Abstract
Background
The proportion of positive patients admitted to acute-care hospitals for reasons other than coronavirus disease-19 (COVID-19) is unknown. These patients potentially put other patients and healthcare workers at risk of infection.
Objective
The objective of this study was to define the proportion of asymptomatic patients admitted with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Secondary objectives were to define the positivity rate, reasons for admission, and the geographic distribution in the region.
Methods
Universal surveillance testing for SARS-CoV-2 was performed on patients admitted to this hospital over a 12-week period from April 9, 2020 to July 1, 2020. Positive patients were categorized as either symptomatic or asymptomatic as defined by the 11 criteria per the Centers for Disease Control and Prevention. The positivity rate, proportion with and without symptoms, reasons for admission, and geographic distribution in the region were recorded.
Results
The positivity rate ranged from 0.8% to 6.2%. The proportion of asymptomatic patients with SARS-CoV-2 was 37%. Asymptomatic patients primarily presented to the hospital because of either trauma or labor. Some clusters in the region were identified of both symptomatic and asymptomatic patients.
Conclusions
The proportion of asymptomatic patients admitted with SARS-CoV-2 was significant. Identifying and isolating asymptomatic patients likely prevented exposure and development of hospital-acquired COVID-19 cases among healthcare workers and other patients, supporting the universal surveillance of all admitted patients.
Key Words: SARS coronaviruses, Symptoms
Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease-19; RT-PCR, reverse transcriptase-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
INTRODUCTION
Hospitals have had to create new infection prevention and control policies, or adapt current policies, for patients infected with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). The spectrum of illness can range from asymptomatic infection to severe pneumonia with acute respiratory distress syndrome and death.1 Different approaches are available for facilities to identify patients with SARS-CoV-2, which include; testing only those patients who have symptoms, testing those with high risk or testing all patients upon admission (universal surveillance testing). The benefits of the latter include knowing who to isolate in order to prevent transmission of SARS-CoV-2 to staff and other patients. The disadvantages include increased cost of testing and use of additional resources that may be limited (eg, laboratory testing supplies and personal protective equipment). Another potential disadvantage is decreased bed capacity if a hospital has semiprivate rooms unless positive patients are cohorted.
At the University of Louisville Hospital (UofL Health), universal surveillance testing for SARS-CoV-2 was implemented in April, 2020. Patients were tested on admission and, if positive, were placed in a specific coronavirus disease-19 (COVID-19) unit depending on what level of care they needed. The presence of asymptomatic patients hospitalized in the facility had been identified justifying the continuation of universal surveillance testing.2 The objective of this study was to define the proportion of asymptomatic patients admitted with SARS-CoV-2. Secondary objectives were to define the positivity rate, reasons for admission and the geographic distribution in the region.
MATERIALS AND METHODS
This was an observational, descriptive study of all patients admitted to the hospital who were positive for SARS-CoV-2 from April 9 to July 1, 2020 (12 weeks) at UofL Health; an academic acute care trauma hospital in Louisville, KY. Patients were identified using an electronic medical record (Cerner, North Kansas City, MO) and an electronic surveillance system (TheraDoc, Charlotte, NC). Geographic datasets were used (ArcMap, Esri, Redlands, CA) to visualize the distribution of asymptomatic and symptomatic patients in the city. A report was generated including all SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) tests with positive and negative results. The list of patients was filtered to remove duplicate results and outpatients. Institutional review board approval was obtained (IRB# 20.0225). Consent was not necessary since data was gathered by retrospective chart review.
Information collected for each record included the COVID-19 RT-PCR test result from a nasopharyngeal swab. One of three RT-PCR instruments were used onsite by the hospital for SARS-CoV-2 detection (BD Max, Becton Dickinson, Franklin Lakes, NJ; Cepheid, Sunnyvale, CA; or Liaison MDX, Diasorin, Saluggia, Italy). Demographics collected included age, sex, race, ethnicity, preferred language, and primary address. Information also collected were comorbidities (pulmonary, cardiovascular, endocrine, renal, oncologic and other), symptoms, as well as physical examination signs.
The Centers for Disease Control and Prevention (CDC) defines 11 symptoms for COVID-19 as fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, or diarrhea.3 Symptomatic patients were defined as having any of the 11 symptoms, where as asymptomatic patients did not report any of the 11 symptoms on admission. Symptoms may have been a patient's chief complaint or discovered in a review of symptoms. Admission diagnoses of asymptomatic patients were also reviewed. All patients admitted to the hospital with positive SARS-CoV-2 RT-PCR tests were identified. The patients who had COVID-19 symptoms on admission were analyzed separately from those who were asymptomatic.
Baseline patient characteristics of asymptomatic and symptomatic SARS-CoV-2 patients were compared using χ2 test for categorical variables and t-tests test for continuous variables. A P value of <.05 was considered statistically significant. Positivity rates and trends were plotted (Microsoft Excel v16.0 (2016), Microsoft, Redmond, WA).
RESULTS
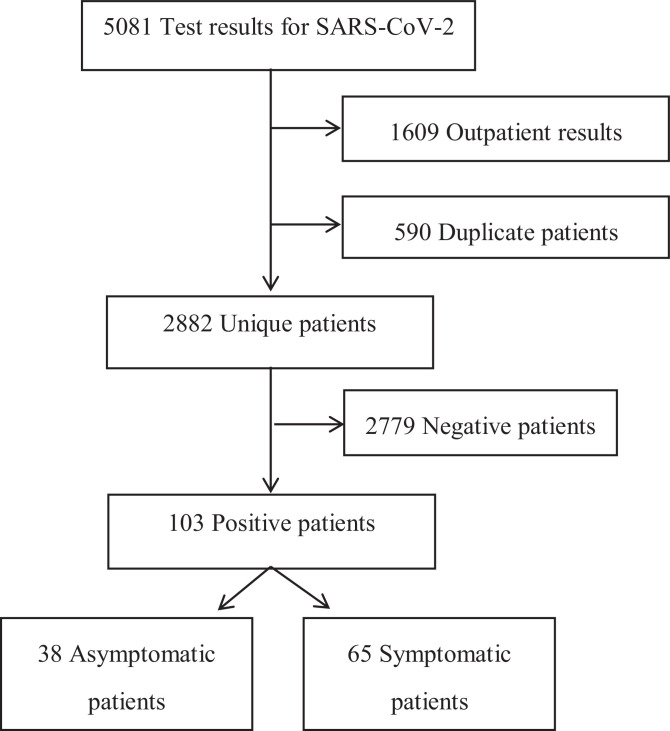
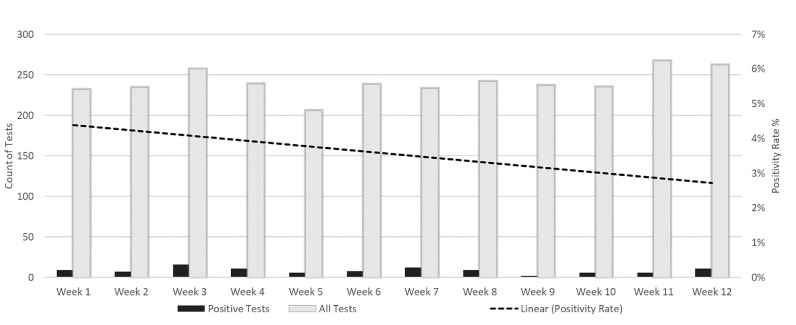
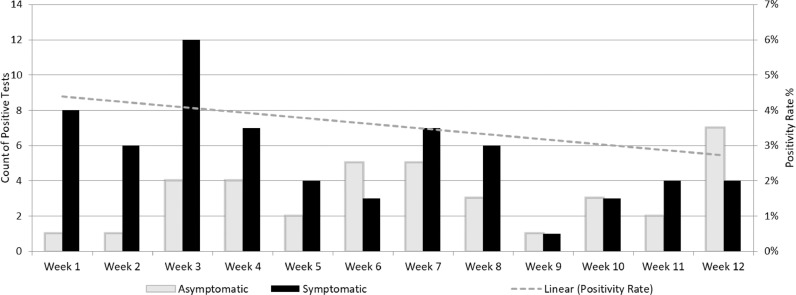
A total of 5,081 SARS-CoV-2 results were reviewed. Of those, 1,609 were excluded because they were outpatients, and 590 duplicate results were removed. Of the 2,882 that remained, 103 were SARS-CoV-2 positive (Fig 1 ) The proportion of positive samples among the 2,882 tested over time varied between 1% and 6%. (Fig 2 ) Among the 103 positive SARS-CoV-2 patients, 65 (63%) patients were symptomatic and 38 (37%) patients were asymptomatic. The proportion of SARS-CoV-2 patients who were asymptomatic varied over the duration of the study but trended up from 20% at the onset of the study period to 60% at the end (Fig 3 ).
Fig 1.
Among all patients who were tested for SARS-CoV-2, 2882 were admitted and 103 were positive. A total of 38 were asymptomatic and 65 were symptomatic.
Fig 2.
The positivity rate of SARS-CoV-2 tests among all hospitalized patients displayed by week.
Fig 3.
Among hospitalized patients who tested positive for SARS-CoV-2 (left y axis), the positivity rate (right y axis) for those who were symptomatic versus asymptomatic is displayed per week.
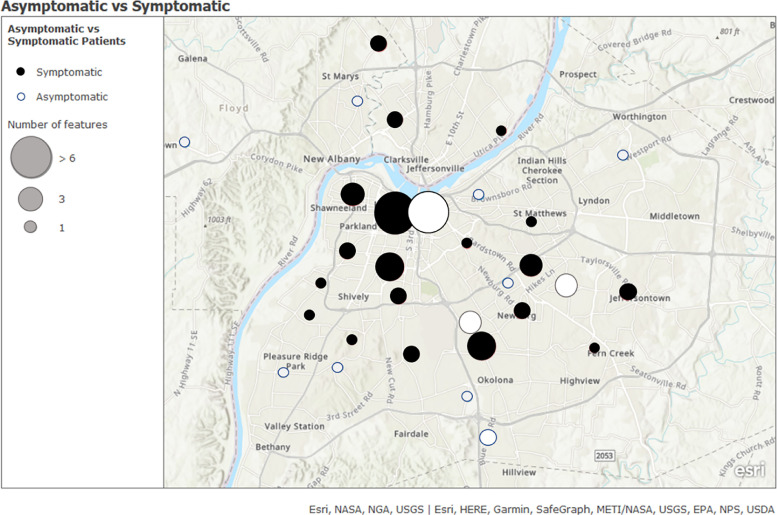
Demographics for the 103 positive patients are identified in Table 1 . Asymptomatic patients were significantly younger and had a lower body mass index (BMI). A higher proportion of pregnant asymptomatic patients spoke Spanish as a preferred language. Reasons for admission of the 38 asymptomatic patients were active labor (55%), trauma (26%), burn/wound (5%), and other (13%). Predominant and comprehensive symptoms of the symptomatic patients were also reviewed. (Table 2 ) Symptomatic patients with COVID-19 were more likely to have COPD, asthma, or any other comorbidity. Geographic datasets identified clusters of SARS-CoV-2 within the city of Louisville, and specific groups of symptomatic and asymptomatic patients. (Fig 4 ) The comparison of symptomatic and asymptomatic patients identified in the geographic dataset showed no obvious differences between the two groups and their residency locations within the city.
Table 1. Demographic information for asymptomatic and symptomatic patients who were admitted with SARS-CoV-2.
| AsymptomaticNo. (%) | SymptomaticNo. (%) | P | |
|---|---|---|---|
| Total | 38 (37) | 65 (63) | - |
| Mean age | 36 ± 20 | 52 ± 21 | <.05 |
| Mean BMI | 28 ± 6 | 21 ± 8 | .024 |
| White | 11 (29) | 26 (40) | .437 |
| Black or African American | 12 (32) | 21 (32) | .433 |
| Female | 29 (76) | 38 (58) | .746 |
| Preferred language | |||
| English | 20 (53) | 44 (68) | .137 |
| Spanish | 15 (39) | 11 (17) | .052 |
| Comorbidities | |||
| Any comorbidity | 12 (29) | 44 (68) | .562 |
| COPD | 0 | 10 (16) | .133 |
| Asthma | 2 (5) | 7 (11) | .769 |
| Other lung disease | 0 | 1 (2) | .635 |
BMI, basic metabolic index; COPD, chronic obstructive pulmonary disease; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
Table 2.
CDC symptoms of COVID-19 which defined 65 of 103 hospitalized patients testing positive as symptomatic
| Symptom | Count of symptoms |
|---|---|
| Fever | 35 |
| Cough | 26 |
| Shortness of breath/difficulty breathing | 35 |
| Fatigue | 11 |
| Muscle/body aches | 11 |
| Headache | 6 |
| Loss taste/smell | 0 |
| Sore throat | 0 |
| Congestion/runny nose | 1 |
| Nausea or vomiting | 18 |
| Diarrhea | 10 |
Most patients had more than one symptom.
Fig 4.
The distribution of where patients were from who were admitted to this facility and positive for SARS-CoV-2. Solid circles represent symptomatic patients and grey circles represent asymptomatic patients. The size of a circle correlates to the number of patients from each area.
DISCUSSION
Approximately one third of admitted patients positive for SARS-CoV-2 during a 12-week period of the 2020 pandemic were asymptomatic. This is the first study to identify the proportion of asymptomatic and symptomatic COVID-19 patients from an acute care setting. Asymptomatic SARS-CoV-2 patients sought care for non-COVID-19 related reasons and were only identified due to universal surveillance testing. The most common reasons for admission of asymptomatic SARS-CoV-2 patients were labor and trauma.
Asymptomatic and symptomatic patients came from all areas of the region and did not appear to be localized. Known risk factors for COVID-19 include persons frequently in congregate settings with an increased likelihood of close contact. The risk factors were exemplified in our population as pregnancy, poverty and crowding. These clusters represented a group of pregnant Hispanic patients from one area, and additional clusters of both symptomatic and asymptomatic patients in densely populated urban parts of the city. This type of information could contribute to outbreak investigations by a health department.
The primary implication of this study is that SARS-CoV-2 is not always clinically detectable based on presentation of signs and symptoms. An important strategy to protect healthcare workers and other patients is to perform a surveillance test on all patients admitted to the hospital. In addition to surveillance testing for SARS-CoV-2, other strategies utilized were standard and transmission-based precautions, as well as universal masking of all staff. In the midst of a pandemic with a local positivity rate of ∼5%, a mortality of ∼3%, a paucity of treatment options, and the absence of a vaccine, the implementation of these practices was supported.
The proportion of asymptomatic patients may change as the positivity rate changes in a local area. If it were to increase, presumably there would be more beds occupied with symptomatic COVID-19 patients, thus decreasing the ratio of asymptomatic to symptomatic patients. The proportion for the subpopulation of trauma and labor and delivery patients, however would likely stay the same, regardless of the positivity rate, since other issues drive them to the hospital. After recovery from a pandemic, the numbers of SARS-CoV-2 patients would likely be too small to be statistically meaningful.
It appears that asymptomatic positive people fueled the pandemic to persist for months.4 , 5 The reason that we currently isolate asymptomatic and symptomatic SARS-CoV-2 patients in the hospital is based on the indirect finding that they shed live virus. Thus, the premise of isolating asymptomatic positive patients is to contain the shedding of live virus. What is known is that a positive RT-PCR test does not necessarily confer that live virus is present. Culturing live virus would confer that, but is burdensome and complicated. A cheaper and quicker, but less accurate, way to determine if someone is shedding live virus is to know how many cycles the RT-PCR instrument took to detect SARS-CoV-2, if positive. Most instruments have a cycle threshold (Ct) for reporting positive as <40 cycles. The more cycles it took an instrument to detect the virus, the less likely there was live virus present.4 At least 3 studies discuss linking the contagiousness of an asymptomatic patient and their cycle threshold values. This is important because they provide data that supports the recommendation from Infection Prevention and Control Departments to isolate asymptomatic patients testing positive for SARS-CoV-2. First, the cycle thresholds of asymptomatic and symptomatic patients on the Diamond Princess were comparable, thus supporting a similar degree of contagiousness and the need to identify and isolate both populations.6 Second, SARS-CoV-2 identified with RT-PCR in asymptomatic patients, detected it with cycle thresholds as low as 21.9.7 Third, 2 of 114 asymptomatic travelers from Wuhan to Frankfurt, Germany tested positive and had viral growth of their samples.8
Studies have identified the proportion of asymptomatic and symptomatic individuals in a variety of populations. A study from Chongqing, China reported 167 patients admitted who were positive for SARS-CoV-2.9 Twenty patients (12%) were asymptomatic, but the study did not report if they were consecutive patients or report why they were tested if they did not have symptoms (clinical suspicion vs surveillance). Surveillance testing was performed in four long-term care facilities by the San Francisco Department of Public Health in response to COVID-19 outbreaks. Between March 30 and April 30 of 2020, the rate of positivity among those tested (residents and employees) was 50% (214/431). Of those, 40% (86/214) were asymptomatic – 63 residents and 23 employees.10 In a similar study, among 76 residents of a long-term care skilled nursing facility who were tested for SARS-CoV-2, 23 were positive with 13 (57%) asymptomatic residents.11
The study reviewing those tested on the Diamond Princess that was isolated in Japanese waters early in the pandemic in February 2020; had 3,711 people on board, of whom 82% were tested and 634 (21%) were positive. Among the positive people, 328 (52%) were asymptomatic.6 The city of Vo’, Italy was the first town to have a death related to COVID-19 in Italy. Of the 3,275 residents, 86% were tested and 73 (2.6%) were positive. Among the positive citizens of Vo’, 29 (40%) were asymptomatic.12 The proportions from these studies are summarized in Table 3 .
Table 3. The proportions of asymptomatic patients with SARS-CoV-2 from a variety of populations.
| Site (Location) | Time period | Proportion asymptomatic of SARS-CoV-2 (+) No. (%) |
|---|---|---|
| Medical Center (Chongqing, China)9 | Jan-Mar 2020 | 20/167 (12) |
| Long-term care facility (King Co., WA)11 | Mar 13, 2020 | 13/23 (57) |
| Long-term care facility (San Francisco, CA)10 | Mar 30-Apr 30, 2020 | 86/214 (40) |
| Cruise liner (Diamond Princess)6 | Feb 5-20, 2020 | 328/634 (52) |
| City (Vo’, Italy)12 | Feb 21-Mar 8, 2020 | 29/73 (40) |
| Airplane (China to Germany)8 | Feb 1, 2020 | 2/114 (<2) |
| Present study | Apr 9, Jul 1, 2020 | 65/103 (63) |
SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
Limitations
The present study was limited by a relatively low number of patients decreasing its generalizability. Multiple PCR testing instruments were used, which range in sensitivities for SARS-CoV-2 detection from 83 to ≥95%. Using the CDC definition of symptoms for COVID-19 may have resulted in some patients being miscategorized due to the inability to adequately attribute signs and symptoms to COVID-19. For example; some patients fulfilled the CDC criteria, but a more significant contributing factor was present, such as a symptomatic patient with shortness of breath who had been kicked in the chest by a horse (false positive). Alternatively, some patients did not meet criteria due to an inability to collect a complete assessment of signs and symptoms, such as an asymptomatic patient with expressive aphasia who was unable to verbalize COVID-19 symptoms (false negative). Considering the retrospective nature of this study, we were reliant on clinician assessments which varied by provider. Therefore, a rigorous chart review for symptoms was performed rather than merely using the chief complaint or billing codes to categorize patients. The study was strengthened by the longitudinal, rather than point-prevalence, assessment of the data over 12 weeks.
Among all the patients admitted to the hospital over 12 weeks that tested positive for SARS-CoV-2, 37% were asymptomatic in this acute care setting. These patients were characterized by lower body mass index, lower age, and were primarily admitted for trauma or labor. Having a universal surveillance testing policy in place to test everyone admitted during the COVID-19 pandemic likely prevented exposure and development of hospital-acquired COVID-19 cases among healthcare workers and other patients. Acute care facilities should consider universal surveillance testing on admission to identify all positive patients – symptomatic or asymptomatic.
Footnotes
Conflicts of interest: None to report.
References
- 1.Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) Centers Dis Control Prev. 2020 https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html Available at: [Google Scholar]
- 2.Arnold FW, Mahmood K, Prabhu A, et al. COPD exacerbation caused by SARS-CoV-2: a case report from the louisville COVID-19 surveillance program. Univ Louisville J Respir Infect. 2020;4:1–3. [Google Scholar]
- 3.Coronavirus Disease 2019 (COVID-19) Symptoms of Coronavirus. Centers for Disease control and prevention. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed July 20, 2020.
- 4.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikolai LA, Meyer CG, Kremsner PG, Velavan TP. Asymptomatic SARS Coronavirus 2 infection: invisible yet invincible. Int J Infect Dis. 2020;100:112–116. doi: 10.1016/j.ijid.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Scola B, Le Bideau M, Andrieani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Europ J Clin Micro Infect Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoehl S, Rabenau H, Berger A, Kortenbusch M, Cinatl J, Bojkova D. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao Y, Cheng P, Chen W, et al. High incidence of asymptomatic SARS-CoV-2 infection, Chongqing, China. Lancet, in press.
- 10.Louie JK, Scott HH, Dubois A, et al. Lessons from mass-testing for COVID-19 in long term care facilities for the elderly in San Francisco. Clin Infect Dis, in press [DOI] [PMC free article] [PubMed]
- 11.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of a SARS=CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]