Abstract
Increasing evidence suggests that abnormal regulation of neurotrophic factors is involved in the etiology and pathogenesis of Autism Spectrum Disorder (ASD). However, clinical data on neurotrophic factor levels in children with ASD were inconsistent. Therefore, we performed a systematic review of peripheral blood neurotrophic factors levels in children with ASD, and quantitatively summarized the clinical data of peripheral blood neurotrophic factors in ASD children and healthy controls. A systematic search of PubMed and Web of Science identified 31 studies with 2627 ASD children and 4418 healthy controls to be included in the meta-analysis. The results of random effect meta-analysis showed that the peripheral blood levels of brain-derived neurotrophic factor (Hedges’ g = 0.302; 95% CI = 0.014 to 0.591; P = 0.040) , nerve growth factor (Hedges’ g = 0.395; 95% CI = 0.104 to 0.686; P = 0.008) and vascular endothelial growth factor (VEGF) (Hedges’ g = 0.097; 95% CI = 0.018 to 0.175; P = 0.016) in children with ASD were significantly higher than that of healthy controls, whereas blood neurotrophin-3 (Hedges’ g = − 0.795; 95% CI = − 1.723 to 0.134; P = 0.093) and neurotrophin-4 (Hedges’ g = 0.182; 95% CI = − 0.285 to 0.650; P = 0.445) levels did not show significant differences between cases and controls. Taken together, these results clarified circulating neurotrophic factor profile in children with ASD, strengthening clinical evidence of neurotrophic factor aberrations in children with ASD.
Subject terms: Neurotrophic factors, Autism spectrum disorders, Diseases
Introduction
Autism Spectrum Disorder (ASD) refer to a group of neurodevelopmental disorders characterized primarily by restrictive, repetitive patterns of behaviors, loof of interest or activity, and social communication impairment. The disease includes autistic disorder, Asperger's syndrome and pervasive developmental disorder-not otherwise specified1,2. According to the Autism and Developmental Disabilities Monitoring Network, the number of children diagnosed with ASD has increased by 150% since 2000, with the overall prevalence of ASD estimated at 16.8 per 1000 children aged 8 years in 20143. Twin studies have shown that the concordance of ASD in monozygotic twins is higher than that in dizygotic twins, suggesting that ASD is highly heritable4. However, the coincidence rate between identical twins and autism and related diseases was less than 100%, indicating that environmental factors also play a role in ASD5.
The neurotrophins are a family of proteins that have been shown to play an important role in the central and peripheral nervous system, which control a number of aspects of survival, development, and function of neurons6. The so-called “classic” neurotrophin family includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4)7. Recently, research findings from analyses of postmortem and peripheral tissue and molecular genetic studies led to the hypothesis that neurotrophins—as crucial regulators of neuroplasticity—impacting on the pathophysiologic features of ASD8. In a trios-based association study, Nishimura et al. showed that the BDNF SNP haplotype combinations significantly associated with ASD9. Moreover, Postmortem studies found elevated BDNF protein levels in the fusiform gyrus tissue10 and elevated NT-3 protein levels in the cerebellum11 from patients with ASD. However, molecular genetic studies and postmortem studies on neurotrophic factors were scarce, and therefore it is difficult to evaluate the robustness of the associations of neurotrophins with ASD.
Increasing number of clinical studies measured peripheral blood levels of neurotrophic factors in children with ASD, due to the easy accessibility of blood and “periphery as a window to the brain” hypothesis12. However, clinical data on neurotrophic factor levels in children with ASD have yielded inconsistent results. One study showed that the serum BDNF levels of children with ASD were significantly higher than that of the control subjects13, whereas Makkonen et al.14 suggested that was no significant difference in serum BDNF concentrations between cases and controls, and another study showed that BDNF serum levels were significantly decreased in ASD children when compared with controls15. For VEGF, results from Emanuele et al. showed that VEGF levels in patients with ASD were lower than that of healthy controls16. In contrast, one study indicated that VEGF levels did not show significantly difference between children with ASD and healthy controls17. Moreover, studies have demonstrated inconsistent data for IGF-1 and IGF-2 levels comparing ASD children and healthy controls18,19. Given the inconsistent findings on neurotrophic factors in children with ASD, a meta-analysis on this subject is necessary.
To clarify neurotrophic factor profile in children with ASD, here we undertook a meta-analysis of studies measuring neurotrophic factor levels in blood of children with ASD and healthy controls.
Results
The initial search generated a total of 1217 records: 545 were searched from PubMed database, 670 from Web of Science and 2 additional records identified from the reference lists of relevant studies. After scanning the titles and abstracts, 62 articles relevant to present subject were identified for full-text scrutiny. Several studies were excluded as they did not have necessary data (11 studies)20–30; lack of healthy controls (6 studies)31–36; sample source is not peripheral blood (5 studies)18,37–40; samples derived from postmortem brain (3 studies)10,11,41; participants were adults (3 study)16,42,43; had patient samples that overlapped with another studies (1 study)44; neurotrophic factors were studied in less than 3 articles (1 study)19 and non-English publication (1 studies)45. Therefore, a total of 31 studies met the criteria were included for this meta-analysis (Fig. 1)8,12,13,15,17,46–71. Demographic and clinical profile of the included studies were presented in Supplementary Table S1.
Figure 1.
PRISMA flowchart of the literature search.
Main association of peripheral blood neurotrophic factor levels with ASD in children
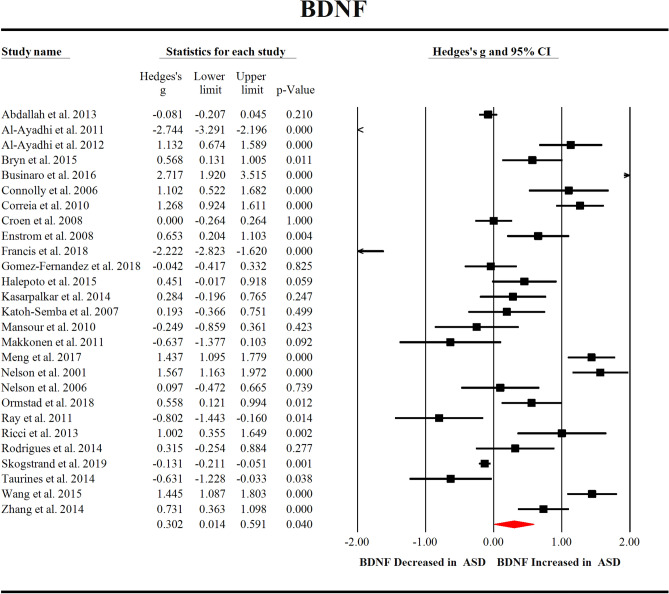
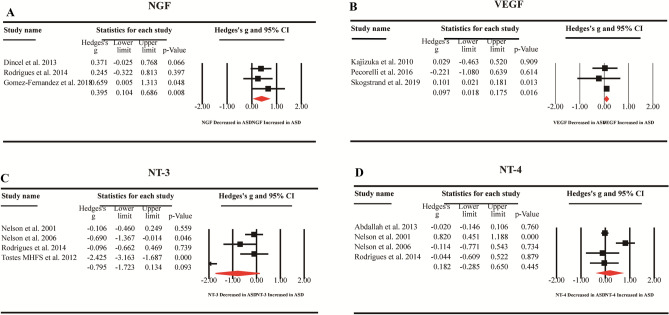
We first compared the peripheral blood BDNF levels between 2380 children with ASD and 4191 healthy controls extracted from 27 studies. Random-effects meta-analysis showed that ASD children had significantly increased levels of BDNF compared with healthy controls (Fig. 2, Hedges’ g = 0.302; 95% CI = 0.014 to 0.591; P = 0.040). Then, we compared blood NGF levels between 100 children with ASD and 84 healthy controls extracted from 3 studies, and the results showed that compared with healthy controls, children with ASD had significantly increased NGF levels (Fig. 3A, Hedges’ g = 0.395; 95% CI = 0.104 to 0.686; P = 0.008). In addition, we compared the blood VEGF levels of 844 children with ASD and 2460 healthy controls extracted from 3 studies, which showed significantly increased VEGF levels in ASD children when compared with healthy controls (Fig. 3B, Hedges’ g = 0.097; 95% CI = 0.018 to 0.175; P = 0.016). However, we did not observe significant differences between children with ASD and healthy controls for peripheral blood NT-3 (Hedges’ g = − 0.795; 95% CI = − 1.723 to 0.134; P = 0.093) and NT-4 (Hedges’ g = 0.182; 95% CI = − 0.285 to 0.650; P = 0.445) levels (Fig. 3C,D).
Figure 2.
Forest plot for random-effects meta-analysis for BDNF. Forest plot for random-effects meta-analysis on differences in blood BDNF concentrations between children with autism spectrum disorder (ASD) and healthy controls. The sizes of the squares are proportional to study weight. Diamond marker indicates pooled effect size. CI, confidence interval.
Figure 3.
Forest plot for random-effects meta-analysis for NGF, VEGF, NT-3 and NT-4. Forest plot for random-effects meta-analysis on differences in blood NGF (A), VEGF (B), NT-3 (C) and NT-4 (D) concentrations between children with autism spectrum disorder (ASD) and healthy controls. The sizes of the squares are proportional to study weight. Diamond marker indicates pooled effect size. CI, confidence interval.
Investigation of heterogeneity
Of the three neurotrophic factors that were significantly associated with ASD, NGF and VEGF did show no significant between-study heterogeneity, whereas BDNF showed high levels of heterogeneity. Therefore, we next performed subgroup analyses to investigate potential source of the high levels of heterogeneity for studies measuring BDNF concentrations.
The sources of heterogeneity include category variables (sample source, assay type and medication status) and continuous variables (sample size, age, sex, publication year, course of disease and disease severity). Due to a substantial lack of information regarding medication status, course of disease and disease severity, we only performed subgroup analyses on sample sources (serum and plasma) and assay types (ELISA and no ELISA), as well as meta-regression analyses based on sample size, age, sex and publication year.
Subgroup analyses suggested that sampling source did not address the between-study heterogeneity, and we still observed significant heterogeneity for studies plasma (Q = 43.966; I2 = 88.628; P < 0.001) and serum (Q = 344.797; I2 = 95.650; P < 0.001) BDNF levels (see Supplementary Fig. S1). Additionally, subgroup analyses stratified by assay type showed increased BDNF levels in ASD children when compared with controls in ELISA group (20 studies, Hedges’ g = 0.404; 95% CI = 0.024 to 0.785; P = 0.037), but not in non-ELISA group (7 studies, Hedges’ g = 0.015; 95% CI = − 0.618 to 0.647; P = 0.964). Again, we observed high levels of heterogeneity among studies for ELISA group (Q = 413.794; I2 = 95.408; P < 0.001) and non-ELISA group (Q = 119.886; I2 = 94.995; P < 0.001) (see Supplementary Fig. S2).
Meta-regression analyses showed that sample size, age, gender and publication year had no moderating effects on the outcome of the meta-analysis (P > 0.05 in all analyses) (see Supplementary Fig. S3).
Visual inspection of funnel plots suggested no risk of publication bias for studies analyzing BDNF, NGF and VEGF levels, and these were confirmed by the results of the Egger’s test (see Supplementary Fig. S4 and Table 1).
Table 1.
Summary of comparative outcomes for measurements of neurotrophic factor levels.
| TF | No. of studies | No. with ASD/controls | Main effect | Heterogeneity | Publication bias | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hedges g (95% CI) | Z score | P value | Q statistic | df | P value | I2 Statistic | Egger intercept | P value | |||
| BDNF | 27 | 2380/4191 | 0.302 (0.014 to 0.591) | 2.054 | 0.040 | 537.149 | 26 | 0.000 | 95.160 | 2.13562 | 0.11851 |
| NGF | 3 | 100/84 | 0.395 (0.104 to 0.686) | 2.662 | 0.008 | 0.909 | 2 | 0.635 | 0.000 | 1.13092 | 0.71027 |
| NT-3 | 4 | 140/114 | − 0.795 (− 1.723 to 0.134) | − 1.678 | 0.093 | 33.086 | 3 | 0.000 | 90.933 | − 7.11871 | 0.26344 |
| NT-4 | 4 | 485/838 | 0.182 (− 0.285 to 0.650) | 0.764 | 0.445 | 18.275 | 3 | 0.000 | 83.585 | 1.37770 | 0.61376 |
| VEGF | 3 | 844/2460 | 0.097 (0.018 to 0.175) | 2.414 | 0.016 | 0.611 | 2 | 0.737 | 0.000 | − 0.59890 | 0.23760 |
df, degrees of freedom; ASD, Autism Spectrum Disorder; BDNF, Brain-Derived Neurotrophic Factor; NGF, Nerve Growth Factor; NT-3, Neurotrophin-3; NT-4, Neurotrophin-4; VEGF, Vascular Endothelial Growth Factor; NTF, Neurotrophic Factor.
Discussion
In this study, we performed a comprehensive investigation on the changes of peripheral neurotrophic factors in children with ASD. We included 31 studies with 2486 ASD children and 4303 healthy controls measuring five neurotrophic factors, and reported the levels of BDNF, NGF and VEGF were elevated in children with ASD. However, no significant associations were found between NT-3 or NT-4 and ASD. In addition, we found high levels of between-study heterogeneity for BDNF, whereas NGF and VEGF did not show between-study heterogeneities. We further performed subgroup analysis based on the sample source and assay type, and meta-regression analysis based on sample size, age, gender and publication year for studies measuring BDNF levels. However, we did not find potential sources of heterogeneity. This is consistent with a previous meta-analysis on blood BDNF levels in ASD children, which included a relatively small number of studies published in 201672. Although studies from the literature in the past decade has produced inconsistent results, and the role of neurotrophic factors in children with ASD is still unclear, this study provides strong clinical evidence that the levels of BDNF, NGF and VEGF in peripheral blood of children with ASD are higher than those in healthy controls, strengthening the clinical evidence that neurotrophic factor plays a critical role in ASD onset and/or development.
Despite the largely unknown etiology and pathogenesis of ASD, researches have consistently demonstrated that children with ASD are accompanied by abnormal brain development73,74. Courchesne et al. investigated the neural basis of brain overgrowth in ASD children at the cellular level and found abnormal increases in the number of neurons in the prefrontal cortex of ASD boys75. Additionally, it has been suggested that that the brain volume overgrowth in early ASD children is mainly due to the increased proliferation of neural progenitor cells76. Neurotrophic factors play a positive role on the proliferation of embryonic neural progenitor cells27, and an in vivo study showed that BDNF increased neurogenesis in the granule cell layer of hippocampus in rats77. Importantly, BDNF levels are temporally regulated during development, which has been suggested to be required for proper neuronal development and functions78. Therefore, it is very likely that in the early stage of ASD children, abnormal regulation of BDNF leads to the subsequent long-term changes in the brain structure and function. Furthermore, it has been reported the excessive brain growth in ASD children occur prior to most clinical manifestations of the disease79, raising the possibility that the observed BDNF aberrations in ASD children found in the meta-analysis contributed to the excessive growth in brains of early ASD children.
We noted that levels of two other neurotrophic factors, NGF and VEGF, also increased compared with healthy controls. VEGF is a key signaling molecule of the central nervous system which is involved in neuroprotection, neuronal survival and axonal growth80. Similarly, NGF is also involved in important aspects of nerve cell growth, differentiation, survival, and regeneration, and thought may represent a serological marker for autistic children23,81. It is possible that that the structural changes in the brains of children with ASD may also be contributed by the abnormalities of NGF and VEGF. Therefore, targeting the neurotrophic factor system may provide a novel strategy for the potential treatment of ASD, and future studies are needed to validate the hypothesis. However, since the studies included in this meta-analysis analyzed NGF levels by ELISA method, and the commercially available ELISA kits could not differentiate between pro and mature forms of NGF, it is unclear whether the levels of the mature form of NGF (the form with neurotrophic activity) were up-regulated in ASD children. Thus, another explanation for the observed NGF aberrations in ASD children is the compromise of proNGF to mature NGF conversion in the disease, which requires further investigations.
Abnormalities of neurotrophic factors were also thought to be associated with other neurological diseases, such as Alzheimer's disease and schizophrenia. Previous meta-analyses have demonstrated that blood BDNF levels were significantly decreased in patients with Alzheimer’s disease, whereas blood NGF and VEGF levels did not show significant differences between patients with Alzheimer’s disease and control subjects82. Additionally, meta-analyses showed that blood BDNF and NGF levels were significantly decreased in schizophrenia patients when compared with controls83,84. In contrast, blood VEGF levels were found not to be significantly different between first-episode schizophrenia patients and controls, whereas medicated multiple-episode schizophrenia had higher levels of VEGF than that of control subjects85. It is considered that ASD shares many molecular pathways with other neuropsychiatric diseases including schizophrenia. However, the present meta-analysis revealed heightened blood BDNF, NGF and VEGF levels in ASD children, it is very likely that patients with ASD have a unique neurotrophic factor profile comparing with other neuropsychiatric diseases, and this may partially explain the pathogenesis of ASD.
Although no significant between-study heterogeneity was found in the analysis of blood NGF and VEGF levels, one explanation for the low heterogeneity is that relatively few studies have been included. Additionally, we found high levels of heterogeneity among studies analyzing BDNF levels. Here we used subgroup and meta-regression analyses to adjust confounders that could explain the between-study heterogeneity. The potential moderators that we have analyzed including sampling source, assay type, sample size, publication year, age and gender did not address the heterogeneity. Obviously, the unexplained heterogeneity may due to other variables that we have not analyzed, such as medication status, disease severity and life style. However, the limited information on these variables in the included studies prevented us from analyzing whether the potential confounders had moderating effects on the outcome of the meta-analysis, therefore highlighting the need to control the variables in future studies.
Despite this work provides strong clinical evidence of the increased blood neurotrophic factor profile in children with ASD, there are still some limitations in this study. Firstly, the meta-analysis of peripheral blood neurotrophic factor levels in children with ASD and healthy controls produced a summary of results mainly from cross-sectional studies. Therefore, it is unclear whether the abnormal levels of neurotrophic factors are the cause or consequence for the development of ASD. Secondly, this meta-analysis analyzed blood neurotrophic factors levels in ASD children, but the neurotrophic factor profile in the brains of ASD children is largely unknown. The third limitation of the meta-analysis is that the effect size is very small, and the number of participants is very small except for BDNF and VEGF. In addition, a small number of studies evaluated NT-3 and NT-4, which may make it difficult to observe significant associations between the two neurotrophins and ASD. It should be noted that our meta-analysis showed a trend of decreased blood NT-3 levels in ASD children when compared with controls (P = 0.093). It is likely that we would observe a significant association between NT-3 and ASD with increased number of studies and sample size from future studies. Lastly, our study included only English articles, which may lead to publication bias. However, considering that we have excluded only one non-English articles, this is unlikely to have a significantly impact on the outcome of our meta-analysis.
In conclusion, the results of our meta-analysis showed the elevated peripheral blood BDNF, NGF and VEGF concentrations as a manifestation of children with ASD, strengthening the clinical evidence of an abnormal neurotrophic factor profile in children with ASD. Thus, future investigators into neurotrophic factors as potential therapeutic targets for the treatment of ASD are warranted.
Method
This was an exploratory meta-analysis, and adhered to the guidelines that are recommended by the PRISMA statement (Preferred Reporting Items for Systematic reviews and Meta-Analysis)86.
Search strategy and study selection
We have conducted a systematic search of peer-reviewed English articles using PubMed and Web of Science databases up to December 23, 2019. Our search strategy was:(neurotrophin OR neurotrophic factor OR brain-derived neurotrophic factor OR BDNF OR nerve growth factor OR NGF OR neurotrophin-3 OR NT-3 OR neurotrophin-4 OR NT-4 OR glial cell-derived neurotrophic factor OR GDNF OR insulin-like growth factor OR IGF OR vascular endothelial growth factor OR VEGF) AND (Autism), without year limitation. Additionally, we checked the reference list of relevant studies.
Original articles were screened according to the title and abstract, and then were scrutinized based on the following criteria: (1) measured peripheral blood neurotrophic factors; (2) neurotrophic factor that were available in three or more studies; (3) studies which provide the neurotrophic factor concentrations and standard deviation, or sample size and P value; (4) compared with matched healthy controls; (5) studies were excluded if the samples were from adult ASD patients, this is because neurotrophic factor levels were altered in adult68, which could have a confounding effect.
Data extraction
Data from each included study was extracted by one investigator, and was verified by another investigator. Any inconsistencies were settled by discussions. Sample sizes, mean neurotrophic factor concentrations, standard deviation and P values were extracted as primary outcomes to generate effective size (ES). For studies that did not report neurotrophic factor concentrations, the sample size and P values were used to calculate the effect size87. Data on author last name, publication year, country of region, age, gender, sample source (serum or plasma sample or dried blood spot), diagnosis and assay type were also extracted.
Statistical analysis
All statistical analyses were performed by comprehensive Meta-Analysis version 2 software. The ES was produced by sample size, mean concentration and standard deviation (SD), or by sample size and P value if the data of mean concentration were not available. The standardized mean difference of neurotrophic factor levels between children with ASD and healthy controls was calculated as ES, and converted into Hedge’s g statistic, which provides an unbiased adjusted ES for sample size88. We calculated ES estimates by evaluating each neurotrophic factor. And we used a random effects model in this meta-analysis, because if between-study heterogeneity is significant, the random effects model can produce a wider 95% confidence interval than the fixed effect model89.
We used the Cochrane Q test and I2 statistics to assess the heterogeneity among studies. P < 0.10 was considered statistically significant. The inconsistent levels among studies was decided by the I2 index to reflect the impact of heterogeneity, and an I2 index of 0.25, 0.50, 0.75 indicated low, moderate and high levels of heterogeneity, respectively90. We used subgroup analysis and unrestricted maximum-likelihood random-effects meta-regressions of ES to evaluate whether theoretically related covariates influence the outcome of meta-analysis. Publication bias was assessed via visual inspection of funnel plots, and Egger’s test was used to estimate the statistical significance.
We set all the statistical significances at P < 0.05 in this study except for where noted.
Supplementary Information
Acknowledgements
This study was supported by the National Science Foundation of China (81703492), Beijing Natural Science Foundation (7182092), the Minzu University Research Fund Q8 (2018CXTD03), and the MUC 111 project.
Authors Contributions
Y.C conceived and designed this study; S-H.L and X-J.S extracted the data; S-H.L and F-C.F performed statistical analyses; All authors analyzed and interpreted the data; S-H.L drafted the manuscript with critical revisions from Y.C.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-79080-w.
References
- 1.Li D, Karnath HO, Xu X. Candidate biomarkers in children with autism spectrum disorder: a review of MRI studies. Neurosci. Bull. 2017;33:219–237. doi: 10.1007/s12264-017-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma SR, Gonda X, Tarazi FI. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol. Ther. 2018;190:91–104. doi: 10.1016/j.pharmthera.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Baio J, et al. Prevalence of Autism Spectrum Disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 2018;67:1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin M, Sur M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 2015 doi: 10.1126/science.aab3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matelski L, Van de Water J. Risk factors in autism: thinking outside the brain. J. Autoimmun. 2016;67:1–7. doi: 10.1016/j.jaut.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skaper SD. Neurotrophic factors: an overview. Methods Mol. Biol. 2018;1727:1–17. doi: 10.1007/978-1-4939-7571-6_1. [DOI] [PubMed] [Google Scholar]
- 7.Keefe KM, Sheikh IS, Smith GM. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int. J. Mol. Sci. 2017 doi: 10.3390/ijms18030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, et al. Increased serum levels of brain-derived neurotrophic factor in autism spectrum disorder. NeuroReport. 2015;26:638–641. doi: 10.1097/wnr.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura K, et al. Genetic analyses of the brain-derived neurotrophic factor (BDNF) gene in autism. Biochem. Biophys. Res. Commun. 2007;356:200–206. doi: 10.1016/j.bbrc.2007.02.135. [DOI] [PubMed] [Google Scholar]
- 10.Garcia KL, et al. Altered balance of proteolytic isoforms of pro-brain-derived neurotrophic factor in autism. J. Neuropathol. Exp. Neurol. 2012;71:289–297. doi: 10.1097/NEN.0b013e31824b27e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sajdel-Sulkowska EM, Xu M, Koibuchi N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum. 2009;8:366–372. doi: 10.1007/s12311-009-0105-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhang QB, Jiang LF, Kong LY, Lu YJ. Serum Brain-derived neurotrophic factor levels in Chinese children with autism spectrum disorders: a pilot study. Int. J. Dev. Neurosci. 2014;37:65–68. doi: 10.1016/j.ijdevneu.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Meng WD, et al. Elevated serum brain-derived neurotrophic factor (BDNF) but not BDNF gene val66met polymorphism is associated with autism spectrum disorders. Mol. Neurobiol. 2017;54:1167–1172. doi: 10.1007/s12035-016-9721-9. [DOI] [PubMed] [Google Scholar]
- 14.Makkonen I, et al. Brain derived neurotrophic factor and serotonin transporter binding as markers of clinical response to fluoxetine therapy in children with autism. J. Pediatr. Neurol. 2011;9:1–8. doi: 10.3233/JPN-2010-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis K, et al. Brain-derived neurotrophic factor (BDNF) in children with ASD and their parents: a 3-year follow-up. Acta Psychiatr. Scand. 2018;137:433–441. doi: 10.1111/acps.12872. [DOI] [PubMed] [Google Scholar]
- 16.Emanuele E, et al. Serum levels of vascular endothelial growth factor and its receptors in patients with severe autism. Clin. Biochem. 2010;43:317–319. doi: 10.1016/j.clinbiochem.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Kajizuka M, et al. Serum levels of platelet-derived growth factor BB homodimers are increased in male children with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:154–158. doi: 10.1016/j.pnpbp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Riikonen R, et al. Cerebrospinal fluid insulin-like growth factors IGF-1 and IGF-2 in infantile autism. Dev. Med. Child Neurol. 2006;48:751–755. doi: 10.1017/S0012162206001605. [DOI] [PubMed] [Google Scholar]
- 19.Mills JL, et al. Elevated levels of growth-related hormones in autism and autism spectrum disorder. Clin. Endocrinol. (Oxf.) 2007;67:230–237. doi: 10.1111/j.1365-2265.2007.02868.x. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia A. Sensory impairment in mental retardation: a potential role for NGF. Arch. Ital. Biol. 2011;149:193–203. doi: 10.4449/aib.v149i2.1362. [DOI] [PubMed] [Google Scholar]
- 21.Bou Khalil R. Is insulin growth factor-1 the future for treating autism spectrum disorder and/or schizophrenia? Med. Hypotheses. 2017;99:23–25. doi: 10.1016/j.mehy.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Das UN. Nutritional factors in the pathobiology of autism. Nutrition. 2013;29:1066–1069. doi: 10.1016/j.nut.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Galvez-Contreras AY, Campos-Ordonez T, Gonzalez-Castaneda RE, Gonzalez-Perez O. Alterations of growth factors in autism and attention-deficit/hyperactivity disorder. Front. Psychiatry. 2017;8:126. doi: 10.3389/fpsyt.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh JY, Lim JS, Byun HR, Yoo MH. Abnormalities in the zinc–metalloprotease–BDNF axis may contribute to megalencephaly and cortical hyperconnectivity in young autism spectrum disorder patients. Mol. Brain. 2014;7:64. doi: 10.1186/s13041-014-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korzeniewski SJ, et al. Elevated protein concentrations in newborn blood and the risks of autism spectrum disorder, and of social impairment, at age 10 years among infants born before the 28th week of gestation. Transl. Psychiatry. 2018;8:115. doi: 10.1038/s41398-018-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li LY, Jiang N, Zhao Y. Could acupuncture have a role in the treatment of autism spectrum disorder via modulation of BDNF expression and activation? Acupunct. Med. 2014;32:503–505. doi: 10.1136/acupmed-2014-010602. [DOI] [PubMed] [Google Scholar]
- 27.Numakawa T, Odaka H, Adachi N. Actions of Brain-derived neurotrophic factor and glucocorticoid stress in neurogenesis. Int. J. Mol. Sci. 2017 doi: 10.3390/ijms18112312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman G. IGF—autism prevention/amelioration. Med. Hypotheses. 2019;122:45–47. doi: 10.1016/j.mehy.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Tsai SJ. Is autism caused by early hyperactivity of brain-derived neurotrophic factor? Med. Hypotheses. 2005;65:79–82. doi: 10.1016/j.mehy.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 30.Steinman G, Mankuta D. Insulin-like growth factor and the etiology of autism. Med. Hypotheses. 2013;80:475–480. doi: 10.1016/j.mehy.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Al-Ayadhi L, El-Ansary A, Bjorklund G, Chirumbolo S, Mostafa GA. Impact of Auditory Integration Therapy (AIT) on the plasma levels of human glial cell line-derived neurotrophic factor (GDNF) in Autism Spectrum Disorder. J. Mol. Neurosci. 2019;68:688–695. doi: 10.1007/s12031-019-01332-w. [DOI] [PubMed] [Google Scholar]
- 32.Moradi H, Sohrabi M, Taheri H, Khodashenas E, Movahedi A. The effects of different combinations of perceptual-motor exercises, music, and vitamin D supplementation on the nerve growth factor in children with high-functioning autism. Complement. Ther. Clin. Pract. 2018;31:139–145. doi: 10.1016/j.ctcp.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Riikonen R, Vanhala R. Levels of cerebrospinal fluid nerve-growth factor differ in infantile autism and Rett syndrome. Dev. Med. Child. Neurol. 1999;41:148–152. doi: 10.1017/s0012162299000328. [DOI] [PubMed] [Google Scholar]
- 34.Skogstrand K, et al. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin. Chem. 2005;51:1854–1866. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 35.Spratt EG, et al. Pilot study and review: physiological differences in BDNF, a potential biomarker in males and females with autistic disorder. Int. Neuropsychiatr. Dis. J. 2015;3:19–26. doi: 10.9734/INDJ/2015/12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukasem C, et al. Pharmacogenetics of risperidone-induced insulin resistance in children and adolescents with autism spectrum disorder. Basic Clin. Pharmacol. Toxicol. 2018;123:42–50. doi: 10.1111/bcpt.12970. [DOI] [PubMed] [Google Scholar]
- 37.Anlar B, et al. Urinary epidermal and insulin-like growth factor excretion in autistic children. Neuropediatrics. 2007;38:151–153. doi: 10.1055/s-2007-990282. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, et al. Transplantation of umbilical cord blood mononuclear cells increases levels of nerve growth factor in the cerebrospinal fluid of patients with autism. Genet. Mol. Res. 2015;14:8725–8732. doi: 10.4238/2015.July.31.21. [DOI] [PubMed] [Google Scholar]
- 39.Makkonen I, Kokki H, Kuikka J, Turpeinen U, Riikonen R. Effects of fluoxetine treatment on striatal dopamine transporter binding and cerebrospinal fluid insulin-like growth factor-1 in children with autism. Neuropediatrics. 2011;42:207–209. doi: 10.1055/s-0031-1291242. [DOI] [PubMed] [Google Scholar]
- 40.Vanhala R, Turpeinen U, Riikonen R. Low levels of insulin-like growth factor-I in cerebrospinal fluid in children with autism. Dev. Med. Child. Neurol. 2001;43:614–616. doi: 10.1017/s0012162201001116. [DOI] [PubMed] [Google Scholar]
- 41.Sheikh AM, et al. BDNF-Akt-Bcl2 antiapoptotic signaling pathway is compromised in the brain of autistic subjects. J. Neurosci. Res. 2010;88:2641–2647. doi: 10.1002/jnr.22416. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto K, et al. Reduced serum levels of brain-derived neurotrophic factor in adult male patients with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:1529–1531. doi: 10.1016/j.pnpbp.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Miyazaki K, et al. Serum neurotrophin concentrations in autism and mental retardation: a pilot study. Brain Dev. 2004;26:292–295. doi: 10.1016/S0387-7604(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 44.Abdallah MW, et al. Amniotic fluid MMP-9 and neurotrophins in autism spectrum disorders: an exploratory study. Autism Res. 2012;5:428–433. doi: 10.1002/aur.1254. [DOI] [PubMed] [Google Scholar]
- 45.Tsukurova LA. A neuroprotective approach to optimizing treatment and correction activities in children with autism spectrum disorders. Zh. Nevrol. Psikhiatr. Im. S S Korsakova. 2018;118:51–56. doi: 10.17116/jnevro20181185251. [DOI] [PubMed] [Google Scholar]
- 46.Skogstrand K, et al. Reduced neonatal brain-derived neurotrophic factor is associated with autism spectrum disorders. Transl. Psychiatry. 2019;9:252. doi: 10.1038/s41398-019-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ormstad H, et al. Serum tryptophan, tryptophan catabolites and brain-derived neurotrophic factor in subgroups of youngsters with autism spectrum disorders. CNS Neurol. Disord. Drug Targets. 2018;17:626–639. doi: 10.2174/1871527317666180720163221. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Fernandez A, et al. Children with autism spectrum disorder with regression exhibit a different profile in plasma cytokines and adhesion molecules compared to children without such regression. Front. Pediatr. 2018;6:264. doi: 10.3389/fped.2018.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Businaro R, et al. Interleukin-18 modulation in autism spectrum disorders. J. Neuroinflam. 2016;13:2. doi: 10.1186/s12974-015-0466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecorelli A, et al. Cytokines profile and peripheral blood mononuclear cells morphology in Rett and autistic patients. Cytokine. 2016;77:180–188. doi: 10.1016/j.cyto.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Bryn V, et al. Brain derived neurotrophic factor (BDNF) and autism spectrum disorders (ASD) in childhood. Eur. J. Paediatr. Neurol. 2015;19:411–414. doi: 10.1016/j.ejpn.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Halepoto DM, Bashir S, Zeina R, Al-Ayadhi LY. Correlation Between Hedgehog (Hh) protein family and brain-derived neurotrophic factor (BDNF) in Autism Spectrum Disorder (ASD) J. Coll. Phys. Surg. Pak. 2015;25:882–885. doi: 10.2015/JCPSP.882885. [DOI] [PubMed] [Google Scholar]
- 53.Taurines R, et al. Altered peripheral BDNF mRNA expression and BDNF protein concentrations in blood of children and adolescents with autism spectrum disorder. J. Neural Transm. (Vienna) 2014;121:1117–1128. doi: 10.1007/s00702-014-1162-x. [DOI] [PubMed] [Google Scholar]
- 54.Kasarpalkar NJ, Kothari ST, Dave UP. Brain-derived neurotrophic factor in children with Autism Spectrum Disorder. Ann Neurosci. 2014;21:129–133. doi: 10.5214/ans.0972.7531.210403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues DH, et al. Circulating levels of neurotrophic factors in autism spectrum disorders. Neuro Endocrinol. Lett. 2014;35:380–384. [PubMed] [Google Scholar]
- 56.Chandley M, et al. Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J. Psychiatry Neurosci. JPN. 2013;38:276–284. doi: 10.1503/jpn.120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dincel N, et al. Serum nerve growth factor levels in autistic children in Turkish population: a preliminary study. Indian J. Med. Res. 2013;138:900–903. [PMC free article] [PubMed] [Google Scholar]
- 58.Abdallah MW, et al. Neonatal levels of neurotrophic factors and risk of autism spectrum disorders. Acta Psychiatr. Scand. 2013;128:61–69. doi: 10.1111/acps.12020. [DOI] [PubMed] [Google Scholar]
- 59.Tostes MH, Teixeira HC, Gattaz WF, Brandao MA, Raposo NR. Altered neurotrophin, neuropeptide, cytokines and nitric oxide levels in autism. Pharmacopsychiatry. 2012;45:241–243. doi: 10.1055/s-0032-1301914. [DOI] [PubMed] [Google Scholar]
- 60.Al-Ayadhi LY. Relationship between Sonic hedgehog protein, brain-derived neurotrophic factor and oxidative stress in autism spectrum disorders. Neurochem. Res. 2012;37:394–400. doi: 10.1007/s11064-011-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khundakar A, Morris C, Oakley A, Thomas A. Cellular pathology within the anterior cingulate cortex of patients with late-life depression: a morphometric study. Psychiatry Res. 2011;194:184–189. doi: 10.1016/j.pscychresns.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Ray B, Long JM, Sokol DK, Lahiri DK. Increased secreted amyloid precursor protein-alpha (sAPPalpha) in severe autism: proposal of a specific, anabolic pathway and putative biomarker. PLoS ONE. 2011;6:e20405. doi: 10.1371/journal.pone.0020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.AL-Ayadhi L. Serum levels of brain-derived neurotrophic factor (BDNF) in autistic children in central Saudi Arabia. The Open Conference Proceedings Journal. 2011;2:36–40. doi: 10.2174/2210289201102010036. [DOI] [Google Scholar]
- 64.Mona Mansour AM, Azam H, Henedy M. Brain derived neurotrophic factor in autism. Curr. Psychiatr. 2010;17:23–29. [Google Scholar]
- 65.Correia CT, et al. Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes Brain Behav. 2010;9:841–848. doi: 10.1111/j.1601-183X.2010.00627.x. [DOI] [PubMed] [Google Scholar]
- 66.Croen LA, et al. Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) Study. Autism Res. 2008;1:130–137. doi: 10.1002/aur.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enstrom A, et al. Peripheral blood leukocyte production of BDNF following mitogen stimulation in early onset and regressive autism. Am. J. Biochem. Biotechnol. 2008;4:121–129. doi: 10.3844/ajbbsp.2008.121.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katoh-Semba R, et al. Age-related changes in BDNF protein levels in human serum: differences between autism cases and normal controls. Int. J. Dev. Neurosci. 2007;25:367–372. doi: 10.1016/j.ijdevneu.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Nelson P, et al. Selected neurotrophins, neuropeptides, and cytokines: developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. Int. J. Dev. Neurosci. 2006;24:73–80. doi: 10.1016/j.ijdevneu.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Connolly AM, et al. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol. Psychiatry. 2006;59:354–363. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Nelson KB, et al. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann. Neurol. 2001;49:597–606. doi: 10.1002/ana.1024. [DOI] [PubMed] [Google Scholar]
- 72.Qin XY, et al. Association of peripheral blood levels of brain-derived neurotrophic factor with autism spectrum disorder in children: a systematic review and meta-analysis. JAMA Pediatr. 2016;170:1079–1086. doi: 10.1001/jamapediatrics.2016.1626. [DOI] [PubMed] [Google Scholar]
- 73.Sparks B, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 74.Dementieva Y, et al. Accelerated head growth in early development of individuals with autism. Pediatr. Neurol. 2005;32:102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Courchesne E, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 76.Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in autism. Mol. Psychiatry. 2017;22:1385–1394. doi: 10.1038/mp.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scharfman H, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 78.Mori T, Shimizu K, Hayashi M. Differential expression patterns of TrkB ligands in the macaque monkey brain. NeuroReport. 2004;15:2507–2511. doi: 10.1097/00001756-200411150-00015. [DOI] [PubMed] [Google Scholar]
- 79.Lainhart J, Lange N. Increased neuron number and head size in autism. JAMA. 2011;306:2031–2032. doi: 10.1001/jama.2011.1633. [DOI] [PubMed] [Google Scholar]
- 80.Yasuhara T, Shingo T, Date I. The potential role of vascular endothelial growth factor in the central nervous system. Rev. Neurosci. 2004;15:293–307. doi: 10.1515/revneuro.2004.15.4.293. [DOI] [PubMed] [Google Scholar]
- 81.Cortesi M, Alfei E, Barale F, Fusar-Poli P. Linking autism, regression and Landau-Kleffner syndrome: integrative role of nerve growth factor. Med. Hypotheses. 2007;68:1178–1179. doi: 10.1016/j.mehy.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 82.Du Y, et al. Postmortem brain, cerebrospinal fluid, and blood neurotrophic factor levels in Alzheimer's disease: a systematic review and meta-analysis. J. Mol. Neurosci. 2018;65:289–300. doi: 10.1007/s12031-018-1100-8. [DOI] [PubMed] [Google Scholar]
- 83.Fernandes BS, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol. Psychiatry. 2015;20:1108–1119. doi: 10.1038/mp.2014.117. [DOI] [PubMed] [Google Scholar]
- 84.Qin XY, Wu HT, Cao C, Loh YP, Cheng Y. A meta-analysis of peripheral blood nerve growth factor levels in patients with schizophrenia. Mol. Psychiatry. 2017;22:1306–1312. doi: 10.1038/mp.2016.235. [DOI] [PubMed] [Google Scholar]
- 85.Misiak B, Stramecki F, Stanczykiewicz B, Frydecka D, Lubeiro A. Vascular endothelial growth factor in patients with schizophrenia: a systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;86:24–29. doi: 10.1016/j.pnpbp.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 88.Qin XY, et al. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer's disease: a meta-analysis study (N=7277) Mol. Psychiatry. 2017;22:312–320. doi: 10.1038/mp.2016.62. [DOI] [PubMed] [Google Scholar]
- 89.Wei Z, Li X, Li X, Liu Q, Cheng Y. Oxidative stress in Parkinson's disease: a systematic review and meta-analysis. Front. Mol. Neurosci. 2018;11:236. doi: 10.3389/fnmol.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei Z, Li X, Li X, Liu Q, Cheng Y. Oxidative stress in Parkinson's disease: a systematic review and meta-analysis. Stat. Med. 2018;21:1539–1558. doi: 10.3389/fnmol.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.