Abstract
Purpose
This trial evaluated the addition of cetuximab to a modified FOLFOXIRI (mFOLFOXIRI: 5‐fluorouracil/folinic acid, oxaliplatin, irinotecan) as conversion therapy in a two‐group, nonrandomized, multicenter, phase II trial in patients with initially technically unresectable colorectal liver‐limited metastases (CLM) and BRAF/RAS wild‐type.
Patients and Methods
Patients were enrolled to receive cetuximab (500 mg/m2) plus mFOLFOXIRI (oxaliplatin 85 mg/m2, irinotecan 165 mg/m2, folinic acid 400 mg/m2, 5‐fluorouracil 2,800 mg/m2 46‐hour infusion, every 2 weeks) (the cetuximab group) or the same regimen of mFOLFOXIRI alone (the control group), in a 2:1 ratio allocation. The primary endpoint was the rate of no evidence of disease (NED) achieved. Secondary endpoints included resection rate, objective response rate (ORR), survival, and safety.
Results
Between February 2014 and July 2019, 117 patients were registered for screening at six centers in China, and 101 of these were enrolled (67 cetuximab group, 34 control group). The rate of NED achieved was 70.1% in the cetuximab group and 41.2% in the control group (difference 29.0%; 95% confidence interval [CI], 9.1%–48.8%; p = .005). Patients in the cetuximab group had improved ORR (95.5% vs. 76.5%; difference 19.1%; 95% CI, 17.4%–36.4%; p = .010) compared with those in control group. Progression‐free survival and overall survival showed the trend to favor the cetuximab group. The incidence of grade 3 and 4 adverse events was similar in the two groups.
Conclusion
Addition of cetuximab to mFOLFOXIRI improved the rate of NED achieved. This combination could be an option of conversion regimen for molecularly selected patients with initially technically unresectable CLM.
Implications for Practice
This trial evaluated the addition of cetuximab to a modified FOLFOXIRI as conversion therapy in a phase II trial in patients with initially technically unresectable colorectal liver‐limited metastases and BRAF/RAS wild‐type. The rate of no evidence of disease achieved was 70.1% in the cetuximab plus modified FOLFOXIRI group and 41.2% in the modified FOLFOXIRI group. Objective response rates, overall survival, and progression‐free survival were improved in the cetuximab group when compared with the modified FOLFOXIRI group. Addition of cetuximab to modified FOLFOXIRI increased the rate of no evidence of disease achieved, and this combination could be an option of conversion regimen for molecularly selected patients with initially technically unresectable colorectal liver‐limited metastasis.
Keywords: Modified FOLFOXIRI, Cetuximab, Conversion therapy, Colorectal cancer, Liver metastasis
Short abstract
The FOCULM study was designed to evaluate whether cetuximab would improve the efficacy of a modified FOLFOXIRI regimen as conversion therapy in patients with RAS/BRAF wild‐type unresectable colorectal liver‐limited metastases. Results are reported here.
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer death worldwide, with an estimated 881,000 deaths in 2018 [1]. Approximately 20% of patients with CRC present with liver metastases (LMs) at the time of diagnosis, and approximately 50% of patients subsequently develop LMs during the disease course [2, 3]. Patients with colorectal liver‐limited metastases (CLM) represent an exceptional subgroup with regard to the possible benefits of potentially curative multidisciplinary strategies, which offer better 5‐year survival rates of 28%–39% than palliative chemotherapy alone [3, 4, 5, 6]. About 80% of these patients are considered unresectable because of the number, size, or location of LMs [3]. Nonetheless, because of the technological advancements and availability of effective chemotherapy regimens and local ablative treatment (LAT), including surgery, thermal ablation, stereotactic ablative body radiotherapy, and embolization techniques, an increasing number of patients with CLM, after conversion therapy, have the possibility of achieving complete removal of all tumor masses and no evidence of disease (NED) [3, 7, 8].
Evidence suggests that the addition of cetuximab to doublet regimens, that is, infused 5‐fluorouracil/folinic acid plus irinotecan (FOLFIRI) or oxaliplatin (FOLFOX), improve response, resectability of LMs, and survival in metastatic CRC [9]. Moreover, it is observed that a triplet chemotherapeutic regimen (FOLFOXIRI) yields higher response and resection rates compared with doublet regimens among patients with CLM [10, 11, 12]. However, studies comparing FOLFOXIRI plus cetuximab with FOLFOXIRI alone as conversion therapy in patients with RAS/BRAF wild‐type CLM are limited. To address this question, the FOCULM study was designed to evaluate whether cetuximab would improve the efficacy of a modified FOLFOXIRI (mFOLFOXIRI) regimen in this setting.
Materials and Methods
Study Design and Patients
FOCULM is a two‐to‐one, nonrandomized, multicenter, phase II study conducted at six centers in China. Eligible patients were aged 18 to 70 years and had Eastern Cooperative Oncology Group performance status score of 0 to 1, histologically confirmed adenocarcinoma of the colon or rectum, presence of LMs that was deemed initially technically unresectable with curative intent by a local multidisciplinary team (MDT), RAS (codons 12, 13, 59, 61, 117, and 146 of KRAS and NRAS) and BRAF (Val600Glu) wild‐type by local assessment of the primary tumor or LMs, no previous chemotherapy for LMs, measurable disease according to RECIST, version 1.1, and adequate functioning of the bone marrow, hepatic, and renal. Main exclusion criteria were (neo)adjuvant treatment completed less than 12 months before enrolment, any extrahepatic metastatic disease, and primary tumors was not deemed resectable with clear surgical margins.
The study was done in accordance with the Declaration of Helsinki and adhered to Good Clinical Practice guidelines. The protocol was approved by the local ethics committees of participating sites. The trial was registered with ClinicalTrials.gov, NCT02063529.
Treatment Procedures
The treatment grouping of either the cetuximab group or the control group was based on the enrolled patients’ ability to afford cetuximab. Patients in the cetuximab group received cetuximab (500 mg/m2 on day 1) plus mFOLFOXIRI (oxaliplatin 85 mg/m2, irinotecan 165 mg/m2, and folinic acid 400 mg/m2 followed by 5‐fluorouracil 2,800 mg/m2 as a 46‐hour continuous infusion on day 1). In the control group, patients received the same regimen of mFOLFOXIRI alone. Treatment in both groups was repeated every 2 weeks until disease progression or unacceptable toxicity or resectability or up to a maximum of 12 cycles. Patients who achieved NED were continued on treatment with the same dose used at the last cycle of the conversion therapy for an overall duration of 6 months (12 cycles), and at least one cytotoxic agent could be discontinued if grade 3 or more adverse event (AE) occurred. Patients with disease progression or failed conversion were treated at the discretion of the investigators. Prophylactic use of granulocyte colony‐stimulating factor was permitted. Surgical resection or tumor ablation (either alone or in combination with resection) was only performed with curative intent. Thermal ablation was allowed for LMs with number of lesions ≤5 and largest lesion size <5 cm. A two‐stage approach for LMs to achieve NED was permitted.
Study Endpoints and Definitions
The primary endpoint was the rate of NED achieved. Secondary endpoints were R0 resection rate, macroscopically complete resection rate, overall resection rate, objective response rate (ORR), depth of response (DpR), progression‐free survival (PFS), overall survival (OS), and safety.
The rate of NED achieved was defined as the proportion of patients with achieving R0 resection, complete remission, or macroscopically complete ablation of all known tumor masses. ORR was defined as the proportion of patients with complete response or partial response as per RECIST version 1.1. OS was defined as time from first study treatment to death from any cause. PFS was defined as time from first study treatment to the first documented progression disease or death, whichever occurred first. DpR was defined as maximum percentage change in tumor size compared with baseline. Nonresectability of LMs was defined as one of the following criteria assessed by a local MDT: no possible upfront macroscopically complete resection of all LM lesions; <30% estimated remnant liver volume after resection; and LMs infiltrated all hepatic liver veins, both hepatic arteries, or both portal vein branches.
Assessments
Tumor response by means of chest and abdominopelvic computed tomography (CT) or magnetic resonance imaging (MRI) of the liver with the same method as baseline was performed every four cycles and was assessed according to RECIST (version 1.1). Surgical resection margin status was defined as R0 for clear resection margin, R1 for microscopic residual tumor less than 1 mm from resection margin, and R2 for macroscopic residual tumor [13]. Complete thermal ablation was defined as ablation zone completely overlapped or encompassed target tumor plus an ablative margin, and entire ablated area presented no enhancement assessed by three‐phase contrast‐enhanced CT or MRI of the liver at the 4‐week follow‐up after ablation [14].
Each of the CT and MRI scans was presented by an independent radiologist to all participating MDT members including at least a colorectal surgeon, medical oncologist, and at least two hepatic surgeons and radiologists, all blinded to the patient's treatment group and clinical outcome data. The patient would be considered resectable if 50% or more of the hepatic surgeons voted for resectability. The determination of NED achieved and radiological tumor response also required more than 50% of the radiologists to vote.
Statistical Analysis
The rate of NED achieved in the control group was assumed to be 25%. In order to demonstrate a relevant increase of 30% in the cetuximab group (up to 55%), a total of 92 patients (61 in the cetuximab group and 31 in the control group) were required at a 2:1 ratio allocation, two‐sided, level α = .05, with a power of 80%.
Comparison of continuous variables was performed using the Wilcoxon test. Categorical variables were compared using the chi‐square test or Fisher's exact test. Cochran‐Mantel‐Haenszel and Mantel‐Haenszel methods were used to adjust imbalance baseline characteristics between two treatment groups. Distributions of time‐to‐event variables were estimated using the Kaplan‐Meier method and were compared by means of the log‐rank test and Cox proportional hazards regression model with hazard ratios (HRs) and 95% confidence intervals (CIs). Stratified Cox regression was used to adjust imbalance baseline characteristics between two treatment groups. All statistical tests were two‐sided, and p < .05 was considered to indicate statistical significance. Statistical analyses were performed using software SPSS version 22.0 (SPSS, Inc., Chicago, IL).
Results
Demographic Characteristics of the Study Population
From February 1, 2014, to July 31, 2019, a total of 117 patients at six sites in China were registered for screening. Of these, 16 patients were excluded for not meeting criteria or withdrawal of consent (Fig. 1), Therefore, 101 patients were included in the primary efficacy analysis (67 in the cetuximab group, 34 in the control group). The demographic characteristics of the patients and the disease characteristics at baseline were generally well balanced between treatment groups (Table 1); however, more patients in the cetuximab group than those in the control group presented five or more LM lesions (86.6% vs. 52.9%, p < .001) and were evaluated as unresectable because of no possible upfront complete resection of hepatic lesions (89.6% vs. 73.5%, p = .037).
Figure 1.

Trial profile.
Table 1.
Demographic and clinicopathological characteristics at baseline
| Characteristic | Patients, n (%) | p value | |
|---|---|---|---|
| mFOLFOXIRI plus cetuximab (n = 67) | mFOLFOXIRI (n = 34) | ||
| Age, years | |||
| Median | 52 | 55 | .122 |
| Range | 28–70 | 29–70 | |
| ≥65 | 6 (9.0) | 4 (11.8) | .655 |
| Sex | .861 | ||
| Male | 58 (86.6) | 29 (85.3) | |
| Female | 9 (13.4) | 5 (14.7) | |
| ECOG performance status at baseline | .473 | ||
| 0 | 51 (76.1) | 28 (82.4) | |
| 1 | 16 (23.9) | 6 (17.6) | |
| Primary tumor site | .074 | ||
| Left (splenic flexure, descending colon, sigmoid colon, and rectum) | 66 (98.5) | 31 (91.2) | |
| Right (cecum, ascending colon, hepatic flexure, and transverse colon) | 1 (1.5) | 3 (8.8) | |
| Primary tumor in situ at baseline | .081 | ||
| Yes | 49 (73.1) | 19 (55.9) | |
| No | 18 (26.9) | 15 (44.1) | |
| Time to metastases | .433 | ||
| Synchronous (metastases present at diagnosis of primary tumor) | 59 (88.1) | 28 (82.4) | |
| Metachronous (metastasis subsequent to diagnosis of primary tumor) | 8 (11.9) | 6 (17.6) | |
| Reasons for unresectability | |||
| No upfront completely resection of hepatic lesions possible | 60 (89.6) | 25 (73.5) | .037 |
| Less than 30% estimated residual liver after resection | 22 (32.8) | 8 (23.5) | .333 |
| Disease in contact with major vessels of remnant liver | 27 (40.3) | 11 (32.4) | .436 |
| Maximum size of liver metastases, cm | .292 | ||
| ≥5 | 31 (46.3) | 12 (35.3) | |
| <5 | 36 (53.7) | 22 (64.7) | |
| Number of liver metastases | <.001 | ||
| ≥5 | 58 (86.6) | 18 (52.9) | |
| <5 | 9 (13.4) | 16 (47.1) | |
| Involved portal or hepatic vein | .078 | ||
| Yes | 40 (59.7) | 14 (41.2) | |
| No | 27 (40.3) | 20 (58.8) | |
| Involved inferior vena cava | .308 | ||
| Yes | 4 (6.0) | 4 (11.8) | |
| No | 63 (94.0) | 30 (88.2) | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; mFOLFOXIRI, modified FOLFOXIRI.
The data cutoff for the current analysis was December 31, 2019, with a median follow‐up time of 22.6 months. The median number of cycles of mFOLFOXIRI chemotherapeutic regimen administered was seven (range, 4–12) in the cetuximab group and six (range, 2–12) in the control group, and the relative dose intensity of chemotherapy drugs were slightly higher in the control group (supplemental online Table 1). Reported second‐line drug therapies are summarized in supplemental online Table 2. Sixty‐four of 68 patients (the cetuximab group, n = 45; the control group, n = 19) with primary tumor in situ had primary resection surgery during the study.
Efficacy Outcomes
NED was achieved in 61 of 101 patients (clinical complete response, n = 4; R0 resection, n = 31; R0 resection plus thermal ablation, n = 8; complete thermal ablation, n = 18): 47 (70.1%; 95% CI, 58.3%–81.9%) of 67 in the cetuximab group compared with 14 (41.2%; 95% CI, 25.5%–56.9%) of 34 in the control group (difference 29.0%; 95% CI, 9.1%–48.8%; p = .005). The median duration of NED achieved was 13.9 (95% CI, 6.5–21.3) months in the cetuximab group and 10.7 (95% CI, 0.6–20.8) months in the control group (p = .944). The overall surgical resection for LMs was done in 37 (55.2%; 95% CI, 43.3%–67.0%) patients in the cetuximab group and 10 (29.4%; 95% CI, 13.7%–45.1%) in the control group (p = .014). Twenty‐four (35.8%) and 7 (10.4%) patients in the cetuximab group and 7 (20.6%) and 1 (2.9%) patients in the control group achieved a R0 resection and R0 resection combined with thermal ablation, respectively. Additionally, macroscopically complete resection rates were significantly higher in the cetuximab group than in the control group (46.3%; 95% CI, 34.5%–58.1% vs. 23.5%; 95% CI, 9.8%–37.2%; p = .027). Furthermore, 20 patients received thermal ablation alone for LMs (eight patients were recommended ablation as the optimal strategy by the MDT, eight patients refused surgery, and four patients had severe comorbidity unfit for surgery), and 18 of 20 patients had complete thermal ablation (Table 2).
Table 2.
Efficacy outcomes
| Variable | mFOLFOXIRI plus cetuximab (n = 67) | mFOLFOXIRI (n = 34) | p value |
|---|---|---|---|
| NED achieved, n (%) | 47 (70.1) | 14 (41.2) | .005 |
| Overall resection, n (%) | 37 (55.2) | 10 (29.4) | .014 |
| R0 resection | 24 (35.8) | 7 (20.6) | .117 |
| R0 resection plus complete thermal ablation | 7 (10.4) | 1 (2.9) | .187 |
| Macroscopically complete resection | 31 (46.3) | 8 (23.5) | .027 |
| R2 resection | 6 (9.0) | 2 (5.9) | .589 |
| Thermal ablation alone, n (%) | 14 (20.9) | 6 (17.6) | .699 |
| Complete thermal ablation | 13 (19.4) | 5 (14.7) | .560 |
| ORR, n (%) | 64 (95.5) | 26 (76.5) | .010 |
| CR | 3 (4.5) | 1 (3.0) | |
| PR | 61 (91.0) | 25 (73.5) | |
| SD | 3 (4.5) | 6 (17.6) | |
| PD | 0 (0.0) | 2 (5.9) | |
| DpR, median (IQR), % | 56.1 (48.4–65.2) | 44.0 (31.7–65.2) | .012 |
| PFS, median (95% CI), months | 15.5 (13.2–17.8) | 14.2 (11.0–17.3) |
.248 .031 a |
| OS, median (95% CI), months | Not reached | 33.2 (8.2–58.2) |
.056 .020 a |
p values were adjusted for the proportion of patients with five or more liver metastases and the proportion of patients without complete upfront resection of liver metastases.
Abbreviations: CI, confidence interval; CR, complete response; DpR, depth of response; IQR, interquartile range; mFOLFOXIRI, modified FOLFOXIRI; NED, no evidence of disease; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
The ORR was higher in the cetuximab group than in the control group (95.5%; 95% CI, 89.6%–100% vs. 76.5%; 95% CI, 62.8%–90.2%; difference 19.1%; 95% CI, 17.4%–36.4%; p = .010). Median DpR was also significantly greater in the cetuximab group (56.1%; interquartile range [IQR], 48.4%–65.2% vs. 44.0%; IQR, 31.7%–65.2%; p = .012) (Fig. 2).
Figure 2.

Change in tumor size assessed by RECIST version 1.1.
Median PFS was 15.5 (95% CI, 13.2–17.8) months in the cetuximab group and 14.2 (95% CI, 11.0–17.3) months in the control group (unadjusted HR, 0.74; 95% CI, 0.45–1.23; p = .248; adjusted HR, 0.54; 95% CI, 0.30–0.95; p = .031) (Fig. 3A). Eight patients in the cetuximab group and 13 in the control group died. Median OS was not reached in the cetuximab group, and it was 33.2 (95% CI, 8.2–58.2) months in the control group (unadjusted p = .056; adjusted p = .020) (Fig. 3B). Patients who achieved NED had a significantly longer OS compared with those who did not achieve NED, both in the cetuximab group (p = .010) and the control group (p = .005) (Fig. 3C, D).
Figure 3.
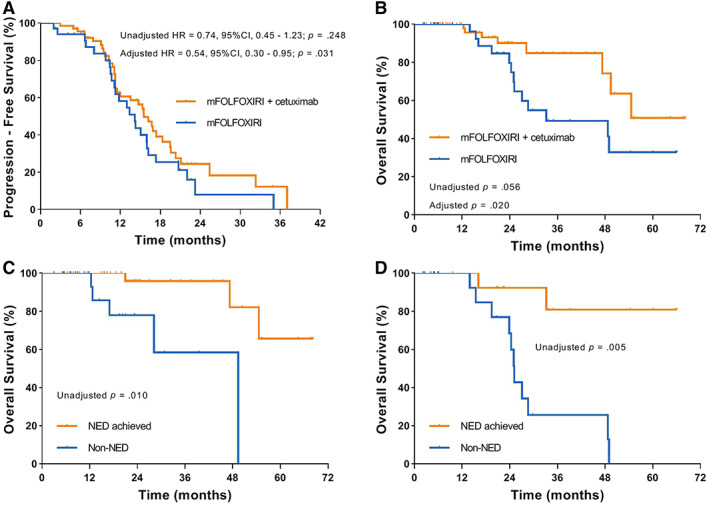
Kaplan‐Meier estimates of survival. (A): Progression‐free survival in all patients. (B): Overall survival in all patients. (C): Overall survival according to NED achieved status in the cetuximab group. (D): Overall survival according to NED achieved status in the control group.
Abbreviations: CI, confidence interval; HR, hazard ratio; NED, no evidence of disease.
Safety Outcomes
Most common AEs were summarized in Table 3. Any‐grade acneiform exanthema/rash was significantly higher in the cetuximab group compared with the control group (55.2% vs. 5.9%, p < .001). The incidence of grade 3 or 4 AEs was similar between treatment groups, and the most frequently occurring grade 3 or 4 AEs were neutropenia (the cetuximab group: 31.3%; the control group: 41.2%; p = .326), diarrhea (the cetuximab group: 7.5%; the control group: 5.9%; p = .768), and increased alanine aminotransferase or aspartate aminotransferase (the cetuximab group: 7.5%; the control group: 5.9%; p = .768). One patient in each group discontinued the treatment because of intestinal obstructions. There was no grade 5 AE in either group. Surgical morbidity is summarized in supplemental online Table 3. The overall morbidity rates were 21.6% and 30.0% in the cetuximab group and the control group, respectively. There was no perioperative death.
Table 3.
Most common adverse events (maximum grade per patient per event)
| Adverse event | Any grade | Grade 3 or 4 | ||||
|---|---|---|---|---|---|---|
| mFOLFOXIRI plus cetuximab (n = 67), n (%) | mFOLFOXIRI (n = 34), n (%) | p value | mFOLFOXIRI plus cetuximab (n = 67), n (%) | mFOLFOXIRI (n = 34), n (%) | p value | |
| Neutropenia | 63 (94.0) | 29 (85.3) | .145 | 21 (31.3) | 14 (41.2) | .326 |
| Diarrhea | 16 (23.9) | 10 (29.4) | .548 | 5 (7.5) | 2 (5.9) | .768 |
| ALT/AST increased | 55 (82.1) | 23 (67.6) | .102 | 5 (7.5) | 2 (5.9) | .768 |
| Peripheral neuropathy | 32 (47.8) | 17 (50.0) | .832 | 3 (4.5) | 2 (5.9) | .859 a |
| Febrile neutropenia | 2 (3.0) | 1 (2.9) | .543 a | 2 (3.0) | 1 (2.9) | .543 a |
| Fatigue | 45 (67.2) | 23 (67.6) | .961 | 1 (1.5) | 1 (2.9) | .793 a |
| Vomiting | 16 (23.9) | 6 (17.6) | .473 | 1 (1.5) | 1 (2.9) | .793 a |
| Intestinal obstructions | 4 (6.0) | 2 (5.9) | .669 a | 1 (1.5) | 1 (2.9) | .793 a |
| Thrombocytopenia | 15 (22.4) | 10 (29.4) | .440 | 1 (1.5) | 0 (0.0) | .728 a |
| Stomatitis | 12 (17.9) | 4 (11.8) | .424 | 1 (1.5) | 0 (0.0) | .728 a |
| Acneiform exanthema/rash | 37 (55.2) | 2 (5.9) | <.001 | 0 (0.0) | 0 (0.0) | — |
Chi‐squared test with continuity correction.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; mFOLFOXIRI, modified FOLFOXIRI.
Discussion
This multicenter phase II study revealed that the combination of cetuximab and mFOLFOXIRI significantly improved the rate of NED achieved in patients with initially technically unresectable CLM and BRAF/RAS wild‐type compared with mFOLFOXIRI alone (70.1% vs. 41.2%). Additionally, our data suggested the NED status can translate into longer OS, and systemic therapy intensification is associated with high conversion rate. To our knowledge, this is the first multicenter, prospective phase II trial to investigate the efficacy of cetuximab plus mFOLFOXIRI as conversion therapy in patients with RAS/BRAF wild‐type CLM.
For patients with CLM, there is a clear need for an effective conversion therapy that yields high tumor response rates leading to secondary resection [15, 16]. However, there is no standardized criteria for resectability, and the decision to resect LMs is probably driven by multiple factors including prognostic considerations, risks of resection, patient's physical condition, and patient preferences. LATs are a reasonable therapeutic option for nonsurgical candidates and associated with a significant improvement in OS [7, 8]. The international treatment guidelines for metastatic CRC recommend that a new treatment goal for patients with CLM is to maximize the possibility of eradicating all visible LM lesions using the best instrument from the toolbox of LATs [17, 18]. Therefore, the rate of NED achieved might be an optimal endpoint to assess the efficacy of conversion therapy in patients with CLM.
Several studies have reported the advantage of targeted therapy in achieving higher secondary resection rate in patients with initially technically unresectable CLM. Addition of cetuximab to mFOLFOX6/FOLFIRI yielded a higher R0 resection rate, response rate, and survival rate compared with chemotherapy alone in patients with CLM metastatic CRC (25% vs. 7%) in the BELIFE trial [9]. Similarly, in the CELIM study, cetuximab plus FOLFOX/FOLFIRI resulted in a R0 resection rate of 28% in the subgroup of patients with technically unresectable LMs [19]. In our trial, only patients with technically unresectable CLM were included, and addition of cetuximab to mFOLFOXIRI resulted in an increased R0 resection rate from 20.6% to 35.8% as well as a high rate of NED achieved from 41.2% to 70.1%. This could plausibly be attributed to more effective triplet‐drug chemotherapy and inclusion of patients with RAS/BRAF wild‐type that contributed to a higher resection rate compared with those reported in BELIFE and CELIM studies [9, 19].
Chemotherapeutic triplet‐drug regimen and doublet‐drug combined with anti‐EGFR antibody are both widely adopted treatments for metastatic CRC with RAS/BRAF wild‐type. However, no study of FOLFOXIRI compared with FOLFOX/FOLFIRI plus cetuximab or panitumumab as conversion therapy for patients with CLM and RAS/BRAF wild‐type has been reported. A single‐arm, phase II study by Ychou et al. showed that FOLFIRINOX led to an ORR of 71% and enabled 27% of patients with technically unresectable CLM to undergo liver R0 resection [20]. In this FOCULM study, the ORR of 76.5% and R0 resection rate of 20.6% in the control group with mFOLFOXIRI alone were observed. The short‐term efficacy of chemotherapeutic triplet‐drug regimen seems to be numerically comparable to FOLFOX/FOLFIRI plus cetuximab with ORRs from 57% to 73% and R0 resection rates from 26% to 28% (supplemental online Table 4) [9, 19, 21]. Future studies are required to compare these two treatment strategies head‐to‐head in this setting.
Additionally, the therapeutic regimens and efficacy outcomes in our study are similar to those of the recently reported VOLFI trial, which enrolled 99 patients with RAS wild‐type metastatic colorectal cancer but compared panitumumab in combination with mFOLFOXIRI with FOLFOXIRI as the first‐line therapy. The primary endpoint of ORR was significantly higher in the panitumumab group than in the control group (87% vs. 60%) [22]. In our study, the ORR in cetuximab plus mFOLFOXIRI was 95.5%, which is comparable to that reported in patients with a chance of secondary resection in the VOLFI study. In addition, our study indicated possibly improved median PFS after adding cetuximab to mFOLFOXIRI, yet the VOLFI study demonstrated no apparent difference in PFS. Our finding concurs with the BELIFE trial investigating cetuximab plus doublet‐drug in patients with CLM, with respect to an increase in the rate of LATs for LMs [9].
In our study, 8 and 18 patients underwent R0 resection plus ablation and complete ablation, respectively. Actually, ablation resulted in adequate local control of LM lesions, and the local recurrence rate does not appear to differ greatly between the sites treated with ablation (2/18) and surgical resection (3/39) (supplemental online Table 5). These results are concurrent with the findings from a pooled analysis of two randomized controlled trials of EORTC 40983 and CLOCC 40004 [23]. It may be argued that thermal ablation is relatively inferior to resection in terms of local recurrence rate and 5‐year OS. We set out a clear definition of complete ablation and the ablation indications for LMs in this study, according to the Image‐Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria, in an attempt to improve the local control rate [19]. Furthermore, ablations allow a wider indication for further LATs of recurrent disease and better treatment compliance of patient. The CLOCC 40004 trial has indicated that patients receiving ablation in addition to chemotherapy have a long‐term survival benefit compared with those treated by chemotherapy only [7]. Our data confirmed the significant difference in survival outcome observed for patients with CLM who underwent complete ablation versus without any LATs (supplemental online Fig. 1).
There was a higher incidence of all grade acneiform exanthema/rash in the cetuximab group than the control group, whereas no grade 3 or 4 event was observed in both treatment groups. The cetuximab in combination with a mFOLFOXIRI regimen had a manageable safety profile, as demonstrated by the occurrence of only the expected toxicities for each agent and no unexpected interactions. The main concerned AEs of the triplet‐drug regimen were severe neutropenia and diarrhea. Our findings showed that grade 3 or 4 neutropenia and diarrhea incidence (31.3% and 7.5% in the cetuximab group, 41.2% and 5.9% in the control group) was relatively lower than that reported in previous studies exploring FOLFOXIRI with or without monoclonal antibody in metastatic CRC by the Gruppo Oncologico Nord Ovest (GONO) (neutropenia, 49%–50%; diarrhea, 14%–35%) [24, 25, 26, 27]. It is important to note that a modified FOLFOXIRI with reduction in 5‐fluorouracil dose to 2,800 mg/m2 46‐hour infusion was used in the FOCULM study. In addition, our study sets the upper age limit of the enrolled patients to 70 years, so the included patients are relatively young, which may also be a potential reason for the better tolerance of the triplet‐drug chemotherapy. This mFOLFOXIRI regimen was feasible and resulted in favorable outcomes, leading us to recommend it in clinical practice, especially for younger patients.
The main limitation of the FOCULM trial is that the treatment grouping is according to enrolled patients’ choice: most cases are based on the patient's financial status. Thus, some unrecognized biases might contribute to the differences reported between the two treatment groups. Patients’ baseline characteristics were generally balanced, except for two variables; namely, fewer patients with five or more LMs, and fewer patients with unresectability because of impossibility to completely resect all LMs, might have favored the control group. After adjustment for the above two covariates, the cetuximab group was also significantly associated with the improved clinical outcomes compared with the control group. Although no patient in either group refused local treatment for LMs because of financial burden, the proportion of patients in the control group using anti–vascular endothelial growth factor or anti‐EGFR agent–based regimen as the second‐line treatment was relatively low (supplemental online Table 2). Other limitations should be acknowledged: there was no standardized approach or training across centers for the determination of resectability and no independent central review of the radiologic images. Moreover, the follow‐up time was short. The comparisons of long‐term outcomes should be interpreted with caution.
Conclusion
Our findings show that the addition of cetuximab to mFOLFOXIRI improved the rate of NED achieved. This effective conversion regimen provides an option of treatment for molecularly selected patients with initially technically unresectable CLM.
Author Contributions
Huabin Hu, Kun Wang, Meijin Huang, Liang Kang, Wei Wang, Hui Wang, Meng Qiu, Rongbo Lin, Haibo Zhang, Ping Lan, Xiaojian Wu, Guangjian Liu, Yunle Wan, Ming Liu, Zhiyang Zhou, Yan Huang, Fangqian Li, Jianwei Zhang, Yue Cai, Tenghui Ma, Jiaming Zhou, Huaiming Wang, Jiayu Ling, Yonghua Cai, Zehua Wu, Shuangling Luo, Li Ling, Yanhong Deng
Conception/design: Huabin Hu, Li Ling, Yanhong Deng
Provision of study material or patients: Kun Wang, Meijin Huang, Liang Kang, Wei Wang, Hui Wang, Meng Qiu, Rongbo Lin, Haibo Zhang, Ping Lan, Xiaojian Wu, Guangjian Liu, Yunle Wan, Tenghui Ma, Jiaming Zhou, Huaiming Wang, Yonghua Cai, Shuangling Luo, Yanhong Deng
Collection and/or assembly of data: Huabin Hu, Ming Liu, Zhiyang Zhou, Yan Huang, Jianwei Zhang, Yue Cai, Jiayu Ling, Zehua Wu
Data analysis and interpretation: Huabin Hu, Kun Wang, Wei Wang, Meng Qiu, Rongbo Lin, Haibo Zhang, Zhiyang Zhou, Fangqian Li, Li Ling, Yanhong Deng
Manuscript writing: Huabin Hu, Kun Wang, Meijin Huang, Liang Kang, Wei Wang, Hui Wang, Meng Qiu, Rongbo Lin, Haibo Zhang, Ping Lan, Xiaojian Wu, Guangjian Liu, Yunle Wan, Ming Liu, Zhiyang Zhou, Yan Huang, Fangqian Li, Jianwei Zhang, Yue Cai, Tenghui Ma, Jiaming Zhou, Huaiming Wang, Jiayu Ling, Yonghua Cai, Zehua Wu, Shuangling Luo, Li Ling, Yanhong Deng
Final approval of manuscript: Huabin Hu, Kun Wang, Meijin Huang, Liang Kang, Wei Wang, Hui Wang, Meng Qiu, Rongbo Lin, Haibo Zhang, Ping Lan, Xiaojian Wu, Guangjian Liu, Yunle Wan, Ming Liu, Zhiyang Zhou, Yan Huang, Fangqian Li, Jianwei Zhang, Yue Cai, Tenghui Ma, Jiaming Zhou, Huaiming Wang, Jiayu Ling, Yonghua Cai, Zehua Wu, Shuangling Luo, Li Ling, Yanhong Deng
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Table S1 The exposure of mFOLFOXIRI regimen in the cetuximab group and the control group.
Table S2. Second‐Line drug therapy
Table S3. Surgical mortality and morbidity.
Table S4. Prospective trials investigating chemotherapy in patients with initially unresectable or unsuitable for resection liver metastases colorectal cancer.
Table S5. Site of first progression in patients who received local and ablative treatment and achieved no evidence of disease
Supplementary Figure S1 Kaplan‐Meier overall survival curves by local and ablative treatments.
Acknowledgments
The study was supported by National Natural Science Foundation of China (81974369), National Science and Technology Major Project of China for “Major New Drugs Innovation and Development” (2018ZX09734003), and CSCO‐Merck Serono Oncology Research Fund (Y‐MX2015‐005). We thank all participating patients and their families. We were supported by Dr. Anuradha Nalli, Ph.D., and Dr. Amit Bhat, Ph.D., Indegene, Bangalore, for providing the necessary writing assistance and editorial support during the development of the manuscript.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Siriwardena AK, Mason JM, Mullamitha S et al. Management of colorectal cancer presenting with synchronous liver metastases. Nat Rev Clin Oncol 2014;11:446–459. [DOI] [PubMed] [Google Scholar]
- 3. Adam R, Delvart V, Pascal G et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long‐term survival. Ann Surg 2004;240:644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fong Y, Fortner J, Sun RL et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg 1999;230:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nordlinger B, Guiguet M, Vaillant JC et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254–1262. [PubMed] [Google Scholar]
- 6. Symonds LK, Cohen SA. Use of perioperative chemotherapy in colorectal cancer metastatic to the liver. Gastroenterol Rep (Oxf) 2019;7:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruers T, Van Coevorden F, Punt CJ et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II trial. J Natl Cancer Inst 2017;109:djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rusthoven KE, Kavanagh BD, Cardenes H et al. Multi‐institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572–1578. [DOI] [PubMed] [Google Scholar]
- 9. Ye LC, Liu TS, Ren L et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild‐type unresectable colorectal liver‐limited metastases. J Clin Oncol 2013;31:1931–1938. [DOI] [PubMed] [Google Scholar]
- 10. Gruenberger T, Bridgewater J, Chau I et al. Bevacizumab plus mFOLFOX‐6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: The OLIVIA multinational randomised phase II trial. Ann Oncol 2015;26:702–708. [DOI] [PubMed] [Google Scholar]
- 11. Tomasello G, Petrelli F, Ghidini M et al. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: A systematic review and pooled analysis. JAMA Oncol 2017;3:e170278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ychou M, Rivoire M, Thezenas S et al. A randomized phase II trial of three intensified chemotherapy regimens in first‐line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann Surg Oncol 2013;20:4289–4297. [DOI] [PubMed] [Google Scholar]
- 13. Pawlik TM, Scoggins CR, Zorzi D et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmed M, Solbiati L, Brace CL et al. Image‐guided tumor ablation: standardization of terminology and reporting criteria‐‐a 10‐year update. Radiology 2014;273:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones RP, Hamann S, Malik HZ et al. Defined criteria for resectability improves rates of secondary resection after systemic therapy for liver limited metastatic colorectal cancer. Eur J Cancer 2014;50:1590–1601. [DOI] [PubMed] [Google Scholar]
- 16. Folprecht G, Grothey A, Alberts S et al. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann Oncol 2005;16:1311–1319. [DOI] [PubMed] [Google Scholar]
- 17. Adam R, Haller DG, Poston G et al. Toward optimized front‐line therapeutic strategies in patients with metastatic colorectal cancer‐‐an expert review from the International Congress on Anti‐Cancer Treatment (ICACT) 2009. Ann Oncol 2010;21:1579–1584. [DOI] [PubMed] [Google Scholar]
- 18. Yoshino T, Arnold D, Taniguchi H et al. Pan‐Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO‐ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018;29:44–70. [DOI] [PubMed] [Google Scholar]
- 19. Folprecht G, Gruenberger T, Bechstein WO et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38–47. [DOI] [PubMed] [Google Scholar]
- 20. Ychou M, Viret F, Kramar A et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): A phase II study in colorectal cancer patients with non‐resectable liver metastases. Cancer Chemother Pharmacol 2008;62:195–201. [DOI] [PubMed] [Google Scholar]
- 21. Ji JH, Park SH, Lee J et al. Prospective phase II study of neoadjuvant FOLFOX6 plus cetuximab in patients with colorectal cancer and unresectable liver‐only metastasis. Cancer Chemother Pharmacol 2013;72:223–230. [DOI] [PubMed] [Google Scholar]
- 22. Modest DP, Martens UM, Riera‐Knorrenschild J et al. FOLFOXIRI plus panitumumab as first‐line treatment of RAS wild‐type metastatic colorectal cancer: The randomized, open‐label, phase II VOLFI study (AIO KRK0109). J Clin Oncol 2019;37:3401–3411. [DOI] [PubMed] [Google Scholar]
- 23. Tanis E, Nordlinger B, Mauer M et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer 2014;50:912–919. [DOI] [PubMed] [Google Scholar]
- 24. Falcone A, Ricci S, Brunetti I et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first‐line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670–1676. [DOI] [PubMed] [Google Scholar]
- 25. Masi G, Loupakis F, Salvatore L et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first‐line treatment for metastatic colorectal cancer: A phase 2 trial. Lancet Oncol 2010;11:845–852. [DOI] [PubMed] [Google Scholar]
- 26. Loupakis F, Cremolini C, Masi G et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–1618. [DOI] [PubMed] [Google Scholar]
- 27. Fornaro L, Lonardi S, Masi G et al. FOLFOXIRI in combination with panitumumab as first‐line treatment in quadruple wild‐type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: A phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol 2013;24:2062–2067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Table S1 The exposure of mFOLFOXIRI regimen in the cetuximab group and the control group.
Table S2. Second‐Line drug therapy
Table S3. Surgical mortality and morbidity.
Table S4. Prospective trials investigating chemotherapy in patients with initially unresectable or unsuitable for resection liver metastases colorectal cancer.
Table S5. Site of first progression in patients who received local and ablative treatment and achieved no evidence of disease
Supplementary Figure S1 Kaplan‐Meier overall survival curves by local and ablative treatments.


