Abstract
Aberrant glycosylation is a universal feature of cancer cells that can impact all steps in tumour progression from malignant transformation to metastasis and immune evasion. One key change in tumour glycosylation is altered core fucosylation. Core fucosylation is driven by fucosyltransferase 8 (FUT8), which catalyses the addition of α1,6-fucose to the innermost GlcNAc residue of N-glycans. FUT8 is frequently upregulated in cancer, and plays a critical role in immune evasion, antibody-dependent cellular cytotoxicity (ADCC), and the regulation of TGF-β, EGF, α3β1 integrin and E-Cadherin. Here, we summarise the role of FUT8 in various cancers (including lung, liver, colorectal, ovarian, prostate, breast, melanoma, thyroid, and pancreatic), discuss the potential mechanisms involved, and outline opportunities to exploit FUT8 as a critical factor in cancer therapeutics in the future.
Keywords: fut8, cancer, glycosylation, fucosylation
1. Glycosylation in Cancer
Glycosylation is the enzymatic process that produces glycosidic linkages of saccharides to other saccharides, proteins or lipids. It is one of the most commonly occurring post-translational modifications and one of the most complex [1,2,3]. Aberrant glycosylation is an established hallmark of cancer that plays a critical role in tumour biology [1,4,5,6,7]. Changes to glycans in cancer were initially described more than 50 years ago [8], and since then technological advances have highlighted cell-surface glycans and changes in glycosylation patterns as key drivers of cell signalling, tumour immunology, metastasis, and how tumours respond to therapy [1,4,9,10,11,12,13]. A wide range of alterations to glycans have been observed in cancer, and tumour cells consistently express specific changes to glycan epitopes, including truncated O-glycans, altered N-glycan branching, increased sialylation (the addition of sialic acid to the terminal end of glycoproteins) and increased fucosylation [4,14,15]. These modifications to glycans can be driven by the altered expression of glycosyltranserases in cancer cells.
2. Fucosylation
Fucosylation is a type of glycosylation, which results in the attachment of a fucose residue to N-glycans, O-glycans, and glycolipids. Fucosylation can be divided into terminal and core fucosylation. Fucosylated glycans are synthesized by a range of fucosyltransferases (Fut), of which 13 have been identified in the human genome (Table 1) [16,17]. FUT8 is unique in that it is the only Fut responsible for core-fucosylation on N-glycoproteins (as most of the other fucosyltransferases are functionally redundant this makes core fucosylation unique) [16,18,19,20].
Table 1.
| Common Name(s) | Abbreviation | Subcellular Location [23] | Representative Major Products |
|---|---|---|---|
| H blood group α2fucosyltransferase |
FUT1 | Membrane |
|
| Secretor (Se) blood group α2fucosyltransferase |
FUT2 | Plasma membrane Cytosol |
|
| Fuc-TII α3/4fucosyltransferase Lewis blood group fucosyltransferase |
FUT3 | Intracellular membrane (different isoforms) |
Sialyl-Lewis Structures Lewis Structures 
|
| Fuc-TIV α3fucosyltransferase ELAM-1 ligand fucosyltransferase |
FUT4 | Vesicles |
|
| Fuc-TV α3fucosyltransferase |
FUT5 | Membrane |
|
| Fuc-VI α3fucosyltransferase |
FUT6 | Golgi apparatus |
|
| Fuc-VII α3fucosyltransferase |
FUT7 | Golgi apparatus |
|
| Fuc-VIII α3fucosyltransferase |
FUT8 | Golgi apparatus Cytosol |
|
| Fuc-TIX α3fucosyltransferase |
FUT9 | Nucleoplasm Endoplasmic reticulum Golgi apparatus |
|
| Fuc-TX α3fucosyltransferase |
FUT10 | Nucleoplasm Endoplasmic reticulum Golgi apparatus |
Unknown |
| Fuc-TXI α3fucosyltransferase |
FUT11 | Nuclear membrane Golgi apparatus |
Unknown |
| Protein O-fucosyltransferase 1 |
POFUT1/FUT12 | Centrosome |
|
| Protein O-fucosyltransferase 2 |
POFUT2/FUT13 | Intracellular |
|
Core Fucosylation
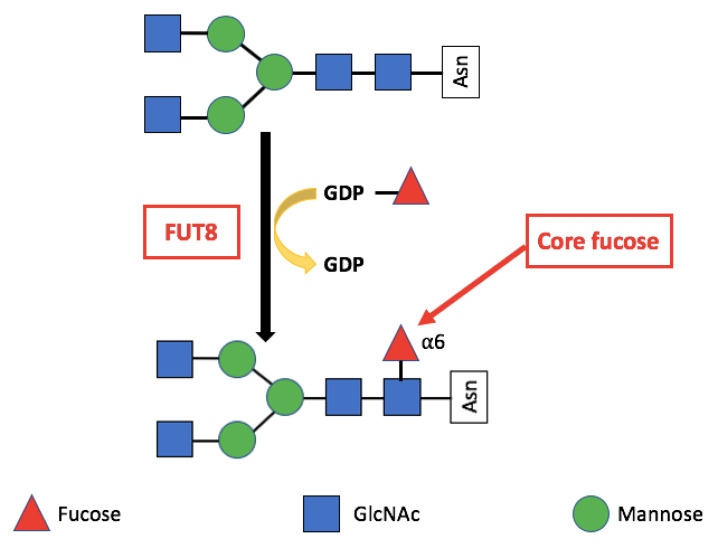
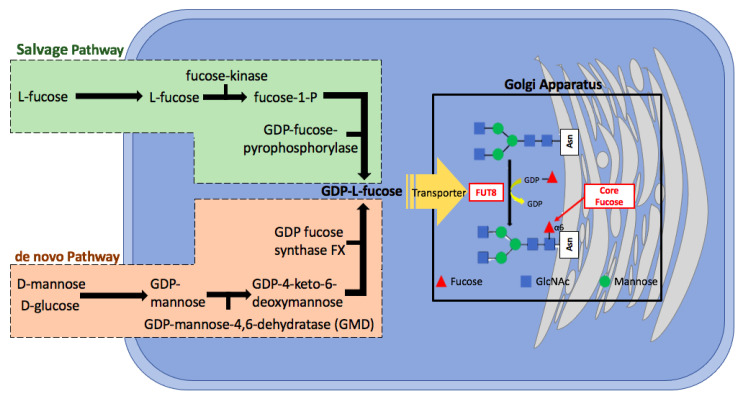
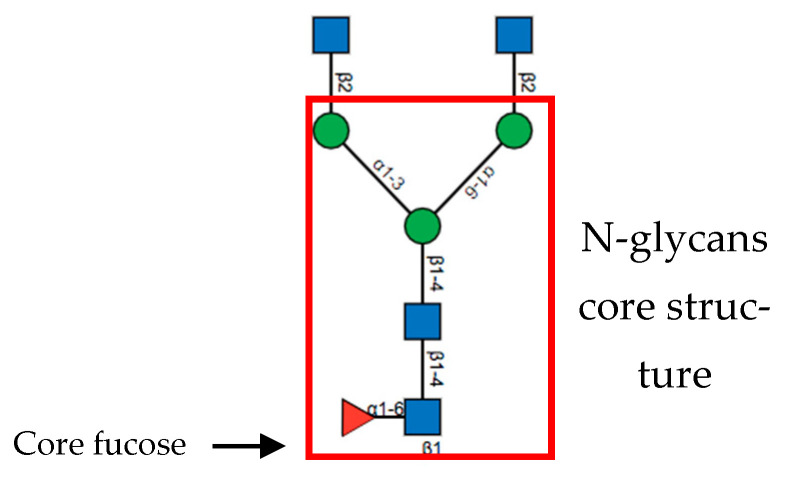
FUT8 mediated core fucosylation is an important post-translational modification, that has been linked to cancer cell invasion and metastasis [24,25,26,27,28,29,30,31]. In the Golgi apparatus FUT8 transfers an l-fucose reside from guanosine diphosphate (GDP-β-l-fucose) (GDP-Fuc) onto the innermost GlcNAc of an N-glycan to form an α-1,6 linkage (Figure 1). Core fucose levels can be affected by the level of GDP-Fuc substrate and/or its transport into the Golgi [16,32,33]. GDP-fuose is generated in the cytosol by two distinct pathways from l-fucose, the more dominate de novo synthesis and the salvage pathway (Figure 2). The de novo pathway transforms GDP-mannose to GDP-fucose via three enzymatic reactions carried out by two proteins, GDP-mannose 4,6-dehydratase (GMD) and a second enzyme, GDP-keto-6-deoxymannose 3,5-epimerase, 4-reductase (otherwise referred to as the FX protein) [3,34]. The de novo pathway accounts for 90% of GDP-fucose synthesis and was discovered over 40 years ago. In contrast the salvage pathway synthesizes GDP-fucose from free fucose derived from extracellular or lysosomal sources [16].
Figure 1.
The reaction catalysed by FUT8. FUT8 transfers an l-fucose reside from GDP-β-l-fucose (GDP-Fuc) onto the innermost GlcNAc of an N-glycan to form an α-1,6 linkage.
Figure 2.
The sythetic pathway for GDP-fucose. GDP-fucose, an essential component of core fucosyaltion is produced by two different pathways within the cell. The predominat de novo pathway relies on GMD and FX proteins. Adapted from [32].
3. Alpha-(1,6)-Fucosyltransferase (FUT8)
The Fucosyltransferase 8 (FUT8) gene is located on chromosome 14q23.3 and encodes an enzyme that catalyses core fucosylation—an essential N-glycan modification [35]. FUT8 is the only enzyme responsible for core-fucosylation on N-glycoproteins [19]. It is expressed in various human tissues with particularly high levels in the brain, placenta, lung, stomach, and small intestine [36].
It is worth noting the physiological role of FUT8 is crucial: homozygous knockout mice experience early postnatal death, severe growth retardation and emphysema-like changes in the lung, suggesting the importance of this fucose modification for growth factor receptor activation [37]. No oligosaccharide structures with core fucose are present in FUT8 knockout mice, and 70–80% of mice die two to three days after birth. This is in contrast to the other FUT enzymes, for which loss of function does not cause lethality [18,32,38,39].
Structure
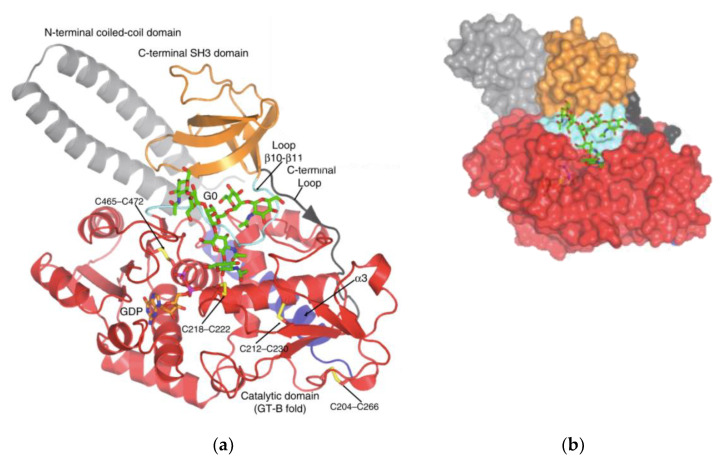
Several glycosyltransferases have been crystallographically analysed to identify two structural superfamilies (GT-A and GT-B) [40]. GT-A enzymes have the DXD or EXD motif, containing a Rossman fold (a nucleotide-sugar binding domain) of two tightly associated domains at the N-terminus. GT-B enzymes have folds involving two similar Rossman folds [41,42]. Even though FUT8 contains one Rossman fold, the catalytic site of FUT8 lacks the characteristic elements typical of GT-A enzymes. FUT8 is thought to have a catalytic region closer to GT-B enzymes. Similar to those of GT-B enzyme, FUT8 also has a DXD motif and is fully active without a metal ion.
FUT8 is a multi-domain enzyme containing an N-terminal coiled-coil domain, a catalytic domain (which adopts a GT-B fold), and a luminal C-terminal Src homology 3 (SH3) domain revealed by the crystal structure (Figure 3) [40]. The acceptor specificity of FUT8 requires that a terminal GlcNAc moiety on the α1,3 arm of the N-glycan is present, with more flexibility on the α1,6 arm [43]. A peptide/protein is not required unless FUT8 is fucosylating a high mannose N-glycan lacking a GlcNAc moiety, in which case a peptide/protein is required [43]. For many years the reaction mechanism for FUT8 remained elusive. Recently, it was discovered that the FUT8 structure allows for conformational changes similar to a SN2 single displacement reaction mechanism. What differentiates FUT8 is that instead of an amino acid acting as a catalytic base already present in the binding site in the absence of ligands, the catalytic base is brought to the binding site in the presence of ligands. It is with this newly provided information on the structure and enzymatic mechanism of FUT8 that the development of inhibitors could be developed for cancer therapy.
Figure 3.
Structure of FUT8. Published by [43] and reproduced with permission. (a) Ribbon structure of HsFUT8 with orange carbon atoms representing GDP and green carbon atoms representing a bi-antennary complex N-glycan (G0). The coiled-coil domain is colored in gray, the catalytic domain in red, and the SH3 domain in orange. The interdomain α3 and loop β10–β11 are colored in blue and aquamarine, respectively. Yellow sulfur atoms indicate disulfide bridges. The C-terminal loop is colored in black. Electron density maps are FO–FC (blue) contoured at 2.2σ for GDP and G0. (b) Surface representation of the HsFUT8-GDP-G0 complex.
The SH3 domain is typical of signal transduction molecules in the cytosol, but is unique to FUT8 compared to other glycosyltransferases (and likely plays are an important role in FUT8’s activity and core fucosylation) [44]. FUT8 is mainly localized to the Golgi with a type II topology, however, Tomida et al. have reported that FUT8 can be partially localized to the cell surface in an SH3-dependent manner [44,45]. Amino acid His535 in the SH3 domain is an essential residue for the enzymatic activity of FUT8, and ribophorin I (RPN1) has been identified as an SH3-dependent binding protein of FUT8, that can stimulate core fucosylation [44].
4. FUT8 Expression in Cancer
Aberrant fucosylation is one of the most important oligiosaccharide modifications involved in cancer and inflammation and is often caused by dysregulated expression of fucosyltransferases (FUTs) [1,12,32,46,47,48]. α1,6-fucosyltransferase, encoded by FUT8, has demonstrated involvement in biological and tumour characteristics and is upregulated in various cancers including lung [20,49], liver [50,51,52], colorectal [24], ovarian [53], prostate [54], breast [30], melanoma [55], thyroid [56], and pancreatic [27] (Table 2). FUT8 has been associated with patient outcomes, and is a suggested prognostic biomarker for patients with lung cancer [31], colorectal cancer [51] and prostate cancer [5].
Table 2.
FUT8 in cancer.
| Lung Cancer [20,28,49] |
|
| Liver Cancer [50,52] |
|
| Colorectal Cancer [24] |
|
| Ovarian Cancer [53] |
|
| Prostate Cancer [5,57] |
|
| Breast Cancer [30] |
|
| Thyroid Cancer [56] |
|
| Melanoma [55] |
|
| Pancreatic Cancer [27] |
|
Fucosylated glycoproteins hold promise as cancer biomarkers [5,32,58]. At present, there is an absence of clinically relevant biomarkers for predicting immunotherapy treatment outcomes. α1-Acid glycoprotein (AGP) is a major serum glycoprotein that possesses a range of immunomodulating effects and has five N-linked complex type glycan structures [59]. During periods of acute and chronic inflammation, particularly in the presence of tumours, the glycan structures on AGP change dramatically. AGP glycoforms containing highly fucosylated triantennary and tetraantennary sugar chains have been detected in the serum of patients with various advanced malignancies and are associated with poor prognosis [60]. Above normal levels of tri- and tetraantennary glycan chains in AGP (FUCAGP) have also been linked to poor prognosis in patients with esophagus, stomach, lung, breast, liver, pancreas, colon and rectum cacinomas [59,61,62]. Moreover, α1,3fucosylated AGP (fAGP) has been proposed as a marker of disease progression and prognosis in various cancers. In patients with advanced lung cancer, fAGP levels have been shown to predict a good and/or poor response to the immune checkpoint inhibitor Nivolumab [59]. Collectively, these examples emphasize the importance of fucosylated glycoproteins as potential biomarkers of cancer.
4.1. FUT8 Expression in Lung Cancer
Lung cancer is the most commonly diagnosed cancer and is the leading cause of cancer death worldwide [63]. Lung cancer can be separated into two main forms which are classified by the type of cells from which the cancer originates [64]. The most common form (affecting 87% of lung cancer patients) is non-small-cell lung cancer which can be further divided into three types—squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma. The less common form but more metastatic is small-cell lung cancer. FUT8 is up-regulated in non-small cell lung cancer (NSCLC) and is correlated with tumour metastasis, disease recurrence and poor survival in patients [49]. One study utilized aggressive lung cancer cell lines (CL1-5 and PC14) and found FUT8 knockdown significantly inhibited their malignant behaviours, including in vitro invasion and cell proliferation, and in vivo metastasis and tumour growth [49]. A study by Honma et al., examined the expression of FUT8 in 129 NSCLCs using immunohistochemistry and identified that over half of the samples exhibited high expression of FUT8, which was also associated with poor survival [31]. The prognostic potential of FUT8 is further supported by its significant association with unfavourable clinical outcomes in patients with potentially curatively resected NSCLCs [31].
Core fucosylation is needed for the proper function of the TGF-β receptor and thus TGF-β–induced epithelial-mesenchymal transition (EMT) [49,65]. In support of this, FUT8 has been shown to be up-regulated during EMT in several cancers suggesting a positive feedback loop that promotes EMT and tumour development [49]. A proposed molecular mechanism linking FUT8 and lung cancer progression involves the nuclear accumulation of β-catenin, which occurs during EMT when cancer cells lose the expression of E-cadherin. The nuclear β-catenin along with lymphoid enhancer binding factor 1 (LEF-1) activates FUT8 expression, causing FUT8 upregulation, globally altering core fucosylation, cancer cells’ response to extracellular matrix, growth factors, and other elements of the tumour microenvironment.
4.2. FUT8 Expression in Liver Cancer
Liver cancer is the second leading cause of cancer death [29]. The most common type of liver cancer is hepatocellular carcinoma (HCC) [52,66]. Unfortunately, the median survival of most patients after diagnosis is 6–9 months, emphasising the necessity for improved diagnostic biomarkers and targets for therapeutic treatments [52,67]. Serum α-fetoprotein (AFP) is a gold standard biomarker for the diagnosis of hepatocellular carcinoma (HCC), however the specificity of AFP for HCC is relatively low and often does not distinguish HCC from other liver diseases. Increased fucosylation is a promising marker for monitoring the progression from chronic liver disease to HCC [52], and levels of FUT8 have been shown to be increased on the cell surface and in the serum samples of HCC patients [68]. A study conducted by Egashira et al., developed and characterized a glycan antibody specific for α1-6 fucosylated AFP which could improve the specificity of AFP to improve HCC diagnosis [69]. Furthermore, when matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS) was applied to profile N-glycans in HCC tissues, FUT8 was significantly up-regulated in HCC compared to adjacent tissues [52]. Work conducted on cell lines found that expression of FUT8 is upregulated in cells with high metastatic potential [29,52]. MicroRNAs may prove valuable in inhibiting fucosylation machinery in HCC. It is believed that miR-26a, miR-34a, and miR-455-3p directly bind and negatively regulate FUT8 mRNA stability thus reducing FUT8-mediated progression of HCC and proposing a new therapeutic intervention for HCC patients [52,70].
4.3. FUT8 Expression in Colorectal Cancer
Although the number of people diagnosed with colon cancer has decreased since the mid-1980’s thanks to increased screening, colon cancer still remains the third leading cause of cancer-related deaths worldwide [71]. FUT8 was recently suggested as a direct transcriptional target of wild-type p53 in HCC cells [72], and this prompted the prediction that p53 status may affect the expression and function of FUT8 in colorectal cancer (CRC). One exploratory study, focused on the prognostic value of FUT8 expression on disease free survival (DFS) in patients with stage II and III CRC after curative surgery, found FUT8 is significantly associated with better DFS in tumours with negative p53 [51]. Contradictory findings come from studies conducted by Muinelo-Romay et al. and Noda et al. who found no association between DFS and the expression of FUT8 in their respective immunohistochemistry (n = 141 and n = 123) and microarray (n = 357) cohorts [51,73]. In tumours without p53 alterations, high levels of FUT8 protein expression were significantly associated with better DFS. This finding could imply that any prognostic value of FUT8 expression may be confined to the patient subgroups with p53-negative tumours. Although these studies have opposing findings, limitations such as variation in p53 detection techniques, and sample size may have influenced their results [51].
4.4. FUT8 Expression in Ovarian Cancer
Epithelial ovarian cancer (EOC) is the most lethal female genital tract cancer in the world (<30% 5-year survival rate), largely due to the 90% of patients who experience chemo-resistance [53]. The exact mechanisms responsible for chemoresistance are not fully understood, however reduced cellular accumulation of platinum-based drugs, enhanced detoxification capability, and aberrant apoptosis pathways are a few of the suggested mechanisms [53]. Studies have indicated a key role for core fucosylation in the progression of EOC [74,75].
The role of core fucosylation in the drug resistance of EOC is a focal point of research. This is due to the association of core fucosylation with TGFβ1, epidermal growth factor, and B cell receptors, as well as the uptake of drugs by cancer cells being dependent on the molecular interaction between cell surface proteins and drugs. In EOC the inhibition of cisplatin (cDDP) uptake is the main cause for cDDp-resistance. Studies show that serum and tissue from cDDP-treated EOC patients have increased levels of core fucosylation, and that the binding of Copper transporter 1 (CTR1) (an important transporter in regulating the uptake of cDDP) is supressed when core fucosylation levels are elevated [53].
4.5. FUT8 Expression in Prostate Cancer
Worldwide it is estimated that there is a total annual number of over 1.2 million cases of prostate cancer [76]. It is the most common male cancer in 91 countries worldwide and claims over 350,000 lives a year [77]. The highly heterogenous nature of prostate cancer leaves large gaps in diagnosis and treatment. Prostate cancer is severely lacking a method of diagnosis that is both sensitive and specific. The current biomarker PSA does not distinguish between indolent and aggressive prostate cancers and therefore does not serve as a prognostic biomarker. Decreased fucosylation on prostate-specific antigen (PSA) [78] and integrins [79], as well as elevated fucosylated haptoglobin are under investigation as potential non-invasive biomarkers for prostate cancer [80]. In a study conducted by Peracaula et al., the biochemical properties of PSA in the LNCaP cell line and from normal seminal fluid was investigated to aid in distinguishing between PSA from normal and tumour origins [58]. The difference reported in the context of fucose suggest the importance of analysing PSA glycosylation to improve its ability to distinguish between benign and malignant prostate cancer.
FUT8 drives increased core fucosylation in prostate cancer and has been linked to disease progression [5]. Overexpression of FUT8 has been positively correlated with the epithelial compartment and high grade prostate cancer [54]. In particular, higher FUT8 expression is present in high grade prostate cancer compared to low grade prostate cancer [54]. In tissue it was observed that a higher percentage of epithelial compartment (cores) were stained strongly in cases with a high grade cancer, suggesting FUT8 epithelial compartments may be associated with more aggressive disease [54]. Additionally, upregulation of FUT8 is correlated with increased fucosylation of glycoproteins in aggressive prostate cancer cells [81]. FUT8 is expressed in PC3 and DU145—prostate cancer cell lines [80], normal prostate epithelial cells (PrECs) [78], and normal prostate stromal cells (PrSCs) [78]. PC3 cells are an androgen-independent cell line with high metastatic potential, while LNCaP cells are an androgen-dependent cancer cell line [81]. It was in these two model prostate cancer cell lines that FUT8 was found to aid in the regulation of cancer cell migration [54]. Furthermore, FUT8 has been shown to be elevated at the protein level in metastatic cancer tissue in comparison to primary cancer tissue (n = 20) [54].
In 2018, the first study was published reporting the functional role of the FUT8 enzyme in castrate-resistant prostate cancer development. It was shown that androgen ablation is essential for FUT8 overexpression in AR-positive prostate cancer cells. Ectopic overexpression of FUT8 in androgen-dependent cells resulted in suppression of PSA production, while an increase in PSA was observed when endogenous FUT8 was knocked down. In accordance with this, patient samples with overexpressed FUT8 have been found to have lower levels of PSA [57]. In a recent study, the systemic impact of altered FUT8 on prostate cancer-derived extracellular vesicles (EVs) was determined using EV characterization and quantitative proteomics [82]. The findings revealed that the number of vesicles secreted by prostate cancer cells was reduced when the cellular expression of FUT8 was increased. In contrast the abundance of proteins associated with cell motility and prostate cancer metastasis increased when FUT8 expression is elevated [82].
4.6. FUT8 Expression in Breast Cancer
Breast cancer is the most common cancer in women worldwide [83]. Even though breast cancer is heterogenous, most subtypes are hormone-related [83]. FUT8 has been linked to the migratory and invasive capabilities of aggressive breast cancer cell lines, and identified as highly upregulated in TGFβ-induced EMT [30]. FUT8 expression has been observed in normal human epithelial cells (MCF-10A), low-metastatic breast cancer cell lines (T-47D), and mesenchymal-like highly invasive breast cancer cell lines (MDA-MB-231 and Hss578T), with protein levels highest in the highly invasive breast cancer cells [30]. Furthermore, a combination of data mining of FUT8 expression at the mRNA level and the analysis of three public microarray datasets revealed high FUT8 levels are associated with cancer cell invasiveness and poor prognosis [30,84,85,86,87]. Tu et al. revealed a role for FUT8 in stimulating breast cancer cell invasion and metastasis. The research also involved a pharmacological proof-of-concept experiment using the fucosylation inhibitor, 2-fluorinated-peracetyl-fucose both in vitro and in vivo. In agreement with genetic inactivation of FUT8, administration of the fucosylation inhibitor repressed the mobility, invasiveness, and lung metastasis of breast cancer cells [30].
4.7. FUT8 Expression in Thyroid Cancer
There are two types of thyroid cancers papillary and follicular, with papillary being the most common [56]. Although thyroid cancer has a slow growth rate, once anaplastic the malignancy is the most rapid and progressive of all human carcinomas [56,88]. The polar characteristic of this cancer has initiated research aimed at elucidating the mechanisms responsible for the transition [56]. One of the few studies examining the role of FUT8 in thyroid cancer utilized immunohistochemistry of 133 thyroid tumours, and found FUT8 is associated with larger tumour volumes and lymph node metastasis [56]. Further studies are needed to elucidate the link between FUT8 and aggressive papillary carcinomas, identify the mechanisms involved, and understand whether FUT8 plays a role in the development of papillary carcinoma prior to anaplastic transformation [56,89].
4.8. FUT8 Expression in Melanoma
Melanoma is considered an incurable disease with incidence increasing twofold every 20 years. To date, very few systematic studies focusing on aberrant glycosylation in melanoma have been conducted. In 2017, a systems-based study by Agrawal et al. highlighted the importance of FUT8 in melanoma metastasis, and validated the criticality of core fucosylation in the adaptation of cancer cells to metastatic sites. Analysis of formalin-fixed embedded patient matched primary and metastatic melanoma tissues (n = 34 total) using lectin microarrays, identified higher levels of core fucose structures (α -1,6 fucose; PSA, LcH) in metastatic tumours [55]. This finding is consistent with publicly available transcriptomic data from The Cancer Genome Atlas (TCGA) [55,90,91]. Agrawal et al. also demonstrated that silencing of FUT8 in vivo reduces metastatic dissemination of melanoma cells to the lungs and inhibits the growth of pre-seeded metastases in liver, brain, and kidney. This study suggests that core fucosylation may be required for the adaptations that disseminated cancer cells undergo to survive in “foreign” tissues, highlighting FUT8 as a potential therapeutic potential for treating metastatic melanoma [55].
4.9. FUT8 Expression in Pancreatic Cancer
The most commonly diagnosed type of pancreatic cancer is pancreatic ductal adenocarcinoma (PDAC). Although chemotherapeutic improvements have been made, surgical resection is the only chance of cure for the disease. The overall survival time for PDAC that cannot be surgically removed is only 8.5 months. FUT8 expression is significantly higher during the development of adenoma into carcinoma compared to expression levels in normal pancreatic duct tissue [92]. High FUT8 expression is also associated with lymph-node metastasis, higher recurrence rate, and poorer prognosis. The function of FUT8 in pancreatic cancer is not fully understood. However, there are indications that FUT8 may stimulate the invasiveness and metastasis of PDAC by inducing EMT, with heavy involvement of core fucosylated TGF-β [27].
The results from multiple pancreatic studies highlight the pronounced elevation of fucosylated proteins in the serum of pancreatic cancer patients in comparison to patients with other cancers such as hepatocellular carcinoma, gastric cancer, and colorectal cancer [93,94]. Furthermore, in pancreatic cancer patients, site-specific fucosylation of bi-antennary glycans in two sites (N207 and N241) increased, while tri-antennary glycans (glycans containing three branches) increased in four haptoglobin N-glycan sites [93]. These findings suggest that fucosylated haptoglobin could serve as a novel marker for pancreatic cancer [93].
5. Molecular Mechanisms of FUT8 in Cancer
The regulatory mechanisms of FUT8 in cancer are not fully understood but important associations have been made. Studies indicate that core fucosylation can regulate the expression of programmed cell death protein 1 (PD-1) [95], and can alter antibody-dependent cellular cytotoxicity (ADCC) [43]. In addition, core fucosylation also regulates transforming growth factor- β1 receptor (TGF-β) [30], epidermal growth factor (EGF) receptor [26,96], α3β1 integrin [25], and E-cadherin [28,97,98].
5.1. Immune Evasion
FUT8 plays a key role in immune evasion in cancer, and has shown promise as a potential therapeutic target to improve responses to immunotherapy. Genetic ablation or pharmacological inhibition of FUT8 reduces cell-surface expression of the immune checkpoint protein PD-1, thus promoting enhanced T cell activation, and resulting in more effective tumour eradication. PD-1 contains two primary N-glycosylation sites that are heavily core fucosylated—these post-translational modifications are key regulators of PD-1 expression, and thus play a critical role in anti-tumoural response optimisation. Additionally, exhausted T cells in tumours also contain highly core-fucosylated structures including PD-1. Together these findings suggest that FUT8 can damper tumour-infiltrating immune cells, and that targeting core fucosylation may inhibit tumour growth, thus reducing PD-1/PD-L1 interactions and weakening tumour immune evasion [95].
5.2. Antibody-Dependent Cellular Cytotoxicity (ADCC)
ADCC is a lytic attack on antibody-targeted cells that is elicited upon binding of lymphocyte receptors (FcRs) to the constant region (Fc) of antibodies [99]. There are five types of immunoglobulin isotypes, of which immunoglobulin G (IgG) is the most abundant in human blood, and is responsible for 10–20% of plasma protein [100]. Therapeutic antibodies of human IgG1 comprised of two biantennary complex-type N-linked oligosaccharides in the constant region can mediate effector functions through the Fc and also influence ADCC [101,102].
Most licensed therapeutic antibodies possess core fucosylated Fc oligiosaccharides—this stems from their production in rodent mammalian cell lines. To achieve optimal ADCC therapeutic antibodies that fully lack core fucosylation can be produced [102,103]. Studies supporting the optimization of ADCC with defucosylated antibodies have primarily been conducted in T-cell leukaemia and lymphoma [104,105,106,107]. Double gene knockout of key oligiosaccharide fucose modifying genes FUT8 and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing Chinese hamster ovary (CHO) cells have previously been achieved [102]. It is believed that converting already established antibody-producing cells to non-fucosylated antibody producers promotes a more potent efficacy [102,103]. Without optimizing the potential of ADCC, antibodies are administered to cancer patients at high dosages and unfortunately high costs [102]. Strategies aimed at creating defucosylated antibodies that enhance ADCC offer the potential for next-generation therapeutic antibodies. Specifically, inhibiting FUT8 activity holds promise to help promote a potent anti-tumoral response [43,102].
5.3. Transforming Growth Factor Beta (TGF-β)
Core fucosylation alters cell surface molecules and the tumour microenvironment inclusive of the extracellular matrix and growth factors, promoting the progression of cancer. The presence of core fucose greatly affects the binding affinity of TGF-β receptor toward TGF-β, making it critical to its function [20]. TGF-β has been shown to have an immunosuppressive effect in cancer, preventing anti-tumour immune mediated killing [108]. FUT8 knockdown results in lessened TGFβ-inducing EMT, while an upregulation of FUT8 is observed during EMT—suggesting a positive feedback loop between FUT8 expression and TGFβ receptor signalling to promote EMT and tumour development [30,65]. EMT is a necessary process that transforms benign tumours to aggressive and highly invasive cancer [109]. In breast cancer, upregulated FUT8 remodels TGF-β RI and RII complexes to promote downstream signalling, and genetic or pharmacological interruption of FUT8 inhibits TGF-β signalling and suppresses breast cancer metastasis in vivo [30].
In lung cancer cells regulation of the β-catenin/ lymphoid enhancer-binding factor-1 (LEF-1) binding (a major signaling event in EMT), is linked to FUT8 upregulation and disease progression, however in breast cancer cell studies suggest that TGF-β mediated upregulation of FUT8 is independent of β-catenin/LEF-1 [30,49]. FUT8 may be regulated by EMT-inducing transcription factors such as TWIST, SNAIL, SLUG, ZEB1, or ZEB2, with specific attention to SNAIL [30,110]. The 5’-flanking promoter region of the FUT8 gene is comprised of several enhancer box (E-box) motifs, which may help explain the involvement of the above E-box binding proteins [111]. Although the precise structural and functional basis underlying the upregulation of FUT8 during TGF-β-induced EMT still remains under investigation, the discovery of the regulatory mechanisms could unveil promising prognostic or therapeutic leads for cancers [30].
5.4. Epidermal Growth Factor (EGF)
Core fucosylation also plays a key role in the regulation of EGF within multiple cell types. In Fut8−/− embryonic fibroblast cells (derived from Fut8 null mice), EGF-induced phosphorylation of EGFR is blocked (while no significant changes in tyrosine phosphatase total activities are observed) [26]. FUT8 overexpression can also increase EGFR fucosylation to enhance EGF stimulus response and decrease sensitivity to tyrosine kinase inhibitors. When A549 cells (a human non-small cell lung cancer cell line) are depleted of FUT8, EGFR fucosylation is reduced, as is EGF-mediated cellular growth response and sensitivity. Consistent with this, overexpression of FUT8 in HEK293 cells (a human embryonic kidney cell line) increases EGF-mediated cellular growth [96].
5.5. α3β1. Integrin
In addition to core fucosylation deficiency being reported to down-regulate the functions of TGF-β receptor and EGF receptor, loss of core fucosylation can also inhibit α3β1 integrin-mediated cell migration and cell signalling. In Fut8−/− cells integrin-mediated migration and cell signalling are decreased and can be partially rescued with the reintroduction of FUT8. Like other glycosyltransferases that play a role in the regulation of integrin functions, it is strongly suggested that FUT8 is essential for the functions of α3β1 [25].
5.6. E-Cadherin
Increased core fucosylation on E-cadherin is known to strengthen cell-cell adhesion and cell migratory processes via regulation of E-cadherin turnover and expression levels [24]. FUT8 and E-cadherin protein levels are significantly increased in primary colorectal cancer samples. A study conducted by Osumi et al., examined E-cadherin in FUT8 transfected human colon carcinoma cells, FUT8 knock down cells, and Fut8 deficient cells from Fut8−/− mice. The results demonstrated that the activity of FUT8 regulates the total amount of E-cadherin [24]. In aggressive lung cancer, E-cadherin is oftern dysregulated. Geng et al., found that in highly metastatic lung cancer cells E-cadherin is core fucosylated while absent in lowly metastatic lung cancer cells. Furthermore, their results confirmed that E-cadherin is the substrate of FUT8 and that core fucosylated E-cadherin is positively correlated with cancer metastasis. Upregulation of FUT8 increases the levels of core fucosylation on E-cadherin and inhibits the function of E-cadherin [28,97,98].
6. Therapeutic Approaches
6.1. Afucosylated Antibodies Production Strategies
Antibody-dependent cellular cytotoxicity (ADCC) plays a critical role in tumour cell eradication. ADCC is controlled almost exclusively by the absence of fucose on IgG1, and IgG1 defucosylation can improve antibody effector function. Afucosylated anti-cancer antibodies with enhanced ADCC are expected to improve the efficacy and reduce the dose and cost of therapeutic antibodies [112,113]. Antibodies completely lacking core fucosylation can be produced in CHO cell lines containing zinc-finger nucleases (ZFNs) that cleave the FUT8 gene [114]. In addition to CHO cells, plant cells have been considered as expression platforms for the production of recombinant antibodies [113,115].
Rat hybridoma YB2/0 cells have lower levels of FUT8 mRNA than CHO cells and have shown promise for the production of afucosylated antibodies. Shinkawa et al. found antibodies produced in YB2/0 cells have lower levels of core fucose and can have a 50-fold higher ADCC than antibodies produced in CHO cells [99,113]. Small molecules that inhibit fucosylation (such as 2-fluorofucose and 5-alkynylfucose) can also be utilised to help produce afucosylated antibodies. These fucosylation inhibitors produce monoclonal antibodies through the proposed mechanistic actions of intracellular GDP-fucose depletion, consequently blocking the de novo pathway (discussed in Section 2) or through the inhibition of FUT8 [113,116].
6.2. Therapeutic Afucosylated Antibody Drugs
Various strategies have been used to produce afucosylated antibodies with improved therapeutic efficacy. To date, three afucosylated antibodies are on the market, and more than 20 are under evaluation in clinical trials [113]. Promising afucosylated antibody drugs include afucosylated Rituximab (an anti-CD20 monoclonal antibody used to treat B-cell malignancies including non-Hodgkin lymphoma), which has greater ADCC and B-cell depletion, and lower complement-dependent cytotoxicity than rituximab [117,118]. The efficacy of CD20 antibody therapy has also been improved by the development of an Fc-engineered type II humanised antibody (known as Obinutuzumab or GA101). Compared to rituximab, GA101 has a <30% reduction in fucosylation and has increased direct and immune effector cell-mediated cytotoxicity and superior antitumour activity [113,119]. GA101 has FDA approval for patients with chronic lymphocytic leukemia (CLL) or follicular lymphoma [113,119], where it promotes increased ADCC, rapid B cell removal in peripheral blood, and prolongs overall and progression-free survival in CLL patients [120,121,122].
Afucosylation has also been used to improve the efficacy of CD19 antibody drugs (which are used for therapy of B cell malignancies). CD19 is a B cell marker, and elevated levels of CD19 are detected in B cell lymphomas. Afucosylated anti-CD19 antibodies have potent B cell depletive activity and inhibit lymphoma growth in vivo [123,124,125,126,127]. Antibodies with reduced fucosylation have also been produced against EGFR, insulin-like growth factor 1 and c-Met. These antibodies have shown enhanced in vivo efficacy and tolerability in animal models and have progressed to human clinical trials [113,128,129,130].
Other notable afucosylated antibodies include mogamulizumab (POTELIGEO®), Ublituximab (TG-1101), TrasGEX (GT-MAB7.3-GEX, Glycooptimized Trastuzumab-GEX) and SEA-CD40. Mogamulizumab is an afucosylated monoclonal antibody that selectively binds to CC chemokine receptor 4 (CCR4). Mogamulizumab is approved in Japan for the treatment of hematologic malignancies and cutaneous T-cell lymphoma (CTCL) [113]), and is in multiple clinical trials (in combination with other drugs) for the treatment of solid tumours, and also for the treatment of human T-lymphotrophic virus 1 (HTLV1)-associated myelopathy [113,131]. Ublituximab (an afucosylated monoclonal antibody that targets a unique epitope on CD20 exprrssing B cells) has been tested in clincal trials for patients with B-cell non-Hodgkin lymphoma or CLL who were previously treated with rituximab [132,133]. TrasGEX (a glyco-optimised anti-HER2 antibody with enhanced ADCC) can induce longstanding remission in HER2+ relapsed metastatic colon cancer patients [134], and SEA-CD40 (a non-fucosylated anti-CD40 antibody) has potent pharmacodynamic activity in preclinical models and patients with advanced solid tumors [135,136,137].
6.3. Fucosylation Inhibitor-2-Fluorofucose
A number of fucosyltransferases inhibitors have been developed employing a variety of strategies. One common approach has been mimicking the natural substrate guanosine diphosphate fucose (GDP-Fuc), while others have developed fucose derivatives metabolized through the salvage pathway, that target fucosyltransferases by competitive inhibition [138]. 2-fluorofucose (SGN-2FF) is one inhibitor that acts via the salvage pathway and has shown promising anticancer effects both in vitro and in vivo [138]. SGN-2FF is a small molecule inhibitor of fucosylation with direct and indirect effects on immune cells, tumour cells, and the tumour microenvironment [139]. The inhibitor is passively transported over the cell membrane, deprotected by esterases, and metabolized to its corresponding GDP-analog [140]. Fucosylation is decreased through feedback inhibition of de novo biosynthesis, as well as competitive inhibition of fucosyltransferases [140]. SGN-2FF is an orally bioavailable inhibitor that has been used in various mouse models [30,116,141,142]. In one study, tumours were implanted in multiple strains of SGN-2FF treated mice to determine how differences in the immune repertoire affect the antitumour activity. It was found that in mice with intact immune systems SGN-2FF is reliant on T cell activity. T cells isolated from SGN-2FF-treated tumour bearing mice were transferred to naïve tumour-bearing mice, delaying tumour growth. The same effect was not observed when T cells from untreated tumour-bearing mice were isolated. The results suggest that the activity of SGN-2FF is influenced by afucosylated immune cells [139].
SGN-2FF has been tested in a phase 1 multicentre clinical trial for patients with advanced solid tumours (NCT# 02952989) [113,143]. Preliminary data supported the biological effects of SGN-2FF. Data suggested that fucosylation on the cell surface of granulocytes, and IgG fucosylation was significantly decreased, while neutrophil count was significantly increased [139]. In addition to the antitumor activity of SGN-2FF as a monotherapy, the study aimed to assess how the drug acts when in combination with the standard approved dose of Pembrolizumab (anti-PD-1) [113,143]. The study had to be terminated after three years due to overall benefit/risk profile, with no further details disclosed [113,143]. The data from the literature indicates promise in using SGN-2FF in cancer therapeutics however, the inhibitory potency requires further investigation [140].
6.4. De novo and Salvage Pathway Inhibition of Cellular Fucosylation
New fucosylation inhibitors that act via the de novo and salvage pathways have been recently developed. The salvage pathway can be inhibited via two anomers of SGN-2FF (A2FF1P and B2FF1P). A2FF1P and B2FF1P enter the metabolic pathway at a later stage and have 4-7 times higher potency than SGN-2FF—believed to be due to better retainment inside the cell, and more efficient conversion of GDP-Fuc2F [140]. Pijnenborg et al. have also developed a class of fucose inhibitors that directly target de novo GDP-fucose biosynthesis. 90% of GDP-fucose is biosynthesized via de novo biosynthesis from GDP-mannose, thus direct inhibition could produce more potent inhibitors than the salvage pathway counterparts. Two new inhibitors, Fucotrim I (P-D-Rha6F2-1P) and Fucotrim II (P-D-Rha6F3-1P) are based on fluorinated mannose 1-phosphate derivatives, and have been shown to be more potent than SGN-2FF (which acts via the salvage pathway) [138]. The potency, specificty, and low toxicity of these inhibitors makes them exciting new candidates for cancer therapeutics.
7. Conclusions and Future Perspective
2020 saw the publication of several pivotal studies focussing on novel FUT8 discoveries. Key publications have ranged from the first in depth description of FUT8’s structural basis [43,44,144,145], identification of FUT8’s substrate specificity [146], its regulatory role amongst various cancers [27,82,147,148,149,150,151,152,153] and the inhibition of fucosylation [138,140]. The literature has vastly expanded upon our understanding and the particular importance of FUT8 in human disease. As FUT8 establishes itself as a critical component of cancer progression, the exciting potential to exploit FUT8 therapeutically will inevitably become an important focal point of the glyco-oncology field. Preclinical data of the FUT8 inhibitor SGN-2FF has demonstrated robust biological effects which have supported one clinical trial (NCT# 02952989). FUT8 is associated to some of the most aggressive and lethal cancers, supporting further the investigation into the effect of targeting FUT8 in combination with already approved antineoplastic drugs or immunotherapies to improve cancer therapy outcomes. The recent discoveries outlined here, have made considerable advancements in our understanding of FUT8’s fundamental biology. Using this information we can move forward, to realise the full potential of FUT8 as a critical factor in cancer diagnosis and therapeutics in the future.
Abbreviations
| ER | Endoplasmic reticulum |
| GALNT | N-acetylgalactosaminyltransferase |
| GalNAc | N-acetylgalactosamine |
| FUT8 | α-(1,6)-fucosyltransferase |
| GLCNAc-TV or MGAT5 | N-acetylglucosaminyltransferase |
| GDP-β-l-fucose | Guanosine diphosphate |
| GMD | GDP-mannose 4,6-dehydrastase |
| FX protein | GDP-keto-6-deoxymannose 3,5-epimerase, 4-reductase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| AFP | α-fetoprotein |
| HCC | Hepatocellular carcinoma |
| LCA | Lens culinaris agglutinin |
| PCa | Prostate Cancer |
| PSA | Prostate Specific Antigen |
| BPH | Benign Prostate Hyperplasia |
| HPLC | High-performance liquid chromatography |
| AAL | Aleuria aurantica lectin |
| AGP | α1-acid glycoprotein |
| CAIE | Crossed affinoimmunoelectrophoresis |
| fAGP | α1,3fucosylated AGP |
| RPN1 | Ribophorin I |
| FUTs | Fucosyltransferases |
| NSCLC | Nonsmall cell lung cancer |
| LEF-1 | Lymphoid enhancer binding factor 1 |
| CRC | Colorectal cancer |
| DFS | Disease free survival |
| EOC | Epithelial Ovarian Cancer |
| cDDP | Cisplatin |
| CTR1 | Copper transporter 1 |
| PrECs | Prostate epithelial cells |
| PrSCs | Prostate stromal cells |
| ADT | Androgen Deprivation Therapy |
| EV | Extracellular Vesicles |
| PDAC | Pancreatic ductal adenocarcinoma |
| ADCC | Antibody-dependent cellular cytotoxicity |
| PD-1 | Programmed cell death |
| TGF-β | Transforming growth factor- β1 |
| EGF | Epidermal growth factor |
| FcRs | Lymphocyte receptors |
| Fc | Constant region |
| IgG | Immunoglobulin G |
| CHO | Chinese hamster ovary |
| EMT | Epithelial-mesenchymal transition |
| E-box | Enhancer box |
| ZFNs | Zinc-finger nucleases |
| SGN-2FF | 2-fluorofucose |
| FDA | US Food and Drug Administration |
| NHL | Non-Hodgkin’s lymphoma |
| CTCL | T-cell lymphoma |
| HTLV1 | T-lymphotrophic virus 1 |
Author Contributions
K.B., E.S., D.J.E. & J.M. discussed the content and wrote the manuscript. All authors edited and/or reviewed the manuscript prior to submission. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Prostate Cancer UK through a Research Innovation Award (RIA16-ST2-011).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pinho S.S., Reis C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 2.Varki A., Lowe J.B. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2009. Biological roles of glycans. [PubMed] [Google Scholar]
- 3.Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2015. [PubMed] [Google Scholar]
- 4.Munkley J., Elliott D.J. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7:35478–35489. doi: 10.18632/oncotarget.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott E., Munkley J. Glycans as Biomarkers in Prostate Cancer. Int. J. Mol. Sci. 2019;20:1389. doi: 10.3390/ijms20061389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakomori S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakomori S.-I. Aberrant Glycosylation In Tumors And Tumor-Associated Carbohydrate Antigens. Adv. Cancer Res. 1989:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 8.Meezan E., Wu H.C., Black P.H., Robbins P.W. Comparative Studies on the Carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by Sephadex chromatography. Biochemistry. 1969;8:2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- 9.Reis C.A., Osorio H., Silva L., Gomes C., David L. Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 2010;63:322–329. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 10.Woods E.C., Kai F., Barnes J.M., Pedram K., Pickup M.W., Hollander M.J., Weaver V.M., Bertozzi C.R. A bulky glycocalyx fosters metastasis formation by promoting G1 cell cycle progression. eLife. 2017;6:e25752. doi: 10.7554/eLife.25752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez E., Schetters S.T.T., Van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 2018;18:204–211. doi: 10.1038/nri.2018.3. [DOI] [PubMed] [Google Scholar]
- 12.Scott E., Elliott D.J., Munkley J. Tumour associated glycans: A route to boost immunotherapy? Clin. Chim. Acta. 2020;502:167–173. doi: 10.1016/j.cca.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Magalhães A., Duarte H.O., Reis C.A. Aberrant Glycosylation in Cancer: A Novel Molecular Mechanism Controlling Metastasis. Cancer Cell. 2017;31:733–735. doi: 10.1016/j.ccell.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Powers T.W., Neely B.A., Shao Y., Tang H., Troyer D.A., Mehta A.S., Haab B.B., Drake R.R. MALDI Imaging Mass Spectrometry Profiling of N-Glycans in Formalin-Fixed Paraffin Embedded Clinical Tissue Blocks and Tissue Microarrays. PLoS ONE. 2014;9:e106255. doi: 10.1371/journal.pone.0106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake R.R., Powers T.W., Jones E.E., Bruner E., Mehta A.S., Angel P.M. Advances in Cancer Research. Elsevier; Amsterdam, The Netherlands: 2017. MALDI Mass Spectrometry Imaging of N-Linked Glycans in Cancer Tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker D.J., Lowe J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology. 2003;13:41–53. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 17.Stanley P., Taniguchi N., Aebi M. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2017. N-glycans. [Google Scholar]
- 18.Schneider M., Al-Shareffi E., Haltiwanger R.S. Biological functions of fucose in mammals. Glycobiology. 2017;27:601–618. doi: 10.1093/glycob/cwx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q., Wang L.-X. Mammalian α-1,6-Fucosyltransferase (FUT8) Is the Sole Enzyme Responsible for theN-Acetylglucosaminyltransferase I-independent Core Fucosylation of High-mannoseN-Glycans. J. Biol. Chem. 2016;291:11064–11071. doi: 10.1074/jbc.M116.720789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., et al. From The Cover: Dysregulation of TGF-β 1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. USA. 2005;102:15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan M., Yang D., Dou H., Zhang L. Progress in Molecular Biology and Translational Science. Academic Press; Cambridge, MA, USA: 2019. Fucosylation in cancer biology and its clinical applications; pp. 93–119. [DOI] [PubMed] [Google Scholar]
- 22.Mehta A.Y., Cummings R.D. GlycoGlyph: A glycan visualizing, drawing and naming application. Bioinformatics. 2020;36:3613–3614. doi: 10.1093/bioinformatics/btaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Human Protein Atlas. Fucosyltransferase. [(accessed on 27 September 2020)];2020 Available online: https://www.proteinatlas.org/search/fucosyltransferase.
- 24.Osumi D., Takahashi M., Miyoshi E., Yokoe S., Lee S.H., Noda K., Nakamori S., Gu J., Ikeda Y., Kuroki Y., et al. Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells. Cancer Sci. 2009;100:888–895. doi: 10.1111/j.1349-7006.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Itoh S., Wang X., Isaji T., Miyoshi E., Kariya Y., Miyazaki K., Kawasaki N., Taniguchi N., Gu J. Deletion of Core Fucosylation on α3β1 Integrin Down-regulates Its Functions. J. Biol. Chem. 2006;281:38343–38350. doi: 10.1074/jbc.M608764200. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Gu J., Ihara H., Miyoshi E., Honke K., Taniguchi N. Core Fucosylation Regulates Epidermal Growth Factor Receptor-mediated Intracellular Signaling. J. Biol. Chem. 2006;281:2572–2577. doi: 10.1074/jbc.M510893200. [DOI] [PubMed] [Google Scholar]
- 27.Tada K., Ohta M., Hidano S., Watanabe K., Hirashita T., Oshima Y., Fujnaga A., Nakanuma H., Masuda T., Endo Y., et al. Fucosyltransferase 8 plays a crucial role in the invasion and metastasis of pancreatic ductal adenocarcinoma. Surg. Today. 2020;50:767–777. doi: 10.1007/s00595-019-01953-z. [DOI] [PubMed] [Google Scholar]
- 28.Shao K., Chen Z.Y., Gautam S., Deng N.H., Zhou Y., Wu X.Z. Posttranslational modification of E-cadherin by core fucosylation regulates Src activation and induces epithelial–mesenchymal transition-like process in lung cancer cells. Glycobiology. 2016;26:142–154. doi: 10.1093/glycob/cwv089. [DOI] [PubMed] [Google Scholar]
- 29.Kang X., Wang N., Pei C., Sun L., Sun R., Chen J., Liu Y. Glycan-related gene expression signatures in human metastatic hepatocellular carcinoma cells. Exp. Ther. Med. 2012;3:415–422. doi: 10.3892/etm.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu C.-F., Wu M.-Y., Lin Y.-C., Kannagi R., Yang R.-B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017;19:1–15. doi: 10.1186/s13058-017-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honma R., Kinoshita I., Miyoshi E., Tomaru U., Matsuno Y., Shimizu Y., Takeuchi S., Kobayashi Y., Kaga K., Taniguchi N., et al. Expression of Fucosyltransferase 8 Is Associated with an Unfavorable Clinical Outcome in Non-Small Cell Lung Cancers. Oncology. 2015;88:298–308. doi: 10.1159/000369495. [DOI] [PubMed] [Google Scholar]
- 32.Miyoshi E., Moriwaki K., Nakagawa T. Biological Function of Fucosylation in Cancer Biology. J. Biochem. 2008;143:725–729. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- 33.A Norton P., Mehta A.S. Expression of genes that control core fucosylation in hepatocellular carcinoma: Systematic review. World J. Gastroenterol. 2019;25:2947–2960. doi: 10.3748/wjg.v25.i23.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonetti M., Sturla L., Bisso A., Benatti U., De Flora A. Synthesis of GDP-l-fucose by the Human FX Protein. J. Biol. Chem. 1996;271:27274–27279. doi: 10.1074/jbc.271.44.27274. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi Y., Fujii J., Inoue S., Uozumi N., Yanagidani S., Ikeda Y., Egashira M., Miyoshi O., Niikawa N., Taniguchi N. Mapping of the α-1,6-fucosyltransferase gene, FUT8, to human chromosome 14q24.3. Cytogenet. Genome Res. 1999;84:58–60. doi: 10.1159/000015215. [DOI] [PubMed] [Google Scholar]
- 36.The Human Protein Atlas. FUT8. [(accessed on 28 April 2020)];2020 Available online: https://www.proteinatlas.org/ENSG00000033170-FUT8.
- 37.Taniguchi N. Experimental Glycoscience. Springer; Berlin/Heidelberg, Germany: 2009. Knockout Mice of α1,6 Fucosyltransferase (Fut 8) pp. 379–380. [Google Scholar]
- 38.Malý P., Thall A.D., Petryniak B., E Rogers C., Smith P.L., Marks R.M., Kelly R.J., Gersten K.M., Cheng G., Saunders T.L., et al. The α(1,3)Fucosyltransferase Fuc-TVII Controls Leukocyte Trafficking through an Essential Role in L-, E-, and P-selectin Ligand Biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/S0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 39.Kudo T., Fujii T., Ikegami S., Inokuchi K., Takayama Y., Ikehara Y., Nishihara S., Togayachi A., Takahashi S., Tachibana K., et al. Mice lacking α1,3-fucosyltransferase IX demonstrate disappearance of Lewis x structure in brain and increased anxiety-like behaviors. Glycobiology. 2007;17:1–9. doi: 10.1093/glycob/cwl047. [DOI] [PubMed] [Google Scholar]
- 40.Ihara H., Ikeda Y., Toma S., Wang X., Suzuki T., Gu J., Miyoshi E., Tsukihara T., Honke K., Matsumoto A., et al. Crystal structure of mammalian α1,6-fucosyltransferase, FUT8. Glycobiology. 2007;17:455–466. doi: 10.1093/glycob/cwl079. [DOI] [PubMed] [Google Scholar]
- 41.Coutinho P.M., Deleury E., Davies G.J., Henrissat B. An Evolving Hierarchical Family Classification for Glycosyltransferases. J. Mol. Biol. 2003;328:307–317. doi: 10.1016/S0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 42.Qasba P.K., Ramakrishnan B., Boeggeman E. Substrate-induced conformational changes in glycosyltransferases. Trends Biochem. Sci. 2005;30:53–62. doi: 10.1016/j.tibs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 43.García-García A., Ceballos-Laita L., Serna S., Artschwager R., Reichardt N.C., Corzana F., Hurtado-Guerrero R. Structural basis for substrate specificity and catalysis of α1,6-fucosyltransferase. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-14794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomida S., Takata M., Hirata T., Nagae M., Nakano M., Kizuka Y. The SH3 domain in the fucosyltransferase FUT8 controls FUT8 activity and localization and is essential for core fucosylation. J. Biol. Chem. 2020;295:7992–8004. doi: 10.1074/jbc.RA120.013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyoshi E., Kawamoto S., Moriwaki K., Nakagawa T., Terao M., Shinzaki S., Yamane-Ohnuki N., Satoh M., Mehta A.S., Block T.M. Overexpression of α1,6-fucosyltransferase in hepatoma enhances expression of Golgi phosphoprotein 2 in a fucosylation-independent manner. Int. J. Oncol. 2011;39:203–208. doi: 10.3892/ijo.2011.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dube D.H., Bertozzi C.R. Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 47.Reily C., Stewart T.J., Renfrow M.B., Novak J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019;15:346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinho S.S., Oliveira P., Cabral J., Carvalho S., Huntsman D., Gärtner F., Seruca R., Reis C.A., Oliveira C. Loss and Recovery of Mgat3 and GnT-III Mediated E-cadherin N-glycosylation Is a Mechanism Involved in Epithelial-Mesenchymal-Epithelial Transitions. PLoS ONE. 2012;7:e33191. doi: 10.1371/journal.pone.0033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C.-Y., Jan Y.-H., Juan Y.-H., Yang C.-J., Huang M.-S., Yu C.-J., Yang P.-C., Hsiao M., Hsu T.-L., Wong C.-H. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc. Natl. Acad. Sci. USA. 2013;110:630–635. doi: 10.1073/pnas.1220425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noda K., Miyoshi E., Uozumi N., Yanagidani S., Ikeda Y., Gao C.-X., Suzuki K., Yoshihara H., Yoshikawa M., Kawano K., et al. Gene expression of α1-6 fucosyltransferase in human hepatoma tissues: A possible implication for increased fucosylation of α-fetoprotein. Hepatology. 1998;28:944–952. doi: 10.1002/hep.510280408. [DOI] [PubMed] [Google Scholar]
- 51.Noda M., Okayama H., Kofunato Y., Chida S., Saito K., Tada T., Ashizawa M., Nakajima T., Aoto K., Kikuchi T., et al. Prognostic role of FUT8 expression in relation to p53 status in stage II and III colorectal cancer. PLoS ONE. 2018;13:e0200315. doi: 10.1371/journal.pone.0200315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng L., Gao S., Song X., Dong W., Zhou H., Zhao L., Jia L. Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs. Oncotarget. 2016;7:61199–61214. doi: 10.18632/oncotarget.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lv X., Song J., Xue K., Li Z., Li M., Zahid D., Cao H., Wang L., Song W., Ma T., et al. Core fucosylation of copper transporter 1 plays a crucial role in cisplatin-resistance of epithelial ovarian cancer by regulating drug uptake. Mol. Carcinog. 2019;58:794–807. doi: 10.1002/mc.22971. [DOI] [PubMed] [Google Scholar]
- 54.Wang X., Chen J., Li Q.K., Peskoe S.B., Zhang B., Choi C., A Platz E., Zhang H. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 2014;24:935–944. doi: 10.1093/glycob/cwu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agrawal P., Fontanals-Cirera B., Sokolova E., Jacob S., Vaiana C.A., Argibay D., Davalos V., McDermott M., Nayak S., Darvishian F., et al. A Systems Biology Approach Identifies FUT8 as a Driver of Melanoma Metastasis. Cancer Cell. 2017;31:804–819.e7. doi: 10.1016/j.ccell.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito Y., Miyauchi A., Yoshida H., Uruno T., Nakano K., Takamura Y., Miya A., Kobayashi K., Yokozawa T., Matsuzuka F., et al. Expression of α1,6-fucosyltransferase (FUT8) in papillary carcinoma of the thyroid: Its linkage to biological aggressiveness and anaplastic transformation. Cancer Lett. 2003;200:167–172. doi: 10.1016/S0304-3835(03)00383-5. [DOI] [PubMed] [Google Scholar]
- 57.Hoti N., Yang S., Hu Y., Shah P., Haffner M.C., Zhang H. Overexpression of α (1,6) fucosyltransferase in the development of castration-resistant prostate cancer cells. Prostate Cancer Prostatic Dis. 2018;21:137–146. doi: 10.1038/s41391-017-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peracaula R. Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology. 2003;13:457–470. doi: 10.1093/glycob/cwg041. [DOI] [PubMed] [Google Scholar]
- 59.Yokobori T., Yazawa S., Asao T., Nakazawa N., Mogi A., Sano R., Kuwano H., Kaira K., Shirabe K. Fucosylated α1-acid glycoprotein as a biomarker to predict prognosis following tumor immunotherapy of patients with lung cancer. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-51021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hashimoto S., Asao T., Takahashi J., Yagihashi Y., Nishimura T., Saniabadi A.R., Poland D.C.W., Van Dijk W., Kuwano H., Kochibe N., et al. α1-Acid glycoprotein fucosylation as a marker of carcinoma progression and prognosis. Cancer. 2004;101:2825–2836. doi: 10.1002/cncr.20713. [DOI] [PubMed] [Google Scholar]
- 61.Asao T., Yazawa S., Nishimura T., Hayashi T., Shimaoka H., Saniabadi A.R., Kuwano H. Development of a Novel System for Mass Spectrometric Analysis of Cancer-Associated Fucosylation in Plasmaα1-Acid Glycoprotein. BioMed Res. Int. 2013;2013:1–9. doi: 10.1155/2013/834790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yazawa S., Takahashi R., Yokobori T., Sano R., Mogi A., Saniabadi A.R., Kuwano H., Asao T. Fucosylated Glycans in α1-Acid Glycoprotein for Monitoring Treatment Outcomes and Prognosis of Cancer Patients. PLoS ONE. 2016;11:e0156277. doi: 10.1371/journal.pone.0156277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.The American Cancer Society . The Cancer Atlas. 2nd ed. The American Cancer Society; Atlanta, GA, USA: 2019. Lung Cancer. [Google Scholar]
- 64.Zugazagoitia J., Enguita A.B., Nuñez J.A., Iglesias L., Ponce S. The new IASLC/ATS/ERS lung adenocarcinoma classification from a clinical perspective: Current concepts and future prospects. J. Thorac. Dis. 2014;6:S526–S536. doi: 10.3978/j.issn.2072-1439.2014.01.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin H., Wang D., Wu T., Dong C., Shen N., Sun Y., Sun Y., Xie H., Wang N., Shan L. Blocking core fucosylation of TGF-β1 receptors downregulates their functions and attenuates the epithelial-mesenchymal transition of renal tubular cells. Am. J. Physiol. Physiol. 2011;300:F1017–F1025. doi: 10.1152/ajprenal.00426.2010. [DOI] [PubMed] [Google Scholar]
- 66.Block T.M., Mehta A.S., Fimmel C.J., Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093–5107. doi: 10.1038/sj.onc.1206557. [DOI] [PubMed] [Google Scholar]
- 67.Attwa M.H. Guide for diagnosis and treatment of hepatocellular carcinoma. World J. Hepatol. 2015;7:1632–1651. doi: 10.4254/wjh.v7.i12.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nie H., Liu X., Zhang Y., Li T., Zhan C., Huo W., He A., Yao Y., Jin Y., Qu Y., et al. Specific N-glycans of Hepatocellular Carcinoma Cell Surface and the Abnormal Increase of Core-α-1, 6-fucosylated Triantennary Glycan via N-acetylglucosaminyltransferases-IVa Regulation. Sci. Rep. 2015;5:16007. doi: 10.1038/srep16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Egashira Y., Suganuma M., Kataoka Y., Higa Y., Ide N., Morishita K., Kamada Y., Gu J., Fukagawa K., Miyoshi E. Establishment and characterization of a fucosylated α-fetoprotein-specific monoclonal antibody: A potential application for clinical research. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-48821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y., Fukuda T., Isaji T., Lu J., Im S., Hang Q., Gu W., Hou S., Ohtsubo K., Gu J. Loss of αl,6-fucosyltransferase inhibits chemical-induced hepatocellular carcinoma and tumorigenesis by down-regulating several cell signaling pathways. FASEB J. 2015;29:3217–3227. doi: 10.1096/fj.15-270710. [DOI] [PubMed] [Google Scholar]
- 71.The American Cancer Society . Key Statistics for Colorectal Cancer. The American Cancer Society; Atlanta, GA, USA: 2020. [Google Scholar]
- 72.Okagawa Y., Takada K., Arihara Y., Kikuchi S., Osuga T., Nakamura H., Kamihara Y., Hayasaka N., Usami M., Murase K., et al. Activated p53 with Histone Deacetylase Inhibitor Enhances l-Fucose-Mediated Drug Delivery through Induction of Fucosyltransferase 8 Expression in Hepatocellular Carcinoma Cells. PLoS ONE. 2016;11:e0168355. doi: 10.1371/journal.pone.0168355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muinelo-Romay L., Villar-Portela S., Alvarez E.C., Gil-Martín E., Fern�Ndez-Briera A. α(1,6)Fucosyltransferase expression is an independent prognostic factor for disease-free survival in colorectal carcinoma. Hum. Pathol. 2011;42:1740–1750. doi: 10.1016/j.humpath.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 74.Schwedler C., Kaup M., Weiz S., Hoppe M., Braicu E.I., Sehouli J., Hoppe B., Tauber R., Berger M., Blanchard V. Identification of 34 N-glycan isomers in human serum by capillary electrophoresis coupled with laser-induced fluorescence allows improving glycan biomarker discovery. Anal. Bioanal. Chem. 2014;406:7185–7193. doi: 10.1007/s00216-014-8168-y. [DOI] [PubMed] [Google Scholar]
- 75.Zhao R., Qin W., Qin R., Han J., Li C., Wang Y., Xu C.-J. Lectin array and glycogene expression analyses of ovarian cancer cell line A2780 and its cisplatin-resistant derivate cell line A2780-cp. Clin. Proteom. 2017;14:1–10. doi: 10.1186/s12014-017-9155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.The Global Cancer Observatory Age standardized (World) incidence rates, prostate, all ages. Int. Agency Res. Cancer. 2018;876:2018–2019. [Google Scholar]
- 77.Bolla M., van Poppel H. Management of Prostate Cancer. 2nd ed. Volume 73 Springer; Berlin/Heidelberg, Germany: 2017. [Google Scholar]
- 78.Fujita K., Hayashi T., Matsuzaki K., Nakata W., Masuda M., Kawashima A., Ujike T., Nagahara A., Tsuchiya M., Kobayashi Y., et al. Decreased fucosylated PSA as a urinary marker for high Gleason score prostate cancer. Oncotarget. 2016;7:56643–56649. doi: 10.18632/oncotarget.10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J.-L., Yang W., Hu Y., Höti N., Liu Y., Shah P., Sun S., Clark D., Thomas S.N., Zhang H. Site-Specific Fucosylation Analysis Identifying Glycoproteins Associated with Aggressive Prostate Cancer Cell Lines Using Tandem Affinity Enrichments of Intact Glycopeptides Followed by Mass Spectrometry. Anal. Chem. 2017;89:7623–7630. doi: 10.1021/acs.analchem.7b01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujita K., Shimomura M., Uemura M., Nakata W., Sato M., Nagahara A., Nakai Y., Takamatsu S., Miyoshi E., Nonomura N. Serum fucosylated haptoglobin as a novel prognostic biomarker predicting high-Gleason prostate cancer. Prostate. 2014;74:1052–1058. doi: 10.1002/pros.22824. [DOI] [PubMed] [Google Scholar]
- 81.Shah P., Wang X., Yang W., Eshghi S.T., Sun S., Hoti N., Chen L., Yang S., Pasay J., Rubin A., et al. Integrated Proteomic and Glycoproteomic Analyses of Prostate Cancer Cells Reveal Glycoprotein Alteration in Protein Abundance and Glycosylation. Mol. Cell. Proteom. 2015;14:2753–2763. doi: 10.1074/mcp.M115.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clark D.J., Schnaubelt M., Hoti N., Hu Y., Zhou Y., Gooya M., Zhang H. Impact of Increased FUT8 Expression on the Extracellular Vesicle Proteome in Prostate Cancer Cells. J. Proteome Res. 2020;19:2195–2205. doi: 10.1021/acs.jproteome.9b00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Cancer Research Fund Diet, Nutrition, Physical Activity and Breast Cancer. [(accessed on 18 July 2020)];2018 Available online: https://www.wcrf.org/sites/default/files/Breast-cancer-report.pdf.
- 84.Yue L., Han C., Li Z., Li X., Liu D., Liu S., Yu H. Fucosyltransferase 8 expression in breast cancer patients: A high throughput tissue microarray analysis. Histol. Histopathol. 2015;31:547–855. doi: 10.14670/HH-11-693. [DOI] [PubMed] [Google Scholar]
- 85.Chanrion M., Negre V., Fontaine H., Salvetat N., Bibeau F., Mac Grogan G., Mauriac L., Katsaros D., Molina F., Theillet C., et al. A Gene Expression Signature that Can Predict the Recurrence of Tamoxifen-Treated Primary Breast Cancer. Clin. Cancer Res. 2008;14:1744–1752. doi: 10.1158/1078-0432.CCR-07-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y., Yau C., Gray J.W., Chew K., Dairkee S.H., Moore D.H., Eppenberger U., Eppenberger-Castori S., Benz C.C. Enhanced NFκB and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y., Sieuwerts A.M., McGreevy M., Casey G., Cufer T., Paradiso A., Harbeck N., Span P.N., Hicks D.G., Crowe J., et al. The 76-gene signature defines high-risk patients that benefit from adjuvant tamoxifen therapy. Breast Cancer Res. Treat. 2008;116:303–309. doi: 10.1007/s10549-008-0183-2. [DOI] [PubMed] [Google Scholar]
- 88.Aldinger K.A., Samaan N.A., Ibanez M., Hill C.S. Anaplastic carcinoma of the thyroid.A review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer. 1978;41:2267–2275. doi: 10.1002/1097-0142(197806)41:6<2267::AID-CNCR2820410627>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 89.Miyoshi E., Ito Y., Miyoshi Y. Involvement of Aberrant Glycosylation in Thyroid Cancer. J. Oncol. 2010;2010:816595. doi: 10.1155/2010/816595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.I Riker A., Enkemann S.A., Fodstad O., Liu S., Ren S., Morris C., Xi Y., Howell P., Metge B., Samant R., et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med. Genom. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu L., Shen S.S., Hoshida Y., Subramanian A., Ross K., Brunet J.-P., Wagner S.N., Ramaswamy S., Mesirov J.P., Hynes R.O. Gene Expression Changes in an Animal Melanoma Model Correlate with Aggressiveness of Human Melanoma Metastases. Mol. Cancer Res. 2008;6:760–769. doi: 10.1158/1541-7786.MCR-07-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watanabe K., Ohta M., Yada K., Komori Y., Iwashita Y., Kashima K., Inomata M. Fucosylation is associated with the malignant transformation of intraductal papillary mucinous neoplasms: A lectin microarray-based study. Surg. Today. 2016;46:1217–1223. doi: 10.1007/s00595-015-1299-8. [DOI] [PubMed] [Google Scholar]
- 93.Okuyama N., Ide Y., Nakano M., Nakagawa T., Yamanaka K., Moriwaki K., Murata K., Ohigashi H., Yokoyama S., Eguchi H., et al. Fucosylated haptoglobin is a novel marker for pancreatic cancer: A detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int. J. Cancer. 2006;118:2803–2808. doi: 10.1002/ijc.21728. [DOI] [PubMed] [Google Scholar]
- 94.Miyoshi E., Nakano M. Fucosylated haptoglobin is a novel marker for pancreatic cancer: Detailed analyses of oligosaccharide structures. Proteomics. 2008;8:3257–3262. doi: 10.1002/pmic.200800046. [DOI] [PubMed] [Google Scholar]
- 95.Okada M., Chikuma S., Kondo T., Hibino S., Machiyama H., Yokosuka T., Nakano M., Yoshimura A. Blockage of Core Fucosylation Reduces Cell-Surface Expression of PD-1 and Promotes Anti-tumor Immune Responses of T Cells. Cell Rep. 2017;20:1017–1028. doi: 10.1016/j.celrep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 96.Matsumoto K., Yokote H., Arao T., Maegawa M., Tanaka K., Fujita Y., Shimizu C., Hanafusa T., Fujiwara Y., Nishio K. N-Glycan fucosylation of epidermal growth factor receptor modulates receptor activity and sensitivity to epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Sci. 2008;99:1611–1617. doi: 10.1111/j.1349-7006.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geng F., Shi B.Z., Yuan Y.F., Wu X.Z. The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: Prognostic implications. Cell Res. 2004;14:423–433. doi: 10.1038/sj.cr.7290243. [DOI] [PubMed] [Google Scholar]
- 98.Hu P., Shi B., Geng F., Zhang C., Wu W., Wu X.Z. E-cadherin core fucosylation regulates nuclear β-catenin accumulation in lung cancer cells. Glycoconj. J. 2008;25:843–850. doi: 10.1007/s10719-008-9144-6. [DOI] [PubMed] [Google Scholar]
- 99.Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., et al. The Absence of Fucose but Not the Presence of Galactose or BisectingN-Acetylglucosamine of Human IgG1 Complex-type Oligosaccharides Shows the Critical Role of Enhancing Antibody-dependent Cellular Cytotoxicity. J. Biol. Chem. 2002;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 100.Vidarsson G., Dekkers G., Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clynes R.A., Towers T.L., Presta L.G., Ravetch J.V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 102.Imai-Nishiya H., Mori K., Inoue M., Wakitani M., Iida S., Shitara K., Satoh M. Double knockdown of alpha1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: A new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol. 2007;7:84. doi: 10.1186/1472-6750-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roy G., Martin T., Barnes A., Wang J., Jimenez R.B., Rice M., Li L., Feng H., Zhang S., Chaerkady R., et al. A novel bicistronic gene design couples stable cell line selection with a fucose switch in a designer CHO host to produce native and afucosylated glycoform antibodies. mAbs. 2018;10:416–430. doi: 10.1080/19420862.2018.1433975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Niwa R. Enhancement of the Antibody-Dependent Cellular Cytotoxicity of Low-Fucose IgG1 Is Independent of Fc RIIIa Functional Polymorphism. Clin. Cancer Res. 2004;10:6248–6255. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 105.Niwa R., Natsume A., Uehara A., Wakitani M., Iida S., Uchida K., Satoh M., Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Methods. 2005;306:151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 106.Niwa R., Sakurada M., Kobayashi Y., Uehara A., Matsushima K., Ueda R., Nakamura K., Shitara K. Enhanced Natural Killer Cell Binding and Activation by Low-Fucose IgG1 Antibody Results in Potent Antibody-Dependent Cellular Cytotoxicity Induction at Lower Antigen Density. Clin. Cancer Res. 2005;11:2327–2336. doi: 10.1158/1078-0432.CCR-04-2263. [DOI] [PubMed] [Google Scholar]
- 107.Niwa R., Shoji-Hosaka E., Sakurada M., Shinkawa T., Uchida K., Nakamura K., Matsushima K., Ueda R., Hanai N., Shitara K. Defucosylated Chimeric Anti-CC Chemokine Receptor 4 IgG1 with Enhanced Antibody-Dependent Cellular Cytotoxicity Shows Potent Therapeutic Activity to T-Cell Leukemia and Lymphoma. Cancer Res. 2004;64:2127–2133. doi: 10.1158/0008-5472.CAN-03-2068. [DOI] [PubMed] [Google Scholar]
- 108.Dahmani A., Delisle J.-S. TGF-β in T Cell Biology: Implications for Cancer Immunotherapy. Cancers. 2018;10:194. doi: 10.3390/cancers10060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thiery J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 110.Puisieux A., Brabletz T., Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 111.Nieto M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 112.Yamane-Ohnuki N., Kinoshita S., Inoue-Urakubo M., Kusunoki M., Iida S., Nakano R., Wakitani M., Niwa R., Sakurada M., Uchida K., et al. Establishment ofFUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng. 2004;87:614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 113.Pereira N.A., Chan K.F., Lin P.C., Song Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs. 2018;10:693–711. doi: 10.1080/19420862.2018.1466767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Malphettes L., Freyvert Y., Chang J., Liu P.-Q., Chan E., Miller J.C., Zhou Z., Nguyen T., Tsai C., Snowden A.W., et al. Highly efficient deletion of FUT8 in CHO cell lines using zinc-finger nucleases yields cells that produce completely nonfucosylated antibodies. Biotechnol. Bioeng. 2010;106:774–783. doi: 10.1002/bit.22751. [DOI] [PubMed] [Google Scholar]
- 115.Loos A., Steinkellner H. IgG-Fc glycoengineering in non-mammalian expression hosts. Arch. Biochem. Biophys. 2012;526:167–173. doi: 10.1016/j.abb.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Okeley N.M., Alley S.C., Anderson M.E., Boursalian T.E., Burke P.J., Emmerton K.M., Jeffrey S.C., Klussman K., Law C.-L., Sussman D., et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc. Natl. Acad. Sci. USA. 2013;110:5404–5409. doi: 10.1073/pnas.1222263110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gasdaska J.R., Sherwood S., Regan J.T., Dickey L.F. An afucosylated anti-CD20 monoclonal antibody with greater antibody-dependent cellular cytotoxicity and B-cell depletion and lower complement-dependent cytotoxicity than rituximab. Mol. Immunol. 2012;50:134–141. doi: 10.1016/j.molimm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Li T., DiLillo D.J., Bournazos S., Giddens J.P., Ravetch J.V., Wang L.-X. Modulating IgG effector function by Fc glycan engineering. Proc. Natl. Acad. Sci. USA. 2017;114:3485–3490. doi: 10.1073/pnas.1702173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mössner E., Brünker P., Moser S., Püntener U., Schmidt C., Herter S., Grau R., Gerdes C., Nopora A., Van Puijenbroek E., et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell–mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cartron G., De Guibert S., Dilhuydy M.-S., Morschhauser F., Leblond V., Dupuis J., Mahe B., Bouabdallah R., Lei G., Wenger M., et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: Final data from the phase 1/2 GAUGUIN study. Blood. 2014;124:2196–2202. doi: 10.1182/blood-2014-07-586610. [DOI] [PubMed] [Google Scholar]
- 121.Goede V., Fischer K., Engelke A., Schlag R., Lepretre S., Montero L.F.C., Montillo M., Fegan C., Asikanius E., Humphrey K., et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: Updated results of the CLL11 study. Leukemia. 2015;29:1602–1604. doi: 10.1038/leu.2015.14. [DOI] [PubMed] [Google Scholar]
- 122.Goede V., Fischer K., Busch R., Engelke A., Eichhorst B., Wendtner C.M., Chagorova T., De La Serna J., Dilhuydy M.-S., Illmer T., et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 123.Cardarelli P.M., Rao-Naik C., Chen S., Huang H., Pham A., Moldovan-Loomis M.-C., Pan C., Preston B., Passmore D., Liu J., et al. A nonfucosylated human antibody to CD19 with potent B-cell depletive activity for therapy of B-cell malignancies. Cancer Immunol. Immunother. 2010;59:257–265. doi: 10.1007/s00262-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ward E., Mittereder N., Kuta E., Sims G.P., Bowen M.A., Dall’Acqua W., Tedder T., Kiener P., Coyle A.J., Wu H., et al. A glycoengineered anti-CD19 antibody with potent antibody-dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br. J. Haematol. 2011;155:426–437. doi: 10.1111/j.1365-2141.2011.08857.x. [DOI] [PubMed] [Google Scholar]
- 125.Herbst R., Wang Y., Gallagher S., Mittereder N., Kuta E., Damschroder M.M., Woods R., Rowe D.C., Cheng L., Cook K., et al. B-Cell Depletion In Vitro and In Vivo with an Afucosylated Anti-CD19 Antibody. J. Pharmacol. Exp. Ther. 2010;335:213–222. doi: 10.1124/jpet.110.168062. [DOI] [PubMed] [Google Scholar]
- 126.Matlawska-Wasowska K., Ward E.S., Stevens S., Wang Y., Herbst R., Winter S.S., Wilson B.S. Macrophage and NK-mediated killing of precursor-B acute lymphoblastic leukemia cells targeted with a-fucosylated anti-CD19 humanized antibodies. Leukemia. 2013;27:1263–1274. doi: 10.1038/leu.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Breton C., Nahimana A., Aubry D., Macoin J., Moretti P., Bertschinger M., Hou S., Duchosal M.A., Back J. A novel anti-CD19 monoclonal antibody (GBR 401) with high killing activity against B cell malignancies. J. Hematol. Oncol. 2014;7:33. doi: 10.1186/1756-8722-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gerdes C.A., Nicolini V.G., Herter S., Van Puijenbroek E., Lang S., Roemmele M., Moessner E., Freytag O., Friess T., Ries C.H., et al. GA201 (RG7160): A Novel, Humanized, Glycoengineered Anti-EGFR Antibody with Enhanced ADCC and Superior In Vivo Efficacy Compared with Cetuximab. Clin. Cancer Res. 2013;19:1126–1138. doi: 10.1158/1078-0432.CCR-12-0989. [DOI] [PubMed] [Google Scholar]
- 129.Grugan K.D., Dorn K., Jarantow S.W., Bushey B.S., Pardinas J.R., Laquerre S., Moores S.L., Chiu M.L. Fc-mediated activity of EGFR x c-Met bispecific antibody JNJ-61186372 enhanced killing of lung cancer cells. mAbs. 2017;9:114–126. doi: 10.1080/19420862.2016.1249079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schanzer J.M., Wartha K., Croasdale R., Moser S., Künkele K.-P., Ries C., Scheuer W., Duerr H., Pompiati S., Pollmann J., et al. A Novel Glycoengineered Bispecific Antibody Format for Targeted Inhibition of Epidermal Growth Factor Receptor (EGFR) and Insulin-like Growth Factor Receptor Type I (IGF-1R) Demonstrating Unique Molecular Properties. J. Biol. Chem. 2014;289:18693–18706. doi: 10.1074/jbc.M113.528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duvic M., Pinter-Brown L.C., Foss F.M., Sokol L., Jorgensen J.L., Challagundla P., Dwyer K.M., Zhang X., Kurman M.R., Ballerini R., et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125:1883–1889. doi: 10.1182/blood-2014-09-600924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beck A., Reichert J.M. Marketing approval of mogamulizumab. mAbs. 2012;4:419–425. doi: 10.4161/mabs.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sawas A., Farber C.M., Schreeder M.T., Khalil M.Y., Mahadevan D., Deng C., Amengual J.E., Nikolinakos P.G., Kolesar J.M., Kuhn J.G., et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br. J. Haematol. 2017;177:243–253. doi: 10.1111/bjh.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eisner F., Pichler M., Goletz S., Stoeger H., Samonigg H. A glyco-engineered anti-HER2 monoclonal antibody (TrasGEX) induces a long-lasting remission in a patient with HER2 overexpressing metastatic colorectal cancer after failure of all available treatment options. J. Clin. Pathol. 2015;68:1044.1–1046. doi: 10.1136/jclinpath-2015-202996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neff-LaFord H., Grilley-Olson J.E., Smith D.C., Curti B., Goel S., Kuzel T.M., Markovic S.N., Rixe O., Bajor D.L., Gajewski T.F., et al. Abstract 5535: SEA-CD40 is a non-fucosylated anti-CD40 antibody with potent pharmacodynamic activity in preclinical models and patients with advanced solid tumors. Immunology. 2020;80:5535. [Google Scholar]
- 136.Grilley-Olson J.E., Curti B.D., Smith D.C., Goel S., Gajewski T., Markovic S., Rixe O., Bajor D.L., Gutierrez M., Kuzel T., et al. SEA-CD40, a non-fucosylated CD40 agonist: Interim results from a phase 1 study in advanced solid tumors. J. Clin. Oncol. 2018;36:3093. doi: 10.1200/JCO.2018.36.15_suppl.3093. [DOI] [Google Scholar]
- 137.Coveler A.L., Bajor D.L., Masood A., Yilmaz E., Shields A.F., Javle M.M., Paluri R.K., Vaccaro G.M., Zalupski M., Grilley-Olson J.E., et al. Phase I study of SEA-CD40, gemcitabine, nab-paclitaxel, and pembrolizumab in patients with metastatic pancreatic ductal adenocarcinoma (PDAC) J. Clin. Oncol. 2020;38:TPS4671. doi: 10.1200/JCO.2020.38.15_suppl.TPS4671. [DOI] [Google Scholar]
- 138.Pijnenborg J., Rossing E., Noga M., Titulaer W., Veizaj R., Lefeber D.J., Boltje T. Fluorinated Mannosides Inhibit Cellular Fucosylation. ChemRxiv. 2020 doi: 10.26434/chemrxiv.13082138.v1. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Okeley N.M., Heiser R.A., Zeng W., Hengel S.M., Wall J., Haughney P.C., Yap T.A., Robert F., Sanborn R.E., Burris H., et al. Abstract 5551: SGN-2FF: A small-molecule inhibitor of fucosylation modulates immune cell activity in preclinical models and demonstrates pharmacodynamic activity in early phase 1 analysis. Clin. Trials. 2018;78:5551. doi: 10.1158/1538-7445.am2018-5551. [DOI] [Google Scholar]
- 140.Pijnenborg J.F., Visser E.A., Noga M., Rossing E., Veizaj R., Lefeber D.J., Büll C., Boltje T.J. Cellular fucosylation inhibitors based on fluorinated fucose-1-phosphates. ChemRxiv. 2020 doi: 10.1002/chem.202005359. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou Y., Fukuda T., Hang Q., Hou S., Isaji T., Kameyama A., Gu J. Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-11911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Park S., Lim J., Chun J.N., Lee S., Kim T.M., Kim D., Kim S., Bae D., Bae S., So I., et al. Altered expression of fucosylation pathway genes is associated with poor prognosis and tumor metastasis in non-small cell lung cancer. Int. J. Oncol. 2020;56:559–567. doi: 10.3892/ijo.2019.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.U.S. National Library of Medicine ClinicalTrials.gov. [(accessed on 10 October 2020)];2020 Available online: https://clinicaltrials.gov/ct2/home.
- 144.Järvå M.A., Dramicanin M., Lingford J.P., Mao R., John A., Jarman K.E., Grinter R., Goddard-Borger E.D. Structural basis of substrate recognition and catalysis by fucosyltransferase 8. J. Biol. Chem. 2020;295:6677–6688. doi: 10.1074/jbc.RA120.013291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ihara H., Okada T., Taniguchi N., Ikeda Y. Involvement of the α-helical and Src homology 3 domains in the molecular assembly and enzymatic activity of human α1,6-fucosyltransferase, FUT8. Biochim. Biophys. Acta Gen. Subj. 2020;1864:129596. doi: 10.1016/j.bbagen.2020.129596. [DOI] [PubMed] [Google Scholar]
- 146.Boruah B.M., Kadirvelraj R., Liu L., Ramiah A., Li C., Zong G., Bosman G.P., Yang J.-Y., Wang L.-X., Boons G.-J., et al. Characterizing human α-1,6-fucosyltransferase (FUT8) substrate specificity and structural similarities with related fucosyltransferases. J. Biol. Chem. 2020;295:17027–17045. doi: 10.1074/jbc.RA120.014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dai Y., Hartke R., Li C., Yang Q., Liu J.O., Wang L.-X. Synthetic Fluorinated l-Fucose Analogs Inhibit Proliferation of Cancer Cells and Primary Endothelial Cells. ACS Chem. Biol. 2020 doi: 10.1021/acschembio.0c00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takei J., Ohishi T., Kaneko M.K., Harada H., Kawada M., Kato Y. A defucosylated anti-PD-L1 monoclonal antibody 13-mG2a-f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Biochem. Biophys. Rep. 2020;24:100801. doi: 10.1016/j.bbrep.2020.100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang C., Wu Q., Huang H., Chen X., Huang T., Li W., Zhang J., Liu Y. Caveolin-1 upregulates Fut8 expression by activating the Wnt/β-catenin pathway to enhance HCC cell proliferative and invasive ability. Cell Biol. Int. 2020;44 doi: 10.1002/cbin.11426. [DOI] [PubMed] [Google Scholar]
- 150.Li F., Zhao S., Cui Y., Guo T., Qiang J., Xie Q., Yu W., Guo W., Deng W., Gu C., et al. 6-Fucosyltransferase (FUT8) regulates the cancer-promoting capacity of cancer-associated fibroblasts (CAFs) by modifying EGFR core fucosylation (CF) in non-small cell lung cancer (NSCLC) Am. J. Cancer Res. 2020;10:816. [PMC free article] [PubMed] [Google Scholar]
- 151.Höti N., Lih T.-S., Pan J., Zhou Y., Yang G., Deng A., Chen L., Dong M., Yang R.-B., Tu C.-F., et al. A Comprehensive Analysis of FUT8 Overexpressing Prostate Cancer Cells Reveals the Role of EGFR in Castration Resistance. Cancers. 2020;12:468. doi: 10.3390/cancers12020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shen L., Xia M., Deng X., Ke Q., Zhang C., Peng F., Dong X., Luo Z. A lectin-based glycomic approach identifies FUT8 as a driver of radioresistance in oesophageal squamous cell carcinoma. Cell. Oncol. 2020;43:695–707. doi: 10.1007/s13402-020-00517-5. [DOI] [PubMed] [Google Scholar]
- 153.Zhang N., Li M., Xu X., Zhang Y., Liu Y., Zhao M., Li P., Chen J., Fukuda T., Gu J., et al. Loss of core fucosylation enhances the anticancer activity of cytotoxic T lymphocytes by increasing PD-1 degradation. Eur. J. Immunol. 2020;50:1820–1833. doi: 10.1002/eji.202048543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.