Abstract
The phylum Thermotogae is composed of a single class (Thermotogae), 4 orders (Thermotogales, Kosmotogales, Petrotogales, Mesoaciditogales), 5 families (Thermatogaceae, Fervidobacteriaceae, Kosmotogaceae, Petrotogaceae, Mesoaciditogaceae), and 13 genera. They have been isolated from extremely hot environments whose characteristics are reflected in the metabolic and phenotypic properties of the Thermotogae species. The metabolic versatility of Thermotogae members leads to a pool of high value-added products with application potentials in many industry fields. The low risk of contamination associated with their extreme culture conditions has made most species of the phylum attractive candidates in biotechnological processes. Almost all members of the phylum, especially those in the order Thermotogales, can produce bio-hydrogen from a variety of simple and complex sugars with yields close to the theoretical Thauer limit of 4 mol H2/mol consumed glucose. Acetate, lactate, and L-alanine are the major organic end products. Thermotagae fermentation processes are influenced by various factors, such as hydrogen partial pressure, agitation, gas sparging, culture/headspace ratio, inoculum, pH, temperature, nitrogen sources, sulfur sources, inorganic compounds, metal ions, etc. Optimization of these parameters will help to fully unleash the biotechnological potentials of Thermotogae and promote their applications in industry. This article gives an overview of how these operational parameters could impact Thermotogae fermentation in terms of sugar consumption, hydrogen yields, and organic acids production.
Keywords: anaerobic bacteria, hydrogen yields, fermentation rate, organic acids, nitrogen, carbon dioxide
1. Introduction
The phylum Thermotogae is comprised of thermophilic, hyperthermophilic, mesophilic, and thermo-acidophilic anaerobic bacteria that originated from geothermally heated environments (Table 1) [1,2]. Recent phylogenetic analyses based on gene markers/core genome inferences, comparative genomics, and whole-genome relatedness have led to a taxonomic revision of the phylum, with a single class (Thermotogae), 4 orders (Thermotogales, Kosmotogales, Petrotogales, Mesoaciditogales), 5 families (Thermatogaceae, Fervidobacteriaceae, Kosmotogaceae, Petrotogaceae, Mesoaciditogaceae), and 13 genera, i.e., Thermotoga (T.) [3], Pseudothermotoga (Pseudot.) [2,4], Fervidobacterium (F.) [5], Thermosipho (Ts.) [6], Kosmotoga (K.) [7], Mesotoga (Ms.) [8], Defluviitoga (D.) [9], Geotoga (G.) and Petrotoga (P.) [10], Marinitoga (Mn.) [11], Oceanotoga (O.) [12], Mesoaciditoga (M.) [13], and Athalassatoga (A.) (Table 1) [2,4,14]. Thermotogae are able to grow under mesophilic (Kosmotogales; Mesoaciditogales, Petrotogales) and thermophilic conditions (Thermotogales), but most species have optimal growth temperatures in the range of 45–80 °C (Table 1). They are Gram-negative bacteria, except for D. tunisiensis, which shows a positive result in Gram staining [9]. Apart from K. shengliensis, whose cells are in a coccoid form, Thermotogae cells are rod-shaped and encapsulated by a unique outer membrane, named “toga” [1,8,15]. Usually, the cells grow singly or in pairs, but it is also possible to observe chains surrounded by a unique toga [1,2]. Cell length is typically less than 20 µm, except for F. gondwanense and some members of the Petrotoga genus, whose cells can reach to 50 µm long (Table 1) [2,10]. Almost all species grow at neutral pH, and NaCl tolerances are high among Geotoga, Oceanotoga, and Petrotoga species (Table 1). Numerous studies have reported that members of the phylum can grow on both simple (e.g., glucose, galactose, fructose, lactose, maltose, mannose, sucrose) and complex carbohydrates (e.g., starch, glycogen, cellulose, keratin) (Table 1). Genes, transcriptional factors, and regulatory mechanisms driving the carbohydrates utilization have been identified for multiple members of the phylum [16,17,18]. ABC transporters for the uptake of a broad list of sugars have also been characterized [19,20,21,22,23].
Table 1.
Physiological and metabolic properties of Thermotogae species. YE: Yeast extract; BHI: Brain heart infusion; CMC: Carboxymethylcellulose; S0 = Elemental sulfur; Thio: Thiosulfate; Cys: Cysteine; AA: Acetic acid; LA: Lactic acid; ALA: Alanine; EPS: Exopolysaccharide; AABA: α-aminobutyrate; EtOH: Ethanol; AQDS: Anthraquinone-2,6-disulfonate; But: Butyrate; Val: Valerate; Glu: Glutamate; BuOH: Butanol; iBut: isobutyrate; iVal: isovalerate; PPA: Propionic Acid; Gly: Glycine; Pro: Proline; Fo: Formate; HPA: Hydroxyphenilacetate; PA: Phenylacetate; 3-IAA: Indole-3-acetate; 2-MeBu: 2-Methylbutyrate.
| Genus | Species | Isolation | Temp. Range/ Optimal (°C) |
pH Range/ Optimal |
Cell Dimension (Long by Wide) (µm) |
Growth Substrates | NaCl Range/ Optimal (%) |
Electron Acceptor |
End Products |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Thermotoga | Thermotoga petrophila | Oil reservoir, Japan | 47–88/ 80 |
5.2–9.0/ 7.0 |
2.0–7.0 by 0.7–1.0 |
YE, peptone, glucose, fructose, ribose, arabinose, sucrose, lactose, maltose, starch, cellulose |
0.1–5.5/ 1.0 |
S0; Thio |
AA, LA, CO2, H2 |
[72] |
| Thermotoga naphthophila | Oil reservoir, Japan | 48–86/ 80 |
5.4–9.0/ 7.0 |
2.0–7.0 by 0.8–1.2 |
YE, peptone, glucose, galactose, fructose, mannitol, ribose, arabinose, sucrose, lactose, maltose, starch |
0.1–6.0/ 1.0 |
S0; Thio |
AA, LA, CO2, H2 |
[72] | |
| Thermotoga maritima | Geotermal vent |
55–90/ 80 |
5.5–9.0/ 6.5 |
1.5–11.0 by 0.6 |
ribose, xylose, glucose, sucrose, maltose, lactose, galactose, starch, glycogen |
0.2–3.8/ 2.7 |
Fe (III) S0; Thio |
AA, LA, CO2, H2, ALA, EPS, AABA | [3] | |
| Thermotoga profunda | Hot spring, Japan |
50–72/ 60 |
6.0–8.6/ 7.4 |
0.8–2.1 by 0.4 |
glucose, trehalose, cellobiose, arabinose, xylose, ribose, pyruvate |
n. d | S0; Thio |
n. d | [73] | |
| Thermotoga caldifontis | Hot spring, Japan |
55–85/ 70 |
6.0–8.6/ 7.4 |
1.2–3.5 by 0.5 |
glucose, maltose, trehalose, cellobiose, arabinose, xylose, ribose, pyruvate, starch |
n. d | Thio | n. d | [73] | |
| Thermotoga neapolitana | Submarine thermal vent | 55–95/ 77 |
6.0–9.0/ 7.5 |
1.5–11.0 by 0.6 |
fructose, fucose, galactose, mannose, rhamnose, pyruvate, glucosamine, lactulose, turanose, glycerol, dextrin, ribose, xylose, glucose, sucrose, maltose, lactose, starch, glycogen | 0.2–6.0/ 2.0 |
S0 | AA, ALA, CO2, H2 | [74] | |
| Pseudothermotoga | Pseudothermotoga lettingae | Thermophilic bioreactor |
50–75/ 65 |
6.0–8.5/ 7.0 |
2.0–3.0 by 0.5–1.0 |
glucose, EtOH, acetate, formate | 0.0–2.8/ 1.0 |
S0; Thio; AQDS; Fe(III) | AA, ALA, LA, EtOH, AA, BA, CO2, H2 |
[75] |
| Pseudothermotoga elfii | Oil reservoir | 50–72/ 66 |
5.5–7.5/ 7.5 |
2.0–3.0 by 0.5–1.0 |
glucose, arabinose, fructose, lactose, maltose, mannose, ribose, sucrose, xylose |
0.0–2.8/ 1.0 |
Thio | AA, CO2, H2 | [76] | |
| Pseudothermotoga hypogea | Oil reservoir, Africa | 56–90/ 70 |
6.1–9.1/ 7.3–7.4 |
2.0–3.0 by 0.5–1.0 |
fructose, galactose, glucose, lactose, maltose, mannose, sucrose, xylose, xylan |
0.0–1.5/ 0.2 |
Thio | AA, ALA, CO2, H2, EtOH | [77] | |
| Pseudothermotoga | Pseudothermotoga subterranea | Oil reservoir, Paris | 50–75/ 70 |
6.0–8.5/ 7.0 |
3.0–10.0 by 0.5 |
YE, peptone, tryptone, casein | 0.0–2.4/ 1.2 |
Cys, Thio |
n.d. | [78] |
| Pseudothermotoga thermarum | Hot spring, Africa |
55–84/ 70 |
6.0–9.0/ 7.0 |
1.5–11.0 by 0.6 |
starch, glucose, maltose | 0.2–0.5/ 0.35 |
S0 | n.d. | [6] | |
| Fervidobacterium | Fervidobacterium nodosum | Hot spring, New Zealand |
40–80/ 65–70 |
6.0–8.0/ 7.0 |
1.0–2.5 by 0.5–0.55 |
glucose, sucrose, starch and lactose | n.d./<1.0 | S0 | AA, LA, CO2, H2, EtOH, But, Val | [5] |
| Fervidobacterium pennavorans | Hot spring, Portugal |
50–80/ 70 |
5.5–8.0/ 6.5 |
2.0–20.0 by 0.5 |
cellobiose, starch, glycogen, pullulan, glucose, fructose, maltose, xylose, native feathers |
0.0–4.0/ 0.4 |
S0; Thio |
AA, CO2, ALA, Glu, EtOH, But, H2, BuOH | [79] | |
| Fervidobacterium islandicum | Icelandic Hot spring |
50–80/ 65 |
6.0–8.0/ 7.2 |
1.0–4.0 by 0.6 |
pyruvate, ribose, glucose, maltose, raffinose, starch, cellulose |
0.0–1.0/ 0.2 |
S0; Thio |
LA, AA, H2, EtOH, CO2, iBut, iVal | [80] | |
| Fervidobacterium riparium | Hot spring, Russia | 46–80/ 65 |
5.7–7.9/ 7.8 |
1.0–3.0 by 0.4–0.5 |
peptone, YE, pyruvate, glucose, xylose, fructose, maltose, sucrose, cellobiose, starch, xylan, CMC, cellulose, filter paper |
0.0–1.0/ 0.0 |
S0 | H2, AA, CO2, PPA, iBut, But | [81] | |
| Fervidobacterium gondwanense | Hot spring, Australia | 45–80/ 65–68 |
5.5–8.5/ 7.0 |
4.0–40.0 by 0.5–0.6 |
cellobiose, amylopectin, maltose, starch, dextrin, xylose, glucose, pyruvate, lactose, fructose, mannose, CMC, galactose |
0.0–0.6/ 0.1 |
S0 | EtOH, AA, LA, CO2, H2 | [82] | |
| Fervidobacterium thailandese | Hot spring, Thailand | 60–88/ 78–80 |
6.5–8.5/ 7.5 |
1.1–2.5 by 0.5–0.6 |
glucose, maltose, sucrose, fructose, cellobiose, CMC, cellulose, starch |
<0.5/0.5 | S0 | n.d. | [83] | |
| Fervidobacterium changbaicum | Hot spring, China | 55–90/ 75–80 |
6.3–8.5/ 7.5 |
1.0–8.0 by 0.5–0.6 |
glucose, lactose, fructose, sucrose, maltose, starch, sorbitol, cellobiose, trehalose, galactose, melibiose, pyruvate, glycerin |
0.0–1.0/ 0.0 |
S0 | n.d. | [84] | |
| Thermosipho |
Thermosipho
africanus |
Hot spring, Africa |
53–77/ 75 |
6.0–8.0/ 7.2 |
3.0–4.0 by 0.5 |
glucose, ribose, maltose, starch, galactose, fructose, sucrose |
0.11–3.6 | S0; Thio |
AA, H2, CO2, EtOH, LA |
[85] |
|
Thermosipho
japonicus |
Hydrothermal vent, Japan | 45–80/ 72 |
5.3–9.3/ 7.2–7.6 |
3.0–4.0 by 0.5 |
YE, peptone, and tryptone, maltose, glucose, galactose, starch, sacharose, ribose, casein | 0.7–7.9/ 4.0 |
S0; Thio |
n.d. | [86] | |
|
Thermosipho
geolei |
Oil reservoir, Russia | 45–75/ 70 |
6.0–9.4/ 7.5 |
2.0–3.0 by 0.4–0.6 |
Glucose, peptone, beef extract, YE | 0.5–7.0/ 2.0–3.0 |
S0 | H2, AA, ALA, CO2, iVal | [87] | |
| Thermosipho |
Thermosipho
affectus |
Hydrothermal vent, Atlantic Ocean |
37–75/ 70 |
5.6–8.2/ 6.6 |
1.2–6.0 by 0.4–0.9 |
YE, beef extract, glucose, maltose, sucrose, starch, dextrin, CMC, cellulose | 1.0–5.5/ 2.0 |
S0 | AA, H2, CO2, EtOH |
[88] |
|
Thermosipho
globiformans |
Hydrothermal vent | 40–75/ 68 |
5.0–8.2/ 6.8 |
2.0–4.0 by 0.5 |
YE, tryptone, starch | 0.2–5.2/ 2.5 |
S0; Fe2O3 |
n.d. | [89] | |
|
Thermosipho
melanesiensis |
Hydrothermal vent, Pacific Ocean |
50–75/ 70 |
4.5–8.5/ 6.5–7.5 |
1.0–3.5 by 0.4–0.6 |
BHI, malt extract, tryptone, sucrose, starch, glucose, maltose, lactose, cellobiose, galactose | 1.0–6.0/ 3.0 |
S0 | H2, AA, ALA, CO2 |
[90] | |
|
Thermosipho
activus |
Riftia sheath, Guaymas Basin |
44–75/ 65 |
5.5–8.0/ 6.0 |
1.5–10.0 by 0.3–0.8 |
glucose, maltose, cellobiose, cellulose, filter paper, chitin, xylan, pectin, xanthan gum, YE, beef extract, tryptone, casein, keratin, arabinose, xylose, gelatin |
0.3–6.0/ 2.5 |
S0, Fe (III) |
AA, H2, CO2 |
[91] | |
|
Thermosipho
atlanticus |
Hydrothermal vent, Atlantic Ocean |
45–80/ 65 |
5.0–9.0/ 6.0 |
1.0–2.6 by 0.2–0.6 |
cellobiose, xylose, starch, LA, maltose, mannose, trehalose, lactose, arabinose, galactose, mannitol, peptone, casamino acids, gelatin, BHI, YE, glucose |
1.5–4.6/ 2.3 |
S0, Thio, Cys |
AA, iVal, H2, Gly, ALA, Pro | [92] | |
| Geotoga |
Geotoga
subterranea |
Oilfields, USA |
30–60/ 45 |
5.5–9.0/ 6.5 |
4.0– 7.5 by 0.5 |
mannose, starch, maltodextrins, glucose, lactose, sucrose, galactose, maltose |
0.5-10/ 4.0 |
S0 | H2, CO2, AA, EtOH |
[10] |
|
Geotoga
petraea |
Oilfields, USA |
30–55/ 50 |
5.5–9.0/ 6.5 |
3.0– 20.0 by 0.6 |
mannose, starch, maltodextrins, glucose, lactose, sucrose, galactose, maltose |
0.5–10/ 3.0 |
S0 | H2, CO2, AA, EtOH |
[10] | |
| Petrotoga |
Petrotoga
miotherma |
Oilfields, USA |
35–65/ 55 |
5.5–9.0/ 6.5 |
2.0– 7.5 by 0.6 |
mannose, starch, maltodextrins, glucose, lactose, sucrose, galactose, maltose, maltodexstrins, xylose |
0.5–10/ 2.0 |
S0 | H2, CO2, AA, EtOH |
[10] |
|
Petrotoga
olearia |
Oil reservoir, Russia | 37–60/ 55 |
6.5–8.5/ 7.5 |
0.9–2.5 by 0.3–0.6 |
arabinose, xylose, cellobiose, dextrin, sucrose, glucose, fructose, maltose, ribose, trehalose, xylan, pyruvate, peptone, starch |
0.5–8.0/ 2.0 |
S0 | H2, AA, LA, ALA, EtOH | [93] | |
|
Petrotoga
sibirica |
Oil reservoir, Russia | 37–55/ 55 |
6.5–9.4/ 8.0 |
0.9–2.5 by 0.3–0.6 |
sucrose, glucose, fructose, maltose, ribose, trehalose, xylan, pyruvate, peptone, galactose | 0.5–7.0/ 1.0 |
S0 | H2, AA, LA, ALA, EtOH | [93] | |
| Petrotoga |
Petrotoga
mobilis |
Oilfield, North Sea |
40–65/ 58–60 |
5.5–8.5/ 6.5–7.0 |
1.0–50.0 by 0.5–1.5 |
starch, xylan, maltodextrin, maltose, cellobiose, sucrose, lactose, glucose, galactose, fructose, arabinose, xylose, ribose, rhamnose |
0.5–9.0/ 3.0–4.0 |
S0, Thio |
H2, CO2, AA, EtOH |
[94] |
|
Petrotoga
halophila |
Offshore oil, Africa |
45–65/ 60 |
5.6–7.8/ 6.7–7.2 |
2.0–45.0 by 0.5–0.7 |
arabinose, cellobiose, fructose, galactose, glucose, lactose, maltose, rhamnose, ribose, starch, sucrose, xylose, xylan, pyruvate |
0.5–9.0/ 4.0–6.0 |
S0 | AA, LA, ALA, H2, CO2 |
[95] | |
|
Petrotoga
mexicana |
Offshore oil, Africa |
25–65/ 55 |
5.8–8.5/ 6.6 |
1.0–30.0 by 0.5–0.7 |
arabinose, cellobiose, fructose, galactose, glucose, lactose, maltose, mannose, raffinose, rhamnose, ribose, starch, sucrose, xylose, xylan, pyruvate. |
1.0–20.0/ 3.0 |
S0, Thio, Sulfite |
AA, LA, H2, CO2, ALA | [96] | |
|
Petrotoga
japonica |
Oil reservoir, Japan | 40–65/ 60 |
6.0–9.0/ 7.5 |
2.5–7.0 by 0.25–0.75 |
starch, xylan, maltose, cellobiose, sucrose, lactose, glucose, galactose, fructose, casamino acids, mannose, arabinose, xylose, ribose | 0.5–9.0/ 0.5–1.0 |
S0, Thio |
AA, H2, CO2, ALA |
[97] | |
| Marinitoga |
Marinitoga
piezophila |
Hydrothermal chimney, Pacific Ocean |
45–70/ 65 |
5.0–8.0/ 6.0 |
1.0–1.5 by 0.5 |
starch, fructose, glucose, galactose, maltose, cellobiose, ribose, acetate |
1.0–5.0/ 3.0 |
S0, Thio, Cys |
n.d. | [98] |
|
Marinitoga
litoralis |
Hot spring, Indian Ocean |
45–70/ 65 |
5.5–7.5/ 6.0 |
1.0–7.0 by 0.8–1.0 |
cellobiose, galactose, glucose, glycogen, lactose, maltose, ribose, starch, BHI, casamino acids, casein, peptone, pyruvate, tryptone, YE |
0.8–4.6/ 2.6 |
S0 | n.d. | [99] | |
|
Marinitoga
okinawensis |
Hydrothermal field, Okinawa | 30–70/ 55–60 |
5.5–7.4/ 5.5–5.8 |
1.5–5.0 by 0.5–0.8 |
YE, tryptone, peptone, starch, glucose, glycerol |
1.0–5.5/ 3.0–3.5 |
S0, Cys |
n.d. | [100] | |
|
Marinitoga
hydrogenitolerans |
Hydrothermal chimney, Atlantic Ocean |
35–65/ 60 |
4.5–8.5/ 6.0 |
1.5–5.0 by 0.5–0.8 |
glucose, starch, glycogen, chitin, YE, BHI, peptone, casein, pyruvate, maltose |
1.0–6.5/ 3.0–4.0 |
S0, Thio, Cys |
AA, EtOH, Fo, H2, CO2 | [101] | |
|
Marinitoga
artica |
Hydrothermal chimney, Norwegian |
45–70/ 65 |
5.0–7.5/ 5.5 |
1.0–5.0 by 0.5–0.8 |
glucose, trehalose, maltose, sucrose, maltodextrin, starch, pectin, meat extract, tryptone, YE, pyruvate, fructose, mannose, cellobiose, cellulose, peptone |
1.5–5.5/ 2.5 |
S0, Cys |
n.d. | [102] | |
|
Marinitoga
camini |
Hydrothermal chimney, Atlantic Ridge |
25–65/ 55 |
5.0–9.0/ 7.0 |
2.0–3.0 by 0.5–1.0 |
BHI, gluten, peptone, tryptone, pyruvate, glucose, fructose, maltose, cellobiose, sucrose, starch, cellulose, CMC, pectin, chitin |
1.0–4.5/ 2.0 |
S0, Cys |
AA, iBut, iVal, H2, 3-IAA, LA CO2, HPA, PA | [11] | |
| Oceanotoga |
Oceanotoga
teriensis |
Offshore oil, India |
25–70/ 55– 58 |
5.5–9.0/ 7.5 |
1.5–1.7 by 0.5–0.7 | glucose, fructose, cellobiose, arabinose, raffinose, rhamnose, sucrose, xylose, ribose, starch, EtOH, formate, acetate, BHI, YE, bio–trypticase |
0.0–12/ 4.3 |
S0, Thio |
AA, H2, CO2, EtOH |
[12] |
| Defluviitoga |
Defluviitog
tunisiensis |
Mesothermic digester | 37–65/ 55 |
6.7–7.9/ 6.9 |
3.0–30.0 by 1.0 |
arabinose, cellobiose, fructose, galactose, glucose, lactose, maltose, mannose, raffinose, ribose, sucrose, xylose, cellulose, xylan |
0.2–3.0/ 0.5 |
S0, Thio |
AA, H2, CO2 |
[9] |
| Mesotoga |
Mesotoga
infera |
Deep aquifer, France | 30–50/ 45 |
6.2–7.9/ 7.4 |
2.0–4.0 by 1.0–2.0 |
arabinose, cellobiose, fructose, galactose, glucose, lactose, LA, mannose, maltose, raffinose, ribose, sucrose, xylose |
0.0–1.5/ 0.2 |
S0 | AA, CO2 | [26] |
|
Mesotoga
prima |
Sediment, USA | 20–50/ 37 |
6.5–8.0/ 7.5 |
1.0 by 0.2 | xylose, fructose, ribose, sucrose, mannose, galactose, maltose, lactose, peptone, tryptone, casamino acids, glucose, arabinose, cellobiose, casein, pyruvate |
2.0–6.0/ 4.0 |
S0, Thio, Sulfite |
AA, But, iBut, iVal, 2–MeBu | [8] | |
| Kosmotoga |
Kosmotoga
arenicorallina |
Hot spring, Japan |
50–65/ 60 |
6.2–8.0/ 7.1 |
1.1–2.7 by 1.1–1.9 |
xylose, maltose, glycerol | 1.0–6.0/ 3.0 |
S0, Cys |
n.d. | [103] |
|
Kosmotoga
pacifica |
Hydrothermal field, Pacific Ocean | 33–78/ 70 |
6.2–8.0/ 7.1 |
1.0 by 0.6 | maltose, YE, peptone, BHI, glycerol, tryptone, xylose, glucose, fructose, cellobiose, trehalose, LA, propionate, glutamate |
0.5–6.0/ n.d. |
S0, Cys |
n.d. | [104] | |
|
Kosmotoga
olearia |
Fluid, North Sea |
20–80/ 65 |
5.5–8.0/ 6.8 |
0.8–1.2 by 0.4–0.7 |
maltose, ribose, sucrose, starch, casamino acids, tryptone, pyruvate |
1.0–6.0/ 2.5–3.0 |
Thio | H2, CO2, AA, EtOH, PPA | [7] | |
|
Kosmotoga
shengliensis |
Oilfield, China |
45–75/ 65 |
6.0–8.0/ 7.0 |
0.7–0.9 | glucose, acetate, mEtOH, galactose, fructose, xylose, sucrose, maltose, sorbitol, lactose, xylan, arabinose, formate, rhamnose, glycerol, pyruvate, starch, LA |
0.0–4.0/ 1.5 |
S0, Thio, Sulfate |
AA, LA, ALA, CO2, H2 |
[15] | |
| Athalassatoga |
Athalassatoga
saccharophila |
Hot spring, Japan |
30–60/ 55 |
4.5–7.5/ 5.5–6.0 |
0.8–2.0 by 0.7–0.8 |
arabinose, fructose, glucose, lactose, maltose, mannose, ribose, sucrose, xylose, starch, glycogen, peptone, YE |
<1/0.0 | Fe (III), Thio, Cys |
AA, iBut, iVal |
[14] |
| Mesoaciditoga |
Mesoaciditoga
lauensis |
Hydrothermal vent, Pacific Ocean |
45–65/ 57–60 |
4.1–6.0/ 5.5–5.7 |
0.8–1.0 by 0.4 |
YE, peptone, maltose, sucrose, glucose, xylose, ribose, starch, tryptone | 0.5–6.0/ 3.0 |
S0; Thio, Cys |
n.d. | [13] |
All species of the phylum, except for Mesotoga spp., have tremendous potentials inbiotechnological production of H2, especially the order Thermotogales, as their hydrogen yields are close to the theoretical maximum value (Thauer limit) of 4 mol H2/mol glucose [1,4,24]. Acetate, lactate, and L-alanine are the major organic products of the sugar fermentation [1]. Ms. prima and Ms. infera produce mainly/only acetate from sugar utilization without H2 formation [8,25,26,27]. Lactate is produced by T. maritima, T. neapolitana, and Mn. camini in variable quantities depending on growth conditions [11,28,29,30,31]. Other significant products include ethanol (has been measured in Geotoga, Petrotoga, Kosmotoga, and Oceanotoga spp.); isovalerate, isobutyrate, and/or propionate (have been measured in Mn. camini and K. olearia); L-glutamate, alpha-aminobutyrate, hydroxyphenyl-acetate, or phenylacetate (have been measured in F. pennavorans) [1,32] (Table 1). Among these fermentation end-products, lactic acid has been widely used in various industries such as food, cosmetic, pharmaceutical, and chemical industries, although its primary application is serving as the building block for the production of biodegradable polylactic acid (PLA) [33]. Ethanol is an important industrial commodity; it is used as a food additive and a renewable biofuel; it is also contained in many cosmetics, households, and sanitizer products [34]. Moreover, a plethora of thermostable enzymes, harbored by most of these bacteria, are valuable components for many industrial and biotechnological applications [17,35,36,37,38,39,40,41,42,43,44].
Hydrogen (H2) is considered a green and sustainable alternative to traditional fossil fuels and is capable of mitigating greenhouse gas emissions. Using hydrogen in fuel cells or combustion engines produces heat and electricity with water as the only waste. As the current abiotic hydrogen production method is energy-consuming and still causes pollution, emphasis must be given to biological production of the energy from renewable sources [45,46]. Biological synthesis of H2 can use a wide range of organic substrates as feedstocks, including agro-industrial wastes and algal biomass, and may operate under various environmental conditions [1,46,47,48,49,50,51,52,53,54]. In addition, high temperatures help to improve the solubilization of substrates, reduce fermentation time, and lower contamination risks [55]. Although hydrogen production by Thermotoga species is considered one of the most challenging biological systems, no application using pure Thermotoga cultures has been reported at the industrial scale.
Releasing hydrogen is an efficient way to dissipate excessive reductants generated during the fermentative conversion of organic substrates. The process is generally referred to as dark fermentation (DF) and is typically influenced by environmental conditions such as pH, cell growth rate, and hydrogen partial pressure [24,56,57].
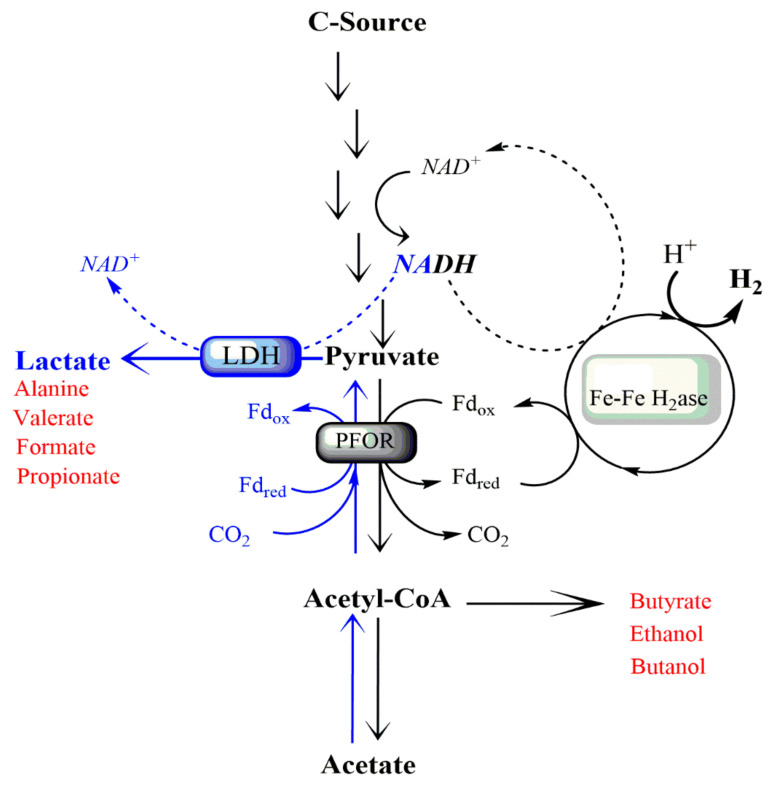
According to the classical model of dark fermentation, theoretically up to 4 mol of hydrogen may be produced from each mole of glucose, which is converted to acetate and CO2 (Thaeur limit Figure 1) [24]. When hydrogen accumulates, pyruvate is diverted away from acetate production. In this case, excessive NADH from glycolysis is not used in the energetically favorable manner to synthesize acetate and H2 but dissipated via synthesizing other metabolic products such as lactic acid, L-alanine, ethanol, butyrate, and valerate (Figure 1) [24]. Synthesis of hydrogen in Thermotogae species is performed by the heterotrimeric [FeFe]-hydrogenase, an electron-bifurcating enzyme that couples the endergonic reduction of H+ to hydrogen by NADH to the exergonic reduction of H+ to hydrogen by reduced ferredoxin (Figure 1) [58]. Because the hydrogenase uses both NADH and reduced ferredoxin as electron donors, hydrogen yield is influenced by factors that affect both reductants.
Figure 1.
Schematic representation of Thermotogae metabolic fermentation. Dark fermentation (black arrows) of glucose leads to the production of H2 and acetate. An increase in CO2 concentration in the reactor headspace induces the recycling of Ac-CoA and CO2 into lactate without impairing the synthesis of biogas (blue arrows). This process is named “Capnophilic lactic fermentation (CLF)” [30,31,56,70]. The main end-products of Thermotogae fermentation are H2, lactate, and acetate. Other fermentation products are reported in red. Fe-Fe H2ase = [Fe-Fe] hydrogenase; PFOR = Pyruvate ferredoxin oxidoreductase; LDH = Lactate dehydrogenase; Fd = Ferredoxin.
The value of these bacteria in biotechnological processes is rising sharply since the discovery of the bifurcating hydrogenase and will probably be enhanced with a full elucidation of the molecular and biochemical properties of the processes. Despite decades of efforts in the development of genetic tools to engineer these species, only a few of thermostable selectable markers and genetic modifications with low stability are reported, which makes it still difficult to perform genetic modifications of these organisms [59,60,61]. However, these difficulties could be offset by their well-known susceptibility to mutations under environmental pressures [62,63].
In recent years, many researchers have been focusing on the optimization of fermentation performance towards the production of hydrogen and other target end-products [30,43,64,65,66,67,68,69,70,71].
Anaerobic fermentation in Thermotogae depends on many cultivation parameters such as hydrogen partial pressure, agitation, gas sparging, culture/headspace ratio, inoculum, pH, temperature, nitrogen sources, sulfur sources, inorganic compounds, and metal ions. The effect of each factor on H2 yield, sugar consumption rate, and formation of biotechnologically interesting end-products are discussed here. Main data are also summarized in extensive tables, citing the most important studies, with the information on their cultivation systems (e.g., reactor type, incubation periods, batch vs. continuous modality).
2. Operating Conditions
2.1. H2 Partial Pressure (PH2)
Since Thermotogae members are hydrogen producers, tolerance to hydrogen produced by the bacteria on its own gaseous production, known as the “hydrogen partial pressure (PH2)” effect, is one of the primary parameters being extensively investigated [51,70,105]. The highest hydrogen tolerance has been observed in the genus Marinitoga. Mn. camini and Mn. piezophila were able to grow with H2 concentrations up to 40% and 60%, respectively. Mn. hydrogenitolerans and Mn. okinawensis can grow under 100% H2 atmosphere with only minor inhibition on growth and fermentation [100,101]. Their remarkable resistance to high H2 levels is probably related to the typical habitats in which Marinotoga species thrive [100]. However, the growth of Thermotogae species is often inhibited by H2 accumulation, and the metabolism of these organisms undergoes a series of rearrangements to suit PH2 levels in the bioreactor headspace. The majority of literature data refers to H2 percentages in gaseous phase, although some studies have been reporting values of PH2. Partial pressure around 607 mbar led to decreased levels of biomass production, glucose consumption rate, and H2 production in both T. neapolitana and T. maritima [106,107]. Boileau et al. [107] highlighted a shift of T. maritima glucose catabolism from acetic acid towards lactic acid when PH2 increased from 7 to 607 mbar (Table 2) [106,107]. In contrast, low PH2 (less than 80 mbar) promoted acetic acid accumulation. Biomass production and glucose consumption rate are unaffected when PH2 is maintained within the range of 7.1–178.5 mbar (Table 2) [105,106]. In fact, PH2 lower than 200 mbar is required for optimal growth in reactors, and PH2 around 2900 mbar completely inhibits growth in T. maritima [1,45,49,108,109].
Table 2.
Effects of operating conditions on Thermotogae fermentation. MOPS: Morpholinopropane-1-sulfonic acid; HEPES: 2-[4-(2-hydroxyethyl) piperazin-1-yl] ethanesulfonic acid; TRIS: tris(idrossimetil)amminometano cloridrato; CDW: Cellular dry weight; AA: Acetic acid; LA: Lactic acid; ALA: Alanine; But: Butyrate; IA: Itaconic acid; GaR: recirculation of H2-rich biogas. Experiments were performed in different bioreactor configurations: B = Batch; CSTR = Continuous-flow Stirred-Tank Reactor; CSABR: Continuously Stirred Anaerobic Bioreactor; SB = Serum bottles. H2 column: a H2 yield = mol H2/mol consumed substrate; b mL/L culture. * Values extrapolated from the graphical representation of data.
| Parameter | Organism | T (°C) | Culture Type | Mixing Speed (rpm) | Reactor/Working Volume (L) | Substrate Loaded (mmol/L) | Operational Parameter |
Substrate Consumed (mmol/L) | Products | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 yielda |
AA (mmol/L) | LA (mmol/L) | ALA (mmol/L) | But (mmol/L) | ||||||||||
| PH2 (mbar) |
T.
maritima |
80 | B | 350 | 1.4/0.1 | Glucose (28) | PH2 = 7.1 ± 0.4 | 19.8 ± 1.1 | 2.34 | 25.0 ± 1.4 | 10.5 ± 0.5 | [107] | ||
| PH2 = 71.4 ± 2.1 | 19.7 ± 1.4 | 2.44 | 24.6 ± 2.4 | 11.0 ± 0.6 | ||||||||||
| PH2 = 178.5 ± 3.5 | 17.2 ± 0.9 | 2.32 | 20.1 ± 1.0 | 9.4 ± 0.5 | ||||||||||
| PH2 = 606.9 ± 18.7 | 13.4 ± 0.7 | n. d. | 13.0 ± 0.7 | 11.0 ± 0.6 | ||||||||||
| Stirring Speed (rpm) |
T.
neapolitana |
75 | CSABR | 300 | 3.0/1.0 | Xylose (33.3) | 300 | 31.43 | 2.13 ± 0.11 | 41.8 ± 2.16 | 1.78 ± 0.11 | [113] | ||
| 400 | 400 | 32.56 | 2.94 ± 0.15 | 50.12 ± 2.5 | 4.0 ± 0.22 | |||||||||
| 500 | 500 | 32.03 | 2.31 ± 0.12 | 44.62 ± 2.16 | 4.84 ± 0.22 | |||||||||
| 600 | 600 | 31.87 | 2.24 ± 0.11 | 41.12 ± 2.0 | 1.89 ± 0.11 | |||||||||
|
T.
neapolitana subsp. capnolactica |
80 | CSTR | 300 | 3.0/2.0 | Glucose (28) | 300 | 22.9 ± 2.7 | 3.0 ± 0.0 | 32.3 ± 4.3 | 10.0 ± 1.0 | 1.1 ± 0.1 | [69] | ||
| 500 | 500 | 24.8 ± 0.4 | 3.2 ± 0.1 | 37.7 ± 2.7 | 8.1 ± 0.2 | 1.0 ± 0.1 | ||||||||
| 300 | 300 + GaR | 24.7 ± 0.2 | 3.5 ± 0.2 | 39.2 ± 1.2 | 4.4 ± 0.1 | 0.9 ± 0.0 | ||||||||
| 500 | 500 + GaR | 24.9 ± 0.2 | 3.3 ± 0.1 | 38.7 ± 2.2 | 5.1 ± 0.5 | 0.8 ± 0.0 | ||||||||
| Gas sparging |
T.
neapolitana |
80 | B | 250 | 3.8/1.0 | Glucose (28) | N2 | 25.9 ± 1.3 | 2.8 | 44.8 ± 5.4 | 12.5 ± 2.9 | 1.3 ± 0.4 | [31] | |
| CO2 | 26.1 ± 1.2 | 2.8 | 35.6 ± 5.8 | 20.0 ± 6.1 | 2.7 ± 0.5 | |||||||||
| 75 | SB | no | 0.12/0.04 | Glycerol (108.6) | w/o | 13 ±0.6 | 1.24 ± 0.06 | 8.71 ± 0.35 | 0.36 ± 0.02 | [115] | ||||
| N2 | 14 ± 0.7 | 2.06 ± 0.09 | 10.04 ± 0.5 | 0.34 ± 0.02 | ||||||||||
| N2 plus pH control |
18 ± 0.9 | 1.98 ± 0.1 | 12.62 ± 0.53 | 0.25 ± 0.01 | ||||||||||
|
Gas
sparging |
T.
neapolitana |
77 | SB | 150 | 0.12/0.04 | Glucose (39) | w/o | - | 1.82 ± 0.09 | 64.28 ± 2.83 | 33.48 ± 1.47 | [110] | ||
| N2 | - | 3.24 ± 0.14 | 81.42 ± 3.49 | 36.77 ± 2.04 | ||||||||||
| Xylose (27) | w/o | - | 1.14 ± 0.07 | 40.30 ± 3.5 | 37.68 ± 1.7 | |||||||||
| N2 | - | 2.20 ± 0.13 | 71.94 ± 3.66 | 50.62 ± 2.38 | ||||||||||
|
T.
neapolitana subsp. capnolactica |
80 | SB | no | 0.12/0.03 | Glucose (28) | N2 | 25.7 ± 0.1 | 2.5 ± 0.06 | 27.3 ± 0.8 | 8.6 ± 0.2 | 2.5 ± 0.2 | [70] | ||
| CO2 | 28.3 ± 1.0 | 2.9 ± 0.1 | 22.1 ± 0.9 | 11.3 ± 0.1 | 3.0 ± 0.3 | |||||||||
|
T.
neapolitana |
80 | SB | no | 0.12/0.03 | Glucose (28) | N2 | 21.7 ± 0.6 | 2.5 ± 0.03 | 30.2 ± 0.4 | 2.2 ± 0.02 | 1.9 ± 0.3 | |||
| CO2 | 20.8 ± 2.3 | 1.9 ± 0.1 | 20.8 ± 0.1 | 1.2 ± 0.06 | 2.4 ± 0.3 | |||||||||
|
T.
maritima |
80 | SB | no | 0.12/0.03 | Glucose (28) | N2 | 23.2 ± 1.0 | 1.9± 0.06 | 25.5 ± 0.5 | 5.3 ± 0.8 | 2.4 ± 0.06 | |||
| CO2 | 19.9 ± 0.6 | 2.0 ± 0.1 | 18.3 ± 0.3 | 1.6 ± 0.2 | 2.3 ± 0.3 | |||||||||
|
T.
naphtophila |
80 | SB | no | 0.12/0.04 | Glucose (28) | N2 | 13.30 ± 1.10 | 2.20 ± 0.20 | 15.70 ± 0.10 | 1.40 ± 0.06 | 0.80 ±0.10 | |||
| CO2 | 20.80 ± 1.70 | 1.60 ± 0.20 | 19.20 ± 0.10 | 5.00 ± 0.02 | 1.80 ±0.05 | |||||||||
|
T.
petrophila |
80 | SB | no | 0.12/0.05 | Glucose (28) | N2 | 9.20 ± 1.30 | 3.00 ± 0.40 | 13.10 ± 0.05 | 2.00 ± 0.01 | 0.00 | |||
| CO2 | 14.20 ± 0.60 | 1.90 ± 0.10 | 12.60 ± 0.10 | 3.80 ± 0.02 | 0.30 ±0.10 | |||||||||
|
T.
caldifontis |
70 | SB | no | 0.12/0.05 | Glucose (28) | N2 | 10.90 ± 1.10 | 2.60 ± 0.10 | 16.70 ± 3.60 | 2.20 ± 0.50 | 3.20 ±0.90 | |||
| CO2 | 15.20 ± 0.90 | 1.80 ± 0.03 | 15.60 ± 1.50 | 2.30 ± 0.40 | 6.60 ±0.70 | |||||||||
|
T.
profunda |
60 | SB | no | 0.12/0.05 | Glucose (28) | N2 | 18.1 0 ±0.40 | 1.50 ± 0.20 | 15.90 ± 0.40 | 5.70 ± 0.10 | 1.40 ±0.06 | |||
| CO2 | 22.60 ± 1.70 | 0.70 ± 0.04 | 5.60 ± 0.20 | 2.3 ± 0.04 | 2.60 ±0.30 | |||||||||
| Pseudot. hypogea | 70 | SB | no | 0.12/0.05 | Glucose (28) | N2 | 8.80 ± 1.10 | 1.10 ± 0.30 | 6.40 ± 0.10 | 0.10 ± 0.00 | 2.90 ±0.10 | |||
| CO2 | 4.30 ± 0.10 | 0.50 ± 0.10 | 3.10 ± 0.20 | 0.10 ± 0.00 | 3.40 ±0.30 | |||||||||
| Pseudot. elfii | 70 | SB | no | 0.12/0.05 | Glucose (28) | N2 | 7.00 ± 0.90 | 2.00 ± 0.20 | 8.30 ± 0.06 | 0.20 ± 0.03 | 4.20 ±0.30 | [70] | ||
| CO2 | 6.70 ± 0.20 | 2.10 ± 0.10 | 7.80 ± 0.30 | 0.10 ± 0.01 | 10.0 ±0.30 | |||||||||
| Pseudot. lettingae | 70 | SB | no | 0.12/0.05 | Glucose (28) | N2 | 9.30 ± 0.50 | 1.20 ± 0.10 | 5.10 ± 0.05 | 0.20 ± 0.00 | 2.70 ±0.05 | |||
| CO2 | 8.10 ± 0.70 | 1.30 ± 0.30 | 4.40 ± 0.10 | 0.05 ± 0.01 | 3.70 ±0.20 | |||||||||
|
Gas
sparging |
Pseudot. subterranea | 70 | SB | no | 0.12/0.05 | Glucose (28) | N2 | 23.10 ± 2.10 | 1.80 ± 0.20 | 30.60 ± 6.90 | 16.20 ± 4.60 | 9.50 ±0.40 | ||
| CO2 | 27.00 ± 1.40 | 1.40 ± 0.10 | 31.90 ± 7.90 | 10.70 ± 4.0 | 20.0 ± 8.0 | |||||||||
| Pseudot. thermarum | 80 | SB | no | 0.12/0.05 | Glucose (28) | N2 | Complete | 1.8 ± 0.02 | 30.00 ± 2.20 | 6.50 ± 0.20 | 1.10 ±0.07 | |||
| CO2 | Complete | 1.50 ± 0.10 | 24.80 ± 0.70 | 5.60 ± 0.60 | 2.20 ±0.20 | |||||||||
|
Biomass
(g CDW/L) |
T.
neapolitana |
80 | Flask | 300 | 0.25/0.2 | Glucose (28) | 0.46 | 3.2 ± 0.04 | 2.39 | 34.3 ± 0.6 | 10.9 ± 0.4 | [68] | ||
| 0.91 | 2.9 ± 0.06 | 2.44 | 32.9 ± 0.8 | 12.2 ± 0.8 | ||||||||||
| 1.33 | 3.4 ± 0.01 | 2.58 | 32.3 ± 0.2 | 11.5 ± 0.5 | ||||||||||
| 1.74 | 3.0 ± 0.04 | 2.37 | 31.4 ± 1.1 | 14.7 ± 0.7 | ||||||||||
| pH |
T.
neapolitana subsp. capnolactica |
80 | SB | no | 0.12/0.03 | Glucose (28) | w/o | 18.54 ± 0.15 | 1.78 ± 0.29 | 22.76 ± 0.40 | 11.35 ± 0.62 | [67] | ||
| 0.01M MOPS | 26.42 ± 0.05 | 3.27 ± 0.18 | 26.65 ± 0.87 | 14.23 ± 0.22 | ||||||||||
| 0.01M TRIS | 25.55 ± 0.06 | 3.10 ± 0.10 | 26.77 ± 0.29 | 12.08 ± 0.89 | ||||||||||
| 0.01M HEPES | 25.99 ± 0.03 | 2.85 ± 0.40 | 25.56 ± 0.49 | 13.58 ± 0.88 | ||||||||||
| 0.01M HCO3− | 25.62 ± 0.10 | 2.20 ± 0.30 | 22.82 ± 0.84 | 14.63 ± 3.23 | ||||||||||
| 0.01M phosphate | 26.17 ± 0.26 | 2.78 ± 0.40 | 24.70 ± 0.59 | 14.92 ± 0.25 | ||||||||||
|
T.
neapolitana |
75 | CSABR | 300 | 3.0/1.0 | Glucose (28) | w/o pH control | 21.98 ± 1.11 | 2.05 ± 0.1 | 30.81 ± 1.5 | 3.33 ± 0.22 | [113] | |||
| plus pH control | 27.47 ± 1.39 | 3.2 ± 0.16 | 38.3 ± 2.0 | 1.77 ± 0.11 | ||||||||||
| Xylose (33.3) | w/o pH control | 29.77 ± 1.46 | 1.84 ± 0.09 | 34.47 ± 1.66 | 3.77 ± 0.22 | |||||||||
| plus pH control | 31.83 ± 1.6 | 2.22 ± 0.11 | 41.8 ± 2.0 | 1.66 ± 0.11 | ||||||||||
| pH |
T.
neapolitana |
75 | CSABR | 300 | 3.0/1.0 | Sucrose (14.6) | w/o pH control | 13.78 ± 0.7 | 3.52 ± 0.18 | 33.13 ± 1.65 | 3.11 ± 0.11 | [113] | ||
| plus pH control | 14.69 ± 0.06 | 4.95 ± 0.25 | 35.47 ± 1.83 | 2.11 ± 0.11 | ||||||||||
| Xylose (33.3) | w/o pH control | 29.44 | 1.85 ± 0.09 | 34.97 ± 1.66 | 3.88 ±0.22 | |||||||||
| pH = 6.5 | 32.57 | 2.71 ± 0.14 | 49.62 ± 2.50 | 3.44 ± 0.11 | ||||||||||
| pH = 7.0 | 32.9 | 2.84 ±0.14 | 50.29 ± 2.50 | 4.00 ± 0.22 | ||||||||||
| pH = 7.5 | 31.77 | 2.23 ± 0.11 | 41.96 ± 2.16 | 1.89 ± 0.11 | ||||||||||
| 75 | SB | no | 0.04/ 0.12 | Glycerol (108.6) | w/o HEPES | 16.96 ± 0.8 | 1.23 ± 0.06 | 9.14 ± 0.45 | [116] | |||||
| 0.05 M HEPES | 28.26 ± 1.4 | 2.73 ± 0.14 | 22.35 ± 1.05 | |||||||||||
|
T.
neapolitana |
80 | B | 250 | 3.8/1.0 | Glucose (28) | w/o NaHCO3 | 25.9 ± 1.3 | 2.8 | 44.5 ± 5.4 | 12.5 ± 2.69 | [31] | |||
| NaHCO3 14 mM | 25.4 ± 2.1 | 1.7 | 30.5 ± 4.9 | 18.0 ± 0.6 | ||||||||||
| NaHCO3 20 mM | 23.2 ± 1.9 | 1.0 | 44.4 ± 8.2 | 9.2 ± 2.7 | ||||||||||
| pH | NaHCO3 40 mM | 6.2 ± 0.8 | 2.7 | 18.0 ± 4.3 | 0.7 ± 1.5 | |||||||||
| 75 | B | no | 0.12/0.04 | Glycerol (108.6) | w/o IA | - | 438 ± 22 b | 7.49 ± 0.33 | 3.55 ± 0.22 * | [122] | ||||
| 1.5 g/L IA | - | 619 ± 30 b | 11.49 ± 0.5 | 1.66 ± 0.0 * | ||||||||||
|
Temp.
(°C) |
T.
neapolitana |
60 | SB | 75 | 0.26/0.05 | Glucose (14) | 60 | 2.2 * | 2.04 ± 0.05 | 2.0 | n. d | [65] | ||
| 65 | 65 | 5.0 * | 3.09 ± 0.3 | 7.0 | 0.05 | |||||||||
| 70 | 70 | 8.5 * | 3.18 ± 0.02 | 11.5 | 0.45 | |||||||||
| 77 | 77 | 11.0 ± 0.5 * | 3.85 ± 0.28 | 16.5 | 0.85 ± 0.1 | |||||||||
| 85 | 85 | 11.0 ± 0.5 * | 3.75 ± 0.49 | 18.0 ± 1.0 | 1.25 ± 0.05 | |||||||||
| Oxygen |
T.
maritima |
80 | B | 150 | 2.30/1.53 | Glucose (20) | w/o O2 | 17.41 | 38.09 b | 18.05 | 4.36 | 1.60 ± 0.2 | [129] | |
| with O2 | 19.30 | 31.75 b | 18.27 | 5.45 | 1.30 ± 0.2 | |||||||||
Hydrogen evolution is driven by a bifurcating hydrogenase (H2ase) that couples the oxidation of reduced ferredoxin (Fd) and NADH with the reduction of protons to H2 (Figure 1) [58]. In dark fermentation, pyruvate is converted to acetate and ATP, which thermodynamically drives the H2-acetate pathway. Under high H2 partial pressure, hydrogenase activity is inhibited, NADH consumption stops, pyruvate is diverted away from acetic acid production, and lactic acid synthesis becomes the only mechanism for recycling reduced electron carriers (Figure 1) [28,29,30,57,64,106,110]. Synthesis of lactic acid by the lactate dehydrogenase (LDH) catalyzes the conversion of pyruvate to lactate with the concomitant conversion of NADH to NAD+ (Figure 1). The depletion of the pyruvate pool, as occurs with the synthesis of lactic acid, negatively affects hydrogen yield, preventing it from reaching the theoretical maximal value (Figure 1) [24]. This problem can be overcome by enhancing the liquid-to-gas mass transfer and keeping H2 concentrations low in experimental conditions (See Section 2.2) or by using mixed cultures with microbial species that are able to oxidize H2 [27,111].
2.2. Shaking Speed, Culture/Headspace Volume Ratio, Gas Sparging, and Inoculum
Growth and metabolism of thermophilic bacteria are reported to be strongly affected by an increase in the hydrogen level, which makes the metabolic reactions thermodynamically unfavorable [112]. Many effective strategies have been developed to overcome the H2 feedback inhibition, such as gas sparging, vigorous stirring, or simply increasing the gas/liquid volume ratio in the reactor. H2 saturation is dependent on the partial pressure of hydrogen in the culture medium and its mass transfer from liquid to gas phase. As a matter of fact, the mass transfer of H2 from liquid to gas can be improved by applying vigorous agitation in bioreactors [69,106]. Increased H2 production rate, glucose consumption rate, and lactic acid synthesis have been observed in T. neapolitana cultures with agitation at 200 rpm, compared to static cultures, although the final H2 yields were similar [106]. Comparable hydrogen yields were also observed when the agitation speed was 300 and 500 rpm, e.g., 3.0 ± 0.0 mol H2/moL glucose at 300 rpm vs. 3.2 ± 0.1 moL H2/moL glucose at 500 rpm, with a mild improvement in fermentation rate (Table 2) [69]. In xylose fermentation, the highest hydrogen and organic acid yields have been reported at 400 rpm when tested in the range of 300–600 rpm [113].
To improve hydrogen liquid-gas mass transfer, Dreschke et al. [69] designed a new method that recirculated the H2-rich biogas (GaR) into the T. neapolitana subs. capnolactica broth with agitation (300, 500 rpm). This combination accelerated the H2 evolution rate and glucose consumption rate during glucose fermentation, compared to the treatments including agitation but excluding GaR. Nonetheless, levels of the end-products, except for H2 yield, were not significantly altered by the combined parameters (Table 2) [69].
Since PH2 depends on the culture/headspace volume ratio in the bioreactors, its impacts on the performance of fermentation have also been investigated, mainly in batch reactors. Nguyen et al. [64] have experimented various culture/headspace volume ratio from 8.3% (10 mL/120 mL) up to 50% (60 mL/120 mL) in T. neapolitana and T. maritima cultures [64]. At 8.3%, the H2 production is the highest for both species (890 mL H2/L medium in T. neapolitana and 883 mL H2/L medium in T. maritima). H2 production gradually diminished, and lactic acid production was promoted with increasing culture volumes [30,64,110]. d’Ippolito et al. [30] found 1:3 culture/headspace volume was the most suitable ratio for high hydrogen yields [30]. When these conditions were optimized, T. neapolitana resulted in H2 yields between 3.46–3.85 mol H2/mol glucose [30,114].
Gas sparging, mainly with N2, is the most common method to reduce hydrogen partial pressure by removing H2 and CO2 produced from sugar fermentation in closed bioreactors [56,108,115,116]. Under nitrogen sparging conditions, the overall yield of H2 in T. neapolitana fermentation was about two-fold of the non-sparged cultures, e.g., 1.82 vs. 3.24 moL H2/moL glucose or 1.14 vs. 2.20 moL H2/moL xylose (Table 2). The levels of acetic acid and butyrate also increased [110]. Moreover, the fermentation performance was remarkably improved when N2- sparging was coupled with pH control in T. neapolitana using pure glycerol as the sole carbon source (Table 2) [116]. Keeping pH close to neutral improved the glucose utilization and H2-acetate production rates. In contrast, lactic acid production was lowered under these conditions (0.255 mmol/L with pH control and sparging vs. 0.36 mmol/L with pH control but no sparging) (Table 2) [116]. The use of a CO2-enriched atmosphere significantly increased both glucose consumption rate and hydrogen production rate, even though the molar yield was comparable to that of N2−sparging (Table 2) [31]. Surprisingly, supplementation of CO2 to T. neapolitana cultures induced an unexpected metabolic shift from acetic to lactic fermentation without any significant change in hydrogen production (3.6 moL/moL glucose) (Table 2) [31]. Experiments with labeled precursors revealed that part of the exogenous CO2 was biologically coupled with acetyl-CoA to give lactic acid when the cultures were sparged with CO2 gas or enriched in sodium bicarbonate (Figure 1) [117]. This process, named Capnophilic Lactic Fermentation (CLF), has the surprising feature to produce more lactic acid than expected from the classical dark fermentation model where H2 production is impaired by the onset of by-passing pathways (Figure 1) [31,56,117,118,119]. In dark fermentation, hydrogen and lactic acid levels competed for a common pool of reducing power. Whereas, in CLF, the H2 level remained high, probably due to additional sources of reductants to sustain NADH-dependent pathways (Figure 1) [118,119,120]. Recently, an additional increase in lactic acid production occurred in a T. neapolitana mutant that was isolated from a culture adapted to continuous exposure to CO2 [62]. Sparging with CO2 was also performed on the culture of other Thermotogales species, whose metabolic response was qualitatively and quantitatively diverse (Table 2) [70]. CO2-enriched conditions promoted glucose consumption rate and lowered biogas production in almost all tested species [70]. T. caldifontis, Pseudot. elfii, Pseudot. thermarum, Pseudot. lettingae, and Pseudot. subterranea did not show substantial variations in the levels of the fermentation products compared to cultures in an N2-enriched atmosphere [70]. T. neapolitana, T. maritima, T. profunda, and Pseudot. hypogea species responded to CO2 by reducing the fermentation rate. T. neapolitana subsp. capnolactica was the only species to increase lactic acid and H2 yield moving from N2-sparging to CO2-sparging [70]. Generally speaking, the supplementation of external gas (N2 or CO2) successfully improves the fermentation performance in most species and lowers the inhibitory effect of H2 accumulation, but it inevitably causes an undesired dilution of hydrogen in evolved gases. In this context, the recirculation of the H2-rich biogas method prevents hydrogen saturation in the bioreactor without negatively affecting the content of the produced biogas [69].
The initial biomass concentration (size of inoculum) also has an unexpected impact on the fermentation of thermophilic bacteria. Using various initial biomass concentrations of T. neapolitana subs. capnolactica (in the range of 0.46–1.74 g CDW/L) under CO2 atmosphere, hydrogen yield and the distribution of end-products were unaffected (Table 2) [68]. However, increasing inoculum size from 0.46 to 1.74 g/L reduced the fermentation time from 7 h to 3 h [68]. Moreover, the hydrogen production rate, glucose consumption rate, and biomass growth rate were increased [49,50,68]. It is worth pointing out that Ngo et al. [116] reported a reverse correlation between hydrogen production rate and inoculum size, stating that high initial biomass corresponded to a mild reduction of hydrogen production rate [116].
2.3. pH
As the fermentation of sugars leads to the production and accumulation of organic acids, the pH is decreasing during the process, which may inhibit bacterial growth before the substrates are completely consumed [30,106,113]. Two factors impose a strong inhibition on bacterial growth and H2 production: rapid decrease in pH due to the accumulation of byproducts and feedback inhibition caused by H2 accumulated in the headspace [65,105,106,107,108,113,121].
Thus, pH is a critical factor to control sugar consumption and direct end-products formation [65,67,117,119,122]. Gradual pH drop causes enzyme activity loss [123]. To overcome pH-induced limitations on Thermotogae fermentation, several studies were performed with pH adjustments [51,67,121]. In pH-controlled cultures (~6.5–7.0), H2 and acetic acid production predominated over lactic acid and peaked around 20 h [113]. In contrast, lactic acid production only started when pH declined to around 5.0 [113].
The addition of NaOH at regular intervals and the use of buffering reagents have been regarded as the best-performing methods with serum bottles [56,66,67,113]. The optimum pH for growth and hydrogen production is 6.5–7.0 in T. maritima and 6.5–7.5 in T. neapolitana depending on substrates and growth conditions [64,113,122]. Moreover, pH 7.0 provides the most promising results in terms of H2 and organic acids production in T. neapolitana [113,122]. A pH shift from 5.5 to 7.0 improved H2 yield from 125 to 198 mL H2/L medium in T. neapolitana [61]. With T. neapolitana cells immobilized on ceramic surfaces using glucose as the carbon source, the highest hydrogen production was observed in the pH range of 7.7–8.5 [51]. Further increase in the range of pH to 8.0–9.0 led to a dramatic decrease in the biogas evolution [64].
Different organic and inorganic buffers have been examined for their effect on anaerobic fermentation under various growth conditions and buffer concentrations [51]. According to Cappelletti et al. [51], 0.1 M HEPES resulted in the best performance, compared to MOPS, PIPES, HPO4−/H2PO4−, or Tris-HCl buffer in T. neapolitana batch cultures growing on glucose under N2 atmosphere [51]. The good buffering properties of HEPES, whose pK (7.55) is near the optimal pH of T. neapolitana, was also demonstrated for T. neapolitana cultures growing on different complex carbon sources (cheese whey, molasses, or waste glycerol) [51,122]. In another study, 0.05 M HEPES was found to be sufficient under N2 sparging atmosphere (Table 2) [113]. Under CLF conditions, 0.01 M MOPS, TRIS, or HEPES buffers provided satisfactory results for both H2 and lactic acid synthesis in T. neapolitana subs. capnolactica (Table 2) [67]. More specifically, H2 synthesis was found to be the highest in MOPS, while TRIS promoted acetic acid formation (Table 2) [67]. The highest value of lactic acid synthesis was 14.9 ± 0.3 mM in phosphate buffer compared to 11.3 ± 0.6 mM in the standard condition (Table 2) [67].
The buffering capacity of HCO3− is sufficient to maintain near to optimal pH for growth (~6.5), facilitating the complete substrate degradation and desired by-product formation (Table 2) [31,56,67].
In other studies, itaconic acid was successfully used as a physiological buffer to enhance hydrogen production in T. neapolitana growing on glucose or glycerol [121,122]. During the cultivation with 1.5 g/L itaconic acid, the pH slowly dropped from 7.5 to 6.8 over 99 h, while the same pH change was reached within 48 h in cultures not buffered [122]. Although itaconic acid is only poorly catabolized, it affected the overall metabolism of T. neapolitana because H2 and acetic acid production were almost 1.4-fold higher than the control, while lactic acid production was reduced by nearly 100% compared to the control (Table 2) [122]. In addition, Ngo and Sim [122] found that the performance of T. neapolitana fermentation growing on waste glycerol was improved by almost 40% by adding itaconic acid into the culture medium [122].
2.4. Temperature
Due to their origin from hot habitats, bacterial species of the phylum Thermotogae can live and grow at temperatures in the range of 40–90 °C (Table 1). Some species such as K. olearia, O. teriensis, Ms. prima, and P. mexicana can thrive at mesophilic temperatures (Table 1) [7,8,96,100], and other species such as F. changbaicum, F. thailandese, T. maritima, Pseudot. hypogea, and T. neapolitana share the ability of growing at temperatures close to 90 °C (Table 1) [3,74,77,83,94]. For a long time, researchers have selected an operating temperature of 70 °C [104,117] or 80 °C [105] to cultivate T. neapolitana and T. maritima without careful investigation of the impacts on fermentation. Nguyen et al. [64] explored changes of H2 production with temperatures ranging from 55 to 90 °C for T. neapolitana and T. maritima. Both cultures showed approximately 100 mL H2/L medium at 55 °C and a maximum of 200 mL H2/L medium at 75–80 °C, with a decrease to 150 H2/L medium at 90 °C [64]. In T. neapolitana, high temperatures (77–85 °C) enhanced glucose uptake (2.2 mmol/L at 60 °C and 11.0 mmol/L at 77–85 °C) and boosted hydrogen yields (2.04 mol H2/moL consumed glucose at 60 °C and 3.85 mol H2/mol at 77 °C) [65]. This positive effect was also found for acetic acid (2.0 mmol/L at 60 °C and 18.0 mmol/L at 85 °C) and lactic acid production (no production at 60 °C and 1.25 mmol/L at 85 °C) (Table 2) [65]. Studies conducted on T. maritima hydrogenase demonstrated that this enzyme is unstable at the ambient temperature and its activity increased considerably with rising temperature (an activity of 25 units/mg at 20 °C and 110 units/mg at 90 °C [123].
2.5. Oxygen (O2)
Thermotogae members occur in various hot ecosystems, including hot springs, deep-sea, and shallow hydrothermal vents, and may also be exposed to O2 in these ecological niches [1254]. Indeed, despite their anaerobic nature, O2 tolerance is variable in the phylum; for example, Thermotoga, Fervidobacterium, and Geotoga genera can grow only under strictly anaerobic conditions, while K. olearia can survive in up to 15% O2 [10]. With elemental sulfur, Ts. atlanticus can grow with up to 8% O2 in the headspace [92]. Geochemical and microbial analyses demonstrated the wide distribution of Thermotogae species in ecosystems that are not only anaerobic but also partially oxygenated [124]. For this reason, the question of O2 tolerance and microaerophilic metabolism of Thermotogae has been addressed by several studies [65,105,106,125,126,127,128,129]. Some researchers have demonstrated that low concentrations of O2 are tolerated by T. neapolitana and T. maritima [127,128]. An O2 insensitive hydrogenase has been described in T. neapolitana, explaining why microaerobic H2 production and O2 tolerance could take place in this bacterium [130]. Additionally, Pseudot. hypogea and T. maritima contain an NADH oxidase that may serve as an O2 detoxification system [131,132]. Lakhal et al. [129] demonstrated O2 consumption over 12 h during the stationary phase of T. maritima in a batch reactor without reducing agent [129]. O2 presence reduced glucose fermentation rate and significantly shifted metabolism towards lactic acid production in T. maritima (Table 2). This change can probably be explained by O2 sensitivity of the hydrogenase [129]. Furthermore, T. maritima overproduced enzymes involved in reactive oxygen species (ROS) detoxification, iron-sulfur cluster synthesis/repair, cysteine biosynthesis, and a flavoprotein homologous to the rubredoxin of Desulfovibrio species that exhibited an oxygen reductase activity [127].
Van Ooteghem et al. [121] reported that O2 concentration decreased during the growth of F. pennavorans, P. miotherma, Ts. africanus, Pseudot. elfii, and T. neapolitana. In these experiments, the H2 yield greatly exceeded the theoretical limit of 4 mol H2/mol glucose in F. pennavorans, Pseudot. elfii, and T. neapolitana fermentation [121]. These surprisingly high H2 yield have led to the hypothesis of an unidentified aerobic pathway using O2 as a terminal electron acceptor in these bacteria which may not be obligate anaerobes [121]. However, aerobic metabolism is not supported by the genomic sequence of T. maritima, although the enzymes involved in the pentose phosphate pathway and an NADPH-reducing hydrogenase have been identified in the genome [16]. To explain the increased yield of H2 by T. neapolitana in microaerobic conditions and the existence of a catabolic process requiring O2, van Ooteghem et al. [121] used malonic acid as an inhibitor of succinate dehydrogenase and thus the O2-dependent metabolism. Even if the coding sequence for succinate dehydrogenase has not been identified in the T. maritima genome, hydrogen generation was completely inhibited for >40 h in the presence of malonate, postulating that malonate in the medium was no longer available to block catabolism [121]. Then, Eriksen et al. [106] demonstrated that malonic acid was not metabolized by T. neapolitana cultures but the exposure to malonic acid clearly affected the metabolism as reduced production of lactic acid and increased H2 yield were observed [106]. Against these findings, other researchers reported a reduction of H2 rate and production in T. neapolitana cultures after the injection of 6% O2 [65,106]. The reduction of O2 consumes reducing equivalents that are then unvailable to produce H2. The total duration of T. maritima fermentation in the batch reactor was delayed about 67 h under O2-induced stress [129]. In addition, the consumption rate of glucose was drastically reduced and the metabolism of T. maritima shifted towards lactic acid production due to inhibition of the O2-sensitive hydrogenase [129].
From a technical point of view, several strategies were adopted to remove dissolved O2 in the bioreactor: [I] sparging the culture with N2, CO2 or a mixture of both gases; [II] heating the medium; [III] adding a reducing agent such as sodium sulfide or cysteine-HCl in the medium; [IV] maintaining a positive pressure in the bioreactor headspace [31,56,62,67,70,105,106,113,121].
3. Nitrogen Containing-Compounds
Nitrogen sources (N-sources) are essential for bacterial life for the synthesis of cellular components like nucleic acids, proteins, and enzymes [133,134]. Yeast extract (YE), tryptone, and ammonium chloride (NH4Cl) have been identified as highly efficient and versatile organic N-sources in laboratory practices. It is widely demonstrated that most of the Thermotogae members can use yeast extract and tryptone to grow and metabolize carbohydrates [1,10,77,108,135,136].
Numerous efforts were made to replace YE by combining casamino acids and amino acids, but Pseudot. elfii failed to grow on these alternative substrates. The biogas yields of cultures grown with other N-sources were about 4–14% of those with YE (Table 3) [108].
Table 3.
Effect of organic nitrogen source and NaCl on Thermotogae fermentation. AA: Acetic acid; LA: Lactic acid; ALA: Alanine; YE: Yeast extract; Tryp: Tryptone; CA: Casamino acids; V: Vitamins solution [108]; aa: Amino acids (cysteine, alanine, asparagine, proline, glutamine, serine, and tryptophan, added at 0.2 g/L each). Experiments were performed in different bioreactor configurations: B = Batch; SB = Serum bottles. H2 column: a % H2 = calculated setting hydrogen production yield on medium with yeast extract to 100%; b mmol H2/L medium; c mL H2/L culture; d mol H2/mol glucose. * Values extrapolated from the graphical representation of data.
| Parameter | Organism | T (°C) | Culture Type | Mixing Speed (rpm) | Reactor/Working Volume (L) | Substrate Loaded (mmol/L) | Operational Parameter | Substrate Consumed (mmol/L) | Products | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | AA (mmol/L) | LA (mmol/L) | ALA (mmol/L) | ||||||||||
| Nitrogen sources (g/L) |
Pseudot.
elfii |
65 | B | 100 | 3.0/1.0 | no | w/o YE | - | 40 a | [108] | |||
| CA + V | - | 4 a | |||||||||||
| CA + V + aa | - | 6 a | |||||||||||
| 65 | B | 100 | 3.0/1.0 | Glucose (22.4) | YE (5) | n.d. | 100 a | ||||||
| CA + V | n.d. | 14 a | |||||||||||
| CA +V + aa | n.d. | 14 a | |||||||||||
| 65 | B | 100 | 3.0/1.0 | no | YE (2) -Tryp (0) | - | 13.9 b | 3.5 | |||||
| YE (2) -Tryp (2) | - | 14.8 b | 3.4 | ||||||||||
| YE (5) -Tryp (0) | - | 14.0 b | 0.0 | ||||||||||
| YE (5) -Tryp (5) | - | 28.8 b | 4.9 | ||||||||||
| 65 | B | 100 | 3.0/1.0 | Glucose (56) | YE (2) -Tryp (0) | 10.3 | 25.8 b | 10.7 | |||||
| YE (2) -Tryp (2) | 18.3 | 78.5 b | 19.7 | ||||||||||
| YE (5) -Tryp (0) | 13.1 | 84.9 b | 26.3 | ||||||||||
| YE (5) -Tryp (5) | 17.9 | 82.5 b | 21.2 | ||||||||||
|
T.
neapolitana |
80 | SB | no | 0.12/0.05 | Glucose (28) | YE (0.5) | 26.6 * | 260 *c | 15 * | [64] | |||
| YE (1.0) | 26 * | 320 *c | 22.5 * | ||||||||||
| YE (2.0) | 25.5 * | 360 *c | 26.6 * | ||||||||||
| YE (4.0) | 25 * | 430 *c | 30 * | ||||||||||
| YE (6.0) | 25 * | 430 *c | 33.3 * | ||||||||||
|
T.
maritima |
80 | SB | no | 0.12/0.05 | Glucose (28.00) | YE (0.5) | 25.5 * | 190 *c | 0.0 * | ||||
| YE (1.0) | 25 * | 260 *c | 20.8 * | ||||||||||
| YE (2.0) | 25 * | 270 *c | 23 * | ||||||||||
| Nitrogen sources (g/L) |
T.
maritima |
80 | SB | no | 0.12/0.05 | Glucose (28.00) | YE (4.0) | 25 * | 335 *c | 27.5 * | [64] | ||
| YE (6.0) | 24 * | 390 *c | 28 * | ||||||||||
|
T.
neapolitana |
77 | B | 75 | 0.12/0.05 | Glucose (28) | no YE | 23 * | 9 *b | 4.2 * | [136] | |||
| YE (0.5) | Completed * | 16 *b | 7.2 * | ||||||||||
| NaCl (g/L) |
T.
neapolitana subsp. capnolactica |
80 | SB | no | 0.12/0.03 | Glucose (28) | w/o | 25.62 ± 0.07 | 2.30 ± 0.50 d | 20.66 ± 0.27 | 2.80 ± 0.26 | 1.28 ± 0.9 | [67] |
| NaCl (5) | 26.00 ± 0.14 | 2.50 ± 1.20 d | 24.59 ± 0.95 | 6.23 ± 3.26 | 1.61 ± 0.58 | ||||||||
| NaCl (10) | 26.12 ± 0.16 | 3.10 ± 0.80 d | 26.05 ± 4.69 | 11.61 ± 2.42 | 2.46 ± 0.24 | ||||||||
| NaCl (20) | 25.96 ± 0.11 | 3.30 ± 0.20 d | 25.58 ± 1.03 | 13.44 ± 0.94 | 2.41 ± 0.09 | ||||||||
| NaCl (30) | 25.68 ± 0.25 | 2.91 ± 0.37 d | 23.22 ± 0.81 | 21.63 ± 6.15 | 2.38 ± 0.10 | ||||||||
Experiments with different concentrations of YE and tryptone were performed to identify their optimal and minimal concentrations in growth media [64,108,122,137,138]. YE and tryptone are sufficient to ensure growth and hydrogen production without additional carbon sources in Pseudot. elfii (Table 3) [108]. van Niel et al. [108] used media with various concentrations of YE and tryptone to ferment glucose by Pseudot. elfii [108]. They discovered that increasing the contents of both YE and tryptone from 2 g/L to 5 g/L improved H2 production (14.8 vs. 28.8 mmol/L) but higher contents did not further improve hydrogen and acetic acid production; high levels of both YE and tryptone only increased acetic acid production in medium lacking other C-sources [108].
When there was a low level of YE (2 g/L) but no tryptone, productions of H2 and acetic acid remained low, suggesting that tryptone served as an energy source like YE (Table 3) [108]. Although the amino acid compositions of the two N-sources are fairly similar, tryptone contains abundant peptides, a preferred form of amino acids by many bacteria [138]. In another study [122], T. neapolitana biomass increased along with the increase of YE concentrations in the range of 1.0–4.0 g/L but not with higher YE concentrations (5.0–6.0 g/L) [122]. The H2 production plateaued at 420 mL/L in T. neapolitana growing on glycerol with 1.0–4.0 g/L YE [122]. Experiments in T. maritima and T. neapolitana revealed that with over 2 g/L YE, there was a clear increase of acetic acid production, and hydrogen counted up to 30-33% of the total gas in the headspace, even though a mild reduction in glucose consumption occurred (Table 3) [64,138].
Nevertheless, low concentrations (2–4 g/L) of YE are still able to support productivity and bacterial growth [64,108,122,138]. d’Ippolito et al. [30] reported that 2 g/L of both tryptone and YE contributed to 10–15% of the total fermentation products in T. neapolitana [30]. Balk et al. [75] demonstrated that Pseudot. lettingae was able to degrade methanol in around 30 days in the presence of 0.5 g/L YE, whereas the substrate degradation did not occur when YE was omitted [75]. In contrast, the fermentation of T. neapolitana with glucose occurred in a medium without YE, even though the total glucose consumption without YE was attained in 30 h rather than 12 h. H2 and acetate amounts were half in the medium without YE, (Table 3) [135].
The impact of an inorganic N-source on Thermotogae fermentation, such as NH4Cl, has not been extensively studied, but the presence of NH4Cl has often been associated with either exopolysaccharide (EPS) formation in T. maritima or alanine production in T. neapolitana [62,129,136,139]. It is not clear how NH4Cl stimulates EPS production, but it might involve processing the surplus of reducing equivalents. For example, some organisms produce EPS as a mechanism to transport reducing equivalents out of the cell [140].
Han and Xu [61] demonstrated that a surplus of NH4Cl could partially substitute YE and tryptone in an optimized medium for auxotrophic Thermotoga sp. RQ7 strain [61].
4. Sodium Chloride and Phosphate
All members of the phylum Thermotogae showed great adaptability to a wide range of salinity levels (Table 1), although the optimal concentrations of NaCl vary among the members. Geotoga, Oceanotoga, and Petrotoga species can survive in environments comprised of 10% NaCl, while P. mexicana can live in up to 20% NaCl (Table 1) [10,12,95]. In contrast, species of the genus Fervidobacterium can tolerate salt concentrations up to 1% [5,79,80,81,83]. Among the species of the genus Mesotoga, Ms. infera exhibited the lowest tolerance of NaCl (Table 1).
NaCl at 20 g/L was reported to be optimal for T. neapolitana growing on either glucose or glycerol when hydrogen production is concerned [64,105,106,108,110,116]. Recently, the effect of different NaCl concentrations (0–35 g/L) on the CLF process was explored in T. neapolitana subs. capnolactica using glucose as the carbon source [67]. H2 synthesis and biomass growth were reduced by 15% and 25%, respectively, when NaCl was increased to 35 g/L (Table 3). Similarly, acetic acid production decreased from 26.1 ± 4.7 mM with 10 g/L NaCl to 23.2 ± 0.8 mM with 35 g/L NaCl. In contrast, high NaCl levels had a positive impact on lactic acid production, which increased 7.5-fold (2.8 ± 0.3 mM at 0 g/L NaCl vs. 21.6 ± 6.2 mM at 35 g/L NaCl), without affecting the overall H2 yields (Table 3) [67]. Pradhan and coworkers [67] suggested a possible involvement of NaCl in a sodium ion gradient that potentially fuels ATP synthesis and transport processes [67]. This creates a bioenergetic balance and supplies necessary reducing equivalents to convert acetic acid into lactic acid under CLF conditions (Figure 1) [67,118,119]. Similarly, another study [141] on H2-producing Vibrionaceae showed that increasing NaCl levels from 9 to 75 g/L enhanced lactic acid synthesis [141].
Regarding phosphate species, they have a strong buffering ability to mitigate pH fluctuation caused by the accumulation of volatile fatty acids [142]. Phosphate deficiency induced an increase in lactic acid production and a small decrease in H2 formation, suggesting a slight shift of the T. maritima metabolism towards lactic acid production. Besides its role as a macro-element, phosphate can also interact with calcium, favoring H2 production [141,143]. Saidi and co-workers [52] showed that T. maritima struggled to produce H2 at the same rate when there was an oversupply of calcium but an undersupply of phosphate in the medium [52]. For unknown reasons, phosphate exceeding 50 mM has been suggested to inhibit Pseudot. elfii growth [108].
5. Sulfur-Containing Compounds
All members of the phylum Thermotogae reduced sulfur-containing compounds such as elemental sulfur (S0), thiosulfate (Thio), and polysulfide to hydrogen sulfide (H2S), which is produced at the expense of H2 (Table 1) [1,4,29,76,144,145]. Sufficient supply of sulfur-containing compounds seems to be critically important; due to a large requirement for Fe-S clusters by the hydrogenase (containing 20 atoms of Fe and 18 atoms of S), PFOR, and other enzymes (Figure 1) [123,146]. In the literature, the effect of sulfur sources has been widely explored. The reduction of S-sources is considered an electron-sink reaction to deplete the surplus of electron power [3,98,107,147]. It is well known that the growth of most anaerobic bacteria of the phylum Thermotogae is stimulated by S-sources, but not dependent on them [1,29,52,53,75,107,125,126,144]. Generally speaking, the substrate consumption rate is benefited from a sulfur supply in the medium, except for the methanol fermentation in Pseudot. lettingae, which is reduced by S-containing compounds (19.7 mmol/L w/o S-source, 18.7 mmol/L with Thio and 10.6 mmol/L with S0) (Table 4). Members of the Mesotoga genus are able to oxidize sugars, although with low efficiency, only when S0 is used as the terminal electron acceptor [26,27,66,148,149]. This process gives acetic acid, CO2, and sulfide (2 mol of acetate and 4 mol of sulfide per mol of glucose), with no or trace amounts of H2 (Table 4) [27]. After 250 days of Ms. prima cultivation, 9.21 ± 0.13 mmol/L of acetate was measured in the presence of S0 rather than 1.67 ± 0.21 mM obtained in its absence (Table 4) [27]. Fadhlaoui and collaborators [27] argued that the metabolic differences between Thermotoga spp. and Ms. prima strains are related to the absence of a bifurcating [FeFe]-hydrogenase and the accumulation of NADH in Ms. prima, leading to growth inhibition in the absence of an external electron acceptor [27]. However, Ms. prima and Ms. infera strains grew more efficiently in a syntrophic association with a hydrogenotrophic microbial partner that serves as a biological electron acceptor compared to growing Mesotoga in a pure culture with sulfur as electron acceptor [26,27]. Boileau et al. [107] investigated the different responses of fermentation performance to different S-sources (Table 4) [107]. Among these compounds (Table 4), thiosulfate, cysteine, and Na2S were the most efficient ones to optimize T. maritima glucose fermentation (Table 4) [107]. Biogas production and glucose utilization increased in the order of no S-source < DMSO < S0 < Thio < Methionine (Met) < Na2S < Cysteine (Cys) (Table 4) [107]. Moreover, Na2S and Cys increased acetic acid production 3-fold and H2 production 2-fold (Table 4). Thiosulfate seemed to promote lactic acid formation (0.8 ± 0.1 mM w/o S-source and 6.3 ± 0.6 mM with Thio) without affecting other products [107]. Surprisingly, lactic acid was dependent on thiosulfate concentration (0.3 mol/mol glucose w/o Thio and 0.6 mol/mol glucose with 0.24 mmol Thio), even though the proportion between lactic and acetic acid yields remained constant (Table 4). DMSO had no significant impact on T. maritima fermentation parameters (Table 4) [107].
Table 4.
: Effect of sulfur compounds on Thermotogae fermentation. AA: Acetic acid; LA: Lactic acid; ALA: Alanine; EtOH: Ethanol; iVal: isovalerate; H2S: Hydrogen sulfide; Glu: Glutamate; DMSO: Dimethyl Sulfoxide; S0: Elemental sulfur; Met: Methionine; Thio: Thiosulfate; Cys: Cysteine; Na2S: Sodium sulfide. * Values extrapolated from the graphical representation of data. ** Concentrations of Sulfur compounds are 0.03 mol equivalent of sulfur. a H2 produced millimolar equivalent; b mmol; c µM.
| Organism | Carbon Source (mM) | Sulfur Source (mM) |
Substrate Consumed (mmol/L) | Products mmol/L Culture | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | AA | LA | ALA | EtOH | iVal | H2S | Glu | |||||
|
T.
maritima |
Glucose (25) | w/o | 7.1 ± 0.4 | 21.3 ± 2.1 | 10.1 ± 0.8 | 0.8 ± 0.1 | - | [107] | ||||
| DMSO ** | 9.2 ± 0.5 | 28.7 ± 2.9 | 13.3 ± 1.1 | 0.8 ± 0.1 | - | |||||||
| S0 ** | 16.6 ± 0.8 | 46.1 ± 4.6 | 23.8 ± 1.9 | 3.4 ± 0.3 | - | |||||||
| Met ** | 18.3 ± 0.9 | 53.3 ± 5.3 | 26.5 ± 2.1 | 3.1 ± 0.3 | - | |||||||
| Thio ** | 17.5 ± 0.9 | 47.3 ± 4.7 | 24.1 ± 1.9 | 6.3 ± 0.6 | - | |||||||
| Cys ** | 20.4 ± 1.0 | 58.5 ± 5.8 | 30.5 ± 2.4 | 4.1 ± 0.4 | - | |||||||
| Na2S ** | 20.4 ± 1.0 | 54.9 ± 5.5 | 30.7 ± 2.5 | 4.7 ± 0.5 | - | |||||||
| Glucose (60) | w/o Thio | 17.7 ± 1.9 | 25.0 ± 2.2 | 12.8 ± 1.0 | 5.4 ± 0.6 | 1.39 ± 0.2 | ||||||
| Thio (0.01) | 20.0 ± 1.1 | 31.0 ± 2.3 | 16.0 ± 0.8 | 10.2 ± 1.1 | - | |||||||
| Thio (0.03) | 28.0 ± 1.5 | 57.9 ± 4.8 | 30.6 ± 1.9 | 8.2 ± 0.7 | - | |||||||
| Thio (0.06) | 38.5 ± 2.0 | 73.3 ± 5.9 | 38.2 ± 2.4 | 18.1 ± 1.8 | - | |||||||
| Thio (0.12) | 45.7 ± 2.5 | 99.7 ± 8.3 | 52.4 ± 3.3 | 15.4 ± 1.6 | 3.8 ± 0.3 | |||||||
| Thio (0.18) | 45.4 ± 2.2 | 86.9 ± 8.2 | 45.0 ± 2.2 | 23.4 ± 2.3 | - | |||||||
| Thio (0.24) | 43.8 ± 2.2 | 88.6 ± 8.9 | 46.1 ± 3.3 | 26.4 ± 1.4 | 3.8 ± 0.2 | |||||||
| Glucose (20) | w/o | 13.70 | 36.09 | 15.62 | 0.70 | n.d. | [145] | |||||
| Thio (20) | 13.55 | 4.02 | 15.99 | 0.80 | 14.45 | |||||||
|
T.
neapolitana |
Glucose (20) | w/o | 14.00 | 31.67 | 18.27 | 0.87 | n.d. | [145] | ||||
| Thio (20) | 13.90 | 16.07 | 16.12 | 0.60 | 7.39 | |||||||
|
Pseudot.
lettingae |
Methanol (20) | w/o | 19.70 | n. d. | 13.70 | - | - | [75] | ||||
| Thio (20) | 18.7 | n. d. | - | 5.8 | 11.2 | |||||||
| S0 (2%) | 10.6 | n. d. | - | 3.1 | 7.3 | |||||||
|
Pseudot.
hypogea |
Glucose (20) | w/o | 8.60 | 29.03 | 4.49 | 1.71 | n. d. | [145] | ||||
| Thio (20) | 14.39 | 2.29 | 19.7 | 1.06 | 15.08 | |||||||
|
Pseudot.
hypogea |
Glucose (20) | w/o | 7.0 | 9.4 a | 5.0 | 1.7 | 1.0 | 0.2 | [77] | |||
| Thio (20) | 13.0 | 0.9 a | 19.8 | 1.0 | 1.6 | 15.1 | ||||||
|
Pseudot.
hypogea |
Xylose (20) | w/o | 12.9 | 19.0 a | 8.9 | 2.4 | 1.0 | 0.2 | [77] | |||
| Thio (20) | 12.0 | 1.8 a | 13.7 | 1.3 | 1.0 | 7.5 | ||||||
|
Pseudot.
elfii |
Glucose (20) | w/o | 3.1 | 8.8 | 4.0 | 0.0 | [77] | |||||
| Thio (20) | 10.4 | 2.0 | 17.9 | 23.00 | ||||||||
| Glucose (20) | w/o | 2.75 | 7.70 | 3.49 | 1.05 | n. d. | [145] | |||||
| Thio (20) | 8.15 | n. d. | 12.63 | 0.41 | 14.55 | |||||||
|
Ts.
geolei |
Glucose (0.28) | w/o | 7.0 b | 9.3 a | 8.5 b | 1.2 b | 0.5 b | [87] | ||||
| S0 (2%) | 6.0 b | 0.0 a | 7.5 b | 0.5 b | 12.5 b | |||||||
|
Ms.
Prima Phos Ac3 |
Glucose (20) | w/o | 1.50 ± 0.20 | <1 c | 1.67 ± 0.21 | 1.05 ± 0.25 | [27] | |||||
| S0 | 6.57 ± 0.19 | <1 c | 9.21 ± 0.13 | 24.40 ± 0.30 | ||||||||
|
Ms.
Prima MesG1Ag4.2T |
Fructose (20) | w/o | 1.00 ± 0.23 | <1 c | 0.70 ± 0.41 | 1.18 ± 0.41 | ||||||
| S0 | 3.27 ± 0.85 | <1 c | 8.48 ± 1.96 | 18.03 ± 5.16 | ||||||||
|
Ts.
africanus |
Glucose (28) | w/o | 7.20 | 16.80 | 7.90 | <0.2 | 0.79 | n.d. | [145] | |||
| Thio (20) | 7.70 | 1.00 | 12.40 | - | - | 14.60 | ||||||
|
Ts.
atlanticus |
Glucose (28) | w/o | 5.6 | 12.5 | 1.7 | 0.14 | - | [92] | ||||
| S0 (1%) | 6.0 | 7.5 | 1.9 | 0.15 | 1.3 | |||||||
|
F.
islandicum |
Glucose (20) | w/o | 14.20 | 21.58 | 6.25 | 3.98 | n.d. | [145] | ||||
| Thio (20) | 16.20 | n. d. | 20.25 | 1.22 | 34.02 | |||||||
|
F.
pennavorans |
Glucose (11) | w/o | - | 0.25 * | 6.7 * | 4.0 ± 0.5 * | 1.3 * | [32] | ||||
| Thio (20) | - | 0.2 * | 6.7 * | 4.50 * | No * | |||||||
In the presence of thiosulfate, the growth and glutamate production of Fervidobacterium is stimulated; however, S0 does not seem to help overcoming the H2-feedback inhibition (Table 4) [32,80,88,144]. P. olearia, P. sibirica, and Ts. Africanus produced small amounts of ethanol (0.17 mM for both Petrotoga species and 0.79 mM for Ts. africanus) only in the absence of S-sources (Table 4) [93,145]. Pseudot. lettingae produced L-alanine, at the expense of acetic acid, only when thiosulfate or S0 was present in the medium using methanol as the substrate (Table 4) [75]. Meanwhile, the presence of thiosulfate or S0 resulted in increased production of acetic acid and decreased production of alanine in Pseudot. hypogea, Ts. melaniensis, Ts. geolei, P. olearia, and P. sibirica cultures, using glucose or xylose as the carbon source (Table 4) [77,87,90,93]. When S0 is available, no hydrogen could be detected in Mn. hydrogenitolerans growing on glucose [101].
Thermotogae members have been widely employed to degrade different organic wastes, and their degradation significantly benefited from the presence of a reducing agent [51,52,53,54,113,116,138]. It is noteworthy to mention that high concentrations of thiosulfinate, a volatile organo-sulfur compound found in organic wastes, has an inhibitory effect on T. maritima growth [54]. Similarly, Tao et al. [150] demonstrated that thiosulfinate inhibited the H2 production by mesophilic seed sludge when co-fermenting food wastes [150].
6. Metal Ions
Typically, hydrothermal ecosystems are enriched with essential micronutrients and trace metals such as soluble and insoluble iron, manganese, cobalt, and molybdenum. Some terrestrial hydrothermal waters are also characterized by chromium and uranium contents of several micrograms per liter [151]. The physiological roles that most of these metals play in microbial metabolism are still largely unknown. It is believed that their functions include energy generation and biosynthesis [151]. In addition, Mn, Fe, Zn, and Co metals are vitally important micro-elements for growth, essential for cellular transport processes, and serve as cofactors for many enzymes [152]. Understanding the physicochemical properties of extreme habitats can help to determine the metal toxicity limits on microbial growth in laboratory settings. Indeed, metal susceptibility tests have been carried out on T. neapolitana, T. maritima, and Ts. africanus, and have identified the following toxicity order: cadmium (1.0–10.0 µM) > zinc (0.01–0.1 mM) > nickel (1.0–5.0 mM) > cobalt (1.0–10.0 mM) [153].
Attention has also been paid to Fe (III) reduction by thermophilic bacteria, since Fe (III) may work as an external electron acceptor in microbial metabolism [154]. Members of the phylum Thermotogae are capable of coupling the reduction of iron with the oxidation of a wide range of organic and inorganic compounds. T. maritima reduced Fe (III) into Fe (II) exclusively with molecular hydrogen as an electron donor [154]. Fe (III) reduction has also been reported to stimulate growth and mitigate H2 inhibition in Pseudot. lettingae, Pseudot. subterranea, Pseudot. elfii, Ts. affectus, Ts. globiformans, and Ts. activus [75,76,88,89,91]. The recently characterized member of the order Mesoaciditogales, A. saccharophila, changed fermentation end-products when growing with Fe (III), favoring the production of small amounts of acetate, isobutyrate, and isovalerate [14].
Ions and metals are generally supplied in Thermotogae growth media through Balch’s oligo-elements solution [155]. The removal of oligo-elements from T. maritima cultures resulted in a minor increase in lactic acid production (1.2 vs. 4.3 mmol/L) and a decrease in H2 productivity (12.4 vs. 8.8 mmol/h/L) [52]. Limitation in iron lowered H2 production by deviating the fermentation pathway towards the production of more reduced end-products such as lactic acid in mixed cultures [156,157]. Another study [139] highlighted how the supplementation of Fe ions to mixed cultures had pronounced effect on hydrogen activity [139]. Similarly, Fe2+ (as well as Co, Ni and Mn) stimulated Pseudot. hypogea alcohol dehydrogenase activity (ADH), an iron-containing enzyme involved in alcohol fermentation, by 10–15%, while Zn2+ completely inhibited the enzyme activity [158]. On the same base, the inclusion of tungsten in the growth medium of T. maritima increased the specific activity of both hydrogenase (by up to 10-fold) and PFOR in cell-free extracts, although the function of tungsten in the metabolism of T. maritima is not clear [123,126].
As for magnesium, potassium, and calcium ions, they not only play critical roles in bacterial growth, but also act as enzyme cofactors and ensure the survival of microorganisms in their hot ecosystems, by protecting double-stranded DNA from degradation [159]. The best cell yields were obtained with a low concentration of Mg2+ and a high concentration of Ca2+ [126]. It would be worthwhile to dig further into the metal ions repercussions on Thermotogae metabolism in future research.
7. Conclusions
Steam reforming of methane (CH4) is currently used to produce hydrogen in the industry, as it is the most economic technology available so far. Producing hydrogen by biological means at an industrial scale remains as a challenge. Within the race to find the best way to generate hydrogen via microbes (e.g., choice of strains, substrates, fermentation conditions), Thermotogae seem to have many unique advantages. Optimization of their cultivation conditions is fundamental to improve the overall productivity of the fermentation system and its profitability, which determine the feasibility of replacing the current methods of hydrogen production.
The phylum Thermotogae comprises a wide collection of species with astonishing and unique features associated to their original habitats. Extensive research has shown tremendous potentials of using these bacteria in biological production of hydrogen, degradation of wastes, and isolation of thermostable enzymes.
Many factors affect the anaerobic metabolism of Thermotogae species, including operating conditions (shaking, inoculum, gas sparging, and culture/headspace volume ratio), temperature, pH, nitrogen, sulfur-containing compounds, sodium chloride, phosphate, and metal ions. Optimization of these fermentation parameters has been intensively pursued with Thermotoga and Pseudothermotoga species, which are the best hydrogen producers in the phylum. In contrast, little is known regarding other species of the phylum, especially their ability to synthesize desirable biological products.
In general, Thermotogae fermentation is affected by the accumulation of produced biogas and organic acids because they increase hydrogen partial pressure inside of the bioreactor and drastically reduce the pH of the cultivation medium. Consequently, the metabolic process stops before the substrate is completely consumed. Gas sparging, stirring, and adjusting culture/headspace volume ratio can help to overcome the inhibition on growth caused by hydrogen accumulation. Implementing these strategies and adjusting pH during the fermentation process can result in high hydrogen yields and efficient consumption of substrates. A reduction of fermentation time by starting with the right inoculum size could cast favorable great perspectives on the economics of the industrial processes.
This review highlights the importance of nitrogen-containing compounds that need to be supplied to the medium to stimulate bacterial growth. Overall, yeast extract and tryptone are the preferred forms of nitrogen. Sulfur-containing compounds not only play a critical role in bacterial growth but also divert reducing power to selectively produce certain end-products in Thermotogae metabolism.
Until now, the impact of metal ions and salts on the fermentation process has not been well investigated even though it has been demonstrated that they could stimulate many key enzymes involved in various metabolic pathways.
In summary, the extensive data collection of this review offers a great reference for the optimization and development of sustainable bioprocesses based on Thermotogae species and helps to generate insightful perspectives for the exploitation of these anaerobic bacteria in biotechnological processes.
Acknowledgments
The authors would like to thank Lucio Caso (CNR-ICB) for the technical support in preparing the manuscript.
Funding
This research was funded by BioRECO2VER Project, through the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 760431.
Conflicts of Interest
The authors declare no conflict of interest. The funding agencies gave their permissions to the publication of manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huber R.H.M. In: Thermotogales. Dworkin M., Falkow S., Rosenberg E., Schleifer K.H., Stackebrandt E., editors. Springer; New York, NY, USA: 2006. pp. 899–922. [Google Scholar]
- 2.Bhandari V., Gupta R.S. The Phylum Thermotogae. In: Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. The Prokaryotes. Springer; Berlin/Heidelberg, Germany: 2014. [Google Scholar]
- 3.Huber R., Langworthy T.A., König H., Thomm M., Woese C.R., Sleytr U.B., Stetter K.O. Thermotoga maritima Sp. Nov. Represents a New Genus of Unique Extremely Thermophilic Eubacteria Growing up to 90 °C. Arch. Microbiol. 1986;144:324–333. doi: 10.1007/BF00409880. [DOI] [Google Scholar]
- 4.Belahbib H., Summers Z.M., Fardeau M., Joseph M., Tamburini C., Dolla A., Ollivier B., Armougom F. Towards a Congruent Reclassification and Nomenclature of the Thermophilic Species of the Genus Pseudothermotoga within the Order Thermotogales. Syst. Appl. Microbiol. 2018;41:555–563. doi: 10.1016/j.syapm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Patel B.K.C., Morgan H.W., Daniel R.M. Fervidobacterium nodosum Gen. Nov. and Spec. Nov., a New Chemoorganotrophic, Caldoactive, Anaerobic Bacterium. Arch. Microbiol. 1985;141:63–69. doi: 10.1007/BF00446741. [DOI] [Google Scholar]
- 6.Windberger E., Huber R., Trincone A., Fricke H., Stetter K.O. Thermotoga thermarum Sp. Nov. and Thermotoga Neapolitana Occurring in African Continental Solfataric Springs. Arch. Microbiol. 1989;151:506–512. doi: 10.1007/BF00454866. [DOI] [Google Scholar]
- 7.DiPippo J.L., Nesbø C.L., Dahle H., Doolittle W.F., Birkland N.K., Noll K.M. Kosmotoga olearia Gen. Nov., Sp. Nov., a Thermophilic, Anaerobic Heterotroph Isolated from an Oil Production Fluid. Int. J. Syst. Evol. Microbiol. 2009;59:2991–3000. doi: 10.1099/ijs.0.008045-0. [DOI] [PubMed] [Google Scholar]
- 8.Nesbø C.L., Bradnan D.M., Adebusuyi A., Dlutek M., Petrus A.K., Foght J., Doolittle W.F., Noll K.M. Mesotoga prima Gen. Nov., Sp. Nov., the First Described Mesophilic Species of the Thermotogales. Extremophile. 2012;16:387–393. doi: 10.1007/s00792-012-0437-0. [DOI] [PubMed] [Google Scholar]
- 9.Ben Hania W., Godbane R., Postec A., Hamdi M., Ollivier B., Fardeau M.L. Defluviitoga tunisiensis Gen. Nov., Sp. Nov., a Thermophilic Bacterium Isolated from a Mesothermic and Anaerobic Whey Digester. Int. J. Syst. Evol. Microbiol. 2012;62:1377–1382. doi: 10.1099/ijs.0.033720-0. [DOI] [PubMed] [Google Scholar]
- 10.Davey M.E., Wood W.A., Key R., Nakamura K., Stahl D.A. Isolation of Three Species of Geotoga and Petrotoga: Two New Genera, Representing a New Lineage in the Bacterial Line of Descent Distantly Related to the “Thermotogales”. Syst. Appl. Microbiol. 1993;16:191–200. doi: 10.1016/S0723-2020(11)80467-4. [DOI] [Google Scholar]
- 11.Wery N., Lesongeur F., Pignet P., Derennes V., Cambon-Bonavita M.A., Godfroy A., Barbier G. Marinitoga camini Gen. Nov., Sp. Nov., a Rod-Shaped Bacterium Belonging to the Order Thermotogales, Isolated from a Deep-Sea Hydrothermal Vent. Int. J. Syst. Evol. Microbiol. 2001;51:495–504. doi: 10.1099/00207713-51-2-495. [DOI] [PubMed] [Google Scholar]
- 12.Jayasinghearachchi H.S., Lal B. Oceanotoga Teriensis Gen. Nov., Sp. Nov., a Thermophilic Bacterium Isolated from Offshore Oil-Producing Wells. Int. J. Syst. Evol. Microbiol. 2011;61:554–560. doi: 10.1099/ijs.0.018036-0. [DOI] [PubMed] [Google Scholar]
- 13.Reysenbach A.L., Liu Y., Lindgren A.R., Wagner I.D., Sislak C.D., Mets A., Schouten S. Mesoaciditoga lauensis Gen. Nov., Sp. Nov., a Moderately Thermoacidophilic Member of the Order Thermotogales from a Deep-Sea Hydrothermal Vent. Int. J. Syst. Evol. Microbiol. 2013;63:4724–4729. doi: 10.1099/ijs.0.050518-0. [DOI] [PubMed] [Google Scholar]
- 14.Itoh T., Onishi M., Kato S., Iino T., Sakamoto M., Kudo T., Takashina T., Ohkuma M. Athalassotoga saccharophila Gen. Nov., Sp. Nov., Isolated from an Acidic Terrestrial Hot Spring, and Proposal of Mesoaciditogales Ord. Nov. and Mesoaciditogaceae Fam. Nov. in the Phylum Thermotogae. Int. J. Syst. Evol. Microbiol. 2016;66:1045–1051. doi: 10.1099/ijsem.0.000833. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y., Cheng L., Zhang X., Li X., Deng Y., Zhang H. Thermococcoides shengliensis Gen. Nov., Sp. Nov., a New Member of the Order Thermotogales Isolated from Oil-Production Fluid. Int. J. Syst. Evol. Microbiol. 2010;60:932–937. doi: 10.1099/ijs.0.013912-0. [DOI] [PubMed] [Google Scholar]
- 16.Nelson K., Clayton R., Gill S., Gwinn M., Dodson R., Haft D., Hickey E., Peterson J., Nelson W., Ketchum K., et al. Evidence for Lateral Gene Transfer between Archae and Bacteria from Genome Sequence of Thermotoga maritima. Nature. 1999;399:323–329. doi: 10.1038/20601. [DOI] [PubMed] [Google Scholar]
- 17.Conners S.B., Mongodin E.F., Johnson M.R., Montero C.I., Nelson K.E., Kelly R.M. Microbial Biochemistry, Physiology, and Biotechnology of Hyperthermophilic Thermotoga Species. FEMS Microbiol. Rev. 2006;30:872–905. doi: 10.1111/j.1574-6976.2006.00039.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodionov D.A., Rodionova I.A., Li X., Ravcheev I., Tarasova Y., Portnoy V.A., Zengler K., Osterman A.L. Transcriptional Regulation of the Carbohydrate Utilization Network in Thermotoga maritima. Front. Microbiol. 2013;4:244. doi: 10.3389/fmicb.2013.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galperin M.Y., Noll K.M., Romano A.H. The Glucose Transport System of the Hyperthermophilic Anaerobic Bacterium Thermotoga neapolitana. Appl. Environ. Microbiol. 1996;62:2915–2918. doi: 10.1128/AEM.62.8.2915-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsen I.T., Nguyen L., Sliwinski M.K., Rabus R., Jr M.H.S. Microbial Genome Analyses: Comparative Transport Capabilities in Eighteen Prokaryotes. J. Mol. Biol. 2000;301:75–100. doi: 10.1006/jmbi.2000.3961. [DOI] [PubMed] [Google Scholar]
- 21.Nanavati D., Thirangoon K., Noll K.M. Several Archaeal Homologs of Putative Oligopeptide-Binding Proteins Encoded by Thermotoga maritima Bind Sugars. Appl. Environ. Microbiol. 2006;72:1336–1345. doi: 10.1128/AEM.72.2.1336-1345.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latif H., Sahin M., Tarasova J., Tarasova Y., Portnoy V.A., Nogales J., Zengler K. Adaptive Evolution of Thermotoga maritima Reveals Plasticity of the ABC Transporter Network. Appl. Environ. Microbiol. 2015;81:5477–5485. doi: 10.1128/AEM.01365-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boucher N., Noll K.M. Substrate Adaptabilities of Thermotogae Mannan Binding Proteins as a Function of Their Evolutionary Histories. Extremophiles. 2016;20:771–783. doi: 10.1007/s00792-016-0866-2. [DOI] [PubMed] [Google Scholar]
- 24.Thauer R.K., Jungermann K., Decker K. Energy Conservation in Chemotrophic Anaerobic Bacteria. Bacteriol. Rev. 1977;41:100–180. doi: 10.1128/BR.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Hania W., Ghodbane R., Postec A., Brochier-Armanet C., Hamdi M., Fardeau M.L., Ollivier B. Cultivation of the First Mesophilic Representative (“Mesotoga”) within the Order Thermotogales. Syst. Appl. Microbiol. 2011;34:581–585. doi: 10.1016/j.syapm.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Hania W.B., Postec A., Aüllo T., Ranchou-Peyruse A., Erauso G., Brochier-Armanet C., Hamdi M., Ollivier B., Saint-Laurent S., Magot M., et al. Mesotoga infera Sp. Nov., a Mesophilic Member of the Order Thermotogales, Isolated from an Underground Gas Storage Aquifer. Int. J. Syst. Evol. Microbiol. 2013;63:3003–3008. doi: 10.1099/ijs.0.047993-0. [DOI] [PubMed] [Google Scholar]
- 27.Fadhlaoui K., Hania W.B., Armougom F., Bartoli M., Fardeau M.L., Erauso G., Brasseur G., Aubert C., Hamdi M., Brochier-Armanet C., et al. Obligate Sugar Oxidation in Mesotoga Spp., Phylum Thermotogae, in the Presence of Either Elemental Sulfur or Hydrogenotrophic Sulfate-Reducers as Electron Acceptor. Environ. Microbiol. 2017;20:281–292. doi: 10.1111/1462-2920.13995. [DOI] [PubMed] [Google Scholar]
- 28.Janssen P.H., Morgan H.W. Heterotrophic Sulfur Reduction by Thermotoga Sp. Strain FjSS3.B1. FEMS Microbiol. Lett. 1992;96:213–218. doi: 10.1016/0378-1097(92)90406-e. [DOI] [PubMed] [Google Scholar]
- 29.Schröder C., Selig M., Schönheit P. Glucose Fermentation to Acetate, CO2 and H2 in the Anaerobic Hyperthermophilic Eubacterium Thermotoga maritima: Involvement of the Embden-Meyerhof Pathway. Arch. Microbiol. 1994;161:460–470. doi: 10.1007/BF00307766. [DOI] [Google Scholar]
- 30.D’Ippolito G., Dipasquale L., Vella F.M., Romano I., Gambacorta A., Cutignano A., Fontana A. Hydrogen Metabolism in the Extreme Thermophile Thermotoga neapolitana. Int. J. Hydrogen Energy. 2010;35:2290–2295. doi: 10.1016/j.ijhydene.2009.12.044. [DOI] [Google Scholar]
- 31.Dipasquale L., d’Ippolito G., Fontana A. Capnophilic Lactic Fermentation and Hydrogen Synthesis by Thermotoga neapolitana: An Unexpected Deviation from the Dark Fermentation Model. Int. J. Hydrogen Energy. 2014;39:4857–4862. doi: 10.1016/j.ijhydene.2013.12.183. [DOI] [Google Scholar]
- 32.Wushke S., Fristensky B., Zhang X.L., Spicer V., Krokhin O.V., Levin D.B., Stott M.B., Sparling R. A Metabolic and Genomic Assessment of Sugar Fermentation Profiles of the Thermophilic Thermotogales, Fervidobacterium pennivorans. Extremophiles. 2018;22:965–974. doi: 10.1007/s00792-018-1053-4. [DOI] [PubMed] [Google Scholar]
- 33.Vijayakumar J., Aravindan R., Viruthagiri T. Lactic Acid and Its Potential Applications in Industries. Bioprod. Biosyst. Eng. 2007;42:101–103. [Google Scholar]
- 34.Roehr M., Kosaric N., Vardar-Sukan F., Pieper H.J., Senn T. The Biotechnology of Ethanol. Classical and Future Applications. Wiley; Hoboken, NJ, USA: 2005. p. 245. [Google Scholar]
- 35.Vieille C., Zeikus G.J. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiol. Mol. Biol. Rev. 2001;65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ul Haq I., Hussain Z., Khan M., Muneer B., Afzal S., Majeed S., Akram F. Kinetic and Thermodynamic Study of Cloned Thermostable Endo-1, 4- b -Xylanase from Thermotoga petrophila in Mesophilic Host. Mol. Biol. Rep. 2012;39:7251–7261. doi: 10.1007/s11033-012-1555-6. [DOI] [PubMed] [Google Scholar]
- 37.Ul Haq I., Tahir S.F., Aftab M.N., Akram F., ur Rehman A., Nawaz A., Mukhtar H. Purification and Characterization of a Thermostable Cellobiohydrolase from Thermotoga petrophila. Protein Pept. Lett. 2018;25:1003–1014. doi: 10.2174/0929866525666181108101824. [DOI] [PubMed] [Google Scholar]
- 38.Colussi F., Viviam M., Ian S., Junio M. Oligomeric State and Structural Stability of Two Hyperthermophilic β—Glucosidases from Thermotoga petrophila. Amino Acids. 2015;47:937–948. doi: 10.1007/s00726-015-1923-3. [DOI] [PubMed] [Google Scholar]
- 39.Pollo S.M.J., Zhaxybayeva O., Nesbø C.L. Insights into Thermoadaptation and the Evolution of Mesophily from the Bacterial Phylum Thermotogae. Can. J. Microbiol. 2015;61:655–670. doi: 10.1139/cjm-2015-0073. [DOI] [PubMed] [Google Scholar]
- 40.Fatima B., Aftab M., Ul Haq I. Cloning, Purifiication, and Characterization of Xylose Isomerase from Thermotoga naphthophila RKU-10. J. Basic Microbiol. 2016;56:949–962. doi: 10.1002/jobm.201500589. [DOI] [PubMed] [Google Scholar]
- 41.Lopes J.L.S., Yoneda J.S., Martins J.M., Demarco R. Environmental Factors Modulating the Stability and Enzymatic Activity of the Petrotoga mobilis Esterase (PmEst) PLoS ONE. 2016;11:e0158146. doi: 10.1371/journal.pone.0158146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Intagun W., Kanoksilapatham W. A Review: Biodegradation and Applications of Keratin Degrading Microorganisms and Keratinolytic Enzymes, Focusing on Thermophiles and Thermostable Serine Proteases. Am. J. Appl. Sci. Rev. 2017;14:1016–1023. doi: 10.3844/ajassp.2017.1016.1023. [DOI] [Google Scholar]
- 43.Hamid A., Aftab M.N. Cloning, Purification, and Characterization of Recombinant Thermostable β -Xylanase Tnap_0700 from Thermotoga naphthophila. Appl. Biochem. Biotechnol. 2019;189:1274–1290. doi: 10.1007/s12010-019-03068-0. [DOI] [PubMed] [Google Scholar]
- 44.Kang E., Jin H., La J.W., Park S., Kim W., Lee W. Identification of Keratinases from Fervidobacterium islandicum AW-1 Using Dynamic Gene Expression pro Fi Ling. Microb. Biotechnol. 2019;13:442–457. doi: 10.1111/1751-7915.13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.European Commission . Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. European Commission; Brussels, Belgium: 2020. A Hydrogen Strategy for a Climate-Neutral Europe; 8.7.2020 COM 301 Final. [Google Scholar]
- 46.Dipasquale L., d’Ippolito G., Gallo C., Vella F.M., Gambacorta A., Picariello G., Fontana A. Hydrogen Production by the Thermophilic Eubacterium Thermotoga neapolitana from Storage Polysaccharides of the CO2-Fixing Diatom Thalassiosira weissflogii. Int. J. Hydrogen Energy. 2012;37:12250–12257. doi: 10.1016/j.ijhydene.2012.05.160. [DOI] [Google Scholar]
- 47.Angenent L., Karim K., Al-Dahhan M., Wrenn B., Domiguez-Espinosa R. Production of Bioenergy and Biochemicals from Industrial and Agricultural Wastewater. Trends Biotechnol. 2004;22:477–485. doi: 10.1016/j.tibtech.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 48.De Vrije T., de Haas G.G., Tan G.B., Keijsers E.R.P., Claassen P.A.M. Pretreatment of Miscanthus for Hydrogen Production by Thermotoga elfii. Hydrog. Energy. 2002;27:1381–1390. doi: 10.1016/S0360-3199(02)00124-6. [DOI] [Google Scholar]
- 49.De Vrije T., Budde M.A.W., Lips S.J., Bakker R.R., Mars A.E., Claassen P.A.M. Hydrogen Production from Carrot Pulp by the Extreme Thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy. 2010;35:13206–13213. doi: 10.1016/j.ijhydene.2010.09.014. [DOI] [Google Scholar]
- 50.Mars A.E., Veuskens T., Budde M.A.W., Van Doeveren P.F., Lips S.J., Bakker R.R., De Vrije T., Claassen P.A.M. Biohydrogen Production from Untreated and Hydrolyzed Potato Steam Peels by the Extreme Thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy. 2010;35:7730–7737. doi: 10.1016/j.ijhydene.2010.05.063. [DOI] [Google Scholar]
- 51.Cappelletti M., Bucchi G., De Sousa Mendes J., Alberini A., Fedi S., Bertin L., Frascari D. Biohydrogen Production from Glucose, Molasses and Cheese Whey by Suspended and Attached Cells of Four Hyperthermophilic Thermotoga Strains. J. Chem. Technol. Biotechnol. 2012;87:1291–1301. doi: 10.1002/jctb.3782. [DOI] [Google Scholar]
- 52.Saidi R., Liebgott P.P., Gannoun H., Ben Gaida L., Miladi B., Hamdi M., Bouallagui H., Auria R. Biohydrogen Production from Hyperthermophilic Anaerobic Digestion of Fruit and Vegetable Wastes in Seawater: Simplification of the Culture Medium of Thermotoga maritima. Waste Manag. 2018;71:474–484. doi: 10.1016/j.wasman.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 53.Saidi R., Liebgott P.P., Hamdi M., Auria R., Bouallagui H. Enhancement of Fermentative Hydrogen Production by Thermotoga maritima through Hyperthermophilic Anaerobic Co-Digestion of Fruit-Vegetable and Fish Wastes. Int. J. Hydrogen Energy. 2018;43:23168–23177. doi: 10.1016/j.ijhydene.2018.10.208. [DOI] [Google Scholar]
- 54.Saidi R., Hamdi M., Bouallagui H. Hyperthermophilic Hydrogen Production in a Simplified Reaction Medium Containing Onion Wastes as a Source of Carbon and Sulfur. Environ. Sci. Pollut. Res. 2020;27:17382–17392. doi: 10.1007/s11356-020-08270-w. [DOI] [PubMed] [Google Scholar]
- 55.Amend J.P., Shock E.L. Energetics of Overall Metabolic Reactions of Thermophilic and Hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 2001;25:175–243. doi: 10.1111/j.1574-6976.2001.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 56.Pradhan N., Dipasquale L., d’Ippolito G., Panico A., Lens P.N.L., Esposito G., Fontana A. Hydrogen Production by the Thermophilic Bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015;16:12578–12600. doi: 10.3390/ijms160612578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin D.B., Pitt L., Love M. Biohydrogen Production: Prospects and Limitations to Practical Application. Int. J. Hydrogen Energy. 2004;29:173–185. doi: 10.1016/S0360-3199(03)00094-6. [DOI] [Google Scholar]
- 58.Schut G.J., Adams M.W.W. The Iron-Hydrogenase of Thermotoga maritima Utilizes Ferredoxin and NADH Synergistically: A New Perspective on Anaerobic Hydrogen Production. J. Bacteriol. 2009;191:4451–4457. doi: 10.1128/JB.01582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu J., Varga M., Mityas C., Noll K.M. Liposome-Mediated DNA Uptake and Transient Expression in Thermotoga. Extremophiles. 2001;5:53–60. doi: 10.1007/s007920000173. [DOI] [PubMed] [Google Scholar]
- 60.Xu H., Han D., Xu Z. Expression of Heterologous Cellulases in Thermotoga Sp. Strain RQ2. BioMed Res. Int. 2015;2015:304523. doi: 10.1155/2015/304523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han D., Xu Z. Development of a PyrE—Based Selective System for Thermotoga sp. Extremophiles. 2017;21:297–306. doi: 10.1007/s00792-016-0902-2. [DOI] [PubMed] [Google Scholar]
- 62.Pradhan N., Dipasquale L., Panico A., Lens P.N.L., Esposito G., d’Ippolito G., Fontana A. Hydrogen and Lactic Acid Synthesis by the Wild-Type and a Laboratory Strain of the Hyperthermophilic Bacterium Thermotoga neapolitana DSMZ 4359 under Capnophilic Lactic Fermentation Conditions. Int. J. Hydrogen Energy. 2017;42:16023–16030. doi: 10.1016/j.ijhydene.2017.05.052. [DOI] [Google Scholar]
- 63.Grogan D.W., Carver G.T., Drake J.W. Genetic fidelity under harsh conditions: Analysis of spontaneous mutation in the thermoacidophilic archaeon Sulfolobus acidocaldarius. Proc. Natl. Acad. Sci. USA. 2001;98:7928–7933. doi: 10.1073/pnas.141113098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen T.A.D., Pyo Kim J., Sun Kim M., Kwan Oh Y., Sim S.J. Optimization of Hydrogen Production by Hyperthermophilic Eubacteria, Thermotoga maritima and Thermotoga neapolitana in Batch Fermentation. Int. J. Hydrogen Energy. 2008;33:1483–1488. doi: 10.1016/j.ijhydene.2007.09.033. [DOI] [Google Scholar]
- 65.Munro S.A., Zinder S.H., Walker L.P. The Fermentation Stoichiometry of Thermotoga neapolitana and Influence of Temperature, Oxygen, and PH on Hydrogen Production. Biotechnol. Prog. 2009;25:1035–1042. doi: 10.1002/btpr.201. [DOI] [PubMed] [Google Scholar]
- 66.Cappelletti M., Zannoni D., Postec A., Ollivier B. Microbial BioEnergy: Hydrogen Production, Advances in Photosynthesis and Respiration. Volume 38. Springer; Dordrecht, The Netherlands: 2014. Members of the Order Thermotogales: From Microbiology to Hydrogen Production; pp. 197–224. [Google Scholar]
- 67.Pradhan N., d’Ippolito G., Dipasquale L., Esposito G., Panico A., Lens P.N.L., Fontana A. Simultaneous Synthesis of Lactic Acid and Hydrogen from Sugars via Capnophilic Lactic Fermentation by Thermotoga neapolitana Cf Capnolactica. Biomass Bioenergy. 2019;125:17–22. doi: 10.1016/j.biombioe.2019.04.007. [DOI] [Google Scholar]
- 68.Dreschke G., d’Ippolito G., Panico A., Lens P.N.L., Esposito G., Fontana A. Enhancement of Hydrogen Production Rate by High Biomass Concentrations of Thermotoga Neapolitana. Int. J. Hydrogen Energy. 2018;43:13072–13080. doi: 10.1016/j.ijhydene.2018.05.072. [DOI] [Google Scholar]
- 69.Dreschke G., Papirio S., Panico A., Lens P.N.L., Esposito G., d’Ippolito G., Fontana A. H2-Rich Biogas Recirculation Prevents Hydrogen Supersaturation and Enhances Hydrogen Production by Thermotoga neapolitana Cf. Capnolactica. Int. J. Hydrogen Energy. 2019;44:19698–19708. doi: 10.1016/j.ijhydene.2019.06.022. [DOI] [Google Scholar]
- 70.Dipasquale L., Pradhan N., d’Ippolito G., Fontana A. Grand Challenges in Marine Biotechnology. Springer; Dordrecht, The Netherlands: 2018. Potential of Hydrogen Fermentative Pathways in Marine Thermophilic Bacteria: Dark Fermentation and Capnophilic Lactic Fermentation in Thermotoga and Pseudothermotoga Species; pp. 217–235. [Google Scholar]
- 71.Nuzzo G., Landi S., Esercizio N., Manzo E., Fontana A., d’Ippolito G. Capnophilic Lactic Fermentation from Thermotoga neapolitana: A Resourceful Pathway to Obtain Almost Enantiopure L-Lactic Acid. Fermentation. 2019;5:34. doi: 10.3390/fermentation5020034. [DOI] [Google Scholar]
- 72.Takahata Y., Nishijima M., Hoaki T., Maruyama T. Thermotoga petrophila sp. Nov. and Thermotoga naphthophila Sp. Nov., Two Hyperthermophilic Bacteria from the Kubiki Oil Reservoir in Niigata, Japan. Int. J. Syst. Evol. Microbiol. 2001;51:1901–1909. doi: 10.1099/00207713-51-5-1901. [DOI] [PubMed] [Google Scholar]
- 73.Mori K., Yamazoe A., Hosoyama A., Ohji S., Fujita N., Ishibashi J.I., Kimura H., Suzuki K.I. Thermotoga profunda Sp. Nov. and Thermotoga caldifontis Sp. Nov., Anaerobic Thermophilic Bacteria Isolated from Terrestrial Hot Springs. Int. J. Syst. Evol. Microbiol. 2014;64:2128–2136. doi: 10.1099/ijs.0.060137-0. [DOI] [PubMed] [Google Scholar]
- 74.Jannasch H.W., Huber R., Belkin S., Stetter K.O. Thermotoga neapolitana Sp. Nov. of the Extremely Thermophilic, Eubacterial Genus Thermotoga. Arch. Microbiol. 1988;150:103–104. doi: 10.1007/BF00409725. [DOI] [Google Scholar]
- 75.Balk M., Weijma J., Stams A.J.M. Thermotoga Lettingae Sp. Nov., a Novel Thermophilic, Methanol-Degrading Bacterium Isolated from a Thermophilic Anaerobic Reactor. Int. J. Syst. Evol. Microbiol. 2002;52:1361–1368. doi: 10.1099/00207713-52-4-1361. [DOI] [PubMed] [Google Scholar]
- 76.Ravot G., Magot M., Fardeau M.L., Patel B.K.C., Prensier G., Egan A., Garcia J.L., Ollivier B. Thermotoga elfii Sp. Nov., a Novel Thermophilic Bacterium from an African Oil-Producing Well. Int. J. Syst. Bacteriol. 1995;45:308–314. doi: 10.1099/00207713-45-2-308. [DOI] [PubMed] [Google Scholar]
- 77.Fardeau M.L., Ollivier B.C., Patel B.K., Magot M., Thomas P., Rimbault A., Rocchiccioli F., Garcia J.L. Thermotoga hypogea Sp. Nov., a Xylanolytic, Thermophilic Bacterium from an Oil-Producing Well. Int. J. Syst. Bacteriol. 1997;147:51–56. doi: 10.1099/00207713-47-4-1013. [DOI] [PubMed] [Google Scholar]
- 78.Jeanthon C., Reysenbach A.L., L’Haridon S., Gambacorta A., Pace N.R., Glénat P., Prieur D. Thermotoga subterranea Sp. Nov., a New Thermophilic Bacterium Isolated from a Continental Oil Reservoir. Arch. Microbiol. 1995;164:91–97. doi: 10.1007/BF02525313. [DOI] [PubMed] [Google Scholar]
- 79.Friedricht A.B., Antranikian G. Keratin Degradation by Fervidobacterium pennavorans, a Novel Thermophilic Anaerobic Species of the Order Thermotogales. Appl. Environ. Microbiol. 1996;62:2875–2882. doi: 10.1128/AEM.62.8.2875-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huber R., Woese C.R., Langworthy T.A., Kristjansson J.K., Stetter K.O. Fervidobacterium islandicum Sp. Nov., a New Extremely Thermophilic Eubacterium Belonging to the “Thermotogales”. Arch. Microbiol. 1990;154:105–111. doi: 10.1007/BF00423318. [DOI] [Google Scholar]
- 81.Podosokorskaya O.A., Merkel Y.A., Kolganova T.V., Chernyh N.A., Miroshnichenko M.L., Bonch-Osmolovskaya E.A., Kublanov I.V. Fervidobacterium riparium Sp. Nov., a Thermophilic Anaerobic Cellulolytic Bacterium Isolated from a Hot Spring. Int. J. Syst. Evol. Microbiol. 2011;61:2697–2701. doi: 10.1099/ijs.0.026070-0. [DOI] [PubMed] [Google Scholar]
- 82.Andrews K.T., Patel B.K.C. Fervidobacterium gondwanense Sp. Nov., a New Thermophilic Anaerobic Bacterium Isolated from Nonvolcanically Heated Geothermal Waters of the Great Artesian Basin of Australia. Int. J. Syst. Bacteriol. 1996;46:265–269. doi: 10.1099/00207713-46-1-265. [DOI] [PubMed] [Google Scholar]
- 83.Kanoksilapatham W., Pasomsup P., Keawram P., Cuecas A., Portillo M.C., Gonzalez J.M. Fervidobacterium thailandense Sp. Nov., an Extremely Thermophilic Bacterium Isolated from a Hot Spring. Int. J. Syst. Evol. Microbiol. 2016;66:5023–5027. doi: 10.1099/ijsem.0.001463. [DOI] [PubMed] [Google Scholar]
- 84.Cai J., Wang Y., Liu D., Zeng Y., Xue Y., Ma Y., Feng Y. Fervidobacterium changbaicum Sp. Nov., a Novel Thermophilic Anaerobic Bacterium Isolated from a Hot Spring of the Changbai Mountains, China. Int. J. Syst. Evol. Microbiol. 2007;57:2333–2336. doi: 10.1099/ijs.0.64758-0. [DOI] [PubMed] [Google Scholar]
- 85.Huber R., Woese C.R., Langworthy T.A., Fricke H., Stetter K.O. Thermosipho Africanus Gen. Nov., Represents a New Genus of Thermophilic Eubacteria within the “Thermotogales”. Syst. Appl. Microbiol. 1989;12:32–37. doi: 10.1016/S0723-2020(89)80037-2. [DOI] [Google Scholar]
- 86.Takai K., Horikoshi K. Thermosipho japonicus Sp. Nov., an Extremely Thermophilic Bacterium Isolated from a Deep-Sea Hydrothermal Vent in Japan. Extremophiles. 2000;4:9–17. doi: 10.1007/s007920050002. [DOI] [PubMed] [Google Scholar]
- 87.L’Haridon S., Miroshnichenko M.L., Hippe H., Fardeau M.L., Bonch-Osmolovskaya E., Stackebrandt E., Jeanthon C. Thermosipho geolei Sp. Nov., a Thermophilic Bacterium Isolated from a Continental Petroleum Reservoir in Western Siberia. Int. J. Syst. Evol. Microbiol. 2001;51:1327–1334. doi: 10.1099/00207713-51-4-1327. [DOI] [PubMed] [Google Scholar]
- 88.Podosokorskaya O.A., Kublanov I.V., Reysenbach A.L., Kolganova T.V., Bonch-Osmolovskaya E.A. Thermosipho affectus Sp. Nov., a Thermophilic, Anaerobic, Cellulolytic Bacterium Isolated from a Mid-Atlantic Ridge Hydrothermal Vent. Int. J. Syst. Evol. Microbiol. 2011;61:1160–1164. doi: 10.1099/ijs.0.025197-0. [DOI] [PubMed] [Google Scholar]
- 89.Kuwabara T., Kawasaki A., Uda I., Sugai A. Thermosipho globiformans Sp. Nov., an Anaerobic Thermophilic Bacterium That Transforms into Multicellular Spheroids with a Defect in Peptidoglycan Formation. Int. J. Syst. Evol. Microbiol. 2011;61:1622–1627. doi: 10.1099/ijs.0.025106-0. [DOI] [PubMed] [Google Scholar]
- 90.Antoine E., Cilia V., Meunier J.R., Guezennec J., Lesongeur F., Barbier G. Thermosipho melanesiensis Sp. Nov., a New Thermophilic Anaerobic Bacterium Belonging to the Order Thermotogales, Isolated from Deep-Sea Hydrothermal Vents in the Southwestern Pacific Ocean. Int. J. Syst. Bacteriol. 1997;47:1118–1123. doi: 10.1099/00207713-47-4-1118. [DOI] [PubMed] [Google Scholar]
- 91.Podosokorskaya O.A., Bonch-Osmolovskaya E.A., Godfroy A., Gavrilov S.N., Beskorovaynaya D.A., Sokolova T.G., Kolganova T.V., Toshchakov S.V., Kublanov I.V. Thermosipho activus Sp. Nov., a Thermophilic, Anaerobic, Hydrolytic Bacterium Isolated from a Deep-Sea Sample. Int. J. Syst. Evol. Microbiol. 2014;64:3307–3313. doi: 10.1099/ijs.0.063156-0. [DOI] [PubMed] [Google Scholar]
- 92.Urios L., Cueff-Gauchard V., Pignet P., Postec A., Fardeau M.L., Ollivier B., Barbier G. Thermosipho atlanticus Sp. Nov., a Novel Member of the Thermotogales Isolated from a Mid-Atlantic Ridge Hydrothermal Vent. Int. J. Syst. Evol. Microbiol. 2004;54:1953–1957. doi: 10.1099/ijs.0.63069-0. [DOI] [PubMed] [Google Scholar]
- 93.L’Haridon S., Miroshnichenko M.L., Hippe H., Fardeau M.L., Stackebrandt E., Jeanthon C. Petrotoga olearia Sp. Nov. and Petrotoga sibirica Sp. Nov., Two Thermophilic Bacteria Isolated from a Continental Petroleum Reservoir in Western Siberia. Int. J. Syst. Evol. Microbiol. 2002;52:1715–1722. doi: 10.1099/00207713-52-5-1715. [DOI] [PubMed] [Google Scholar]
- 94.Lien T., Madsen M., Rainey F.A., Birkeland N.K. Petrotoga mobilis Sp. Nov., from a North Sea Oil-Production Well. Int. J. Syst. Bacteriol. 1998;48:1007–1013. doi: 10.1099/00207713-48-3-1007. [DOI] [PubMed] [Google Scholar]
- 95.Miranda-Tello E., Fardeau M.L., Joulian C., Magot M., Thomas P., Tholozan J.L., Olivier B. Petrotoga halophila Sp. Nov., a Thermophilic, Moderately Halophilic, Fermentative Bacterium Isolated from an Offshore Oil Well in Congo. Int. J. Syst. Evol. Microbiol. 2007;57:40–44. doi: 10.1099/ijs.0.64516-0. [DOI] [PubMed] [Google Scholar]
- 96.Miranda-Tello E., Fardeau M.L., Thomas P., Ramirez F., Casalot L., Cayol J.L., Garcia J.L., Ollivier B. Petrotoga mexicana Sp. Nov., a Novel Thermophilic, Anaerobic and Xylanolytic Bacterium Isolated from an Oil-Producing Well in the Gulf of Mexico. Int. J. Syst. Evol. Microbiol. 2004;54:169–174. doi: 10.1099/ijs.0.02702-0. [DOI] [PubMed] [Google Scholar]
- 97.Purwasena I.A., Sugai Y., Sasaki K. Petrotoga japonica Sp. Nov., a Thermophilic, Fermentative Bacterium Isolated from Yabase Oilfield in Japan. Arch Microbiol. 2014;196:313–321. doi: 10.1007/s00203-014-0972-4. [DOI] [PubMed] [Google Scholar]
- 98.Alain K., Marteinsson V.T., Miroshnichenko M.L., Bonch-Osmolovskaya E.A., Prieur D., Birrien J.-L. Marinitoga piezophila Sp. Nov., a Rod-Shaped, Thermo-Piezophilic Bacterium Isolated under High Hydrostatic Pressure from a Deep-Sea Hydrothermal Vent. Int. J. Syst. Evol. Microbiol. 2002;52:1331–1339. doi: 10.1099/00207713-52-4-1331. [DOI] [PubMed] [Google Scholar]
- 99.Postec A., Ciobanu M., Birrien J.L., Bienvenu N., Prieur D., Le Romancer M. Marinitoga litoralis Sp. Nov., a Thermophilic, Heterotrophic Bacterium Isolated from a Coastal Thermal Spring on Île Saint-Paul, Southern Indian Ocean. Int. J. Syst. Evol. Microbiol. 2010;60:1778–1782. doi: 10.1099/ijs.0.017574-0. [DOI] [PubMed] [Google Scholar]
- 100.Nunoura T., Oida H., Miyazaki M., Suzuki Y., Takai K., Horikoshi K. Marinitoga okinawensis Sp. Nov., a Novel Thermophilic and Anaerobic Heterotroph Isolated from a Deep-Sea Hydrothermal Field, Southern Okinawa Trough. Int. J. Syst. Evol. Microbiol. 2007;57:467–471. doi: 10.1099/ijs.0.64640-0. [DOI] [PubMed] [Google Scholar]
- 101.Postec A., Le Breton C., Fardeau M.L., Lesongeur F., Pignet P., Querellou J., Ollivier B., Godfroy A. Marinitoga hydrogenitolerans Sp. Nov., a Novel Member of the Order Thermotogales Isolated from a Black Smoker Chimney on the Mid-Atlantic Ridge. Int. J. Syst. Evol. Microbiol. 2005;55:1217–1221. doi: 10.1099/ijs.0.63550-0. [DOI] [PubMed] [Google Scholar]
- 102.Steinsbu B.O., Røyseth V., Thorseth I.H., Steen I.H. Marinitoga arctica Sp. Nov., a Thermophilic, Anaerobic Heterotroph Isolated from a Mid-Ocean Ridge Vent Field. Int. J. Syst. Evol. Microbiol. 2016;66:5070–5076. doi: 10.1099/ijsem.0.001472. [DOI] [PubMed] [Google Scholar]
- 103.Nunoura T., Hirai M., Imachi H., Miyazaki M., Makita H., Hirayama H., Furushima Y., Yamamoto H., Takai K. Kosmotoga arenicorallina Sp. Nov. a Thermophilic and Obligately Anaerobic Heterotroph Isolated from a Shallow Hydrothermal System Occurring within a Coral Reef, Southern Part of the Yaeyama Archipelago, Japan, Reclassification of Thermococcoides shengliensis. Arch. Microbiol. 2010;192:811–819. doi: 10.1007/s00203-010-0611-7. [DOI] [PubMed] [Google Scholar]
- 104.L’Haridon S., Jiang L., Alain K., Chalopin M., Rouxel O., Beauverger M., Xu H., Shao Z., Jebbar M. Kosmotoga pacifica Sp. Nov., a Thermophilic Chemoorganoheterotrophic Bacterium Isolated from an East Pacific Hydrothermal Sediment. Extremophiles. 2014;18:81–88. doi: 10.1007/s00792-013-0596-7. [DOI] [PubMed] [Google Scholar]
- 105.Van Ooteghem S.A., Beer S.K., Yue P.C. Hydrogen Production by the Thermophilic Bacterium Thermotoga neapolitana. Appl. Biochem. Biotechnol. 2002;98–100:177–189. doi: 10.1385/ABAB:98-100:1-9:177. [DOI] [PubMed] [Google Scholar]
- 106.Eriksen N.T., Nielsen T.M., Iversen N. Hydrogen Production in Anaerobic and Microaerobic Thermotoga neapolitana. Biotechnol. Lett. 2008;30:103–109. doi: 10.1007/s10529-007-9520-5. [DOI] [PubMed] [Google Scholar]
- 107.Boileau C., Auria R., Davidson S., Casalot L., Christen P., Liebgott P.P., Combet-Blanc Y. Hydrogen Production by the Hyperthermophilic Bacterium Thermotoga maritima Part I: Effects of Sulfured Nutriments, with Thiosulfate as Model, on Hydrogen Production and Growth. Biotechnol. Biofuels. 2016;9:1–17. doi: 10.1186/s13068-016-0678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Niel E.W.J., Budde M.A.W., De Haas G., van der Wal F.J., Claassen P.A.M., Stams A.J.M. Distinctive Properties of High Hydrogen Producing Extreme Thermophiles, Caldicellulosiruptor saccharolyticus and Thermotoga elfii. Int. J. Hydrogen Energy. 2002;27:1391–1398. doi: 10.1016/S0360-3199(02)00115-5. [DOI] [Google Scholar]
- 109.Hawkes F., Hussy I., Kyazze G., Dinsdale R., Hawkes D. Continuous Dark Fermentative Hydrogen Production by Mesophilic Microflora: Principles and Progress. Int. J. Hydrogen Energy. 2007;32:172–184. doi: 10.1016/j.ijhydene.2006.08.014. [DOI] [Google Scholar]
- 110.Nguyen T.A.D., Han S.J., Kim J.P., Kim M.S., Sim S.J. Hydrogen Production of the Hyperthermophilic Eubacterium, Thermotoga neapolitana under N2 Sparging Condition. Bioresour. Technol. 2010;101:S38–S41. doi: 10.1016/j.biortech.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 111.Karadagli F., Marcus A.K., Rittmann B.E. Role of Hydrogen (H2) Mass Transfer in Microbiological H2-Threshold Studies. Biodegradation. 2019;30:113–125. doi: 10.1007/s10532-019-09870-1. [DOI] [PubMed] [Google Scholar]
- 112.Nath K., Das D. Improvement of Fermentative Hydrogen Production: Various Approaches. Appl. Microbiol. Biotechnol. 2004;65:520–529. doi: 10.1007/s00253-004-1644-0. [DOI] [PubMed] [Google Scholar]
- 113.Ngo T.A., Mi-sun K., Sim S.J. Thermophilic Hydrogen Fermentation Using Thermotoga neapolitana DSM 4359 by Fed-Batch Culture. Int. J. Hydrogen Energy. 2011;36:14014–14023. doi: 10.1016/j.ijhydene.2011.04.058. [DOI] [Google Scholar]
- 114.Pradhan N., Dipasquale L., d’Ippolito G., Fontana A., Panico A., Lens P.N.L., Pirozzi F., Esposito G. Kinetic modeling of fermentative hydrogen production by Thermotoga neapolitana. Int. J. Hydrogen Energy. 2016;41:1–10. doi: 10.1016/j.ijhydene.2016.01.107. [DOI] [Google Scholar]
- 115.Mizuno O., Dinsdale R., Hawkes F.R., Hawkes D.L. Enhancement of Hydrogen Production from Glucose by Nitrogen Gas Sparging. Bioresour. Technol. 2000;73:59–65. doi: 10.1016/S0960-8524(99)00130-3. [DOI] [Google Scholar]
- 116.Ngo T.A., Kim M.S., Sim S.J. High-Yield Biohydrogen Production from Biodiesel Manufacturing Waste by Thermotoga neapolitana. Int. J. Hydrogen Energy. 2011;36:5836–5842. doi: 10.1016/j.ijhydene.2010.11.057. [DOI] [Google Scholar]
- 117.D’Ippolito G., Dipasquale L., Fontana A. Recycling of Carbon Dioxide and Acetate as Lactic Acid by the Hydrogen-Producing Bacterium Thermotoga neapolitana. ChemSuschem. 2014;7:2678–2683. doi: 10.1002/cssc.201402155. [DOI] [PubMed] [Google Scholar]
- 118.Pradhan N., Dipasquale L., d’Ippolito G., Fontana A., Panico A., Pirozzi F., Lens P.N.L., Esposito G. Model Development and Experimental Validation of Capnophilic Lactic Fermentation and Hydrogen Synthesis by Thermotoga neapolitana. Water Res. 2016;99:225–234. doi: 10.1016/j.watres.2016.04.063. [DOI] [PubMed] [Google Scholar]
- 119.Dipasquale L., Adessi A., d’Ippolito G., Rossi F., Fontana A., De Philippis R. Introducing Capnophilic Lactic Fermentation in a Combined Dark-Photo Fermentation Process: A Route to Unparalleled H2 Yields. Appl. Microbiol. Biotechnol. 2015;99:1001–1010. doi: 10.1007/s00253-014-6231-4. [DOI] [PubMed] [Google Scholar]
- 120.D’Ippolito G., Landi S., Esercizio N., Lanzilli M., Vastano M., Dipasquale L., Pradhan N., Fontana A. CO2-Induced Transcriptional Reorganization: Molecular Basis of Capnophillic Lactic Fermentation in Thermotoga neapolitana. Front. Microbiol. 2020;11:171. doi: 10.3389/fmicb.2020.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Van Ooteghem S.A., Jones A., Van Der Lelie D., Dong B., Mahajan D. H2 Production and Carbon Utilization by Thermotoga neapolitana under Anaerobic and Microaerobic Growth Conditions. Biotechnol. Lett. 2004;26:1223–1232. doi: 10.1023/B:BILE.0000036602.75427.88. [DOI] [PubMed] [Google Scholar]
- 122.Ngo T.A., Sim S.J. Dark Fermentation of Hydrogen from Waste Glycerol Using Hyperthermophilic Eubacterium Thermotoga neapolitana. Environ. Prog. Sustain. Energy. 2011;31:466–473. doi: 10.1002/ep.10578. [DOI] [Google Scholar]
- 123.Juszczak A., Aono S., Adams M.W.W. The Extremely Thermophilic Eubacterium, Thermotoga maritima Contains a Novel Iron-Hydrogenase Whose Cellular Activity Is Dependent Upon Tungsten. J. Bacteriol. Chem. 1991;226:13834–13841. [PubMed] [Google Scholar]
- 124.Rusch A., Walpersdorf E., DeBeer D., Gurrier S., Amend P.J. Microbial Communities near the Oxic/Anoxic Interface in the Hydrothermal System of Vulcano Island, Italy. Chem. Geol. 2005;224:169–182. doi: 10.1016/j.chemgeo.2005.07.026. [DOI] [Google Scholar]
- 125.Belkin S., Wirsen C.O., Jannasch H.W. A New Sulfur-Reducing, Extremely Thermophilic Eubacterium from a Submarine Thermal Vent. Appl. Environ. Microbiol. 1986;51:1180–1185. doi: 10.1128/AEM.51.6.1180-1185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Childers S.E., Vargas M., Noll K.M. Improved Methods for Cultivation of the Extremely Thermophilic Bacterium Thermotoga neapolitana. Appl. Environ. Microbiol. 1992;58:3949–3953. doi: 10.1128/AEM.58.12.3949-3953.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Le Fourn C., Fardeau M.L., Ollivier B., Lojou E., Dolla A. The Hyperthermophilic Anaerobe Thermotoga maritima Is Able to Cope with Limited Amount of Oxygen: Insights into Its Defence Strategies. Environ. Microbiol. 2008;10:1877–1887. doi: 10.1111/j.1462-2920.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- 128.Tosatto S.C.E., Toppo S., Carbonera D., Giacometti G.M., Costantini P. Comparative Analysis of [FeFe] Hydrogenase from Thermotogales Indicates the Molecular Basis of Resistance to Oxygen Inactivation. Int. J. Hydrogen Energy. 2008;33:570–578. doi: 10.1016/j.ijhydene.2007.10.010. [DOI] [Google Scholar]
- 129.Lakhal R., Auria R., Davidson S., Ollivier B., Dolla A., Hamdi M., Combet-Blanc Y. Effect of Oxygen and Redox Potential on Glucose Fermentation in Thermotoga maritima under Controlled Physicochemical Conditions. Int. J. Microbiol. 2010;2010:896510. doi: 10.1155/2010/896510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaslin S.A., Childers S.E., Noll K.M. Membrane-Associated Redox Activities in Thermotoga neapolitana. Arch. Microbiol. 1998;170:297–303. doi: 10.1007/s002030050645. [DOI] [PubMed] [Google Scholar]
- 131.Yang X., Ma K. Purification and Characterization of an NADH Oxidase from Extremely Thermophilic Anaerobic Bacterium Thermotoga hypogea. Arch. Microbiol. 2005;183:331–337. doi: 10.1007/s00203-005-0777-6. [DOI] [PubMed] [Google Scholar]
- 132.Yang X., Ma K. Characterization of an Exceedingly Active NADH Oxidase from the Anaerobic Hyperthermophilic Bacterium Thermotoga maritima. J. Bacteriol. 2007;189:3312–3317. doi: 10.1128/JB.01525-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mangayil R., Aho T., Karp M., Santala V. Improved Bioconversion of Crude Glycerol to Hydrogen by Statistical Optimization of Media Components. Renew. Energy. 2015;75:583–589. doi: 10.1016/j.renene.2014.10.051. [DOI] [Google Scholar]
- 134.Abdullah M.F., Md Jahim J., Abdul P.M., Mahmod S.S. Effect of Carbon/Nitrogen Ratio and Ferric Ion on the Production of Biohydrogen from Palm Oil Mill Effluent (POME) Biocatal. Agric. Biotechnol. 2020;23:101445. doi: 10.1016/j.bcab.2019.101445. [DOI] [Google Scholar]
- 135.Munro S.A., Choe L., Zinder S.H., Lee K.H., Walker L.P. Proteomic and Physiological Experiments to Test Thermotoga neapolitana Constraint-Based Model Hypotheses of Carbon Source Utilization. Biotechnol. Prog. 2012;28:312–318. doi: 10.1002/btpr.735. [DOI] [PubMed] [Google Scholar]
- 136.Rinker K.D., Kelly R.M. Effect of Carbon and Nitrogen Sources on Growth Dynamics and Exopolysaccharide Production for the Hyperthermophilic Archaeon Thermococcus Litoralis and Bacterium Thermotoga maritima. Biotechnol. Bioeng. 2000;69:537–547. doi: 10.1002/1097-0290(20000905)69:5<537::AID-BIT8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 137.Van Niel E.W.J., Hahn-Hägerdal B. Nutrient Requirements of Lactococci in Defined Growth Media. Appl. Microbiol. Biotechnol. 1999;52:617–627. doi: 10.1007/s002530051569. [DOI] [Google Scholar]
- 138.Maru B.T., Bielen A.A.M., Kengen S.W.M., Constantí M., Medinaa F. Biohydrogen Production from Glycerol Using Thermotoga Spp. Energy Procedia. 2012;29:300–307. doi: 10.1016/j.egypro.2012.09.036. [DOI] [Google Scholar]
- 139.Kengen S.W.M., Stams A.J.M. Formation of L-Alanine as a Reduced End Product in Carbohydrate Fermentation by the Hyperthermophilic Archaeon Pyrococcus Furiosus. Arch. Microbiol. 1994;161:168–175. doi: 10.1007/BF00276479. [DOI] [Google Scholar]
- 140.Weiner R., Langille S., Quintero E. Structure, Function, and Immu- Nochemistry of Bacterial Exopolysaccharides. J. Ind. Microbiol. 1995;15:339–346. doi: 10.1007/BF01569989. [DOI] [PubMed] [Google Scholar]
- 141.Pierra M., Trably E., Godon J.J., Bernet N. Fermentative Hydrogen Production under Moderate Halophilic Conditions. Int. J. Hydrogen Energy. 2014;39:7508–7517. doi: 10.1016/j.ijhydene.2013.08.035. [DOI] [Google Scholar]
- 142.Liu Q., Chen W., Zhang X., Yu L., Zhou J., Xu Y., Qian G. Phosphate Enhancing Fermentative Hydrogen Production from Substrate with Municipal Solid Waste Composting Leachate as a Nutrient. Bioresour. Technol. 2015;190:431–437. doi: 10.1016/j.biortech.2015.01.139. [DOI] [PubMed] [Google Scholar]
- 143.Chang F., Lin C. Calcium Effect on Fermentative Hydrogen Production in an Anaerobic Up-Flow Sludge Blanket System. Water Sci. Technol. 2006;54:105–112. doi: 10.2166/wst.2006.867. [DOI] [PubMed] [Google Scholar]
- 144.Ravot G., Ollivier B., Magot M., Patel B.K.C., Crolet J.L., Fardeau M.L., Garcia J.L. Thiosulfate Reduction, an Important Physiological Feature Shared by Members of the Order Thermotogales. Appl. Environ. Microbiol. 1995;61:2053–2055. doi: 10.1128/AEM.61.5.2053-2055.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ravot G., Ollivier B., Fardeau M.L., Patel B.K.C., Andrews K.T., Magot M., Garcia J.L. L-Alanine Production from Glucose Fermentation by Hyperthermophilic Members of the Domains Bacteria and Archaea: A Remnant of an Ancestral Metabolism? Appl. Environ. Microbiol. 1996;62:2657–2659. doi: 10.1128/AEM.62.7.2657-2659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ainala S.K., Seol E., Kim J.R., Park S. Effect of Culture Medium on Fermentative and CO-Dependent H2 Production Activity in Citrobacter Amalonaticus Y19. Int. J. Hydrogen Energy. 2016;41:6734–6742. doi: 10.1016/j.ijhydene.2016.03.088. [DOI] [Google Scholar]
- 147.Huber R., Stetter K.O. The Prokaryotes. Springer; New York, NY, USA: 1992. The Order Thermotogales; pp. 93809–93815. [Google Scholar]
- 148.Hania W.B., Fadhlaoui K., Brochier-Armanet C., Persillon C., Postec A., Hamdi M., Dolla A., Ollivier B., Fardeau M.L., Le Mer J., et al. Draft Genome Sequence of Mesotoga Strain PhosAC3, a Mesophilic Member of the Bacterial Order Thermotogales, Isolated from a Digestor Treating Phosphogypsum in Tunisia. Stand. Genom. Sci. 2015;10:1–7. doi: 10.1186/1944-3277-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nesbø C.L., Charchuk R., Pollo S.M.J., Budwill K., Kublanov I.V., Haverkamp T.H.A., Foght J. Genomic Analysis of the Mesophilic Thermotogae Genus Mesotoga Reveals Phylogeographic Structure and Genomic Determinants of Its Distinct Metabolism. Environ. Microbiol. 2019;21:456–470. doi: 10.1111/1462-2920.14477. [DOI] [PubMed] [Google Scholar]
- 150.Tao Z., Yang Q., Yao F., Huang X., Wu Y., Du M., Chen S., Liu X., Li X., Wang D. The Inhibitory Effect of Thiosulfinate on Volatile Fatty Acid and Hydrogen Production from Anaerobic Co-Fermentation of Food Waste and Waste Activated Sludge. Bioresour. Technol. 2020;297:122428. doi: 10.1016/j.biortech.2019.122428. [DOI] [PubMed] [Google Scholar]
- 151.Slobodkin A.I. Thermophilic Microbial Metal Reduction. Microbiologia. 2005;74:581–595. doi: 10.1007/s11021-005-0096-6. [DOI] [PubMed] [Google Scholar]
- 152.Gomez-Romero J., Gonzalez-Garcia A., Chairez I., Torres L., García-Peña E.I. Selective Adaptation of an Anaerobic Microbial Community: Biohydrogen Production by Co-Digestion of Cheese Whey and Vegetables Fruit Waste. Int. J. Hydrogen Energy. 2014;39:12541–12550. doi: 10.1016/j.ijhydene.2014.06.050. [DOI] [Google Scholar]
- 153.Llanos J., Capasso C., Parisi E., Prieur D., Jeanthon C. Susceptibility to Heavy Metals and Cadmium Accumulation in Aerobic and Anaerobic Thermophilic Microorganisms Isolated from Deep-Sea Hydrothermal Vents. Curr. Microbiol. 2000;41:0201–0205. doi: 10.1007/s00284431056. [DOI] [PubMed] [Google Scholar]
- 154.Vargas M., Kashefi K., Blunt-harris E.L., Lovley D.R. Fe (III) Reduction on Early Earth. Nature. 1998;395:65–67. doi: 10.1038/25720. [DOI] [PubMed] [Google Scholar]
- 155.Balch W.E., Fox G.E., Magrum L.J., Woese C.R., Wolfe R.S. Methanogens: Reevaluation of a Unique Biological Group. Microbiol. Rev. 1979;43:260–296. doi: 10.1128/MR.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee Y.J., Miyahara T., Noike T. Effect of Iron Concentration on Hydrogen Fermentation. Bioresour. Technol. 2001;80:227–231. doi: 10.1016/S0960-8524(01)00067-0. [DOI] [PubMed] [Google Scholar]
- 157.Zhang Y., Shen J. Effect of Temperature and Iron Concentration on the Growth and Hydrogen Production of Mixed Bacteria. Int. J. Hydrogen Energy. 2006;31:441–446. doi: 10.1016/j.ijhydene.2005.05.006. [DOI] [Google Scholar]
- 158.Ying X., Wang Y., Badiei H.R., Karanassios V., Ma K. Purification and Characterization of an Iron-Containing Alcohol Dehydrogenase in Extremely Thermophilic Bacterium Thermotoga hypogea. Arch. Microbiol. 2007;187:499–510. doi: 10.1007/s00203-007-0217-x. [DOI] [PubMed] [Google Scholar]
- 159.Trivedi S., Rao S.R., Gehlot H.S. Nucleic Acid Stability in Thermophilic Prokaryotes: A Review Nucleic Acid Stability in Thermophilic Prokaryotes: A Review. J. Cell Mol. Biol. 2005;4:61–69. [Google Scholar]