Abstract
Background
The benefits of neoadjuvant therapy for patients with locally advanced gastric cancer (GC) are increasingly recognized. The 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual first proposed ypTNM staging, but its accuracy is controversial. This study aims to develop a modified ypTNM staging.
Patients and Methods
Clinicopathological data of 1,791 patients who underwent curative‐intent gastrectomy after neoadjuvant therapy in the Surveillance, Epidemiology, and End Results database, as the development cohort, were retrospectively analyzed. Modified ypTNM staging was established based on overall survival (OS). We compared the prognostic performance of the AJCC 8th edition ypTNM staging and the modified staging for patients after neoadjuvant therapy.
Results
In the development cohort, the 5‐year OS for AJCC stages I, II, and III was 58.8%, 39.1%, and 21.6%, respectively, compared with 69.9%, 54.4%, 34.4%, 24.1%, and 13.6% for modified ypTNM stages IA, IB, II, IIIA, and IIIB. The modified staging had better discriminatory ability (C‐index: 0.620 vs. 0.589, p < .001), predictive homogeneity (likelihood ratio chi‐square: 140.71 vs. 218.66, p < .001), predictive accuracy (mean difference in Bayesian information criterion: 64.94; net reclassification index: 35.54%; integrated discrimination improvement index: 0.032; all p < .001), and model stability (time‐dependent receiver operating characteristics curves) over AJCC. Decision curve analysis showed that the modified staging achieved a better net benefit than AJCC. In external validation (n = 266), the modified ypTNM staging had superior prognostic predictive power (all p < .05).
Conclusion
We have developed and validated a modified ypTNM staging through multicenter data that is superior to the AJCC 8th edition ypTNM staging, allowing more accurate assessment of the prognosis of patients with GC after neoadjuvant therapy.
Implications for Practice
The 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual first proposed ypTNM staging, but its accuracy is controversial. Based on multi‐institutional data, this study developed a modified ypTNM staging, which is superior to the AJCC 8th edition ypTNM staging, allowing more accurate assessment of the prognosis of patients with gastric cancer after neoadjuvant therapy.
Keywords: Gastric cancer, ypTNM staging, Neoadjuvant therapy, Modified, Validation
Short abstract
The 8th edition of the AJCC Staging Manual first proposed ypTNM staging for gastric cancer, but its accuracy is controversial. Modified ypTNM staging is needed. This article reports a modified staging system that allows for a more accurate assessment of the prognosis of gastric cancer patients after neoadjuvant therapy.
Introduction
Globally, gastric cancer (GC) is the fifth most frequently diagnosed cancer and the third leading cause of cancer‐related deaths [1]. GC is often diagnosed at an advanced stage in China, Europe, and the U.S. [2, 3]. Despite the development of radical surgery techniques and perioperative chemotherapy, the survival of patients with advanced GC is still poor. The 5‐year overall survival (OS) is mostly less than 50% [4]. In recent years, surgeons in the East and West have gradually realized that patients with locally advanced GC can benefit from preoperative (neoadjuvant) treatment, and more and more patients receive neoadjuvant therapy [5, 6, 7]. How to effectively stratify the prognosis of these patients has become a hot topic of current research. Because there are no staging criteria specifically for patients who undergo surgical resection and are given neoadjuvant therapy before surgery, prognostic evaluation of such patients has used the American Joint Committee on Cancer (AJCC) pTNM staging system in the past [8], but this application has not been validated and has neglected the possible downstage effects of neoadjuvant therapy. The 8th edition of the AJCC manual, released in 2017, proposed the post‐neoadjuvant treatment staging (ypTNM) system for the first time [9], filling the gap in clinical application.
The 8th edition of the AJCC manual first described the staging system for patients who receive neoadjuvant therapy, which is undoubtedly a major advancement in precision therapy and lays a solid foundation for patient evaluation after neoadjuvant treatment. However, although it meets the clinical needs to a certain extent, the current staging only divides patients with nonmetastatic GC after neoadjuvant therapy into three stages, stage I, stage II, and stage III, and it does not make a more detailed distinction, which is also the limitation of the ypTNM staging mentioned in the manual. In addition, a limited number of patients were available for this analysis (n = 683), with a median follow‐up of only 23 months [10]. It is urgent to find a larger sample of patients with longer follow‐up times to verify and recalibrate the ypTNM staging. Neoadjuvant therapy often affects the status of the primary tumor and lymph nodes; however, comparing the 8th edition of the AJCC ypTNM staging and the 7th edition of the AJCC pTNM staging (supplemental online Fig. 1), there was no difference in stages I–III between the two classifications. In other words, the AJCC 8th edition ypTNM staging is a simple integration of the AJCC 7th edition pTNM staging, which may not be very suitable for prognostic evaluation of patients after neoadjuvant therapy. Although mostly because of the lack of sufficient clinical data, a modified ypTNM staging system for patients with GC after neoadjuvant therapy is needed. Therefore, this study aims to establish a modified ypTNM staging through a large data sample from the U.S. and to validate the modified staging through data from China and Italy to accurately assess the prognosis of patients with GC after neoadjuvant therapy.
Materials and Methods
Population and Covariates
Data were obtained from the Surveillance, Epidemiology, and End Results (SEER) 18 Regs Research database (registration number 11994‐Nov2018), which covers approximately 27.8% of the U.S. population (based on the 2010 census) [11]. Because of changes in coding (specifically the AJCC staging) and the requirement of at least 1 year of follow‐up, data were extracted from the SEER database from 2004 to 2015. A value of 6 for the category “CS Lymph Nodes Eval” was used to select patients with GC who received neoadjuvant therapy before surgery in the SEER database, which meant the patient met criteria for AJCC y‐pathologic (yp) staging. All the cases were restaged according to the criteria described in the AJCC cancer staging manual (8th edition).
The inclusion criteria were defined as follows: the presence of primary GC; no combined malignancy, preoperative chemotherapy, or preoperative radiotherapy; no distant metastasis; and complete ypT category and ypN category information. Exclusion criteria were defined as follows: histology showing a tumor type other than adenocarcinoma and remnant GC. The selection scheme of the SEER database is shown in supplemental online Figure 2. The remaining 1,791 patients who underwent curative‐intent resection after neoadjuvant therapy were included as the development cohort in the present study.
Clinicopathological data were routinely collected. The tumor site was divided into four subsites: lower third (C16.3 and C16.4), upper third (C16.0 and C16.1), middle third (C16.2, C16.5, and C16.6), and overlapping (C16.8) [12]. The tumors were pathologically categorized as low grade (well and moderately differentiated), high grade (poorly differentiated and undifferentiated), or Gx (grade could not be evaluated). The histological types were categorized into general (8140–8389: adenomas and adenocarcinomas) and special (8440–8499: cystic, mucinous, and serous neoplasms). Tumor size was assessed on the basis of the largest diameter of resected specimens.
Multi‐institutional data from the following centers that satisfied the aforementioned inclusion criteria were included in the validation analysis: Fujian Medical University Union Hospital (FMUUH) from 2000 to 2015 in China (n = 111), Qinghai University Affiliated Hospital (QUAH) from 2012 to 2014 in China (n = 58), and the International Study Group on Minimally Invasive Surgery for GC (IMIGASTRIC) between 2000 and 2014 in Italy (n = 97). The institutional review boards of all the participating institutions approved the study.
In the validation cohort we studied, the regimen of neoadjuvant chemotherapy included the selective use of fluorouracil, oxaliplatin, paclitaxel, and other drugs when necessary. The efficacy of neoadjuvant therapy was evaluated every two cycles of treatment using enhanced computed tomography and ultrasound endoscopy, and the therapy was prematurely terminated in cases of disease progression. In all resectable cases, elective gastrectomies were scheduled for 2 to 4 weeks after neoadjuvant therapy. Fluorouracil‐based adjuvant chemotherapy was recommended for patients with advanced GC after surgery in the validation cohort.
The cause of death among the SEER cohorts was defined using the cause‐of‐death codes [13]. All patients from validation centers received standard postoperative follow‐up, including visits every 3 to 6 months for the first 2 years, every 6 to 12 months from the third to the fifth year, and once per year thereafter. All the patients were observed until death or the final follow‐up date of June 2019 in the validation cohort.
Statistical Analysis
Overall survival was defined as the time from surgery to death from any cause. Survival curves were estimated using the Kaplan‐Meier method, and the log‐rank test was used to determine significance. Variables associated with OS were selected using univariate and multivariate Cox regression models. To investigate which staging system was more suitable for prognostic assessment, a two‐step multivariate analysis was performed [14]. In step 1 of multivariate analysis, all significantly important prognostic factors in univariate analysis were considered, except for the modified ypTNM staging system. In step 2 of multivariate analysis, the modified staging system was also considered, together with the AJCC 8th edition staging system and other significantly important prognostic factors in univariate analysis. In addition, we employed a univariate Cox analysis at later time points (time‐dependent Cox analysis) to assess the prognostic system for survival among patients who were alive after a certain number of years [15].
The performance of a prognostic system has been shown to be related to discriminatory ability (greater differences in survival among patients in different stages within each system) and homogeneity (small differences in survival among patients in the same class within each system). Harrell's C‐index was used to measure the discriminatory ability of different staging systems [16, 17]. The likelihood ratio chi‐square score was calculated using Cox regression to measure homogeneity; a higher likelihood ratio chi‐square score indicates better homogeneity [18]. The Akaike information criterion (AIC) within the Cox regression model was used to compare performance between two staging systems; smaller AIC values represent more optimistic prognostic stratification [19]. We then calculated the relative likelihood of two models using the formula exp([AIC(model A) − AIC(model B)]/2) [20]. The relative likelihood can be interpreted as a p value for the comparison of both AIC values. The Bayesian information criterion (BIC) was used to assess the overall prognostic performance of different prognostic systems via bootstrap‐resampling analysis [21]. The BIC and 95% confidence intervals indicate significantly different predictive capability of two staging systems if the zero value is not included. Net reclassification index (NRI) [22] and integrated discrimination improvement (IDI) index [23] were used to quantify the improvement from the new staging system for prediction of the patient's survival. We also performed time‐dependent receiver operating characteristics (ROC) analysis to assess the discriminatory power of the prognostic model for time‐dependent disease outcomes [24]. Decision curve analysis was used to evaluate the clinical usefulness of the prediction models [25]. An internal validation procedure using 1,000 bootstraps was applied to different staging systems. External validation was performed using the data from the multi‐institutional cohort.
All data were processed using SPSS 19.0 (SPSS Inc., Chicago, IL) and R software (version 3.5.3). The data are presented as means ± SD for the continuous variables and as a number for the categorical variables. The differences between the groups were calculated by using Fisher's exact test, the t test, or the chi‐square test, as appropriate. All the tests were two‐sided, with a significance level set to p < .05.
Results
Demographic and Clinicopathological Characteristics
The clinicopathological features of the development cohort (n = 1,791) and the validation cohort (n = 266) are shown in Table 1. The mean ages of the development and validation cohorts were 60.2 ± 11.5 and 59.4 ± 11.3, respectively (p = .315), and the mean number of lymph node (LN) dissections was 19.8 ± 12.7 and 29.4 ± 12.8, respectively (p < .001). The patients in the two groups were significantly different in race, tumor site, histological type, grade, tumor size, surgical procedure, ypT category, and ypN category distribution (all p < .05). There was no significant difference in the distribution of sex or AJCC 8th edition ypTNM staging (both p > .05).
Table 1.
Sociodemographic and clinicopathologic characteristics of the development and the validation cohort
| Variable | Development cohort (n = 1,791), n (%) | Validation cohort (n = 266), n (%) | p value |
|---|---|---|---|
| Region | |||
| U.S. | 1,791 (100) | ||
| China | 169 (63.5) | ||
| Italy | 97 (36.5) | ||
| Year of operation | |||
| 2004–2009 | 527 (29.4) | 75 (28.2) | .681 |
| 2010–2015 | 1,264 (70.6) | 191 (71.8) | |
| Age, years, mean ± SD | 60.2 ± 11.5 | 59.4 ± 11.3 | .315 |
| Race | <.001 | ||
| White | 1,376 (76.8) | 0 (0.0) | |
| Black | 173 (9.7) | 0 (0.0) | |
| Other a | 238 (13.3) | 169 (63.5) | |
| Unknown | 4 (0.2) | 97 (36.5) | |
| Sex | .438 | ||
| Female | 464 (25.9) | 63 (23.7) | |
| Male | 1,327 (74.1) | 203 (76.3) | |
| Site | <.001 | ||
| Upper | 1,046 (58.4) | 114 (42.9) | |
| Middle | 312 (17.4) | 93 (35.0) | |
| Lower | 217 (12.1) | 47 (17.7) | |
| Overlapping | 131 (7.3) | 12 (4.5) | |
| NOS | 85 (4.7) | 0 (0.0) | |
| Histological type | .009 | ||
| General types | 1,364 (76.2) | 183 (68.8) | |
| Special types | 427 (23.8) | 83 (31.2) | |
| Size | <.001 | ||
| ≤2 cm | 248 (13.8) | 22 (8.3) | |
| >2 cm, ≤5 cm | 669 (37.4) | 112 (42.1) | |
| >5 cm | 545 (30.4) | 116 (43.6) | |
| Linitis plastica | 37 (2.1) | 6 (2.3) | |
| Unknown | 292 (16.3) | 10 (3.8) | |
| Surgical procedure | <.001 | ||
| Partial gastrectomy | 973 (54.3) | 90 (33.8) | |
| Total gastrectomy | 553 (30.9) | 176 (66.2) | |
| Gastrectomy, NOS | 265 (14.8) | 0 (0.0) | |
| Grade | <.001 | ||
| High | 463 (25.9) | 107 (40.2) | |
| Low | 1,216 (67.9) | 143 (53.8) | |
| Gx | 112 (6.3) | 16 (6.0) | |
| ypT category | .010 | ||
| T1 | 153 (8.5) | 23 (8.6) | |
| T2 | 222 (12.4) | 52 (19.5) | |
| T3 | 727 (40.6) | 87 (32.7) | |
| T4a | 560 (31.3) | 88 (33.1) | |
| T4b | 129 (7.2) | 16 (6.0) | |
| ypN category | .001 | ||
| N0 | 486 (27.1) | 84 (31.6) | |
| N1 | 474 (26.5) | 53 (19.9) | |
| N2 | 446 (24.9) | 50 (18.8) | |
| N3 | 385 (21.5) | 79 (29.7) | |
| LNs examined | <.001 | ||
| Mean ± SD | 19.8 ± 12.7 | 29.4 ± 12.8 | |
| <15 | 690 (38.5) | 21 (7.9) | |
| ≥15 | 1,101 (61.5) | 245 (92.1) | |
| ypTNM staging (AJCC 8th) | .614 | ||
| I | 227 (12.7) | 39 (14.7) | |
| II | 598 (33.4) | 90 (33.8) | |
| III | 966 (53.9) | 137 (51.5) | |
| Modified ypTNM staging | .118 | ||
| IA | 93 (5.2) | 17 (6.4) | |
| IB | 265 (14.8) | 45 (16.9) | |
| II | 555 (31.0) | 70 (26.3) | |
| IIIA | 515 (28.8) | 66 (24.8) | |
| IIIB | 363 (20.3) | 68 (25.6) | |
| Adjuvant radiotherapy | <.001 | ||
| None | 1,532 (85.5) | 261 (98.1) | |
| Yes | 259 (14.5) | 5 (1.9) | |
| Adjuvant chemotherapy b | |||
| None | NA | 97 (36.5) | |
| Yes | NA | 169 (63.5) | |
| Neoadjuvant radiotherapy | <.001 | ||
| None | 1,016 (56.7) | 258 (96.6) | |
| Yes | 775 (43.3) | 9 (3.4) | |
| Neoadjuvant chemotherapy b | |||
| None | NA | 2 (0.8) | |
| Yes | NA | 264 (99.2) |
Abbreviations: AJCC 8th, American Joint Committee on Cancer 8th edition; Gx, grade could not be evaluated; LN, lymph node; NA, not applicable; NOS, not otherwise specified.
American Indian/Alaska Native, Asian/Pacific Islander.
In the SEER database, category record “Chemotherapy recode (yes, no/unk)” does not distinguish between preoperative and postoperative chemotherapy in detail.
Development of a Modified Staging System
Supplemental online Table 1 shows the 5‐year OS of each ypTNM subgroup in the development cohort. We found that the 5‐year OS of ypT2N0M0 patients was higher than that of ypT1N1M0 patients belonging to AJCC 8th edition ypTNM stage I (54.9% vs. 42.4%), and the 5‐year OS of patients with ypT3N0M0 was significantly higher than that of patients with ypT1N3M0 belonging to AJCC 8th edition ypTNM stage II (54.3% vs. 20.0%), whereas the 5‐year OS of ypT4bN0M0 patients was also higher than that of ypT4bN3M0 patients belonging to the AJCC 8th edition ypTNM stage III (26.2% vs. 15.7%). Therefore, we reorganized each of the ypTNM subgroups with similar 5‐year OS and established a modified ypTNM staging, which clearly identified five subgroups with different prognoses (Table 2). Table 2 also shows the changes in AJCC 8th edition ypTNM staging and modified ypTNM staging in the development and validation cohorts.
Table 2.
The AJCC 8th edition ypTNM staging definitions and the modified ypTNM staging definitions for gastric cancer after neoadjuvant therapy, with cross‐tabulation of stage distributions
| Definitions for ypT, ypN | |
|---|---|
| ypT category (primary tumor) | |
| T1 | Tumor invades the lamina propria, muscularis mucosae, or submucosa |
| T2 | Tumor invades the muscularis propria |
| T3 | Tumor penetrates the subserosal connective tissue without invasion of the visceral peritoneum or adjacent structures |
| T4 | Tumor invades the serosa (visceral peritoneum) or adjacent structures |
| T4a | Tumor invades the serosa (visceral peritoneum) |
| T4b | Tumor invades adjacent structures/organs |
| ypN category (regional lymph nodes) | |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in one or two regional lymph nodes |
| N2 | Metastasis in three to six regional lymph nodes |
| N3 | Metastasis in seven or more regional lymph nodes |
| N3a | Metastasis in seven to 15 regional lymph nodes |
| N3b | Metastasis in 16 or more regional lymph nodes |
| ypTNM staging (AJCC 8th) | Modified ypTNM staging | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N0 | N1 | N2 | N3 | N0 | N1 | N2 | N3 | ||
| T1 | I | I | II | II | T1 | IA | II | IIIA | IIIA |
| T2 | I | II | II | III | T2 | IB | II | IIIA | IIIA |
| T3 | II | II | III | III | T3 | IB | II | IIIA | IIIB |
| T4a | II | III | III | III | T4a | II | II | IIIA | IIIB |
| T4b | III | III | III | III | T4b | IIIA | IIIA | IIIA | IIIB |
| Development cohort | Validation cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Systems | IA | IB | II | IIIA | IIIB | IA | IB | II | IIIA | IIIB |
| I | 93 | 92 | 42 | 0 | 0 | 17 | 19 | 3 | 0 | 0 |
| II | 0 | 173 | 364 | 61 | 0 | 0 | 26 | 50 | 14 | 0 |
| III | 0 | 0 | 149 | 454 | 363 | 0 | 0 | 17 | 52 | 68 |
Abbreviation: AJCC 8th, American Joint Committee on Cancer 8th edition.
Survival of Two Staging Systems
The median follow‐up time of the development cohort was 60.0 months (1–155 months), and death was observed in 1,097 (61.3%) patients. The median follow‐up time was 68.0 months (1–156 months) in the validation cohort, and 148 (55.6%) patients died during the follow‐up. Figure 1 depicts the overall survival of the two staging systems. In the development cohort, the 5‐year OS of the AJCC ypTNM stages I, II, and III were 58.8%, 39.1%, and 21.6% (p < .001). Applying the modified ypTNM staging, the 5‐year OS was 69.9% for stage IA, 54.4% for stage IB, 34.4% for stage II, 24.1% for stage IIIA, and 13.6% for stage IIIB (p < .001). In the validation cohort, the 5‐year OS of the AJCC ypTNM staging was 62.3% for stage I, 55.8% for stage II, and 27.4% for stage III (p < .001). The 5‐year OS of the modified ypTNM staging was 79.1%, 61.6%, 49.7%, 37.8%, and 16.1% for IA, IB, II, IIIA, and IIIB, respectively (p < .001). According to the number of LN dissections (supplemental online Fig. 3), histological grade (supplemental online Fig. 4), and histological type (supplemental online Fig. 5), the modified ypTNM staging could separate the OS of each staged patient well (all p < .001).
Figure 1.
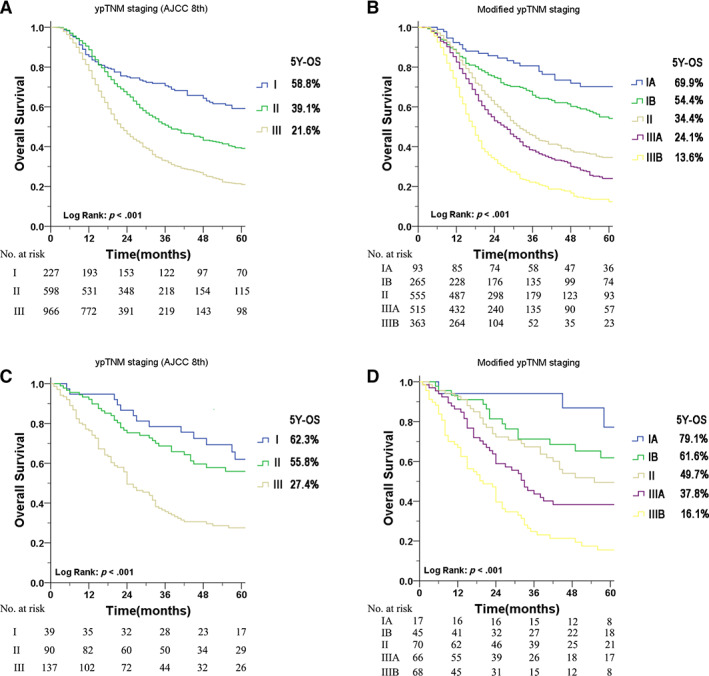
Kaplan‐Meier survival curves of the different staging systems for patients after neoadjuvant therapy. (A): The AJCC 8th edition ypTNM staging system in the development cohort. (B): The modified ypTNM staging system in the development cohort. (C): The AJCC 8th edition ypTNM staging system in the validation cohort. (D): The modified ypTNM staging system in the validation cohort. Abbreviations: AJCC 8th, American Joint Committee on Cancer 8th edition; OS, overall survival.
Univariate and Multivariate Analysis
In the development cohort, univariate Cox regression analysis (supplemental online Table 2) showed that age, race, tumor size, histological type, tumor size, surgical procedure, histological grade, number of LN dissections, AJCC 8th edition ypTNM stage, and modified ypTNM stage were associated with OS (all p < .05). Then, a two‐step multivariate Cox analysis was used to identify the independent prognostic factors of OS (Table 3). The multivariate analysis in the first step included all OS‐related prognostic factors except the modified ypTNM stage, and the AJCC 8th edition ypTNM stage was an independent prognostic factor affecting OS (p < .001). The second‐step multivariate analysis included all OS‐related prognostic factors, including modified ypTNM stage. The results showed that modified ypTNM stage was an independent prognostic factor for OS (p < .001), whereas the AJCC 8th edition ypTNM stage disappeared (p = .807). Similar results were observed in the validation cohort. Univariate analysis revealed that tumor site, tumor size, histological grade, AJCC 8th edition ypTNM stage, and modified ypTNM stage were correlated with OS (p < .05). Multivariate analysis in the first step showed that the AJCC 8th edition ypTNM stage was an independent factor affecting OS (p < .001), whereas in the second‐step multivariate analysis, the AJCC ypTNM stage (p = .868) was replaced by modified ypTNM stage (p < .001).
Table 3.
Two‐step multivariate analysis of the prognostic factors for patients with gastric cancer after neoadjuvant therapy
| Variables | Relative risk (95% CI) | p value |
|---|---|---|
| Development cohort | ||
| Step 1 | ||
| Age | 1.161 (1.075–1.253) | <.001 |
| Race | 0.987 (0.962–1.013) | .325 |
| Site | 1.001 (0.999–1.003) | .190 |
| Histological type | 1.300 (1.133–1.491) | <.001 |
| Size | 1.000 (0.998–1.001) | .696 |
| Surgical procedure | 1.169 (1.079–1.266) | <.001 |
| Grade | 0.998 (0.995–1.000) | .087 |
| LN examined | 0.751 (0.665–0.848) | <.001 |
| ypTNM staging (AJCC 8th) | 1.717 (1.561–1.888) | <.001 |
| Step 2 | ||
| Age | 1.164 (1.078–1.257) | <.001 |
| Race | 0.988 (0.960–1.016) | .388 |
| Site | 1.001 (0.999–1.002) | .355 |
| Histological type | 1.260 (1.098–1.446) | .001 |
| Size | 1.000 (0.998–1.001) | .850 |
| Surgical procedure | 1.14 (1.061–1.245) | .001 |
| Grade | 0.998 (0.995–1.000) | .106 |
| LN examined | 0.661 (0.583–0.749) | <.001 |
| ypTNM staging (AJCC 8th) | 1.018 (0.880–1.179) | .807 |
| Modified ypTNM staging | 1.540 (1.403–1.691) | <.001 |
| Validation cohort | ||
| Step 1 | ||
| Site | 0.889 (0.725–1.089) | .255 |
| Size | 1.008 (1.000–1.016) | .047 |
| Grade | 1.001 (0.994–1.008) | .748 |
| ypTNM staging (AJCC 8th) | 1.892 (1.463–2.446) | <.001 |
| Step 2 | ||
| Site | 0.924 (0.755–1.131) | .445 |
| Size | 1.007 (0.999–1.015) | .080 |
| Grade | 1.002 (0.995–1.009) | .590 |
| ypTNM staging (AJCC 8th) | 0.965 (0.630–1.476) | .868 |
| Modified ypTNM staging | 1.636 (1.278–2.096) | <.001 |
Step 1, with consideration of all significantly important prognostic factors in univariate analysis except for the modified ypTNM stage; step 2, with consideration of all significantly important prognostic factors in univariate analysis, including the modified ypTNM stage.
Abbreviations: AJCC 8th, American Joint Committee on Cancer 8th edition; CI, confidence interval; LN, lymph node.
Time‐dependent Cox analysis (supplemental online Table 3) showed that the modified ypTNM staging system could differentiate patients’ prognoses over time among patients with GC after neoadjuvant therapy in both the development and validation cohorts.
Comparing the Prognostic Performance of the Two Staging Systems
Table 4 compares the prognostic predictive power of the two staging systems. In the development cohort (internal validation), the prognostic discriminating ability (C‐index) of the modified ypTNM staging was better than that of the AJCC 8th edition system (0.620 vs. 0.589, p < .001). AIC analysis showed that the modified staging had a better goodness of fit than the AJCC staging (14,793.55 vs. 14,867.50, p < .001). The modified ypTNM staging had a better survival predictive homogeneity (higher likelihood ratio chi‐square) compared with the AJCC ypTNM staging. BIC was used to compare the prognostic performance of the different staging systems. It accurately considered the number of parameters included in the staging system. The results showed that the modified ypTNM staging had a significant advantage over the AJCC ypTNM staging (mean difference in BIC: 64.94). With the NRI or the IDI index, the survival prediction performance of the modified ypTNM staging was improved compared with the AJCC 8th edition staging (NRI: 35.54%, p < .001; IDI: 0.032, p < .001). Similarly, in external validation, the modified ypTNM staging was superior to the AJCC ypTNM staging in various indicators, reflecting the prognostic predictive power of the staging system (all p < .05).
Table 4.
Comparison of the prognostic performance of the AJCC 8th edition ypTNM staging system and the modified ypTNM staging system
| Variable | ypTNM staging (AJCC 8th) | Modified ypTNM staging | p value |
|---|---|---|---|
| Development cohort (internal validation) | |||
| Harrell's C‐index a | 0.589 (0.572–0.605) | 0.620 (0.602–0.638) | <.001 |
| AIC b | 14,867.50 | 14,793.55 | <.001 |
| Likelihood ratio chi‐square c | 140.71 | 218.66 | <.001 |
| Mean difference in BIC (95% CI) d | 64.94 (25.55–96.46) | ||
| NRI (95% CI) | 35.54% (14.04%–43.12%) | <.001 | |
| IDI (95% CI) | 0.032 (0.012–0.053) | .002 | |
| Validation cohort (external validation) | |||
| Harrell's C‐index a | 0.631 (0.591–0.671) | 0.668 (0.625–0.712) | .014 |
| AIC b | 1,461.06 | 1,443.6 | <.001 |
| Likelihood ratio chi‐square c | 28.75 | 45.15 | <.001 |
| Mean difference in BIC (95% CI) d | 17.50 (1.98–32.88) | ||
| NRI (95% CI) e | 44.26% (10.00%–66.18%) | <.001 | |
| IDI (95% CI) e | 0.048 (0.000–0.086) | .048 | |
Abbreviations: AIC, Akaike information criterion; AJCC 8th, American Joint Committee on Cancer 8th edition; BIC, Bayesian information criteria; CI, confidence interval; IDI, integrated discrimination improvement index; NRI, net reclassification index.
A higher Harrell's C‐index indicates higher discriminative ability.
Smaller AIC values indicate better optimistic prognostic stratification.
A higher likelihood ratio chi‐square score indicates better homogeneity.
The BIC was used to assess the overall prognostic performance of different prognostic systems via bootstrap‐resampling analysis.
NRI and IDI quantify the improvement by the new staging system in predicting the patient's 5‐year survival.
The stratified analysis showed that the prognostic performance of the modified ypTNM staging was higher than that of the AJCC ypTNM staging in the development cohort, regardless of whether the number of LNs was adequate (supplemental online Table 4). We also performed a stratified analysis based on the histological grade (supplemental online Table 5) and the histological type (supplemental online Table 6). The results showed that in the development cohort, regardless of grade and histological type, the prognostic performance of the modified ypTNM staging was better than that of the AJCC ypTNM staging. In the external validation cohort, although the number of patients was limited, regardless of grade and histological type, the prognostic performance of the modified ypTNM staging showed a trend toward superiority of the AJCC ypTNM staging.
Time‐Dependent ROC Curves and Decision Curve Analysis
Time‐dependent ROC curves were used to compare the continuity trends of hazard ratios across systems. As shown in Figure 2A and B, the modified ypTNM staging was superior to the AJCC ypTNM staging over time in both the development and validation cohorts. We also used the decision curve to intuitively evaluate and compare the clinical applicability of the staging systems (Fig. 2C, D). The results showed that the modified staging could achieve better net benefits at the same probability threshold compared with the AJCC staging in both groups. According to the number of LN dissections (supplemental online Fig. 6), histological grade (supplemental online Fig. 7), and histological type (supplemental online Fig. 8), stratified analysis indicated that the modified staging was superior to the AJCC ypTNM staging in both the decision curve and the time‐dependent ROC curve.
Figure 2.
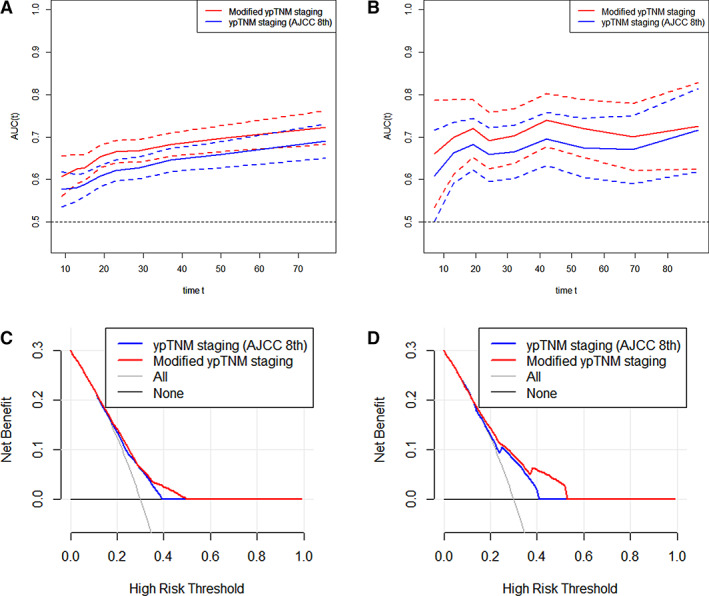
Comparison of the clinical usefulness of the AJCC 8th edition ypTNM staging system and the modified ypTNM staging system. Time‐dependent receiver operating characteristics (ROC) curves for the AJCC 8th edition ypTNM staging system and the modified ypTNM staging system in the development cohort (A) and the validation cohort (B). The x‐axis represents the years after surgery, and the y‐axis represents the estimated area under the ROC curve for survival at the time of interest. Decision curve analysis for overall survival after surgery in the development cohort (C) and the validation cohort (D). The y‐axis measures the net benefit. Abbreviations: AJCC 8th, American Joint Committee on Cancer 8th edition; AUC, area under the curve.
Discussion
GC is still a major global health problem, although with the popularity of upper gastrointestinal endoscopy screening, most patients with GC are diagnosed at an advanced stage [2, 26]. In recent years, based on clinical research on neoadjuvant therapy for GC [7, 27, 28, 29], preoperative therapy for GC has been increasingly recommended by Eastern and Western scholars. The National Comprehensive Cancer Network and European Society for Medical Oncology guidelines also emphasize that neoadjuvant therapy can be used as a routine recommended treatment for patients with locally advanced GC [30, 31]. In contrast to the previous application of pTNM directly to the prognostic evaluation of patients with GC after receiving neoadjuvant therapy, the 8th edition of the AJCC manual provided the first standard for prognostic evaluation after neoadjuvant chemotherapy, and the ypTNM staging came into being. However, because of the limited number of cases included in the analysis of the AJCC manual, patients with nonmetastatic GC were only divided into three stages, I, II, and III, and our results showed that the 5‐year OSs of patients in the same AJCC 8th edition stage could be very different. More detailed staging is the direction of the next edition of AJCC ypTNM staging. Our study confirmed the worth of the modification of the existing ypTNM staging and the more detailed classification of patients into five groups, which achieved more accurate prognostic discrimination. A detailed distinction between the prognosis of patients after surgery will facilitate postoperative adjuvant treatment options and follow‐up surveillance. Moreover, time‐dependent Cox regression showed that the modified ypTNM staging system differentiated the patients’ prognoses well, with prolonged postoperative survival time. This modified ypTNM staging will help doctors provide patients with longer‐term, more accurate counseling.
We used a variety of prognostic model evaluation indicators to compare the modified ypTNM staging system and the AJCC 8th edition ypTNM staging system in 1,791 patients who had undergone surgery after neoadjuvant chemotherapy from the SEER database. The discriminatory ability, predictive homogeneity, predictive accuracy, and model stability of the modified staging were superior to those of the AJCC 8th edition staging. At the same time, the two‐step Cox regression analysis further indicated that the modified staging was significantly better than the AJCC 8th edition staging in evaluating the OS. Retrospective analysis showed that ≥15 LN dissections had a positive effect on the survival of patients with GC [32, 33]. The current guidelines also recommend ≥15 LN dissection for a more accurate staging [30, 34]. Our stratified analysis showed that the modified ypTNM staging was superior to the AJCC 8th edition ypTNM staging, regardless of whether the number of LN dissections was more than 15, which showed the stability of the modified staging. The study also used a validation cohort from China and Italy (mean LN examined: 29.4; D2 LN dissections: 94.0%) to confirm that the modified staging could still be used to evaluate the prognosis of patients after neoadjuvant therapy in Asian and European populations better than the existing AJCC ypTNM staging.
Previous studies have shown that different histological grades and histological types may have different responses to neoadjuvant therapy [35, 36]. Therefore, according to different histological grades and histological types, further stratified analysis was carried out. Our results show that the prognostic predictive performance of modified staging was still superior to the that of existing AJCC ypTNM staging for patients with well differentiated or poorly differentiated tumors, common adenocarcinomas, or special type adenocarcinomas, such as signet‐ring cell carcinoma.
To maintain consistency in postoperative pathological judgment, ypT and ypN categories were defined with reference to pT and pN categories. A closer look at the AJCC TNM staging reveals that the 8th edition of the AJCC ypTNM staging is identical to the 7th edition of the AJCC pTNM staging in the broad classification. However, neoadjuvant therapy often has a downstaging effect on patients with cancer. Different patients have different therapeutic sensitivities to neoadjuvant therapy. Cancers that respond poorly to neoadjuvant therapy often exhibit stronger tumor invasion and metastatic ability [37]. We used a large sample of data to reorganize subgroups with similar 5‐year survival rates to establish the new staging. In the modified staging, we found that the ypN category had a greater weighted effect on the prognosis of patients with GC receiving neoadjuvant therapy than ypT category. Further survival analysis under the ypT category and ypN category confirmed these findings (supplemental online Table 7; supplemental online Fig. 9). This may reflect that the primary tumor and metastatic LN respond differently to neoadjuvant therapy. Previous studies have also shown that LN status is more likely to affect the prognosis of patients receiving neoadjuvant therapy than the primary tumor state [38]. Our proposal has a quite different structure, suggesting stronger significance of the ypN category than the ypT category. Our modified staging allows us to assess the prognosis of patients receiving neoadjuvant therapy based on the response of the tumor to treatment, to a certain extent, and can provide a reference for future management and postoperative adjuvant treatment options for these patients.
The 5‐year OS of patients between 2005–2009 and 2010–2015 in the development cohort was 33.6% and 31.1%, respectively (supplemental online Fig. 10). There was no significant difference between the two groups (p = .654). We also analyzed the OS of patients in the validation cohort according to the year of operation. The results showed that there was no significant difference between the two groups in 5‐year OS (47.1% vs. 39.7%, p = .538). At the same time, we analyzed the OS of different centers in the validation cohort (supplemental online Fig. 11) and found that the 5‐year OSs of FMUUH, IMIGASTRIC, and QUAH were 39.9%, 47.1%, and 39.6% respectively, and there was no significant statistical difference among the three groups (p = .596). The results of the univariate analysis showed that different years of operation or different centers are not independent prognostic factors of OS. We believe that although the patterns of neoadjuvant therapy are different in different periods, and with the progress of medical treatment, the neoadjuvant therapy has been continuously optimized, but the main factors that affect the prognosis may be ypT category and ypN category. The effect of the optimization of neoadjuvant treatment mode on the improvement of prognosis may be more reflected in its more obvious effect of downstaging. The modified ypTNM staging we established is based on the ypT category and ypN category, so it is more applicable and may be suitable for patients with GC who receive different neoadjuvant treatment schemes. We look forward to further validation of the modified ypTNM staging in the future through prospective studies of different neoadjuvant therapies.
The AJCC 8th edition ypTNM staging did not include patients with pathological complete response (ypCR) in the staging system. Because SEER did not describe ypCR or depth of invasion in detail, to ensure the reliability of staging, our modified ypTNM staging did not include patients with ypCR. In addition, although five patients in the validation cohort reached ypCR, their 5‐year OS was 80.0%, which was similar to that of patients with stage IA. Because of the small number of patients, we did not include ypCR in the modified staging. A larger‐sample analysis is still needed to further explore the prognosis of patients with ypCR and the best strategy for treating patients with ypCR. Previous studies have shown that the pathologic tumor response may affect the prognosis of patients with GC after neoadjuvant therapy [39]. However, studies such as the MAGIC trials have shown that the degree of tumor regression is not an independent prognostic factor [38]. This study is the largest study on the staging of patients with GC after neoadjuvant therapy. The SEER database provides a large amount of data from the U.S. population. Compared with data from one or two large centers, the SEER database can better reflect the overall prognosis of patients with GC receiving neoadjuvant therapy. The results are more universal and practical. However, the SEER database did not record the frequency of neoadjuvant therapy; we cannot know how many cycles of chemotherapy a given patient received, what regimen was given, etc., so we were unable to conduct further stratified analysis. There is no denying that the SEER data set may not be able to evaluate the ypN category very accurately, as fewer than 15 LNs were examined in 38.5% of the cases. The purpose of this study was to develop and validate a modified ypTNM staging, and the results showed that the modified staging was better than the AJCC 8th edition staging in both the development and validation cohorts. Supplemental online Table 1 also shows the 5‐year OS of each ypTNM subgroup in the validation cohort. However, we found that because of the limited number of cases in the validation cohort, there were not enough cases in each ypTNM subgroup. In the validation cohort, one of two patients with ypT1N2M0 disease and one of two patients with ypT4bN1M0 disease were censored. Therefore, the 5‐year OS of ypT1N2M0 and ypT4bN1M0 was not calculated. There may be some selection biases. AJCC TNM staging should be international, reflecting as many expert facilities as possible worldwide. The data used for AJCC 8th edition TNM staging was international, yet there were gaps and limitations as well. Future iterations need to overcome limitations from individual databases like SEER that have only a selected cohort of patients. We look forward to further validation of this modified staging through global big data sets in the future, especially data from East Asia.
Conclusion
We have developed and validated a modified ypTNM staging through global multicenter data that is superior to the AJCC 8th edition ypTNM staging, allowing for a more accurate assessment of the prognosis of patients with GC after neoadjuvant therapy. It may provide a reference for the next edition of the AJCC ypTNM staging.
Author Contributions
Conception/design: Qing Zhong, Qi‐Yue Chen, Ping Li, Chao‐Hui Zheng, Chang‐Ming Huang
Provision of study material or patients: Qing Zhong, Qi‐Yue Chen, Amilcare Parisi, Yu‐Bin Ma, Guang‐Tan Lin, Jacopo Desiderio, Su Yan, Jian‐Wei Xie, Jia‐Bin Wang, Jun‐Fang Hou, Jian‐Xian Lin, Jun Lu, Long‐Long Cao, Mi Lin, Ru‐Hong Tu, Ze‐Ning Huang, Ju‐Li Lin, Zhi‐Yu Liu, Si‐Jin Que, Ping Li, Chao‐Hui Zheng, Chang‐Ming Huang
Collection and/or assembly of data: Qing Zhong, Qi‐Yue Chen, Amilcare Parisi, Yu‐Bin Ma, Guang‐Tan Lin, Jian‐Wei Xie, Jia‐Bin Wang, Jian‐Xian Lin, Jun Lu, Long‐Long Cao, Mi Lin, Ru‐Hong Tu, Ze‐Ning Huang, Ju‐Li Lin, Ping Li, Chao‐Hui Zheng, Chang‐Ming Huang
Data analysis and interpretation: Qing Zhong, Qi‐Yue Chen, Amilcare Parisi, Yu‐Bin Ma, Ping Li, Chao‐Hui Zheng, Chang‐Ming Huang
Manuscript writing: Qing Zhong, Qi‐Yue Chen, Chang‐Ming Huang
Final approval of manuscript: Qing Zhong, Qi‐Yue Chen, Amilcare Parisi, Yu‐Bin Ma, Guang‐Tan Lin, Jacopo Desiderio, Su Yan, Jian‐Wei Xie, Jia‐Bin Wang, Jun‐Fang Hou, Jian‐Xian Lin, Jun Lu, Long‐Long Cao, Mi Lin, Ru‐Hong Tu, Ze‐Ning Huang, Ju‐Li Lin, Zhi‐Yu Liu, Si‐Jin Que, Ping Li, Chao‐Hui Zheng, Chang‐Ming Huang
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.
Acknowledgments
We thank Dr. Xiao‐Yan Li for help in the past 3 years. This study was supported by Scientific and Technological Innovation Joint Capital Projects of Fujian Province (2017Y9011, 2017Y9004, 2018Y9041), Construction Project of Fujian Province Minimally Invasive Medical Center ([2017]171), National Natural Science Foundation of China (81802312), China Scholarship Council (201908350095) and Funded Project of Fujian Medical University Innovation and Entrepreneurship Training Program (S202010392006).
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 4. Ajani JA, Lee J, Sano T et al. Gastric adenocarcinoma. Nat Rev Dis Primers 2017;3:17036. [DOI] [PubMed] [Google Scholar]
- 5. Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol 2015;21:7343–7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newton AD, Datta J, Loaiza‐Bonilla A et al. Neoadjuvant therapy for gastric cancer: Current evidence and future directions. J Gastrointest Oncol 2015;6:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stahl M, Walz MK, Stuschke M et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851–856. [DOI] [PubMed] [Google Scholar]
- 8. Edge SB. AJCC cancer staging manual. JAMA 2010;304:1726–1727. [Google Scholar]
- 9. Doescher J, Veit JA, Hoffmann TK. The 8th edition of the AJCC cancer staging manual [in German]. HNO 2017;65:956–961 [DOI] [PubMed] [Google Scholar]
- 10. In H, Ravetch E, Langdon‐Embry M et al. The newly proposed clinical and post‐neoadjuvant treatment staging classifications for gastric adenocarcinoma for the American Joint Committee on Cancer (AJCC) staging. Gastric Cancer 2018;21:1–9. [DOI] [PubMed] [Google Scholar]
- 11. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database . Incidence ‐ SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases (with additional treatment fields), Nov 2018 Sub (1975‐2016 varying). Bethesda, MD: Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, released April 2019, based on the November 2018 submission. [Google Scholar]
- 12. Chen QY, Zhong Q, Wang W et al. Prognosis of young survivors of gastric cancer in China and the U.S.: Determining long‐term outcomes based on conditional survival. The Oncologist 2019;24:e260–e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu C, Xing Y, Cormier JN et al. The validity of cancer specific mortality within the Surveillance, Epidemiology, and End Results Registry. J Surg Res 2011;165:P270a. [Google Scholar]
- 14. Sun Z, Zhu GL, Lu C et al. A novel subclassification of pT2 gastric cancers according to the depth of muscularis propria invasion: Superficial muscularis propria versus deep muscularis propria/subserosa. Ann Surg 2009;249:768–775. [DOI] [PubMed] [Google Scholar]
- 15. Margonis GA, Buettner S, Andreatos N et al. Prognostic factors change over time after hepatectomy for colorectal liver metastases: A multi‐institutional, international analysis of 1099 patients. Ann Surg 2019;269:1129–1137. [DOI] [PubMed] [Google Scholar]
- 16. Jr HF, Califf RM, Pryor DB et al. Evaluating the yield of medical tests. JAMA 1982;247:2543–2546. [PubMed] [Google Scholar]
- 17. Jr HF, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 18. Yoon HM, Ryu KW, Nam BH et al. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg 2012;214:88–96. [DOI] [PubMed] [Google Scholar]
- 19. Awad AM. Properties of the Akaike information criterion. Microelectronics Reliability 1996;36:457–464. [Google Scholar]
- 20. Edeline J, Blanc JF, Johnson P et al. A multicenter comparison between Child Pugh and albumin‐bilirubin scores in patients treated with sorafenib for hepatocellular carcinoma. Liver Int 2016;36:1821–1828. [DOI] [PubMed] [Google Scholar]
- 21. Neath AA, Cavanaugh JE. The Bayesian information criterion: Background, derivation, and applications. Wiley Interdiscip Rev Comput Stat 2012;4:199–203. [Google Scholar]
- 22. Alba AC, Agoritsas T, Walsh M et al. Discrimination and calibration of clinical prediction models. JAMA 2017;318:1377–1384. [DOI] [PubMed] [Google Scholar]
- 23. van Smeden M, Moons KGM. Event rate net reclassification index and the integrated discrimination improvement for studying incremental value of risk markers. Stat Med 2017;36:4495–4497. [DOI] [PubMed] [Google Scholar]
- 24. Heagerty PJ, Lumley T, Pepe MS et al. Time‐dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–344. [DOI] [PubMed] [Google Scholar]
- 25. Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tey J, Lu JJ. Gastric cancer In: Lee NY, Riaz N, Lu JJ, eds. Target Volume Delineation for Conformal and Intensity‐Modulated Radiation Therapy. Medical Radiology. Cham, Switzerland: Springer, 2014:261–273. [Google Scholar]
- 27. van Hagen P, Hulshof MC, van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 28. Cats A, Jansen EPM, van Grieken NCT et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open‐label, randomised phase 3 trial. Lancet Oncol 2018;19:616–628. [DOI] [PubMed] [Google Scholar]
- 29. Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 30. Wang XZ, Zeng ZY, Ye X et al. Interpretation of the development of neoadjuvant therapy for gastric cancer based on the vicissitudes of the NCCN guidelines. World J Gastrointest Oncol, 2020, 12: 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okines A, Verheij M, Allum W et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2010;21(suppl 5):v50–v54. [DOI] [PubMed] [Google Scholar]
- 32. Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317. [DOI] [PubMed] [Google Scholar]
- 33. Biondi A, D'Ugo D, Cananzi FC et al. Does a minimum number of 16 retrieved nodes affect survival in curatively resected gastric cancer? Eur J Surg Oncol 2015;41:779–786. [DOI] [PubMed] [Google Scholar]
- 34. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu SB, Liu CH, Wang X et al. Pathological evaluation of neoadjuvant chemotherapy in advanced gastric cancer. World J Surg Oncol 2019;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vošmik M, Laco J, Sirák I et al. Histopathologic features are more important prognostic factors than primary tumour location in gastro‐oesophageal adenocarcinoma treated with preoperative chemoradiation and surgery. Pathol Oncol Res 2018;24:373–383. [DOI] [PubMed] [Google Scholar]
- 37. Karen B, Rupert L, Daniel R et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: A summary of 480 cases. Ann Surg 2011;253:934. [DOI] [PubMed] [Google Scholar]
- 38. Smyth EC, Fassan M, Cunningham D et al. Effect of pathologic tumor response and nodal status on survival in the Medical Research Council adjuvant gastric infusional chemotherapy trial. J Clin Oncol 2016;34:2721–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Becker K, Reim D, Novotny A et al. Proposal for a multifactorial prognostic score that accurately classifies 3 groups of gastric carcinoma patients with different outcomes after neoadjuvant chemotherapy and surgery. Ann Surg 2012;256:1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.


