Abstract
Heritable microbes are an important component of invertebrate biology, acting both as beneficial symbionts and reproductive parasites. Whilst most previous research has focussed on the ‘Wolbachia pandemic’, recent work has emphasised the importance of other microbial symbionts. In this study, we present a survey of odonates (dragonflies and damselflies) for torix group Rickettsia, following previous research indicating that this clade can be common in other aquatic insect groups. PCR assays were used to screen a broad range of odonates from two continents and revealed 8 of 76 species tested were infected with Rickettsia. We then conducted further deeper screening of UK representatives of the Coenagrionidae damselfly family, revealing 6 of 8 UK coenagrionid species to be positive for torix Rickettsia. Analysis of Rickettsia gene sequences supported multiple establishments of symbiosis in the group. Some strains were shared between UK coenagrionid species that shared mtDNA barcodes, indicating a likely route for mitochondrial introgression between sister species. There was also evidence of coinfecting Rickettsia strains in two species. FISH analysis indicated Rickettsia were observed in the ovarioles, consistent with heritable symbiosis. We conclude that torix Rickettsia represent an important associate of odonates, being found in a broad range of species from both Europe and South America. There is evidence that coinfection can occur, vertical transmission is likely, and that symbiont movement following hybridisation may underpin the lack of ‘barcoding gap’ between well-established species pairs in the genus. Future work should establish the biological significance of the symbioses observed.
Electronic supplementary material
The online version of this article (10.1007/s00248-020-01568-9) contains supplementary material, which is available to authorized users.
Keywords: Torix, Rickettsia, Odonates, Endosymbionts
Introduction
Animals and plants commonly form associations with microbes, either by interacting with environmental microbes on their surface, in their gut, or with microbes living inside the organism’s tissues as endosymbionts. A subset of these may pass vertically from a female to her offspring and are termed heritable symbionts. Vertical transmission aligns the fitness interest of host and symbiont and has selected for these microbes to play important roles in host function. Carrying a symbiont can influence a host individual’s reproductive success [1–3], modulate its ability to defend against natural enemies [4], or alter digestion and nutrient production [5, 6]. However, the maternal inheritance of symbionts creates a dependence of symbiont fitness solely on the production and survival of daughters, leading to the evolution of reproductive parasitic phenotypes [2, 7].
The best-known example of a heritable symbiont is Wolbachia, which is estimated to infect over 50% of insect species [8]. Wolbachia is most commonly known as a reproductive manipulator, which drives itself into a population through cytoplasmic incompatibility, feminisation, male killing and induced parthenogenesis [3, 7, 9]. In some cases, like the butterfly Acraea encedon, it can result in highly female-biassed population sex ratios that alter mating behaviour [10]. Wolbachia can also act as a nutritional symbiont for blood-feeding insects by synthesising B vitamins that the host cannot make on its own or obtain from its diet, and can enhance tolerance to RNA virus infection in diverse species [11]. This covers just a few examples of Wolbachia impacts and is not an exhaustive list.
Whilst Wolbachia is not the only bacterial symbiont of insects, it is the best studied associate of terrestrial and, to a lesser extent, freshwater taxa [12]. The documentation for endosymbionts of freshwater insects is particularly poor when compared with terrestrial insects, with the notable exception of mosquitoes [13]. Recently, the presence of torix group Rickettsia (hereafter referred to as torix Rickettsia) has been noted in a variety of aquatic invertebrate taxa. First discovered in Torix leeches [14, 15], hotspots of torix Rickettsia have been observed in Culicoides biting midges [16], deronectid diving beetles [17] and dolichopodid flies [18]. To date, the impact of symbionts from this group on host biology is unclear, with the exception of bark lice, in which Rickettsia infection is associated with parthenogenetic reproduction by the host [19]. Analysis of the symbiont genome sequences from midges found no evidence for B vitamin synthesis capacity [16]. However, the symbiont infection is a potentially important aspect of biology that has generally been overlooked in many aquatic insects.
The cosmopolitan insect order Odonata (dragonflies and damselflies, generally referred to as ‘odonates’) are associated with freshwater habitats. This ecologically important taxon of insects is easily identifiable, enabling their use in citizen science and in conservation as indicator species for monitoring the health of freshwater habitats [20]. They have also been identified as model organisms in ecological and evolutionary research [21]. Odonates are predatory insects with aquatic larvae and aerial adults, which depend on freshwater habitats in all stages of life. These insects have recently been revealed as hosts for Wolbachia [22–24], but surveys for other members of the Rickettsiales have yet to be completed. Investigating other symbiotic interactions in these ecologically important species could help enrich biological and ecological knowledge of both symbiotic bacteria and odonate hosts. Exploratory research will hopefully encourage further studies in this aspect of insect-endosymbiont evolution.
In this study, we first present an analysis of the incidence of Rickettsia infection in odonates through PCR assays. The screened species combined a broad sweep of biogeographical and taxonomic diversity. We also explored infection in-depth with a greater number of individuals in the damselfly family Coenagrionidae in the UK, which were readily available for collection. We performed FISH analysis of Rickettsia tropism in Coenagrion puella to establish if the symbiont is present in developing oocytes and thus determine the likelihood of vertical transmission.
Methods
Sample Collection and DNA Preparation
Existing odonate DNA from previous studies [25–32] and freshly collected leg material were tested for the presence of Rickettsia. Where leg material was obtained, a Promega Wizard® Genomic DNA Purification kit was used for DNA preparation. The analysed material covered a total of 284 individuals from 76 species within 8 families, from the UK, South America, mainland Europe and the Azores (Table 1). To enable a view of the commonness within species and any sex bias in presence, a focussed screening of 112 individuals belonging to 8 damselfly species within the family Coenagrionidae from the UK was executed in further depth, which included 5 additional species than the broad screen (Table 1).
Table 1.
Screening results split according to screen type. The broad screen includes species from across South America, continental Europe, the Azores and the UK. The UK species included in the broad screen were tested prior to the focused screen and were used as the basis for doing the focussed screen. The focused screen covers coenagrionid species from the UK in greater breadth and depth
| No. | Species | Family | Location | Number infected (number tested) |
|---|---|---|---|---|
| Broad global screen results | ||||
| Suborder Anisoptera (dragonflies) | ||||
| 1 | Anax imperator | Aeshnidae | Italy, Spain, Azores and continental Portugal | 0 (14) |
| 2 | Oxygastra curtisii | Corduliidae | Tojal, Portugal | 0 (1) |
| 3 | Cannaphila vibex | Libellulidae | Maquipucuna, Ecuador | 0 (1) |
| 4 | Erythrodiplax amazonica | Libellulidae | Tiputini Ecuador | 0 (1) |
| 5 | E. kimminsi | Libellulidae | Tiputini Ecuador | 0 (3) |
| 6 | E. unimaculata | Libellulidae | Tiputini Ecuador | 0 (1) |
| 7 | Libellula depressa | Libellulidae | Cheshire, UK | 1 (1) |
| 8 | Orthemis cultriformis | Libellulidae | Tiputini, Ecuador | 0 (1) |
| 9 | Sympetrum fonscolombii | Libellulidae | Azores, Portugal; Sardinia, Italy | 2 (22) |
| 10 | Trithemis annulata | Libellulidae | Pontevedra, Spain | 0 (1) |
| Suborder Zygoptera (damselflies) | ||||
| 11 | Calopteryx haemorrhoidalis | Calopterygidae | Italy, Portugal, Spain | 0 (8) |
| 12 | C. splendens | Calopterygidae | Frosinone, Italy | 0 (2) |
| 13 | Haetarina sp. | Calopterygidae | Peru | 0 (1) |
| 14 | Aeolagrion sp. | Coenagrionidae | Pará, Brazil | 0 (1) |
| 15 | A. axine | Coenagrionidae | Napo, Ecuador | 0 (3) |
| 16 | A. quadratum | Coenagrionidae | Xalapa Mexico | 0 (3) |
| 17 | A. inca | Coenagrionidae | Pacaya-Samiria, Loreto, Peru | 0 (1) |
| 18 | Argia joergenseni | Coenagrionidae | Argentina | 0 (2) |
| 19 | A. kokama | Coenagrionidae | Tiputini, Ecuador | 0 (1) |
| 20 | Bromeliagrion sp. | Coenagrionidae | Pará, Brazil | 0 (1) |
| 21 | B. fernandezianum | Coenagrionidae | Tiputini Ecuador | 0 (1) |
| 22 | B. renhi | Coenagrionidae | Tiputini, Ecuador | 0 (1) |
| 23 | Coenagrion puella | Coenagrionidae | Cheshire, UK | 28 (28) |
| 24 | Enallagma cyathigerum | Coenagrionidae | Cheshire, UK | 7 (7) |
| 25 | Ischnura elegans | Coenagrionidae | Cheshire, UK | 0 (10) |
| 26 | I. graellsii | Coenagrionidae | Galicia | 0 (18) |
| 27 | I. hastata | Coenagrionidae | Azores (Portugal), Dominican Republic, Jamaica, Cuba, Mexico, Florida | 0 (43) |
| 28 | Leptobasis vacillans | Coenagrionidae | Santiago de Cuba, Cuba | 0 (2) |
| 29 | Metaleptobasis brysonima | Coenagrionidae | Pará, Brazil | 0 (1) |
| 30 | M. mauffrayi | Coenagrionidae | Tiputini, Ecuador | 0 (3) |
| 31 | M. quadricornis | Coenagrionidae | Pará, Brazil | 0 (1) |
| 32 | Phoenicagrion karaja | Coenagrionidae | Pará, Brazil | 0 (3) |
| 33 | Pyrrhosoma nymphula | Coenagrionidae | Cheshire, UK | 1 (2) |
| 34 | Telebasis carmesina | Coenagrionidae | Minas Gerais, Brazil | 0 (1) |
| 35 | T. dominicana | Coenagrionidae | Represa Chalons, Cuba | 0 (3) |
| 36 | T. salva | Coenagrionidae | Morelos, México | 0 (2) |
| 37 | Heteragrion bariai | Megapodagrionidae | Napo, Ecuador | 0 (1) |
| 38 | Hypolestes clara | Megapodagrionidae | Jamaica | 0 (12) |
| 39 | H. hatuey | Megapodagrionidae | Arroyo Bermejo, Dominican Republic | 0 (10) |
| 40 | H. trinitatis | Megapodagrionidae | Cuba | 0 (10) |
| 41 | Oxystigma sp. | Megapodagrionidae | Pará, Brazil | 0 (1) |
| 42 | Philogenia sp. | Megapodagrionidae | Napo, Ecuador | 0 (1) |
| 43 | Chalcopteryx rutilans | Polythoridae | Trocha Quebrada, Peru | 0 (1) |
| 44 | Cora sp. | Polythoridae | Panguana, Peru | 0 (1) |
| 45 | Polythore aurora | Polythoridae | Iquitos, Peru | 0 (1) |
| 46 | P. lamerceda | Polythoridae | Peru | 1 (3) |
| 47 | P. ornata | Polythoridae | Pampa Hermosa, Peru | 0 (6) |
| 48 | P. picta | Polythoridae | Pozuzo, Peru | 1 (7) |
| 49 | P. spaeteri | Polythoridae | Panguana, Peru | 0 (4) |
| 50 | P. victoria | Polythoridae | Pozuzo, Peru | 0 (9) |
| 51 | Drepanoneura sp. | Protoneuridae | Napo, Ecuador | 0 (3) |
| 52 | D. muzoni | Protoneuridae | Tiputini, Ecuador | 1 (2) |
| 53 | Epipleoneura metallica | Protoneuridae | Mato Grosso, Brazil | 0 (3) |
| 54 | E. fuscaenea | Protoneuridae | Guyana | 0 (2) |
| 55 | E. humeralis | Protoneuridae | Tiputini, Ecuador | 0 (4) |
| 56 | E. machadoi | Protoneuridae | Mato Grosso, Brazil | 0 (2) |
| 57 | E. williamsoni | Protoneuridae | Minas Gerais, Brazil | 0 (1) |
| 58 | Neoneura sp. | Protoneuridae | Pará, Brazil | 0 (2) |
| 59 | N. amelia | Protoneuridae | Veracruz Mexico | 0 (1) |
| 60 | N. bilinearis | Protoneuridae | Guyana | 0 (1) |
| 61 | N. confudens | Protoneuridae | Guyana | 0 (2) |
| 62 | N. denticulata | Protoneuridae | Pará, Brazil | 0 (1) |
| 63 | N. joana | Protoneuridae | Guyana | 0 (2) |
| 64 | N. myrthea | Protoneuridae | Guyana | 0 (2) |
| 65 | N. maria | Protoneuridae | Cuba | 0 (3) |
| 66 | N. sylvatica | Protoneuridae | Mato Grosso, Brazil | 1 (2) |
| 67 | Phasmoneura sp. | Protoneuridae | Mato Grosso, Brazil | 0 (1) |
| 68 | P. exigua | Protoneuridae | Mato Grosso, Brazil | 0 (1) |
| 69 | Protoneura sp. | Protoneuridae | Pará, Brazil | 0 (1) |
| 70 | P. caligata | Protoneuridae | Topes de Collantes, Cuba | 0 (1) |
| 71 | P. capillaris | Protoneuridae | Dos Bocas, Cuba | 0 (1) |
| 72 | P. klugi | Protoneuridae | Tiputini, Ecuador | 0 (1) |
| 73 | P. sanguinipes | Protoneuridae | Dominican Republic | 0 (3) |
| 74 | P. viridis | Protoneuridae | Jamaica | 0 (1) |
| 75 | Psaironeura sp. | Protoneuridae | Pará, Brazil | 0 (1) |
| 76 | P. tenuissima | Protoneuridae | Tiputini, Ecuador | 0 (4) |
| Additional UK coenagrionid damselflies screened | ||||
| 1 | Coenagrion mercuriale | Coenagrionidae | Hampshire, UK | 19 (30) |
| 2 | C. pulchellum | Coenagrionidae | Norfolk, UK | 15 (20) |
| 3 | Ceriagrion tenellum | Coenagrionidae | Hampshire, UK | 0 (5) |
| 4 | Erythromma najas | Coenagrionidae | Cheshire, UK | 1(5) |
| 5 | Pyrrhosoma nymphula | Coenagrionidae | Cheshire, UK | 4 (7) |
Species positive for Rickettsia in the PCR assays are highlighted in bold
General PCR Screening for Rickettsia
DNA was first quality checked (QC) to confirm that the samples contained amplifiable DNA template after storage/preparation. DNA QC was performed using the mtDNA barcoding primer pairs LCO_2190 (5′-GGT CAA CAA ATC ATC AAG ATA TTG G-3′)/HCO_2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) [33] and C1J_1718 (5′-GGA GGA TTT GGA AAT TGA TTA GT-3′)/C1N_2191 (5’-CAG GTA AAA TTA AAA TAT AAA CTT CTC G-3′) [34]. These primers amplify a fragment of approximately 680 and 470 bp of the cytochrome oxidase subunit 1 (COI) gene, respectively. Cycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation (94 °C, 30 s), annealing (54 °C, 30 s), extension (72 °C, 120 s) and a final extension at 72 °C for 7 min.
For samples passing QC, Rickettsia presence was assayed using Rickettsia-specific primers amplifying (a) a section of the bacterial 16S rRNA gene: Ri170_F (5′-GGG CTT GCT CTA AAT TAG TTA GT-3′)/Ri1500_R (5’-ACG TTA GCT CAC CAC CTT CAG G-3′) designed by Küchler et al. [17], and (b) the citrate synthase gene (gltA); RiGltA405_F (5′-GAT CAT CCT ATG GCA-3′)/RiGltA1193_R (5’-TCT TTC CAT TGC CCC-3′) designed by Pilgrim et al. [16]. These primers have been shown to amplify across currently known Rickettsia groups but not cross amplify other alphaproteobacteria. Cycling conditions were the same as described above for the COI. Nuclease-free water was used as a negative control to ensure there were no false positive amplifications, and genomic DNA of Culicoides newsteadi, obtained from Pilgrim et al. [16] was used as a positive control. For each species where a positive amplicon was obtained, amplicons were cleaned of primer and unincorporated nucleotides, and Sanger sequenced from a subset of individuals. The obtained sequence was then used (a) to confirm that the amplicon was a Rickettsia gene product, and (b) to allow estimation of the relatedness of the strains found. These verified positive samples were also selected as positive control in subsequent screenings.
Focussed Study of the UK Coenagrionid Species
Five additional UK coenagrionid species were collected from Cheshire, Hampshire and Norfolk. These samples were prepared and screened as described above to obtain Rickettsia sequences. Additionally, host mitochondrial barcodes were sequenced to confirm species identity, alongside additional markers to distinguish between the sister species Coenagrion puella and C. pulchellum. For distinction between C. puella/pulchellum, fragments of the Myosin light chain (MLC), Arginine methyltransferase (PRMT) and Phosphoglucose isomerase (PGI) nuclear genes were amplified and sequenced, following Ferreira et al. [32].
To allow a more in-depth study of Rickettsia diversity in the UK coenagrionid group, Rickettsia infections detected were further characterised by sequencing three additional loci; ATP-synthase (atpA), 17 kDa antigenic protein (ompA) and COI loci, to create a five loci allelic profile, allowing multi-locus sequence typing (MLST). The PCR conditions and primers used to amplify these genes were based on Pilgrim et al. [16].
Evidence for heritable symbiosis was investigated in C. puella by using fluorescence in situ hybridization (FISH) to ascertain the presence/absence of Rickettsia in ovarian tissues. Methods were adapted from Sakurai et al. [35]. Briefly, internal organs of three female C. puella (target species, Rickettsia positive) and three female Ischnura elegans (non-Rickettsia-infected species) were dissected and fixed in Carnoy’s solution (chloroform:ethanol:acetic acid, 6:3:1) overnight. Tissues were then cleared with 6% H2O2 in ethanol for 12 h or until the tissue were translucent (whichever was longer). Ovary material was then selected, and hybridisation conducted through incubating the tissues overnight in a hybridisation buffer (20 mM Tris-HCl pH 8.0, 0.9 M NaCl, 0.01% sodium dodecyl sulphate 30% formamide) with 10 pmol/ml of rickettsial rRNA-specific probe, 5'-CCA TCA TCC CCT ACT ACA-[ATTO 633]-3' [19]. After incubation, tissues were washed in buffer (0.3 M NaCl, 0.03 M sodium citrate, 0.01% sodium dodecyl sulphate), mounted onto a slide using VECTASHIELD® Antifade with DAPI as a mounting medium, and visualised under a confocal microscope (880 BioAFM).
Diversity of Rickettsia Infections
The phylogenetic relatedness of Rickettsia strains found in odonates based on 16S rRNA and gltA genes was estimated using MEGA X [36]. We selected several published sequences of Rickettsia from NCBI GenBank, including representatives varying in range from close to far distance relations to the strains in this study, based on BLAST homology. The far relative group consisted of several vertebrate pathogenic Rickettsia and other insect endosymbionts which are known belonging to other clades. Occidentia massiliensis was chosen as the outgroup for this Rickettsia topology. Sequences were manually checked and aligned using MUSCLE algorithm with default settings [37]. The relationships between these strains were estimated through the maximum likelihood approach using MEGA X, under the K2+I and T92+G+I model for 16S rRNA and gltA gene, respectively. Support for individual nodes was tested with 1000 bootstrap replicates.
Results
The initial broad screen of odonate material detected Rickettsia amplicons in 8 of the 76 species screened (Table 1), which represented nearly 50% of the families included in the screening. Positive material was derived from the UK, South America, mainland Europe and the Azores, indicating a broad geographic basis to the symbiosis. Four further Rickettsia symbioses were detected in the five additional UK species of Coenagrionidae tested in the focused screening (Table 1), resulting in a total of 6 of 8 UK coenagrionids testing positive.
In those cases where infection was detected in a species, the fraction of individuals testing positive for Rickettsia varied from 9 to 100% (Table 2). In two of the species with more than 1 sample, C. puella and Enallagma cyathigerum, 100% of the screened individuals were infected (Table 2). In cases where the individual sex was known (i.e., template derived from adults), there was no evidence of Rickettsia infection being biased to one host sex (Table 2).
Table 2.
Summary of Rickettsia-positive species, partitioned by host sex, identified across the broad and focused screens. Those listed as “unknown” correspond to non-sexed nymphs (n) and adults (a). Inside the brackets is the number of screened individuals, and outside is the number of infected individuals. Where multiple locations specified, the origin of the positive sample is marked with a superscript number indicating that the number of infected was found there. Asterisks indicate those UK coenagrionid species where the Rickettsia strains were successfully sequenced for all five MLST loci
| No. | Species | Location | Male | Female | Unknown | % infected |
|---|---|---|---|---|---|---|
| UK | ||||||
| 1 | Coenagrion puella* | Cheshire, UK | 8 (8) | 4 (4) | 16 (16 n) | 100 |
| 2 | C. mercuriale* | Hampshire, UK | 12 (20) | 7 (10) | - | 95 |
| 3 | C. pulchellum* | Norfolk, UK | - | - | 15 (20 a) | 75 |
| 4 | Enallagma cyathigerum* | Cheshire, UK | 6(6) | 1 (1) | - | 100 |
| 5 | Erythromma najas | Cheshire, UK | 1 (5) | - | - | 25 |
| 6 | Libellula depressa | Cheshire, UK | - | - | 1 (1 n) | 100 |
| 7 | Pyrrhosoma nymphula* | Cheshire, UK | 2 (4) | 2 (3) | - | 57 |
| South America | ||||||
| 8 | Drepanoneura muzoni | Tiputini, Ecuador | 1 (1) | 0 (1) | - | 50 |
| 9 | Neoneura sylvatica | Minas Gerais, Brazil | 1 (2) | - | - | 50 |
| 10 | Polythore lamerceda | Peru | 0 (1) | 1 (2) | - | 33 |
| 11 | P. picta | Pozuzo, Peru | 1 (6) | 0 (1) | - | 14 |
| Mainland Europe and the Azores | ||||||
| 12 | Sympetrum fonscolombii | Azores, Portugal2 Villasimius, Italy | 0 (6) | 1 (4) | 1 (12 n) | 9 |
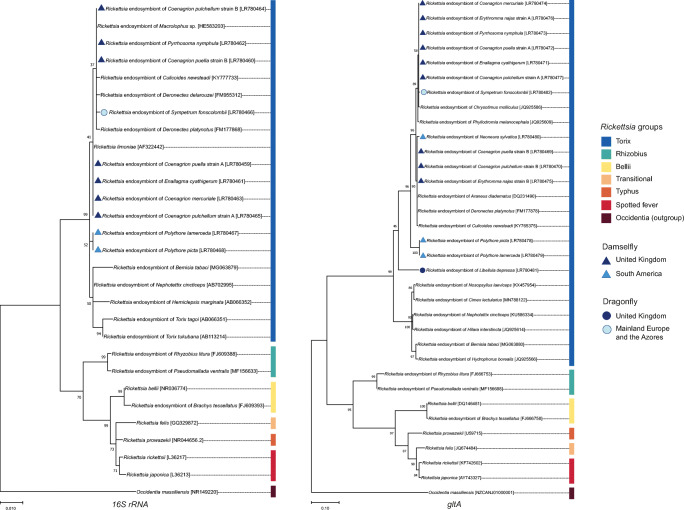
The Rickettsia strains from all 12 infected odonate species successfully produced gltA amplicons and 16S amplicons could be observed from 9 of 12 infected species. All the sequenced amplicons were used in phylogenetic analysis, except the Rickettsia strain from Drepanoneura muzoni, which produced a low quality of DNA sequence for both genes (Fig. 1). The Rickettsia infections detected all belong to the torix subclade of Rickettsia. The infections were diverse, with multiple strains found in odonates, all of them closely allied to Rickettsia strains found in other invertebrate taxa (Fig. 1).
Fig. 1.
Phylogenetic analysis of torix Rickettsia based on 16S rRNA and gltA gene sequences from screened odonate species, marked with coloured shapes, alongside reference DNA sequences of other Rickettsia groups obtained from GenBank (accession numbers in brackets). The tree was constructed in MEGA X by maximum likelihood, with K2+I and T92+G+I model for 16S and gltA, respectively. Numbers above branches indicate bootstrap values from 1000 resampling events. Labels indicate the host species from which the symbiont amplicon was obtained
MLST of the Rickettsia infecting UK coenagerionid species revealed the presence of four closely related Rickettsia strains falling into two clusters, as established in the MLST profiles (Table 3). The data also revealed that the sister species C. puella and C. pulchellum, which share a mtDNA COI haplotype but are distinct at nuclear loci (data not shown), share two Rickettsia strains, A and B, (Table 3). In these two species, there was a mix of double (strain A and B) and single (only strain A) Rickettsia-infected damselflies (coinfection was observed in five of ten C. puella, and two of three C. pulchellum). There were no individuals of either species infected with single Rickettsia strain B. Focussed analysis of 10 C. puella and 3 C. pulchellum individuals revealed an individual was either repeatedly monomorphic, or repeatedly polymorphic, across five loci (five individuals of each type; see Supplementary Table). The polymorphisms observed were largely at synonymous sites, indicating retained functionality of the gene product.
Table 3.
MLST allelic profiles of the Rickettsia found infecting five coenagrionid species from the UK. For any MLST gene locus, sequences with the same number are identical. A strain is defined as identity across all MLST loci
| Species | MLST allelic profiles | |||||
|---|---|---|---|---|---|---|
| 16S rRNA | gltA | ompA | atpA | coxA | Strain | |
| Coenagrion puella strain A | 1 | 1 | 1 | 1 | 1 | A |
| C. puella strain B | 2 | 2 | 2 | 2 | 2 | B |
| C. pulchellum strain A | 1 | 1 | 1 | 1 | 1 | A |
| C. pulchellum strain B | 2 | 2 | 2 | 2 | 2 | B |
| C. mercuriale | 1 | 1 | 1 | 1 | 1 | A |
| Pyrrhosoma nymphula | 2 | 3 | 2 | 2 | 2 | C |
| Enallagma cyathigerum | 1 | 1 | 1 | 3 | 1 | D |
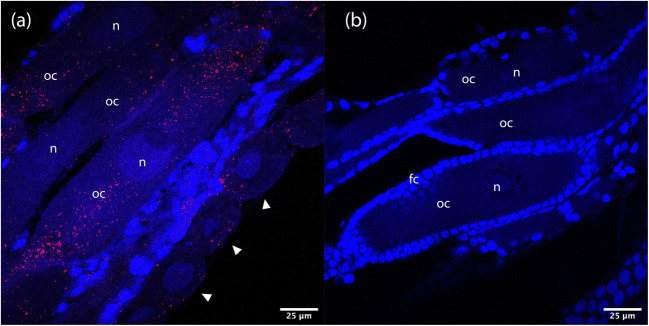
The tissue-mounted fluorescence in situ hybridization revealed a cellular tropism of torix Rickettsia in C. puella. The signal of Rickettsia (ATTO-633 fluorophore) was detected throughout the ovary tissues of C. puella, mostly in the nuclei and cytoplasmic area of both mature and early developing oocytes, while the signal was absent in the non-infected species, I. elegans (Fig. 2).
Fig. 2.
Fluorescence in situ hybridization (FISH) images showing the localisation of torix Rickettsia in a C. puella (Rickettsia positive) and b I. elegans (Rickettsia negative) oocytes. Red colour (ATTO633 label) represents Rickettsia signal and blue areas (DAPI) damselfly nuclei. Infection is observed throughout the ovary tissue of C. puella, mostly in oocytes (oc) and early differentiated oocytes (white arrowhead), but no signal of the symbiont was observed in the ovary of the Rickettsia-negative species, I. elegans; fc, follicular epithelial cells; n, nucleus of oocyte
Discussion
There are numerous heritable microbe taxa that circulate in insects which play important roles as partners and antagonists. While the majority of studies have focused on the ‘global pandemic’ of Wolbachia and its consequences for host biology, ecology and evolution [38]; other heritable symbionts remain less well studied, particularly in freshwater insects. Here, we examined odonates for just one such symbiont—torix group Rickettsia.
Within the global screen, we detected Rickettsia in 8 of 76 odonate species (10.5%) and for the focussed UK screen, in 6 of 8 (75%) species from the coenagrionid family. The Rickettsia infections discovered all fall into the torix group, a basal group of Rickettsia with high levels of diversity, previously highlighted as common in other aquatic invertebrates [14, 16, 17]. The fraction of infected species in our screen is likely to be an underestimate, as there are two systematic biases likely to produce false negative results. First, symbiont infections usually vary in prevalence within species, and can infect a minority of individuals. The limited number of individuals tested for some of the species screened could therefore miss some species with low or intermediate levels of infection. Second, the material available for testing was commonly derived from legs. Symbiont infection that is strongly localised within a host individual (and not present in hemocytes) will appear as negative when leg material is screened. Furthermore, although our data record more infections in species of the UK coenagrionids than elsewhere, this could also be a product of a greater sampling intensity. What is clear, however, is that whilst odonates are hosts to Rickettsia, and they carry torix group strains like other freshwater invertebrates, they do not appear to be a particular hotspot for Rickettsia, when compared with other freshwater insects [16].
The study of torix Rickettsia/insect symbioses is a relatively young field of research, with this diverse group only first described in 2002 [15]. Thus, despite now being known to be widespread, data on the biology of these symbioses is absent or extremely limited. For instance, within host titres are unknown, meaning that we do not know how many cells have to be present for us to be able to detect an infection. However, Rickettsia distribution in insect tissues are commonly diffuse, including haemocytes, Malpighian tubules, gut lining, and in oocytes, where they seem to invade through the follicular epithelium and, unusually, they have also been found in sperm [39].
The symbioses in our study were found in representative species from the two odonate suborders: Zygoptera (damselflies) and Anisoptera (dragonflies). These species belong to four different families from both Europe and South America (Tables 1 and 2). Sequence analysis revealed a wide diversity in Rickettsia infections, suggesting the Rickettsia-odonate symbiosis has multiple origins. The odonate Rickettsia grouped together with strains found in other host species e.g., Deronectes water beetle, Araneus orb-weaving spider, Culicoides biting midge and Cimex common bedbug (Fig. 1). There also appeared to be a hotspot in the UK coenagrionids, in which four MLST strains from two clusters were observed, with two of these strains present in several species. The MLST study of Rickettsia is a recent initiative, introduced by Pilgrim et al. in 2017 [16]. Therefore, more fine-scale comparisons between the Rickettsia strains in our study with those found in other insect orders are limited in scope, due to lack of multilocus data from other taxa. However, this geographically confined clade may reflect symbiont movement between co-occurring odonate species or derivation from a common local source [40].
The presence of double peaks in sequences of Rickettsia marker genes in C. puella and C. pulchellum provide evidence of coinfection, where a single individual carries two strains of Rickettsia. Individuals either show one sequence of strain A at all markers, or two sequences mixing of strain A and B at all markers (with two strains identified). Variable loci can either be the product of two infecting symbiont strains, or a single symbiont alongside a symbiont genome insertion into the insect chromosome [41]. That the amplicons represent two symbionts, rather than a symbiont and a nuclear insertion of symbiont genetic material, is implied by the nature of the variants. The majority of variable sites observed are synonymous differences (e.g., in GltA gene has 16 SNP in 715 bps, of which 14 are synonymous and 2 non-synonymous) that indicate retained functionality of the gene. Retained functionality is expected for a symbiont copy (where function is required) rather than a nuclear insert (which is expected to pseudogenize). Coinfections are well known for Wolbachia [42] but are less commonly recorded for other symbionts; however, they are clear in this system.
Within the UK group, we observed a pair of Rickettsia strains shared by the sister species pair C. puella and C. pulchellum. This species pair is robustly supported in analysis of nuclear markers [32, 43], but shares a mtDNA barcode [40]. Shared mtDNA barcodes for otherwise distinct species pairs commonly reflects introgression of the mtDNA across the species boundary [44]. This process is known to be driven by Wolbachia in other cases [45, 46]. Whilst hybridization is considered very uncommon between these species [47], mitochondrial introgression requires only a single hybridization event, and it is likely that the shared mtDNA and symbiont in this case reflect a history of symbiont movement across the species barrier, along with accompanying mtDNA. This process produces distinct species, divergent at nuclear markers, that have no mtDNA ‘barcoding gap’, as observed in the case of C. puella and C. pulchellum. An implication of our results is that screening for Wolbachia alone is not sufficient to rule out symbiont-mediated introgression of mtDNA.
Torix Rickettsia are considered likely to show maternal inheritance, and in some cases also show paternal transmission [39]. In our system, Rickettsia were visible in C. puella ovarioles under FISH microscopy, making maternal inheritance very likely. Additionally, infection was detected in both larvae and adults, which implies vertical transmission (Table 2). Thus, our data supports the idea Rickettsia is a heritable symbiont in odonates, as inferred for other taxa [16, 39, 48, 49].
The significance of the symbiosis is uncertain. Vertical transmission through eggs ties Rickettsia transmission to odonate survival and reproduction, and thus selects for symbiont contribution to host function. Heritable symbionts are commonly important contributors to organismal function but the impact of torix Rickettsia on their host is unknown in all but one system. In the parasitoid wasp, Pnigalio soemius [3], torix Rickettsia are associated with the induction of parthenogenesis. However, sex-ratio distortion mediated by Rickettsia is unlikely in the case of odonates, as there were no obvious male/female host biases in species where large numbers of individuals were collected. Indeed, the symbionts were absent in the only odonate species known to have thelytokous parthenogenesis (I. hastata from the Azores islands) [50]. These data, by exclusion, indicate that symbionts are likely retained in odonate hosts by some other means, which should be explored further.
Electronic supplementary material
(DOCX 37 kb)
Acknowledgements
We wish to thank Dr. Jack Pilgrim and Dr. Emily Hornett for comments on the manuscript. We thank Adolfo Cordero Rivera (University of Vigo) for allowing us to use specimens and DNA templates from his personal Odonata collection. MOL-C thanks David Outomuro, Beatriz Willink, Pablo Pessacq and Rosser W. Garrison, who kindly provided odonate specimens from their collections. The project was supported by the Development and Promotion of Science and Technology Talents Project (DPST) of the Institute for the Promotion of Teaching Science and Technology, Thailand to PT; a Harry Smith Vacation studentship (Microbiology society) to HRD and a grant from the Spanish Ministry with competences in Science, including FEDER funds (CGL2008-02799).
Availability of Data and Material
Gene sequences are available from EMBL (accession numbers LR778303-LR778309 and LR780445-LR780482).
Authors’ Contributions
The project was devised by PT, HD and GH. Material was collected/DNA extracted by PT, HD, DJT and MOL-C. PCR screening and sequencing was performed by PT and HD. Analysis was performed by PT, HD and GH. PT, HD and GH wrote the manuscript. All authors commented on the manuscript draft.
Funding Information
The project was supported by the Development and Promotion of Science and Technology Talents Project (DPST) of the Institute for the Promotion of Teaching Science and Technology, Thailand to PT; a Harry Smith Vacation Studentship (Microbiology Society) to HRD; and a grant from the Spanish Ministry with competences in Science, including FEDER funds (CGL2008–02799). Microscopy was conducted at the Centre for Cell Imaging (CCI), using equipment funded from BBSRC grant BB/M012441/1.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
Specimens from the UK were collected under a permit from Natural England (C. mercuriale) and with permission of the Norfolk Wildlife Trust. Specimens from AC-R personal collection were collected under relevant permits as follows: Ecuador Ministry of Environment capture permit 007–201 2-IC-FAU-MAE-DPO-PNY and export permit 007- EXP-IC-FAU·OPO/MA; Instituto Nacional de Recursos Naturales (INRENA) of Peru (Authorization #62–2008-INRENA-IFFS-DCB and #016 C/C-2008-INRENA-IANP); Government of Brazil (permit no 45256–1); Wildlife Research Application, Jamaica (Ref. #18/27); Dominican Republic Government; Regional Government of Galicia; Instituto para a Conservação da Natureza and Secretaría Regional do Ambiente (Portugal and Azores).
Statement of Informed Consent
All authors approve the final draft of the publication and consent to submission.
References
- 1.Curry MM, Paliulis LV, Welch KD, Harwood JD, White JA. Multiple endosymbiont infections and reproductive manipulations in a linyphiid spider population. Heredity. 2015;115(2):146–152. doi: 10.1038/hdy.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurst GDD, Majerus MEN. Why do maternally inherited microorganisms kill males? Heredity. 1993;71(1):81–95. [Google Scholar]
- 3.Giorgini M, Bernardo U, Monti MM, Nappo AG, Gebiola M. Rickettsia symbionts cause parthenogenetic reproduction in the parasitoid wasp Pnigalio soemius (Hymenoptera: Eulophidae) Appl Environ Microbiol. 2010;76(8):2589–2599. doi: 10.1128/AEM.03154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendry TA, Hunter MS, Baltrus DA (2014) The facultative symbiont Rickettsia protects an invasive whitefly against entomopathogenic Pseudomonas syringae strains. Appl Environ Microbiol 80(23). 10.1128/AEM.03179-14 [DOI] [PMC free article] [PubMed]
- 5.Wilkinson T, Koga R, Fukatsu T. Role of host nutrition in symbiont regulation: impact of dietary nitrogen on proliferation of obligate and facultative bacterial endosymbionts of the pea aphid Acyrthosiphon pisum. Appl Environ Microbiol. 2007;73(4):1362–1366. doi: 10.1128/AEM.01211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet. 2002;32(3):402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 7.Werren JH, Hurst GD, Zhang W, Breeuwer JA, Stouthamer R, Majerus ME. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata) J Bacteriol. 1994;176(2):388–394. doi: 10.1128/jb.176.2.388-394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?--a statistical analysis of current data. FEMS Microbiol Lett. 2008;281(2):215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter MS, Perlman SJ, Kelly SE. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc Biol Sci. 2003;270(1529):2185–2190. doi: 10.1098/rspb.2003.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiggins FM, Hurst GD, Majerus ME. Sex-ratio-distorting Wolbachia causes sex-role reversal in its butterfly host. Proc Biol Sci. 2000;267(1438):69–73. doi: 10.1098/rspb.2000.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. PNAS. 2010;107(2):769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstädter J et al (2008) The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6(27) [DOI] [PMC free article] [PubMed]
- 13.Johnson KN (2015) The Impact of Wolbachia on virus infection in mosquitoes. Viruses 7(11). 10.3390/v7112903 [DOI] [PMC free article] [PubMed]
- 14.Kikuchi Y, Fukatsu T. Rickettsia infection in natural leech populations. Microb Ecol. 2005;49:265–271. doi: 10.1007/s00248-004-0140-5. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi Y, Sameshima S, Kitade O, Kojima J, Fukatsu T. Novel clade of Rickettsia spp. from leeches. Appl Environ Microbiol. 2002;68:999–1004. doi: 10.1128/AEM.68.2.999-1004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilgrim J, Ander M, Garros C, Baylis M, Hurst GDD, Siozios S. Torix group Rickettsia are widespread in Culicoides biting midges (Diptera: Ceratopogonidae), reach high frequency and carry unique genomic features. Environ Microbiol. 2017;19(10):4238–4255. doi: 10.1111/1462-2920.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Küchler SM, Kehl S, Dettner K. Characterization and localization of Rickettsia sp. in water beetles of genus Deronectes (Coleoptera: Dytiscidae) FEMS Microbiol Ecol. 2009;68(2):201–211. doi: 10.1111/j.1574-6941.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin OY, Puniamoorthy N, Gubler A, Wimmer C, Bernasconi MV. Infections with Wolbachia, Spiroplasma, and Rickettsia in the Dolichopodidae and other Empidoidea. Infect Genet Evol. 2013;13:317–330. doi: 10.1016/j.meegid.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Perotti MA, Clarke HK, Turner BD, Braig HR. Rickettsia as obligate and mycetomic bacteria. FASEB J. 2006;20:E1646–E1656. doi: 10.1096/fj.06-5870fje. [DOI] [PubMed] [Google Scholar]
- 20.Chovanec A (2000) Dragonflies (Insecta: Odonata) as indicators of the ecological integrity of aquatic systems – a new assessment approach. SIL Proceedings, 1922–2010. 27(2):887–90. 10.1080/03680770.1998.11901366
- 21.Córdoba-Aguilar A (ed) (2008) Dragonflies and damselflies: model organisms for ecological and evolutionary research. Oxford University Press, UK
- 22.Salunkhe RC, Dhotre DP, Salunke BK, Patil VS, Mahale V, Andrew RJ et al (2015) Distribution and molecular characterization of Wolbachia endosymbionts in Odonata (Insecta) from Central India by multigene approach. Curr Sci 108(5):971–978
- 23.Thipaksorn A, Jamnongluk W, Kittayapong P. Molecular evidence of Wolbachia infection in natural populations of tropical odonates. Curr Microbiol. 2003;47(4):0314–0318. doi: 10.1007/s00284-002-4010-4. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzo-Carballa MO, Torres-Cambas Y, Heaton K, Hurst GDD, Charlat S, Sherratt TN, van Gossum H, Cordero-Rivera A, Beatty CD. Widespread Wolbachia infection in an insular radiation of damselflies (Odonata, Coenagrionidae) Sci Rep. 2019;9(1):11933. doi: 10.1038/s41598-019-47954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swaegers J, Janssens SB, Ferreira S, Watts PC, Mergeay J, McPeek MA et al (2014) Ecological and evolutionary drivers of range size in Coenagrion damselflies. J Evol Biol 27(11):2386–2395. 10.1111/jeb.12481 [DOI] [PubMed]
- 26.Lorenzo-Carballa MO, Torres-Cambas Y, Ferreira S, Trapero-Quintana AD, Cordero-Rivera A. Microneura is a junior synonym of Protoneura (Zygoptera, Coenagrionidae) Int J Odonatol. 2016;19(1–2):13–22. [Google Scholar]
- 27.Torres-Cambas Y, Ferreira S, Cordero-Rivera A, Lorenzo-Carballa MO. Mechanisms of allopatric speciation in an Antillean damselfly genus (Odonata, Zygoptera): Vicariance or long-distance dispersal? Mol Phylogenet Evol. 2019;137:14–21. doi: 10.1016/j.ympev.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez Herrera M, Kuhn WR, Lorenzo-Carballa MO, Harding KM, Ankrom N, Sherratt TN, Hoffmann J, van Gossum H, Ware JL, Cordero-Rivera A, Beatty CD. Mixed signals? Morphological and molecular evidence suggest a color polymorphism in some neotropical Polythore damselflies. PLoS One. 2015;10(4):e0125074. doi: 10.1371/journal.pone.0125074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzo-Carballa MO, Hadrys H, Cordero-Rivera A, Andrés JA. Population genetic structure of sexual and parthenogenetic damselflies inferred from mitochondrial and nuclear markers. Heredity. 2012;108(4):386–395. doi: 10.1038/hdy.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva-Méndez G, Lorenzo-Carballa MO, Cordero-Rivera A, Watts PC. Microsatellite loci for two threatened dragonfly (Odonata: Anisoptera) species: Oxygastra curtisii (Dale, 1834) and Macromia splendens (Pictet, 1843) Conserv Genet Resour. 2013;5(4):1171–1174. [Google Scholar]
- 31.Lorenzo-Carballa MO, Watts PC, Cordero-Rivera A. Hybridization between Calopteryx splendens and C. haemorrhoidalis confirmed by morphological and genetic analyses. Int J Odonatol. 2014;17(2–3):149–160. [Google Scholar]
- 32.Ferreira S, Lorenzo-Carballa MO, Torres-Cambas Y, Cordero-Rivera A, Thompson DJ, Watts PC. New EPIC nuclear DNA sequence markers to improve the resolution of phylogeographic studies of coenagrionids and other odonates. Int J Odonatol. 2014;17(2–3):135–147. [Google Scholar]
- 33.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3(5):294–299. [PubMed] [Google Scholar]
- 34.Gibson JF, Kelso S, Jackson MD, Kits JH, Miranda GFG, Skevington JH. Diptera-specific polymerase chain reaction amplification primers of use in molecular phylogenetic research. Ann Entomol Soc Am. 2011;104(5):976–997. [Google Scholar]
- 35.Sakurai M, Koga R, Tsuchida T, Meng X-Y, Fukatsu T. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl Environ Microbiol. 2005;71(7):4069–4075. doi: 10.1128/AEM.71.7.4069-4075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stouthamer R. Chapter 269 - Wolbachia. In: Resh VH, Cardé RT, editors. Encyclopedia of insects. 2. San Diego: Academic Press; 2009. pp. 1061–1063. [Google Scholar]
- 39.Watanabe K, Yukuhiro F, Matsuura Y, Fukatsu T, Noda H. Intrasperm vertical symbiont transmission. PNAS. 2014;111(20):7433–7437. doi: 10.1073/pnas.1402476111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeland JR, Conrad KF. Genetic similarity within and among populations of the variable and azure damelflies (Coenagrion pulchellum and C. puella) Hydrobiologia. 2002;479(1):69–73. [Google Scholar]
- 41.Dunning Hotopp JC, Clark ME, Oliveira DC, Foster JM, Fischer P, Munoz Torres MC, et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science. 2007;317(5845):1753–1756. doi: 10.1126/science.1142490. [DOI] [PubMed] [Google Scholar]
- 42.Ant TH, Sinkins SP. A Wolbachia triple-strain infection generates self-incompatibility in Aedes albopictus and transmission instability in Aedes aegypti. Parasit Vectors. 2018;11(1):295. doi: 10.1186/s13071-018-2870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira S, Boudot J-P, El Haissoufi M, Alves PC, Thompson DJ, Brito JC, et al. Genetic distinctiveness of the damselfly Coenagrion puella in North Africa: an overlooked and endangered taxon. Conserv Genet. 2016;17(4):985–991. [Google Scholar]
- 44.Hurst GDD, Jiggins FM. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proc R Soc B. 2005;272:1525–1534. doi: 10.1098/rspb.2005.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller GP, Windsor DM, Saucedo JM, Werren JH. Reproductive effects and geographical distributions of two Wolbachia strains infecting the Neotropical beetle, Chelymorpha alternans Boh. (Chrysomelidae, Cassidinae) Mol Ecol. 2004;13(8):2405–2420. doi: 10.1111/j.1365-294X.2004.02213.x. [DOI] [PubMed] [Google Scholar]
- 46.Jiggins FM. Male-killing Wolbachia and mitochondrial DNA: selective sweeps, hybrid introgression and parasite population dynamics. Genetics. 2003;164(1):5–12. doi: 10.1093/genetics/164.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowe CD, Harvey IF, Thompson DJ, Watts PC. Strong genetic divergence indicates that congeneric damselflies Coenagrion puella and C. pulchellum (Odonata: Zygoptera: Coenagrionidae) do not hybridise. Hydrobiologia. 2008;605(1):55–63. [Google Scholar]
- 48.Weinert LA. The diversity and phylogeny of Rickettsia. In: Morand S, Krasnov BR, Littlewood DTJ, editors. Parasite diversity and diversification evolutionary ecology meets phylogenetics. UK: Cambridge University Press; 2015. p. 150. [Google Scholar]
- 49.Perlman SJ, Hunter MS, Zchori-Fein E. The emerging diversity of Rickettsia. Proc R Soc B Biol Sci. 2006;273:2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorenzo-Carballa MO, Cordero-Rivera A. Thelytokous parthenogenesis in the damselfly Ischnura hastata (Odonata, Coenagrionidae): genetic mechanisms and lack of bacterial infection. Heredity. 2009;103:377–384. doi: 10.1038/hdy.2009.65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 37 kb)
Data Availability Statement
Gene sequences are available from EMBL (accession numbers LR778303-LR778309 and LR780445-LR780482).