 20]. A right‐sided primary tumor site in microsatellite stable mCRC was associated with increased mutations, and enrichment of oncogenic alterations in KRAS, BRAF, PIK3CA, AKT1, RNF43, and SMAD4 compared with left‐sided primaries [21]; this provides a biological explanation behind the difference in response to EGFR inhibitors.
The present study had several limitations. First, BRAF‐mutated patients were not excluded, given that the examination of BRAF status was approved in August 2018 in Japan. Second, the primary endpoint of this study was DCR, whereas the primary endpoint of a phase II study is usually the response rate. Panitumumab rarely causes severe toxicities expect for skin toxicities. In addition, the RR of the standard therapy of capecitabine plus bevacizumab was low (19%) despite the 74% DCR [12]. Thus, we set the primary endpoint as DCR to compare with historical data. Third, some elderly patients with good PS might have benefited from intensive chemotherapy. Therefore, the criteria of this trial included patients aged ≥76 or ≥ 65 years who were not considered eligible for intensive chemotherapy, as there is no established method to identify frailty prior to the beginning of treatment. Fourth, the data regarding PFS and OS were immature and preliminary during this analysis. The ongoing follow‐up of PFS and OS rates is expected to confirm the efficacy of panitumumab as first‐line treatment. Fifth, the present trial is single‐arm phase II trial of which sample size is relatively small.
In conclusion, panitumumab monotherapy showed favorable efficacy and feasibility in the treatment of frail or elderly patients with RAS WT unresectable CRC. It is especially effective for left‐sided tumors and, therefore, panitumumab as first‐line treatment could be an additional therapeutic option for frail elderly patients, particularly in those who are unsuitable for upfront oxaliplatin‐based or irinotecan‐based combination regimens. The survival analysis including OS, PFS, and time to treatment failure is still in progress.
Disclosures
Hironaga Satake: Bayer Co., Ltd., Bristol‐Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly Japan Co., Ltd., Merck Bio Pharma Co., Ltd., MSD Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Co., Ltd., Yakult Honsha Co., Ltd. (H), Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Co., Ltd. (RF); Hisato Kawakami: Bristol‐ Myers Squibb Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Daiichi‐Sankyo Co. Ltd., Taiho Pharmaceutical Co. Ltd; (C/A), Bristol‐Myers Squibb Co. Ltd., AstraZeneca K.K., Bayer Vakuhin Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Takeda Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd. (H), Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd, Eisai Co. Ltd. (RF); Masahito Kotaka: Chusai Pharmaceutical, Yakut Honsya, Taiho (H); Yukinori Kurokawa: Taiho Pharmaceutical, Yakult Honsha, Ono Pharmaceutical, MSD, Daiichi Sankyo, Takeda Pharmaceutical, Kaken Pharmaceutical (H), Taiho Pharmaceutical, Ono Pharmaceutical, MSD (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures
Acknowledgments
The authors thank all the patients and their families. The authors also thank Akemi Morita, Mieko Nakai, Nami Yoshida, Chihiro Sawano, and Yoshiro Matsubara for data management and Takeda Pharmaceutical Company Limited. for providing funding.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- UMIN Trial ID: UMIN000024528
- Sponsor: Takeda Pharmaceutical Company Limited
- Principal Investigators: Takeshi Kato, Masahiro Goto
- IRB Approved: Yes
Contributor Information
Tetsuji Terazawa, Email: terasawat@osaka-med.ac.jp.
Takeshi Kato, Email: ken-kato@momo.so-net.ne.jp.
References
- 1. Ferlay J, Colombet M, Soerjomataram I et al. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int J Cancer 2019;144:1941–1953. [DOI] [PubMed] [Google Scholar]
- 2. Schwartzberg LS, Rivera F, Karthaus M et al. PEAK: A randomized, multicenter phase ii study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mfolfox6 in patients with previously untreated, unresectable, wild‐type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014;32:2240–2247. [DOI] [PubMed] [Google Scholar]
- 3. Heinemann V, von Weikersthal LF, Decker T et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): A randomised, open‐label, phase 3 trial. Lancet Oncol 2014;15:1065–1075. [DOI] [PubMed] [Google Scholar]
- 4. Kopetz S, Chang GJ, Overman MJ et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fakih MG. Metastatic colorectal cancer: Current state and future directions. J Clin Oncol 2015;33:1809–1824. [DOI] [PubMed] [Google Scholar]
- 6. Sorbye H, Pfeiffer P, Cavalli‐Björkman N et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer 2009;115:4679–4687. [DOI] [PubMed] [Google Scholar]
- 7. Moore KJ, Sussman DA, Koru‐Sengul T. Age‐specific risk factors for advanced stage colorectal cancer, 1981‐2013. Prev Chronic Dis 2018;15:E106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papamichael D, Audisio RA, Glimelius B et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol 2015;26:463–476. [DOI] [PubMed] [Google Scholar]
- 9. Berger NA, Savvides P, Koroukian SM et al. Cancer in the elderly. Trans Am Clin Climatol Assoc 2006;117:147–155; discussion 155–146. [PMC free article] [PubMed] [Google Scholar]
- 10. Aarts MJ, Lemmens VE, Louwman MW et al. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer 2010;46:2681–2695. [DOI] [PubMed] [Google Scholar]
- 11. van Leersum NJ, Janssen‐Heijnen ML, Wouters MW et al. Increasing prevalence of comorbidity in patients with colorectal cancer in the south of the Netherlands 1995‐2010. Int J Cancer 2013;132:2157–2163. [DOI] [PubMed] [Google Scholar]
- 12. Cunningham D, Lang I, Marcuello E et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open‐label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–1085. [DOI] [PubMed] [Google Scholar]
- 13. Seymour MT, Thompson LC, Wasan HS et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open‐label, randomised factorial trial. Lancet 2011;377:1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sastre J, Massuti B, Pulido G et al. First‐line single‐agent panitumumab in frail elderly patients with wild‐type KRAS metastatic colorectal cancer and poor prognostic factors: A phase II study of the Spanish cooperative group for the treatment of digestive tumours. Eur J Cancer 2015;51:1371–1380. [DOI] [PubMed] [Google Scholar]
- 15. Pietrantonio F, Cremolini C, Aprile G et al. Single‐agent panitumumab in frail elderly patients with advanced RAS and BRAF wild‐type colorectal cancer: Challenging drug label to light up new hope. The Oncologist 2015;20:1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnold D, Lueza B, Douillard JY et al. Prognostic and predictive value of primary tumour side in patients with RAS wild‐type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boehm S, Rothermundt C, Hess D et al. Antiangiogenic drugs in oncology: A focus on drug safety and the elderly ‐ A mini‐review. Gerontology 2010;56:303–309. [DOI] [PubMed] [Google Scholar]
- 18. Holch JW, Ricard I, Stintzing S et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta‐analysis of first‐line clinical trials. Eur J Cancer 2017;70:87–98. [DOI] [PubMed] [Google Scholar]
- 19. Sunakawa Y, Tsuji A, Fujii M et al. No benefit from the addition of anti‐EGFR antibody in all right‐sided metastatic colorectal cancer? Ann Oncol 2017;28:2030–2031. [DOI] [PubMed] [Google Scholar]
- 20. Boeckx N, Koukakis R, Op de Beeck K et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: Results from two randomized first‐line panitumumab studies. Ann Oncol 2017;28:1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yaeger R, Chatila WK, Lipsyc MD et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 2018;33:125–136.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]


