Abstract
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that is essential for the regulation of food intake and approved for the treatment of type 2 diabetes mellitus and obesity in humans. More recently, GLP-1 has been investigated for its ability to modulate motivation for food and drugs. Reward behavior can be divided into two components: ‘motivational’ (i.e., approach and consummatory behaviors) and ‘affective’ (i.e., perceived palatability). Studies show that GLP-1 analogs reduce the motivation to approach and consume palatable food, but the impact on affective responding is unknown. Thus, the present study tested the effect of the GLP-1 analog, Exendin-4 (Ex-4), on the appetitive response to intraorally delivered sucrose and quinine. Results showed that Ex-4 (2.4ug/kg ip) failed to alter passive drip, appetitive reactions (i.e., mouth movements, tongue protrusions, and lateral tongue protrusions) or aversive reactions (i.e., gapes) to sucrose. Paw-licking, however, was significantly reduced by Ex-4. Treatment with Ex-4 also failed to influence passive drip to quinine, but increased the latency to gape and reduced the total number of gapes emitted. In addition, Ex-4 reduced intake of quinine in water restricted rats, but did not reduce conditioned aversion (i.e., gapes) or avoidance (i.e., reduced intake) of a LiCl-paired saccharin cue. Thus, while Ex-4 had no effect on a learned aversion, it reduced approach and ingestion of sweet and bitter solutions, while leaving the appetitive affective response to the sweet almost intact, and the aversive affective response to the bitter reduced. Treatment with Ex-4, then, differentially modulates appetitive and consummatory components of reward, depending on the valence of the stimulus and whether its valence is learned or innate.
Keywords: GLP-1, Exendin-4, Reward, Aversion, Wanting, Liking
1. Introduction
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted by the gut1 and a neurohormone released by neurons located in the nucleus tractus solitarius (NTS) 2–5. GLP-1 is essential for the regulation of food intake, as both central and peripheral administration 6–8 reduce food consumption. Consequently, GLP-1 is thought to act as a ‘satiety’ agent, and GLP-1 analogs have been approved for the treatment of type II diabetes and obesity 9–11. More recently, in addition to its homeostatic effects, GLP-1 and its analogs (e.g., Liraglutide, Exendin-4) have been investigated for their ability to modulate the reward system. In this context, the ‘satiety’ effects of GLP-1 on reward-driven eating12 is extended to inhibition of reward-driven behaviors for drugs of abuse as well13–18.
How GLP-1 modulates reward-associated intake behaviors is not fully understood. It was first thought that the anorexigenic effects of GLP-1 were mediated by visceral illness, as doses that reduced food intake in animals also supported conditioned-taste aversion/avoidance (CTA) 19, 20. It was later discovered, however, that the aversive and anorexigenic effects of GLP-1 may be dissociable21. Indeed, while GLP-1 receptor activation can reliably support CTA19, the induction of pica (i.e., the eating of clay or kaolin found to be anti-emetic in humans) varies as a function of the type of GLP-1R agonist employed and the route of administration12, 20. These data imply more nuanced mechanisms by which GLP-1 modulates both reward and aversion.
Reward-associated intake behavior has two components: the motivational component inferred from the willingness of the animal to approach and initiate consumption; and the affective component inferred from the behavior driven by the sensory feedback of the stimulus 22, 23. Evidence suggests that the reduction in food intake induced by GLP-1 or its analogs is due, at least in part, to a reduction in the motivation to obtain food24, 25. Thus, it has been observed that Ex-4 can reduce self-administration and conditioned-place preference for sugar pellets12. A microstructural analysis showed that central Ex-4 decreases licking of sucrose by sham feeding rats, and blockade of GLP-1 receptors in the nucleus accumbens shell increases sucrose consumption26, 27. Importantly, each of these protocols (operant conditioning, conditioned place preference, and intake) requires that the rat approach and initiate consumption, suggesting that GLP-1 and its analogs reduce the motivational/consummatory aspect of reward.
The Taste Reactivity Test is a reliable method to detect ingestion and rejection responses to sapid stimuli when administered directly into the oral cavity 28, 29. Mouth movements following intraoral delivery of a sweet match those made during normal licking behavior, occurring at a rate about 7/sec30, and they increase in frequency monotonically with increasing concentrations of the stimulus 28. Rejection behaviors, such as gapes, are important, particularly for non-emetic animals like rats, as gapes are part of a complex set of behaviors that allow the animal to expel any potentially harmful substance from the oral cavity28. Like mouth movements, the number of gapes emitted also increases as a function of the concentration of the bitter substance 31. Collectively, these behaviors are thought to reflect the valence of the stimulus (i.e., whether the stimulus is perceived as rewarding or aversive) and, in some cases, to reflect the “affective” response of the organism 22, 23.
That said, it is not at all clear how GLP-1 receptor agonists modulate the affective component of food reward. This question pertains to both rewarding and aversive gustatory stimuli. Thus, while GLP-1 and its analogs reduce intake of a sweet, it is not known whether the same GLP-1 analog will reduce ingestive taste reactivity behavior (i.e., mouth movements, tongue protrusions, lateral tongue protrusions) when the sweet is infused directly into the oral cavity. This question is addressed in Experiment 1 of the present study. We predict that treatment with the GLP-1 receptor agonist, exendin-4 (Ex-4), will reduce ingestive responding following the intraoral delivery of sucrose indicating a corresponding reduction in the perceived palatability of the stimulus. Previous results support the hypothesis that GLP-1 affects not only satiety, but perceived palatability as well26.
In contrast to the effects of GLP-1 and its analogs on responding to rewarding stimuli, little data addresses the impact of a GLP-1 agonist on responding for aversive stimuli. As a result, Experiment 1 also tests whether Ex-4 will alter the aversive taste reactivity behavior (i.e., gapes) emitted following the intraoral delivery of a quinine (QHCl) solution. Experiment 2 examines the effect of Ex-4 on the motivation to approach and ingest QHCl in water restricted rats. We predict that Ex-4 will increase the perceived aversive properties of QHCl leading to an increase in gaping behavior following intraoral delivery in Experiment 1 and a probable reduction in QHCl intake by thirsty rats in Experiment 2. Finally, Experiment 3 tests the effect of Ex-4 on the conditioned aversive taste reactivity behavior (i.e., gapes) elicited by intraoral delivery of a LiCl-paired saccharin cue. Given that GLP-1 and its analogs can, themselves, induce a CTA19, 20, we predict that treatment with the GLP-1 analog, Ex-4, will increase aversive taste reactivity behavior following intraoral delivery of the LiCl-paired cue. Together, this set of experiments explores the role of a GLP-1 receptor agonist on the different aspects of reward behavior (consummatory and appetitive) to innate and learned rewarding and aversive stimuli.
2. Methods
2.1. Subjects
The subjects were 43 naive, male Sprague-Dawley rats delivered from Charles River Laboratories (Wilmington, MA) weighing between 300 – 400g at the start of the experiment. All subjects were housed individually in standard, suspended, stainless steel cages. The environment in the animal care facility had controlled humidity and temperature (21 °C), with a 12/12 h light/dark cycle, with lights on at 7:00 am. All experimental manipulations were conducted 2 hours into the light phase of the cycle. After one week of acclimation to their home cages, rats were habituated to experimenter handling by daily weighing. Food and water were available ad-libitum except where noted otherwise.
2.2. Experiment 1:
Taste Reactivity Test
The taste reactivity test takes advantage of the fact that affective responses (ingestion and rejection responses) to sweet and bitter solutions are conserved across species. Humans, chimpanzees, and even rats demonstrate immediate stereotypic oromotor responses when a tastant is placed in the oral cavity28, 32. In such experiments, facial expressions are recorded and analyzed during and after the taste is infused into the oral cavity in an effort to measure ingestion and rejection responses28, 33. To do so here, two intraoral cannulas were custom-made 34 and, under ketamine (70 mg/kg, im) and xylazine (14 mg/kg, im) anesthesia, surgically implanted on both sides between the cheek and the jaw, lateral to the first maxillary molar. Once recovered from surgery, all rats were adapted to 15 min intraoral infusions (0.2 ml delivered over 3.5 s once/min) of either 0.5M sucrose or 0.003M QHCl in operant chambers with transparent Plexiglas sides and floors (MED Associates, St. Albans, VT) for seven consecutive days. On the eighth day, rats received an intraperitoneal (ip) injection of vehicle (saline), and 15 min later, were placed in the chamber where they received a 15-min intraoral infusion of either 0.5M sucrose (n=8) or 0.003M QHCl (n=9) while a video camera recorded their orofacial behavior as described 34. On the ninth day, the same procedure was performed but a 2.4 μg/kg ip injection of Ex-4 was administered 15 minutes prior to the intraoral delivery of the tastants. The Ex-4 dose chosen was a dose proven to reduce the rewarding effects of palatable food when administered peripherally 12.
An angled mirror was located below the floor, allowing for a view of the ventral surface of the rat. A high-speed camera was positioned below the floor of the chamber facing the mirror to record the behavior. Taste reactivity behaviors were manually scored after the conclusion of the experiment. The responses were scored according to the categories described by Grill and Norgren28. An appetitive bout included tongue protrusions, lateral tongue protrusions and rhythmic mouth movements. An appetitive bout was counted when any of these behaviors started and was considered finished after a period of 4 seconds (minimum inter-bout interval) without any of these behaviors. In addition, total number of tongue protrusions (TP) and lateral tongue protrusions (LTP), and the total duration of paw licking (PL) behavior were counted separately. Aversive responses were scored as the total number of gapes, the latency in sec to emit the first gape, and the latency or number of passive drips.
2.3. Experiment 2:
Quinine intake
This experiment was performed in behavioral chambers (MED Associates, Inc., St. Albans, VT) and QHCl intake was measured using a lickometer. Since rats will not voluntarily drink strong concentrations of QHCl, a different set of 12 naïve male Sprague-Dawley rats underwent a water restriction protocol in which they had 15 min access to fluid during the daily session and to 20mL of water overnight. On the first two training days the rats drank water for 15 min daily from the spout in the chamber. The availability of the spout was signaled by a cue light located above. The rats then had 4 daily sessions of 15 min access to a lower concentration of quinine (0.0003M). On the test day, rats were injected ip with either 2.4μg/kg Ex-4 (n=6) or vehicle (n=6). Fifteen min later, they were placed in the chamber, were given 15 min access to a more concentrated 0.003M quinine solution, and latency to lick, number of licks, and inter lick intervals were measured.
2.4. Experiment 3: Conditioned Taste Aversion/Avoidance
Intraoral cannulas were placed as described above in a naïve set of 14 adult male Sprague Dawley. After recovering from surgery, the rats were subjected to a water restriction protocol in which they had 15 min access to water each morning and 20ml overnight. The rats had 2 consecutive days of habituation to the taste reactivity chamber where they received intraoral infusions of water for 5 minutes. Then, they were subjected to two saccharin-LiCl pairings. On pairing days, two hours into the light cycle, rats were given 15 min access to 0.15% saccharin via an inverted graduated cylinder with a spout affixed to the front of the home cage. Immediately after, all rats received an ip injection of 0.15M LiCl (1.33 ml/100 g body weight). The pairings were followed by a single test day, with 48 hours between each conditioning and test trial. On the test day, rats were injected ip with either 2.4μg/kg Ex-4 (n=7) or vehicle (n=7) fifteen minutes prior being placed in the Taste Reactivity Chamber. Once in the chamber, all rats received intraoral infusions of 0.15% saccharin (0.2 ml delivered over 3.5 s once/min) and their oromotor behavior was recorded as previously described across a 15 min test period. Once the session was over, rats were returned to their home cage where they were presented with 0.15% saccharin and water in a 24 h, two-bottle test. Placement of water and saccharin was counterbalanced across rats, and total intake was analyzed 24 hours later.
2.5. Data Analysis
All data were analyzed using Student’s t-test or mixed factorial ANOVA. The Newman-Keuls post-hoc test, with α=0.05, was used when appropriate.
3. Results
3.1. Experiment 1:
Sucrose.
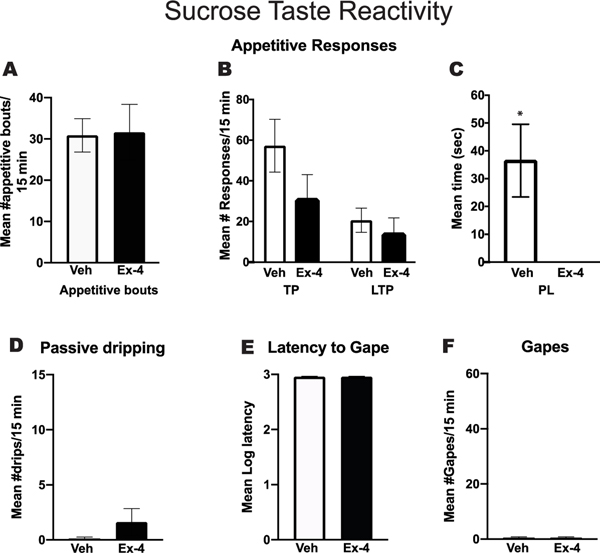
Figure 1 shows the responses emitted during the Taste Reactivity Test for vehicle (Veh) or Ex-4 treated rats during the intraoral infusions of sucrose. The Veh-treated group showed a high number of appetitive bouts in the taste reactivity test following the infusion of sucrose, and pretreatment with Ex-4 fifteen min prior the intraoral delivery of sucrose had no effect (Figure1A). Further, appetitive bouts were divided into number of tongue protrusions, number of lateral tongue protrusions and time spent paw-licking. While, there was a trend for Ex-4 to reduce the number of tongue protrusions emitted for sucrose, the difference was not significant [p>0.05]. In addition, no difference was observed in the number of lateral tongue protrusions [p>0.05] (Figure 1B). The Veh-treated group, however, did spend in average 36.5 seconds licking their paws after the intraoral infusion of sucrose, behavior that was completely suppressed after Ex-4 treatment (Figure 1C) [t(8) = 2.79, p<0.05]. Finally, Veh-treated rats showed a low number of passive drips (Figure1D), and no gaping behavior (Figure1F). The latency to gape was, consequently, the entire session (Figure 1E). Pretreatment with Ex-4 fifteen min prior the intraoral delivery of sucrose had no effect on these behaviors.
Figure 1. Taste Reactivity Test.
Rats (n=8) were subjected to this test across two days. On the first day they were treated with vehicle (Veh) and the second day with 2.4μg/kg Exendin-4 (Ex-4). A. Mean number of appetitive bouts/15 min intraoral (IO) delivery of 0.5 M sucrose in rats treated with Veh or Ex-4.B. Mean number of tongue protrusions (TP) or lateral tongue protrusions (LTP) during the 15 min IO delivery of 0.5 M sucrose in rats treated with Veh or Ex-4.C. Mean time (seconds) of paw-licking (PL) during the 15 min IO delivery of 0.5 M sucrose in rats treated with Veh or Ex-4. D. Mean number of passive drips emitted during the 15 min IO delivery of 0.5 M sucrose for Veh and Ex-4 treated rats. E. Mean log 10 latency (in sec) to elicit the 1st gape to sucrose for Veh and Ex-4 treated rats F. Mean number of aversive responses (gapes) elicited by intraoral infusion of 0.5 M sucrose in Veh vs. Ex-4 treated rats. *p<0.005
Quinine.
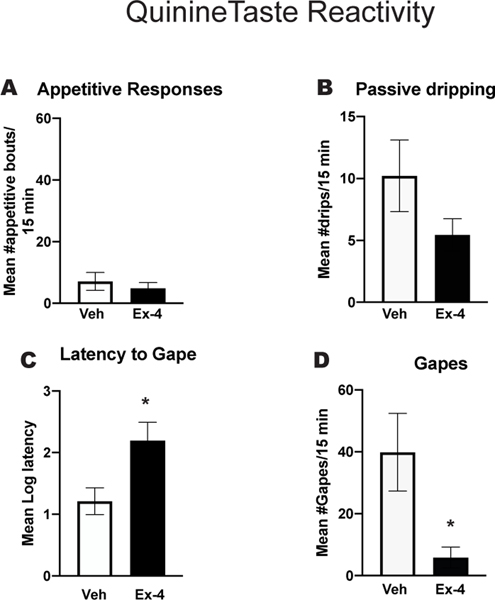
Figure 2 shows the orofacial responses in the Taste Reactivity Test for Veh or Ex-4 treated rats during the intraoral delivery of 0.003M QHCl. As expected, this group showed a low number of appetitive bouts during the session, whether treated with Veh or Ex-4 (Figure 2A). Intraoral delivery of quinine was associated with a relatively high number of passive drips (Figure 2B), but passive dripping was not significantly affected by treatment with Ex-4 [p>0.05]. Veh treated rats infused with QHCl showed a short latency to gape (Figure 2C) and a high number of gapes throughout the session (Figure 2D). Treatment with Ex-4 fifteen minutes prior to infusion of the tastant, however, significantly increased the latency to initiate the 1st gape [t(8)=2.58; p<0.05] (Figure 2C) and significantly reduced the number of gapes elicited by QHCl compared with the number emitted by the Veh-treated QHCl infused rats [t(8)=2.6; p<0.005] (Figure 2D).
Figure 2. Taste Reactivity Test.
Rats (n=9) were subjected to this test on two consecutive days. On the first day they were treated with vehicle (Veh) and the second day with 2.4μg/kg Exendin-4 (Ex-4). A. Mean number of appetitive responses/15 min IO infusion of 0.003 M quinine in rats treated with Veh or Ex-4. B. Mean number of passive drips/15 min IO infusion of 0.003 M quinine in rats treated with Veh or Ex-4. C. Mean log 10 latency (sec) to elicit the 1st gape following the IO infusion of quinine in Veh and Ex-4 treated rats. D. Mean number of aversive responses (gapes)/15 min IO infusion of quinine in Veh and Ex-4 treated rats. *p<0.005
3.2. Experiment 2:
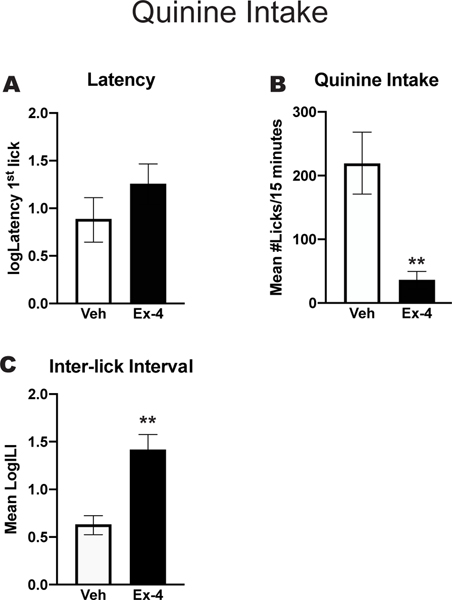
Figure 3 shows behaviors associated with QHCl intake in water deprived rats. There was no significant difference in the latency to initiate the 1st lick of quinine between Ex-4 and Veh-treated rats, [p>0.05] (Figure 3A). Nevertheless, intake of the aversive solution was different between the groups. While Veh-treated rats made over 200 licks/15 min for the 0.003 M quinine solution, rats treated with Ex-4 made less than 50 licks/15 min for the same solution (Figure 3B) [t(10)=3.6; p<0.001]. In addition, the reduced number of licks emitted by the Ex-4-treated group for the 0.003 M quinine solution was associated with a significantly longer inter-lick interval (ILI)[t(10)=4.35, p=0.001] (Figure 3C).
Figure 3. Intake of quinine.
Water deprived rats were trained to drink 0.0003M QHCl. On the test day they were treated with either vehicle (Veh; n=6) or 2.4 μg/kg Ex-4 (n=6) 15 min before being placed in the chamber where they had access to 0.003M QHCl. A Mean log 10 latency (sec) to emit the 1st lick for 0.003M quinine. B. Mean number of licks/15 min emitted for 0.003 M Quinine. C. Mean log 10 Inter-lick interval. Vehicle: white bars, n=6. Ex-4: black bars, n=6. **p<0.001.
3.3. Experiment 3:
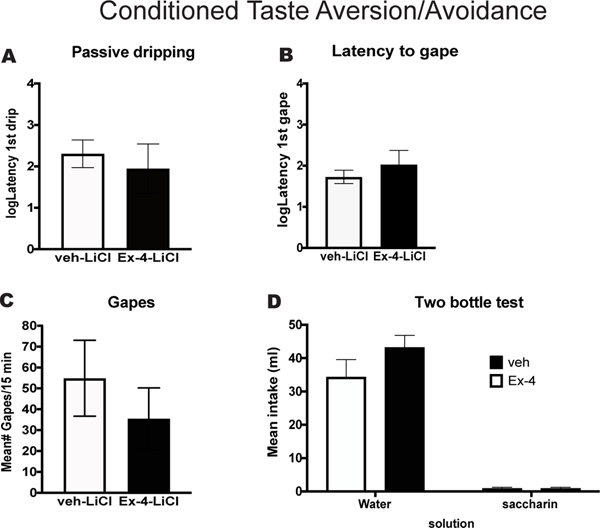
In Experiment 3 we tested the effect of Ex-4 on the aversive taste reactivity behavior elicited by a LiCl-paired saccharin cue, and on intake during a subsequent 24 h two-bottle test. The results of the Taste Reactivity Test confirmed that treatment with Ex-4 had no effect on either the latency to passive drip or on the latency to gape following intraoral infusion of the LiCl-paired saccharin cue, [p>0.05] (see Figures 4A and 4B). Moreover, Veh-treated rats in the saccharin-LiCl group showed a high number of gapes during the session. While numerically lower (Figure 4C), treatment with Ex-4 did not significantly reduce the number of gapes emitted following intraoral infusion of the LiCl-paired saccharin cue (35.5 gapes/15min) relative to the Veh-treated controls (54.8 gapes/15 min) [p>0.05]. Finally, in the Two-bottle Test no differences were observed. All rats, Veh and Ex-4 treated, showed high 24-h water consumption, while none of the rats consumed the LiCl-paired saccharin cue (Figure 4D).
Figure 4. Conditioned Taste Aversion/Avoidance.
All rats had 2 pairings of 0.15% saccharin with LiCl. On the test day, rats were treated with either vehicle (Veh; n=7) or 2.4μg/kg Ex-4 (n=7) 15 minutes prior test. Conditioned taste aversion was measured using the Taste Reactivity Test and conditioned taste avoidance was assessed using a Two-bottle Test. Taste Reactivity Test: A. Mean log 10 latency (sec) to 1st passive drip following intraoral delivery of the LiCl-paired saccharin cue in Veh and Ex-4 treated rats. B. Mean log 10 latency (sec) to elicit the 1st gape following the IO infusion of the LiCl-paired saccharin cue in Veh and Ex-4 treated rats. C. Mean number of aversive responses (gapes/15 min) during IO delivery of the LiCl-paired saccharin cue in Veh or Ex-4 treated rats. D. Two-bottle Test. Mean intake (ml/24 h) of water vs. saccharin in rats treated with Veh or Ex-4. Vehicle: white bars, n=7. Ex-4: black bars, n=7.
4. Discussion
In the present study, we showed that treatment with Ex-4 had no effect on taste reactivity responses following intraoral delivery of 0.5M sucrose, except for paw-licking behavior. In here, Ex-4 did not reduce the total number of appetitive bouts comprised by mouth movements, tongue protrusions and lateral tongue protrusions elicited when the palatable stimulus was placed directly into the oral cavity. While mouth movements were originally described as appetitive reactions, it also has been suggested that they may be categorized as neutral, as mouth movements are still present in response to water and lower concentrations of quinine and dilute sucrose35. For this reason, more highly positive hedonic reactions such as tongue protrusions, lateral tongue protrusions and paw-licking were scored individually. When these behaviors were analyzed separately, no differences were observed. There was a tendency for a reduction in tongue protrusions following treatment with Ex-4, but this effect was not statistically significant. Paw-licking was the only behavior significantly affected by the administration of Ex-4 prior test. While Veh-treated rats spent around 36.5 sec performing this behavior, paw-licking was not observed in any of the rats after treatment with Ex-4. Although this particular result may suggest a reduction in the perceived palatability of the sucrose solution, Ex-4 also has been shown to produce locomotor depression, which may potentially affect paw-licking behavior13. Indeed, the lack of effect on the other behaviors, and the fact that rats treated with Ex-4 did show, in general, less movement in our recordings, suggests that the reduction in paw licking may be due to an overall reduction in general activity rather than a shift in perceived palatability.
Thus, while GLP-1R agonists reduce conditioned-place preference for a context paired with a sweet and intake of a sweet 12, 24, 36, in this study Ex-4 did not reduce the number of appetitive bouts, the number of tongue protrusions or the number of lateral tongue protrusions elicited by the palatable stimulus when placed directly into the oral cavity. Altogether, these data suggest that GLP-1 analogs reduce the motivation to seek and consume sucrose. Once the solution is in the mouth, however, Ex-4 does not appear to significantly alter the apparent perceived intensity or palatability of the stimulus. This finding is consistent with an earlier report showing that, while treatment with Ex-4 reduced intake of sucrose in a two-bottle test, it did not alter the concentration-response function for sucrose in a brief-lick test, a test that is more sensitive to the sensory properties of the stimulus but relatively insensitive to post-ingestive feedback 37. That said, it has been observed that Ex-4 reduced sham intake of sucrose by decreasing the lick rate, burst size, and cluster size26. Given that sham-feeding rats lack most post-ingestive feedback, this reduction in licking behavior by Ex-4 was thought to reflect a disruption in the perceived orosensory value of sucrose rather than an increase in satiety. Even so, the rather long test duration used in the sham feeding study (45 min) may not allow for a separate assessment of the motivational vs. the affective components of food intake because longer test periods require the rats to approach and initiate consumption repeatedly. The advantage afforded by the taste reactivity test is that it by-passes approach and hence the motivational aspect, allowing for an independent assessment of the affective component. As such, we conclude that Ex-4 reduces the willingness to seek and consume the sweet, without affecting its perceived palatability.
We also found that Ex-4 treatment can delay the onset of gaping behavior and reduce the number of gapes emitted following an intraoral infusion of 0.003M quinine. When placed directly into the oral cavity, rats treated with Ex-4 exhibited an 84% reduction in gapes to quinine and a 1-fold delay in the start of this usually robust rejection behavior. These results are remarkable as 0.003M quinine typically is perceived as a potent aversive stimulus, and few substances are known to reduce the apparent aversion by rats when a bitter solution is introduced into the oral cavity38, 39. In two studies, pretreatment with either morphine (2mg/kg sc) or low doses of d-amphetamine (0.25–0.5mg/kg ip), both 30 min prior, reduced gaping behavior elicited by 0.005% or 0.05% quinine. It was suggested that these drugs reduced the perceived aversiveness of QHCl via an increase in dopaminergic transmission. Nevertheless, amphetamine infused directly into the nucleus accumbens shell40 failed to alter taste reactivity to either sucrose or QHCl, complicating the simpler interpretation. Further research is needed to identify the structure(s), and the mechanism(s), by which the GLP-1R agonist so effectively reduces the apparent perceived aversiveness of QHCl.
These data raised the question as to whether the Ex-4-induced reduction in aversive taste reactivity behavior to quinine would be accompanied by an increase in intake of the stimulus. This hypothesis was tested in Experiment 2 and the results showed that ip administration of Ex-4 reduced, rather than increased, intake of the aversive concentration of quinine in thirsty rats. These results mimic those observed with intake of other substances such as salt, water, sweets, and chow 12, 41–43. Thus, our data suggest that the GLP-1R agonist reduces overall motivation, not only for rewarding, but also for aversive gustatory stimuli. Rats, then, are less likely to approach and consume the quinine solution with Ex-4 in the system but, if placed directly in the oral cavity, exhibit markedly less aversive TR behavior for the stimulus.
Along with gaping following intraoral delivery of an innately aversive gustatory stimulus, such as QHCl, rats also gape following the intraoral delivery of an otherwise palatable gustatory cue following pairings with the illness-inducing agent, LiCl 44, 45. Consequently, we tested whether the GLP-1R agonist also would block a LiCl-induced CTA using both taste reactivity to study the affective response, and a two-bottle test to study motivation. The results verified that systemic administration of Ex-4 does not affect the expression of a conditioned taste aversion elicited by LiCl. Specifically, compared with the Veh-treated controls, Ex-4 did not delay passive dripping, the onset of gaping behavior, or significantly reduce the number of gapes to the LiCl-paired cue. In addition, Ex-4 did not affect consumption of the LiCl-paired saccharin solution (which was nil) in a subsequent 24 h two-bottle test. A GLP-1 agonist, then, can reduce rejection of an innately aversive gustatory stimulus, without affecting learned aversion. Further research is needed to assess the neural substrates to better understand how Ex-4 differentially modulates motivated vs. affective behaviors and innate vs. learned aversion.
Taken together, these findings shed light on how GLP-1 modulates both reward and aversion, motivated and affective behavior, and innate and learned aversion. Specifically, while GLP-1 receptor activation reduces the motivation to seek and ingest a palatable foodstuff12, 27, it does not appear to markedly reduce the perceived hedonic-value of that stimulus, at least in the case of sustained appetitive taste reactivity behaviors for 0.5 M sucrose. GLP-1 receptor activation also reduces the motivation to seek and ingest a normally rejected stimulus, such as QHCl, but it somewhat paradoxically reduces gaping to that stimulus once placed in the oral cavity. Finally, peripheral administration of Ex-4 had no effect on either the motivation to consume or the perceived aversiveness of a LiCl-paired saccharin solution. If these data provide insight into the function of endogenous GLP-1, then the satiety mediated by this peptide reduces intake without reducing the perceived value of an otherwise palatable foodstuff and without supporting conditioned aversion. This may differ slightly from ‘natural’ satiety induced by eating or gavage which can reduce appetitive taste reactivity behavior to a sweet with, again, no change in aversive taste reactivity behavior46
Highlights.
Exendin-4 reduces aversive affective responses (i.e. Liking) to quinine
Exendin-4 does not alter affective responses (i.e. Liking) to sucrose
Exendin-4 reduces motivation (i.e. ‘wanting’) to consume quinine
Exendin-4 does not affect motivation (i.e. wanting) or affective responses (i.e. Liking) during LiCl conditioned-taste aversion/avoidance
Acknowledgements:
We thank Sarah Ballard and Nickhil Acharya for their technical assistance.
Funding: This work was supported by the National Institutes of Health [grant numbers DA009815, DA050325]; the Pennsylvania Department of Health, Tobacco Settlement Funds Research [grant numbers SAP# 410007972].
Footnotes
Declarations of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joaquin E. Douton, Department of Neural and Behavioral Sciences Penn State College of Medicine 500 University Drive, H181 Hershey, PA 17033.
Ralph Norgren, Department of Neural and Behavioral Sciences Penn State College of Medicine Hershey, PA 17033.
Patricia Sue Grigson, Department of Neural and Behavioral Sciences Penn State College of Medicine Hershey, PA 17033
References
- 1.Novak U; Wilks A; Buell G; McEwen S, Identical mRNA for preproglucagon in pancreas and gut. Eur J Biochem 1987, 164 (3), 553–8. [DOI] [PubMed] [Google Scholar]
- 2.Merchenthaler I; Lane M; Shughrue P, Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999, 403 (2), 261–80. [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ, The physiology of glucagon-like peptide 1. Physiol Rev 2007, 87 (4), 1409–39. [DOI] [PubMed] [Google Scholar]
- 4.Baggio LL; Drucker DJ, Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132 (6), 2131–57. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez E; Roncero I; Chowen JA; Thorens B; Blazquez E, Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem 1996, 66 (3), 920–7. [DOI] [PubMed] [Google Scholar]
- 6.Abbott CR; Monteiro M; Small CJ; Sajedi A; Smith KL; Parkinson JR; Ghatei MA; Bloom SR, The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 2005, 1044 (1), 127–31. [DOI] [PubMed] [Google Scholar]
- 7.Tang-Christensen M; Larsen PJ; Goke R; Fink-Jensen A; Jessop DS; Moller M; Sheikh SP, Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol 1996, 271 (4 Pt 2), R848–56. [DOI] [PubMed] [Google Scholar]
- 8.Turton MD; O’Shea D; Gunn I; Beak SA; Edwards CM; Meeran K; Choi SJ; Taylor GM; Heath MM; Lambert PD; Wilding JP; Smith DM; Ghatei MA; Herbert J; Bloom SR, A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379 (6560), 69–72. [DOI] [PubMed] [Google Scholar]
- 9.Shukla AP; Buniak WI; Aronne LJ, Treatment of obesity in 2015. Journal of cardiopulmonary rehabilitation and prevention 2015, 35 (2), 81–92. [DOI] [PubMed] [Google Scholar]
- 10.Lovshin JA; Drucker DJ, Incretin-based therapies for type 2 diabetes mellitus. Nature reviews. Endocrinology 2009, 5 (5), 262–9. [DOI] [PubMed] [Google Scholar]
- 11.Blonde L; Rosenstock J; Triplitt C, What are incretins, and how will they influence the management of type 2 diabetes? Journal of Managed Care Pharmacy 2006, 12 (7), S2–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson SL; Shirazi RH; Hansson C; Bergquist F; Nissbrandt H; Skibicka KP, The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 2012, 32 (14), 4812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorensen G; Reddy IA; Weikop P; Graham DL; Stanwood GD; Wortwein G; Galli A; Fink-Jensen A, The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav 2015, 149, 262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egecioglu E; Engel JA; Jerlhag E, The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One 2013, 8 (7), e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt HD; Mietlicki-Baase EG; Ige KY; Maurer JJ; Reiner DJ; Zimmer DJ; Van Nest DS; Guercio LA; Wimmer ME; Olivos DR; De Jonghe BC; Hayes MR, Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology 2016, 41 (7), 1917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez NS; Ige KY; Mietlicki-Baase EG; Molina-Castro GC; Turner CA; Hayes MR; Schmidt HD, Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology 2018, 43 (10), 2000–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomsen M; Dencker D; Wortwein G; Weikop P; Egecioglu E; Jerlhag E; Fink-Jensen A; Molander A, The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacol Biochem Be 2017, 160, 14–20. [DOI] [PubMed] [Google Scholar]
- 18.Graham DL; Erreger K; Galli A; Stanwood GD, GLP-1 analog attenuates cocaine reward. Mol Psychiatry 2013, 18 (9), 961–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiele TE; Van Dijk G; Campfield LA; Smith FJ; Burn P; Woods SC; Bernstein IL; Seeley RJ, Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol 1997, 272 (2 Pt 2), R726–30. [DOI] [PubMed] [Google Scholar]
- 20.Kanoski SE; Rupprecht LE; Fortin SM; De Jonghe BC; Hayes MR, The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 2012, 62 (5–6), 1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinzig KP; D’Alessio DA; Seeley RJ, The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 2002, 22 (23), 10470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berridge KC, Food reward: brain substrates of wanting and liking. Neurosci Biobehav R 1996, 20 (1), 1–25. [DOI] [PubMed] [Google Scholar]
- 23.Berridge KC; Robinson TE; Aldridge JW, Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 2009, 9 (1), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhadeff AL; Grill HJ, Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol 2014, 307 (4), R465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richard JE; Anderberg RH; Goteson A; Gribble FM; Reimann F; Skibicka KP, Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One 2015, 10 (3), e0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asarian L; Corp ES; Hrupka B; Geary N, Intracerebroventricular glucagon-like peptide-1 (7–36) amide inhibits sham feeding in rats without eliciting satiety. Physiology & Behavior 1998, 64 (3), 367–372. [DOI] [PubMed] [Google Scholar]
- 27.Dossat AM; Diaz R; Gallo L; Panagos A; Kay K; Williams DL, Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab 2013, 304 (12), E1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grill HJ; Norgren R, The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 1978, 143 (2), 263–79. [DOI] [PubMed] [Google Scholar]
- 29.Steiner JE, Human facial expressions in response to taste and smell stimulation. Adv Child Dev Behav 1979, 13, 257–95. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan JM; Baird JP; Grill HJ, Dissociation of licking and volume intake controls in rats ingesting glucose and maltodextrin. Behav Neurosci 2001, 115 (1), 188–95. [DOI] [PubMed] [Google Scholar]
- 31.Travers JB; Norgren R, Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci 1986, 100 (4), 544–55. [DOI] [PubMed] [Google Scholar]
- 32.Brightman VJ; Segal AL; Werther P; Steiner J, Facial Expression and Hedonic Response to Taste Stimuli. J Dent Res 1977, 56, B161-B161. [Google Scholar]
- 33.Grill HJ; Norgren R, The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res 1978, 143 (2), 281–97. [DOI] [PubMed] [Google Scholar]
- 34.Colechio EM; Imperio CG; Grigson PS, Once is too much: Conditioned aversion develops immediately and predicts future cocaine self-administration behavior in rats. Behavioral Neuroscience 2014, 128 (2), 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King CT; Hashimoto K; Blonde GD; Spector AC, Unconditioned oromotor taste reactivity elicited by sucrose and quinine is unaffected by extensive bilateral damage to the gustatory zone of the insular cortex in rats. Brain research 2015, 1599, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhadeff AL; Rupprecht LE; Hayes MR, GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012, 153 (2), 647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathes CM; Bueter M; Smith KR; Lutz TA; le Roux CW; Spector AC, Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am J Physiol Regul Integr Comp Physiol 2012, 302 (6), R751–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker L; Leeb K, Amphetamine-induced modification of quinine palatability: analysis by the taste reactivity test. Pharmacol Biochem Behav 1994, 47 (3), 413–20. [DOI] [PubMed] [Google Scholar]
- 39.Clarke SN; Parker LA, Morphine-induced modification of quinine palatability: effects of multiple morphine-quinine trials. Pharmacol Biochem Behav 1995, 51 (2–3), 505–8. [DOI] [PubMed] [Google Scholar]
- 40.Wyvell CL; Berridge KC, Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 2000, 20 (21), 8122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang-Christensen M; Larsen PJ; Göke R; Fink-Jensen A; Jessop DS; Møller M; Sheikh SP, Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol 1996, 271 (4 Pt 2), R848–56. [DOI] [PubMed] [Google Scholar]
- 42.McKay NJ; Kanoski SE; Hayes MR; Daniels D, Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am J Physiol Regul Integr Comp Physiol 2011, 301 (6), R1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKay NJ; Daniels D, Glucagon-like peptide-1 receptor agonist administration suppresses both water and saline intake in rats. J Neuroendocrinol 2013, 25 (10), 929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berridge K; Grill HJ; Norgren R, Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. J Comp Physiol Psychol 1981, 95 (3), 363–82. [DOI] [PubMed] [Google Scholar]
- 45.Barker LM; Smith JC, A comparison of taste aversions induced by radiation and lithium chloride in CS-US and US-CS paradigms. J Comp Physiol Psychol 1974, 87 (4), 644–54. [DOI] [PubMed] [Google Scholar]
- 46.Berridge KC, Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite 1991, 16 (2), 103–20. [DOI] [PubMed] [Google Scholar]