Abstract
Childhood obesity is a global public health problem. Understanding the molecular mechanisms that underlie early origins of childhood obesity can facilitate interventions. Consistent phenotypic and genetic correlations have been found between childhood obesity traits and birth weight (a proxy for in-utero growth), suggesting shared genetic influences (pleiotropy). We aimed to (1) investigate whether there is significant shared genetic influence between birth weight and childhood obesity traits, and (2) to identify genetic loci with shared effects. Using a statistical approach that integrates summary statistics and functional annotations for paired traits, we found strong evidence of pleiotropy (P < 3.53 × 10–127) and enrichment of functional annotations (P < 1.62 × 10–39) between birth weight and childhood body mass index (BMI)/obesity. The pleiotropic loci were enriched for regulatory features in skeletal muscle, adipose and brain tissues and in cell lines derived from blood lymphocytes. At 5% false discovery rate, 6 loci were associated with birth weight and childhood BMI and 13 loci were associated with birth weight and childhood obesity. Out of these 19 loci, one locus (EBF1) was novel to childhood obesity and one locus (LMBR1L) was novel to both birth weight and childhood BMI/obesity. These findings give evidence of substantial shared genetic effects in the regulation of both fetal growth and childhood obesity.
Subject terms: Genetics, Risk factors
Introduction
Childhood overweight or obesity is a global public health problem1–3. Complex interactions of genetic susceptibility4–6, environmental exposures7,8 and in-utero programming9–12 contribute to childhood obesity. Fetal growth and development during the intrauterine period shape physiological and structural processes that impact risk for childhood obesity (COB)9. Birth weight, a marker of fetal growth and prenatal environment, has been consistently associated with COB13–20. Notably, a significant positive genetic correlation has been found between birth weight and COB/childhood body mass index (CBMI)21, suggesting the possibility for genetic links these phenotypes may share. Insight into the shared genetic underpinnings of these early life phenotypes facilitates knowledge on the potential common molecular pathways and intervention targets of abnormal birth weight and COB/CBMI. However, pleiotropic evidence on these traits remains scarce.
Recent genome wide association studies (GWAS) have reported that shared genetic effects (pleiotropy) might explain a substantial proportion of correlations between complex human traits22–26. In an analysis of the GWAS catalog27 it was found that 16.9% of the reported genes were associated with multiple traits28. With the advent of large scale independent GWASs on multiple early life traits and functional annotation database29–31, innovative statistical approaches can be applied to identify and test for novel pleiotropic loci.
In addition, accounting for shared genetic effects improves statistical power for detecting genetic variants that are associated with complex traits32. Both birth weight and COB/CBMI are under strong genetic control10,11,33–35, with relatively high heritability estimates of 25–40% for birth weight35 and 30–70% for COB/CBMI34. However, the heritability of the traits explained by genetic variants identified using published GWAS for birth weight36, COB37 and CBMI38–40 was less than 10%. Therefore, detecting novel genetic loci via pleiotropic analysis can help close the gap in missing heritability.
To date, pleiotropic associations of birth weight and COB/CBMI have not been exclusively investigated. In the current study, we used an innovative statistical approach that integrates pleiotropy and functional annotation data with the following aims: (1) to investigate whether there is significant shared genetic influence between birth weight and COB/CBMI (pleiotropy enrichment test), (2) to examine whether the shared risk variants are functionally more enriched in birth weight and COB/CBMI as compared to only considering single traits in models that incorporate a variety of genetic annotations in different tissues and cell types (annotation enrichment test), and (3) to identify genetic loci with shared genetic effects on birth weight and COB/CBMI. Our analysis found abundant evidence of pleiotropy and significant enrichment of functional annotations for shared risk variants associated with birth weight and COB/CBMI. We identified genetic loci with overlapping influence on both birth weight and COB/CBMI. Most of those loci have been associated with childhood BMI/obesity in previous GWAS but not with birth weight, whereas one locus (EBF1) was novel to childhood obesity and one locus (LMBR1L) was novel to both birth weight and childhood BMI/obesity.
Results
Data on summary statistics including P values and direction of effects in GWAS meta-analysis of birth weight, CBMI and COB was obtained from publicly available data of the Early Growth Genetics (EGG) consortium. Two sets of GWAS summary statistics were available for birth weight, one involving European ancestry individuals (BWEU) and a second from trans-ancestry meta-analysis (BWTR). The GWAS summary statistics for CBMI and COB were from European ancestry individuals. Therefore, tests of pleiotropy were conducted on four trait pairs: BWEU-CBMI, BWEU-COB, BWTR-CBMI, and BWTR-COB.
Pleiotropy analysis was performed via the R package GPA41 which implements a statistical approach to explore the genetic architecture of complex traits by integrating pleiotropy and functional annotation information, including prioritizing risk genetic variants. GPA performs hypothesis test for evaluating enrichment of pleiotropy and functional annotations using a likelihood ratio test approach. For each trait pair, we performed tests of pleiotropy and enrichment of functional annotations based on 4 functional categories: combined annotation dependent depletion (CADD) (http://www.cadd.gs.washington.edu)42, expression quantitative trait loci (eQTL) (https://gtexportal.org/home/datasets)43, transcription factor binding sites (TFBS) (ftp://ccg.epfl.ch/snp2tfbs/mapped_files/annotated)44, and DNase I hypersensitivity sites (DHS) (https://github.com/joepickrell/1000-genomes)45.
Evidence of pleiotropy between birth weight and childhood obesity traits
Under each functional category, we found evidence of significant pleiotropic genetic effects for all tested trait pairs. Pleiotropic genetic effects had enrichment fold ranging from 2.6 to 5.4 (1.17 × 10–277 < P < 3.51 × 10–127) under CADD annotation, 2.6 to 5.2 (1.18 × 10–277 < P < 3.51 × 10–127) under eQTL annotation, 2.6 to 5.1 (2.52 × 10–276 < P < 6.52 × 10–127) under DHS annotation, and 2.6 to 5.1 (1.18 × 10–277 < P < 6.52 × 10–127) under TFBS annotation (Table 1).
Table 1.
Genetic pleiotropy and enrichment of functional deleteriousness among genetic loci associated with birth weight and childhood obesity traits.
| Trait pair | Annotation | Genetic pleiotropy | Functional annotation enrichment | ||
|---|---|---|---|---|---|
| Enrichment fold (s.e) | P value | q11/q00 (s.e) | P value | ||
| BWEU and CBMI | CADD | 2.683 (0.05) | 1.17 × 10–277 | 1.69 (0.08) | 2.69 × 10–38 |
| eQTLs | 2.679 (0.05) | 1.18 × 10–277 | 2.10 (0.04) | ≤ 1 × 10–300 | |
| DHSs | 2.67 (0.05) | 2.52 × 10–276 | 1.17 (0.01) | ≤ 1 × 10–300 | |
| TFBS | 2.685 (0.05) | 1.18 × 10–277 | 1.09 (0.03) | 0.03 | |
| BWEU and COB | CADD | 5.413 (0.19) | 9.79 × 10–144 | 1.54 (0.18) | 1.42 × 10–27 |
| eQTLs | 5.396 (0.29) | 9.79 × 10–144 | 1.73 (0.09) | ≤ 1 × 10–300 | |
| DHSs | 5.379 (0.24) | 1.45 × 10–143 | 1.15 (0.03) | ≤ 1 × 10–300 | |
| TFBS | 5.383 (0.24) | 9.79 × 10–144 | 1.01 (0.06) | 0.21 | |
| BWTR and CBMI | CADD | 2.653 (0.05) | 9.97 × 10–274 | 1.79 (0.08) | 1.62 × 10–39 |
| eQTLs | 2.648 (0.05) | 9.98 × 10–271 | 2.15 (0.04) | ≤ 1 × 10–300 | |
| DHSs | 2.641 (0.05) | 2.18 × 10–269 | 1.17 (0.02) | ≤ 1 × 10–300 | |
| TFBS | 2.655 (0.05) | 9.98 × 10–271 | 1.10 (0.03) | 0.01 | |
| BWTR and COB | CADD | 5.209 (0.17) | 3.51 × 10–127 | 1.68 (0.18) | 1.94 × 10–29 |
| eQTLs | 5.204 (0.18) | 3.51 × 10–127 | 1.80 (0.10) | ≤ 1 × 10–300 | |
| DHSs | 5.186 (0.20) | 6.52 × 10–127 | 1.12 (0.03) | ≤ 1 × 10–300 | |
| TFBS | 5.197 (0.18) | 3.51 × 10–127 | 0.99 (0.07) | 0.04 | |
q11/q00 is the ratio of the probability of jointly associated SNPs being functionally annotated to the probability of a null SNP (not associated with either trait) being functionally annotated.
BWEU and CBMI European birth weight and childhood body mass index, BWEU and COB European birth weight and childhood obesity, BWTR and CBMI trans-ethnic birth weight and childhood body mass index, BWTR and COB trans-ethnic birth weight and childhood obesity, CADD combined annotation dependent depletion, eQTLs expression quantitative loci, DHSs DNase I hypersensitivity sites, TFBS transcription factor binding sites.
Enrichment of functional annotations
Next, we assessed whether genetic loci with regulatory functional annotations had higher likelihood of being associated with both birth weight and CBMI/COB as compared to genetic loci without functional annotations. Functional annotation of the 2.4 million single-nucleotide polymorphisms (SNPs) tested was done using CADD42, eQTL43, TFBS44 , and DHS45. Out of the ~ 2.4 million SNPs tested, approximately 2% of the SNPs were annotated as CADD related, 7% as eQTL related, 20% as TFBS related and 51% as DHS related (Table S1). Enrichment of each of the four functional categories was tested for four trait pairs using the GPA model41, resulting in 16 tests of functional enrichment.
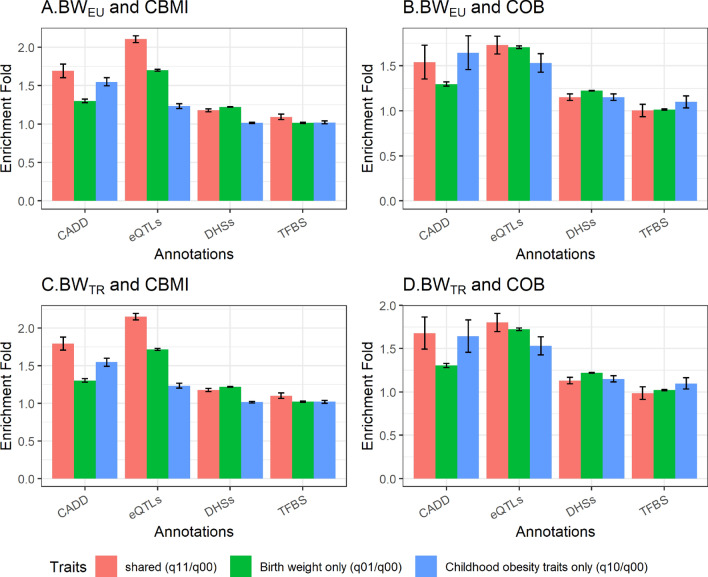
To compare the functional deleteriousness of the pleiotropic SNPs with non-pleiotropic SNPs, each variant in our analysis was annotated to be deleterious or non-deleterious based on CADD score ≥ 15 or CADD score < 15, respectively42. Out of the 16 tests, SNPs associated with pairs of birth weight and childhood obesity traits were more likely to be functionally deleterious than SNPs associated with neither trait in 15 tests (enrichment fold ranging from 0.99 to 2.15 and Bonferroni corrected P values ranging from 1 × 10–300 ≤ P < 1.62 × 10–39); however, 1 test under TFBS category for BWEU-COB pair was not significant (P = 0.21) (Table 1). Enrichment of eQTL annotation was consistently higher for SNPs associated with both birth weight and COB traits compared to SNPs associated with only birth weight or CBMI/COB (Fig. 1).
Figure 1.
Enrichment of four functional annotations among loci jointly associated with birth weight and childhood obesity traits. (A) European birth weight and childhood body mass index (BWEU-CBMI), (B) European birth weight and childhood obesity (BWEU-COB), (C) Trans-ethnic birth weight and childhood body mass index (BWTR-CBMI), (D) Trans-ethnic birth weight and childhood obesity (BWTR-COB). The bars denote the enrichment fold for variants that are associated jointly with BW and childhood obesity traits (red), only with birth weight (green), and only with childhood obesity traits (blue), respectively.
We further examined the specific tissues, cell types and transcription factors that had relatively higher functional enrichment among the common variants between birth weight and childhood obesity traits. For each trait pair, we performed annotation enrichment tests of 49 tissues with eQTL annotations43, 402 tissues/cell lines/cell types with DHSs annotations45, and 195 transcription factors in the TFBS database44, one at a time. The most significant eQTL enrichments (P < 0.05 and lowest in the joint trait association) were observed in tissues from skeletal muscle, adipose, brain, heart, esophagus, thyroid, adrenal, colon, small intestine and whole blood. The most significant DHS enrichments were observed in cell lines derived from blood lymphocytes (e.g. CD4+), skin (e.g. iPS), cancer (e.g. HeLa), embryo (e.g. embryonic stem cells) and in tissues from fetal brain. The most significant transcription factor enrichments were Interferon Regulatory Factor 1, AT-Rich Interaction Domain 3A and Testicular receptor 4 (Table S2–S3).
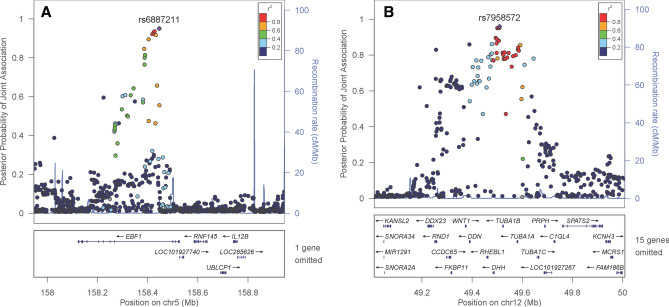
At 5% false discovery rate, 19 loci (consisting 509 SNPs, with each locus having correlated SNPs with linkage disequilibrium r2 > 0.5) were jointly associated with birth weight and childhood obesity traits (Figs. S1–S4). Among these 19 loci, 5 loci were jointly associated with European BW and COB traits, 1 locus was jointly associated with transethnic BW and COB traits and 13 loci were jointly associated with both European and transethnic BW and COB traits (Table S4–S5). Additionally, out of these 19 loci, 17 loci were novel to birth weight but not childhood obesity traits at the genome-wide significance level (P < 5 × 10–8, in the NHGRI-EBI GWAS catalogue: www.ebi.ac.uk/gwas/), one locus (rs6887211 in EBF1) was novel to childhood obesity traits but not birth weight, and one locus (rs7958572 4 Kb upstream to LMBR1L) was novel to both birth weight and childhood obesity traits (Fig. 2). The EBF1 locus was only suggestively associated with childhood obesity traits (P = 8.44 × 10–5) and the LMBR1L locus was only suggestively associated with birth weight (P = 2.4 × 10–3) and childhood obesity traits (P = 7.3 × 10–6) in previous GWAS36,39.
Figure 2.
Regional plots of novel pleiotropic loci associated with birthweight and childhood obesity traits. (A) rs6887211 (EBF1) locus association with both birth weight and childhood body mass index (B) rs7958572 (LMRB1L) locus associated with both birthweight and childhood obesity or childhood body mass index. The horizontal axes cover a region 500 kb upstream and downstream from the reference SNP. The vertical axes denote the joint association probabilities of the SNPs with both birthweight and childhood obesity traits. The purple triangles denote the index SNPs (rs6887211 in EBF1 and rs7958572 in LMRB1L). All other colored points denote the surrounding SNPs in that region, and they are colored based on their linkage disequilibrium (r2) with the reference SNP. The box at the bottom shows genes that fall in the region.
For most of the jointly associated loci, the alleles associated with decreased birth weight were associated with lower CBMI/COB risk. In contrast, for three loci (EBF1, NCOA1 and SEC16B), nearly all alleles associated with decreased birth weight were associated with higher CBMI/COB risk (Table S4–S5). Furthermore, we evaluated our findings using the European-ancestry summary statistics in a recent childhood obesity GWAS46. Out of the 342 SNPs in our study found to be associated with both birthweight and childhood obesity, 98.5% reached > 95% posterior probability when we used this validation childhood obesity GWAS (Table S6).
Pathway analysis
To further understand the functional relevance of the genetic loci jointly associated with birth weight and childhood obesity traits, we performed pathway enrichment analysis of the 25 genes that are near the pleiotropic SNPs using the Ingenuity Pathway Analysis (IPA) tool (QIAGEN Inc, https://digitalinsights.qiagen.com/). The top enriched IPA Canonical Pathways included RAR Activation and Estrogen Receptor Signaling (Table S7) and the top enriched IPA Disease or Function annotations included brain size and morphology (Table S8).
Colocalization analysis
To determine whether the signals identified represent horizontal pleiotropy (influence birthweight and childhood obesity via independent biological pathways) or vertical pleiotropy (influence via shared mechanism in a causal pathway) we performed colocalization analysis using the coloc R package47. Only the EBF1 locus showed evidence of colocalization with a posterior probability of 0.87 for being associated with both traits and share a single causal variant (Tables S9 and S10).
Discussion
There is growing evidence that several genetic variants can influence two or more traits28,48,49. Investigating such effects in early life traits can facilitate our understanding of their complex genetic architecture and in developing early life interventions to promote long-term health50,51. The present study revealed substantial genetic pleiotropic effects between birth weight and childhood obesity traits. We also found that biologically functional SNPs are more likely to be associated with both birth weight and COB traits compared to SNPs that are not functional, consistent with evidence of high evolutionary conservation of pleiotropic genes and their consequences52. Lastly, we identified 19 genetic loci with pleiotropic effects, including loci in EBF1 and LMRBL1 that have not been associated with birth weight and/or COB phenotypes in previous GWAS. With the majority (68.4%) of the 19 loci reaching the significance threshold in analyses involving European-only or trans-ethnic birthweight GWAS and the remaining loci exhibiting high posterior probability of association (0.85–0.93), the results suggest that the effects of many pleiotropic loci are likely shared across ancestries. In all, these findings facilitate our understanding of the genetic mechanism that may underlie associations of early life growth with COB traits.
The birth weight-decreasing allele of the novel pleiotropic SNP rs6887211 (EBF1) was found to be associated with increased risk of COB traits. The association of EBF1 with birth weight and gestational duration is well-recognized36,53–55. EBF1 is highly expressed in adipocytes (Fig. S5) and plays a crucial role in adipogenesis and development of B lymphocytes. Dysregulated expression of EBF1 is associated with adipose hypertrophy, adipose inflammation, variation in body fat distribution and altered adipose morphology through impaired adipogenesis, which in turn has been implicated as a key factor in the development of obesity related traits56–59. EBF1 has a well-known association with cardiometabolic traits in adults. SNPs near EBF1 have been associated with blood pressure60 and hip circumference61. Notably, a multi-ethnic GWAS in adults has found an association between rs1650505 (near EBF1) and pericardial adipose tissue volume56. Previous studies found substantial overlaps between childhood and adult obesity GWAS loci62. While there is relatively weak LD between the lead SNP showing pleiotropic effect in our study (rs6887211) and rs1650505 (r2 = 0.06 in CEU), we acknowledge that the novelty of the locus in childhood BMI may be in part due to the small study power of the source GWAS used for childhood BMI. Furthermore, our novel SNP and its LD proxies (r2 ≥ 0.8) overlapped with DNase I hypersensitivity sites of blood lymphocytes and fetal muscle further indicating that many components of the metabolic and inflammatory pathways are positively and directly regulated by EBF1 as emphasized in a prior study63. These findings collectively indicate EBF1’s role in fetal growth and general adiposity which highlights that it should be considered as a key player in understanding the association of early life growth with the development of COB.
The birth weight-decreasing allele of the novel pleiotropic SNP in LMBR1L (rs7958572) was found to be associated with decreased risk of COB. LMBR1L has regulatory effects on the canonical Wnt signaling pathway. It stabilizes the beta-catenin destruction complex64 that is required for regulating CTNNB1 expression, and is responsible in making the beta-catenin protein which plays a key role in the canonical Wnt signaling pathway65–68. The Wnt signaling pathway is conserved in various organisms and plays important roles in development, cellular proliferation, and differentiation69. Previous studies have demonstrated that the Wnt signaling pathway regulates adipogenesis and maintains the undifferentiated state of pre-adipocytes by inhibiting adipogenic gene expression 70,71. In addition, the Wnt signaling cascade plays fundamental roles in placental development by regulating trophoblast differentiation and invasion, and aberrant Wnt signaling activation can have downstream consequences on fetal growth 72–74. Our finding here for LMBR1L, together with the regulatory impact of LMBR1L on Wnt signaling and the functional relevance of the Wnt pathway in placental function, suggests a novel hypothetical mechanism by which LMBR1L contributes to the link between fetal growth and development of COB. Future studies such as eQTLs in tissues relevant to early life traits can facilitate better inference from the pleiotropic SNP to a potential functional gene.
In agreement with previous observational studies which reported that small for gestational age (SGA) or low birth weight is associated with both lower and higher risk of COB13–20, we observed that birth weight-decreasing alleles of pleiotropic SNPs may be associated with either increased or decreased risk of COB. We found that the birth weight-reducing alleles of most of the pleiotropic loci we found (e.g. FTO, FAIM2, TMEM18) have previously been associated with decreased risk of COB, suggesting that concurrent genetic mechanisms may play an important role in the well-recognized positive correlation between low birth weight and low risk of COB. We also found that in 3 out of our 19 pleiotropic loci (i.e., EBF1, SEC16B, NCOA1) the birth weight-decreasing alleles were associated with increased risk of COB. Genetic loci near EBF1and SEC16B genes have been associated with birth weight, SGA and adiposity traits 53–56,75,76; however, the association of NCOA1 with birth weight or COB traits remains elusive. Interestingly, co-activators like NCOA1 are fundamental in uterine growth by regulating placental morphogenesis, embryo survival and interacting with estrogen receptors in the human placenta to enhance estrogen signaling which has downstream consequences on birth weight77,78. NCOA1 is also highly expressed in the hypothalamus region of the brain (Fig. S6) which is implicated in appetite control, weight loss and shaping the metabolic landscape of an individual79 and any disruption of NCOA1′s function can consequently lead to several metabolic disorders which may explain its association with COB79,80. Therefore, the mixed direction of effects found in our study highlights the complex relationship of in-utero growth and COB traits and may provide new scopes to understand the mechanisms of development of COB.
Functional enrichment tests and pathway analysis revealed three key biological processes that may jointly contribute to the association of early life growth and development of COB. Our study found that SNPs associated with both birth weight and COB traits mapped to genes that were significantly enriched in immune system pathways and regulated the signaling of immune cells and estrogen. Estrogen plays an essential role in regulating immune response, through its interactions with the receptors on the immune cells and their functioning81. Previous studies82,83 have demonstrated that the cell‑mediated immune response is impaired in obese children along with an over‑representation of the immune and inflammatory response in adipose tissues of children. We also found the pleiotropic SNPs to be significantly enriched in Retinoic Acid (RA) signaling pathway and have cis-regulatory effects on gene expression in adipose tissue and muscle skeletal. Multiple studies have documented that RA regulates adipogenesis84–86. RA acts as a high affinity ligand for the nuclear receptor peroxisome proliferation-activated receptor β/δ which is a master regulator of lipid metabolism and glucose homeostasis. Deactivation of these receptors decreases lipid catabolism in adipose tissue and skeletal muscle, increasing the risk of obesity. Lastly, we found the pleiotropic SNPs to be significantly enriched in intracerebral signaling pathway by having regulatory effects on several cerebral tissues. Brain-derived neurotrophic factor (BDNF), a key neurotrophin with multipotent impact on brain signaling87 plays an important role in regulating energy homeostasis and metabolism88–90. BDNF acts on hypothalamic PVN and VMH neurons to suppress appetite and mediate the anorexigenic effects of MSH acting on the MCH-4 receptor89. Deficit in BDNF levels or signaling is attributed to the development of obesity91,92, indicating that pathways of intracerebral signaling may be related to COB. Our results suggest that these same causal biologic pathways likely influence early life growth, but further research is needed to understand how these pathways that influence COB also influence birth weight.
We recognize that our study has limitations. First, despite the large sample sizes of the consortia-based meta-analysis GWAS studies included in our study, there were differences in sample sizes and number of SNPs among the various GWAS studies. These contrasts may contribute to power differences in identifying pleiotropic loci. Additionally, some of the observed associations might be due to independent associations of the locus on birth weight and COB traits, due to the correlation of the traits in a causal pathway or some unmeasured characteristics. Second, the inference on joint associations of the variants in our study is based on estimates from the GPA model which performs optimally under low to moderate genetic correlation between the traits; however, under complex and weaker correlations the estimates of the GPA model can be biased. Third, some cohorts contributed to both childhood BMI and birthweight GWAS. Since GPA does not account for sample overlaps, we do not know whether the estimates are biased. Fourth, our analysis did not find functional enrichment of the pleiotropic loci between birth weight and COB under the TFBS annotation set, which requires further investigation in larger samples. An important strength of our study is the integrated modelling of functional annotations and GWAS summary statistics data from pairs of traits. This multi-trait approach has been most conducive in testing for functional enrichment and identifying novel genetic loci with their shared impacts on multiple traits, expanding our understanding of the genetic links between fetal growth and COB traits.
In conclusion, this study found that pleiotropic genetic influences and enrichment of functional annotations are substantially pervasive in the genetic architectures of birth weight and COB traits. The novel loci found in the analysis and the pathways through which the associated genes act have the potential to unravel the genetic basis that underlines associations between early growth and development of COB.
Materials and methods
Birth weight, childhood BMI and childhood obesity GWAS summary statistic data
Summary statistic data including P values and direction of effects in GWAS meta-analysis of birth weight36, CBMI39 and COB37 was obtained from the publicly available data of EGG (Table S2). The GWAS meta-analysis of CBMI was performed on 20 combined studies with a total sample size of 35,668 children. Children aged 2–10 years with European ancestry were included in the study39. The GWAS meta-analysis of COB combined 14 studies of European children aged 2–10 years with a total sample size of 5530 cases and 8318 controls. Children who had a BMI > 95th percentile were considered to be obese cases while children of BMI < 50th percentile were considered to be controls37. The GWAS meta-analysis of birth weight included neonates of European ancestry (BWEU, n = 298,142) and transethnic (African, South Asian, European) meta-analysis (BWTR, n = 321,223). Individuals who were part of multiple births, who reported their birth weight in multiple visits with the mean difference in their birth weight being > 1 kg and individuals whose birth weight was < 2.5 kg or > 4.5 kg were excluded36. The results from the GWAS of own birth weight (as opposed to offspring birth weight), and without adjustment for maternal genotype, were used in this analysis.
Functional annotation data
We used 4 annotation databases CADD42, eQTL43, TFBS44 and DHSs45 to functionally annotate the SNPs. Under CADD framework, implemented in CADD v1.2 (http://www.cadd.gs.washington.edu)42, a deleteriousness score (combined SVM score; c-score) is generated using the integrated functional and evolutionary importance of each variant from 63 annotation sources. Phred-like scores (ranged 1–99) are further generated based on the rank of each variant relative to 8.6 billion substitutions in human reference genome (-10*log10[rank/total]). Each variant in our analysis receiving a score ≥ 15 was assigned an annotation of 1 (deleterious) while scores < 15 were assigned an annotation of 0 (non-deleterious)42. In addition to CADD, which comprises a composite score, we used annotations from a variety of tissues and cell lines to elucidate the regulatory mechanisms of the risk variants. The eQTL annotations was obtained from dbGaP accession number phs000424.vN.pN on 02/11/20 (https://gtexportal.org/home/datasets)43 which consisted of cis-eQTL files on 49 different tissues. We took the intersection of these eQTL with the common variants of BW and COB/CBMI and the variants that overlapped were annotated as 1 and others as 0. The TFBS annotation (ftp://ccg.epfl.ch/snp2tfbs/mapped_files/annotated/)44 had data on 195 different transcription factors. Similar to eQTL, we took the intersection of the TFBS to annotate the common variants of birth weight and COB/CBMI. The DHSs annotation data, which has also been used in other studies45,93, was downloaded from (https://github.com/joepickrell/1000-genomes)45 and comprised of 402 binary annotations that included maps of DNase-I hypersensitive sites from different primary tissues, cell lines and cell types94,95.
Statistical Analysis
We used a unified statistical approach that integrates summary statistics with functional annotations for paired traits using probabilistic models implemented in genetic analysis incorporating pleiotropy and annotation (GPA)41. For convenience, we briefly introduce the GPA model and its notations below.
Suppose the P values from two GWAS have been collected in an M × 2 matrix, p = [pjk], where pjk denotes the P value of the jth SNP in the kth GWAS, k = 1,2 (in our case) and M is the number of SNPs. In the GPA model, these P values are assumed to come from a mixture of null (un-associated) and non-null (associated), with probability π0 and π1 = 1 − π0, respectively. GPA uses the Uniform distribution on [0,1] and the Beta distribution with parameters (α,1) to model the P values from the null and non-null groups, respectively.
Let Zj ∈ {00,10,01,11} indicate the association between the jth SNP and the two traits: Zj = 00 means the jth SNP is associated with neither of them, Zj = 10 means it is only associated with the first trait, Zj = 01 means it is only associated with the second trait, and Zj = 11 means it is associated with both trait. Thus, the four-group model is represented as:
where pj1 and pj2 is the P value of the jth SNP in GWAS 1 and 2. GPA further incorporates functional annotation as follows. Let an M-dimensional vector A collect functional information from an annotation source, where Aj ∈ {0,1} indicates whether the jth SNP is a functional unit according to the annotation source. For example, given an eQTL data, if the jth SNP is an eQTL, then Aj = 1, otherwise Aj = 0. The relationship between Zj and Aj is described as:
where q00 is the probability of a null SNP being annotated, q10 is the probability of the first trait-associated SNP being annotated, q01 is the probability of the second trait-associated SNP being annotated, and q11 is the probability of jointly associated SNP being annotated. GPA then implements an efficient EM-algorithm to obtain the estimates of the model parameters: {π00, π10, π01, π11, q00, q10, q01, q11, α}.
To assess the significance of enrichment for pleiotropy between two traits it uses the likelihood ratio test (LRT) with H0: π11 = (π10 + π11)( π01 + π11), versus H1: not H0. Similarly, to assess the significance of enrichment for annotation it uses LRT with H0: q00 = q11, versus H1: q00 ≠ q11. GPA also calculates the standard errors for the model parameters along with their covariance matrix based on an empirically observed information matrix.
After estimating the parameters, GPA assigns each SNP four posterior probabilities (PP) (estimated values of {π00, π10, π01, π11}) and controls for false discovery based on the local false discovery rate (FDR). The local FDR is defined as the probability that the jth SNP is either not associated with any trait (Fdr0) or is associated with the first trait (Fdr1), second trait (Fdr2) or both trait (Fdr1,2) given its P value and annotation information.
Finally, to infer associations at the variant level and to control the FDR at 5% we then select those SNPs with any of the four PP > 95% and FDR < 0.05. From the selected set, SNPs that achieve PP > 95% under the PP categories of {π00, π10, π01, π11} are concluded to be associated with neither, first, second or both traits. In our study, we conducted all the tests after controlling for FDR at 0.05 level and used 10,000 EM iterations.
Pathway analysis
Pathway analysis elucidates the underlying biological processes in which genes are related by common functionality. To detect such relationships of the identified pleiotropic genetic loci in our study, we used QIAGEN’s IPA tool (https://digitalinsights.qiagen.com/). IPA is a web-based software application that allows analysis of data obtained from several sequencing platforms. IPA enables for targeted search of information on genes, proteins, chemicals, diseases, and drugs. IPA’s data analysis provides clarity in understanding the significance of data or targets of interest in relation to larger biological systems. Statistical significance of overrepresented canonical pathways was determined using Fisher’s exact test after adjustment for multiple testing using the Benjamini–Hochberg method. Statistical significance was based on P < 0.05 in pathways with at least two molecules.
Colocalization analysis
To evaluated whether birthweight and childhood obesity traits share common genetic causal variant(s) in a region, we performed colocalization analysis for each of the 19 pleiotropic loci using the European ancestry GWAS summary statistics. For each genetic locus, the lead SNP with the highest posterior probability of joint association with birthweight and childhood obesity traits as well as SNPs within 500 kb window on either side of the lead SNP defined a colocalization region. Analysis was performed using the coloc R package47.
Ethical approval
The NIH Office of Human Subjects Research Program granted the study an exemption from IRB review (OHSRP ID Number: 18-NICHD-00412) per 45 CFR 46 and NIH policy for the use of specimens/data.
Supplementary Information
Author contributions
F.T.-A. and S.C. conceived and designed this study; S.C. gathered and managed data; S.C. analyzed the data; S.C. wrote the draft paper; F.T.-A. supervised the work and interpreted the results. M.O. provided critical intellectual content. All authors approved the final manuscript.
Funding
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Additional support was obtained from the National Institute on Minority Health and Health Disparities and the National Institute of Diabetes and Digestive and Kidney Diseases. This work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Data availability
The data analyzed in this study are available online. Table S1 lists the URL of the data sources.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-80084-9.
References
- 1.de Onis M, Blossner M. Prevalence and trends of overweight among preschool children in developing countries. Am. J. Clin. Nutr. 2000;72:1032–1039. doi: 10.1093/ajcn/72.4.1032. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 2017;92:251–265. doi: 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Elks CE, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front. Endocrinol. (Lausanne) 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrera BM, Keildson S, Lindgren CM. Genetics and epigenetics of obesity. Maturitas. 2011;69:41–49. doi: 10.1016/j.maturitas.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am. J. Clin. Nutr. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- 7.Silventoinen K, Rokholm B, Kaprio J, Sorensen TI. The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int. J. Obes. (Lond.) 2010;34:29–40. doi: 10.1038/ijo.2009.177. [DOI] [PubMed] [Google Scholar]
- 8.Tounian P. Programming towards childhood obesity. Ann. Nutr. Metab. 2011;58(Suppl 2):30–41. doi: 10.1159/000328038. [DOI] [PubMed] [Google Scholar]
- 9.Dietz WH. Critical periods in childhood for the development of obesity. Am. J. Clin. Nutr. 1994;59:955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 10.Hui LL, et al. Birth weight, infant growth, and childhood body mass index: Hong Kong's children of 1997 birth cohort. Arch. Pediatr. Adolesc. Med. 2008;162:212–218. doi: 10.1001/archpediatrics.2007.62. [DOI] [PubMed] [Google Scholar]
- 11.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics. 2005;116:1329–1338. doi: 10.1542/peds.2004-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 13.Abe Y, et al. Lower birth weight associated with current overweight status is related with the metabolic syndrome in obese Japanese children. Hypertens. Res. 2007;30:627–634. doi: 10.1291/hypres.30.627. [DOI] [PubMed] [Google Scholar]
- 14.Baird J, et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binkin NJ, Yip R, Fleshood L, Trowbridge FL. Birth weight and childhood growth. Pediatrics. 1988;82:828–834. doi: 10.1542/peds.82.6.828. [DOI] [PubMed] [Google Scholar]
- 16.Hirschler V, Bugna J, Roque M, Gilligan T, Gonzalez C. Does low birth weight predict obesity/overweight and metabolic syndrome in elementary school children? Arch. Med. Res. 2008;39:796–802. doi: 10.1016/j.arcmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Mei Z, Grummer-Strawn LM, Scanlon KS. Does overweight in infancy persist through the preschool years? An analysis of CDC pediatric nutrition surveillance system data. Soz. Praventivmed. 2003;48:161–167. doi: 10.1007/s00038-003-2022-x. [DOI] [PubMed] [Google Scholar]
- 18.Qiao Y, et al. Birth weight and childhood obesity: a 12-country study. Int. J. Obes. Suppl. 2015;5:S74–79. doi: 10.1038/ijosup.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacco MR, de Castro NP, Euclydes VL, Souza JM, Rondo PH. Birth weight, rapid weight gain in infancy and markers of overweight and obesity in childhood. Eur. J. Clin. Nutr. 2013;67:1147–1153. doi: 10.1038/ejcn.2013.183. [DOI] [PubMed] [Google Scholar]
- 20.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 21.Peng S, et al. Genetic regulation of the placental transcriptome underlies birth weight and risk of childhood obesity. PLoS Genet. 2018;14:e1007799. doi: 10.1371/journal.pgen.1007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulik-Sullivan B, et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cross-Disorder Group of the Psychiatric Genomics C Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horikoshi M, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat. Genet. 2013;45:76–82. doi: 10.1038/ng.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakoda LC, Jorgenson E, Witte JS. Turning of COGS moves forward findings for hormonally mediated cancers. Nat. Genet. 2013;45:345–348. doi: 10.1038/ng.2587. [DOI] [PubMed] [Google Scholar]
- 26.Vattikuti S, Guo J, Chow CC. Heritability and genetic correlations explained by common SNPs for metabolic syndrome traits. PLoS Genet. 2012;8:e1002637. doi: 10.1371/journal.pgen.1002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacArthur J, et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2017;45:D896–D901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivakumaran S, et al. Abundant pleiotropy in human complex diseases and traits. Am. J. Hum. Genet. 2011;89:607–618. doi: 10.1016/j.ajhg.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolae DL, et al. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6:e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schork AJ, et al. All SNPs are not created equal: genome-wide association studies reveal a consistent pattern of enrichment among functionally annotated SNPs. PLoS Genet. 2013;9:e1003449. doi: 10.1371/journal.pgen.1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 2011;43:519–525. doi: 10.1038/ng.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreassen OA, et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am. J. Hum. Genet. 2013;92:197–209. doi: 10.1016/j.ajhg.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouchard C. Childhood obesity: are genetic differences involved? Am. J. Clin. Nutr. 2009;89:1494S–1501S. doi: 10.3945/ajcn.2009.27113C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesi A, Grant SFA. The genetics of pediatric obesity. Trends Endocrinol. Metab. 2015;26:711–721. doi: 10.1016/j.tem.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000;107:375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 36.Warrington NM, et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat. Genet. 2019;51:804–814. doi: 10.1038/s41588-019-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradfield JP, et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat. Genet. Nat. Genet. 2012;44:526–531. doi: 10.1038/ng.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couto Alves A, et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv. 2019;5:3095. doi: 10.1126/sciadv.aaw3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felix JF, et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum. Mol. Genet. 2016;25:389–403. doi: 10.1093/hmg/ddv472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helgeland O, et al. Genome-wide association study reveals dynamic role of genetic variation in infant and early childhood growth. Nat. Commun. 2019;10:4448. doi: 10.1038/s41467-019-12308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung D, Yang C, Li C, Gelernter J, Zhao H. GPA: a statistical approach to prioritizing GWAS results by integrating pleiotropy and annotation. PLoS Genet. 2014;10:e1004787. doi: 10.1371/journal.pgen.1004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Consortium GT. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Ambrosini G, Bucher P. SNP2TFBS—a database of regulatory SNPs affecting predicted transcription factor binding site affinity. Nucleic Acids. Res. 2017;45:D139–D144. doi: 10.1093/nar/gkw1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pickrell JK. Joint analysis of functional genomic data and genome-wide association studies of 18 human traits. Am. J. Hum. Genet. 2014;94:559–573. doi: 10.1016/j.ajhg.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradfield JP, et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 2019;28:3327–3338. doi: 10.1093/hmg/ddz161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giambartolomei C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10:e1004383. doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickrell JK, et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016;48:709–717. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stearns FW. One hundred years of pleiotropy: a retrospective. Genetics. 2010;186:767–773. doi: 10.1534/genetics.110.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tekola-Ayele F, Lee A, Workalemahu T, Sanchez-Pozos K. Shared genetic underpinnings of childhood obesity and adult cardiometabolic diseases. Hum. Genom. 2019;13:17. doi: 10.1186/s40246-019-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tekola-Ayele F, et al. Genetic overlap between birthweight and adult cardiometabolic diseases has implications for genomic medicine. Sci. Rep. 2019;9:4076. doi: 10.1038/s41598-019-40834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He X, Zhang J. Toward a molecular understanding of pleiotropy. Genetics. 2006;173:1885–1891. doi: 10.1534/genetics.106.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaumont RN, et al. Genome-wide association study of offspring birth weight in 86 577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum. Mol. Genet. 2018;27:742–756. doi: 10.1093/hmg/ddx429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horikoshi M, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538:248–252. doi: 10.1038/nature19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang G, et al. Genetic associations with gestational duration and spontaneous preterm birth. N. Engl. J. Med. 2017;377:1156–1167. doi: 10.1056/NEJMoa1612665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu AY, et al. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat. Genet. 2017;49:125–130. doi: 10.1038/ng.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao H, et al. Early B cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab. 2014;19:981–992. doi: 10.1016/j.cmet.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol. Cell. Biol. 2007;27:743–757. doi: 10.1128/MCB.01557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Longo M, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019;20:1. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.International Consortium for Blood Pressure Genome-Wide Association S, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh A, et al. Gene by stress genome-wide interaction analysis and path analysis identify EBF1 as a cardiovascular and metabolic risk gene. Eur. J. Hum. Genet. 2015;23:854–862. doi: 10.1038/ejhg.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogelezang S, et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 2020;16:e1008718. doi: 10.1371/journal.pgen.1008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griffin MJ, et al. Early B-cell factor-1 (EBF1) is a key regulator of metabolic and inflammatory signaling pathways in mature adipocytes. J. Biol. Chem. 2013;288:35925–35939. doi: 10.1074/jbc.M113.491936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi JH, et al. LMBR1L regulates lymphopoiesis through Wnt/beta-catenin signaling. Science. 2019;364:1. doi: 10.1126/science.aau0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol. Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- 66.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat. Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 67.Deng C, Dai R, Li X, Liu F. Genetic variation frequencies in Wilms' tumor: a meta-analysis and systematic review. Cancer Sci. 2016;107:690–699. doi: 10.1111/cas.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia J, et al. beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J. Dermatol. Sci. 2006;41:67–75. doi: 10.1016/j.jdermsci.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Teo JL, Kahn M. The Wnt signaling pathway in cellular proliferation and differentiation: a tale of two coactivators. Adv. Drug Deliv. Rev. 2010;62:1149–1155. doi: 10.1016/j.addr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009;20:16–24. doi: 10.1016/j.tem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 72.Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140:803–813. doi: 10.1530/REP-10-0294. [DOI] [PubMed] [Google Scholar]
- 73.Nayeem SB, Arfuso F, Dharmarajan A, Keelan JA. Role of Wnt signalling in early pregnancy. Reprod. Fertil. Dev. 2016;28:525–544. doi: 10.1071/RD14079. [DOI] [PubMed] [Google Scholar]
- 74.Red-Horse K, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Comuzzie AG, et al. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PLoS ONE. 2012;7:e51954. doi: 10.1371/journal.pone.0051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan AR, et al. Obesity and diabetes genes are associated with being born small for gestational age: results from the Auckland birthweight collaborative study. BMC Med. Genet. 2010;11:125. doi: 10.1186/1471-2350-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X, Liu Z, Xu J. The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Mol. Endocrinol. 2010;24:1917–1934. doi: 10.1210/me.2010-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim SC, Park MN, Lee YJ, Joo JK, An BS. Interaction of steroid receptor coactivators and estrogen receptors in the human placenta. J. Mol. Endocrinol. 2016;56:239–247. doi: 10.1530/JME-15-0248. [DOI] [PubMed] [Google Scholar]
- 79.Rollins DA, Coppo M, Rogatsky I. Minireview: nuclear receptor coregulators of the p160 family: insights into inflammation and metabolism. Mol. Endocrinol. 2015;29:502–517. doi: 10.1210/me.2015-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruan XZ, Varghese Z, Powis SH, Moorhead JF. Nuclear receptors and their coregulators in kidney. Kidney Int. 2005;68:2444–2461. doi: 10.1111/j.1523-1755.2005.00721.x. [DOI] [PubMed] [Google Scholar]
- 81.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015;294:63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obes. Rev. 2001;2:131–140. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 83.Tam CS, et al. An early inflammatory gene profile in visceral adipose tissue in children. Int. J. Pediatr. Obes. 2011;6:e360–363. doi: 10.3109/17477166.2011.575152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pairault J, Quignard-Boulange A, Dugail I, Lasnier F. Differential effects of retinoic acid upon early and late events in adipose conversion of 3T3 preadipocytes. Exp. Cell Res. 1988;177:27–36. doi: 10.1016/0014-4827(88)90022-5. [DOI] [PubMed] [Google Scholar]
- 85.Sato M, Hiragun A, Mitsui H. Preadipocytes possess cellular retinoid binding proteins and their differentiation is inhibited by retinoids. Biochem. Biophys. Res. Commun. 1980;95:1839–1845. doi: 10.1016/s0006-291x(80)80113-6. [DOI] [PubMed] [Google Scholar]
- 86.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol. Cell. Biol. 1997;17:1552–1561. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kowianski P, et al. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cordeira J, Rios M. Weighing in the role of BDNF in the central control of eating behavior. Mol. Neurobiol. 2011;44:441–448. doi: 10.1007/s12035-011-8212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu B, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann. N. Y. Acad. Sci. 2012;1264:49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han JC, Muehlbauer MJ, Cui HN, Newgard CB, Haqq AM. Lower brain-derived neurotrophic factor in patients with prader-willi syndrome compared to obese and lean control subjects. J. Clin. Endocrinol. Metab. 2010;95:3532–3536. doi: 10.1210/jc.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeo GS, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 93.Chen W, McDonnell SK, Thibodeau SN, Tillmans LS, Schaid DJ. Incorporating functional annotations for fine-mapping causal variants in a bayesian framework using summary statistics. Genetics. 2016;204:933–958. doi: 10.1534/genetics.116.188953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maurano MT, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thurman RE, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study are available online. Table S1 lists the URL of the data sources.