See Bayram and Litvan (doi:10.1093/brain/awaa287) for a scientific commentary on this article.
Patients with diabetes appear to be at increased risk of Parkinson’s disease. In a longitudinal study of approximately 100,000 patients with diabetes, Brauer et al. reveal a lower risk of incident Parkinson’s disease in those treated with GLP-1 mimetics or DPP-4 inhibitors compared to users of other oral antidiabetic drugs.
Keywords: Parkinson’s disease, electronic health records, diabetes, glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase 4 inhibitors
Abstract
The elevated risk of Parkinson’s disease in patients with diabetes might be mitigated depending on the type of drugs prescribed to treat diabetes. Population data for risk of Parkinson’s disease in users of the newer types of drugs used in diabetes are scarce. We compared the risk of Parkinson’s disease in patients with diabetes exposed to thiazolidinediones (glitazones), glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP4) inhibitors, with the risk of Parkinson’s disease of users of any other oral glucose lowering drugs. A population-based, longitudinal, cohort study was conducted using historic primary care data from The Health Improvement Network. Patients with a diagnosis of diabetes and a minimum of two prescriptions for diabetes medications between January 2006 and January 2019 were included in our study. The primary outcome was the first recording of a diagnosis of Parkinson’s disease after the index date, identified from clinical records. We compared the risk of Parkinson’s disease in individuals treated with glitazones or DPP4 inhibitors and/or GLP-1 receptor agonists to individuals treated with other antidiabetic agents using a Cox regression with inverse probability of treatment weighting based on propensity scores. Results were analysed separately for insulin users. Among 100 288 patients [mean age 62.8 years (standard deviation 12.6)], 329 (0.3%) were diagnosed with Parkinson’s disease during the median follow-up of 3.33 years. The incidence of Parkinson’s disease was 8 per 10 000 person-years in 21 175 patients using glitazones, 5 per 10 000 person-years in 36 897 patients using DPP4 inhibitors and 4 per 10 000 person-years in 10 684 using GLP-1 mimetics, 6861 of whom were prescribed GTZ and/or DPP4 inhibitors prior to using GLP-1 mimetics. Compared with the incidence of Parkinson’s disease in the comparison group (10 per 10 000 person-years), adjusted results showed no evidence of any association between the use of glitazones and Parkinson’s disease [incidence rate ratio (IRR) 1.17; 95% confidence interval (CI) 0.76–1.63; P = 0.467], but there was strong evidence of an inverse association between use of DPP4 inhibitors and GLP-1 mimetics and the onset of Parkinson’s disease (IRR 0.64; 95% CI 0.43–0.88; P < 0.01 and IRR 0.38; 95% CI 0.17–0.60; P < 0.01, respectively). Results for insulin users were in the same direction, but the overall size of this group was small. The incidence of Parkinson’s disease in patients diagnosed with diabetes varies substantially depending on the treatment for diabetes received. The use of DPP4 inhibitors and/or GLP-1 mimetics is associated with a lower rate of Parkinson’s disease compared to the use of other oral antidiabetic drugs.
See Bayram and Litvan (doi:10.1093/brain/awaa287) for a scientific commentary on this article.
Introduction
There is evidence of an increased risk of Parkinson’s disease among patients with diabetes (De Pablo-Fernandez et al., 2018; Jeong et al., 2019). This finding is interesting in light of the growing disease burden of Parkinson’s disease; the prevalence is estimated to rise to more than one million people by 2030 in the USA alone (Marras et al., 2018). The incidence of Parkinson’s disease in the general population is low at 0.03% but the lifetime risk of Parkinson’s disease is expected to rise by 18% in the next 7 years (Parkinson’s UK, 2018). As such there is mounting interest in the potential relationship between glucose control and neurodegeneration and how this may relate to novel treatment opportunities for patients with Parkinson’s disease (Cereda et al., 2011; Athauda and Foltynie, 2016).
Glucagon-like peptide-1 (GLP-1) receptor agonists, or incretin mimetics, were introduced in 2005 [National Institute for Health and Care Excellence (NICE), 2015]. GLP-1 receptor agonists lower blood glucose levels by stimulating the secretion of insulin from pancreatic beta cells and by inhibiting glucagon secretion in the liver (Tran et al., 2017). GLP-1 is degraded by the enzyme dipeptidyl peptidase 4 (DPP4), and the use of either DPP4 inhibitors or GLP-1 mimetics enhances insulin levels and results in lower blood glucose and haemoglobin A1c (HbA1c) levels (Erbil et al., 2019). Animal studies have shown that GLP-1 mimetics may have neuroprotective effects, potentially by decreasing type 2 diabetes mellitus induced neuronal inflammatory processes and/or by enhancing insulin signalling processes (Holscher, 2012). Several small clinical studies have been conducted exploring the potential of repurposing GLP-1 mimetics to slow down the progression of Parkinson’s disease (Aviles-Olmos et al., 2013; Athauda et al., 2017). A recent randomized trial has shown that 48 weeks of exenatide use had positive effects on motor scores in Parkinson’s disease compared to placebo (Athauda et al., 2017).
Based on our previous work, which used routinely collected UK electronic health care records to investigate the potential neuroprotective effects of glitazones (GTZ), we further studied the potential neuroprotective association between GLP-1 mimetics and DPP4 inhibitors on the risk of Parkinson’s disease in the general UK population (Brauer et al., 2015). We used The Health Improvement Network (THIN) database to compare the prevalence of Parkinson’s disease in individuals diagnosed with diabetes to those without diabetes, and to assess the risk of Parkinson’s disease according to diabetes treatment regime. Our aim was to compare the risk of Parkinson’s disease in individuals treated with GLP-1 receptor agonists, DPP4 inhibitors or glitazones to individuals treated with other glucose lowering agents.
Materials and methods
The study protocol and analysis plan were approved by the THIN Scientific Review Committee (SRC). Reference Number: 19THIN001, January 2019 (Supplementary material). Further ethics approval was not required for anonymous healthcare database research.
Study design and data source
We conducted a longitudinal population-based cohort study using the THIN database; an archive of anonymized, demographically representative, primary care data in the UK (Blak et al., 2011). This study used the medical records of patients registered at 808 participating practices, comprising 15 622 569 patients who met accepted data quality criteria in March 2019 (Horsfall et al., 2013). The study cohort was drawn from the entire population of THIN, diagnosed with type 2 diabetes mellitus and with a follow-up time from 1 January 2006 onwards. Subjects were selected for inclusion if they were 18 years of age or older and received two or more consecutive prescriptions for a glucose lowering agent [Chapter 6.1 of the British National Formulary (Joint Formulary Committee, 2019)], with the first prescription recorded at least 6 months after the patient’s initial follow-up at their primary care practice to ensure incident prescribing. The risk of Parkinson’s disease in insulin users was analysed separately. Follow-up ended at the earliest of the following points: the date the patient left the practice, the date of death, the first diagnosis of Parkinson’s disease, or the last date of data collection (9 January 2019).
Exposure to drugs used in diabetes
Exposure was determined from prescribing records, using drug codes for individual glucose lowering agents (Supplementary material). Prescription duration was calculated by dividing the numeric total quantity by the recommended dose per day. Missing duration (∼15% of the total) was replaced by the median duration of every antidiabetic drug. We excluded potential participants from our analysis if they had any diagnosis of Parkinson’s disease prior to their first prescription for a drug used in diabetes or a record of secondary parkinsonism, i.e. patients suspected to have vascular parkinsonism or drug induced parkinsonism.
Exposure to GLP-1 receptor agonist, GTZ and DDP-4 inhibitors
The index date for each patient in the exposed group of interest was the first prescription for study entry (e.g. first exposure to a GTZ, GLP-1 mimetic or DPP4 inhibitor). GLP-1 mimetics are prescribed if treatment with metformin and two other oral drugs is not effective, contraindicated and/or the patient has a body mass index (BMI) >35 kg/m2 [National Institute for Health and Care Excellence (NICE), 2015]. As such, patients could be exposed to GLP-1 mimetics after exposure to glitazones and/or DPP4 inhibitors. We censored GLP-1 receptor agonist users who were exposed to GTZ and/or DPP4 inhibitors on the day of their first prescription for a GLP-1 receptor agonist. In the primary analysis, exposure was characterized as ‘ever exposed’ versus ‘never exposed’ to GLP-1 mimetics, GTZ or DDP-4 agonists; exposed individuals were classified as ever exposed for the duration of the study, regardless of any subsequent changes in therapy.
Selection of the comparison group
Individuals prescribed GTZ, DPP4 inhibitors or GLP-1 mimetics, on their own or in combination with metformin or sulfonylureas, were compared to individuals prescribed any other oral combination therapy for diabetes (control medication). The index date for patients in the comparator group was the date a second drug was prescribed in addition to, or replacing, the first-line treatment.
Parkinson’s disease outcome
The primary outcome was the first recording of a diagnosis of Parkinson’s disease after the index date, as identified from clinical records using Read codes (Supplementary material).
Propensity score weighting
Propensity scores are used to control for bias caused by non-random treatment allocation by measuring the probability that a patient received a certain treatment given their observed characteristics (Austin, 2011). We conducted a propensity score weighted analysis (Olmos, 2015). Propensity scores were estimated by logistic regression with the dependent variable being the treatment of interest (GTZ or DPP4 or GLP-1 exposure) and the following covariates at, or closest recording prior to, the index date: age, sex and calendar year; smoking, alcohol consumption and BMI; medical risk factors for Parkinson’s disease listed in Table 1; use of calcium channel blockers or hormone replacement therapy; time between first diabetes mellitus diagnosis and index date, and HbA1c level. Standardized differences were used to assess the differences in patient characteristics and a value of <0.1 was considered negligible.
Table 1.
Patient characteristics
| Comparison group (n = 38 393) | Exposed GTZ (n = 21 175) | Exposed DPP4 (n = 36 897) | Exposed GLP-1 (n = 10 684) | Maximum standardized difference |
||
|---|---|---|---|---|---|---|
| Before weighting | After weighting | |||||
| Age, mean (SD) | 64.4 (13.1) | 61.9 (11.8) | 63.3 (12.5) | 56.8 (10.7) | −0.671 | 0.031 |
| Gender, male (%) | 22 932 (59.7) | 12 615 (59.6) | 21 526 (58.3) | 5 755 (53.9) | −0.13 | 0.016 |
| Medical diagnoses (%) | ||||||
| Head injury | 1676 (4.4) | 683 (3.2) | 1530 (4.1) | 360 (3.4) | −0.057 | 0.024 |
| Heart failure | 2071 (5.4) | 545 (2.6) | 1949 (5.3) | 392 (3.7) | −0.145 | 0.031 |
| Myocardial Infarction | 2950 (7.7) | 1181 (5.6) | 2795 (7.6) | 590 (5.5) | −0.082 | 0.03 |
| Cerebrovascular disease | 3279 (8.5) | 1387 (6.6) | 3038 (8.2) | 568 (5.3) | −0.124 | −0.028 |
| Arrhythmias | 3699 (9.6) | 1241 (5.9) | 3436 (9.3) | 675 (6.3) | −0.139 | 0.022 |
| Renal disease | 6618 (17.2) | 3536 (16.7) | 7602 (20.6) | 1562 (14.6) | 0.095 | 0.036 |
| Hypertension | 23 477 (61.1) | 13 801 (65.2) | 24 003 (65.1) | 6937 (64.9) | 0.080 | −0.024 |
| Smoking (%) | ||||||
| Current smoker | 6598 (17.2) | 3 640 (17.2) | 5959 (16.2) | 1835 (17.2) | −0.024 | 0.017 |
| Ex-smoker | 18 881 (49.2) | 10 357 (48.9) | 19 029 (51.6) | 5403 (50.6) | 0.043 | −0.027 |
| Non-smoker | 12 749 (33.2) | 7112 (33.6) | 11 873 (32.2) | 3401 (31.8) | −0.041 | 0.014 |
| BMI, kg/m2 (%) | ||||||
| <18.5 | 114 (0.3) | 30 (0.1) | 75 (0.2) | 2 (0.0) | −0.072 | 0.03 |
| 18.5–24.9 | 5490 (14.3) | 2174 (10.3) | 3575 (9.7) | 76 (0.7) | −0.537 | 0.055 |
| 25–29.9 | 12 694 (33.1) | 6686 (31.6) | 10 540 (28.6) | 843 (7.9) | −0.668 | 0.034 |
| ≥30 | 19 531 (50.9) | 11 716 (55.3) | 21 758 (59.0) | 9743 (91.2) | 0.992 | −0.08 |
| Drinking status | ||||||
| Current drinker | 19 843 (51.7) | 11 518 (54.4) | 19 462 (52.7) | 5854 (54.8) | 0.048 | −0.011 |
| Ex-drinker | 6572 (17.1) | 3307 (15.6) | 7485 (20.3) | 1921 (18.0) | 0.082 | 0.007 |
| Non-drinker | 5196 (13.5) | 3089 (14.6) | 4488 (12.2) | 1468 (13.7) | −0.044 | 0.018 |
| Excessive drinker | 4982 (13.0) | 957 (10.9) | 4371 (11.8) | 1064 (10.0) | −0.103 | 0.013 |
| Calcium channel inhibitor use (%) | ||||||
| Current user | 7602 (19.8) | 3988 (18.8) | 7493 (20.3) | 2157 (20.2) | −0.028 | −0.001 |
| Past user | 7873 (20.5) | 4400 (20.8) | 8378 (22.7) | 2177 (20.4) | 0.054 | −0.001 |
| Non-user | 22 918 (59.7) | 12 787 (60.4) | 21 026 (57.0) | 6350 (59.4) | −0.057 | 0.002 |
| Hormone replace therapy use (%) | ||||||
| Current user | 3205 (8.3) | 2058 (9.7) | 3948 (10.7) | 1273 (11.9) | 0.11 | −0.008 |
| Past user | 219 (0.6) | 200 (0.9) | 239 (0.6) | 125 (1.2) | 0.077 | −0.007 |
| Non-user | 34 969 (91.1) | 18 917 (89.3) | 32 710 (88.7 | 9286 (86.9) | −0.13 | 0.01 |
| Time between first DM diagnosis and index date, median (IQR) | 3.4 (1.0–6.6) | 5.1 (2.4–8.6) | 5.3 (2.8–8.5) | 5.3 (2.6–8.9) | 0.456 | −0.21 |
| HbA1c, mm/mmol, mean (SD) | 8.78 (2.1) | 8.75 (1.8) | 8.68 (1.8) | 9.12 (1.8) | 0.175 | −0.011 |
DM = diabetes mellitus; HbA1c = haemoglobin A1c.
Statistical analyses
We measured an incidence rate ratio (IRR) with a 95% confidence interval (CI) for the association between the index drug use and Parkinson’s disease using a Cox regression with inverse probability of treatment weighting using propensity scores. A two-sided P < 0.05 was considered statistically significant. Multiple imputation was used to replace missing HbA1c measurements, BMI, smoking and drinking status. The full analysis procedure was applied to 10 imputed datasets separately, and the results were combined using Rubin’s rules to obtain an overall estimate. This approach accounts for the variability between imputed datasets (Leyrat et al., 2019). All covariates, including the outcome variable, were included in the imputation model to minimize bias and enhance the precision of estimates. Stata version 16 (College Station, TX) was used for conducting statistical analyses.
Additional analyses
We compared the prevalence of Parkinson’s disease in patients with diabetes to those without diabetes using the entire adult THIN population (age >18 years) until March 2019. Separate analyses were conducted for patients with treated versus untreated diabetes.
In a separate analysis, the risk of Parkinson’s disease in individuals receiving insulin before receipt of and in combination with GLP-1 mimetics, GTZ and DDP-4 agonists was compared to the risk of Parkinson’s disease in individuals who received other oral drugs used in diabetes in combination with insulin.
To test for the robustness of the main study results, we also conducted several per protocol additional analyses. First, we censored patients upon discontinuation of the index medication to account for cessation of therapy. This subanalysis allowed us to investigate current and past use of the index drug separately. Next, we identified patients with continuous use of the index drug for minimum durations of: up to 12 months, 12–36 months, and ≥3 years. We also used a stricter definition of Parkinson’s disease in which at least two prescriptions for an anti-Parkinson’s disease drug were required in addition to a Read code indicating Parkinson’s disease. Lastly, users of antidiabetic agents were limited to those aged 40 and older and, in a separate analysis, were restricted to non-smokers. Post hoc, we limited the cohort to obese patients (BMI > 30 kg/m2).
Data availability
Data may be obtained from a third party (IQVIA) and are not publicly available. After SRC review of our research protocol, we obtained anonymized data exclusively for this work without authorization to share the data or to conduct any secondary analysis.
Results
Patient characteristics
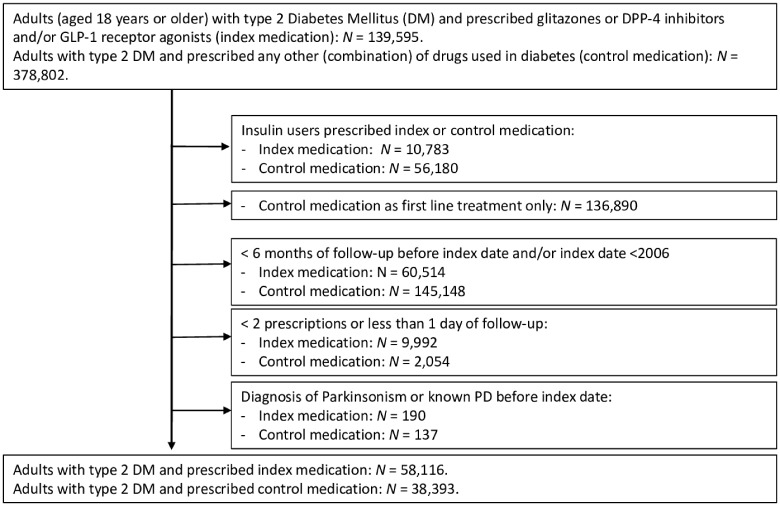
There were 100 288 patients diagnosed with type 2 diabetes mellitus in the THIN database who had two or more consecutive prescriptions for GTZ, DPP4 inhibitors and/or GLP-1 mimetics on or after 1 January 2006. Of these, 61 895 individuals met the inclusion criteria (Fig. 1), and were compared to 38 393 users of other oral glucose lowering agents; 21 175 individuals used GTZ; 36 897 individuals used DPP4 inhibitors; and 10 684 individuals used GLP-1 mimetics, 6861 of whom were prescribed GTZ and/or DPP4 prior to using GLP-1 mimetics.
Figure 1.
Flowchart study population.
The median follow-up time of patients in (i) the group using GTZ, DPP4 inhibitors and/or GLP-1 mimetics; and (ii) those in the comparison group was 3.6 years [interquartile range (IQR) = 1.7–6.4] and 2.81 years (IQR = 1.2–5.3), respectively. A total of 329 of 100 288 patients (0.3%) developed Parkinson’s disease during follow-up. Less than 16% of all Parkinson’s disease diagnoses were recorded in the first year of follow-up. Forty-two per cent of all patients in the group using GTZ, DPP4 inhibitors and/or GLP-1 mimetics were diagnosed within 3 years of the start of follow-up, compared to 52% in the comparison group. The crude incidence rate of Parkinson’s disease per 1000 person-years was 30% lower in the exposure group of interest compared with the comparison group (7 versus 10 per 10 000 person-years).
Patients characteristics are shown in Table 1. Patients in the GLP-1 exposed group were younger and were more likely than those prescribed other antidiabetic agents to be female. Patients in all index exposure groups were more likely to be obese, with GLP-1 mimetic users almost exclusively obese. Patients in the GTZ exposed group and GLP-1 exposed group were less likely to be diagnosed with any cardiovascular disease. After propensity score weighting, differences in baseline characteristics between the index and comparison group were considered minor (value of <0.1; Table 1).
Primary analysis
A total of 109 users of GTZ (0.5%), 66 users of DPP4 inhibitors (0.2%) and 17 users of GLP-1 mimetics (0.2%), compared to 137 users of other oral drugs used in diabetes (0.4%) developed Parkinson’s disease during follow-up (Table 2).
Table 2.
Event rates in the primary and secondary analyses
| GTZ and DPP4 (n = 58 072) |
GTZ (n = 21 175) |
DPP4 inhibitors (n = 36 897) |
GLP-1 receptor agonists (n = 10 684) |
|||||
|---|---|---|---|---|---|---|---|---|
| Type of analysis | Cases, n/ PY | Incidence per 10 000 PY | Cases, n/ PY | Incidence per 10 000 PY | Cases, n/ PY | Incidence per 10 000 PY | Cases, n/ PY | Incidence per 10 000 PY |
| Primary analysis | ||||||||
| Control medication | 137/136 228 | 10 | 137/136 228 | 10 | 137/136 228 | 10 | 137/136 228 | 10 |
| Index medication | 175/251 331 | 7 | 109/130 790 | 8 | 66/120 541 | 5 | 17/42 464 | 4 |
| Secondary analyses | ||||||||
| Censored follow-up time | ||||||||
| Control medication | 132/130 702 | 10 | 132/130 702 | 10 | 132/130 702 | 10 | 132/130 702 | 10 |
| Current use index medication | 115/203 367 | 6 | 50/75 216 | 7 | 49/101 427 | 4 | 7/28 607 | 2 |
| Past use index medication | 60/47 995 | 6 | 59/55 590 | 11 | 17/19 113 | 9 | 10/13 857 | 7 |
| Duration use | ||||||||
| Control medication | 137/136 228 | 10 | 137/136 228 | 10 | 137/136 228 | 10 | 137/136 228 | 10 |
| Use up to 12 months | 32/53 219 | 6 | 23/31 166 | 7 | 9/23 060 | 4 | 7/20 107 | 3 |
| 12–36 months | 25/34 504 | 7 | 14/20 688 | 7 | 11/13 680 | 8 | a | a |
| >36 months | 118/163 609 | 7 | 72/78 936 | 9 | 46 /83 801 | 5 | 10/22308 | 4 |
| Age >40 years | ||||||||
| Control medication | 137/132 395 | 10 | 137/132 395 | 1 | 137/132 395 | 10 | 137/132 395 | 10 |
| Index medication | 175/244 105 | 7 | 109/127 006 | 9 | 66/117100 | 6 | 17/39 918 | 4 |
| Non-smokers | ||||||||
| Control medication | 55/46 287 | 12 | 55/46 287 | 12 | 55/46 287 | 12 | 55/46 287 | 12 |
| Index medication | 60/84 920 | 7 | 40/45 148 | 5 | 20/39 772 | 5 | 7/13 777 | 5 |
| Secondary definition Parkinson’s disease | ||||||||
| Control medication | 104/136 284 | 8 | 104/136 284 | 8 | 104/136 284 | 8 | 104/136 284 | 8 |
| Index medication | 135/251 455 | 6 | 86/130 722 | 7 | 54/120 821 | 4 | 12/42 477 | 3 |
| BMI >30 kg/m2 | ||||||||
| Control medication | 59/67 486 | 9 | 59/67 486 | 9 | 59/67 486 | 9 | 59/67 486 | 9 |
| Index medication | 76/143 186 | 5 | 51/71 325 | 7 | 25/71 861 | 3 | 16/39 106 | 4 |
PY = person-years.
Less than five patients were diagnosed with Parkinson’s disease, to comply with THIN anonymization procedures, the results for users 12–36 months were merged with users >36 months
The incidence of Parkinson’s disease in all three index groups was lower than the incidence in the propensity weighted comparison cohort [5 (GLP-1/DPP4) and 8 (GTZ) versus 10 per 10 000 person-years; Table 2).
Compared to users of metformin, sulfonylureas or any other oral drug used in diabetes, the crude relative risk for the incidence of Parkinson’s disease was 0.83 (0.64–1.07), 0.54 (0.41–0.73), and 0.40 (0.24–0.66), for users of GTZ, DPP4 inhibitors and GLP-1 mimetics respectively. There was no evidence of an association between the use of GTZ and Parkinson’s disease in the adjusted analysis (IRR 1.17; 95% CI 0.76–1.63, P = 0.467), but there was strong evidence of an inverse association between use of DPP4 inhibitors and GLP-1 mimetics and the onset of Parkinson’s disease (IRR = 0.64; 95% CI 0.43–0.88; P < 0.01 and IRR 0.38; 95% CI 0.17–0.60; P < 0.01, respectively; Table 3).
Table 3.
Results of the primary and secondary analyses (GTZ, DPP4 and GLP-1 drugs versus other oral drugs used in diabetes)
| GTZ and DPP-4 (n = 58 072) |
GTZ (n = 21 175) |
DPP4 inhibitors (n = 36 897) |
GLP-1 receptor agonists (n = 10 684) |
|||||
|---|---|---|---|---|---|---|---|---|
| Type of analysis | Crude IRR (95% CI), P-value | Adjusted IRR (95% CI), P-value | Crude IRR (95% CI), P-value | Adjusted IRR (95% CI), P-value | Crude IRR (95% CI), P-value | Adjusted IRR (95% CI), P-value | Crude IRR (95% CI), P-value | Adjusted IRR (95% CI), P-value |
| Primary analysis | 0.69 (0.55–0.87), <0.01 | 0.85 (0.66–1.08), 0.206 | 0.83 (0.64–1.07), 0.143 | 1.17 (0.76–1.63), 0.467 | 0.54 (0.41–0.73), <0.01 | 0.64 (0.43–0.88), <0.01 | 0.40 (0.24–0.66), <0.01 | 0.38 (0.17–0.60), <0.01 |
| Additional analyses | ||||||||
| Follow-up time censored | 0.56 (0.44–0.72), <0.01 | 0.58 (0.45–0.76), <0.01 | 0.66 (0.46–0.91), 0.012 | 0.52 (0.32–0.73), <0.01 | 0.48 (0.34–0.66), <0.01 | 0.52 (0.33–0.74), <0.01 | 0.24 (0.11–0.52), <0.01 | 0.16 (0.03–0.3), <0.01 |
| Past use | 1.24 (0.92–1.68), 0.160 | 0.54 (0.40–0.70), <0.01 | 1.06 (0.78–1.43), 0.729 | 0.93 (0.50–1.40), 0.777 | 0.88 (0.53–1.46), 0.633 | 0.29 (0.14–0.45), <0.01 | 0.72 (0.38–1.36), 0.311 | 0.61 (0.07–1.17), 0.179 |
| Duration use | ||||||||
| Up to 12 months | 0.6 (0.41–0.88), <0.01 | 0.75 (0.44–1.08), 0.152 | 0.73 (0.47–1.14), 0.170 | 0.75 (0.38–1.14), 0.208 | 0.39 (0.20–0.76), <0.01 | 0.44 (0.10–0.78), 0.003 | 0.35 (0.16–0.74), <0.01 | 0.26 (0.04–0.48), <0.01 |
| 12–36 months | 0.72 (0.47–1.10), <0.01 | 0.89 (0.50–1.31), 0.607 | 0.67 (0.39–1.17), 0.158 | 0.68 (0.25–1.13), 0.172 | 0.80 (0.43–1.48), 0.475 | 1.20 (0.31–2.14), 0.651 | a | a |
| >36 months | 0.72 (0.56–0.92), 0.132 | 0.86 (0.65–1.10), 0.269 | 0.91 (0.68–1.21), 0.502 | 1.34 (0.76–1.96), 0.248 | 0.55 (0.39–0.76), <0.01 | 0.63 (0.37–0.90), 0.015 | 0.44 (0.23–0.85), 0.01 | 0.45 (0.11–0.79), <0.01 |
| Age >40 years | 0.69 (0.55–0.87), <0.01 | 0.87 (0.67–1.09), 0.256 | 0.83 (0.64–1.07), 0.145 | 1.19 (0.77–1.67), 0.890 | 0.54 (0.41–0.73), <0.01 | 0.65 (0.44–0.89), 0.011 | 0.41 (0.25–0.68), <0.01 | 0.40 (0.18–0.63), <0.01 |
| Non-smokers | 0.59 (0.41–0.86), <0.01 | 0.82 (0.54–1.16), 0.294 | 0.74 (0.50–1.12), 0.158 | 1.16 (0.65–1.77), 0.543 | 0.42 (0.25– 0.71), <0.01 | 0.54 (0.23–0.88), 0.022 | 0.42 (0.19–0.94), 0.034 | 0.42 (0.07–0.80), 0.01 |
| Secondary definition PD | 0.73 (0.57–0.94), 0.02 | 0.87 (0.65–1.13), 0.333 | 0.86 (0.65–1.15) 0.309 | 1.14 (0.66–1.67), 0.578 | 0.59 (0.42–0.81), <0.01 | 0.69 (0.43–0.92), 0.06 | 0.37 (0.20–0.67), <0.01 | 0.28 (0.13–0.64), <0.01 |
| BMI>30 kg/m2 | 0.61 (0.43–0.85), <0.01 | 0.70 (0.47–0.99), 0.072 | 0.82 (0.56–1.19), 0.293 | 0.93 (0.55–1.40), 0.759 | 0.40 (0.25–0.64), <0.01 | 0.41 (0.21–0.64), <0.01 | 0.47 (0.27–0.81), <0.01 | 0.65 (0.28–1.08), 0.13 |
PD = Parkinson’s disease.
Less than five patients were diagnosed with Parkinson’s disease, to comply with THIN anonymization procedures, the results for users 12–36 months were merged with users >36 months.
Prior to the index date, recorded information on smoking behaviour (<1%), alcohol consumption (4%), BMI (2%) and HbA1c (3%) was missing for up to 3848 patients and multiple imputation was used. Complete case analyses yielded similar results.
Additional analyses
We found a lower prevalence of Parkinson’s disease in a cohort of 13 331 246 individuals without a recorded diagnosis of diabetes (prevalence = 3.8 per 1000 patients), compared to the prevalence of Parkinson’s disease in a cohort of 537 306 individuals diagnosed with diabetes (prevalence = 10.7 per 1000 patients). Within the group of patients with diabetes, the prevalence of Parkinson’s disease was highest in a cohort of 139 756 patients diagnosed with diabetes but without any recorded prescription for antidiabetic agents (untreated diabetes; prevalence = 13.9 per 1000 patients).
A total of 49 of 15 683 insulin users developed Parkinson’s disease during the follow-up period. The incidence rates of Parkinson’s disease in insulin users by type of oral antidiabetic drug (co-)prescribed were similar in direction to those found in the main analysis (Supplementary Tables 1 and 2). However, there was no evidence of a significant association between Parkinson’s disease and the use of insulin in combination with GTZ, DPP4 inhibitors or GLP-1 mimetics compared to other insulin combination users.
When the follow-up time was censored at time of the end of the last recorded prescription (both index and comparison medication), the adjusted results were comparable to those found in the primary analyses. The IRR of current GTZ use now showed a strong protective association (IRR 0.52; 95% CI 0.32–0.73).
When use of the index drug was stratified by duration of use, a strong protective association was shown in short and long term (>3 years) users of DPP4 inhibitors and GLP-1 mimetics, but not GTZ.
The results of the analyses using a secondary definition of Parkinson’s disease, and the results of the analyses restricted to subjects aged 40 years and older and, in a separate analysis, non-smokers, were comparable to the results of the main analyses (Table 3).
The results of the post hoc analyses restricted to users of antidiabetic agents with a BMI of ≥30 kg/m2, were in the same direction as the main results. However, there was weaker evidence for a protective association between the use of GLP-1 mimetics and the risk of Parkinson’s disease.
Discussion
In this large population-based cohort study we have shown that the incidence of Parkinson’s disease in patients diagnosed with type 2 diabetes varies substantially depending on the treatment for diabetes received. The rate of Parkinson’s disease was 36–60% lower in users of DPP4 inhibitors and GLP-1 receptor agonists compared to users of other oral antidiabetic drugs. The estimated association was adjusted for established risk factors such as age, smoking and duration of diabetes prior to the index date. Insulin users were excluded from the main analyses. A separate analysis in which the risk of Parkinson’s disease in insulin users in combination with the index drugs was underpowered, but the overall results were in the same direction as those found in the main analyses. Results for additional analyses in which follow-up time was censored at time of cessation of the index and comparison drugs, showed strong evidence for a protective association between current GTZ, DPP4 and GLP-1 exposure and Parkinson’s disease compared with other antidiabetic drug exposure. Adjusted results suggest that the protective association was seen even after short periods of exposure, and might continue after cessation of DPP4 use.
The incidence of Parkinson’s disease reported in this study is in line with the reported incidence of Parkinson’s disease in the UK. Previous studies have shown that diagnoses of Parkinson’s disease in UK primary health care have a positive predictive value of 81% (Hernan et al., 2004; Brauer et al., 2015; Parkinson’s UK, 2018). To our knowledge, only one Swedish population-based case control study has explored the epidemiological associations between the risk of Parkinson’s disease among patients treated with DPP4 inhibitors and/or GLP-1 receptor agonists. Svenningsson et al. (2016) found strong evidence of a protective effect of DPP4 inhibitors on the risk of Parkinson’s disease (OR 0.23; 95% CI 0.07–0.73). The point estimate for the risk of Parkinson’s disease in patients treated with GLP-1 receptor agonists was <1 (i.e. protective); however, the small sample size meant that confidence intervals were large and this could not be interpreted as evidence of a protective effect. The positive markers from this population study seem to be further supported by the findings of potential disease modifying effects of two single centre phase 2 interventional studies (Aviles-Olmos et al., 2014; Athauda et al., 2017). Outcomes of upcoming larger, long term randomized trials exploring these agents will, however, be crucial in providing certainty and a multicentre phase 3 trial is currently underway to explore the disease modifying effect of exenatide in Parkinson’s disease (Exenatide-PD3; EudraCT: 2018-003028-35).
Evidence was found of an association between the use of GTZ and the onset of Parkinson’s disease in the secondary analysis whereby the follow-up period was censored at the end of antidiabetic drug use. Several observational studies conducted since 2015 have reported strong evidence of a protective effect of glitazones on the risk of Parkinson’s disease in individuals with diabetes, whilst other studies found no such effect (Brauer et al., 2015; Connolly et al., 2015; Brakedal et al., 2017). The results of the first clinical trial in which the potential of pioglitazone as Parkinson’s disease treatment was investigated were negative. This could potentially be attributed to the brief 44-week follow-up period [NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators, 2015].
Type 2 diabetes is an established risk factor for Parkinson’s disease and antidiabetic drugs may lower the excess risk of Parkinson’s disease in patients with diabetes (Svenningsson et al., 2016). Indeed, when we compared the prevalence of Parkinson’s disease in patients with diabetes to subjects without diabetes, we found a lower prevalence of Parkinson’s disease in a cohort of individuals without a recorded diagnosis of diabetes. Within the group of patients with diabetes, the prevalence of Parkinson’s disease was lower in a cohort of patients treated for diabetes compared to patients with diabetes, but no GP recorded antidiabetic treatment. Given the inherent difficulty involved in performing cross-study comparisons of anti-glycaemic drugs, there are few head-to-head studies comparing glycaemic control of different drug classes. Therefore, it may simply be that the addition of GTZs, DPP-IVs or GLP-1 drugs may achieve more effective glycaemic control and/or weight loss in combination, thus limiting the damaging effects of excess glycation on overall brain function. However, our propensity weighted scores have accounted for the severity of glucose control and the patients’ BMI thereby adjusting for these potential confounders. This suggests that this is not simply a reflection of peripheral glucose level or weight loss. GTZ, DPP4 and GLP-1 drugs have been shown to have anti-inflammatory effects as well as a number of other neuroprotective properties. It has previously been hypothesized that GLP-1 receptor stimulation in the CNS may impact on insulin receptor signalling pathways, which may enhance neuronal survival pathways (Athauda and Foltynie, 2016). An alternative mechanistic explanation relates to the anti-inflammatory effects mediated through GLP-1 receptor stimulation on microglia (Yun et al., 2018). Either or both mechanisms may have contributed to the observed reduction in Parkinson’s disease incidence rates reported.
Lastly, small vessel disease is more common in patients with diabetes and may contribute to the appearance of ‘vascular parkinsonism’, which may have been mistakenly diagnosed as Parkinson’s disease. Diabetes is one of the main risk factors for microvascular disease which is often seen in the brain (Fang et al., 2018). We deliberately excluded all individuals with a diagnosis of ‘secondary parkinsonism’ (which extends to patients with diagnoses of vascular parkinsonism) in an attempt to minimize this potential bias; however, a small number of individuals with Parkinson’s disease diagnoses (∼6%) may have in fact had vascular parkinsonism (Schrag et al., 2002). Furthermore, small vessel disease has been associated with the severity of motor impairment in Parkinson’s patients (Schwartz et al., 2018). A recent small study showed that post-mortem diagnosed small vessel disease in 77 patients with Parkinson’s disease was related to the degree of motor impairment (Schwartz et al., 2018). We therefore must acknowledge that the use of DPP4 inhibitors and/or GLP-1 receptor agonists in the management of diabetes may have a different microvascular effect in the brain compared to other oral antidiabetic agents.
Strengths and limitations
Our large study population comprised >100 000 patients diagnosed with diabetes and receiving treatment with GTZ, DPP4 inhibitors and/or GLP-1 mimetics or similar from 2006 onwards. GTZ, DPP4 inhibitors and/or GLP-1 mimetics are most often prescribed as a second-line or third-line treatment to patients who cannot tolerate or have responded less successfully to other oral treatments for diabetes [National Institute for Health and Care Excellence (NICE), 2015). We compared these patients to other oral users of second-line treatment at the date of prescribing the add-on treatment. Missing patient information with regard to potential confounders such as smoking status and alcohol use were recorded for <5% and we imputed missing values with the outcome included in the imputation model. This is recommended as the preferred approach in the literature (Leyrat et al., 2019).
Using electronic prescribing data may cause some exposure misclassification or non-adherence issues if patients did not collect or use their prescribed medicines as intended. Furthermore, some patients may have been under care of a specialist care provider. We believe that potential misclassification of exposure would be non-differential with respect to Parkinson’s disease status and would cause bias towards the null, or a potential underestimation of the protective association.
Less than 16% of all patients were diagnosed with Parkinson’s disease during the first 12 months of index drug use. Patients with Parkinson’s disease are likely to have experienced symptoms before the date of their clinical diagnosis. The patient's decision to treat their diabetes with a change in type or number of medications may have coincided with an increase in symptoms of Parkinson’s disease. Moreover, we cannot exclude the possibility that patients may have been given GTZ based on previous evidence that GTZ use is associated with a decreased risk of Parkinson’s disease.
The results of our study are of value in clinical practice when presented with a choice of oral pharmacological treatment options for diabetes. Our results cannot inform on the usefulness of specific drug classes on the rate of progression of Parkinson’s disease after diagnosis nor on their efficacy among patients with Parkinson’s disease in the absence of diabetes. Clinical trials are currently being undertaken to inform us of the effect of these drug classes on disease progression after diagnosis and in patients with Parkinson’s disease who do not have diabetes. Until completion of these phase 3 trials we are not making any recommendations about their use in treating Parkinson’s disease.
Conclusion
The incidence of Parkinson’s disease in patients diagnosed with type 2 diabetes varies substantially depending on the treatment for diabetes received. The use of DPP4 inhibitors and/or GLP-1 receptor agonists is associated with a lower rate of Parkinson’s disease compared to the use of other oral antidiabetic drugs. Our study provides unique evidence to support further investigation of DPP4 inhibitor and GLP-1 receptor agonist use in Parkinson’s disease.
Funding
This study was funded by the The Cure Parkinson's Trust (CPT project code: LW011). The Cure Parkinson’s Trust had no role in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Competing interests
All authors declare they received no support from any organization for the submitted work. T.F. has received honoraria from Profile Pharma, BIAL, AbbVie, Genus, Medtronic, and St Jude Medical, outside the submitted work. I.W. has received grants from The Research Grants Council (Hong Kong), Innovative Medicines Initiative, Amgen, Shire, Janssen-Cilag, Eli-Lily, Pfizer, GSK, Bayer Novartis and the European Union FP7 programme, outside the submitted work.
Supplementary Material
Glossary
- BMI =
body mass index
- GLP-1 =
glucagon-like peptide 1
- GTZ =
glitazones/thiazolidinediones
- IRR =
incidence rate ratio
- THIN =
The Health Improvement Network
References
- Athauda D, Foltynie T. Insulin resistance and Parkinson's disease: a new target for disease modification? Prog Neurobiol 2016; 145–146: 98–120. [DOI] [PubMed] [Google Scholar]
- Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 1664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest 2013; 123: 2730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson's disease. J Parkinsons Dis 2014; 4: 337–44. [DOI] [PubMed] [Google Scholar]
- Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011; 19: 251–5. [DOI] [PubMed] [Google Scholar]
- Brakedal B, Flones I, Reiter SF, Torkildsen O, Dolle C, Assmus J, et al. Glitazone use associated with reduced risk of Parkinson's disease. Mov Disord 2017; 32: 1594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer R, Bhaskaran K, Chaturvedi N, Dexter DT, Smeeth L, Douglas I. Glitazone treatment and incidence of Parkinson’s disease among people with diabetes: a retrospective cohort study. PLoS Med 2015; 12: e1001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda E, Barichella M, Pedrolli C, Klersy C, Cassani E, Caccialanza R, et al. Diabetes and risk of Parkinson's disease: a systematic review and meta-analysis. Diabetes Care 2011; 34: 2614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JG, Bykov K, Gagne JJ. Thiazolidinediones and Parkinson disease: a cohort study. Am J Epidemiol 2015; 182: 936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablo-Fernandez E, Goldacre R, Pakpoor J, Noyce AJ, Warner TT. Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology 2018; 91: e139–42. [DOI] [PubMed] [Google Scholar]
- Erbil D, Eren CY, Demirel C, Kucuker MU, Solaroglu I, Eser HY. GLP-1's role in neuroprotection: a systematic review. Brain Inj 2019; 33: 734–819. [DOI] [PubMed] [Google Scholar]
- Fang F, Zhan YF, Zhuo YY, Yin DZ, Li KA, Wang YF. Brain atrophy in middle-aged subjects with Type 2 diabetes mellitus, with and without microvascular complications. J Diabetes 2018; 10: 625–32. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Logroscino G, Rodriguez LA. A prospective study of alcoholism and the risk of Parkinson's disease. J Neurol 2004; 251 (Suppl 7): vII14–7. [DOI] [PubMed] [Google Scholar]
- Holscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs 2012; 26: 871–82. [DOI] [PubMed] [Google Scholar]
- Horsfall L, Walters K, Petersen I. Identifying periods of acceptable computer usage in primary care research databases. Pharmacoepidemiol Drug Saf 2013; 22: 64–9. [DOI] [PubMed] [Google Scholar]
- Jeong SM, Han K, Kim D, Rhee SY, Jang W, Shin DW. Body mass index, diabetes, and the risk of Parkinson's disease. Mov Disord 2019; 35: 236–44. [DOI] [PubMed] [Google Scholar]
- Joint Formulary Committee. British National Formulary 77: March-September; 2019. London: Pharmaceutical Press. [Google Scholar]
- Leyrat C, Seaman SR, White IR, Douglas I, Smeeth L, Kim J, et al. Propensity score analysis with partially observed covariates: how should multiple imputation be used? Stat Methods Med Res 2019; 28: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, et al. Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis 2018; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE). NICE Guideline. Type 2 Diabetes in Adults: Management. 2015. www.nice.org.uk/guidance/ng28 (24 July 2020, date last accessed). [PubMed]
- NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators. Pioglitazone in early Parkinson's disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol 2015; 14: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos A, Priyalatha G. A practical guide for using propensity score weighting in R. Practical Assessment, Research, and Evaluation 2015; 20: 13.. doi: 10.7275/jjtm-r398.
- Parkinson’s UK. The Incidence and Prevalence of Parkinson’s in the UK. Results from the Clinical Practice Research Datalink Summary Report. 2018. https://www.parkinsons.org.uk/sites/default/files/2018-01/CS2960%20Incidence%20and%20prevalence%20report%20branding%20summary%20report%20Published%202017.pdf (24 July 2020, date last accessed).
- Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson's disease in the community? J Neurol Neurosurg Psychiatry 2002; 73: 529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RS, Halliday GM, Soh D, Cordato DJ, Kril JJ. Impact of small vessel disease on severity of motor and cognitive impairment in Parkinson's disease. J Clin Neurosci 2018; 58: 70–4. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Wirdefeldt K, Yin L, Fang F, Markaki I, Efendic S, et al. Reduced incidence of Parkinson's disease after dipeptidyl peptidase-4 inhibitors-A nationwide case-control study. Mov Disord 2016; 31: 1422–3. [DOI] [PubMed] [Google Scholar]
- Tran KL, Park YI, Pandya S, Muliyil NJ, Jensen BD, Huynh K, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits 2017; 10: 178–88. [PMC free article] [PubMed] [Google Scholar]
- Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med 2018; 24: 931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party (IQVIA) and are not publicly available. After SRC review of our research protocol, we obtained anonymized data exclusively for this work without authorization to share the data or to conduct any secondary analysis.