Abstract
Background
Ascertaining involvement of left ventricular assist device (LVAD) in a patient presenting with bloodstream infection (BSI) can be challenging, frequently leading to use of chronic antimicrobial suppressive (CAS) therapy. We aimed to assess the efficacy of CAS therapy to prevent relapse of BSI from LVAD and non-LVAD sources.
Methods
We retrospectively screened adults receiving LVAD support from 2010 through 2018, to identify cases of BSI. Bloodstream infection events were classified into LVAD-related, LVAD-associated, and non-LVAD BSIs.
Results
A total of 121 episodes of BSI were identified in 80 patients. Of these, 35 cases in the LVAD-related, 14 in the LVAD-associated, and 46 in the non-LVAD BSI groups completed the recommended initial course of therapy and were evaluated for CAS therapy. Chronic antimicrobial suppressive therapy was prescribed in most of the LVAD-related BSI cases (32 of 35, 91.4%) and 12 (37.5%) experienced relapse. Chronic antimicrobial suppressive therapy was not prescribed in a majority of non-LVAD BSI cases (33, 58.9%), and most (31, 93.9%) did not experience relapse. Chronic antimicrobial suppressive therapy was prescribed in 9 of 14 (64.2%) cases of LVAD-associated BSI and none experienced relapse. Of the 5 cases in this group that were managed without CAS, 2 had relapse.
Conclusions
Patients presenting with LVAD-related BSI are at high risk of relapse. Consequently, CAS therapy may be a reasonable approach in the management of these cases. In contrast, routine use of CAS therapy may be unnecessary for non-LVAD BSIs.
Keywords: bloodstream infections, chronic antimicrobial suppressive therapy, left ventricular assist device
Routine use of CAS therapy may be unnecessary for cases of non-LVAD BSIs. In contrast, CAS therapy seems a reasonable approach to prevent relapse of BSI in LVAD-related cases.
Bloodstream infections (BSIs) are a frequent and serious complication in patients receiving left ventricular assist device (LVAD) therapy and are associated with high rates of morbidity and mortality [1–5]. Factors predisposing LVAD recipients to BSI include disruption of the skin barrier with the driveline, presence of intravascular catheters, and comorbid medical conditions [4]. Furthermore, in vitro experiments have also suggested that the biomaterials in these devices, when exposed to the bloodstream, may impair the cellular immune response and increase the susceptibility to infection [6].
International Society of Heart and Lung Transplantation (ISHLT) [7] guidelines suggest that the source of BSI in LVAD recipients can be determined by a process of elimination [8]. Once catheter-related BSI (CRBSI) has been excluded, then LVAD-related BSI—involving blood-contacting surfaces of the device—or an alternative site of infection should be investigated. However, in clinical practice, identification of the precise source of BSI in LVAD recipients can be quite challenging. Unlike cardiovascular implantable electronic device infections [9], removal of an LVAD for source control and microbiologic confirmation is not feasible without concurrent cardiac transplantation [7, 10, 11]. Moreover, imaging of the internal surfaces of the LVAD is technically difficult due to interference of metal components [5]. Therefore, even in cases where an alternative source of BSI is established, secondary hematogenous seeding of the device remains a concern [12].
The aforementioned uncertainties represent a major challenge in establishing the LVAD as primarily, secondarily, or noninfected during a BSI episode, frequently leading to the use of chronic antimicrobial suppressive (CAS) therapy to prevent relapse of BSI while the device remains in place [5]. However, these management decisions are not standardized or evidence-based and are left to the discretion of the treating physician.
The current investigation aimed to describe and analyze management of BSI episodes in LVAD recipients and assess the safety and efficacy of CAS therapy to prevent relapse of BSI from LVAD and non-LVAD sources.
METHODS
Study Design
We retrospectively screened all adults in our institutional LVAD registry who had continuous-flow LVAD support from January 1, 2010 through December 31, 2018, to identify cases of BSI irrespective of the underlying source. Cases of contaminated blood culture specimens and those on CAS therapy for reasons other than BSI were excluded.
Patient demographics, comorbid conditions, and LVAD features were recorded. Multiple episodes of BSI reported during the study period in the same patient after clinical improvement and microbiologic resolution were considered distinct events. We recorded clinical presentation, source evaluation, microbiologic data, and management of all episodes of BSI and analyzed impact of CAS therapy on patient outcomes.
Patient Consent Statement
The study was exempt from patient consent and the Mayo Clinic Institutional Review Board approved the study.
Definitions and Outcomes
A case of BSI was considered as “confirmed” after applying the Bekeris et al’s [13] definition of blood culture contamination to all blood culture specimens with reported microbial growth with excluding cases of contaminants. Persistent BSI was defined as bacteremia or fungemia for ≥72 hours after initiation of adequate pathogen-directed antimicrobial therapy [8]. Because current ISHLT definitions for BSI in LVAD recipients [7] do not distinguish between LVAD-related and LVAD-associated BSIs, study definitions listed in Table 1 to classify confirmed episodes of BSI in LVAD recipients were used. Accordingly, cases were classified as LVAD-related, LVAD-associated, and non-LVAD BSIs. All cases were reviewed by 2 infectious diseases providers (Z.E.G. and P.V.) to confirm the accurate classification of all episodes of BSI. Disagreements were resolved by discussion with the principal investigator (M.R.S.).
Table 1.
Study Definitions for BSI in LVAD Recipients
| LVAD classification | Definition | Radiographic Criteria | Microbiologic Criteria | Operative or Histopathology Criteria |
|---|---|---|---|---|
| LVAD-associated | Episodes involving all “indeterminate” cases of BSI where infection of the internal surface of the LVAD is suspected but cannot be confirmed and after an alternative site of infection has been ruled out based on review of clinical findings, imaging studies, and microbiologic and laboratory data. | No definitive evidence of LVAD infection on imaging. | 2 or more positive blood cultures of indeterminate source. | No inflammation or purulence of LVAD surrounding tissues. |
| LVAD-related | Episode of BSI arising from an infection on the external or internal surface of the device components including driveline, pocket, pump or cannula, and/or surrounding tissues | Any imaging modality showing fluid collection, gas, soft tissue inflammation, or increased uptake along the device components. | 2 or more positive blood cultures± local swab from driveline, surgical site, or operative cultures demonstrating the same organism and susceptibility pattern. | Intraoperative evidence of tissue inflammation or purulence. |
| Evidence of LVAD infection on physical examination (local inflammatory signs) or imaging. | Histopathology with acute or chronic inflammation and/or positive microbial stains. | |||
| Non-LVAD related | BSI episodes secondary to a confirmed alternative source of infection other than the internal or external surfaces of the LVAD or surrounding tissues (ie, pneumonia, catheter-related BSI, CIED infection). | No radiographic signs of LVAD infection. Imaging supporting infection elsewhere. | Blood cultures and local cultures with same organism and susceptibility pattern. | N/A |
Abbreviations: BSI, bloodstream infection; CIED, cardiovascular implantable electronic device; LVAD, left ventricular assist device; N/A, not applicable.
Definitions of CRBSI [8] and other infectious diseases syndromes were made in alignment with the Infectious Diseases Society of America [14–16], the American Heart Association [9, 17], and the Centers for Disease Control and Prevention (CDC)/National Healthcare Safety Network criteria [18]. An appropriate course of initial therapy for index BSI was defined as pathogen-directed antimicrobial therapy guided by susceptibility testing for the minimum recommended duration, in accordance with published guidelines or expert consensus for a specific infectious diseases syndrome.
Subsequent episodes of BSI in a given patient, after resolution of an index episode, were categorized into relapsing or recurrent BSI based on clinical, imaging, microbiologic, histopathology, or operative data. Relapsing BSI was defined as BSI with the same microorganism (based on identification and susceptibility pattern) originating from the same site of infection as the index episode, whereas recurrent BSI was defined as an episode of BSI from a different site of infection with either the same or a different microorganism than the one identified as the cause of index episode.
Multidrug-resistant (MDR) and extensively drug-resistant (XDR) microorganisms were defined per CDC criteria [19]. Source control measures included all interventions aimed to control the site of infection such as drainage of infected fluid collections, tissue debridement, LVAD exchange, and catheter removal.
Chronic antimicrobial suppressive therapy was defined as daily pathogen-directed antimicrobial therapy (oral or intravenous), guided by susceptibility testing, indicated life-long or until cardiac transplantation, after completion of the initial course of therapy given for acute BSI episode.
Our primary outcome of interest was occurrence and timing of relapse of BSI. Secondary outcomes were adverse events related to CAS therapy, isolation of resistant organisms during subsequent BSI episodes, and Clostridiodes difficile infection.
Statistical Analysis
Categorical variables of LVAD patients with BSI were reported as frequencies and percentages, and continuous variables were reported as medians and interquartile ranges (IQRs). The χ 2 test was used to compare categorical variables, and Kruskal-Wallis tests were used to analyze continuous variables among LVAD-related, LVAD-associated, and non-LVAD BSI cases. Statistical tests were 2-tailed with P < .05 considered statistically significant.
RESULTS
Patient Characteristics
Over the 8-year study period among 241 LVAD patients, 80 (33.2%) had confirmed episodes of BSI. Baseline characteristics of these patients are presented in Table 2. Median age at the time of LVAD implantation was 61 years (IQR, 50–67.1), and the majority of patients were male (65, 81.2%). The median body mass index in this population was 49.3 (IQR, 42.4–56.6).
Table 2.
Baseline Characteristics of 80 LVAD Recipients With Bloodstream Infections
| LVAD Classification | Total (n = 80) |
|---|---|
| Demographic, Patient Characteristics, and Device Features | |
| Age at implantation, years median (IQR) | 61 (50–67.1) |
| Ethnicity | |
| White | 72 (90%) |
| African American | 3 (4%) |
| Other | 5 (6%) |
| Male | 65 (81.2%) |
| BMI, kg/m2 median (IQR) | 49.3 (42.4–56.6) |
| Diabetes mellitus | 21 (26.5%) |
| Heart failure, ischemic | 41 (51.2%) |
| Chronic kidney disease | 30 (37.5%) |
| Hemodialysis | 6/30 (20%) |
| Autoimmune disorder | 4 (5%) |
| LVAD Characteristics | |
| Duration of device support, days median (IQR) | 857 (447–1569.2) |
| Destination therapy | 48 (60%) |
| HeartMate II device HeartWare | 68 (85%) 12 (15%) |
| Presence of CIED at implantation prosthetic valve or vascular graft | 51 (63.7%) 29 (36.2%) |
Abbreviations: BMI, body mass index; CIED, cardiovascular implantable electronic device; IQR, interquartile range; LVAD, left ventricular assist device.
Bloodstream Infection Episodes
A total of 121 distinct episodes of BSI were identified in 80 patients. Of these, 46 (38%) were classified as LVAD-related, 19 (15.7%) as LVAD-associated, and 56 (46.2%) as non-LVAD BSIs (Table 3). Of the total 121 BSI episodes, 80 (66.1%) were initial and 41 (33.8%) represented subsequent episodes.
Table 3.
Analysis of 121 Distinct Episodes of Bloodstream Infection Identified in 80 LVAD Recipients
| Variable | LVAD-Related (n = 46) |
LVAD-Associated (n = 19) | Non-LVAD (n = 56) | P Value |
|---|---|---|---|---|
| Primary Source | ||||
| Pump, cannula, pocket and/or driveline | 46 | -- | -- | -- |
| CRBSI | -- | 23 (41%) | -- | |
| CIED infection, right-sided IE and non-LVAD mediastinitis | -- | 9 (16%) | -- | |
| Pneumonia | -- | 3 (5.3%) | -- | |
| GI tract including biliary | -- | 12 (21.4%) | -- | |
| Renal and urinary tract | -- | 6 (10.7%) | -- | |
| Other | -- | -- | 3 (5.3%) | -- |
| Time to clearance of positive blood culturesa, days median, (IQR) | 3 (1–5) | 1 (1–3) | 1 (1–2) | .006 |
| BSI due to MDR or XDRb organisms | 18 (39.1%) | 1 (5.2%) | 4 (7.4%) | <.001 |
| Time from implant to BSI event, days median (IQR) | 416.5 (67.7–697.5) | 218 (35–411) | 171 (27–639.7) | <.001 |
Bold text indicates values that are statistically significant (P <.05).
Abbreviations: BSI, bloodstream infection; CIED, cardiovascular implantable electronic device; CRBSI, catheter-related bloodstream infection; GI, gastrointestinal; IE, infective endocarditis; IQR, interquartile range; LVAD, left ventricular assist device; MDR, multidrug resistant; XDR, extensively drug resistant.
aAfter the start of antimicrobial therapy.
bFive LVAD-related and 1 LVAD-associated BSI were due to XDR organisms.
The most common primary site of infection in LVAD-related cases was the driveline (31 of 46, 67.3%), whereas in non-LVAD cases the most frequent source of infection included intravascular catheters (23 of 56, 41.3%). Compared with LVAD-associated and LVAD-related BSIs, cases of non-LVAD BSI occurred earlier after LVAD implantation (P < .001). It is notable that the median time to blood-culture clearance after the start of effective antimicrobial therapy was significantly longer for cases of LVAD-related BSI compared with LVAD-associated and non-LVAD BSIs (P < .001).
The most prevalent group of microorganisms identified in blood cultures in all 3 study groups were Gram-positive cocci other than Staphylococcus aureus, primarily coagulase-negative staphylococci (CoNS) and enterococcal spp. Infections due to MDR and XDR organisms occurred more frequently in the LVAD-related BSI group (P < .001). Details of clinical presentation, sources of BSI, microbiologic data, management, and outcome of all 121 episodes of BSI are included in Supplementary Table 1.
Primary Outcome
Left Ventricular Assist Device-Related Bloodstream Infection
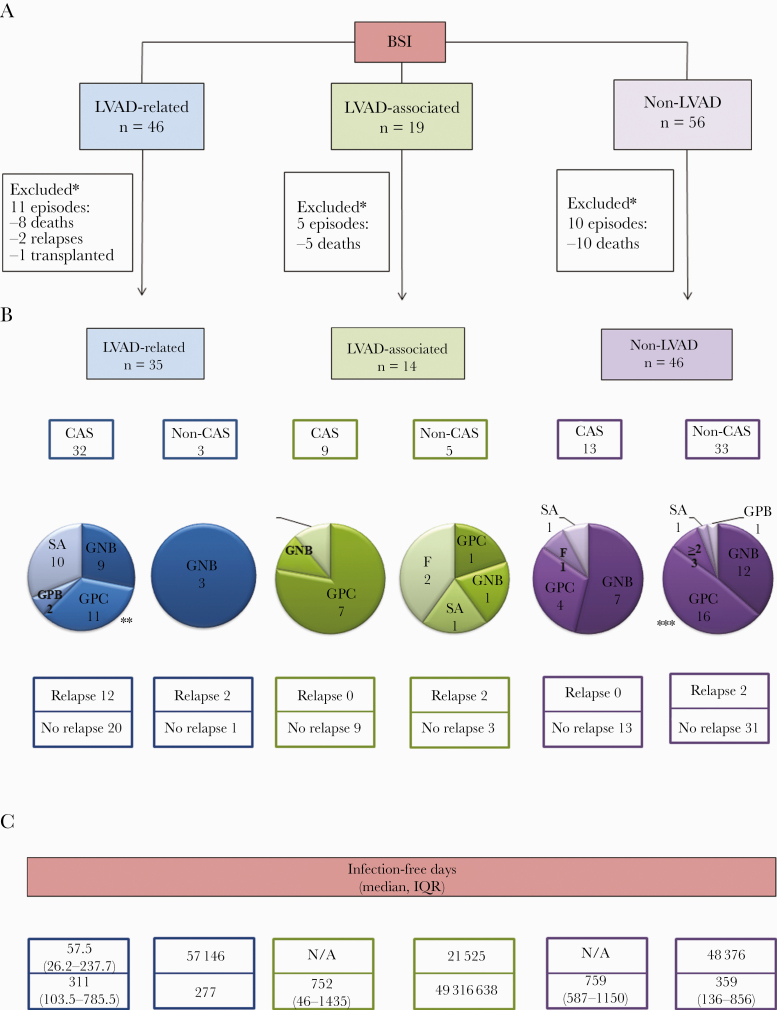
Primary outcome (relapse of BSI) and associated microbiology is summarized in Figure 1. Of 46 episodes of LVAD-related BSI, 35 (76%) completed the recommended initial course of therapy and were assessed for CAS candidacy at follow-up. Of the remaining 11 cases, 8 died, 1 was transplanted, and 2 relapsed before being evaluated for CAS therapy.
Figure 1.
Outcomes of 120 distinct episodes of bloodstream infection (BSI) among 80 left ventricular assist device (LVAD)-supported patients. *, Cases excluded from analysis if death, transplanted, or loss of follow-up before completion of initial course of antimicrobial therapy and evaluation for chronic antimicrobial suppression (CAS) therapy candidacy. **, Of the 11 cases due to Gram-positive cocci (GPC) other than Staphylococcus aureus, 4 were due to coagulase-negative staphylococci, 3 were due to Enterococcus spp, and 4 were due to Streptococcus spp. ***, Of the 16 cases due to GPC other than S aureus, 9 were due to coagulase-negative staphylococci, 5 due to Enterococcus spp, and 2 due to Streptococcus spp. Details of individual cases are provided in Supplementary Table 1. F, fungal; GNB, Gram-negative bacilli; GPB, Gram-positive bacilli; GPC, Gram-positive cocci other than S aureus; IQR, interquartile range; ≥2, more than 2 organisms.
With 3 exceptions, CAS therapy was prescribed in all cases of LVAD-related BSI after completion of initial course of antimicrobial therapy (32, 91.4%). Oral administration was the selected route in the majority of suppressed cases (24, 68.5%). The remaining cases were treated with parenteral therapy due to a lack of active oral options. Relapsing BSI was seen in 12 (37.5%) cases on CAS therapy, with a median infection-free survival of 57.5 days (IQR 26.2–237.7), and in 2 cases managed without CAS. Both of these cases were due to MDR-Gram-negative bacilli (Klebsiella pneumoniae and Pseudomonas aeruginosa) and developed relapse at 57 and 146 days, respectively (cases 11 and 34 of Supplementary Table 1). Further CAS therapy was not feasible in these 2 cases due to lack of available antimicrobial options.
The most common pathogen in patients who received CAS therapy and later relapsed was Staphylococcus aureus (5 of 12, 41.6%), in contrast to patients who remained suppressed where Gram-positive cocci other than S aureus were the most prevalent (9 of 20, 45%). Twenty (62.5%) patients remained on CAS therapy until the time of transplantation, death or last follow-up, with a median of 311 infection-free days (IQR, 103.5–785.5). Partial source control measures were performed in 8 and 2 cases in the CAS and non-CAS therapy groups, respectively (cases 2, 4, 6, 12, 15, 16, 17, 24, 34, and 39).
Left Ventricular Assist Device-Associated Bloodstream Infection
Of 19 cases, 5 died during the index episode of BSI while receiving initial course of antimicrobial therapy and before CAS could be considered (Figure 1). Of the remaining 14 cases, the majority received CAS therapy (9, 64.2%), and none experienced relapse during the suppression period, with a median of 752 infection-free days (IQR, 46–1435). A large proportion of these suppressed cases were due to Gram-positive cocci other than S aureus (7, 77.7%).
Despite an undefined source of infection in these cases, reasons for prescribing CAS therapy included (1) strong clinical suspicion for LVAD involvement in 8 patients and (2) suspicion for possible device seeding due to recurrent BSI in 1 case (same organism that was previously isolated from respiratory and blood specimens [cases 63 and 79]).
Of the 5 cases managed without CAS, 2 experienced infection relapse. These cases were due to S aureus and Enterococcus faecalis without an obvious source of infection. Infection-free days in these patients were 21 and 565, respectively, after the initial episode (cases 49 and 55).
Nonleft Ventricular Assist Device Bloodstream Infection
A total of 46 of 56 (82.1%) episodes of non-LVAD BSI completed the recommended initial course of pathogen-directed therapy and were evaluated for CAS (Figure 1). The remaining 10 episodes occurred in patients who died during index hospitalization while receiving initial course of therapy. Of 46 episodes of non-LVAD BSI, CAS therapy was prescribed for 13 (23.2%) with no relapses and a median of 759 infection-free days (IQR, 587–1150). In the remaining 33 cases (58.9%) in which CAS therapy was not prescribed and antimicrobials were discontinued after an appropriate initial course and source control, most (31 of 33, 93.9%) did not experience relapse (median of 359 infection-free days, IQR, 136–856). Of the 2 patients in the non-CAS group who experienced relapse of BSI, an intra-abdominal source of infection (adenocarcinoma of the colon) was identified at the time of relapse in 1 case (case 81). The other patient presented with CRBSI and exit-site infection. The patient developed relapse of BSI despite catheter removal at the time of index hospitalization and insertion of a new catheter into the ipsilateral vein (case 106).
Gram-negative bacilli were the most frequent pathogens isolated from blood cultures in cases that were suppressed, whereas for the cases managed without CAS therapy, Gram-positive cocci other than S aureus were the most common, mainly CoNS. There was no statistically significant difference in the median duration of positive blood cultures between CAS and non-CAS therapy groups (1 [IQR, 1–2] vs 1 [IQR, 1–2], P = .738). Of note, surveillance blood cultures were performed for 7 cases managed without CAS therapy collected at 1, 2, and 4 weeks after discontinuing antimicrobial therapy, and all were negative.
Secondary Outcomes at 6 Months
Chronic antimicrobial suppressive therapy was overall well tolerated, with only 3 patients experiencing serious adverse events that required a change in choice of CAS. Four cases in the CAS therapy group and 2 in the non-CAS therapy developed C difficile infection. Infection due to MDR or XDR organisms in subsequent episodes of BSI occurred in 7 cases managed with CAS therapy and in 3 cases managed without CAS therapy.
DISCUSSION
Our study findings suggest that with adequate source control and appropriate pathogen-directed antimicrobial therapy, relapse of BSI from non-LVAD sources is rare and routine use of CAS therapy is probably not necessary. Although no correlation between persistent BSI and a decision to use suppressive therapy was observed in our study cohort, CAS therapy may be considered in patients with non-LVAD BSI who have persistent bacteremia, especially with organisms that are associated with higher risk of hematogenous seeding of cardiac devices (such as S aureus or P aeruginosa) [12]. In contrast, routine use of CAS therapy may be a reasonable approach to prevent relapse of BSI secondary to LVAD-related bacteremia, considering that LVAD-specific infections cannot be cured without hardware removal and, treatment failures, particularly due to S aureus, are expected. Although CAS therapy effectively suppressed BSI episodes in 20 (62.5%) patients with a median infection-free of 311 days, 12 (37.5%) of them developed relapse relatively soon after index BSI (57.5 days; IQR, 26.2–237.7). Given the lack of a non-CAS therapy comparator group for LVAD-related BSI, we are unable to evaluate whether other strategies, such as a prolonged (4 to 6 weeks) course of parenteral antimicrobial therapy ± surgical debridement or exchange would be effective.
Optimal management of patients who present with LVAD-associated infections (BSI with no clear non-LVAD primary source) is yet to be defined. Considering earlier publications [12] that suggest higher risk of device seeding with S aureus or P aeruginosa, CAS therapy seems reasonable in such cases in which infection of the blood-contacting surface of the device is suspected, but not confirmed, and no other alternative source is identified. A multicenter study with a larger sample size could be useful in demonstrating an optimal management strategy.
Although CAS therapy was overall well tolerated, selection of MDR/XDR organisms in subsequent episodes of BSI and C difficile infection may occur on CAS therapy. Due to the potential harms associated with CAS therapy, other preventive strategies, such as close monitoring of clinical status and surveillance blood cultures, may be considered in LAVD-associated and non-LVAD BSI in individual cases using clinical judgment. In our patient cohort, surveillance blood cultures were recommended in a small proportion of cases with non-LVAD BSIs managed without CAS therapy and none were positive. However, optimal timing of obtaining surveillance blood cultures after completion of initial course of therapy is unclear.
A previous investigation by Maskarinec et al [12] showed that the risk of underlying cardiac device infection in patients with bacteremia due to S aureus, P aeruginosa, and Serratia marcescens was higher than that observed for other organisms. In our investigation, S aureus was the most frequent pathogen involved in LVAD-related infections. However, it was not a major pathogen in LVAD-associated and non-LVAD BSI cases. In all cases of BSI due to this organism, CAS therapy was recommended by the Infectious Diseases team. Likewise, with the exception of 2 cases (1 LVAD-related and 1 LVAD-associated), providers elected to suppress all cases of BSI due to P aeruginosa; relapse occurred exclusively in those with LVAD-related BSI. There was only 1 case of S marcesens in the non-LVAD group, which was not suppressed and relapse was not observed. More importantly, in cases in which other organisms were involved, the rationale behind the decision to suppress was not documented in detail and appeared mostly related to the clinician’s judgment and overall assessment of the case, rather than the organism involved or days of positive blood cultures.
The role of CAS therapy has only been examined in a previous investigation by Jennings et al [20]. This study described the clinical outcomes of 16 patients with LVAD-specific infections treated with CAS therapy. In this investigation, CAS was defined as continuation of therapy for longer than 6 weeks after index infection. Five of 16 cases (32%) failed suppression at 175 days (range, 10–598). The most common organisms isolated from local cultures in these cases were Gram-negative bacilli and S aureus. Similar to our findings, the rates of adverse events and C difficile infection were low [20].
The limitations of our study are primarily related the retrospective design and small sample size. Classification and confirmation of cases was heavily dependent on documentation provided by treating physicians. For instance, before positron emission tomography was available at our institution for the diagnosis of LVAD infections (2016), imaging studies obtained to define whether a device was infected were frequently limited to ultrasound and computed tomography exams. Modified definitions were used, rather than those proposed by the ISHLT, because their classification scheme does not distinguish between LVAD-related and LVAD-associated BSI. In our opinion, the distinction is important in individual patient management, especially the need for CAS therapy. Finally, without microbial sequencing, we were unable to ascertain whether subsequent episodes of BSI were relapses or recurrences. Our study definitions considered BSI relapse as an episode due to the same organism and same source identified during index episode. Nonetheless, organisms known to cause metastatic infection (ie, S aureus) may have accounted for some of the cases classified as “recurrent BSI.” Classification certainty cannot be confirmed in these cases without molecular testing. Finally, our study did not include contemporary devices approved by the US Food and Drug Administration in 2018, which may harbor a lower risk for LVAD infections, in general, including BSI. Despite these limitations, our study provides novel insights into the role of CAS therapy in the management of BSI in LVAD recipients and provides framework for future multicenter collaborative studies.
CONCLUSIONS
Routine use of CAS therapy may be unnecessary for cases of non-LVAD BSIs. However, it may be considered in non-LVAD BSI cases with prolonged bacteremia and those due to S aureus. In contrast, CAS therapy seems to be a reasonable approach to prevent relapse of BSI in LVAD-related cases. Chronic antimicrobial suppressive therapy is generally well tolerated, although selection of MDR/XDR organisms on subsequent episodes of BSI and treatment failures may be observed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This study was made possible using a research grant from Mayo Clinic Center for Clinical and Translational Science, which is supported by the National Institutes of Health (NIH) under Award Number UL1TR002377. In addition, NIH grant support (UL1 TR000135) was available for REDCap use.
Potential conflicts of interest. M. R. S. reports receiving funds from Medtronic, for prior research unrelated to this study, and unrelated honoraria/consulting fees from Medtronic, Spectranetics, Boston Scientific, and Aziyo Biologics, Inc. (all <$20 000). L. M. B. has received payment from Boston Scientific for consultant duties (<$20 000), and royalty payments (<$25 000) from Wolters Kluwer (“UpToDate”), and both activities are unrelated to the work described herein. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Aggarwal A, Gupta A, Kumar S, et al. Are blood stream infections associated with an increased risk of hemorrhagic stroke in patients with a left ventricular assist device? ASAIO J 2012; 58:509–13. [DOI] [PubMed] [Google Scholar]
- 2. Kyvernitakis A, Pappas O, Farmakiotis D, et al. Bloodstream infections in continuous flow left ventricular assist device recipients: diagnostic and clinical implications. ASAIO J 2019; 65:798–805. [DOI] [PubMed] [Google Scholar]
- 3. O’Horo JC, Abu Saleh OM, Stulak JM, et al. Left ventricular assist device infections: a systematic review. ASAIO J 2018; 64:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon SM, Schmitt SK, Jacobs M, et al. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann Thorac Surg 2001; 72:725–30. [DOI] [PubMed] [Google Scholar]
- 5. Nienaber J, Wilhelm MP, Sohail MR. Current concepts in the diagnosis and management of left ventricular assist device infections. Expert Rev Anti Infect Ther 2013; 11:201–10. [DOI] [PubMed] [Google Scholar]
- 6. Kimball PM, Flattery M, McDougan F, Kasirajan V. Cellular immunity impaired among patients on left ventricular assist device for 6 months. Ann Thorac Surg 2008; 85:1656–61. [DOI] [PubMed] [Google Scholar]
- 7. Hannan MM, Husain S, Mattner F, et al. ; International Society for Heart and Lung Transplantation. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant 2011; 30:375–84. [DOI] [PubMed] [Google Scholar]
- 8. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baddour LM, Epstein AE, Erickson CC, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–77. [DOI] [PubMed] [Google Scholar]
- 10. Esquer Garrigos Z, Castillo Almeida NE, Gurram P, et al. Management and outcome of left ventricular assist device infections in patients undergoing cardiac transplantation. Open Forum Infect Dis 2020; 7:ofaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nienaber JJ, Kusne S, Riaz T, et al. ; Mayo Cardiovascular Infections Study Group. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 2013; 57:1438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maskarinec SA, Thaden JT, Cyr DD, et al. The risk of cardiac device-related infection in bacteremic patients is species specific: results of a 12-year prospective cohort. Open Forum Infect Dis 2017; 4:ofx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bekeris LG, Tworek JA, Walsh MK, Valenstein PN. Trends in blood culture contamination: a College of American Pathologists Q-Tracks study of 356 institutions. Arch Pathol Lab Med 2005; 129:1222–5. [DOI] [PubMed] [Google Scholar]
- 14. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 15. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109. [DOI] [PubMed] [Google Scholar]
- 17. Baddour LM, Wilson WR, Bayer AS, et al. ; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf
- 19. Weiner-Lastinger LM, Abner S, Benin AL, et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Cont Hosp Ep 2020; 41:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jennings DL, Chopra A, Chambers R, Morgan JA. Clinical outcomes associated with chronic antimicrobial suppression therapy in patients with continuous-flow left ventricular assist devices. Artif Organs 2014; 38:875–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.