Abstract
Oxidative stress is a major contributor to muscle aging and loss of muscle tissue. Jakyakgamcho-tang (JGT) has been used in traditional Eastern medicine to treat muscle pain. Here, we compared the total phenolic and flavonoid contents in 30% ethanol and water extracts of JGT and tested the preventive effects against oxidative stress (hydrogen peroxide)-induced cell death in murine C2C12 skeletal muscle cells. The total phenolic content and total flavonoid content in 30% ethanol extracts of JGT were higher than those of water extracts of JGT. Ethanol extracts of JGT (JGT-E) had stronger antioxidant activities of 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and 2,2′-diphenyl-1-picrylhydrazyl-scavenging activity (DPPH) than water extracts of JGT (JGT-W). JGT-E contained 19–53% (1.8 to 4.9-fold) more active compounds (i.e., albiflorin, liquiritin, pentagalloylglucose, isoliquiritin apioside, isoliquiritin, liquiritigenin, and glycyrrhizin) than JGT-W. The ethanol extracts of JGT inhibited hydrogen peroxide-induced cell death and intracellular reactive oxygen species generation more effectively than the water extract of JGT in a dose-dependent manner. For the first time, these results suggest that ethanol extract of JGT is relatively more efficacious at protecting against oxidative stress-induced muscle cell death.
Keywords: antioxidant, C2C12 cell, Jakyakgamcho-tang, muscle atrophy, oxidative stress
1. Introduction
Sarcopenia, or aging-associated muscle loss, affects 10% of all adults aged over 50 years old. Muscle aging is characterized by a decline in function and a loss of tissue. Oxidative stress and chronic inflammation are the main mechanisms of skeletal muscle senescence and are associated with increased protein breakdown, decreased protein synthesis, mitochondrial dysfunction, and apoptosis [1,2]. Oxidative stress induces muscle aging. Reactive oxygen species (ROS) are naturally and constantly formed during normal cellular activity and act as mediator of cellular dysfunctions under condition of hypoxia, inflammation or in the impaired antioxidant defenses in vivo [3]. The increase in ROS levels has harmful effects on cell homeostasis, structure, and functions, and results in oxidative stress. Hydrogen peroxide (H2O2) induces atrophy and loss in myotubes, and it is used as an exogenous oxidant treatment to promote free radical generation in both isolated skeletal muscle and in in vitro myotubes [3].
Herbs are sources of important remedies and supplements used in the traditional medicine of Eastern Asia. Nearly 65% of the global population uses herbs as part of their primary health care modality [4]. About 25% of all modern medicines such as aspirin and ephedrine are derived from plants [5]. Natural antioxidants occur in herbal medicines and foods. Several studies have assessed the impacts of supplement and nutrients on muscle strength, mass, and physical performance [6]. It was found that dietary antioxidants such as vitamins C and E inhibited oxidation, induced antioxidant enzymes, and improved positive work function in the chronically loaded muscles of aged rats [7]. Vitamin C regenerates vitamin E in cell membranes, and the latter inhibits free radical production [6]. The study in [8] evaluated the free radical-scavenging abilities of natural products via in vitro assays, specifically their scavenging activities against 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), superoxide, and hydroxyl and nitric oxide radicals as well as their influences on lipid peroxidation levels and antioxidant enzyme activation. The oxidation of ABTS generates preformed radical mononcation of ABTS (ABTS·+) with potassium persulfate, and it reduces in the presence of hydrogen-donating antioxidants [8].
Jakyakgamcho-tang (JGT; Shaoyao-gancao-tang in Chinese; Shakuyaku-kanzo-to in Japanese) is a herbal medicine that is widely prescribed in traditional Eastern medicine and is used to alleviate muscle pain. JGT consists of Paeonia lactiflora Pallas radix (root) and Glycyrrhiza uralensis Fisch radix and rhizome. In a clinical trial, JGT reduced muscle pain and improved muscle damage after exercise [9]. JGT and its major component Paeonia lactiflora have potent antiglycation properties [10]. Glycation is a spontaneous nonenzymatic reaction of free reducing sugars and amino groups with protein and lipids in blood and tissues. Glycation is highly accelerated under oxidative stress and is implicated in the pathogenesis of diabetes and age-related diseases.
More recently, ethanol or ethanol with water has been used as an extraction solvent for traditional Chinese or herbal medicines for pharmaceuticals and dietary supplements [9,10]. However, to the best of our knowledge, no studies have assessed the efficacy of 30% ethanol extract of JGT (JGT-E) against aging-related muscular atrophy in vitro. Thus, we aim to analyze and evaluate the antioxidant properties of water extracts of JGT (JGT-W) and JGT-E. In addition, we evaluate the effect of JGT-E and JGT-W on H2O2-induced cell death in murine C2C12 skeletal muscle cells.
2. Materials and Methods
2.1. Preparation of 30% Ethanol and Water Extracts of Jakyakgamcho-Tang
Paeonia lactiflora and Glycyrrhiza uralensis roots were purchased from an oriental herbal market (Omniherb, GyeongsangBuk-Do, Korea) (http://www.omniherb.com/) that only handles herbs certified by the Korean Pharmacopoeia. The Jakyakgamcho-tang was prepared using a 2:1 (w/w) ratio of P. lactiflora root to G. uralensis root. The JGT extracts were prepared by accurately weighing out 15 g of P. lactiflora root and 7.5 g of G. uralensis root and by mixing them according to the Oriental Medicine Advanced Searching Integrated System (http://oasis.kiom.re.kr) of the Korea Institute of Oriental Medicine. Distilled water or 30% (v/v) ethanol was added to the roots, and the mixture was extracted for 1 h in a reflux extractor (MS-DM607; M-TOPS, Seoul, Korea). The extracts were then filtered, evaporated under reduced pressure in a rotary evaporator (N-1200A; Eyela, Tokyo, Japan) at 50 °C, and freeze-dried (FDU-2100; Eyela, Tokyo, Japan) at −80 °C for 72 h to obtain either a 30% (v/v) ethanol extract (JGT-E; yield 29.6%) or water extract (JGT-W; yield 34.8%). For ultra-performance liquid chromatography (UPLC), 20 mg of each extract was dissolved in 2 mL dimethyl sulfoxide (DMSO) and diluted tenfold with distilled water. The solutions were passed through a 0.45-μm syringe filter (Whatman; Clifton, NJ, USA) before injection.
2.2. UPLC-Quadruple Time-of-Flight Mass Spectrometry (UPLC-Q-Tof/MS) Analysis
UPLC was performed in a Waters Acquity UPLC system (Waters Corp., Milford, MA, USA) coupled to a Q-Tof Premier ESI/mass spectrometer (Q-Tof Premier™, Waters Corp., Milford, MA, USA). Three-microliter aliquots per sample were injected into a BEH C18 column (100 mm × 2.1 mm; i.d. 1.7 μm) at a flow rate of 0.4 mL min−1 and eluted with a chromatographic gradient, comprising mobile phases A (water containing 0.1% (v/v) formic acid) and B (acetonitrile containing 0.1% (v/v) formic acid). The linear gradient was optimized as follows: 0 min, 10% B; 0–9 min, 10–20% B; 9–11 min, 20–30% B; 11–12 min, 30–90% B; 12–13 min, 90–100% B; 13–14 min, 100% B; and 14–15 min, 10% B. The Q-Tof was operated in negative ion mode under the following conditions: capillary voltage, 2.3 kV; cone voltage, 50 V; source temperature, 110 °C; and desolvation temperature, 350 °C. A sprayer with a leucine-enkephalin reference solution ([M−H] − m/z 554.2615) served as the lock mass. Full scan data and MS/MS spectra were collected with MassLynx 4.1 software (Waters Corp., Milford, MA, USA).
2.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in JGT-W and JGT-E
The total phenolic content (TPC) in various extracts of JGT and its constituent herbs were determined by a modified Folin–Ciocalteu method [11]. Briefly, 0.125 mL of each extract was transferred to a test tube containing 1.8 mL Folin–Ciocalteu reagent. After 5 min, 1.2 mL of 15% (w/v) Na2CO3 was added, and the mixture was kept in the dark at 25 °C for 2 h. Absorbance was measured at 765 nm in a SpectraMax M2 multimode microplate reader (Molecular Devices, Sunnyvale, CA, USA). A linear calibration curve (R2 = 0.998) was plotted using gallic acid, and the results were expressed in mg gallic acid equivalents (GAE) per g extract sample.
Total flavonoid content (TFC) was determined by an aluminum chloride colorimetric assay [11]. Quantification was expressed by reporting the absorbance in the quercetin calibration graph used as the flavonoid standard (R2 = 0.999). A 1 mL sample of prepared extract or standard was mixed with 4 mL distilled water and 0.3 mL of 5% (w/v) NaNO2. After 5 min and 1 min, respectively, 0.3 mL of 10% (w/v) AlCl3 and 2 mL of 1 M NaOH were added. Then, 2.4 mL distilled water was added, and the mixture was shaken. The resultant pink color was read in a SpectraMax M2 multimode microplate reader at 510 nm, and the results were expressed in mg quercetin equivalents (QE) per g extract sample.
2.4. Scavenging of 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS)
ABTS-scavenging activity was assessed according to a modified version of the method described by Re et al. [12]. ABTS (7.0 mM) and potassium persulfate (2.45 mM) were mixed in water and stored at room temperature for 12 h in the dark to produce ABTS·+. The aqueous ABTS·+ solution was then diluted to an absorbance of 1.50 at 405 nm. Various concentrations of each extract solution (1 mL) were added to 2 mL diluted ABTS·+ solution, and the absorbance were measured at 405 nm.
2.5. 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Free Radical Assay
Water and 30% (v/v) ethanol extracts of the P. lactiflora and G. uralensis roots (50 μg mL−1, 100 μg mL−1, 250 μg mL−1, 500 μg mL−1, and 1000 μg mL−1) were prepared for the DPPH assay. The DPPH-scavenging activity of the extracts was measured according to a previously reported method [8]. Here, 1 mm aliquots of the various concentrations of the extract solutions were added to 2 mm of a DPPH-methanol solution (5 mg 100 mL−1). The decrease in absorbance at 517 nm was measured with a SpectraMax M2 multimode microplate reader. The DPPH-scavenging activity (%) was calculated according to the following equation:
| (1) |
where Asample is the steady-state absorbance of the sample solution, and A0 is the absorbance of the DPPH solution before the addition of the extract.
2.6. Cell Culture
The immortalized mouse myoblast cell line C2C12 was purchased from the American Type Culture Collection (ATCC CRL-1772; Manassas, VA, USA) and cultured in the Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (w/v) penicillin-streptomycin (P/S) at 37 °C in a humidified 5% CO2 incubator as per previously described methods [13]. To induce myotube formation, the media were replaced with differentiation media (DMEM with 2% (v/v) FBS and 1% (w/v) P/S) and cultured for an additional 7 d.
2.7. Cell Viability Assay in H2O2-Induced C2C12 Cells
C2C12 viability was determined by a cell counting kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Kumamoto, Japan). C2C12 cells (0.5 × 104 cells/well) were seeded in each well of a 96-well plate containing DMEM plus 10% (v/v) FBS and incubated for 24 h. After attachment, the cells were pretreated with JGT extract in a serum-free medium for 24 h and then with 250 μM H2O2 for 3 h. After incubation, 10 μL CCK-8 was added to each well, and the plate was incubated at 37 °C and 5% CO2 in a humidified incubator for 2 h. Absorbance was measured at 450 nm in a multidetection microplate reader (Synergy HT; BioTek, Winooski, VT, USA).
2.8. Intracellular ROS Measurements in H2O2-Induced C2C12 Cells
C2C12 cells were pretreated with JGT-E and JGT-W for 24 h and then subjected to 250 μM H2O2 for 30 min. N-acetylcysteine (NAC, 1 mM) served as a positive control. Intracellular ROS levels were measured by the DCF-DA method, wherein the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA, Molecular Probes Inc., Eugene, OR, USA) is converted by intracellular esterases to 2′,7′-dichlorodihydrofluorescein, which in turn is oxidized by intracellular ROS to highly fluorescent 2′,7′-dichlorofluorescein (DCF). The treated cells were then washed with a Hank’s Balanced Salt Solution (HBSS) buffer and incubated in the dark for 30 min in a HBSS buffer containing 50 μM H2DCF-DA. DCF fluorescence was measured in a Synergy HT spectrofluorometer (BioTek, Winooski, VT, USA) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. All experiments were repeated at least thrice.
2.9. Statistical Analysis
Data are means ± SD or mean ± SE. Here, ANOVA with Tukey’s test was used for multiple treatment means comparisons in Prism v. 7.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Qualitative and Quantitative Analyses of JGT Components by UPLC-PDA-ESI-QTOF-MS
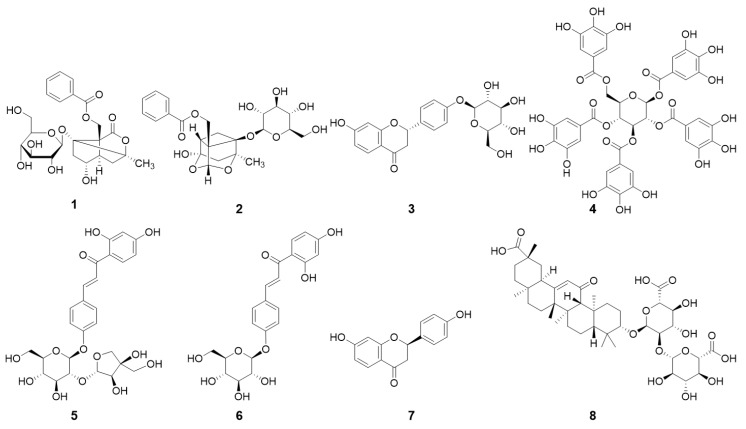
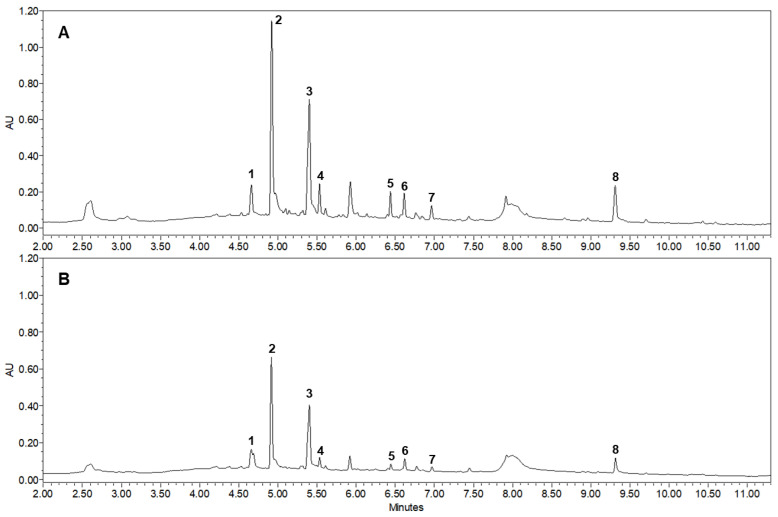
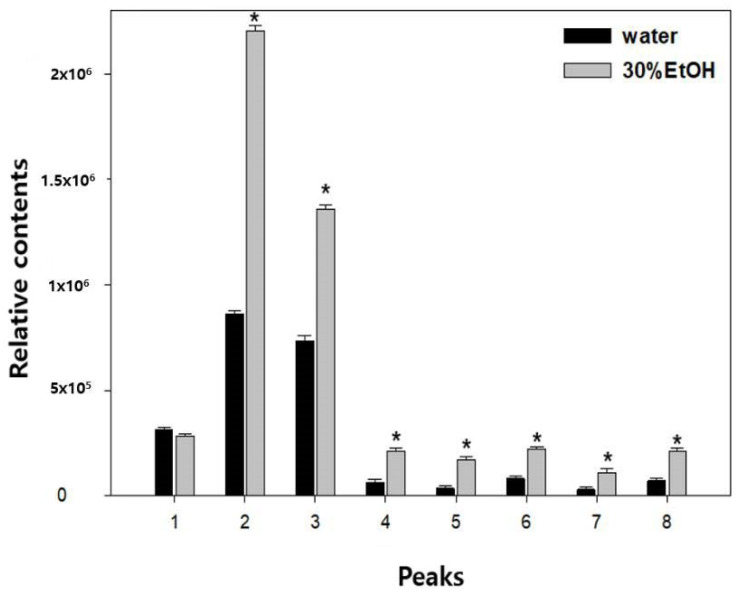
As shown in Figure 1 and Figure 2, the major active compounds in JGT-E and JGT-W were albiflorin (1), paeoniflorin (2), liquiritin (3), pentagalloylglucose (4), isoliquiritin apioside (5), isoliquiritin (6), liquiritigenin (7), and glycyrrhizin (8). JGT-E contained 19–53% more of the aforementioned constituents than those in JGT-W, except for albiflorin (1) (Figure 3).
Figure 1.
Chemical structures of Jakyakgamcho-tang (JGT) components: albiflorin (1), paeoniflorin (2), liquiritin (3), pentagalloylglucose (4), isoliquiritin apioside (5), isoliquiritin (6), liquiritigenin (7), and glycyrrhizin (8).
Figure 2.
Ultra-performance liquid chromatography profiles for JGT-E (ethanol extracts of JGT) and JGT-W (water extracts of JGT). (A) JGT-E, 30% (w/v) EtOH extract of Jakyakgamcho-tang; (B) JGT-W, water extract of Jakyakgamcho-tang. Albiflorin (1), paeoniflorin (2), liquiritin (3), pentagalloylglucose (4), isoliquiritin apioside (5), isoliquiritin (6), liquiritigenin (7), and glycyrrhizin (8).
Figure 3.
Comparative changes in individual peak marker levels from two extraction solvents. Calculations were based on the area determined from UPLC spectra. Mean ± SD of measurements determined for three independent samples analyzed thrice (n = 3). * p < 0.001 vs. water extract. Albiflorin (1), paeoniflorin (2), liquiritin (3), pentagalloylglucose (4), isoliquiritin apioside (5), isoliquiritin (6), liquiritigenin (7), and glycyrrhizin (8).
3.2. Effects of JGT-E and JGT-W on ABTS- and DPPH-Scavenging Activities
The relative total phenolic content (TPC) and total flavonoid content (TFC) are shown in Table 1. The TPC and TFC in the 30% (w/v) ethanol extracts of P. lactiflora and G. uralensis roots were higher than those in the water extracts. The contents of TPC and TFC were higher than those in JGT-W.
Table 1.
Total phenolic content (TPC) and total flavonoid content (TFC) in Jakyakgamcho-tang (JGT) and its constituents.
| Sample | TPC (mg GAE g−1) | TFC (mg QE g−1) |
|---|---|---|
| Water extract of P. lactiflora root | 17.17 ± 0.6 | 3.25 ± 0.3 |
| 30% (w/v) ethanol extract of P. lactiflora root | 29.70 ± 1.0 * | 8.11 ± 0.1 * |
| Water extract of G. uralensis root | 18.37 ± 0.1 | 3.72 ± 0.1 |
| 30% (w/v) ethanol extract of G. uralensis root | 23.10 ± 1.6 * | 7.02 ± 0.2 * |
| JGT-W | 25.49 ± 0.8 | 3.94 ± 0.5 |
| JGT-E | 34.01 ± 2.6 ** | 7.32 ± 0.6 ** |
Data indicate mean ± SD (n = 3). All extracts were analyzed in triplicate. * p < 0.05 vs. water extract; ** p < 0.01 vs. JGT-W.
Table 2 shows the antioxidant properties of water and 30% (w/v) ethanol extracts evaluated by in vitro ABTS+ − and DPPH-scavenging assays. The antioxidant activity of JGT-E was higher than that of JGT-W. The ABTS+− and DPPH-scavenging capacities JGT-E are commensurate with the phenolic compound content.
Table 2.
Relative ABTS and DPPH activities of Jakyakgamcho-tang (JGT).
| Samples | ABTS | DPPH | ||
|---|---|---|---|---|
| Inhibition (%) |
IC50
(µg mL−1) |
Inhibition (%) |
IC50
(µg mL−1) |
|
| JGT-W | 72.8 ± 1.2 | 60.5 ± 0.9 | 25.1 ± 1.3 | 742.8 ± 2.1 |
| JGT-E | 80.8 ± 1.1 | 34.2 ± 0.4 *** | 38.9 ± 1.1 | 436.1 ± 3.7 *** |
Data indicate mean ± SD (n = 3). All extracts were analyzed in triplicate. *** p < 0.0001 vs. JGT-W. ABTS, 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid; DPPH, 2,2-diphenyl-1-picrylhydrazyl; IC50, 50% inhibitory concentration; JGT-E, 30% (w/v) ethanol extract of Jakyakgamcho-tang, JGT-W, Jakyakgamcho-tang water extract.
3.3. JGT-E and JGT-W Protect C2C12 Myocytes against H2O2-Induced Decrease in Cell Viability
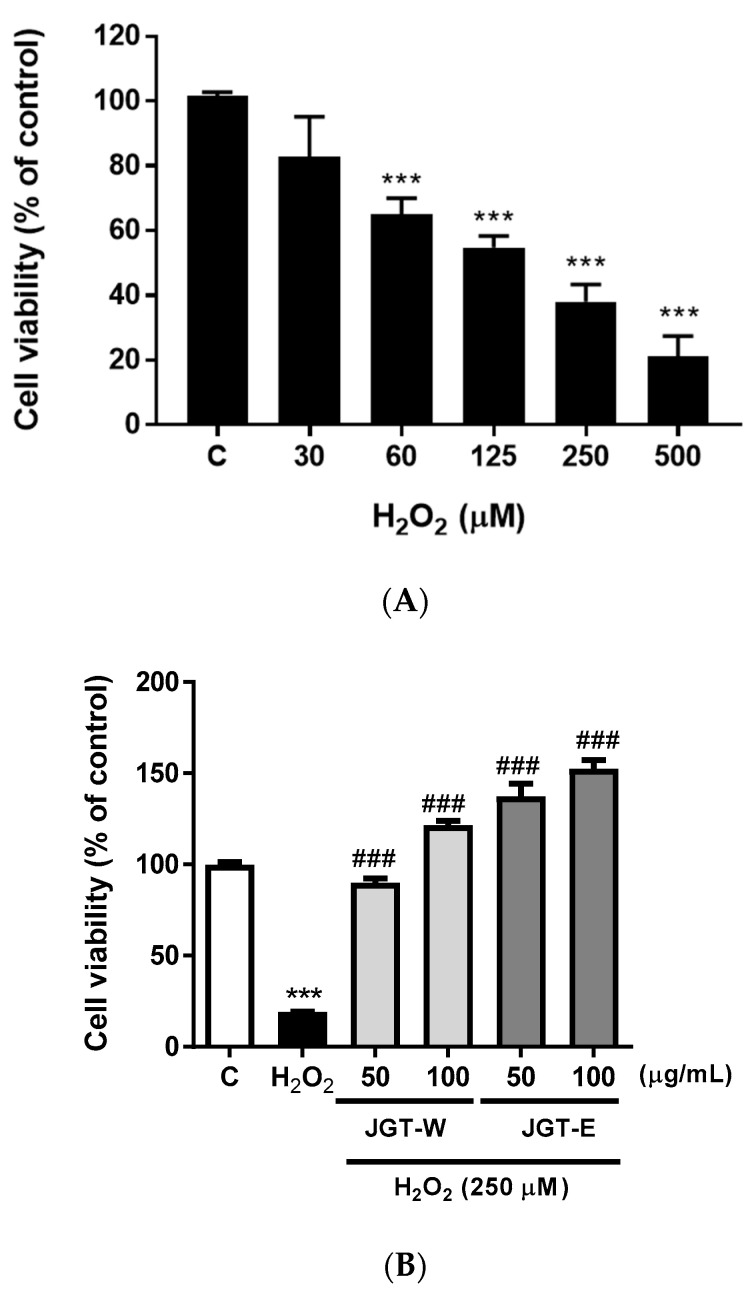
To determine whether hydrogen peroxide (H2O2) induced C2C12 death, we subjected the cells to various H2O2 concentrations for 24 h (Figure 4A). H2O2 decreased C2C12 viability in a dose-dependent manner. To assess the efficacies of JGT-E and JGT-W at preventing H2O2-induced C2C12 death, we pretreated the cells with JGT-E and JGT-W, subjected them to H2O2, and evaluated cell viability by CCK-8. Figure 4B shows that JGT-E and JGT-W preserve C2C12 proliferative activity in a dose-dependent manner.
Figure 4.
Effects of JGT-W and JGT-E on growth of H2O2-treated C2C12 cells. (A) H2O2-induced C2C12 death. (B) Protective effects of JGT-W and JGT-E in C2C12 against H2O2-induced cytotoxicity. Data are representative of three independent experiments and expressed as mean ± standard error of mean (n = 7). *** p < 0.001 vs. control; ### p < 0.001 vs. viability of H2O2-treated cells. JGT-E, 30% (w/v) ethanol extract of Jakyakgamcho-tang, JGT-W, Jakyakgamcho-tang water extract.
3.4. JGT-E and JGT-W Protect C2C12 Myocytes from H2O2-Induced Damage
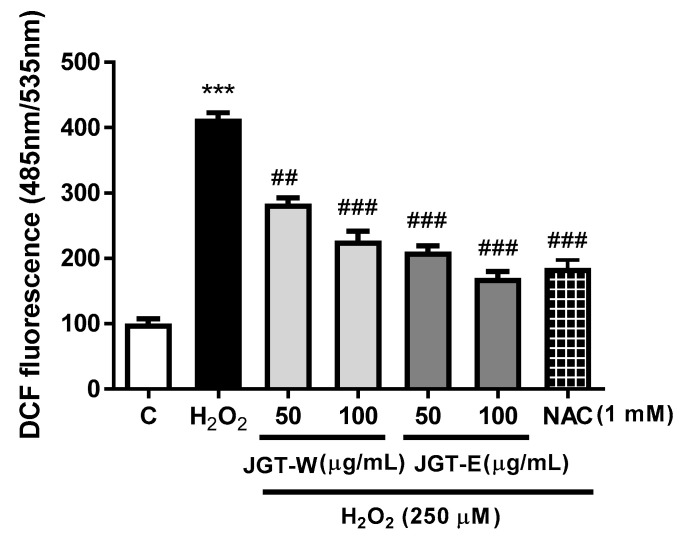
The H2O2 treatment was cytotoxic to C2C12, but both JGT-W and JGT-E helped prevent cell death. To determine whether this protection was related to the antioxidant effects of JGT-W and JGT-E in C2C12, we evaluated whether JGT-W and JGT-E could inhibit intracellular ROS generation in H2O2-treated cells. Figure 5 shows that the nearly fourfold increase in intracellular ROS levels induced by H2O2 was significantly inhibited by JGT-W and JGT-E pretreatment in a dose-dependent manner. JGT-E reduced DCF fluorescence by >27.75% (100 μg mL−1) and by 25.50% (50 μg mL−1) compared to JGT-W. NAC pretreatment also attenuated H2O2-stimulated increase in intracellular ROS levels in C2C12.
Figure 5.
Effects of JGT-W and JGT-E extracts on viability of H2O2-treated C2C12 cells. NAC was positive control. Data are representative of three independent experiments and expressed as mean ± standard error of mean (n = 7). *** p < 0.001 vs. control; ## p < 0.01, ### p < 0.001 vs. viability of H2O2-treated cells. JGT-E, 30% (w/v) ethanol extract of Jakyakgamcho-tang; JGT-W, Jakyakgamcho-tang water extract.
4. Discussion
During aging, oxidative stress plays an important role in the progression of muscle mass loss or skeletal muscle atrophy [14]. Dietary supplementation with exogenous antioxidants such as herbal medicines exerts health-promoting effects and helps to prevent muscle aging [15]. Jakyakgamcho-tang (JGT), a herbal medicine consisting of a 2:1 (w/w) mixture of Paeonia lactiflora and Glycyrrhiza uralensis roots, has been used both clinically and pharmacologically in the treatment of muscle and acute abdominal pain and backache [16,17]. Here, we analyzed the composition of a water extract (JGT-W) and a 30% (w/v) ethanol extract of JGT (JGT-E) and confirmed whether the efficacies of JGT-W and JGT-E could prevent muscle cell damage under oxidative stress for the first time.
JGT is a herbal medicine widely prescribed in Eastern Asia and has recently been known for various effects, such as the inhibitory effect of formation of advanced glycation end products, the inhibitory effect of nitric oxide, and anti-inflammatory effect [9,10]. Here, Figure 3 showed the antioxidant properties of JGT-W and JGT-E by in vitro ABTS- and DPPH-scavenging assay, and the antioxidant activity of JGE-E was higher than that of JGT-W. In addition, JGT-E had higher total phenolic and flavonoid content than those in JGT-W (Table 1). Plant phenolic and flavonoid compounds are secondary metabolites, and these compounds scavenge free radicals and act as antioxidants [11,18,19]. JGT-E was relatively more effective at scavenging ABTS·+ and DPPH-inhibiting intracellular ROS generation in skeletal muscle cells as it contained comparatively more TPC and TFC. A previous study showed that phenolic compounds strongly inhibit H2O2-induced apoptosis in HepG2 cells [20]. To date, no studies have investigated the contents of plant phenolic and flavonoid compounds in JGT-W and JGT-E.
One previous study showed that the relative amounts of effective components such as paeoniflorin, benzoic acid, glycyrrhizin, and isoliquiritin in JGT is higher in the 70% ethanol extracts than in water-extracted JGT [21]. Given the herbal combination of JGT and with chromatographic conditions, various compounds in JGT can be isolated [22]. As shown as Figure 3 and Table 1, the levels of all measured phenolic compounds except albiflorin were also higher in JGT-E than JGT-W. Paeoniflorin was found to be the most abundant component, and its concentration markedly differed between JGT-E and JGT-W. This compound is a pinnae monoterpene glycoside with various pharmacological effects, such as antioxidant, anti-inflammatory, and immunoregulatory effects [23,24]. Liquiritin has an antioxidant effect on neuroblastoma cells [25,26]. Pentagalloylglucose prevents acute lung injury in lipopolysaccharide-induced rat models and has antioxidant properties [27,28,29,30,31]. Isoliquiritin apioside shows marked modulatory activities against oxidative stress-induced genotoxicity [31]. Isoliquiritin presents with antioxidant activity, while liquiritigenin is also a potent antioxidant and shows various efficacy against multidrug-resistant bacteria [32,33]. Glycyrrhizin reduces the formation of ROS by inducing AMPK phosphorylation and nuclear transfer NRF2, resulting in an upregulation of antioxidant enzymes and anti-inflammatory efficacy [34,35].
The accumulation of oxidative stress leads to cell transformation and causes tissue injury, loss of function, enhanced senescence, and loss of potential associated with degenerative diseases associated with aging such as sarcopenia [36]. Excess cellular levels of ROS cause damage to cell membrane and organelles, which leads to activation of cell death [37]. Here, ROS-induced cell death was prevented in JGT-E or JGT-W-pretreated C2C12 cells via the inhibition of oxidation (Figure 4B). The inhibition of oxidative stress by JGT-E is thought to contribute to promoting cell growth. Exogenous antioxidants that reduce intracellular ROS levels promote cell growth in tumor progression [38].
Generally, traditional Chinese and oriental herbal medicines are water extracts. The Korea Food and Drug Administration either exempts or requires minimum toxicity test data for the approval of oriental herbal medicines when the ethanol content in the extraction solvent is ≤30% and the balance is water. The present study demonstrated that an ethanol-water solvent mixture was relatively more effective at extracting the bioactive components in JGT, thereby increasing its biological activity and reducing the dosage required for optimal efficacy. JGT-E showed the preventive effect on H2O2-induced cell death via the inhibition of oxidation. To the best of our knowledge, this is the first report on the effectiveness of JGT-E against aging-related muscular atrophy in vitro.
5. Conclusions
The aim of this study was to compare the relative antioxidant principles of JGT-W and JGT-E derived from the traditional herbal blend, JGT. JGT-E had strong antioxidant activity and prevented oxidative stress-induced skeletal muscle cell death. The results of the present study suggest that low doses of JGT-E may help prevent skeletal muscle aging via the inhibition of oxidation. Moreover, 30% (v/v) ethanol effectively extracts JCT and substantially enhances its antioxidant and other biological activities compared with traditional water extracts that have been prepared to reduce the dosages for muscle atrophy.
Author Contributions
Y.S.K. designed the in vitro cell experiment and wrote the manuscript; H.J.Y. confirmed the HPLC chromatogram and antioxidant assay and wrote the manuscript; D.S.K. designed all experiments and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Oriental Medicine (Grant Nos. KSN1911310 and KSN2012330).
Data Availability Statement
All the data generated and analyzed in this study are mentioned in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meng S.J., Yu L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010;7:1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson S., Cooper C., Sayer A.A. Nutrition and sarcopenia: A review of the evidence and implications for preventive strategies. J. Aging. Res. 2012;2012:510801. doi: 10.1155/2012/510801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scicchitano B.M., Pelosi L., Sica G., Musarò A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Aging Dev. 2018;170:37–44. doi: 10.1016/j.mad.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Farnsworth N.R., Akerele O., Bingel A.S., Soejarto D.D., Guo Z. Medicinal plants in therapy. Bull. World Health Org. 1985;63:965–981. doi: 10.1016/0378-8741(87)90016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niki E. Interaction of ascorbate and alpha-tocopherol. Ann. N. Y. Acad. Sci. 1987;498:186–199. doi: 10.1111/j.1749-6632.1987.tb23761.x. [DOI] [PubMed] [Google Scholar]
- 7.Ryan M.J., Dudash H.J., Docherty M., Geronilla G.B., Baker B.A., Haff G.G., Cutlip R.G., Always S.E. Vitamin E and C Supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp. Gerontol. 2010;45:882–895. doi: 10.1016/j.exger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo J., Lee S., Elam M.L., Johnson S.A., Kang J., Arjmandi B.H. Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci Nutr. 2014;2:174–180. doi: 10.1002/fsn3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han K., Kwon O., Jung S.-Y., Park I.-H., Hwang M.-S., Park S.-Y., Hwang E.-H., Lee J.-H. Jakyakgamcho-Tang in the relief of delayed-onset muscle soreness in healthy adults: Study protocol for a randomized, double-blind, placebo-controlled, crossover design clinical trial. Trials. 2020;21:211. doi: 10.1186/s13063-020-4119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J., Kim C.K., Kim Y.S., Lee I.S., Kim J.S. Jakyakgamcho-tang and its major component, Paeonia lactiflora, exhibit potent anti-glycation properties. J. Exerc. Nutr. Biochem. 2016;20:60–64. doi: 10.20463/jenb.2016.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra S., Khan S., Avula B., Lata H., Yang M.H., Elsohly M.A., Khan I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid. Based Complement. Alternat. Med. 2014;2014:253875. doi: 10.1155/2014/253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 13.Jun I., Jeong S., Shin H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials. 2009;30:2038–2047. doi: 10.1016/j.biomaterials.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 14.Gomes M.J., Martinez P.F., Pagan L.U., Damatto R.L., Cezar M.D.M., Lima A.R.R., Okoshi K., Okoshi M.P. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget. 2017;8:20428–20440. doi: 10.18632/oncotarget.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouayed J., Bohn T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinoshita F., Ogura Y., Suzuki Y., Hara S., Yamada A., Tanaka N., Yamashita A., Marumo F. Effect of orally administered Shao-Yao-Gan-Cao-Tang (Shakuyaku-Kanzo-To) on muscle cramps in maintenance hemodialysis patients: A preliminary study. Am. J. Chin. Med. 2003;31:445–453. doi: 10.1142/S0192415X03001144. [DOI] [PubMed] [Google Scholar]
- 17.Hidaka T., Shima T., Nagira K., Ieki M., Nakamura T., Aono Y., Kuraishi Y., Arai T., Saito S. Herbal Medicine Shakuyaku-Kanzo-To reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur. J. Pain. 2009;13:22–27. doi: 10.1016/j.ejpain.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 20.Najafabad A.M., Jamei R. Free radical scavenging capacity and antioxidant activity of methanol and ethanol extracts of plum (Prunus domestica L.) in both fresh and dried samples. Avicenna J. Phytomed. 2014;4:343–353. [PMC free article] [PubMed] [Google Scholar]
- 21.Kwak D., Lee C., Kong I., Choi D., Na C. Comparison of the spasmolytic effects of Jakyak-Gamcho decoctions derived via different extractants. Evid. Based Complement. Alternat. Med. 2015;2015:270380. doi: 10.1155/2015/270380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.-H., Shin H.-K., Seo C.-S. Chemical interaction between Paeonia lactiflora and Glycyrrhiza uralensis, the components of Jakyakgamcho-tang, using a validated high-performance liquid chromatography method: Herbal combination and chemical interaction in a decoction. J. Sep. Sci. 2014;37:2704–2715. doi: 10.1002/jssc.201400522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu J., Guo Y., Hong W., Fang Y., Han D., Zhang P., Wang X., Körner H., Wie W. The regulatory effects of paeoniflorin and its derivative paeoniflorin-6′-o-benzene sulfonate CP-25 on inflammation and immune diseases. Front. Pharmacol. 2019;10:57. doi: 10.3389/fphar.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su J., Zhang P., Zhang J.-J., Qi X.-M., Wu Y.-G., Shen J.-J. Effects of total glucosides of paeony on oxidative stress in the kidney from diabetic rats. Phytomedicine. 2010;17:254–260. doi: 10.1016/j.phymed.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani Y., Kobe A., Kuriya M., Hiroki Y., Yahagi T., Sakakibara I., Matsuzaki K., Amano T. Neuroprotective effect of liquiritin as an antioxidant via an increase in glucose-6-phosphate dehydrogenase expression on B65 neuroblastoma cells. Eur. J. Pharmacol. 2017;815:381–390. doi: 10.1016/j.ejphar.2017.09.040. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima H., Matsui T., Yoshida O., Isowa Y., Kido Y., Motoki Y., Ito M., Shigeta S., Mori T., Yamamoto N. A new anti-human immunodeficiency virus substance, glycyrrhizin sulfate; endowment of glycyrrhizin with reverse transcriptase-inhibitory activity by chemical modification. Jpn. J. Cancer Res. 1987;78:767–771. [PubMed] [Google Scholar]
- 27.Fu Y., Hsieh T., Guo J., Kunicki J., Lee M.Y.W.T., Darzynkiewicz Z., Wu J.M. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2004;322:263–270. doi: 10.1016/j.bbrc.2004.07.094. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q., Nie J., Chen S.-J., Li Q. Protective effects of ethyl gallate and pentagalloylglucose, the active components of Qingwen Baidu decoction, against lipopolysaccharide-induced acute lung injury in rats. Drug Des. Devel. Ther. 2019;13:71–77. doi: 10.2147/DDDT.S186029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piao M., Kang K., Zhang R., Ko D., Wang Z., Lee K., Chang W., Chae S., Jee Y., Shin T., et al. Antioxidant properties of 1,2,3,4,6-penta-o-galloyl-β-d-glucose from Elaeocarpus sylvestris var. ellipticus. Food Chem. 2009;115:412–418. doi: 10.1016/j.foodchem.2008.12.020. [DOI] [Google Scholar]
- 30.Patnaik S.S., Simionescu D.T., Goergen C.G., Hoyt K., Sirsi S., Finol E.A. Pentagalloyl glucose and its functional role in vascular health: Biomechanics and drug-delivery characteristics. Ann. Biomed. Eng. 2019;47:39–59. doi: 10.1007/s10439-018-02145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaur P., Kaur S., Kumar N., Singh B., Kumar S. Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L. Toxicol. In Vitro. 2009;23:680–686. doi: 10.1016/j.tiv.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Alrushaid S., Davies N.M., Martinez S.E., Sayre C.L. Pharmacological characterization of liquiritigenin, a chiral flavonoid in licorice. Res. Pharm. Sci. 2016;11:355–365. doi: 10.4103/1735-5362.192484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman H., Khan I., Hussain A., Shahat A.A., Tawab A., Qasim M., Adnan M., Al-Said M.S., Ullah R., Khan S.N. Glycyrrhiza glabra HPLC fractions: Identification of aldehydo isoophiopogonone and liquiritigenin having activity against multidrug resistant bacteria. BMC Complement Altern. Med. 2018;18:140. doi: 10.1186/s12906-018-2207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J.C. Mechanism of action of glycyrrhizic acid in inhibition of epstein-barr virus replication in vitro. Antiviral Res. 2003;59:41–47. doi: 10.1016/S0166-3542(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Chen C., Zhu X., Li Y., Yu R., Xu W. Glycyrrhizin suppresses RANKL-induced osteoclastogenesis and oxidative stress through inhibiting NF-κB and MAPK and activating AMPK/Nrf2. Calcif Tissue Int. 2018;103:324–337. doi: 10.1007/s00223-018-0425-1. [DOI] [PubMed] [Google Scholar]
- 36.Shyh-Chang N., Daley G.Q., Cantley L.C. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu T., Dohl J., Elenberg F., Chen Y., Deuster P. Curcumin induces concentration-dependent alterations in mitochondrial function through ROS in C2C12 mouse myoblasts. J. Cell Physiol. 2019;234:6371–6381. doi: 10.1002/jcp.27370. [DOI] [PubMed] [Google Scholar]
- 38.Cockfield J.A., Schafer Z.T. Antioxidant defenses: A context-specific vulnerability of cancer cells. Cancers. 2019;11:1208. doi: 10.3390/cancers11081208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated and analyzed in this study are mentioned in this manuscript.