Abstract
Annual gains in BMC and areal bone mineral density (aBMD) in children vary with age, pubertal status, height-velocity, and lean body mass accrual (LBM velocity). Evaluating bone accrual in children with bone health-threatening conditions requires consideration of these determinants. The objective of this study was to develop prediction equations for calculating BMC/aBMD velocity SD scores (velocity-Z) and to evaluate bone accrual in youth with health conditions. Bone and body compositions via DXA were obtained for up to six annual intervals in healthy youth (n = 2014) enrolled in the Bone Mineral Density in Childhood Study (BMDCS) . Longitudinal statistical methods were used to develop sex- and pubertal-status-specific reference equations for calculating velocity-Z for total body less head-BMC and lumbar spine (LS), total hip (TotHip), femoral neck, and 1/3-radius aBMD. Equations accounted for (1) height velocity, (2) height velocity and weight velocity, or (3) height velocity and LBM velocity. These equations were then applied to observational, single-center, 12-month longitudinal data from youth with cystic fibrosis (CF; n = 65), acute lymphoblastic leukemia (ALL) survivors (n = 45), or Crohn disease (CD) initiating infliximab (n = 72). Associations between BMC/aBMD-Z change (conventional pediatric bone health monitoring method) and BMC/aBMD velocity-Z were assessed. The BMC/aBMD velocity-Z for CF, ALL, and CD was compared with BMDCS. Annual changes in the BMC/aBMD-Z and the BMC/aBMD velocity-Z were strongly correlated, but not equivalent; LS aBMD-Z = 1 equated with LS aBMD velocity-Z = −3. In CF, BMC/aBMD velocity-Z was normal. In posttherapy ALL, BMC/aBMD velocity-Z was increased, particularly at TotHip (1.01 [−.047; 1.7], p < 0.0001). In CD, BMC/aBMD velocity-Z was increased at all skeletal sites. LBM-velocity adjustment attenuated these increases (eg, TotHip aBMD velocity-Z: 1.13 [0.004; 2.34] versus 1.52 [0.3; 2.85], p < 0.0001). Methods for quantifying the BMC/aBMD velocity that account for maturation and body composition changes provide a framework for evaluating childhood bone accretion and may provide insight into mechanisms contributing to altered accrual in chronic childhood conditions.
Keywords: BONE MINERAL CONTENT/DENSITY, BONE ACCRUAL/ACCRETION, PEDIATRIC ENDOCRINOLOGY, GROWTH, PUBERTY, LEAN BODY MASS, CYSTIC FIBROSIS, CROHN’S DISEASE, ACUTE LYMPHOBLASTIC LEUKEMIA
Introduction
Numerous childhood diseases and their therapies threaten bone mineral accretion during peak bone mass development. Children with such conditions often have poor growth, delayed maturation, and altered body composition: All of which impact bone mineral accrual. DXA measures of BMC and areal bone mineral density (aBMD) are a critical component of bone health assessment and monitoring for such children.(1)
SD scores (Z) based upon pediatric BMC and aBMD reference data are necessary for interpreting a child’s measures,(2) and methods are available to account for the effects of short stature on DXA outcomes.(3) Presently, monitoring bone health is limited to examining the change in bone-Z obtained at discrete points over time. An important clinical question is whether BMC or aBMD changes over a given interval are appropriate for the amount of growth and maturation achieved. Currently, no such method exists. This issue is particularly relevant in children with chronic illness who may fail to accrue bone mineral at a pace similar to same-age peers caused, for example, by growth faltering, or for whom pharmacological treatments or improved nutritional status result in catch-up growth and rapid bone accrual.
Annual changes in BMC and aBMD (hereafter referred to as BMC/aBMD velocity) differ by age, sex, pubertal stage, and population ancestry.(4) BMC/aBMD velocity typically accelerates during puberty in sex-specific ways,(5,6) and is driven by linear growth and body composition changes. The functional muscle–bone unit model posits that bone mineral deposition is responsive to muscle forces on bone.(7–10) The amount of lean tissue is widely used as a surrogate for this mechanism of bone accretion. Thus, assessment of BMC/aBMD velocity should consider sex, age, pubertal status, and gains in height and lean mass.(3,11–14)
Our goal was to develop a method for evaluating BMC/aBMD changes over time accounting for important determinants of BMC/aBMD accrual (age, pubertal status, linear growth, body composition). We then applied these equations to three pediatric disease models for which DXA data from previously published studies were available for secondary analyses: cystic fibrosis (CF), posttreatment acute lymphoblastic leukemia (ALL), and Crohn disease (CD).
Subjects and Methods
BMDCS Study participants for the development of prediction equations
The Bone Mineral Density in Childhood Study (BMDCS), a multicenter longitudinal study to develop pediatric BMC and aBMD reference ranges,(15) included 2014 study participants (1022 female) from different ethnic groups in the United States. Participants were classified as African-American or non-African-American based on parental report. Participants were evaluated annually for up to 6 years (seven measurements maximum).
Detailed inclusion/exclusion criteria and study procedures for BMDCS have been published previously.(15) Briefly, healthy typically developing individuals aged 5 to 19 years were recruited. Subjects were retained in the study regardless of pubertal timing, height-percentile, weight-percentile, or BMI-percentile.
We developed aBMD and BMC velocity-Z calculation equations using data from subjects aged ≤22 years with ≥2 annual measurements. Participants diagnosed with significant chronic illness during the study were excluded from analyses.
Pediatric disease participants
We applied these aBMD and BMC velocity-Z calculation equations to data available for secondary analyses from three prospective studies conducted at the Children’s Hospital of Philadelphia: (1) CF patients participating in a nutritional intervention study,(16) (2) ALL patients within 2 years of posttherapy completion,(17) and (3) CD patients participating in an observational study following infliximab therapy initiation.(18) Details of the three cohorts including their DXA data have previously been published,(16–18) and are summarized in Supplemental Table 1.
Anthropometry
Weight was measured on a digital scale with subjects wearing light clothing. Standing height was measured in triplicate using a wall-mounted stadiometer and recorded to the nearest 0.1 cm. Z-scores for height (height Z), weight (weight Z), and BMI (BMI Z) were calculated for subjects 5.0 to 19.9 years old using Centers for Disease Control and Prevention 2000 growth charts.(19) Ages of individuals aged > 19.9 years were converted to 19.9 to permit calculation of height Z, weight Z, and BMI Z. for individuals aged ≥20 years were calculated using age 19.9 years. Weight velocity and height velocity were calculated as the difference in weight or height, divided by the difference in age between consecutive annual study visits. Height velocity <0 cm/year was assumed to reflect a measurement error after reaching adult height and was recoded to 0. Height velocity Z was calculated using published reference data.(20)
Pubertal assessment
For the BMDCS and ALL study participants, sexual maturation was determined by physical examination. The pubertal stage of breast (females) was assessed according to physical maturation Tanner criteria,(21) and testicular volume (males) was measured by orchidometer.(22) For CF and CD cohorts, pubertal status was assessed by validated self-assessment questionnaire.(23,24)
DXA
Whole-body, anteroposterior lumbar spine, nondominant forearm, and left proximal femur DXA scans were acquired with Hologic (Bedford, MA, USA) bone densitometers (QDR4500A, QDR4500W, and Delphi A models) and were analyzed using Hologic software versions 12.3 or 12.6 for quantification of total body less head BMC (TBLH BMC), lumbar spine aBMD (Spine aBMD), total hip aBMD (TotHip aBMD), femoral neck aBMD (HipNeck aBMD), 1/3-radius aBMD (Radius aBMD), whole-body lean body mass excluding BMC (LBM), and whole-body fat mass (FM). Coefficients of variation were <1.4% for TBLH BMC, <1% Spine aBMD, and <1.7% for aBMD of all other sites.(15)
DXA results were corrected for clinical center differences, longitudinal drift, and QDR4500 FM underestimation.(25) Age and height Z-adjusted BMC/aBMD-Zs were calculated as previously described.(2,3) The LBM index-Z (LBMI-Z) was calculated for LBMI [LBM (kg)/height (m)2] using 1999 to 2004 NHANES data.(26) Annualized BMC/aBMD, LBM, and FM velocities were calculated as the difference in outcome divided by the difference in age between consecutive visits. Mid-age was calculated as the midpoint between consecutive visits. BMC/aBMD velocities were included if the interval between consecutive visits was ≥11 months and ≤13 months. Velocity outliers (>99% or <1%) were identified by graphical inspection of BMC/aBMD velocity versus age, reviewed for potential measurement or data entry error, and excluded from the data used to develop Fig. 1A, B if the pattern of BMC/aBMD raw data over consecutive years in a participant revealed juxtaposition of positive and negative extremes (n = 7). Because we could not confirm error, these data were not excluded from prediction equation development.
Fig. 1.
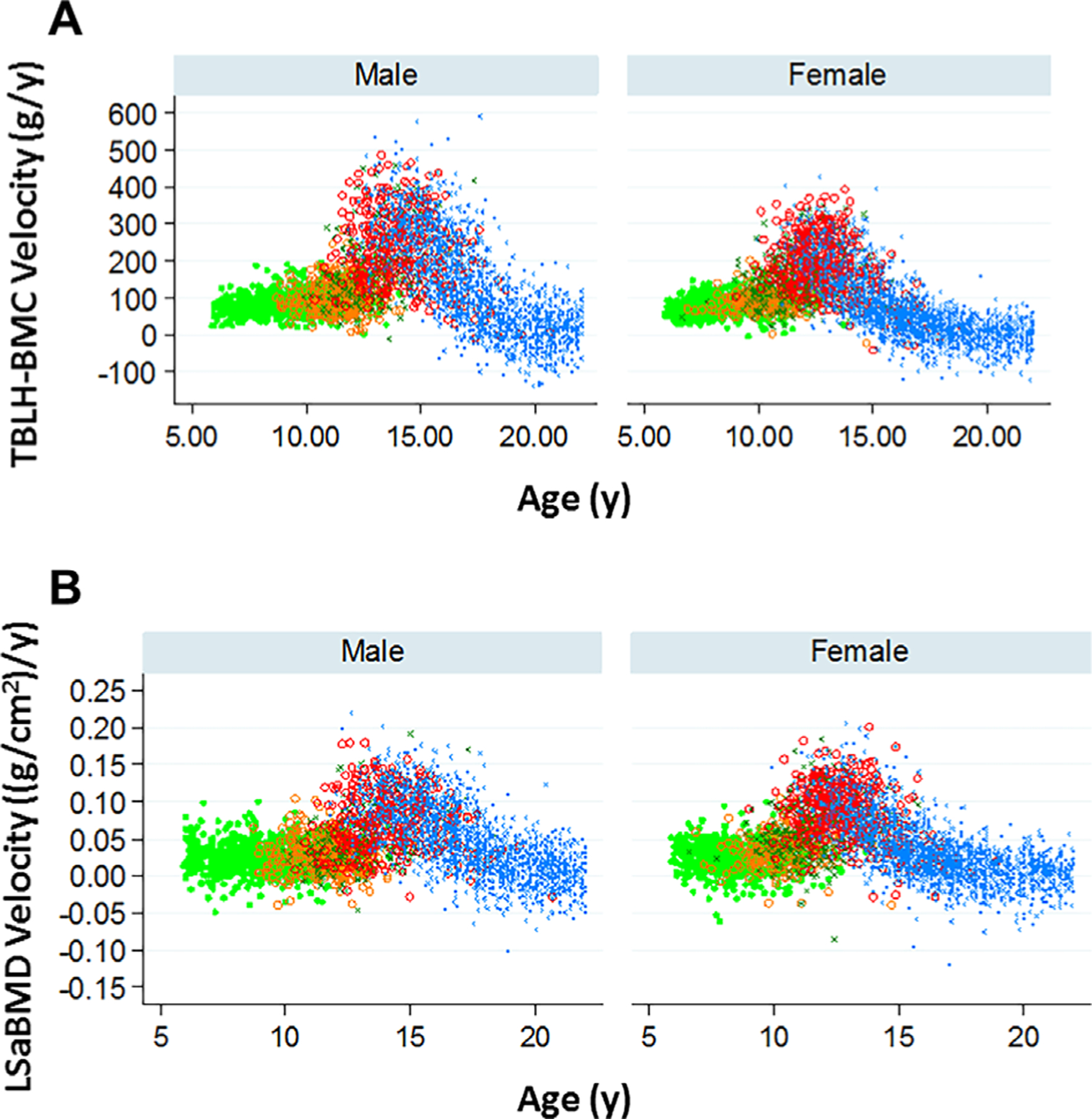
Relationship of annualized (A) total body less head-BMC velocity and (B) lumbar spine areal bone mineral density velocity to age by pubertal status (pubertal stage 1 light green, 2 orange, 3 dark green, 4 red, 5 blue) in males and females.
Statistical analyses
Statistical analyses were conducted with Stata 15 (StataCorp, College Station, TX, USA). Generalized estimating equations were used to develop sex-specific, longitudinal models of annualized velocities from the BMDCS cohort for each DXA outcome: TBLH BMC, Spine aBMD, TotHip aBMD, HipNeck aBMD, and Radius aBMD. Initial models included age, age2, height, weight or LBM and FM, and BMC/aBMD at the beginning of the interval. The last was done as expected changes were conditional on baseline measurement.(18,27) African-American or non-African-American status, pubertal stage at interval end, height velocity, and weight velocity, or LBM velocity and FM velocity were also included. After including these covariates, BMC/aBMD velocity did not vary by African-American ancestry or FM and FM velocity. Therefore, these were not included in subsequent models. The effects of pubertal status identified in initial models were used to develop puberty-specific models. The smallest quasi-likelihood,(28) bias-adjusted quasi-likelihood,(29,30) and correlation information(31) criterion and the Rotniztky-Jewell criteria(32,33) closest to 0 were used to identify the model with the best fit and correlation structure.
Three velocity-Z-score calculation equations were developed for each bone outcome based on: Equation (1): height velocity only; Equation (2): weight velocity and height velocity; and Equation (3): LBM velocity and height velocity. Comparison of Equation (1) and Equation (3) was intended to differentiate the impact of LBM on BMC/aBMD velocity (bone accrual may be “normal” for the increase in LBM over the interval, but nonetheless suboptimal if LBM accrual is compromised); Equation 2 is a surrogate for LBM accrual in the event that LBM was not or could not be determined. Model β-coefficients and SD were used to calculate BMC/aBMD velocity-Z (BMC/aBMD velocity-Z) at each site.
| Velocity Z-score = (measured velocity – predicted velocity)/SD |
These BMC/aBMD Velocity-Z were then compared with the results generated using the standard approach of assessing bone density change, ie, determining the difference in height Z-adjusted BMC/aBMD-Z obtained over the same interval using 1) graphical inspection and 2) Spearman correlation. Linear regression was used to quantify the relationship between a unit change in BMC/aBMD velocity-Z versus the change in aBMD/BMC-Z over the same interval.
For the three patient populations, we compared baseline anthropometric and BMC/aBMD Z-scores, as well as BMC/aBMD velocity-Z results with the BMDCS cohort using the Kruskal-Wallis rank test. Within the patient populations, BMC/aBMD velocity-Z results derived from the three equations were compared using a paired t test; the relationship of differences in these BMC/aBMD velocity-Z results with height velocity-Z and LBM velocity results was assessed using Spearman correlation.
The institutional review board at each institution approved the protocols. For subjects aged <18 years, consent was obtained from each participant’s parent or guardian and assent was obtained from the study participants. Consent was obtained directly from participants if age was ≥18 years.
Results
Sample characteristics
Each study has been published(15–18) (Supplemental Table 1). Baseline characteristics of participants for whom velocities were determined are listed in Table 1. The BMDCS sample was comprised primarily of children of European or African ancestry. Consistent with the increased prevalence of overweight children in the United States, average weight-Z and BMI-Z were >0 in the BMDCS cohort. The CF, ALL, and CD groups exhibited growth and body composition patterns consistent with their diagnosis and treatment.
Table 1.
Study Participants (Median, Interquartile Range for Continuous Data)
| BMDCS (n = 1837) | CF (n = 65) | ALL (n = 45) | CD (n = 72) | |
|---|---|---|---|---|
| Baseline age, years | 10.9 (6.7,13.6) | 9.9 (8.3, 12.3) p = 0.002 | 7.7 (6.6, 12.4) p = 0.0003 | 14.3 (11.8, 16.3) p < 0.0001 |
| Sex, % female | 50.7 | 44 | 62 | 37 |
| Ancestry, % AA | 16.7 | 0 | 11 | 12 |
| Baseline Wt-Z | 0.39 (−0.25, 0.95) | −0.42 (−0.87, 0.04) p = 0.0001 | 0.92 (0.45, 1.52) p = 0.0001 | −0.39 (−1.27, 0.49) p < 0.0001 |
| Baseline Ht-Z | 0.13 (−0.42, 0.73) | −0.40 (−0.96, 0.08) p = 0.0001 | 0.46 (−0.16–1.14) p = 0.02 | −0.73 (−1.33, −0.028) p = 0.0001 |
| Baseline BMI-Z | 0.37 (−0.26, 0.96) | −0.15 (−0.56, 0.47) p = 0.0001 | 1.05 (0.31, 1.44) p = 0.0001 | −0.04 (−0.82, 0.82) p = 0.001 |
| Baseline LBMI-Z (n = 1418; for age ≥8 years) | −0.15 (−0.68, 0.34) | −0.52 (−0.94, −0.02) p < 0.0001 | −0.26 (−0.80, 0.72) p = 0.9 | −1.03 (−1.91, −0.6) p < 0.0001 |
| Wt-Velocity (kg/year) | 4.1 (2.13, 5.49) | 3.63 (2.03–5.16) | 6.02 (2.87–12.2) | |
| Ht-Velocity (cm/year) | 5.63 (4.98, 6.82) | 5.72 (3.71, 6.54) | 4.77 (1.2–8.12) | |
| Ht-Velocity-Z | 0.09 (−0.53, 0.080) p = 0.6; n = 70 | 0.02 (−0.75, 1.02) p = 0.3; n = 43 | 1.05 (−0.15–1.68) p < 0.0001; n = 66 | |
| Baseline TBLH BMC-Z | 0.04 (−0.50, 0.56) | −0.13 (−0.63, 0.47) p = 0.41; n = 65 | 0.13 (−0.36, 0.72) p = 0.4 | −0.42 (−1.09, 0.24) p = 0.0001 |
| Baseline LS aBMD-Z | 0.03 (−0.59, 0.64) | −0.27 (−0.89, 0.38) p = 0.02; n = 62 | −0.33 (−0.75, 0.32) p = 0.13 | −0.70 (−1.34, 0.04) p = 0.0001 |
| Baseline TotHip aBMD-Z | 0.08 (−0.67, 0.75) | −0.68 (−1.51–0.16) p = 0.0001 | −0.69 (−1.32, −1.21) p = 0.0001 | |
| Baseline FN aBMD-Z | −0.005 (−0.67, 0.71) | −0.38 (−1.21, 0.17) p = 0.003 | −0.81 (−1.59, −0.41) p = 0.0001 | |
| Baseline 1/3-Radius BMD-Z | −0.05 (−0.69, 0.71) | −0.24 (−0.81, 0.51) p = 0.4 |
DXA BMC/aBMD-Z data are height-for-age-Z score adjusted.
AA = African American; aBMD = areal bone mineral density; ALL = acute lymphoblastic leukemia; BMC = bone mineral content; BMDCS = Bone Mineral Density in Childhood Study; BMI = body mass index; CD = Crohn’s disease; CF = cystic fibrosis; FN = femoral neck; HT = height; LBMI = lean body mass index; LS = lumbar spine; TBLH = total body less head; TotHip = total hip; Wt = weight.
Baseline BMC/aBMD Z-scores are given in Table 1. TBLH BMC-Z was significantly reduced in CD (p = 0.0001), whereas Spine aBMD-Z was reduced in both CF (p = 0.02) and CD (p = 0.0001), compared with the BMDCS sample. TotHip aBMD-Z and HipNeck aBMD-Z results were also reduced in ALL (p < 0.0001 and p = 0.003, respectively) and CD patients (p = 0.0001 and p = 0.0001, respectively).
Prediction equation development
Figure 1 illustrates TBLH BMC velocity and Spine aBMD velocity according to age and pubertal stage in the BMDCS cohort. Bone accrual is relatively constant in prepubertal/early puberty, increases in midlate puberty, and slows at later ages when bone loss may occur. BMC/aBMD velocities were available for TBLH BMC (n = 7243), Spine aBMD (n = 7535), TotHip aBMD (n = 7527), HipNeck aBMD (n = 7527), and radius aBMD (n = 7428) to develop sex-specific velocity-Z-score equations. Potential determinants of BMC/aBMD velocity were considered: age, age2, pubertal status, height velocity, race (African-American versus non-African-American), and either weight velocity or LBM velocity and FM velocity. As examples of how regression results are used, corresponding prediction equations for TBLH BMC and Spine aBMD are provided in Table 2. African-American ancestry and FM velocity were not significant after adjustment for other covariates and were excluded from the models. BMC/aBMD velocity was not different in pubertal stages 1 to 2 for females and pubertal stages 1 to 3 for males, except for the latter in whom TBLH BMC velocity in pubertal stage 3 was more rapid (p < 0.0001). Covariates that were relevant for some, but not all statistical models, were maintained in prediction equations. An independent correlation structure provided the best overall fit and was, thus, used for prediction equations. β-coefficients for each of the three prediction equations for each skeletal site can be found in Supplemental Table 2.
Table 2.
β-Coefficients and SD for Mixed Effects Models Predicting TBLH BMC-Velocity and Lumbar Spine aBMD Velocity Using HT and HT-Velocity, With Corresponding Equations for Calculating Predicted Annualized BMD/aBMD-Vel-Z –Score
| Model 1: HT & HT-Vel-adjusted | ||||
|---|---|---|---|---|
| Female |
Male |
|||
| TBLH BMC-Vel-Z | Tanner stage 1–2 | Tanner stage 3–5 | Tanner stage 1–2 | Tanner stage 3–5 |
| Age at beginning of interval | 12.1 | 7.8 | 8.0** | 76.1*** |
| (Age at beginning of interval)2 | −0.9 | −0.6 | −0.6 | −2.9 |
| TBLH-BMC at beginning of interval | 0.12*** | −0.02*** | 0.14*** | 0.01 |
| HT at beginning of interval | 0.22 | 3.6*** | 0.17 | 3.4*** |
| HV-Vel (cm/year) | 7.1*** | 21.4*** | 10.5*** | 28.4*** |
| Constant | −104** | −472.1*** | −110.7*** | −1,006*** |
| SD | 25.69 | 53.32 | 30.1 | 76.11 |
| Prediction equations: | ||||
| Females Tanner stage 1–2: [TBLH-BMC-Vel – ((12.1*age0) + (−0.9*age02) + (0.1*TBLH-BMC0) + (0.2*Ht0) + (7.1*Ht-Vel) − 104)]/26 Females Tanner stage 3–5: [TBLH-BMC-Vel – ((7.8*age0) + (−0.6*age02) + (−0.02*TBLH-BMC0) + (3.6*Ht0) + (21.4* Ht-Vel) − 472)]/53.3 Males Tanner stage 1–2: [TBLH-BMC-Vel – ((8.0*age0) + (−0.6*age02) + (0.1*TBLH-BMC0) + (0.2*Ht0) + (10.5*Ht-Vel) − 111)]/30.1 Males Tanner stage 3–5: [TBLH-BMC-Vel – ((76.1*age0) + (−2.9*age02) + (0.01*TBLH-BMC0) + (3.4*Ht0) + (28.4*Ht-Vel) – 1006)]/76.1 | ||||
| Female |
Male |
|||
| LS aBMD-Vel-Z | Tanner stage 1–2 | Tanner stage 3–5 | Tanner stage 1–3 | Tanner stage 4–5 |
| Age at beginning of interval | −0.0105* | 0.0087*** | −0.0127*** | 0.0315*** |
| (Age at beginning of interval)2 | 0.0007* | −0.0004*** | 0.0007*** | −0.0012*** |
| LSaBMD at beginning of interval | 0.0119 | −0.0093 | 0.0154 | 0.0085 |
| HT at beginning of interval | 0.0002 | 0.0011*** | 0.0004*** | 0.0007*** |
| HV-Vel (cm/year) | 0.0035*** | 0.0089*** | 0.0046*** | 0.0075*** |
| Constant | 0.0118 | −0.1770*** | −0.0057 | −0.3052*** |
| SD | 0.0212 | 0.0304 | 0.0214 | 0.0328 |
| Prediction equations: | ||||
| Females Tanner stage 1–2: [LSaBMD-Vel – ((−0.0105*age0) + (0.0007*age02) + (0.0119*LSABMD0) + (0.0002*Ht0) + (0.0035*Ht-Vel) + 0.0118)]/ 0.0212 Females Tanner stage 3–5: [LSaBMD-Vel – ((0.0087*age0) + (−0.0004*age2) + (−0.0093*LSaBMD0) + (0.0011*Ht0) + (0.0089*Ht-Vel) −0.177)]/0.0304 Males Tanner stage 1–3: [LSaBMD-Vel – ((−0.0127*age0) + (0.0007*age02) + (0.01544*LSaBMD0) + (0.0004*Ht0) + (0.0046*Ht-Vel) −0.0057)]/0.0214 Males Tanner stage 4–5: [LSaBMD-Vel – ((0.0315*age0) + (−0.0012*age02) + (0.0085*LSaBMD0) + (0.0007*Ht0) + (0.0075*Ht-Vel) −0.3052)]/0.0328 | ||||
aBMD = areal bone mineral density; BMC = bone mineral content; HT = height; HV = height velocity; LS = lumbar spine; TBLH = total body less head; Vel = velocity; Wt = weight.
p < 0.1
p < 0.05
p < 0.01.
Comparison of BMC/aBMD velocity-Z with change in BMC/aBMD-Z
The relationships of BMC/aBMD velocity-Z adjusted for height velocity [Eq. (1)] to changes in BMC/aBMD-Z over 1 year are shown in Supplemental Fig. 1. In general, a 1 SD change in TBLH BMC-Z over 1 year is associated with a TBLH BMC velocity-Z of 3.00 (95% CI, 2.93 to 3.06); a 1 SD change in Spine aBMD-Z is associated with a Spine aBMD velocity-Z of 2.99 (95% CI, 2.95 to 3.03). In other words, for a child’s Spine aBMD-Z to decline by 1 SD over a year, his/her Spine aBMD velocity-Z would be −3.00.
Impact of additional adjustment for lean body mass velocity or weight velocity upon height velocity-only-adjusted BMC/aBMD velocity-Z
Within BMDCS participants, LBM velocity-adjusted BMC/ aBMD velocity-Z [(Eq. (3)] was on average higher than height velocity-adjusted BMC/aBMD velocity-Z [Eq. (1)] at the femoral neck and 1/3-radius (Supplemental Table 3). These differences varied across individuals at all skeletal sites (eg, 1/3-radius 0.02 [−0.65 to 0.87] and femoral neck (FN) 0.01 [−1.49 to 1.34]). These differences correlated negatively with LBM accrual, eg, in the setting of more rapid LBM accrual, adjusting for LBM velocity lessened the difference between the LBM velocity-adjusted BMC/Abmd velocity-Z [Eq. (3)] and height velocity-adjusted BMC/aBMD velocity-Z (Supplemental Table 3).
Application of the BMD/BMC velocity-Z equations in youth with health conditions
BMC/aBMD velocity-Z equations were calculated using the three sex- and pubertal status-specific prediction equations as plotted in Table 2. For comparison, the change in BMC/aBMD-Z over the same interval for which the velocity-Z was calculated is also shown (Fig. 2).
Fig. 2.
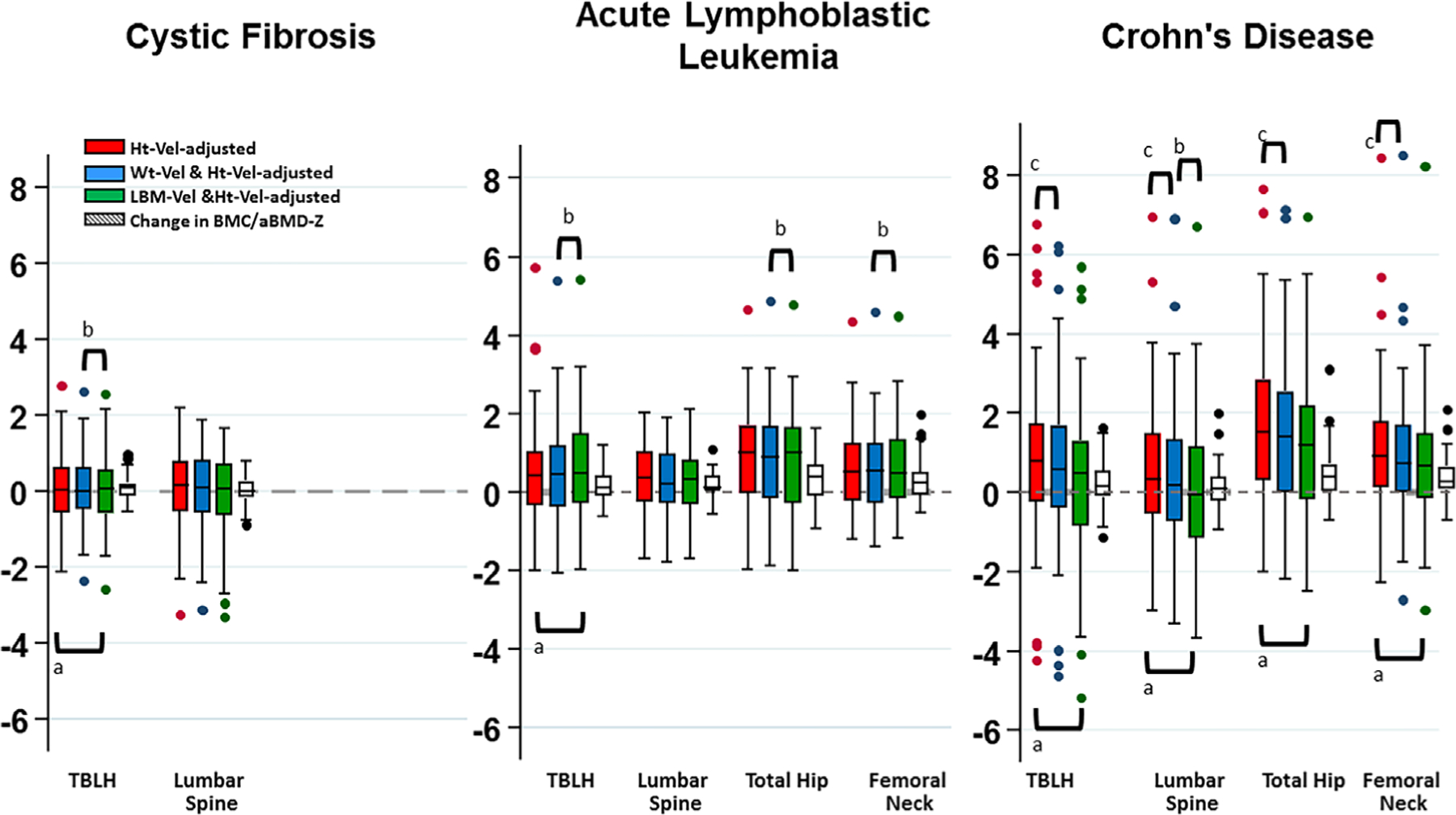
Boxplots comparing bone mineral content/ areal bone mineral density (BMC/aBMD) velocity-Z adjusted for height velocity (red), height velocity and weight velocity (blue), and height velocity and lean body mass accrual (LBM) velocity (green), as well as the BMD/aBMD-Z change over 1 year (conventional approach for monitoring bone health) in youth with cystic fibrosis (CF), postacute lymphoblastic leukemia (ALL) therapy, and in the year following infliximab initiation for Crohn disease (CD). aHeight velocity adj-velocity-Z versus height velocity and LBM velocity adj-velocity-Z. CF: total body less head (TBLH), p = 0.0002; ALL: TBLH, p = 0.0012; CD: TBLH, p = 0.0002; spine, p < 0.0001; total hip, p < 0.0001; femoral neck, p < 0.0001. bHeight velocity and weight velocity adj-velocity-Z versus height velocity and LBM velocity adj-velocity-Z. CF: spine, p < 0.0001; ALL: lumbar spine, p < 0.0001; total hip, p = 0.0026; femoral neck, p = 0.049; CD: spine, p = 0.0001. cHeight velocity versus height velocity and weight velocity adj-velocity-Z. CD: TBLH, p = 0.0001; lumbar spine, p = 0.002; total hip, p < 0.0001; femoral neck, p < 0.0001.
Cystic fibrosis
In CF youth, TBLH BMC velocity-Z and Spine aBMD velocity-Z did not differ from the BMDCS cohort (Table 3). However, TBLH BMC velocity-Z was greater with adjustment for LBM velocity (Fig. 2).
Table 3.
BMC/aBMD Velocity-Z in Three Childhood Conditions
| CF (n = 65) | ALL (n = 46) | CD (n = 72) | |
|---|---|---|---|
| TBLH BMC-Vel-Z | |||
| Ht-Vel-adjusted | 0.02 (−0.57, 0.64) p = 0.33 | 0.43 (−0.34, 1.04) p = 0.002 | 0.80 (−0.24, 1.75) p < 0.0001 |
| Ht-Vel & Wt-Vel-adjusted | 0.002 (−0.48, 0.63) p = 0.27 | 0.46 (−0.38, 1.2) p = 0.005 | 0.57 (−0.4, 1.7) p < 0.0001 |
| Ht-Vel & LBM-Vel-adjusted | 0.24 (−0.45, 0.76) p = 0.09 | 0.68 (−0.12, 1.7) p = 0.0001 | 0.68 (−0.63, 1.53) p = 0.004 |
| Spine aBMD Vel-Z | n = 62 | n = 70 | |
| Ht-Vel-adjusted | 0.15 (−0.56, 0.78) p = 0.30 | 0.36 (−0.26, 1.03) p = 0.012 | 0.33 (−0.53, 1.50) p = 0.004 |
| Ht-Vel & Wt-Vel-adjusted | 0.10 (−0.58, 0.8) p = 0.33 | 0.19 (−0.29, 0.97) p = 0.028 | 0.20 (−0.74, 1.34) p = 0.033 |
| Ht-Vel & LBM-Vel-adjusted | 0.17 (−0.61, 0.78) p = 0.24 | 0.35 (−0.28, 0.92) p = 0.015 | −0.05 (−1.06, 1.22) p = 0.49 |
| TotHip aBMD-Vel-Z | |||
| Ht-Vel-adjusted | 1.01 (−0.047, 1.70) p < 0.0001 | 1.52 (0.30, 2.85) p < 0.0001 | |
| Ht-Vel & Wt-Vel-adjusted | 0.89 (−0.16, 1.68) p < 0.0001 | 1.42 (0.005, 2.55) p < 0.0001 | |
| Ht-Vel & LBM-Vel-adjusted | 1.08 (−0.17, 1.83) p < 0.0001 | 1.13 (0.004, 2.34) p < 0.0001 | |
| FN aBMD-Vel-Z | |||
| Ht-Vel-adjusted | 0.51 (−0.24, 1.26) p = 0.0002 | 0.92 (0.13, 1.8) p < 0.0001 | |
| Ht-Vel & Wt-Vel-adjusted | 0.53 (−0.30, 1.24) p = 0.0005 | 0.75 (−0.008, 1.72) p < 0.0001 | |
| Ht-Vel & LBM-Vel-adjusted | 0.47 (−0.17, 0.47) p = 0.0002 | 0.78 (−0.04, 1.64) p < 0.0001 | |
| 1/3-Radius aBMD-Vel-Z | |||
| Ht-Vel-adjusted | 0.39 (−0.39, 0.92) p = 0.016 | ||
| Ht-Vel & Wt-Vel-adjusted | 0.39 (−0.29, 0.95) p = 0.023 | ||
| Ht-Vel & LBM-Vel-adjusted | 0.40 (−0.26, 1.07) p = 0.005 | ||
Median (interquartile range); p values represent BMC/aBMD-velocity-Z versus Bone Mineral Density in Childhood Study reference.
aBMD = areal bone mineral density; ALL = acute lymphoblastic leukemia; BMC = bone mineral content; CD = Crohn’s disease; CF = cystic fibrosis; FN = femoral neck; HT = height; LBM = lean body mass; TBLH = total body less head; TotHip = total hip; Wt = weight.
Posttherapy ALL (6- to 24-months posttherapy completion)
In ALL survivors, BMC/aBMD velocity-Z were higher than the BMDCS BMC/aBMD velocity-Z at all skeletal sites (all ps < 0.03; Table 3). The TBLH BMC velocity-Z-adjusted for LBM velocity [Eq. (3)] was greater than the TBLH BMC velocity-Z adjusted for height velocity (p = 0.0012). Multiple differences were found between LBM and weight velocity-adjusted velocity-Z scores (Fig. 2).
Crohn’s disease with infliximab
BMC/aBMD velocity-Z was increased at all skeletal sites except for Spine aBMD velocity-Z adjusted for height velocity and LBM velocity [Eq. (3)] in the year following infliximab initiation (all ps < 0.01; Table 3). On average, BMC/aBMD velocity-Z adjusted for height velocity and LBM velocity [Eq. (3)] was lower than BMC/aBMD velocity-Z adjusted for height velocity only [Eq. (1)] and height velocity and weight velocity [Eq. (2); Fig. 2].
Discussion
Childhood is a time of rapid bone accrual. The rate of bone accrual varies with age, pubertal status, linear growth, and with weight gain or LBM accrual. Consideration of these issues is particularly important for pediatric chronic conditions in which disease progression and treatments can affect growth, body composition, maturation, and bone accrual simultaneously. Quantifying the appropriateness of bone accrual given these changes is essential for clinicians and researchers who monitor these children.
In adults, absolute or percent changes in DXA-measured aBMD are used to monitor bone health.(34,35) However, in children, absolute or percent changes in BMC/aBMD are not informative, given the dynamic changes in bone accrual during different growth phases (Fig. 1). Presently, the only available strategy for monitoring bone accrual in children is to compare BMC/aBMD-Z at two time points. Even after adjusting for the effects of short or tall stature on BMC/aBMD-Z, this strategy does not account for pubertal development and weight gain or LBM accrual occurring during this same interval. In addition, BMC/aBMD-Z “track” very strongly (measurements between visits are highly correlated),(12) so the health significance of modest changes in BMC/aBMD-Z-scores is difficult to interpret.
Here we show an approach for the assessment of DXA-derived measures of BMC and aBMD annual velocities. Sex- and pubertal status-specific equations accounted for baseline age, BMC/ aBMD, and height and height velocity over the interval. Additional equations included either LBM or weight, the latter potentially useful when whole-body DXA is not available to quantify LBM. The equations were applied to youth with conditions that place them at increased risk for compromised bone health and interventions that have the potential to augment bone accrual.
In healthy children, BMC/aBMD velocity-Z at the various skeletal sites was highly correlated with absolute changes in BMC/aBMD-Z over the same time interval, but they were not equivalent. BMC/aBMD velocity-Z provided more granularity in detecting changes over 1 year. For example, a decline of 1 SD in Spine aBMD-Z over 1 year was equivalent to a Spine aBMD velocity-Z of −3. Additionally, these two measures likely provide complementary clinical information: BMC/aBMD-Z change may indicate fracture risk as well as peak bone mass trajectory, whereas BMC/aBMD velocity-Z indicates the impact of events over the previous year on bone accretion.
Osteoporosis occurs in approximately 30% of adults with CF.(36) Reports of low BMD in youth with CF have been inconsistent, likely reflecting differences in methods to account for height and LBM.(37,38) In this study of generally healthy CF youth enrolled in an intervention study that was not expected to impact bone accretion, we found normal TBLH BMC velocity-Z and Spine aBMD velocity-Z. This contrasts with the other two chronic disease groups who were studied during (1) a period of recovery from treatment (ALL), or (2) active treatment with an agent (infliximab) expected to reduce inflammation and improve disease course (CD).
Recovery of bone after ALL therapy has been reported.(35) The BMC/aBMD velocity-Z data in our ALL cohort confirm enhanced bone accrual in individuals studied 6 to 24 months from completion of ALL therapy. The total hip was particularly responsive to recovery, with 50% of ALL participants experiencing height velocity-adjusted TotHip-aBMD velocity-Z >1 SD. Moreover, enhanced accrual was apparent even after adjustment for height velocity and lean body mass accrual, suggesting additional mechanisms beyond improved linear growth and body composition are operative in bone recovery in the 6 to 24 months following completion of ALL therapy. These mechanisms could include recovery from toxic effects of glucocorticoids, beneficial effects of increased physical activity, or improved skeletal muscle function. More fascinating was that TBLH BMC velocity-Z adjusted for LBM velocity and height velocity was greater than the TBLH BMC velocity-Z adjusted for weight velocity and height velocity. The TBLH BMC site largely reflects cortical bone. Children with ALL tend to gain excess weight and experience a higher prevalence of obesity, likely attributable to the high-dose glucocorticoid therapy of the ALL protocol.(39,40) Increased BMI-Z in the ALL cohort persisted at the 12-month follow-up (data not shown; p = 0.54). The mechanism underlying the “magnification” of TBLH BMC velocity-Z with adjustment for LBM-velocity versus weight gain is not clear, but the proposed models have the potential to quantitate effects of physical activity, improved muscle function, and body composition changes in future studies.
Poor bone accrual in CD has been attributed to poor linear growth, delayed maturation, inflammation, malabsorption, and compromised lean mass.(41–43) Infliximab targets inflammation and is associated with improvements in disease activity, growth, and body composition.(18,44) Our sample of children with CD treated with infliximab had positive BMC/aBMD velocity-Z. Positive BMC/aBMD velocity-Z indicates that bone accrual was greater than the BMDCS age-matched norms based upon their linear growth and lean body mass accumulation. This augmented bone accretion rate suggests a role for decreased inflammation in bone recovery.
A number of considerations in the use of these prediction equations are worth mentioning. First, these equations are applicable to measurements obtained on Hologic DXA devices, over an interval of 12 months. Although the BMDCS reference dataset is robust, children beyond the extremes of normal puberty (precocious or delayed) were excluded. Thus, how these equations operate in such extremes is not known. Additionally, the disease conditions presented generally had normal or enhanced bone accrual. The utility of these equations in disease conditions in which poor bone accrual or bone loss occurs was not evaluated. These velocity Z-scores do not account for the least significant change that can be detected given scan precision. However, DXA scan precision in children is similar to adults, and expected changes in BMC/aBMD far exceed measurement error until later adolescence.(45) Importantly, poor nutritional status and inadequate LBM accrual and muscle function feature in many childhood conditions (eg, Duchenne muscular dystrophy, mitochondrial disorders, neurologic disorders)— normal bone accrual relative to LBM accrual is not synonymous with having a healthy skeleton in the context of lean mass deficits. Nonetheless, comparing velocity-Z from equations that only include height velocity versus height and LBM velocity can provide insight into the contribution of LBM to poor or enhanced bone accrual, as depicted by the CD and ALL cohorts here. Additionally, as used here, LBM is a surrogate for skeletal muscle. Whether accrual of appendicular lean mass, a DXA-derived measure of skeletal muscle, better predicts bone accretion was not tested. Because LBM is readily available in a DXA report and Dorsey et al. reported appendicular lean mass was worse than LBM in predicting BMC,(46) we elected to rely on LBM rather than appendicular mass to generate prediction equations. Finally, though many of the BMC/aBMD-velocity-Z comparisons within each health condition and between the health condition and BMDCS reference group were highly statistically significant, adjustment for multiple comparisons was not pursued. Our goal was largely to describe how these equations performed and compared, but we did not have any a priori hypotheses regarding how the velocity-Z would compare.
The bone velocity prediction equations proposed here enable precise evaluation of bone accrual in childhood. Their use in health and in chronic illness provides greater insight into the extent to which improving or worsening bone health can be ascribed to linear growth, weight, and lean body mass.
Supplementary Material
Acknowledgments
This project was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Contracts NO1-HD-1–3228, NO1-HD-1–3329, NO1-HD-1–3330, NO1-HD-1–3331, NO1-HD-1–3332, and NO1-HD-1–3333, the Clinical and Translational Research Center Grants 5-MO1-RR-000240 and UL1RR-026314, and the Cystic Fibrosis Foundation. We acknowledge our collaborators in the pediatric endocrine divisions of each Bone Mineral Density in Childhood Study Clinical Center. We also acknowledge the contributions of the subjects and families who volunteered to make this study possible.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosure
The authors have nothing to disclose.
References
- 1.Bianchi ML, Leonard MB, Bechtold S, et al. Bone health in children and adolescents with chronic diseases that may affect the skeleton: the 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):281–94. [DOI] [PubMed] [Google Scholar]
- 2.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack SE, Cousminer DL, Chesi A, et al. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr. 2017;171(9):e171769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole TJ, Pan H, Butler GE. A mixed effects model to estimate timing and intensity of pubertal growth from height and secondary sexual characteristics. Ann Hum Biol. 2014;41(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole TJ, Cortina-Borja M, Sandhu J, Kelly FP, Pan H. Nonlinear growth generates age changes in the moments of the frequency distribution: the example of height in puberty. Biostatistics. 2008;9(1):159–71. [DOI] [PubMed] [Google Scholar]
- 7.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219(1):1–9. [DOI] [PubMed] [Google Scholar]
- 8.Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2(2):73–85. [PubMed] [Google Scholar]
- 9.Schoenau E, Frost HM. The “muscle-bone unit” in children and adolescents. Calcif Tissue Int. 2002;70(5):405–7. [DOI] [PubMed] [Google Scholar]
- 10.Schoenau E, Neu CM, Beck B, Manz F, Rauch F. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res. 2002;17(6):1095–101. [DOI] [PubMed] [Google Scholar]
- 11.Kalkwarf HJ, Gilsanz V, Lappe JM, et al. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab. 2010;95(4):1690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wren TA, Kalkwarf HJ, Zemel BS, et al. Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr. 2014;164(6):1280–5, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Short DF, Zemel BS, Gilsanz V, et al. Fitting of bone mineral density with consideration of anthropometric parameters. Osteoporos Int. 2011;22(4):1047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilsanz V, Chalfant J, Kalkwarf H, et al. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr. 2011;158(1): 100–5, 5 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6): 2087–99. [DOI] [PubMed] [Google Scholar]
- 16.Groleau V, Schall JI, Dougherty KA, et al. Effect of a dietary intervention on growth and energy expenditure in children with cystic fibrosis. J Cyst Fibros. 2014;13(5):572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostoufi-Moab S, Brodsky J, Isaacoff EJ, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012;97(10):3584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin LM, Thayu M, Baldassano RN, et al. Improvements in bone density and structure during anti-TNF-alpha therapy in pediatric Crohn’s disease. J Clin Endocrinol Metab. 2015;100(7):2630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002(246):1–190. [PubMed] [Google Scholar]
- 20.Kelly A, Winer KK, Kalkwarf H, et al. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99(6):2104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner JM. Growth at adolescence. 2nd ed. Oxford, UK: Blackwell Scientific; 1962. [Google Scholar]
- 22.Zachmann M, Prader A, Kind HP, Hafliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29(1):61–72. [PubMed] [Google Scholar]
- 23.Schall JI, Semeao EJ, Stallings VA, Zemel BS. Self-assessment of sexual maturity status in children with Crohn’s disease. J Pediatr. 2002;141(2):223–9. [DOI] [PubMed] [Google Scholar]
- 24.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3): 271–80. [DOI] [PubMed] [Google Scholar]
- 25.Schoeller DA, Tylavsky FA, Baer DJ, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81(5):1018–25. [DOI] [PubMed] [Google Scholar]
- 26.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard MB, Shults J, Long J, et al. Effect of low-magnitude mechanical stimuli on bone density and structure in pediatric Crohn’s disease: a randomized placebo-controlled trial. J Bone Miner Res. 2016;31(6):1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–5. [DOI] [PubMed] [Google Scholar]
- 29.Hurvich C, Tsai C. Bias of the corrected AIC criterion for underfitted regression and time series models. Biometrika. 1991;78(3):499–509. [Google Scholar]
- 30.Hardin JW, Hilbe JM. Generalized linear models and extensions. 3rd ed. College Station, TX: Stata Press; 2012. xxiv, 455 p. [Google Scholar]
- 31.Hin LY, Wang YG. Working-correlation-structure identification in generalized estimating equations. Stat Med. 2009;28(4):642–58. [DOI] [PubMed] [Google Scholar]
- 32.Rotnitzky A, Jewell N. Hypothesis testing of regression parameters in semi-parametric generalized linear models for cluster correlated data. Biometrika. 1990;77(3):485–97. [Google Scholar]
- 33.Shults J, Sun W, Tu X, et al. A comparison of several approaches for choosing between working correlation structures in generalized estimating equation analysis of longitudinal binary data. Stat Med. 2009;28(18):2338–55. [DOI] [PubMed] [Google Scholar]
- 34.Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom. 2013;16 (4):455–66. [DOI] [PubMed] [Google Scholar]
- 35.Inaba H, Cao X, Han AQ, et al. Bone mineral density in children with acute lymphoblastic leukemia. Cancer. 2018;124(5):1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cystic Fibrosis Foundation. 2015. Patient Registry Annual Data Report. [cited 2016 April 17]. Available from: https://www.cff.org/Our-Research/CF-Patient-Registry/2015-Patient-Registry-Annual-Data-Report.pdf
- 37.Conway SP, Morton AM, Oldroyd B, et al. Osteoporosis and osteopenia in adults and adolescents with cystic fibrosis: prevalence and associated factors. Thorax. 2000;55(9):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly A, Schall JI, Stallings VA, Zemel BS. Deficits in bone mineral content in children and adolescents with cystic fibrosis are related to height deficits. J Clin Densitom. 2008;11(4):581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atkinson HC, Marsh JA, Rath SR, et al. Increased body mass index during therapy for childhood acute lymphoblastic leukemia: a significant and underestimated complication. Int J Pediatr. 2015;2015:386413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang FF, Liu S, Chung M, Kelly MJ. Growth patterns during and after treatment in patients with pediatric ALL: a meta-analysis. Pediatr Blood Cancer. 2015;62(8):1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsampalieros A, Lam CK, Spencer JC, et al. Long-term inflammation and glucocorticoid therapy impair skeletal modeling during growth in childhood Crohn disease. J Clin Endocrinol Metab. 2013;98(8): 3438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward LM, Ma J, Rauch F, et al. Musculoskeletal health in newly diagnosed children with Crohn’s disease. Osteoporos Int. 2017; 28(11): 3169–77. [DOI] [PubMed] [Google Scholar]
- 43.Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136(1):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thayu M, Leonard MB, Hyams JS, et al. Improvement in biomarkers of bone formation during infliximab therapy in pediatric Crohn’s disease: results of the REACH study. Clin Gastroenterol Hepatol. 2008;6(12):1378–84. [DOI] [PubMed] [Google Scholar]
- 45.Shepherd JA, Wang L, Fan B, et al. Optimal monitoring time interval between DXA measures in children. J Bone Miner Res. 2011;26(11): 2745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorsey KB, Thornton JC, Heymsfield SB, Gallagher D. Greater lean tissue and skeletal muscle mass are associated with higher bone mineral content in children. Nutr Metab (Lond). 2010;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


