Abstract
Approximately 30 years ago, endoglin was identified as a transforming growth factor (TGF)-β coreceptor with a crucial role in developmental biology and tumor angiogenesis. Its selectively high expression on tumor vessels and its correlation with poor survival in cancer patients led to the exploration of endoglin as a therapeutic target for cancer. The endoglin neutralizing antibody TRC105 (Carotuximab®, Tracon Pharmaceuticals (San Diego, CA, USA) was subsequently tested in a wide variety of preclinical cancer models before being tested in phase I-III clinical studies in cancer patients as both a monotherapy and in combination with other chemotherapeutic and anti-angiogenic therapies. The combined data of these studies have revealed new insights into the role of endoglin in angiogenesis and its expression and functional role on other cells in the tumor microenvironment. In this review, we will summarize the preclinical work, clinical trials and biomarker studies of TRC105 and explore what these studies have enabled us to learn and what questions remain unanswered.
Keywords: endoglin, TRC105, angiogenesis, clinical trials, biomarkers
1. Introduction
1.1. Introduction and Endoglin History
Endoglin is a 180 kDa RGD-domain-containing transmembrane glycoprotein, which was first identified on endothelial cells in 1990, whose expression is increased by hypoxia [1]. Later, its role as a part of the transforming growth factor (TGF)-β receptor signaling complex [2] was shown, followed by the discovery of two endoglin isoforms, L-endoglin and S-endoglin [3]. Endoglin itself does not possess kinase activity but modulates signaling by forming a heterotypic receptor complex with other TGF-β signaling receptors [4]. Mutations in the endoglin gene cause hereditary hemorrhagic telangiectasia (HHT or Osler-Weber-Rendu syndrome), a disease characterized by arteriovenous malformations [5]. Studies in endoglin heterozygous and knockout mice further confirmed the crucial role that endoglin plays in developmental angiogenesis [6,7].
The high and selective expression of endoglin on newly formed tumor blood vessels established endoglin as a predictor of poor survival in patients with various solid tumors, as reviewed in [8,9,10]. Although initially studies on endoglin were focused on its role in endothelial cells and angiogenesis, early results provided data that showed endoglin expression on macrophages [11], leukocytes, syncytiotrophoblasts in the placenta [12] and fibroblasts in culture [13]. More recent work has revealed a crucial role for endoglin expression on other non-endothelial cells, which we have recently reviewed [14]. In both endothelial and non-endothelial cells, endoglin regulates TGF-β family member signaling.
1.2. Endoglin Signaling
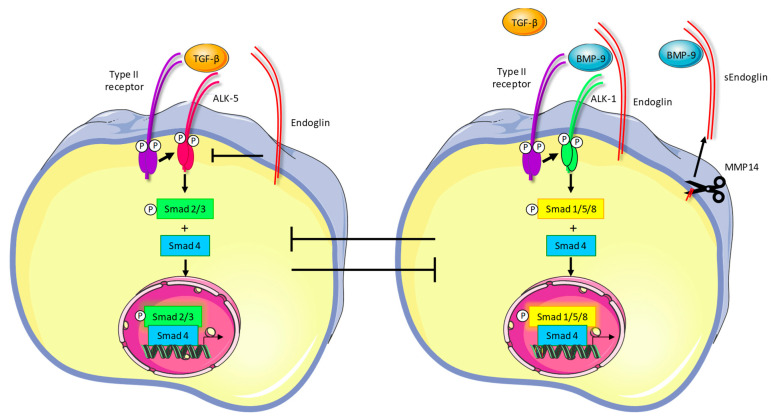
The most important and well-described ligands for endoglin are TGF-β and bone morphogenetic protein (BMP)-9. The binding of these ligands regulates endoglin expression in endothelial cells [15,16,17]. TGF-β or BMP-9 binding to endoglin in a heterotypic complex with the type-II TGF-β or BMP receptor leads to the recruitment of the activin-receptor like kinase (ALK)-1 receptor [10]. Transphosphorylation of ALK-1 by the type-II receptor leads to downstream phosphorylation of the receptor-regulated Smads-1/5/8. Subsequent complex formation with Smad4 leads to the nuclear translocation of the Smad complex and the transcription of target genes, resulting in the regulation of angiogenesis (Figure 1). Activation of the endoglin-Smad1/5/8 pathway indirectly inhibits the canonical TGF-β signaling pathway, which involves another type-I receptor, ALK-5 (Figure 1). ALK-5-dependent signaling leads to canonical TGF-β signaling and vessel maturation [18]. Ultimately, endoglin stimulates angiogenesis both directly through Smad1/5/8 signaling and indirectly by inhibition of ALK-5-mediated pathways.
Figure 1.
Endoglin signaling. When TGF-β binds to its type-II receptor in the absence of endoglin, the type-I receptor ALK-5 is recruited and transphosphorylated. This subsequently leads to phosphorylation of Smad2/3, which then forms a complex with Smad4, translocates to the nucleus and regulates target genes involved in vessel maturation. The presence of endoglin on the cell membrane inhibits this signaling pathway (left panel). In the presence of endoglin, binding of BMP-9 or TGF-β to the type-II receptor results in recruitment and transphosphorylation of ALK-1, which phosphorylates Smad1/5/8, resulting in regulation of angiogenic target genes (right panel). Endoglin can be cleaved from the cell membrane by MMP-14, creating a soluble form of the receptor which may function as a ligand trap.
In addition to ligand binding, endoglin signaling is regulated by matrix metalloproteinase (MMP)-14-dependent cleavage of the extracellular domain, which generates a soluble endoglin (sEng) variant [19]. Endoglin can also be shed through MMP-12-dependent cleavage [20]. Soluble endoglin can inhibit angiogenesis and its levels have been shown to be upregulated in pre-eclampsia [21], metabolic disorders [22] and in cancer [15], although for the latter, conflicting reports have been published. Interestingly, recent work showed that the effects of sEng (i.e., activating or inhibiting signaling) are strongly dependent on whether it circulates in the mono- or dimeric form [23]. This might provide new insights into the role and mechanisms of sEng in regulating signaling and eventually angiogenesis.
1.3. Targeting Endoglin for Imaging and Therapy
Given its high and selective expression on tumor blood vessels, endoglin was exploited as a target for tumor imaging. Specific tumor labeling can be accomplished by using conjugated TRC105 with PET, SPECT and NIRF imaging modalities [10,24,25]. Next to its potential for tumor imaging, endoglin was given attention as a selective tumor target for cancer therapy. Although several endoglin-targeting antibodies and peptides have been developed, the most extensive clinical development has been done using TRC105, which also was the subject of multiple preclinical studies. Below, we will summarize preclinical studies with TRC105, its clinical translation and associated biomarker studies. Finally, we will summarize the findings and discuss what we have learned to date and what questions remain.
2. Preclinical Studies on Endoglin Targeting
2.1. Development of the Anti-Endoglin Antibody Sn6j/TRC105
The first study describing the endoglin antibody Sn6j was published by Matsuno et al. in 1999 [26]. This study showed binding of Sn6j to human umbilical vein endothelial cells (HUVECs) in vitro and to tumor vasculature in severe combined immunodeficient (SCID) mice bearing human tumors. Treatment with drug conjugates of Sn6j or other endoglin antibodies showed long-lasting tumor remission of human breast cancer xenografts and the inhibition of tumor angiogenesis in vivo. Moreover, synergistic anti-tumor effects and complete regression of established MCF-7 human breast tumor nodules in mice were observed when endoglin antibodies were combined with chemotherapy [27]. The heavy and light chains of the Sn6j antibody were humanized to generate a human murine chimeric antibody that was safely administered to monkeys [28].
2.2. TRC105 Acts Through Immune-Dependent Mechanisms
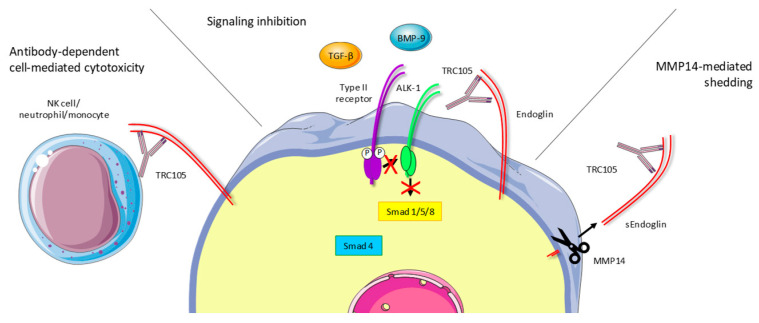
Initial preclinical studies showed evidence that Sn6j worked through immune-dependent mechanisms. In mice bearing subcutaneous tumors, TRC105 demonstrated higher efficacy in suppressing tumor growth in immune-competent BALB/c mice compared to immunodeficient SCID mice [29]. Moreover, the combination of Sn6j with immunostimulants (CpG ODN) synergistically reduced syngeneic CT26 mouse colon tumor growth and improved the survival of BALB/c mice. These effects seemed to partly depend on CD4+, but mostly on CD8+ T-cells, since their depletion abrogated the observed anti-tumor responses [30]. Additionally, TRC105 induced apoptosis via antibody-dependent cell-mediated cytotoxicity (ADCC, Figure 2) [31]. Recent work from our groups indeed confirmed these findings, showing loss of therapeutic efficiency of TRC105 in mice lacking the Fc gamma receptors I-IV that are therefore unable to induce ADCC [32]. This study revealed that TRC105 targets a subset of intratumoral, endoglin-expressing regulatory T cells (Tregs). TRC105 appeared to eliminate this immunosuppressive population from the tumor microenvironment. These data indicate that targeting endoglin with TRC105 plays a role in regulating immune effector cells.
Figure 2.
Proposed mechanisms of action of TRC105. TRC105 exerts its actions through different mechanisms. Firstly, binding to endoglin induces antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by NK cells, neutrophils and monocytes, resulting in target cell killing. Secondly, TRC105 prevents BMP-9 binding to endoglin, thereby inhibiting receptor complex formation and endoglin-mediated downstream signaling. Lastly, MMP-14-mediated endoglin shedding is induced upon TRC105 binding, generating soluble endoglin (sEng) which can function as a ligand trap and thus reduces endoglin signaling.
2.3. TRC105 Inhibits BMP-9-Induced Signaling
In addition to immune-dependent mechanisms, TRC105 also inhibits endoglin-dependent BMP-9 signaling (Figure 2). Strikingly, although clear anti-tumor effects in syngeneic mouse models have been shown, the binding affinity of TRC105 for mouse endoglin is significantly lower compared to human endoglin. To better study the contribution of endoglin-dependent BMP-9 signaling in preclinical models, a mouse endoglin-targeting antibody was developed, M1043 [33]. TRC105 and M1043 both very efficiently inhibit BMP-9-induced Smad1 phosphorylation in human and mouse endothelial cells, respectively, leaving TGF-β-induced, ALK-5-dependent Smad2/3 phosphorylation unaltered. Additional studies showed increased basal Smad2 phosphorylation and decreased Smad1 phosphorylation upon TRC105 treatment in HUVECs [34,35]. Although M1043 is a stronger inhibitor of BMP-9 binding to mouse endoglin than it is to human endoglin, it does appear to be less potent than TRC105 in certain in vivo colorectal cancer models [32]. The fact that the IgG subtype of M1043 (rat IgG1) does not induce ADCC in mice further strengthens the idea that ADCC is an important contributor to the therapeutic effects of TRC105.
The exact molecular mechanism of how TRC105 disturbs BMP-9 binding was more recently shown when the crystal structure of the human endoglin ectodomain and its complex with the ligand BMP-9 was published. BMP-9 interacts with a hydrophobic surface on the N-terminal orphan domain of endoglin, involving the residues mutated in HHT patients and overlapping with the TRC105 binding site [36]. As well as competing with BMP-9 binding, TRC105 induces endoglin shedding from the cell surface of endothelial cells in an MMP-14-dependent manner. This generates high levels of soluble endoglin which can act as a ligand trap and function to inhibit angiogenesis. These mechanisms of action of TRC105 might well contribute to its anti-angiogenic effects [34].
2.4. TRC105 and Crosstalk with the Vascular Endothelial Growth Factor (VEGF) Pathway
Acquired resistance to anti-angiogenic therapies has been reported in a multitude of studies and is mostly the result of the activation of alternative pro-angiogenic pathways in response to treatment [37,38,39]. Considerable crosstalk between the endoglin and vascular endothelial growth factor (VEGF) pathways has been reported. In colorectal cancer patient samples, it was observed that endothelial Smad1 phosphorylation was increased upon treatment with anti-VEGF therapy [35]. Furthermore, in vitro, TRC105 increased VEGF-induced ERK1/2 phosphorylation, while hardly affecting HUVEC viability [40]. In an orthotopic pancreatic cancer xenograft model, endoglin is one of three genes that underwent significant upregulation in response to VEGF blockage [41]. Combining endoglin and VEGF targeting increased efficiency in inhibiting HUVEC cord formation, fetal bone metatarsal angiogenesis assays [42] and developmental angiogenesis in zebrafish embryos [35]. In the mouse KEP1-11 breast cancer model, the combination treatment of TRC105 and VEGF targeting resulted in decreased tumor vessel density, surprisingly without significantly affecting primary tumor growth [35]. These data were confirmed in another study, where TRC105 enhanced the anti-angiogenic effects of the VEGF-A-targeting antibody bevacizumab. The combination of bevacizumab with TRC105 was shown to inhibit VEGF signaling and tip cell formation in vitro and to inhibit tumor growth, metastasis and tumor-associated angiogenesis in a murine breast cancer model [43]. In renal cancer-derived endothelial cells, the combination of TRC105 and the multi-tyrosine kinase inhibitor sunitinib induced phosphorylation of Smad2/3 to promote endothelial cell death. Moreover, TRC105 enhanced the inhibitory effect of sunitinib on VEGF signaling and reduced VEGFR2-Akt-Creb activation, suggesting a molecular cooperation between the two drugs [44]. Together, these data show significant crosstalk between endoglin and the VEGF pathway and provided the basis for combination studies in patients, as discussed later in this review.
2.5. TRC105 and Anti-Tumor Effects in Preclinical Cancer Models
TRC105 has been tested in a wide variety of preclinical cancer models, most of them reporting the inhibition of tumor growth and sometimes even complete tumor regression, although resistant cancer types have also been reported, like the KPC3 pancreatic cancer model (Schoonderwoerd et al., submitted). TRC105 was shown to inhibit the growth of syngeneic CT26 and MC38 mouse colon cancer and MCF7 and 4T1 breast cancer models [45].
TRC105 was additionally tested in multiple other cancer mouse models. Dourado and colleagues [46] showed that TRC105 treatment prevented human acute myeloid leukemia (AML) blast engraftment in irradiated NOD SCID gamma (NSG) mice, and, when administered at disease onset, inhibited AML progression in vivo. In acute B-lymphoblastic leukemia (B-ALL), TRC105 alone was ineffective in inducing a long-term response, which might be explained by the observation that B-ALL blasts produce high levels of sEng which could result in a sink effect for TRC105, preventing effective target binding on the tumor cells. However, in both B-ALL and AML, TRC105 synergized with chemotherapy to inhibit leukemia development or disease progression, respectively.
In mouse models for renal cancer, TRC105 impaired the ability of tumor endothelial cells (TECs) and cancer stem cell-derived TECs to organize into tubular structures, whereas it did not limit proliferation or survival. The combination of TRC105 with sunitinib synergistically reduced tube formation, proliferation and survival of CSC-TECs and tumor-derived TECs to a similar extent [44].
In mice bearing ovarian cancer cells, TRC105 treatment resulted in decreased metastatic spread of high-grade serous ovarian cancer, reduced ascites and delayed growth and spread of abdominal tumor cells, thus extending overall survival in vivo [47].
The anti-tumor effects of TRC105 were initially mostly assigned to targeting of the tumor blood vessels, restricting oxygen and nutrient availability, thereby reducing tumor growth. However, more recent studies revealed additional targets of TRC105 therapy apart from endothelial cells.
In the KEP1-11 breast cancer model, treatment with TRC105 reduced the presence of cancer-associated fibroblasts (CAFs) in primary tumors and reduced metastatic spread in an adjuvant setting [35]. In prostate cancer models, TRC105 treatment significantly potentiated the anti-tumor effects of radiation therapy, when compared to irradiation alone [48]. Additional research showed that the combination of androgen deprivation therapy (ADT) and TRC105 reduced castration-resistant prostate cancer progression through interruption of the communication between endoglin-expressing CAFs and prostate cancer cells [49].
Moreover, in colorectal cancer (CRC), endoglin-expressing CAFs seem to contribute to metastatic potential and the formation of liver metastasis in an experimental model of CRC-derived liver metastases in nude mice. These pro-metastatic effects of CAF-specific endoglin could be inhibited by TRC105, indicating CAFs as an additional TRC105 target, in addition to the angiogenic endothelial cells [50].
Finally, two studies reported on the combined effects of TRC105 and immunotherapy. In a neuroblastoma study in immunodeficient NSG mice, it was shown that immunotherapy with the anti-GD2 antibody dinutuximab combined with activated NK cells is suppressed by endoglin-positive cells in the tumor microenvironment. This suppression could be overcome by targeting endoglin on the human mesenchymal stem cells (MSCs) and murine endothelial cells and macrophages using TRC105 and M1043, respectively, and resulted in improved survival [51]. Furthermore, the combination of TRC105 with the checkpoint inhibitor PD1 was shown to be effective in inhibiting tumor initiation in a chemically induced model for colitis-associated CRC and subcutaneous and orthotopic syngeneic MC38 and CT26 CRC models. Importantly, sustained anti-tumor and memory responses were reported [32].
Taken together, these studies show that TRC105 treatment, alone or as a combination therapy, seems effective in inhibiting tumor growth and preventing metastatic spread in preclinical cancer models. The effects are partly from targeting tumor angiogenesis, but also extend to other cell types, including CAFs, Tregs and other cells suppressing therapeutic responses like myeloid-derived suppressor cells. The expression of endoglin on a variety of cells increases the potential for TRC105 to be used as a cancer therapy and might even extend beyond the field of oncology, looking, for example, at fibrotic diseases.
3. TRC105 Clinical Trials
3.1. Biomarker Findings in Clinical Trials
Consistent with what has been noted for the entire class of anti-angiogenic agents, not all patients respond to TRC105. To enrich patients most likely to benefit, as well as to investigate the molecular impact of the drug, blood-based biomarkers were analyzed using multiplex ELISA to quantitatively assess circulating proteins related to angiogenesis, inflammation and immunity. This panel, termed the angiome [52], is a protein multiplex array that was consistently applied to several TRC105 clinical trials in order to explore potential prognostic, predictive and pharmacodynamic biomarkers. A list of all the clinical trials testing TRC105 is shown in Table 1. Below, we discuss some of the trials and their biomarker findings.
Table 1.
TRC105 clinical trials.
| Trial Description | NCT Number | Trial Phase | Patients Enrolled | Primary Outcome |
|---|---|---|---|---|
| Open label phase 1 dose-finding study of TRC105 in patients with solid cancer | NCT00582985 | 1 | 51 | Safety |
| Preoperative combination of letrozole, everolimus and TRC105 in postmenopausal hormone receptor-positive and Her2-negative breast cancer | NCT02520063 | 1/2 | 14 | MTD |
| Open label dose-finding study of TRC105 plus capecitabine for metastatic breast cancer (TRC105) | NCT01326481 | 1/2 | 19 | MTD |
| A phase I/II study of TRC105 in metastatic castration-resistant prostate cancer (CRPC) | NCT01090765 | 1/2 | 21 | MTD |
| Sorafenib and TRC105 in hepatocellular cancer | NCT01306058 | 1/2 | 27 | MTD and TTP |
| Open label continuation study of TRC105 for patients who have completed a prior TRC105 trial | NCT02354612 | 1/2 | 50 | Long-term TRC105 response |
| Bevacizumab with or without anti-endoglin monoclonal antibody TRC105 in treating patients with recurrent glioblastoma multiforme | NCT01648348 | 1/2 | 116 | MTD, safety and PFS |
| Study of carotuximab (TRC105) plus nivolumab in patients with metastatic NSCLC | NCT03181308 | 1b | 11 | Safety |
| Study of TRC105 combined with standard-dose bevacizumab for advanced solid tumors for which bevacizumab is indicated | NCT01332721 | 1b | 38 | MTD |
| A study of TRC105 in combination with paclitaxel/carboplatin and bevacizumab in non-squamous cell lung cancer | NCT02429843 | 1b | 16 | Change in medical management |
| A phase 1B dose-escalation and phase 2a study of carotuximab (TRC105) in combination with pazopanib in patients with advanced soft tissue sarcoma | NCT01975519 | 1b, 2 | 30, 89 | Safety, PFS, ORR |
| Trial of TRC105 and sorafenib in patients with hepatocellular carcinoma (HCC) | NCT02560779 | 1b/2 | 27 | Safety and ORR |
| TRC105 for recurrent glioblastoma | NCT01778530 | 2 | 2 | Radiographic response rate |
| TRC105 combined with standard-dose bevacizumab for two patients with metastatic and refractory choriocarcinoma | NCT02396511 | 2 | 2 | PFS and ORR |
| Study of TRC105 and bevacizumab in patients with refractory gestational trophoblastic neoplasia (GTN) | NCT02664961 | 2 | 3 | ORR |
| TRC105 for liver cancer that has not responded to sorafenib | NCT01375569 | 2 | 11 | TTP |
| Study of TRC105 with abiraterone and with enzalutamide in prostate cancer patients progressing on therapy | NCT03418324 | 2 | 11 | Disease stabilization or improvement at 2 months |
| TRC105 in adults with advanced/metastatic urothelial carcinoma | NCT01328574 | 2 | 13 | PFS |
| Study of TRC105 + paclitaxel/carboplatin and bevacizumab in patients with NSCLC | NCT03780010 | 2 | 15 | Safety |
| A phase 2 evaluation of TRC105 in combination with bevacizumab in patients with glioblastoma (105GM201) | NCT01564914 | 2 | 22 | OS |
| Evaluation of TRC105 in the treatment of recurrent ovarian, fallopian tube or primary peritoneal carcinoma | NCT01381861 | 2 | 23 | PFS, ORR, safety |
| Bevacizumab with or without TRC105 in treating patients with metastatic kidney cancer | NCT01727089 | 2 | 59 | PFS |
| Randomized phase 2 trial of axitinib and TRC105 versus axitinib alone in patients renal cell carcinoma | NCT01806064 | 2 | 150 | Safety and PFS |
| Trial of TRC105 and pazopanib versus pazopanib alone in patients with advanced angiosarcoma (TAPPAS) | NCT02979899 | 3 | 128 | PFS |
MTD: maximal tolerated dose; TTP: time to tumor progression; PFS: progression free survival; OS: overall survival; ORR: objective or overall response rate.
3.2. TRC105 First-in-Human Clinical Trial
Based on the anti-tumor potency in various preclinical models, as described above, TRC105 was first tested in a phase I trial (NCT00582985) in 2008 that enrolled 50 cancer patients to assess safety and efficacy [53]. The recommended phase II dose was established to be 10 mg/kg weekly or 15 mg/kg every two weeks. In this trial, stable disease or better was achieved in 21 of 45 evaluable patients. Two patients demonstrated prolonged responses. One prostate cancer patient showed a complete prostate-specific antigen (PSA) response with bone scan normalization for more than 5 years, and a heavily pretreated uterine carcinosarcoma patient was progression free for 1.5 years.
The angiome was tested in plasma samples from 32 of 50 patients (64%) at baseline, during treatment and the end of the study [54]. Several VEGF family members, such as PlGF, VEGF and VEGF-D decreased significantly following TRC105 treatment, suggesting an overall suppression of angiogenesis. Interestingly, this effect is distinct from bevacizumab, which increases the level of these markers [54]. Soluble endoglin (sEng) also increased in a dose-dependent manner. sEng induction in response to TRC105 could be recaptured in endothelial cell culture [34,40] and represents the strongest pharmacodynamic effect elicited by TRC105. Taken together, the first-in-human study for TRC105 showed that treatment was safe, with indications of clinical activity and formed the basis for further clinical development.
3.3. Crosstalk with the VEGF Pathway: TRC105 Plus VEGF Inhibitors in Phase Ib Trials
Given the considerable crosstalk between endoglin and VEGF pathways, as described above, targeting both angiogenic pathways simultaneously was expected to exert more potent anti-angiogenic and therefore anti-tumor effects. Between 2011 and 2013, a phase Ib, dose-finding study of TRC105 in combination with the VEGF antibody bevacizumab was conducted (NCT01332721) [55]. Both drugs were well tolerated at their recommended single agent doses (10 mg/kg/week for TRC105 and 10 mg/kg/2 weeks for bevacizumab).
Impressively, combining TRC105 with bevacizumab demonstrated durable activity in a VEGF inhibitor refractory patient population [55]. Drug activity was observed in 18 cancer patients (47%), including two partial responses (PR) and 16 patients with prolonged stable disease (SD). Approximately 25% of patients experienced a 10–25% tumor volume reduction and remained progression free for periods longer than experienced on prior anti-VEGF therapy. CT scans also revealed favorable tumor morphology change, such as decreased tumor density [56]. The angiome biomarker panel was assessed across 37 patients (97%) at baseline, after bevacizumab lead-in monotherapy, after co-administration of both drugs for 3 weeks and at the end of treatment [57]. The modulation of certain pharmacodynamic biomarkers, such as bevacizumab-induced PlGF increases and TRC105-induced sEng increases, were observed and not affected by the presence of the other drug. Additionally, novel biomarker modulations were identified when the two drugs were used in combination. For example, bevacizumab treatment increased VEGF-D levels [58], while TRC105 monotherapy decreased VEGF-D [54]. In response to the combination of drugs, VEGF-D levels did not statistically differ, indicating compensatory VEGF-D signaling was inhibited, possibly accounting for delayed drug resistance [57].
3.4. TRC105 Plus VEGF Inhibitors in a Series of Phase Ib Trials
The dense expression of endoglin on blood vessels in highly vascularized tumors led to the study of TRC105 in glioblastoma [59], renal cell carcinoma (RCC) [60], hepatocellular carcinoma [61] and breast cancer [62]. In addition, endoglin expression on RCC cells [63] and certain sarcoma subtypes [64,65] made these tumor types highly relevant for endoglin targeting.
TRC105 was therefore studied in combination with VEGF inhibitors in glioblastoma [66], sarcoma [67,68], RCC [69,70], breast cancer [71], metastatic castration-resistant prostate cancer [48,72], hepatocellular carcinoma [73,74] and urothelial carcinoma [75]. The angiome was tested in four of these phase 1b trials, with three trials combining TRC105 with VEGF inhibitors and one trial testing TRC105 in combination with chemotherapy alone.
3.4.1. Glioblastoma (NCT01648348)
In this study, TRC105 at a dose of 10 mg/kg/week was given to 22 recurrent or progressive GBM patients with or without bevacizumab. For patients receiving dual therapy, TRC105 began after one week of bevacizumab lead-in monotherapy. Median progression-free survival (PFS) for the five patients receiving only TRC105 was 1.38 months, while median PFS for the 14 patients receiving both TRC105 and bevacizumab was 1.81 months (95% CI: 1.25—2.07). The angiome analysis revealed a marked increase of sEng. However, an increase in PlGF and VEGF, as well as a decrease in VEGF-R2, typical changes following bevacizumab treatment, were not observed, suggesting biomarker modulation may be different in this disease type.
3.4.2. Sarcoma (NCT01975519)
TRC105 (8 or 10 mg/kg/week) combined with pazopanib was given to 19 patients with advanced soft tissue sarcoma after one cycle of pazopanib lead-in monotherapy. Six patients responded according to Choi criteria, reaching a response rate of 32%. In the six responders, baseline levels of ICAM-1 and TSP-2 were significantly lower. Typical biomarker modulations in response to VEGF inhibitors, such as upregulation of PlGF, VEGF and VEGF-D, as well as downregulation of VEGF-R2 and TSP-2, were noted as significant on-treatment changes.
3.4.3. Renal Cell Carcinoma (NCT01806064)
TRC105 (8 or 10 mg/kg/week) together with axitinib was administrated to 18 patients with advanced or metastatic renal cell carcinoma (mRCC). Five patients exhibited a more than 30% reduction in tumor size, demonstrating an ORR of 28%. In the five responders, the baseline level of osteopontin (OPN) was lower, while TGFbeta (β)-R3 was higher [56]. Consistently, we noted an increase in sEng as a TRC105-specific effect and an increase in PlGF, VEGF and VEGF-D, as well as a decrease in TSP-2 and VEGF-R2, as a VEGF inhibitor-specific effect.
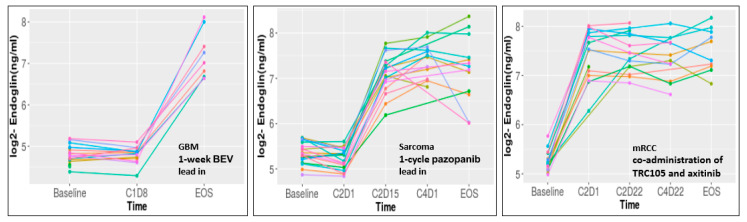
Across the three trials, VEGF monotherapy (bevacizumab for 1 week in GBM, pazopanib for 1 cycle in sarcoma) had little impact on sEng levels. In contrast, sEng increased in all three patient populations following TRC15 treatment, validating sEng as an on-target pharmacodynamic marker (Figure 3).
Figure 3.
TRC105 increases sEng levels in circulation of patients. Each individual patient is represented by a colored line.
Beyond representing a pure pharmacodynamic marker, sEng has important biological functions, such as attenuating the proliferation, migration and tube formation of human umbilical vein endothelial cells [76]. In addition, sEng has been shown to reduce inflammation in mouse models [77]. Alternatively, it has been reported that increased circulating sEng may preferentially direct BMP-9 signaling via cell surface endoglin at the endothelium rather than being an inhibitory ligand trap [23]. The exact function of sEng in patients receiving TRC105 remain to be clarified.
No other consistent, TRC105-dependent modulations were noted for other markers within the endoglin signaling pathway, including upstream ligands (BMP-9, TGF-β1), receptors (TGFβ-R3) and downstream effectors (PDGF-AA, PDGF-BB).
Specific biomarker changes could be identified in response to monotherapy with VEGF inhibitors. Increases in PlGF, VEGF and VEGF-D, as well as decreases in TSP-2 and VEGF-R2, are all noted pharmacodynamic changes in response to VEGF inhibitors, consistent with the literature [58,78,79]. In contrast, VEGF-R3, a receptor playing crucial roles in lymphangiogenesis, significantly decreased in response to both VEGF inhibitors and TRC105, suggesting VEGF-R3 modulation is not specific to VEGF inhibitors and may represent a broader effect of anti-angiogenic therapies.
3.4.4. TRC105 and Capecitabine in Breast Cancer Patients (NCT01326481)
In this trial, TRC105 (7.5 or 10 mg/kg/week) was combined with capecitabine and administrated to 19 progressive or recurrent metastatic HER2-negative breast cancer patients. There were four responders in this study. Angiome analyses revealed that baseline sEng levels were noted to be higher in the responders compared to the non-responders; however, this difference did not reach statistical significance (p = 0.078). In addition to a well-defined upregulation of sEng levels, the endoglin ligand BMP-9 was significantly increased in the circulation of most patients (71%), potentially reflecting a compensatory mechanism. No significant changes in PlGF, VEGF, VEGF-D, TSP-2 or VEGF-R2, were detected in this trial, offering further evidence that changes in these five markers are specific to the combination of TRC105 plus VEGF inhibitors.
3.5. TRC105 Biomarkers in Randomized Trials
Encouraged by these promising phase 1b studies, randomized phase 2/3 trials were next performed in mRCC and angiosarcoma. In the TRAXAR trial, 150 mRCC patients were randomized in a 1:1 ratio to standard dose axitinib or TRC105 combined with axitinib (NCT01806064) [80]. In the TAPPAS trial, 128 angiosarcoma patients were randomized to pazopanib or TRC105 and pazopanib (NCT02979899) [81]. In both studies, no improvement in PFS was observed by the addition of TRC105 to VEGF inhibitors, leading to termination of the further development of TRC105 [80,81].
While several circulating biomarkers were shown to be prognostic for outcome in earlier studies, the identification of a biomarker that could identify patients most likely to respond to TRC105 remains an unmet need. The randomized design of the two studies mentioned above enables the discovery of predictive biomarkers for TRC105. While the angiome was not tested in samples collected from patients in the TAPPAS trial, it has been assessed in patients from the TRAXAR trial. Interestingly, VEGF was identified as a potential predictive marker [80]. Other analyses in randomized trials of TRC105 also indicated potential predictors of efficacy. In a separate phase 2b randomized trial, mRCC patients were treated with bevacizumab alone or bevacizumab + TRC105. PFS was not improved by the addition of TRC105. The authors reported that lower TGF-β levels (<10.6 ng/mL) are associated with better PFS at the 12- or 24-week landmarks [70]. It remains possible that a sub-population of patients, guided by proper biomarkers, would benefit from the addition of TRC105 to VEGF inhibitors.
3.6. Biomarker Conclusions
By now, the angiome has been assessed in seven phase 1-2 trials featuring TRC105 monotherapy or a combination with VEGF inhibitors. In short, TRC105 induces distinct biomarker modulations from VEGF inhibitors. sEng has been identified as a strong pharmacodynamic marker, exhibiting a direct drug effect of TRC105. sEng is an important marker in other diseases, such as pre-eclampsia and Osler-Weber-Rendu syndrome [82]. The identification of VEGF as a potential predictive marker in the randomized mRCC trial emphasizes the importance of patient pre-selection to achieve precision medicine. The lesson learned from the angiome analysis across all TRC105 trials will be appliable to novel anti-angiogenic drugs.
4. Discussion
4.1. Lessons Learned, Question to Be Answered
During the last 20 years, many studies on targeting endoglin, as either monotherapy or combined with other (anti-angiogenic) therapies, have been performed. Although initial encouraging results were reported, the pivotal trials for TRC105 did not demonstrate clinical benefit to warrant further clinical development. Despite this disappointing result, many of these studies have revealed valuable knowledge on endoglin biology, endoglin expression on target cells and crosstalk with other pro-angiogenic pathways.
Endoglin-targeting therapy does not seem to fit the “classical” anti-angiogenic therapies. Although clear crosstalk between the endoglin and VEGF pathways has been shown, combined TRC105/anti-VEGF therapy appeared to be effective in VEGF therapy refractory patients and in preclinical models. These observations might be explained by the binding of TRC105 to additional target cells. Endoglin expression has been reported on tumor-infiltrating Tregs, macrophages, CAFs and cancer (stem) cells in human and mouse samples. This could contribute to the efficiency of TRC105, since targeting those cells might enhance anti-tumor responses. Intriguingly, a decrease in Tregs was also observed in patients dosed with TRC105 [53], showing additional evidence for the targeting of endoglin-expressing Tregs by TRC105. Furthermore, targeting endoglin on fibroblasts might also extend beyond oncology. Endoglin expression has been shown on activated fibroblasts in cardiac fibrosis, where targeting endoglin with TRC105 in preclinical models reduced cardiac fibrosis and improved outcomes [83,84]. Since several studies have investigated the role of endoglin and its ligand BMP-9 in the progression of liver fibrosis, this might be an interesting field of study.
The regulation of angiogenesis by BMP-9 and TGF-β has been a subject of debate for quite some years since their effects seem very concentration and receptor dependent. Despite this fact, the inhibition of endoglin-dependent BMP-9 signaling via TRC105, sEng or by using the BMP-9-binding ligand trap ALK1-Fc [85,86] reduces angiogenesis. However, the inhibition of BMP-9 signaling only seems partly responsible for the therapeutic effects. The immune-dependent effects and induction of ADCC seem to be of crucial importance for the anti-tumor effects of TRC105. This was further emphasized by preclinical studies showing that immunomodulatory therapies increase TRC105 efficiency. Unfortunately, a clinical trial with TRC105 and the PD1 inhibitor nivolumab (NCT03181308) was terminated prematurely. Therefore, it remains unknown if this also holds true for cancer patients.
4.2. Looking Forward
All studies have increased our knowledge on endoglin biology, its expression and regulation, crosstalk with other pathways and appropriateness as a therapeutic target in, and beyond, oncology. Obviously, it is disappointing that despite very promising preclinical studies, the clinical development is only ongoing for acute macular degeneration (AMD). One could argue that if TRC105 was tested in the right setting or in earlier stages of disease, it could prove more effective. Alternatively, immunologically “hot” tumors might also be more responsive given the strong involvement of the immune system and the increased efficiency when combined with checkpoint inhibition in mouse models. One questions that still remains is how on a functional level endoglin exerts its pro-tumorigenic effects. This might be the regulation of immune cell infiltration or affecting the migratory properties of cells. Current in vitro models poorly recapitulate these processes, while studies in mice are hampered by the differences in mechanisms of action and binding of TRC105. Many more years of research should shed light on this and reveal if a novel approach for endoglin targeting would be valuable.
Acknowledgments
We thank our research teams for performing many years of innovative research work on endoglin targeting in oncology. We thank Charles Theuer, Bonne Adams and the entire team from Tracon Pharmaceuticals for their valuable discussions over the years. We thank Charles Theuer for critically reviewing the manuscript.
Abbreviations
| BMP | Bone morphogenetic protein |
| sEng | Soluble endoglin |
| ICAM-1 | Inter-cellular adhesion molecule 1 |
| OPN | Osteopontin |
| PDGF | Platelet-derived growth factor |
| PlGF | Placenta growth factor |
| TGFβ | Transforming growth factor-β |
| TSP2 | Thrombospondin 2 |
| VEGF | Vascular endothelial growth factor |
Author Contributions
Writing—original draft preparation, L.J.A.C.H., M.P., Y.L., A.B.N.; writing—review and editing, L.J.A.C.H., M.P., Y.L., A.B.N.; visualization, M.P.; supervision, L.J.A.C.H., A.B.N. All authors have read and agreed to the published version of the manuscript.
Funding
Our work on endoglin and TRC105 was supported by the Dutch Cancer Society (UL2011-5051), Stichting Fond Oncologie Holand (SFOH) and Tracon Pharmaceuticals.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the review nature of this publication.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data previously exist in the literature. No new data was generated for this review.
Conflicts of Interest
Y.L. and M.P. declare no conflict of interest. A.B.N. has received research funding from Seattle Genetics, MedPatco, Genentech, Tracon Pharma, Acceleron Pharma, Leadiant Biosciences and Sanofi-Aventis and has received consultant/advisory compensation from Tracon Pharma, Leap Therapeutics and Eli Lilly. L.J.H. has received research funding from Tracon Pharma and is a co-inventor on patent TRC105/PD1 combination. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gougos A., Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J. Biol. Chem. 1990;265:8361–8364. [PubMed] [Google Scholar]
- 2.Cheifetz S., Bellon T., Cales C., Vera S., Bernabeu C., Massague J., Letarte M. Endoglin Is a Component of the Transforming Growth-Factor-Beta Receptor System in Human Endothelial-Cells. J. Biol. Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 3.Bellon T., Corbi A., Lastres P., Cales C., Cebrian M., Vera S., Cheifetz S., Massague J., Letarte M., Bernabeu C. Identification and expression of two forms of the human transforming growth factor-beta-binding protein endoglin with distinct cytoplasmic regions. Eur. J. Immunol. 1993;23:2340–2345. doi: 10.1002/eji.1830230943. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita H., Ichijo H., Grimsby S., Moren A., ten Dijke P., Miyazono K. Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-beta. J. Biol. Chem. 1994;269:1995–2001. [PubMed] [Google Scholar]
- 5.McAllister K.A., Grogg K.M., Johnson D.W., Gallione C.J., Baldwin M.A., Jackson C.E., Helmbold E.A., Markel D.S., McKinnon W.C., Murrell J., et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 6.Goumans M.J., Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int. J. Dev. Biol. 2000;44:253–265. [PubMed] [Google Scholar]
- 7.Arthur H.M., Ure J., Smith A.J., Renforth G., Wilson D.I., Torsney E., Charlton R., Parums D.V., Jowett T., Marchuk D.A., et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev. Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 8.Ollauri-Ibanez C., Lopez-Novoa J.M., Pericacho M. Endoglin-based biological therapy in the treatment of angiogenesis-dependent pathologies. Expert Opin. Biol. 2017;17:1053–1063. doi: 10.1080/14712598.2017.1346607. [DOI] [PubMed] [Google Scholar]
- 9.Rosen L.S., Gordon M.S., Robert F., Matei D.E. Endoglin for targeted cancer treatment. Curr. Oncol. Rep. 2014;16:365. doi: 10.1007/s11912-013-0365-x. [DOI] [PubMed] [Google Scholar]
- 10.Paauwe M., ten Dijke P., Hawinkels L.J. Endoglin for tumor imaging and targeted cancer therapy. Expert. Opin. Ther. Targets. 2013;17:421–435. doi: 10.1517/14728222.2013.758716. [DOI] [PubMed] [Google Scholar]
- 11.O’Connell P.J., McKenzie A., Fisicaro N., Rockman S.P., Pearse M.J., d’Apice A.J. Endoglin: A 180-kD endothelial cell and macrophage restricted differentiation molecule. Clin. Exp. Immunol. 1992;90:154–159. doi: 10.1111/j.1365-2249.1992.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gougos A., St Jacques S., Greaves A., O’Connell P.J., d’Apice A.J., Buhring H.J., Bernabeu C., van Mourik J.A., Letarte M. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int. Immunol. 1992;4:83–92. doi: 10.1093/intimm/4.1.83. [DOI] [PubMed] [Google Scholar]
- 13.St-Jacques S., Cymerman U., Pece N., Letarte M. Molecular characterization and in situ localization of murine endoglin reveal that it is a transforming growth factor-beta binding protein of endothelial and stromal cells. Endocrinology. 1994;134:2645–2657. doi: 10.1210/endo.134.6.8194490. [DOI] [PubMed] [Google Scholar]
- 14.Schoonderwoerd M.J.A., Goumans M.T.H., Hawinkels L. Endoglin: Beyond the Endothelium. Biomolecules. 2020;10:289. doi: 10.3390/biom10020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernabeu C., Lopez-Novoa J.M., Quintanilla M. The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim. Biophys. Acta. 2009;1792:954–973. doi: 10.1016/j.bbadis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Dallas N.A., Samuel S., Xia L., Fan F., Gray M.J., Lim S.J., Ellis L.M. Endoglin (CD105): A Marker of Tumor Vasculature and Potential Target for Therapy. Clin. Cancer Res. 2008;14:1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 17.Duff S.E., Li C., Garland J.M., Kumar S. CD105 is important for angiogenesis: Evidence and potential applications. FASEB J. 2003;17:984–992. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- 18.Goumans M.J., Lebrin F., Valdimarsdottir G. Controlling the angiogenic switch: A balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc. Med. 2003;13:301–307. doi: 10.1016/S1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 19.Hawinkels L.J., Kuiper P., Wiercinska E., Verspaget H.W., Liu Z., Pardali E., Sier C.F., ten Dijke P. Matrix Metalloproteinase-14 (MT1-MMP)-Mediated Endoglin Shedding Inhibits Tumor Angiogenesis. Cancer Res. 2010;70:4141–4150. doi: 10.1158/0008-5472.CAN-09-4466. [DOI] [PubMed] [Google Scholar]
- 20.Aristorena M., Gallardo-Vara E., Vicen M., de Las Casas-Engel M., Ojeda-Fernandez L., Nieto C., Blanco F.J., Valbuena-Diez A.C., Botella L.M., Nachtigal P., et al. MMP-12, Secreted by Pro-Inflammatory Macrophages, Targets Endoglin in Human Macrophages and Endothelial Cells. Int. J. Mol. Sci. 2019;20:3107. doi: 10.3390/ijms20123107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armaly Z., Jadaon J.E., Jabbour A., Abassi Z.A. Preeclampsia: Novel Mechanisms and Potential Therapeutic Approaches. Front. Physiol. 2018;9:973. doi: 10.3389/fphys.2018.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vicen M., Igreja Sa I.C., Tripska K., Vitverova B., Najmanova I., Eissazadeh S., Micuda S., Nachtigal P. Membrane and soluble endoglin role in cardiovascular and metabolic disorders related to metabolic syndrome. Cell Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03701-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawera A., Tong Z., Thorikay M., Redgrave R.E., Cai J., van Dinther M., Morrell N.W., Afink G.B., Charnock-Jones D.S., Arthur H.M., et al. Role of soluble endoglin in BMP9 signaling. Proc. Natl. Acad. Sci. USA. 2019;116:17800–17808. doi: 10.1073/pnas.1816661116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q., Bindokas V., Shen J., Fan H., Hoffman R.M., Xing H.R. Time-course imaging of therapeutic functional tumor vascular normalization by antiangiogenic agents. Mol. Cancer. 2011;10:1173–1184. doi: 10.1158/1535-7163.MCT-11-0008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Yang Y., Hong H., Cai W. Multimodality molecular imaging of CD105 (Endoglin) expression. Int. J. Clin. Exp. Med. 2011;4:32–42. [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuno F., Haruta Y., Kondo M., Tsai H., Barcos M., Seon B.K. Induction of lasting complete regression of preformed distinct solid tumors by targeting the tumor vasculature using two new anti-endoglin monoclonal antibodies. Clin. Cancer Res. 1999;5:371–382. [PubMed] [Google Scholar]
- 27.Takahashi N., Haba A., Matsuno F., Seon B.K. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res. 2001;61:7846–7854. [PubMed] [Google Scholar]
- 28.Shiozaki K., Harada N., Greco W.R., Haba A., Uneda S., Tsai H., Seon B.K. Antiangiogenic chimeric anti-endoglin (CD105) antibody: Pharmacokinetics and immunogenicity in nonhuman primates and effects of doxorubicin. Cancer Immunol. Immunother. 2006;55:140–150. doi: 10.1007/s00262-005-0691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsujie M., Uneda S., Tsai H., Seon B.K. Effective anti-angiogenic therapy of established tumors in mice by naked anti-human endoglin (CD105) antibody: Differences in growth rate and therapeutic response between tumors growing at different sites. Int. J. Oncol. 2006;29:1087–1094. doi: 10.3892/ijo.29.5.1087. [DOI] [PubMed] [Google Scholar]
- 30.Tsujie M., Tsujie T., Toi H., Uneda S., Shiozaki K., Tsai H., Seon B.K. Anti-tumor activity of an anti-endoglin monoclonal antibody is enhanced in immunocompetent mice. Int. J. Cancer. 2008;122:2266–2273. doi: 10.1002/ijc.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seon B.K., Haba A., Matsuno F., Takahashi N., Tsujie M., She X., Harada N., Uneda S., Tsujie T., Toi H., et al. Endoglin-targeted cancer therapy. Curr. Drug Deliv. 2011;8:135–143. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoonderwoerd M.J.A., Koops M.F.M., Angela R.A., Koolmoes B., Toitou M., Paauwe M., Barnhoorn M.C., Liu Y., Sier C.F.M., Hardwick J.C.H., et al. Targeting Endoglin-Expressing Regulatory T Cells in the Tumor Microenvironment Enhances the Effect of PD1 Checkpoint Inhibitor Immunotherapy. Clin. Cancer Res. 2020;26:3831–3842. doi: 10.1158/1078-0432.CCR-19-2889. [DOI] [PubMed] [Google Scholar]
- 33.Nolan-Stevaux O., Zhong W., Culp S., Shaffer K., Hoover J., Wickramasinghe D., Ruefli-Brasse A. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti-endoglin antibodies. PLoS ONE. 2012;7:e50920. doi: 10.1371/journal.pone.0050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S., Pan C.C., Bloodworth J.C., Nixon A.B., Theuer C., Hoyt D.G., Lee N.Y. Antibody-directed coupling of endoglin and MMP-14 is a key mechanism for endoglin shedding and deregulation of TGF-beta signaling. Oncogene. 2014;33:3970–3979. doi: 10.1038/onc.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paauwe M., Heijkants R.C., Oudt C.H., van Pelt G.W., Cui C., Theuer C.P., Hardwick J.C., Sier C.F., Hawinkels L.J. Endoglin targeting inhibits tumor angiogenesis and metastatic spread in breast cancer. Oncogene. 2016;35:4069–4079. doi: 10.1038/onc.2015.509. [DOI] [PubMed] [Google Scholar]
- 36.Saito T., Bokhove M., Croci R., Zamora-Caballero S., Han L., Letarte M., de Sanctis D., Jovine L. Structural Basis of the Human Endoglin-BMP9 Interaction: Insights into BMP Signaling and HHT1. Cell Rep. 2017;19:1917–1928. doi: 10.1016/j.celrep.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergers G., Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kieran M.W., Kalluri R., Cho Y.J. The VEGF pathway in cancer and disease: Responses, resistance, and the path forward. Cold Spring Harb. Perspect. Med. 2012;2:a006593. doi: 10.1101/cshperspect.a006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Tian H., Blobe G.C., Theuer C.P., Hurwitz H.I., Nixon A.B. Effects of the combination of TRC105 and bevacizumab on endothelial cell biology. Invest. New Drugs. 2014;32:851–859. doi: 10.1007/s10637-014-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bockhorn M., Tsuzuki Y., Xu L., Frilling A., Broelsch C.E., Fukumura D. Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin. Cancer Res. 2003;9:4221–4226. [PubMed] [Google Scholar]
- 42.Liu Z., Lebrin F., Maring J.A., van den Driesche S., van der Brink S., van D.M., Thorikay M., Martin S., Kobayashi K., Hawinkels L.J., et al. ENDOGLIN is dispensable for vasculogenesis, but required for vascular endothelial growth factor-induced angiogenesis. PLoS ONE. 2014;9:e86273. doi: 10.1371/journal.pone.0086273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian H., Huang J.J., Golzio C., Gao X., Hector-Greene M., Katsanis N., Blobe G.C. Endoglin interacts with VEGFR2 to promote angiogenesis. FASEB J. 2018;32:2934–2949. doi: 10.1096/fj.201700867RR. [DOI] [PubMed] [Google Scholar]
- 44.Brossa A., Buono L., Bussolati B. Effect of the monoclonal antibody TRC105 in combination with Sunitinib on renal tumor derived endothelial cells. Oncotarget. 2018;9:22680–22692. doi: 10.18632/oncotarget.25206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uneda S., Toi H., Tsujie T., Tsujie M., Harada N., Tsai H., Seon B.K. Anti-endoglin monoclonal antibodies are effective for suppressing metastasis and the primary tumors by targeting tumor vasculature. Int. J. Cancer. 2009;125:1446–1453. doi: 10.1002/ijc.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dourado K.M.C., Baik J., Oliveira V.K.P., Beltrame M., Yamamoto A., Theuer C.P., Figueiredo C.A.V., Verneris M.R., Perlingeiro R.C.R. Endoglin: A novel target for therapeutic intervention in acute leukemias revealed in xenograft mouse models. Blood. 2017;129:2526–2536. doi: 10.1182/blood-2017-01-763581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai S., Zhu W., Coffman L., Vlad A., Schwartz L.E., Elishaev E., Drapkin R., Buckanovich R.J. CD105 Is Expressed in Ovarian Cancer Precursor Lesions and Is Required for Metastasis to the Ovary. Cancers. 2019;11:1710. doi: 10.3390/cancers11111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madhav A., Andres A., Duong F., Mishra R., Haldar S., Liu Z., Angara B., Gottlieb R., Zumsteg Z.S., Bhowmick N.A. Antagonizing CD105 enhances radiation sensitivity in prostate cancer. Oncogene. 2018;37:4385–4397. doi: 10.1038/s41388-018-0278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato M., Placencio-Hickok V.R., Madhav A., Haldar S., Tripathi M., Billet S., Mishra R., Smith B., Rohena-Rivera K., Agarwal P., et al. Heterogeneous cancer-associated fibroblast population potentiates neuroendocrine differentiation and castrate resistance in a CD105-dependent manner. Oncogene. 2019;38:716–730. doi: 10.1038/s41388-018-0461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paauwe M., Schoonderwoerd M.J.A., Helderman R., Harryvan T.J., Groenewoud A., van Pelt G.W., Bor R., Hemmer D.M., Versteeg H.H., Snaar-Jagalska B.E., et al. Endoglin Expression on Cancer-Associated Fibroblasts Regulates Invasion and Stimulates Colorectal Cancer Metastasis. Clin. Cancer Res. 2018;24:6331–6344. doi: 10.1158/1078-0432.CCR-18-0329. [DOI] [PubMed] [Google Scholar]
- 51.Wu H.W., Sheard M.A., Malvar J., Fernandez G.E., DeClerck Y.A., Blavier L., Shimada H., Theuer C.P., Sposto R., Seeger R.C. Anti-CD105 Antibody Eliminates Tumor Microenvironment Cells and Enhances Anti-GD2 Antibody Immunotherapy of Neuroblastoma with Activated Natural Killer Cells. Clin. Cancer Res. 2019;25:4761–4774. doi: 10.1158/1078-0432.CCR-18-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Lyu J., Bell Burdett K., Sibley A.B., Hatch A.J., Starr M.D., Brady J.C., Hammond K., Marmorino F., Rossini D., et al. Prognostic and Predictive Biomarkers in Patients with Metastatic Colorectal Cancer Receiving Regorafenib. Mol. Cancer. 2020;19:2146–2154. doi: 10.1158/1535-7163.MCT-20-0249. [DOI] [PubMed] [Google Scholar]
- 53.Rosen L.S., Hurwitz H.I., Wong M.K., Goldman J., Mendelson D.S., Figg W.D., Spencer S., Adams B.J., Alvarez D., Seon B.K., et al. A phase I first-in-human study of TRC105 (Anti-Endoglin Antibody) in patients with advanced cancer. Clin. Cancer Res. 2012;18:4820–4829. doi: 10.1158/1078-0432.CCR-12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y., Starr M.D., Brady J.C., Dellinger A., Pang H., Adams B., Theuer C.P., Lee N.Y., Hurwitz H.I., Nixon A.B. Modulation of circulating protein biomarkers following TRC105 (anti-endoglin antibody) treatment in patients with advanced cancer. Cancer Med. 2014;3:580–591. doi: 10.1002/cam4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon M.S., Robert F., Matei D., Mendelson D.S., Goldman J.W., Chiorean E.G., Strother R.M., Seon B.K., Figg W.D., Peer C.J., et al. An open-label phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) with bevacizumab in patients with advanced cancer. Clin. Cancer Res. 2014;20:5918–5926. doi: 10.1158/1078-0432.CCR-14-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi H., Charnsangavej C., Faria S.C., Macapinlac H.A., Burgess M.A., Patel S.R., Chen L.L., Podoloff D.A., Benjamin R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y., Starr M.D., Brady J.C., Rushing C., Pang H., Adams B., Alvarez D., Theuer C.P., Hurwitz H.I., Nixon A.B. Modulation of Circulating Protein Biomarkers in Cancer Patients Receiving Bevacizumab and the Anti-Endoglin Antibody, TRC105. Mol. Cancer. 2018;17:2248–2256. doi: 10.1158/1535-7163.MCT-17-0916. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y., Starr M.D., Bulusu A., Pang H., Wong N.S., Honeycutt W., Amara A., Hurwitz H.I., Nixon A.B. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med. 2013;2:234–242. doi: 10.1002/cam4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maxwell M., Naber S.P., Wolfe H.J., Hedley-Whyte E.T., Galanopoulos T., Neville-Golden J., Antoniades H.N. Expression of angiogenic growth factor genes in primary human astrocytomas may contribute to their growth and progression. Cancer Res. 1991;51:1345–1351. [PubMed] [Google Scholar]
- 60.Shen C., Kaelin W.G., Jr. The VHL/HIF axis in clear cell renal carcinoma. Semin. Cancer Biol. 2013;23:18–25. doi: 10.1016/j.semcancer.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morse M.A., Sun W., Kim R., He A.R., Abada P.B., Mynderse M., Finn R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. 2019;25:912–920. doi: 10.1158/1078-0432.CCR-18-1254. [DOI] [PubMed] [Google Scholar]
- 62.Lin L., Chen Y.S., Yao Y.D., Chen J.Q., Chen J.N., Huang S.Y., Zeng Y.J., Yao H.R., Zeng S.H., Fu Y.S., et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget. 2015;6:34758–34773. doi: 10.18632/oncotarget.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandlund J., Hedberg Y., Bergh A., Grankvist K., Ljungberg B., Rasmuson T. Endoglin (CD105) expression in human renal cell carcinoma. BJU Int. 2006;97:706–710. doi: 10.1111/j.1464-410X.2006.06006.x. [DOI] [PubMed] [Google Scholar]
- 64.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 65.Tolar J., Nauta A.J., Osborn M.J., Panoskaltsis Mortari A., McElmurry R.T., Bell S., Xia L., Zhou N., Riddle M., Schroeder T.M., et al. Sarcoma derived from cultured mesenchymal stem cells. Stem. Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 66.Ahluwalia M. A phase 2 trial of TRC105 with bevacizumab for bevacizumab refractory glioblastoma. J. Clin. Oncol. 2016;34 doi: 10.1200/JCO.2016.34.15_suppl.2035. [DOI] [Google Scholar]
- 67.Ravi V. TAPPAS: An adaptive enrichment phase 3 trial of TRC105 and pazopanib versus pazopanib alone in patients with advanced angiosarcoma. J. Clin. Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_suppl.TPS11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attia S. A phase 1B/phase 2A study of TRC105 (Endoglin Antibody) in combination with pazopanib (P) in patients (pts) with advanced soft tissue sarcoma (STS) J. Clin. Oncol. 2016;34 doi: 10.1200/JCO.2016.34.15_suppl.11016. [DOI] [Google Scholar]
- 69.Choueiri T.K., Michaelson M.D., Posadas E.M., Sonpavde G.P., McDermott D.F., Nixon A.B., Liu Y., Yuan Z., Seon B.K., Walsh M., et al. An Open Label Phase Ib Dose Escalation Study of TRC105 (Anti-Endoglin Antibody) with Axitinib in Patients with Metastatic Renal Cell Carcinoma. Oncologist. 2019;24:202–210. doi: 10.1634/theoncologist.2018-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dorff T.B., Longmate J.A., Pal S.K., Stadler W.M., Fishman M.N., Vaishampayan U.N., Rao A., Pinksi J.K., Hu J.S., Quinn D.I., et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer. 2017;123:4566–4573. doi: 10.1002/cncr.30942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levine E. A phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) in combination with capecitabine for advanced solid tumors (including patients with progressive or recurrent HER2-negative metastatic breast cancer) J. Clin. Oncol. 2013;31 doi: 10.1200/jco.2013.31.15_suppl.3057. [DOI] [Google Scholar]
- 72.Karzai F.H., Apolo A.B., Cao L., Madan R.A., Adelberg D.E., Parnes H., McLeod D.G., Harold N., Peer C., Yu Y., et al. A phase I study of TRC105 anti-endoglin (CD105) antibody in metastatic castration-resistant prostate cancer. BJU Int. 2015;116:546–555. doi: 10.1111/bju.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duffy A.G., Ma C., Ulahannan S.V., Rahma O.E., Makarova-Rusher O., Cao L., Yu Y., Kleiner D.E., Trepel J., Lee M.J., et al. Phase I and Preliminary Phase II Study of TRC105 in Combination with Sorafenib in Hepatocellular Carcinoma. Clin. Cancer Res. 2017;23:4633–4641. doi: 10.1158/1078-0432.CCR-16-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duffy A.G., Ulahannan S.V., Cao L., Rahma O.E., Makarova-Rusher O.V., Kleiner D.E., Fioravanti S., Walker M., Carey S., Yu Y., et al. A phase II study of TRC105 in patients with hepatocellular carcinoma who have progressed on sorafenib. United Eur. Gastroenterol. J. 2015;3:453–461. doi: 10.1177/2050640615583587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Apolo A.B., Karzai F.H., Trepel J.B., Alarcon S., Lee S., Lee M.J., Tomita Y., Cao L., Yu Y., Merino M.J., et al. A Phase II Clinical Trial of TRC105 (Anti-Endoglin Antibody) in Adults With Advanced/Metastatic Urothelial Carcinoma. Clin. Genitourin. Cancer. 2017;15:77–85. doi: 10.1016/j.clgc.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang X., Yao J., He Q., Liu M., Duan T., Wang K. Exosomes From Women With Preeclampsia Induced Vascular Dysfunction by Delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to Endothelial Cells. Hypertension. 2018;72:1381–1390. doi: 10.1161/HYPERTENSIONAHA.118.11706. [DOI] [PubMed] [Google Scholar]
- 77.Ruiz-Remolina L., Ollauri-Ibanez C., Perez-Roque L., Nunez-Gomez E., Perez-Barriocanal F., Lopez-Novoa J.M., Pericacho M., Rodriguez-Barbero A. Circulating soluble endoglin modifies the inflammatory response in mice. PLoS ONE. 2017;12:e0188204. doi: 10.1371/journal.pone.0188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y., Starr M.D., Brady J.C., Rushing C., Bulusu A., Pang H., Honeycutt W., Amara A., Altomare I., Uronis H.E., et al. Biomarker signatures correlate with clinical outcome in refractory metastatic colorectal cancer patients receiving bevacizumab and everolimus. Mol. Cancer. 2015;14:1048–1056. doi: 10.1158/1535-7163.MCT-14-0923-T. [DOI] [PubMed] [Google Scholar]
- 79.Murukesh N., Dive C., Jayson G.C. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br. J. Cancer. 2010;102:8–18. doi: 10.1038/sj.bjc.6605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choueiri T.K., Kocsis J., Pachynski R.K., Poprach A., deShazo M., Zakharia Y., Lara P.N., Pal S.K., Geczi L., Ho T.H., et al. Results of the Phase 2 TRAXAR Study: A Randomized Phase 2 Trial of Axitinib and TRC105 (TRAX) versus AXitinib (AX) Alone in Patients with Advanced or Metastatic Renal Cell Carcinoma (mRCC) Ann. Oncol. 2019;30(Suppl. 5):v356–v402. doi: 10.1093/annonc/mdz249.011. [DOI] [Google Scholar]
- 81.Mehta C.R., Liu L., Theuer C. An adaptive population enrichment phase III trial of TRC105 and pazopanib versus pazopanib alone in patients with advanced angiosarcoma (TAPPAS trial) Ann. Oncol. 2019;30:103–108. doi: 10.1093/annonc/mdy464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lenato G.M., Guanti G. Hereditary Haemorrhagic Telangiectasia (HHT): Genetic and molecular aspects. Curr. Pharm. Des. 2006;12:1173–1193. doi: 10.2174/138161206776361291. [DOI] [PubMed] [Google Scholar]
- 83.Chen K., Mehta J.L., Li D., Joseph L., Joseph J. Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ. Res. 2004;95:1167–1173. doi: 10.1161/01.RES.0000150369.68826.2f. [DOI] [PubMed] [Google Scholar]
- 84.Kapur N.K., Wilson S., Yunis A.A., Qiao X., Mackey E., Paruchuri V., Baker C., Aronovitz M.J., Karumanchi S.A., Letarte M., et al. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation. 2012;125:2728–2738. doi: 10.1161/CIRCULATIONAHA.111.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hawinkels L.J., Garcia de Vinuesa A., ten Dijke P. Activin receptor-like kinase 1 as a target for anti-angiogenesis therapy. Expert. Opin. Investig. Drugs. 2013;22:1371–1383. doi: 10.1517/13543784.2013.837884. [DOI] [PubMed] [Google Scholar]
- 86.Cunha S.I., Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood. 2011;117:6999–7006. doi: 10.1182/blood-2011-01-330142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data previously exist in the literature. No new data was generated for this review.