Abstract
Titanium dioxide (TiO2) is used as a food additive (E171) and can be found in sauces, icings, and chewing gums, as well as in personal care products such as toothpaste and pharmaceutical tablets. Along with the ubiquitous presence of TiO2 and recent insights into its potentially hazardous properties, there are concerns about its application in commercially available products. Especially the nano-sized particle fraction (<100 nm) of TiO2 warrants a more detailed evaluation of potential adverse health effects after ingestion. A workshop organized by the Dutch Office for Risk Assessment and Research (BuRO) identified uncertainties and knowledge gaps regarding the gastrointestinal absorption of TiO2, its distribution, the potential for accumulation, and induction of adverse health effects such as inflammation, DNA damage, and tumor promotion. This review aims to identify and evaluate recent toxicological studies on food-grade TiO2 and nano-sized TiO2 in ex-vivo, in-vitro, and in-vivo experiments along the gastrointestinal route, and to postulate an Adverse Outcome Pathway (AOP) following ingestion. Additionally, this review summarizes recommendations and outcomes of the expert meeting held by the BuRO in 2018, in order to contribute to the hazard identification and risk assessment process of ingested TiO2.
Keywords: titanium dioxide, TiO2, E171, food additive, food safety, nanomaterial, nano size, oral exposure, mode of action, adverse health effects, toxicity, review
1. Background of TiO2 as a Food Additive
Titanium dioxide (TiO2) is a widely used white pigment and opacifying agent, with applications in paints, pharmaceuticals, cosmetics, and food [1]. When used as a food additive in the European Union (EU), it is listed as E171 to refer to a specified food-grade form of TiO2, which has no nutritional value and is used to attain a white color, shade other pigments, or in pharmaceuticals [2]. The whitening is best achieved with TiO2 particles within a size range of 200–300 nm, due to their light scattering effects [3]. TiO2 occurs in nature in three distinct crystal structures—anatase, rutile, and brookite, but only anatase and rutile are allowed as a food additive [4,5,6]. The European Union allows E171 (anatase and rutile in uncoated, no surface treatment forms) in quantum satis (without limitations), based on its low absorption and subsequent low toxicity, presumed inertness, and low solubility [5,7,8]. Its low toxicity and inertness, however, are being debated, as long-term inhalation studies over two years have shown the development of lung tumors in rats, following exposure to high concentrations of TiO2 [9,10]. As a consequence of these findings, the International Agency for Research and Cancer (IARC) has classified TiO2 as “possibly carcinogenic to humans after inhalation” [10]. In 2017 the Risk Assessment Committee (RAC) of the European Chemical Agency (ECHA) published an opinion that proposed the classification of TiO2 as a category 2 carcinogen after inhalation, according to the criteria of the Classification, Labelling and Packaging (CLP) Regulation [11]. On the 18 February 2020, the EU took over ECHA’s opinion and published the classification of TiO2 as a suspected carcinogen (category 2) by inhalation in powder form with at least 1% particles with aerodynamic diameter ≤ 10 μm, under the CLP Regulation (EC No 1272/2008). The classification will apply on 1 October 2021 after an 18-month transition period [12]. What the observed toxicity and hazard classification following inhalation mean for oral toxicity is of yet not clear.
Over the last years, an increasing number of studies investigated the behavior and effects of E171 and nano-sized TiO2 after ingestion and discovered potential adverse effects, including the induction of inflammation, the formation of reactive oxygen species (ROS), and co-genotoxic effects [13]. Sub-acute and sub-chronic studies also revealed the induction of epithelial hyperplasia and preneoplastic lesions in the colon of rats and mice after the ingestion of E171, while other oral toxicological studies did not confirm such effects [14,15,16,17,18]. For the oral intake of food additive E171, the European Commission requested a re-assessment of TiO2 by the European Food Safety Authority (EFSA), following the publication of studies by ANSES in 2017. EFSA concluded that the results of these studies did not merit a re-opening of the existing opinion but suggested to fill in the existing data gaps, reduce uncertainties and evaluate new findings carefully in regard to their adverse effects and physicochemical properties of the TiO2 particles used [7,8,19,20,21]. The re-assessment of TiO2 has recently been opened and was initiated in 2020 by the EFSA [22].
Parallel to the EFSA activities, the Office of Risk Assessment and Research (BuRO) at the Netherlands Food and Consumer Product Safety Authority (NVWA) organized a workshop that was held in July 2018, regarding the “potential health effects of the food additive titanium dioxide (E171)”, on which BuRO based its opinion that was published in 2019 [23].
In response to signals in the scientific literature about potentially harmful effects after ingestion of E171 in rodents and the widespread use of this substance in foods, BuRO identified the following questions in the process of risk assessment of E171 that have to be addressed:
Does oral exposure to E171 or nano-sized TiO2 reveal a relevant toxicological hazard?
How reliable are these in-vitro and in-vivo studies?
Are the animal models, the exposure conditions, and the effects observed in these studies relevant to humans?
Can the data from in-vitro and in-vivo studies with TiO2 be extrapolated to humans?
Are there epidemiological studies on the effects of E171 in humans after oral exposure?
Since this workshop in 2018, more studies have been published that investigated the concerns of adverse effects arising from E171 ingestion. This literature review integrates the main conclusions of the expert meeting initiated by BuRO, with recently published studies in order to present an overview of relevant findings regarding E171 toxicity after oral intake. The literature search on PubMed and EmBase was conducted from June 2020–September 2020 and included the search criteria “TiO2”, “titanium dioxide”, “E171” with publication dates from 2018–2020. Previous scientific papers in the field, as well as references in these publications were evaluated. The present literature review aims to shed light on the importance of complete particle characterization, on the effects of matrices, and highlight toxicological relevant pathways potentially involved in the induction of adverse health effects following E171 ingestion. Additionally, it provides approaches to decrease uncertainties concerning the health effects of E171 consumption, and finally formulates recommendations for future studies and follow-up actions regarding the risk assessment of E171.
2. Physicochemical Properties and Characterization of E171
Titanium is one of the most abundant elements in the earth’s crust, which occurs in nature only in its oxidized form as titanium dioxide or Ti(IV) oxide. Once processed, TiO2 is a white, odorless powder that is poorly soluble in aqueous solutions [2,5]. The anatase form TiO2 is most frequently used as a whitening agent in foodstuff, despite its high surface reactivity and ability to generate ROS in an aqueous solution after UV irradiation [2,24]. Food-grade TiO2/E171 consists of micro-and nanoparticles with a primary particle size ranging from 60–300 nm [25]. Around 10–40% of the pristine TiO2 particles in E171 are estimated to be smaller than 100 nm and can therefore be considered as nanoparticles [25,26,27,28]. However, according to the Commission’s recommendation in 2011 (2011/696/EU), a nanomaterial must contain over 50% of nanoparticles, which excludes E171 of this category [28,29]. Based on information reported in the literature the EFSA Panel on Food Additives and Nutrient Sources suggest that the food additive E171 mainly consists of micronized TiO2 particles ranging from 104–166 nm and a percentage of particles < 100 nm ranging from 5.4–45.6% [21,30].
Recently published work by Verleysen et al. (2020) showed that 12 out of 15 pristine E171 materials purchased from manufacturers consist of more than 50% TiO2 particles that are smaller than 100 nm and that commercially available anatase E171 materials constitute of 18–74% (TEM) or 32–64% (sp-ICP-MS) nanoparticles [30]. This examination assigns a larger fraction of TiO2 particles present in pristine E171 to the nano-sized fractions than previously assumed. Analysis of food samples containing E171, via ICP-MS and Raman spectroscopy, showed anatase type TiO2 particles in the range of 26.9–463.2 nm, with 21.3–53.7% of the particles in the nano-size fraction [31]. The determination of the nanoparticle fraction (Figure 1) within E171 is of importance since the size of particles is considered to be an important factor influencing toxicokinetics, toxicodynamics, and thus toxicity [8,21,32]. Nanoparticles display a higher surface to volume reactivity, translocation properties, bioavailability, and increased cellular interactions than larger particles [33].
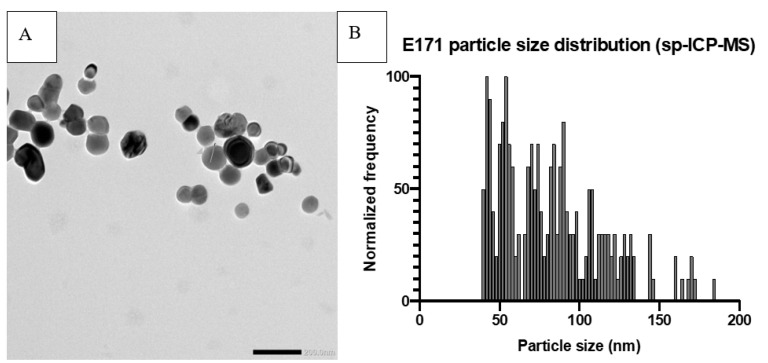
Figure 1.
Example of E171 particle characterization. Prior analysis the samples were dispersed according to the NanoGenotox dispersion protocol at a final concentration of 2.56 mg/mL in 0.05% BSA solution and probe sonicated on ice for 16 min (4 W). (A) Transmission Electron Microscope picture of E171. (B) Size distribution of E171 particles, measured by single-particle ICP-MS, with a median particle size of 79 nm and 72% of particles < 100 nm.
The shape, size, and state of agglomeration and aggregation are important properties regarding the effects of food-grade TiO2. Generally, it is assumed that the round and spherical crystal forms of TiO2 contribute to a lower extent to the induction of adverse effects, when ingested [34]. The size of food-grade TiO2 particles, on the other hand, plays an important role regarding their toxicity. Nano-sized TiO2 particles are suspected to induce more adverse effects, including ROS formation, cytotoxicity, and increased release of inflammatory cytokines, compared to micro-sized TiO2 particles [32,35,36]. Proquin et al. (2018) demonstrated that a mixture of nano- and micro-sized TiO2 particles, as they are present in E171, induce more adverse effects than the single fractions alone. This emphasizes the importance of testing food-grade TiO2 particles as a whole, rather than its nano- and micro-sized fraction [16].
The interaction of E171 with its direct environment and colloidal stability are other factors that need to be considered during its characterization [37]. Suspended TiO2 particles tend to agglomerate or aggregate, according to their isoelectric point and the pH of the milieu, leading to the formation of larger clusters. Aggregation describes the assembly of primary particles through covalent or metallic bindings, while agglomeration results from van-der-Waals interactions, hydrogen bonds, adhesion by surface tension, or electrostatic attraction [2,38]. The determination of agglomeration and aggregation status is crucial because it can significantly alter hydrodynamic diameter, size, and the stability of particle-complexes, thus affecting uptake, reactivity, and toxicity [39].
The high surface area, charge, and chemical properties of TiO2 particles provide the possibility of many biomolecules to be adsorbed. The formation of a protein corona can change the physicochemical properties of TiO2 particles, e.g., their reactivity and the interactions of these particles with their environment, including cellular uptake, accumulation, intracellular localization, distribution, and release [40]. The variability of protein coronas is dependent on the different molecules present at each location and can influence their interaction with cells [41]. The presence of transferrin in the protein corona, for example, can affect the clathrin-mediated endocytosis via the transferrin-receptor and result in significantly altered particle internalization [42].
The formation of protein coronas can also lead to conformational changes of the proteins themselves, resulting in irreversible changes to secondary protein structures and leading to protein dysfunction [43]. Additional interactions of TiO2 nanoparticles with non-protein components might be harmful too. Bianchi et al. (2017) showed that the endotoxic effect of lipopolysaccharides (LPS) is increased when bound to TiO2 nanoparticles, resulting in the potentiation of pro-inflammatory effects including induced expression of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-kB) and interferon regulatory factor 3 (IRF-3) dependent cytokines [44]. The consideration of TiO2-protein-corona-complexes in the characterization and determination of physiochemical properties and adverse effects of food-grade TiO2 is important for an adequate safety evaluation.
For this reason, it is important to carefully examine and analyze the physicochemical characteristics of TiO2 particles in its vehicle, as well as in its surrounding matrix as their final milieu, to guarantee a profound assessment of potential adverse health effects of E171 and to adequately compare different studies in the process of risk assessment.
3. Exposure to E171
E171 is used in products such as candy, coffee creamer, chewing gum, sauces, nutritional supplements, toothpaste, and pharmaceuticals. Although both the anatase and rutile forms of TiO2 are authorized for foods, the characterization of European and American food samples showed that anatase is the predominant TiO2 crystalline structure used as food additive E171 and thus the main source of exposure for the general population [27,45,46,47,48]. The intake of E171 varies between different age groups and countries, while children, in general are the most highly exposed group, due to their lower body mass and disproportionally higher consumption of E171-containing products [5,49]. Table 1 shows the estimated daily intake of E171 per kg body weight (bw) in different countries and age-groups [49,50].
Table 1.
Mean and 95th percentile estimation of daily oral intake of TiO2 from food products (E171), food supplements and toothpaste in different age groups and countries in mg/kg bw/day (n/a = data not available, * mg/person/day).
| Author | Year | Country | Mean (mg/kg bw/Day) | 95th Percentile (mg/kg bw/Day) |
|---|---|---|---|---|
| Wu [50] | 2020 | USA | 0.15–3.9 * (PCP survey and usage patterns, no food included) | n/a |
| EFSA [21] | 2016 | Europe | <11 months: 0.2–0.8 1–3 years: 0.6–4.6 3–9 years: 0.9–5.5 10–17 years: 0.4–4.1 18–64 years: 0.3–4.0 >65 years: 0.2–2.8 |
<11 months: 0.7–3.9 1–3 years: 2.0–6.8 3–9 years: 2.4–14.8 10–17 years: 1.3–10.8 18–64 years: 1.1–9.7 >65 years: 0.5–7.0 |
| Rompelberg [49] | 2016 | NL | 2–6 years: 0.66–0.70 7–69 years: 0.16–0.18 >69 years: 0.05–0.07 |
2–6 years: 1.19–1.40 7–69 years: 0.47–0.54 >69 years: 0.20–0.28 |
| Bachler [51] | 2015 | DE | “Other Children”: ~2 Toddlers, adolescents, adults, elderly: 0.5–1 |
“Other Children”: ~0.7–7.2 Toddlers, adolescents, adults, elderly: ~0.1–4.2 |
| Sprong [52] | 2015 | NL | 2–6 years: 1.3–1.5 7–69 years: 0.6–0.7 >70 years: 0.5–0.6 |
2–6 years: 4.5–5.6 7–69 years: 2.6–3.0 >70 years: 1.7–2.2 |
| Christensen [53] | 2015 | DK | Children: 2 Adults: 1 |
n/a |
| Weir [27] | 2012 | UK | <10 years 2–3 >10 years: 1 |
n/a |
| Weir [27] | 2012 | US | <10 years: 1–2 >10 years: 0.2–0.7 |
n/a |
| Powell [54] | 2010 | UK | 5 * | n/a |
The highest concentrations of E171 are found in chewing gum, candies, and powder sugar toppings such as icings. Chewing gums contain between 1.1 mg (±0.3 mg) to 17.3 mg (±0.9 mg) TiO2 particles per piece of gum with a mean average weight per piece of 1416 mg (±27 mg) to 2240 mg (±86 mg) [26]. TiO2 nanoparticles account for up to 19% (±4) of all particles present in these gums [26]. The accidental ingestion of toothpaste, while brushing teeth is another major source of E171 intake, that can result in an exposure of 0.15 to 3.9 mg/day, when 10% of toothpaste is ingested [50]. Additional release of TiO2 particles (70–200 nm) from food packaging materials or food-related products, such as frying pans, may also contribute to TiO2 ingestion [55]. The focus of oral TiO2 exposure estimation should potentially be extended from the food additive E171 to personal care products, packaging, and coating of household items [28,33,55]. Daily dietary intake of E171 can reach several hundred milligrams, of which at least 10–40% are in the form of TiO2 nanoparticles. The long-term exposure to such quantities of nano- and micro-sized TiO2 raises concerns about the risk of potential accumulation in organs and potentially harmful effects on human health [27].
4. Toxicokinetics of Ingested E171
Following the oral ingestion of E171, the key question is how much of the E171 and which portion of each size fraction will be absorbed along the oro-gastrointestinal route before it is exerting local effects. E171 is expected to be systemically distributed via the blood circulation or the lymphatic system to various organs and tissues. Digestive enzymes and pH levels in the mouth, stomach, and small intestine may alter and change physicochemical properties of E171, including the protein corona formation, and therefore have the potential to affect the absorption in vivo.
The absorption of different sized TiO2 particles (148, 36, 28 nm) in a porcine buccal model showed that all investigated particles permeate the mucosa layer and enter the oral epithelium. Penetration depth varied with particle size, with smaller particles penetrating deeper. This ex-vivo model demonstrated that TiO2 particles can also enter the buccal mucosa under physiological conditions, which included digestive enzymes e.g., mucins, and relevant pH levels [56,57]. Absorption and internalization of E171 have been studied in human epithelial colorectal adenocarcinoma cells (Caco-2 cells) and various other in vitro models. Nanosized TiO2 particles were effectively entrapped by Caco-2 cell monolayers and stored in the affected enterocytes. The internalized TiO2 nanoparticles showed a tendency to agglomerate or aggregate in the cytosol, but nanoparticles and enveloped nanoparticles in cytoplasmic vesicles could also be observed. This internalization of TiO2 nanoparticles in differentiated Caco-2 cell monolayers after 4 h of exposure indicates a transcellular absorption [58]. The exposure of differentiated Caco-2 cell monolayers to TiO2 nanoparticles resulted in increased epithelial permeability, indicating a disruption of the cytoskeletal integrity, increased tight-junction (TJ) permeability, and downregulation of genes encoding for tight junction proteins [58,59].
In vivo, TiO2 can cross the regular ileum and follicle-associated epithelium before it translocates and enters the Peyer’s patch in the colon [60]. Comera et al. (2020) recently showed that TiO2 is mainly taken up by crossing the regular epithelium of the small bowel villi. This process is facilitated by goblet cell-associated passage and passive diffusion through the paracellular tight junction spaces between the enterocytes, without displaying epithelial transcytosis patterns [61]. This indicates that the translocation of TiO2 nanoparticles in the ileum is mainly facilitated through paracellular resorption, transepithelial absorption, and potentially through the impairment of paracellular junctions [58,59,60]. Studies in rats showed that only a very small fraction of 0.007 to 0.6% ingested E171 is absorbed and enters the circulation [18,62]. These observations are consistent through various species, including rats, mice, and Drosophila melanogaster [14,63,64]. When TiO2 nanoparticles (25 or 75 nm) enter the circulation, in laboratory rats, they deposit in the liver and spleen, where they exhibit a half-life time of more than 30 days, resulting in a high risk of bioaccumulation given the chronic daily exposure [18,62,65]. Increased TiO2 tissue levels have been found in the spleen and ovaries of rats, along with sex-related histological changes in the thyroid, adrenal medulla and adrenal cortex (female) and thyroid function (male). These findings indicate the possibility of endocrine and reprotoxic effects after the ingestion of E171 [66]. While a majority of the published oral in vivo studies identify a minor absorption of E171 in rats and mice, others showed that certain forms of TiO2 (rutile) did not migrate from the gastrointestinal tract [18,67].
Studies in humans on orally administrated TiO2 showed a low bioavailability [68,69,70]. Basal titanium blood levels ranged between 5.9–18.1 µg/L (mean 11.1 µg/L) and peaked after 8–12 h at 37.4–49.7 µg/L after ingestion of 22.9 mg TiO2 in a gelatin capsule. Administration of 380 nm sized TiO2 (anatase) showed lower absorption than 160 nm sized TiO2 (anatase). The highest titanium blood concentration was detected at 109.9 µg/L, after the ingestion of 45.8 mg TiO2 in a gelatin capsule, after 8 h, showing large differences in absorption among the group of six male volunteers [68]. The ingestion of 100 mg food-grade TiO2 (E171) increased total titanium blood levels after 6–8 h, with peak titanium blood concentrations reaching 10 ppb in comparison to 1.5 ppb basal levels [70]. Contrary to these findings, the study of Jones et al. (2015), which used different sized TiO2, showed no statistically significant absorption of TiO2, after the ingestion of 5 mg/kg bw TiO2 [69]. Even though the absorption of ingested TiO2 over a healthy intestinal barrier seems to be very low, it is important to take into consideration factors like net volume of translocated particles through the gut barrier, possibly impaired intestinal barrier function that facilitates TiO2 particles translocation and bioaccumulation in systemic organs, when accurately assessing potential health hazards.
Heringa et al. (2016) and Rompelberg et al. (2016) published an overview of studies examining the absorption of ingested TiO2 nanoparticles [1,49]. Following their physiologically based pharmacokinetic (PBPK) modeling, these researchers concluded that TiO2 nanoparticles can be absorbed, although at a very small rate of approximately 0.02 to 0.05% [51,71,72,73]. The translocation into lymphatic and blood circulation can lead to the deposition of TiO2 nanoparticles within tissues and organs after ingestion [71]. The deposition of TiO2 in humans was observed in the Peyer’s patch, especially in patients suffering from inflammatory bowel diseases (IBD) [74,75].
Based on the oral exposure estimation of the Dutch population, using external dosage, no risk of adverse effects is expected in humans, except potential effects on ovaries [1]. However, if toxicokinetic information based on internal organ concentration and accumulation over time of TiO2 nanoparticles was included, the potential additional risk for liver and testis was identified [1]. Additional work of Heringa et al. (2018) and Brand et al. (2020) showed that post-mortem collected human liver (median 0.03 mg/kg), jejunum (median 0.14 mg/kg), ileum (median (0.26 mg/kg), kidney (median 0.06 mg/kg) and spleen (median 0.04 mg/kg) contain titanium particles and that they accumulated both micro-and nanosized TiO2 [13,76]. The quantities detected in these organs were partially higher than levels that are considered safe for humans, after applying conventional safety factors [76]. ICP-MS and TEM-EDX analysis of human placentae and meconium (first stool of newborns) collected from normal pregnancies suggest a maternofetal passage of TiO2, which does not provide information on the source of TiO2 particles in these organs and routs for maternal exposure. However, placenta perfusion experiments with E171 suspension confirmed a low transfer of food-grade TiO2 particles to the fetal side. The diameter of the TiO2 particles recovered in the fetal exudate showed that 70 to 100% of particles were in the nanosized range [77]. These findings suggest that the human placenta barrier is not able to completely prevent the passage of TiO2 nanoparticles to the fetus and emphasized the need to assess the risk of TiO2 nanoparticles during pregnancy [77].
Independent from the extent of TiO2 absorption, a considerable amount of TiO2 (approximately 99%) is retained and accumulated in the intestinal lumen, before it is excreted via the feces, without undergoing any alteration or metabolization [78,79]. Due to accumulation in the intestinal lumen before excretion, interactions of TiO2 with the gut microbiota are possible, which may lead to a modification of intestinal homeostasis and which could possibly impact the health of the host [80].
Uncertainties remain concerning possible effects of the food matrix, on E171 absorption, distribution, metabolism, and excretion. The matrix can potentially alter the physicochemical properties of E171 substantially and influence the degree of absorption. The various influences of digestive processes through saliva, stomach acid, and intestinal pH on E171, its protein corona and its physicochemical properties, bioavailability, and potential adverse effects, are currently poorly understood.
5. Health Effect of Ingested E171
Potential health risks resulting from the ingestion of E171 are still under discussion. Here, an overview of in-vivo, in-vitro, and ex-vivo toxicity studies with TiO2 nanoparticles and food-grade E171 is provided.
5.1. In Vivo Toxicity of E171
In 1979, NTP concluded that the in vivo carcinogenicity studies in rats and mice, they performed demonstrated that TiO2 can be considered as safe as a food additive [81]. These studies were carried out in Fisher 344 rats (male 1125/2250 mg/kg/bw and female 1450/2900 mg/kg/bw/day) and B6C3F1 mice (male 3250/6500 kg/bw/day and female 4175/8350 mg/kg/bw/day) via daily dietary administration of pigment grade anatase [2,81] Microscopical images suggested a mean particle diameter between 200–300 nm, but no specific size characterization was conducted. The test material was included in the diet of the mice, without considerable effects on the survival of male mice. The female mice in the highest dose group, showed a survival rate of 66% at the end of the 104-weeks study, in comparison to 90% survival in the control group. Histopathological examination showed a dose-dependent increase in hepatocellular carcinoma in male mice from 17% in the control group to 29% in the high-dose group. These effects remained in the range of historical control data. Histopathological examination showed an increase in hyperplastic bile ducts in male rats in low- and high-dose groups after 103-weeks of exposure. Female rats showed an overall increased incidence in c-cell adenomas and carcinomas of the thyroid from 2% in the control to 14% in the high-dose group. No adenomas or carcinomas have been detected in the low-dose group. The statistical analysis led to the conclusion that the incidence is not statistically significant nevertheless does the occurrence of thyroid tumors need to be carefully considered [2,81,82].
Table 2 shows an overview of recent in vivo studies, assessing various adverse health effects of TiO2 nanoparticles and E171 following ingestions e.g., genotoxic effects, inflammation, oxidant-antioxidant-balance, and mortality. Some studies with E171 and TiO2 nanoparticles showed no adverse effects, even at extremely high doses of up to 24,000 mg/kg bw/day [18,67,83,84]. Dietary administration of E171 in rats for 7 and 100 days showed no effect on histopathology of the small and large intestine, liver, spleen, lungs, or testes and no effects on aberrant crypt formation, goblet cell number, or colonic gland length. Administration of E171 via the food did not result in any effects on immune parameters, including interleukins, INF-γ, or TNF-α, nor tissue morphological changes [17]. Other studies in rats and mice showed intestinal inflammation, hepatotoxic effects, changes in levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (ASP), and effects on oxidants and antioxidants, including reduced and oxidized forms of glutathione (GSH/GSSG), glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) [14,35,85,86,87,88,89]. Intestinal inflammation was often accompanied by alterations in gene expression and activity TNF-α, IFN-γ, IL-2, IL-8, IL-10, NF-kB, cytochrome p450 (CYP450), cyclooxygenase-2 (COX-2), Ki67, and T-helper cells 1 (Th-1) [15,86,88,90]. Some studies reported increased genotoxicity in the form of DNA damage, micronuclei, and dysplastic alterations of tissues including the distal colon and liver, while others did not [15,35,90,91,92,93,94]. Markers indicating the progression of tumors (COX-2, Ki68, p65, TNF-α, β-catenin), alteration of tumor-related pathways mitogen-activated protein kinase (MAPK) and olfactory/G-protein-coupled receptor family (GPCR) have been measured [15,88,92,95]. Changes in metabolic function, telomere shortening, TJP-1 gene expression, insulin resistance, endoplasmic reticulum (ER) stress, impaired cell cycle, and increased mitotic indices are other reported adverse effects [85,88,91,93,94,95]. Additional studies in vivo and ex vivo following i.v. and i.p. injections and ex vivo experiments in rats and mice, allowing for higher systemic absorption, confirm genotoxic effects e.g., formation of micronuclei (bone marrow), inflammatory responses in the liver, and secretion of IL-β in bone marrow-derived macrophages from mice [96,97,98].
Table 2.
Overview of in vivo studies assessing adverse health effects following TiO2 ingestion related to acute, sub-chronic and chronic toxicity, genotoxicity, inflammation, histopathological changes and other adverse health outcomes in rats and mice. Abbreviations: BW = bodyweight, ACF = aberrant crypt foci, IS = immune system, OS = oxidative stress, ER = endoplasmic reticulum, A = adult, Y = young, WT = wild-type, * mg/kg/week, ** µg/mL, *** administration from Monday to Friday (5 days a week), **** see original document, due to variety of particles).
| Reference | Testing | Material | Ø Primary Size (nm) | Hydrodynamic Diameter (nm) | Zeta Potential (mV) | Species/Sex ♀♂ | Duration (days) | Dose (mg/kg bw/day) |
Administration | Observation |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2019 [99] | 90-days repeated dose | TiO2 NP Spherical anatase (purity 99.90%) |
29 ± 9 | In water ~30 In gastric juices ~105 In intestinal juices ~110 |
In water ~+10 In gastric juices ~+5 In intestinal juices ~−15 |
Rats Sprague-Dawley |
90 | 0, 2, 10, 50 | Oral gavage in ultra-pure water |
|
| Moradi 2019 [86] | 14-days repeated dose | TiO2 NP 80% anatase, 20% rutile |
20 | Not assessed | Not assessed | Rats ♂ Wistar |
14 | 300 | Oral gavage in bi-distilled water |
|
| Jensen 2019 [94] | 70-day repeated exposure | E171 99.8% anatase, 0.2% rutile |
135 ± 6 305 ± 61 900 ± 247 |
In filtered water, 2% FBS 270 ± 25 |
Not assessed | Rats ♀ Zucker |
70 | 50 *, 500 * | Oral gavage in sterile water 2% FBS |
|
| Blevins 2019 [17] | 100-days repeated dose | E171 85% anatase 25% rutile |
110, 115 | In food, no assessment of E171 characteristics in food | In food, no assessment of E171 characteristics in food | Rats ♂ Wistar (immune response and DMH colon carcinogenesis model) |
7100 | 40, 400, 5000 | Ingestion via food in Purina 5002R33 diet |
|
| Bettini 2017 [14] | 100-days repeated dose (E171), 7-days repeater dose (E171 and NM-105) | E171 (45% nanosized by particles number), NM-105 85% anatase 25% rutile |
E171: 118 ± 53 NM-105: 22 ± 1 |
In purified water E171: 373 ± 20 NM-105: 192 ± 2 |
In purified water E171: −23.9 ± 2.4 NM-105: +5.03 ± 0.02 |
Rats ♂ Wistar (immune response) |
7100 | 10 | Oral gavage in purified water |
|
| Bettini 2017 [14] | 100-days repeated dose | E171, | E171: 118 ± 53 |
In purified water E171: 373 ± 20 |
In purified water E171: −23.9 ± 2.4 |
Rats ♂ Wistar (normal wild type and DMH colon carcinogenesis model) Wistar |
7100 | 0.2, 10 | Oral gavage in purified water |
|
| Martins 2017 [18] | 45-days repeated dose | TiO2 NP | 41.99 ± 1.63 | Sodium citrate buffer 0.1M, pH 4.5 447.67 ± 6.43 |
Not assessed | Rats ♂ Wistar |
45 | 0.5 | Oral gavage in sodium citrate buffer |
|
| Donner 2016 [67] | OECD 474 | TiO2 **** | **** | **** | **** | Rats ♂♀ Sprague-Dawley |
2, 3 | 500, 1000, 2000 | Single dose oral gavage in water |
|
| Warheit 2015 [83] | OECD 407 | TiO2 2 types of rutile |
173 | In 0.01% tetrasodium hexametaphosphate + MilliQ 253 |
**** | Rats ♂ Sprague-Dawley |
28 | 24000 | Oral gavage in sterile water |
|
| Warheit 2015 [6] | OECD 414 | TiO2 **** | 20–206 | **** | **** | Rats ♀ Sprague-Dawley, Wistar |
14 | 100, 300, 1000 | Oral gavage in sterile water |
|
| Orazizadeh 2014 [87] | 14-days repeated dose | TiO2 NP | 50–100 | Not assessed | Not assessed | Rats ♂ Wistar |
14 | 300 | Oral gavage in milli-Q water |
|
| Hu 2020 [95] | 56-, 182-days repeated dose | TiO2 NP | 25.37 ± 4.17 | In PBS 34.34 ± 6.33 |
In PBS “negative value” |
Mice ♂ ICR (Y/A) |
56, 182 | 50 | Oral gavage in PBS |
|
| Ali 2019 [35] | 5-days repeated dose | TiO2 NP | 21, 80 | Not assessed | Not assessed | Mice ♂ Swiss-Albino |
5 | 50, 250, 500 | Oral gavage in 0.9% physiological saline solution |
|
| Chakrabarti2019 [91] | OECD 408 | TiO2 NP | 58.25± 8.11 | Not assessed | Not assessed | Mice ♂♀ Swiss-Albino |
90 | 200, 500 | “orally” in water |
|
| Proquin 2018 [92] | 21-days repeated dose | E171 | 535 | In water at 1 mg/mL 316.8 ± 282.4 |
In water at 1 mg/mL −12.78 ± 0.52 |
Mice ♂♀ BALB/c |
2, 7, 14, 21 | 5 | Oral gavage in water |
|
| Hu 2018 [88] | 182-days repeated exposure | TiO2 NP | 26.42± 7.73 | In PBS 42.15 ± 6.71 |
In PBS “negative values” |
Mice ICR |
182 | 10, 20, 50, 100, 200 | Oral gavage in PBS |
|
| Ruiz 2017 [89] | 8-days repeated dose | TiO2 rutile |
30–50 | Not assessed | Not assessed | Mice ♀ C57BL/6 NLRP3− (DSS colitis model) |
8 | 50, 500 | Oral gavage in water |
|
| Urrutia-Ortega 2016 [15] | 77-days repeated dose | E171 (purity > 99%) | 382 502 626 |
In water pH7 300 |
In water pH7 -30 |
Mice ♂♀ BALB/c+ (CAC model) |
45 *** | 5 | Oral gavage in water |
|
| Sycheva 2011 [93] | 7-days repeated dose | TiO2 MP TiO2 NP |
MP: 160 ± 59.4 NP: 33 ± 16.7 |
Not assessed | Not assessed | Mice ♂♀ CBAB6F1 |
7 | 40, 200, 1000 | Oral gavage in distilled water |
|
| Trouiller 2009 [90] | 5-days repeated exposure | TiO2 NP 75% anatase, 25% rutile (purity 99.5%) |
21 | In water 160 ± 5 |
Not assessed | Mice C57B1/6Jpun/pun |
5 | 60, 120, 300, 600 ** | Orally in drinking water |
|
There is increasing evidence that the exposure of E171 can alter gut microbiota in laboratory animals, resulting in changes of colonic pH and abundance of certain commensal bacteria, which in turn can result in increasing levels of LPS, potentially increasing lipid peroxidation processes and a significant increase in oxidative stress [85]. Dietary exposure to E171 has been linked to effects on gut microbiota and intestinal health in experimental animals, where even low exposure interferes with the gut microbiome, causing low-grade intestinal inflammation and exacerbating existing intestinal health conditions [85,100,101,102,103]. Decreased crypt length, infiltration of CD8+ cells, and macrophages, as well as increased expression of inflammatory cytokines, indicate impacts on gut homeostasis and colonic inflammation in vivo [104]. Alterations of the microbiota-immune axis have been associated with IBD, metabolic disorder, and colorectal cancer (CRC) [105,106,107]. Chronic exposure to TiO2 and its effects on intestinal health, especially in relationship with impaired intestinal barrier function, seen as in IBD patients and their potential risk of increased TiO2 absorption due to their impaired intestinal barrier integrity, have to be carefully investigated since they may represent a population with a higher risk of E171 related adverse health effects [80].
TiO2 nanoparticles can cross the blood-brain-barrier (BBB) in rats and mice and accumulate in the brain, leading to an increase of oxidative stress and nitric oxide (NO) levels. TiO2 accumulation leads to histopathological changes of the brain, inflammation, decreased acetylcholinesterase levels, decreased expression of inflammatory markers such as TNF-⍺, IL-6, and GSH depletion. TiO2 toxicity on the brain might increase the risk of Parkinson’s disease, through the destruction of dopaminergic neurons [108,109,110].
Examination of cardiotoxic effects of E171 and TiO2 nanoparticles revealed effects on vasomotor function, including the increase of acetylcholine-induced vasorelaxation, serotonin-induced vasoconstriction, and nitroglycerin levels [111,112].
Reprotoxic and developmental toxic effects have been shown for TiO2 nanoparticles. Exposure decreased testis weight, serum testosterone levels, and induced histopathological changes and anomalies in the sperm of mice [113]. Pregnant mice, which have been exposed to E171, showed altered gene expression related to apoptosis, brain development, and oxidative stress in their newborn pups [114]. The intragastric administration of TiO2 nanoparticles in rats showed an increase in gamma-glutamyltransferase (gamma-GT), decreased testicular steroidogenic regulatory protein (StAR), c-kit gene expression, serum testosterone level, and sperm count. Exposed animals also exhibited prostatic and testicular altered GSH levels, elevated TNF-⍺ concentration, up-regulated Bax, Fas, and caspase-3 gene expression, downregulation of B-cell lymphoma-2 (BCL-2) gene expression and enhanced prostatic lipid peroxidation. Sperm malformation elevated testicular acid phosphatase activity and MAD levels, serum prostatic acid phosphatase activity, prostate-specific antigen (PSA), gonadotrophin, and estradiol levels occurred after 2 and 3 weeks administration [115]. Chronic TiO2 nanoparticle exposure in zebrafish showed a significant impairment of their reproduction, resulting in reduced numbers of eggs laid, changes in ovary histology, and altered gene expression [116]. Caenorhabditis elegans exposed to E171 showed a concentration-dependent effect on worm reproduction, brood size, and overall display a reduced life span as well as TiO2 accumulation in their intestine [117]. E171 exposure to D. melanogaster showed an increase in pupation time, changes in the development of larvae, and altered overall reproductive activity, which was accompanied by gene expression changes of CAT and SOD [118,119].
While some meta-analyses report on a publication bias of TiO2 toxicity, it is noteworthy that the majority of the conducted studies are performed with nanomaterial models and are not executed according to OECD guidelines, including an insufficient number of test animals, as well as missing particle characterization, or relevant route of exposure.
Some of the publications summarized above show organ-specific toxic effects on the liver, ovaries, and brain, especially in studies conducted with TiO2 nanoparticles. These studies reported the onset of inflammation and changes of gene expression related to the immune system, oxidative stress as well as alterations in the oxidant-antioxidant balance system. In vivo studies also show genotoxic effects, including single- and double-strand DNA-damage, micronuclei, and telomere shortening. Other studies, with similar experimental set-up, do not confirm these results, questioning whether the observed effects in vivo do subsequently result in irreversible adverse health effects.
5.2. In Vitro and Ex Vivo Toxicity of E171
Table 3 shows an overview of recent publications summarizing the effects of TiO2 nanoparticles and E171 on various cell models along the oro-gastrointestinal route, as well as cell types found in organs, following the systemic distribution of these particles. Some studies on TiO2 showed the ability to decrease cell viability and induce the formation of ROS, while others do not detect such effects [16,57,58,120,121,122,123,124,125,126,127,128,129,130,131]. In some cases, the increase in ROS was accompanied by elevated oxidative stress levels and lipid peroxidation, which may lead to the induction of DNA damage and micronuclei [16,123,127,129,130]. These events were accompanied by alterations of antioxidant enzymes, such as SOD, GSH, CAT, and glutathione reductase (GR) [121,122,124]. It has been shown that exposure to TiO2 can impair cell membrane integrity, decrease mitochondrial membrane potential, and affect tight junctions [57,58,125]. Other publications reported membrane permeabilization, lysosomal dysfunction, and the initiation of autophagic processes, including a decrease in phagocytic rate and index and changes in the gene expression for autophagy proteins 1A/1B-light-chain-3 (LC-3) and Beclin-I [128,132]. Additional alterations on gene expression related to inflammatory pathways including extracellular signaling regulated kinase (ERK 1/2), Akt, as well as tumor and inflammation-related proteins e.g., p53, BAX, Cytochrome-c, Apaf-1, COX-2, transcription factors such as NF-kB, Nuclear factor erythroid 2-related factor 2 (Nrf2) and caspase-3, 9 have been published and suggest the onset of a tumor-like phenotype [120,128,129,133]. The stimulation of inflammatory processes is indicated by increased production and release of pro-inflammatory cytokines such as TNF-⍺ and IL-8 [58].
Table 3.
Overview of in vitro and ex vivo studies assessing molecular biological effects, cytotoxicity, genotoxicity and gene expression changes following TiO2 exposure to relevant cellular model system Abbreviations: ROS = reactive oxygen species, OS = oxidative stress, ER = endoplasmic reticulum, * various NP mixtures/sizes (see original publication), ** µg/cm2, *** ppm).
| Reference | Material | Ø Primary Size (nm) | Hydrodynamic Diameter (nm) | Zeta-Potential (mV) | Cell Type | Duration (h) | Conc. (µg/mL) | Observation |
|---|---|---|---|---|---|---|---|---|
| Teubl 2015 [57] | NM-103 rutile dimethicone coated NM-104 rutile glycerol coated |
NM-103: 20 NM-104: 20 |
In PBS NM-103: 1819 ± 61.56 NM-104: 1539 ± 489 In artificial saliva NM-103: 3061 ± 134.4 NM-104: 2597 ± 426 |
In PBS NM-103: −25.2 ± 8.2 NM-104: −21 ± 4.3 Inartificial saliva NM-103: −9.5 ± 3.7 NM-104: −9.1 ± 3.3 |
Human buccal epithelial TR146 | 4 | 1–200 |
|
| Teuble 2015 [56] | NM-100 anatase NM-101 anatase NM-105 80% anatase, 20% rutile |
NM-100: 148 ± 45/34 ± 15 NM-101: 28 ± 8 NM-105: 36 ± 10 |
In PBS (d0.5) NM-100: 1346 ± 76 NM-101: 627 ± 5 NM-105: 868 ± 16 In artificial saliva (d0.5) NM-100: 2850 ± 482 NM-101: 1095 ± 28 NM-105: 1183 ± 74 |
In PBS (d0.5) NM-100: −10.3 ± 8.2 NM-101: −28.7 ± 4.3 NM-105: −24.0 ± 3.4 Inartificial saliva (d0.5) NM-100: −12.0 ± 5.7 NM-101: −8.46 ± 4.8 NM-105: −11.7 ± 5.6 |
Human buccal epithelial TR146 | 4, 24 | 1–200 |
|
| Kim 2019 [120] | TiO2 NPs anatase 99%anatase | 15 | n/a | n/a | Periodontal Ligament cells | 0.5, 1, 3, 8, 24, 48 | 2.5–50 |
|
| Gerloff 2012 [121] | TiO2 NPs * (anatase/rutile) | 4–215 * | Was assessed * | Was assessed * | Caco-2 | 4, 24 | 20, 80 ** |
|
| Botelho 2014 [133] | E171 80% anatase, 20% rutile |
21 | In milli-Q water or RPMI supplemented with 10% FBS, or 2% BSA in PBS 420.7 |
In milli-Q water or RPMI supplemented with 10% FBS, or 2% BSA in PBS −27.8 |
AGS human gastric epithelial | 3, 6, 24 | 20–150 |
|
| Dorier 2015 [123] | TiO2 NPs A12 R20 |
A12: 12 R20: 20 |
In water A12: 132 ± 1 R20: >1000 In exposure medium A12: 320 R20: >1000 |
In water A12: −20,0 ± 0.6 R20: −19.5 ± 0.9 In exposure medium A12: −10.8 ± 0.6 R20: −11.7 ± 0.8 |
Caco-2 | 6, 24, 48 | 50 |
|
| Dorier 2017 [122] | A12 E171 * P25 (anatase/rutile) |
A12: 12 ± 3 P25: 24 ± 6 E171: 118 ± 53 * |
In water A12: 85 ± 2.9 E171 *: 415.6 ± 69.5 P25: 157.6 ± 1.0 In cell culture medium A12: 447.9 ± 0.3 E171 *: 739.3 ± 355.3 P25: 439.9 ± 6.7 |
In cell culture medium A12: −10.8 ± 0.6 E171 *: −19 ± 0.7 P25: −11.2 ± 0.8 |
Caco-2/HT29-MTX (co-culture) | 6, 48, 3 weeks |
0–200 |
|
| Proquin 2017 [16] | E171 TiO2 MPs TiO2 NPs 99.5% anatase |
E171: 50–250 TiO2 MPs: 535 TiO2 NPs: 10–25 |
In DMEM 0.05% BSA E171: 669.62 ± 30.13 TiO2 MPs: 1385.83 ± 38.85 TiO2 NPs: > 1000 HBSS E171: > 1000 TiO2 MPs: > 1000 TiO2 NPs: > 1000 McCoy + 10% FBS at 1 mg/mL E171: 316 ± 282.4 |
In DMEM 0.05% BSA E171: −12.97 ± 0.29 TiO2 MPs: −14.10 ±0.56 TiO2 NPs: −13.12 ± 0.44 HBSS E171: −4.39 ± 0.12 TiO2 MPs: −5.62 ± 0.9 TiO2 NPs: −6.08 ± 0.09 McCoy +10% FBS at 1 mg/mL E171: −12.56 ± 8.3 |
Caco-2, HCT116 | 24 | 0.001–1000 |
|
| Popp 2018 [132] | TiO2 NPs NanoAmor, mkNano MKN-TiO2-A100 (purity > 98%) |
NanoAmor:15 mkNano: 50 MKN-TiO2-A100: 100 |
In DMEM at 25 µg/mL NanoAmor: ~300 mkNano: ~800 MKN-TiO2-A100: ~500 |
In DMEM at 25 µg/mL NanoAmor: ~−15 mkNano: ~−12 MKN-TiO2-A100: ~−12 |
HeLa | 24, 48, 72 | 0–500 |
|
| Pedata 2019 [58] | TiO2 NPs P25 | P25: 21 | Not assessed | Not assessed | Caco-2 (differentiated) | 4, 24, 48 | 42, 84 |
|
| Dorier 2019 [124] | E171 A12 NM-105 |
E171: 118 ± 53 A12: 12 ± 3 NM-105: 24 ± 6 |
In ultrapure sterile water at 10 mg/mL E171:415 ± 69 A12: 85 ± 3 NM-105: 158 ± 1 In DMEM + 10% FBS E171:739 ± 355 A12: 448 ± 1 NM-105: 440 ± 7 |
In ultrapure sterile water at 10 mg/mL E171: −19 ± 1 A12: −11 ± 1 NM-105: −11 ± 1 |
Caco-2/HT29-MTX (co-culture) | 6, 24, 48 | 0–200 |
|
| Cao 2019 [125] | E171 | E171: 113.4 ± 37.2 | Not assessed | Not assessed | Caco-2/HT29-MTX (co-culture), + Raji B | 6, 24 | 10, 150 *** |
|
| Li 2020 [126] | TiO2 NPs | 25 | Not assessed | Not assessed | HCT116, NCM4660 | 24 | 2, 30 |
|
| Ghosh 2013 [127] | TiO2 NPs | 100 | In PBS 6180.73 |
Not assessed | Human lymphocyte, human erythrocytes | 3 | 25, 50, 100 |
|
| Dai 2019 [128] | TiO2 NPs rutile |
30–50 50–100 |
Not assessed | Not assessed | RAW 264.7 | 24 | 50, 100, 200, 300, 400 |
|
| Brzicova 2019 [131] | TiO2 NPs | 20–60 * | Was assessed* | Was assessed * | THP-1 | 24 | 32, 128, 256 |
|
| Shukla 2013 [129] | TiO2 NPs Anatase (purity 99.7%) |
30–70 * | In Milli-Q water 192.5 ± 2.00 In CMEM 124.9 ± 3.20 |
In Milli-Q water −11.4 ± 0.25 In CMEM −17.6 ± 0.48 |
HepG2 | 6, 24, 48 | 1, 10, 20, 40, 80 |
|
| Shi 2015 [130] | TiO2 NPs Anatase (purity 99.7%) |
10–25 | DMEM + 1% FCS 366.5 ± 30 |
DMEM + 1% FCS −26.93 ± 0.43 |
HepG2 | 24 | 0.1, 1, 10 |
|
6. Mode of Action
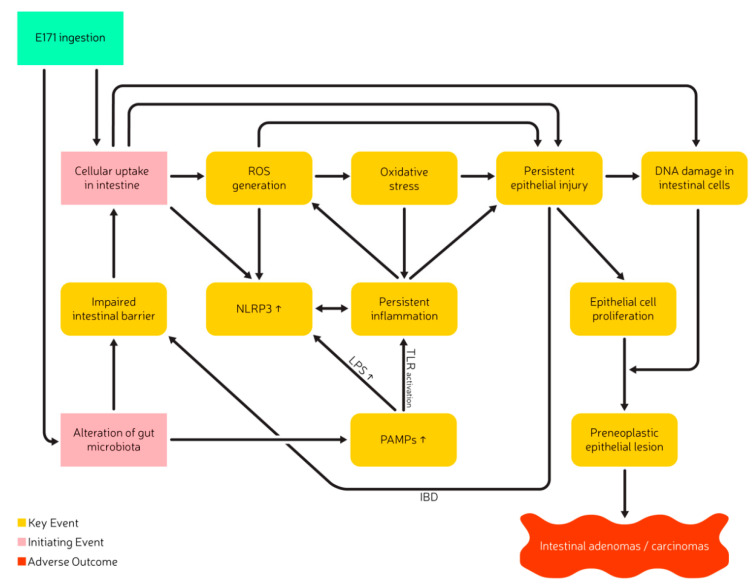
Many in vivo and in vitro studies showed that exposure to TiO2 can result in the formation of ROS and the induction of genetic damage and that E171 has the potential to initiate and stimulate inflammation, promote tumors and impair the overall intestinal health through a similar mode of action. The molecular mechanisms potentially leading to the initiation of E171-induced adverse health effects are still under investigation but are presumed to be closely related to the potential of TiO2 to induce ROS formation and to promote inflammation. Mechanistic studies in vitro and in vivo, mostly performed with TiO2 nanoparticles suggest that the absorption of TiO2/E171 can induce the formation of ROS and could lead to a misbalance of the oxidative-antioxidative system. Two postulated initiating events (IE) and several key events (KE) related to adverse effects after TiO2 ingestion have been identified. KEs include ROS generation, oxidative stress, persistent inflammation, persistent epithelial injury, increased cell proliferation, and DNA damage, resulting in preneoplastic lesions and ultimately intestinal adenomas/carcinomas as seen in Figure 2 [3,85].
Figure 2.
Postulated Adverse Outcome Pathway of TiO2 after ingestion and related to inflammation and carcinogenicity, adapted and extended from Braakhuis et al. [3]. Abbreviation: ROS, reactive oxygen species; PAMPs, Pathogen-Associated-Molecular-Patterns; LPS, Lipopolysaccharides; IBD, Inflammatory Bowel Disease; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3, TLR, Toll-like receptor.
E171 ingestion and its absorption into intestinal enterocytes (IE1) could result in the formation of ROS, due to the semiconducting properties of TiO2 that can lead to the formation of radicals and a misbalance of the oxidative-antioxidative system [33,134,135,136,137,138]. The absorption of E171 into the intestinal enterocytes could furthermore lead to the direct induction of inflammation via lysosomal membrane permeabilization and eventually induce direct DNA damage to intestinal epithelial cells [132,139].
The presence of E171 in the intestine possibly alters the composition of the commensal gut microbiota (IE2), which may result in changes in the metabolic function and result in an increase in pathogen-associated-molecular-patterns (PAMPs), such as LPS [85]. The presence of LPS, the internalization of TiO2 particles itself, and TiO2 related alterations of the adenosine triphosphate (ATP) flux are potential triggers for the activation of the NLPR-3 inflammasome, leading to the subsequent release of IL-18 and IL-1β [85,104,140,141,142,143]. Inflammatory conditions have also been shown in M-cells of the Peyer’s patch. E171 ingestion and absorption led to a decreased T-cell population in Peyer’s patches, which may result in local immunosuppression and pro-inflammatory conditions [14,103]. T-cell receptor suppression associated increase in COX-2 gene expression elevates the production and release of prostaglandin-2 and acts as another mediator for inflammation and GPCR stimulation [120]. Gene expression analysis in vitro and in vivo showed significant alterations in GPCR-family, olfactory receptors, and DNA repair. Molecular pathways relevant for tumor development, including oxidative stress, immune response, inflammation, and cancer signaling showed changes after E171 exposure. Even though the genes affected in these different models were not identical, they showed similarities in their processes. This included increased expression of NF-kB and IRF-3, as well as phosphorylation of tumor suppressor gene p38, along with an increase in MAPK, STAT-1 pathways which are associated with inflammation and cellular stress [44,92]. Additional clustering analysis of RNA-sequences showed the most significantly enriched gene ontology terms and Kyoto Encyclopedia of Genes and Genome (KEGG) pathways relate to Endoplasmic reticulum (ER) stress and initiate an increased cytochrome P450 (CYP450) expression. Such events eventually lead to the activation of MAPK and NF-kB pathways that may further contribute to the induction of inflammation in the intestine after E171 ingestion [88]. Persistent inflammatory processes may weaken the cell membranes and decrease barrier integrity, resulting in impaired barrier function. It is furthermore speculated that general alteration of the gut microbiota (e.g., intestinal dysbiosis) may impair overall intestinal health and increases intestinal permeability [144]. Exposure to E171 can result in a decrease of TJP-1 gene expression, further weakening the intestinal barrier. These processes potentially increase E171 absorption over time and worsen the conditions or accelerate the adverse health effects [94]. A decrease in intestinal barrier integrity and the consequent increase in E171 absorption elevates the systemic TiO2 levels and eventually increases the risk for organ-specific toxicity e.g., neurotoxicity, cardiac toxicity, hepatotoxicity, and reprotoxicity [13,94,108,109,113,145,146,147,148].
Interactions of ROS with DNA and persistent epithelial injury can result in DNA damage and oxidative DNA-base modifications such as 8-Oxoguanine [90,93]. The formation of pre-neoplastic epithelial lesions, intestinal adenomas/carcinomas, or alterations in the size of tumors has been shown to be accompanied by downregulation of epidermal growth factor receptor (EGFR) and AKT, as well as downregulation of tumor suppressor genes e.g., p53 and p21 [129,149,150]. An up-and downregulation of p53 has been observed, which probably displayed different stages within the onset of adverse effects, triggered by E171 exposure. In an earlier stage of adverse effect onset, the upregulation of p53 tumor suppressor gene initiates an increased binding of p53 to DNA to stimulate p21/cdk2 complex building in case of DNA damage, to stop cell division. E171 also appears to increase Apaf-1 and BAX gene expression [129]. Apaf-1 is a cytoplasmic protein initiating apoptosis. BAX is an apoptotic regulator, which binds with the BCL-2 gene to initiate apoptosis and is closely related to the p53 pathway and the formation of colon cancer [129,150]. Upregulation of the master regulator for lysosomal biogenesis and autophagy transcriptional factor EB (TfEB) as well as the increased expression of LC-3 and Beclin-1 (predictive markers for colorectal cancer) contribute to the assumption that the exposure to E171 and the resulting induction of persistent inflammation could be associated with an increased risk for colorectal cancer [128].
As E171 is suspected to impair intestinal barrier functions through dysregulation of inflammatory cytokines, immunosuppression, and alteration of the commensal gut microbiota. It may be expected that patients already suffering from IBD could be a group of people more susceptible to E171 related adverse health outcomes. The examination of blood titanium levels in a swiss IBD cohort support this hypothesis and showed significantly increased blood titanium levels in patients suffering from an active phase of ulcerative colitis and potentially contribute to a worsening of the existing intestinal inflammation [89].
It is currently unclear if E171 is chemically active and a mutagen, or if its mode of action is limited to the formation of free radicals, that promote the growth of tumors. Very limited information regarding the direct interaction of TiO2 with DNA is reported, but it is under discussion if TiO2 can enter the nucleus. A recent study from Du et al. showed no positive mutagenic response of TiO2 nanoparticles in the mouse lymphoma assay nor the Ames test [151]. Though the Ames test is not considered to be suitable for an assessment of the mutagenic potential of nanomaterials, due to the presumed inability of bacterial cells to take up particles [2]. Investigation of human responses to E171 exposure and the comparison of molecular biological processes observed in vivo and in vitro may help to better understand potentially harmful effects following the ingestion of TiO2 as a food additive [23].
7. Recommendation and Outlook
The experts at the workshop on potential health effects of the food additive titanium dioxide (E171) formulated recommendations for future studies, that aim to reduce uncertainties and fill in existing knowledge gaps [23]. These recommendations have been updated and extended to include recent literature. Good Laboratory Practice (GLP) and following OECD guidelines for in vitro and in vivo studies, including dose-response relationships, adequate controls are a given and will aid in the comparability of studies and the compliance with international scientific standards.
7.1. Particle Characterization
The identification of the crystal structure and a precise particle characterization to acquire information about particle size and potential matrix effects are crucial. The characterization of morphological or physiological changes of E171 due to their environment is especially important in both the in vitro and in vivo assessment. Complex biological and physiological cell culture media can contain electrolytes, lipids, or proteins, and vary in their pH values, which potentially alters initial particle characteristics. Examination of the state of aggregation and colloidal stability in a time-based manner, could therefore provide valuable insights into particle behavior, help to better understand particle uptake, and eventually lead to adjustments regarding dosimetry [37,152]. As E171 is brought into commerce in different crystal forms, additional information is needed in which crystal form it is manufactured and how this may affect toxicity. As previously described, the particle size distribution is thought to be a major factor in the toxicity of E171 and can have significant effects on particle properties and reactivity. The TiO2 nanoparticles and microparticles can be characterized by Transmission/Scanning Electron Microscopy (T/SEM), Dynamic Light Scattering (DLS), Nanoparticle Tracking Analysis (NTA), Fluorescence Correlation Spectroscopy (FCS), as well as single-particle ICP-MS or high-resolution MS. As the characterization of any nanomaterial, and also TiO2 and thus E171, depends on the manufacturing conditions as well as on its immediate environment, E171 should be characterized in its pristine form and other matrices, such as in a vehicle/dispersion before dosing in toxicity studies, in the food matrix, or inside the gastrointestinal environment where protein coronas may be formed. In the characterization process, the hydrodynamic diameter including the state of agglomeration and aggregation should be identified, preferable following standardized protocols to enable that results of various studies are comparable and to reduce confounding effects due to variations in the preparation of the samples. A potential approach for such a standardized protocol was made by Kobayashi et al. (2019) and the Joint Research Center. These protocols include recommendations for sonication time, volume, and appropriate vehicles to achieve long-term stability of the particle liquid-phase dispersion [153,154,155,156]. An ever-growing number of studies, assessing the potentially toxic effects of E171, has been published, that associate exposure to E171 with a variety of adverse health outcomes. The majority of these studies evaluated the hazard caused by nanosized TiO2 models (<100 nm). However, to adequately assess the food additive E171 the micro-sized fraction, is also of importance. To assess the risk of E171 via ingestion, it is therefore required to conduct an in-depth characterization of the particle and to compare these test materials with commercially available and used E171 [43,157]. To determine the exact composition of TiO2 used as food additive E171 is not an easy task, due to its various interaction and alterations within food matrices [28]. But it is necessary to further investigate E171 and to evaluate products circulating on the market, that are known to contain E171, to give an approximation of the E171 composition that is found in those products.
7.2. In Vitro Models
In vitro experiments with E171 should consider potential effects on the state of agglomeration or aggregation of E171 following digestive processes in the mouth cavity, stomach and intestine before exposing cellular systems to E171. Protocols for a successful in vitro digestion have been published and are already in use in the assessment of E171 [158,159]. E171 is presumed to be very persistent, it shows a very low dissolution rate in simulated gastrointestinal fluids and tends to agglomerate over time in the digestive cascade [160,161]. Recent studies nevertheless demonstrated that the digestion of TiO2 nanoparticles can have an impact on in vitro testing. The application of the INFOGEST 2.0 in vitro digestion method in Caco-2 and HT29-MTX-E12 cells showed a pronounced increase in cytotoxicity after digestion of certain types of tested TiO2 nanoparticles, probably due to slight changes in the nanomaterial characteristics [162]. Therefore, it is advisable to include digestive protocols in the in vitro toxicity assessment of E171, if possible [158,159]. Another improvement in the testing of E171 would be the use of advanced in vitro cell models, such as 3D colon organoids. Traditional 2D cell culture models such as differentiated Caco-2 monolayers are not sufficient to adequately assess transport or adverse effects of E171, due to their limited presence of intestinal cells types like mucus-secreting HT29/MTX cells, which are responsible for the transport of TiO2 to a significant degree [163]. Differentiated co-cultures of Caco-2/HT29-MTX mucus-producing cells or Caco-2/Raji B cells are an improvement but still do not mimic the complex microarchitecture of the intestine accordingly [164,165]. The intestine is an important and complex organ, which is not properly represented in common cell culture models, due to their lack of organ-specific microarchitecture and a physiological extracellular matrix microenvironment. The proximity of 3D colon organoid cell models to the human colon, with critical self-renewal and maintenance function, reassembles the intestinal tissue to a higher degree and can provide a more realistic model for in vitro experiments. Although immune intraepithelial cells are lacking in human gut organoids, the origin of 3D colon organoids from induced pluripotent stem cells results in the presence of a large variety of physiological cells, such as intestinal stem cells, enterocytes, enteroendocrine and Paneth cells, goblets (mucus-secreting) cells and mesenchymal cells [166,167,168,169].
7.3. Rodent Studies
Future research should include a carcinogenicity study (OECD 451), with well-characterized particles and TiO2 reassembling commercially used E171. Whereas 2-year carcinogenicity studies are considered the gold standard in identifying carcinogens, they require many animals to obtain sufficient sensitivity for the detection of adverse health effects with tumor formation as the final endpoint. The development of certain adverse health outcomes, such as colon cancer, in untreated rats, is highly unusual [170]. Such studies may have limitations, as healthy animals for instance may not represent the best model for more susceptible populations with a compromised barrier function and a higher predisposition for developing colon cancer [23]. As reported by Ruiz et al. (2017), IBD patients with an active UC have significantly elevated levels of blood titanium concentration, making this ever-growing group of patients particularly vulnerable to the potentially harmful effects of TiO2 [89]. These findings may require having a closer look at potential pre-existing conditions, with additional testing in special animal models, e.g., colitis-induced mouse models. Another issue arising regarding long-term carcinogenicity studies is the route of administration. A physiological administration via the diet closely resembles real-life scenarios but may result in a potential underestimation of the risk, due to insufficient systemic exposure, while administration via oral gavage may lead to a potential overestimation of the risk due to exposure to highly dispersed particles. Additional in vivo studies in rodents should be promoted to confirm effects as they have been identified so far, e.g., oriented towards tumorigenesis and the possible underlying mechanism of immune suppression. Gut-associated lymphoid tissues as well as the intestinal microbiota should be considered as possible factors for (pre)tumor formation. Given the possible adverse health effects of ingesting E171, the trade associations intended to conduct an additional laboratory animal study on demand of EFSA, e.g., an Extended One-Generation Reproductive Toxicity Study (EORGTS), in which cohorts are included for reprotoxicological and immunotoxicological tests. BuRO recommended that this research also includes analyses of parameters that are important for the development of colon cancer, for which indications were found in the previously mentioned studies [23]. Future studies should also provide information on internal dose-response relationships, starting from relevant levels for the general population. Studies to be carried out should include parameters that have been shown to be affected by TiO2 or that are important early markers of tumor formation, including putative immune and bacterial factors.
7.4. Non-Rodent Studies and Human Intervention Study
So far, in vivo studies have been performed in rodents. To substantiate the relevance of these effects, testing in other species, better-mimicking humans would be advisable. A candidate could be the mini pig, due to its closer resembling of the human cardiovascular system and immunological processes [171,172,173]. Even though the options to test in humans are more limited and will require ethical examination, testing in humans would be highly preferred. It is recommended to evaluate if a human intervention study can be undertaken, that will assess key parameters as identified in the in vitro and animal studies, and in which subjects are exposed to E171 in concentrations that are sufficient to allow effects on the outcome parameters to occur. In analogy with animal studies evaluating gene expression changes, conducting gene expression analysis in humans may be valuable, as these parameters could easily be assessed and may demonstrate the potential activation of crucial processes related to oxidative stress, inflammation, and tumorigenesis under relevant human exposure conditions [23]. It is especially important to also focus on differences in intestinal permeability between humans. Inclusion of IBD patients that might be more prone to TiO2 related adverse health effects, could potentially contribute to the identification of adverse health effects or populations most at risk. It is recommended that if such studies be carried out, they should be performed in close collaboration with risk assessors, risk managers, as well as clinicians.
8. Summarizing Conclusions of the Workshop
Below, the summarized questions initially raised by the NVWA are answered, based on the workshop discussion and publications released after the workshop. Additional recommendations for future research are outlined to guide the further assessment of potential adverse health effects of ingested E171.
Does oral exposure to E171 reveal a relevant toxicological hazard in in-vitro and in-vivo studies and how reliable are these studies?
Adverse effects were identified including the generation of ROS, alterations of the gut microbiota, persistent inflammation, and other effects on the immune system. These conditions can result in persistent epithelial cell injury and potentially lead to DNA damage and exert a tumor-promoting effect of E171. The findings are inconsistent throughout different species and independent research groups. Some studies are revealing a promotion of colonic precancerous lesions or promotion of tumor formation in mice, using models that involve the chemical induction of colon cancer, while others do not. These models are mainly used as research models and a proper investigation of a dose-response relationship was not performed. With the existing findings, it is not possible to carry out a risk assessment. Currently, in vitro studies are insufficient to form the sole base for risk assessment too. Nevertheless, the results of independent researchers point in a similar direction and should be further investigated.
-
2.
Are the animal models, the exposure conditions, and the effects observed in these studies relevant to humans?
It is not clear to what extent the effects observed in animal models are relevant to humans since it is unknown if the mechanisms that lead to these effects occur in humans. Although laboratory animal models have their limitations, they are still toxicologically relevant. For this reason, the extrapolation of dose-related effects on the colon of rats to humans is considered to be valid. Doses used in laboratory animal studies of 5 and 10 mg/kg bw/day led to pre-neoplastic lesions and 5 mg/kg bw/day already led to the promotion of colon cancer. These doses are in the same order of magnitude as the 95th percentile of 14.8 mg/kg bw/day for children in Europe. Because of the variety of E171 forms and their different physiochemical properties e.g., particle size distribution, the studies involving mice and rats may not represent all forms of E171 on the market. Furthermore, it is not clear whether the absorption of TiO2 increases with higher intake, due to its effects on microbiotic health and intestinal barrier integrity. It is therefore important to determine tissue titanium concentrations in relevant organs in both rodents and humans.
-
3.
Can the data from in vitro and in vivo studies with TiO2 be extrapolated to humans?
The extrapolation of animal testing data to humans is commonly used in risk assessment. Differences between laboratory animals and humans (interspecies) and between human individuals (intraspecies) are considered by using uncertainty factors. Therefore, it is possible to extrapolate the data from laboratory animals exposed to E171 to humans. However, there is no reliable dose-response data in this case.
-
4.
Are there epidemiological studies on the effects of E171 in humans after oral exposure?
There are very few epidemiological data on the adverse effects of E171 in humans available. Except for a limited number of oral absorption experiments and the observation of increased blood TiO2 levels in UC patients from a Swiss IBD cohort. Yet no associations between E171 exposure and the induction inflammatory responses, alteration of gut microbiota, and colon cancer have been reported in humans [68,69,70,89].
9. Conclusions
After reviewing the literature on the potential risks of oral exposure to TiO2, we concluded that the existing body of evidence raises concern for human health regarding the long-term ingestion of E171. The wide-spread human exposure in combination with the reported tumor-promoting and pro-inflammatory responses in animal experiments indicates the necessity to fill in the identified knowledge gaps that are crucial in the hazard identification and risk assessment process. A particular concern was identified for children due to their proportionally higher TiO2 intake, and patients with IBD, given their potential risk of increased absorption, as a consequence of impaired intestinal health.
Animal experiments have shown that chronic exposure to E171 can lead to translocation and bioaccumulation of TiO2 via the bloodstream, in various organs, including the liver, kidney, placenta, and brain. Across different types of models, gene expression patterns have been reported that are associated with inflammation and tumor development. In-vivo, ex-vivo, and in-vitro experiments, mainly conducted with TiO2 nanoparticles, show that TiO2 can result in the formation of ROS, which is associated with the induction of genetic damage, the initiation, and stimulation of inflammation, and the promotion of tumor formation. The endocrine and reprotoxic effects found in rodent studies indicate the need for additional research to reduce uncertainties. These complex interplays of molecular mechanisms involving local persistent inflammation, disturbance of the oxidative–antioxidative balance, immune suppression, apoptosis, changes in microbiotic health, and colon cancer-related pathways need to be further investigated to better understand the molecular biological process, their interaction, and involvement following the chronic exposure to E171.
At the workshop, it was noted that chronic carcinogenicity studies in laboratory animals might have limitations in identifying influences on the incidence of rare tumors, such as colon cancer in rats. For this purpose, specific disease models may provide complementary information. Furthermore, it was concluded that proper characterization of the TiO2 particles is crucial for future studies and that the type of crystal form and particle size used, both in the commercially available E171 and in experimental toxicity studies, should be well described. While oral exposure of TiO2 via drinking water (oral gavage) and via the diet is both relevant, the effect of the food matrix on bioavailability and adverse health effects is still poorly understood and potentially has an influence on particle properties and toxicokinetics, hence should be considered in the hazard identification of E171. Finally, human dietary intervention studies are needed to demonstrate or discard adverse responses to E171, under relevant exposure conditions, and to better understand the potential cellular and molecular mechanism of action in humans.
Acknowledgments
Special thanks go to Ruud Woutersen for participating in the workshop and giving feedback on this review. The authors also want to thank Dirk van Aken and Hubert Noteborn for participating in the workshop, and we thank Nikita Zhukovskiy (WRX) for the excellent graphics and visual communication. We thank Anna K. Undas at Business Unit Contaminants and Toxicology, Wageningen Food Safety Research, The Netherlands, for providing data and images regarding E171 particle characterization.
Abbreviations
| TiO2 | Titanium dioxide |
| BuRO | Dutch Office for Risk Assessment and Research |
| AOP | Adverse Outcome Pathway |
| EU | European Union |
| IARC | International Agency for Research and Cancer |
| RAC | Risk Assessment Committee |
| ECHA | European Chemical Agency |
| CLP | Classification, Labelling and Packaging |
| ROS | Reactive Oxygen Species |
| EFSA | European Food Safety Authority |
| ANSES | French Agency for Food, Environmental and Occupational Health |
| NVWA | Netherlands Food and Consumer Products Safety Authority |
| TEM | Transmission Electron Microscopy |
| Sp-ICP-MS | Single Particle Inductively Couple Plasma Mass Spectrometry |
| LPS | Lipopolysaccharides |
| NF-kB | Nuclear Factor Kappa Light-Chain-Enhancer of activated B-cell |
| IRF-3 | Interferon Regulatory-Factor 3 |
| Bw | Body weight |
| TJ | Tight junction |
| PBPK | Physiologically Based Pharmacokinetics |
| TEM-EDX | Transmission Electron Microscopy -Energy Dispersive X-Ray Analysis |
| IFN-γ | Interferon-gamma |
| TNF-⍺ | Tumor Necrosis Factor alpha |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| ASP | Aspartate Aminotransferase |
| GSH | Glutathione |
| GSSG | Glutathione Disulfide |
| GPx | Glutathione Peroxidase |
| SOD | Superoxid Dismutase |
| CAT | Catalase |
| IL | Interleukin |
| CYP450 | Cytochrome P450 |
| COX-2 | Cyclooxygenase-2 |
| Ki67 | Antigen Ki67 |
| Th-1 | T-helper-1 |
| P65 | Transcription factor p65 |
| MAPK | Mitogen-Activated Protein Kinase |
| GPCR | G-Protein Coupled Receptor |
| ER | Endoplasmic Reticulum |
| I.V. | Intravenous |
| I.P. | Intraperitoneal |
| IBD | Inflammatory Bowel Disease |
| CRC | Colorectal Cancer |
| BBB | Brain-Blood-Barrier |
| NO | Nitric Oxide |
| GT | Glutamyltransferase |
| StAR | Steroidogenic Regulatory Protein |
| BCL-2 | B-cell Lymphoma-2 |
| MAD | Malondialdehyde |
| PSA | Prostate-Specific Antigen |
| OECD | Organization for Economic Cooperation and Development |
| GR | Glutathione Reductase |
| LC-3 | 1A/1B-Light-Chain-3 |
| ERK | Extracellular Signaling Regulated Kinase |
| BAX | Bcl-2-Associated X protein |
| Apaf-1 | Apoptotic Peptidase Activating Factor 1 |
| Nrf2 | Nuclear Factor Erythroid 2-related Factor 2 |
| IE | Initiating Event |
| KE | Key Event |
| PAMP | Pathogen-Associated-Molecular-Pattern |
| ATP | Adenosine Triphosphate |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| EGFR | Epidermal Growth Factor Receptor |
| Cdk2 | Cyclin Dependent Kinase 2 |
| TfEB | Transcriptional Factor EB |
| GLP | Good Laboratory Practice |
| T/SEM | Transmission/Scanning Electron Microscopy |
| DLS | Dynamic Light Scattering |
| NTA | Nanoparticle Tracking Analysis |
| FCS | Fluorescence Correlation Spectroscopy |
Author Contributions
Writing—original draft preparation, N.S.B.; writing—review and editing, N.S.B., T.M.d.K., D.T.H.M.S., S.G.v.B., J.J.B., J.J.M.C., A.O., Y.I.C., H.D., D.G., E.H., A.G.O., M.P., G.R. and H.v.L.; supervision, T.M.d.K., D.T.H.M.S., S.G.v.B., J.J.B. and H.v.L.; project administration, T.M.d.K., D.T.H.M.S., S.G.v.B., J.J.B. and H.v.L.; funding acquisition, D.T.H.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The Office of Risk Assessment and Research of Netherlands Food and Consumer Product Safety Authority (NVWA) is acknowledged for funding the expert meeting.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heringa M.B., Geraets L., van Eijkeren J.C., Vandebriel R.J., de Jong W.H., Oomen A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology. 2016;10:1515–1525. doi: 10.1080/17435390.2016.1238113. [DOI] [PubMed] [Google Scholar]
- 2.Winkler H.C., Notter T., Meyer U., Naegeli H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol. 2018;16:1–19. doi: 10.1186/s12951-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braakhuis H.M., Gosens I., Heringa M.B., Oomen A.G., Vandebriel R.J., Groenewold M., Cassee F.R. Mechanism of Action of TiO2: Recommendations to reduce uncertainties related to carcinogenic potential. Annu. Rev. Pharmacol. Toxicol. 2020;61 doi: 10.1146/annurev-pharmtox-101419-100049. [DOI] [PubMed] [Google Scholar]
- 4.Warheit D.B. How to measure hazards/risks following exposures to nanoscale or pigment-grade titanium dioxide particles. Toxicol. Lett. 2013;220:193–204. doi: 10.1016/j.toxlet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warheit D.B., Donner E.M. Risk assessment strategies for nanoscale and fine-sized titanium dioxide particles: Recognizing hazard and exposure issues. Food. Chem. Toxicol. 2015;85:138–147. doi: 10.1016/j.fct.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 7.EFSA . EFSA Statement on the Review of the Risk Related to the Exposure to the Food Additive Titanium Dioxide (E171) Performed by the French Agency for Food, Environment and Occupational Health and Safety (ANSES) EFSA; Parma, Italy: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EFSA. Additives PoF. Food NSat. Younes M., Aggett P., Aguilar F., Crebelli R., Dusemund B., Filipič M., Frutos M.J., et al. EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) EFSA J. 2019:5714. doi: 10.2903/j.efsa.2019.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K.P., Trochimowicz H.J., Reinhardt C.F. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol. Appl. Pharmacol. 1985;79:179–192. doi: 10.1016/0041-008X(85)90339-4. [DOI] [PubMed] [Google Scholar]
- 10.IARC . Carbon Black, Titanium Dioxide and Talc. Volume 93. IARC; Lyon, France: 2010. IARC monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 11.ECHA . Opinion Proposing Harmonised Classification and Labelling a EU Level of Titanium Dioxide. ECHA; Helsinki, Finland: 2017. [Google Scholar]
- 12.European Parliament . Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. European Parliament; Brussels, Belgium: 2020. [Google Scholar]
- 13.Brand W., Peters R.J.B., Braakhuis H.M., Maslankiewicz L., Oomen A.G. Possible effects of titanium dioxide particles on human liver, intestinal tissue, spleen and kidney after oral exposure. Nanotoxicology. 2020;14:985–1007. doi: 10.1080/17435390.2020.1778809. [DOI] [PubMed] [Google Scholar]
- 14.Bettini S., Boutet-Robinet E., Cartier C., Comera C., Gaultier E., Dupuy J., Naud N., Tache S., Grysan P., Reguer S., et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017;7:40373. doi: 10.1038/srep40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urrutia-Ortega I.M., Garduno-Balderas L.G., Delgado-Buenrostro N.L., Freyre-Fonseca V., Flores-Flores J.O., Gonzalez-Robles A., Pedraza-Chaverri J., Hernandez-Pando R., Rodriguez-Sosa M., Leon-Cabrera S., et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food. Chem. Toxicol. 2016;93:20–31. doi: 10.1016/j.fct.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Proquin H., Rodriguez-Ibarra C., Moonen C.G., Urrutia Ortega I.M., Briede J.J., de Kok T.M., van Loveren H., Chirino Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis. 2017;32:139–149. doi: 10.1093/mutage/gew051. [DOI] [PubMed] [Google Scholar]
- 17.Blevins L.K., Crawford R.B., Bach A., Rizzo M.D., Zhou J., Henriquez J.E., Khan D., Sermet S., Arnold L.L., Pennington K.L., et al. Evaluation of immunologic and intestinal effects in rats administered an E 171-containing diet, a food grade titanium dioxide (TiO2) Food Chem. Toxicol. 2019;133:110793. doi: 10.1016/j.fct.2019.110793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martins A.D.C., Jr., Azevedo L.F., de Souza Rocha C.C., Carneiro M.F.H., Venancio V.P., de Almeida M.R., Antunes L.M.G., de Carvalho Hott R., Rodrigues J.L., Ogunjimi A.T., et al. Evaluation of distribution, redox parameters, and genotoxicity in Wistar rats co-exposed to silver and titanium dioxide nanoparticles. J. Toxicol. Environ. Health A. 2017;80:1156–1165. doi: 10.1080/15287394.2017.1357376. [DOI] [PubMed] [Google Scholar]
- 19.ANSES . OPINION of the French Agency for Food, Environmental and Occupational Health and Safety on the Risk Associated with Ingestion of the Food Additive E171. ANSES; Maisons-Alfort, France: 2019. [Google Scholar]
- 20.Bundesinstitut für Berufsbildung . Titanium Dioxide: Further Research Is Still Needed. Bundesinstitut für Berufsbildung; Bonn, Germany: 2020. [Google Scholar]
- 21.EFSA Re-Evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016;14 doi: 10.2903/j.efsa.2016.4545. [DOI] [Google Scholar]
- 22.EFSA Scientific Panel on food additives and flavourings; Proceedings of the Minutes of the 16th Pleenary Meeting; Parma, Italy. 21–23 September 2020. [Google Scholar]
- 23.BuRO . Opinion on Possible Health Effects of the Food Additive Titanium Dioxide (E171) Netherlands Food and Consumer Product Safety Authority; The Hague, The Netherlands: 2019. [Google Scholar]
- 24.Sayes C.M., Wahi R., Kurian P.A., Liu Y., West J.L., Ausman K.D., Warheit D.B., Colvin V.L. Correlating nanoscale titania structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol. Sci. 2006;92:174–185. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- 25.Peters R.J., van Bemmel G., Herrera-Rivera Z., Helsper H.P., Marvin H.J., Weigel S., Tromp P.C., Oomen A.G., Rietveld A.G., Bouwmeester H. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014;62:6285–6293. doi: 10.1021/jf5011885. [DOI] [PubMed] [Google Scholar]
- 26.Dudefoi W., Terrisse H., Popa A.F., Gautron E., Humbert B., Ropers M.H. Evaluation of the content of TiO2 nanoparticles in the coatings of chewing gums. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:211–221. doi: 10.1080/19440049.2017.1384576. [DOI] [PubMed] [Google Scholar]
- 27.Weir A., Westerhoff P., Fabricius L., Hristovski K., von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012;46:2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geiss O., Ponti J., Senaldi C., Bianchi I., Mehn D., Barrero J., Gilliland D., Matissek R., Anklam E. Characterisation of food grade titania with respect to nanoparticle content in pristine additives and in their related food products. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2020;37:239–253. doi: 10.1080/19440049.2019.1695067. [DOI] [PubMed] [Google Scholar]
- 29.Commission T.E. Definition of nanomaterial. Off. J. Eur. Union. 2011:38–40. doi: 10.3000/19770677.L_2011.317.eng. [DOI] [Google Scholar]
- 30.Verleysen E., Waegeneers N., Brassinne F., De Vos S., Jimenez I.O., Mathioudaki S., Mast J. Physicochemical Characterization of the Pristine E171 Food Additive by Standardized and Validated Methods. Nanomaterials. 2020;10:592. doi: 10.3390/nano10030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim J.H., Bae D., Fong A. Titanium Dioxide in Food Products: Quantitative Analysis Using ICP-MS and Raman Spectroscopy. J. Agric. Food Chem. 2018;66:13533–13540. doi: 10.1021/acs.jafc.8b06571. [DOI] [PubMed] [Google Scholar]
- 32.Xiong S., George S., Ji Z., Lin S., Yu H., Damoiseaux R., France B., Ng K.W., Loo S.C.J. Size of TiO(2) nanoparticles influences their phototoxicity: An in vitro investigation. Arch. Toxicol. 2013;87:99–109. doi: 10.1007/s00204-012-0912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musial J., Krakowiak R., Mlynarczyk D.T., Goslinski T., Stanisz B.J. Titanium dioxide nanoparticles in food and personal care products-what do we know about their safety? Nanomaterials. 2020;10:1110. doi: 10.3390/nano10061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warheit D.B., Brown S.C. What is the impact of surface modifications and particle size on commercial titanium dioxide particle samples? A review of in vivo pulmonary and oral toxicity studies—Revised 11-6-2018. Toxicol. Lett. 2019;302:42–59. doi: 10.1016/j.toxlet.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Ali S.A., Rizk M.Z., Hamed M.A., Aboul-Ela E.I., El-Rigal N.S., Aly H.F., Abdel-Hamid A.Z. Assessment of titanium dioxide nanoparticles toxicity via oral exposure in mice: Effect of dose and particle size. Biomarkers. 2019;24:492–498. doi: 10.1080/1354750X.2019.1620336. [DOI] [PubMed] [Google Scholar]
- 36.Posgai R., Cipolla-McCulloch C.B., Murphy K.R., Hussain S.M., Rowe J.J., Nielsen M.G. Differential toxicity of silver and titanium dioxide nanoparticles on Drosophila melanogaster development, reproductive effort, and viability: Size, coatings and antioxidants matter. Chemosphere. 2011;85:34–42. doi: 10.1016/j.chemosphere.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 37.Moore T.L., Rodriguez-Lorenzo L., Hirsch V., Balog S., Urban D., Jud C., Rothen-Rutishauser B., Lattuada M., Petri-Fink A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015;44:6287–6305. doi: 10.1039/C4CS00487F. [DOI] [PubMed] [Google Scholar]
- 38.Janer G., Mas del Molino E., Fernandez-Rosas E., Fernandez A., Vazquez-Campos S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2014;228:103–110. doi: 10.1016/j.toxlet.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Murugadoss S., Brassinne F., Sebaihi N., Petry J., Cokic S.M., Van Landuyt K.L., Godderis L., Mast J., Lison D., Hoet P.H., et al. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo. Part. Fibre Toxicol. 2020;17:10. doi: 10.1186/s12989-020-00341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foroozandeh P., Aziz A.A. Merging worlds of nanomaterials and biological environment: Factors governing protein corona formation on nanoparticles and its biological consequences. Nanoscale Res. Lett. 2015;10:221. doi: 10.1186/s11671-015-0922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan A.O., Di Maio A., Guggenheim E.J., Chetwynd A.J., Pencross D., Tang S., Belinga-Desaunay M.A., Thomas S.G., Rappoport J.Z., Lynch I. Surface chemistry-dependent evolution of the nanomaterial corona on TiO2 nanomaterials following uptake and sub-cellular localization. Nanomaterials. 2020;10:401. doi: 10.3390/nano10030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazzolini J., Weber R.J., Chen H.S., Khan A., Guggenheim E., Shaw R.K., Chipman J.K., Viant M.R., Rappoport J.Z. Protein corona modulates uptake and toxicity of nanoceria via clathrin-mediated endocytosis. Biol. Bull. 2016;231:40–60. doi: 10.1086/689590. [DOI] [PubMed] [Google Scholar]
- 43.Saptarshi S.R., Duschl A., Lopata A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013;11:26. doi: 10.1186/1477-3155-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianchi M.G., Allegri M., Chiu M., Costa A.L., Blosi M., Ortelli S., Bussolati O., Bergamaschi E. Lipopolysaccharide adsorbed to the bio-corona of TIO2 nanoparticles powerfully activates selected pro-inflammatory transduction pathways. Front. Immunol. 2017;8:866. doi: 10.3389/fimmu.2017.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X.X., Cheng B., Yang Y.X., Cao A., Liu J.H., Du L.J., Liu Y., Zhao Y., Wang H. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small. 2013;9:1765–1774. doi: 10.1002/smll.201201506. [DOI] [PubMed] [Google Scholar]
- 46.Dudefoi W., Moniz K., Allen-Vercoe E., Ropers M.H., Walker V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017;106:242–249. doi: 10.1016/j.fct.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 47.Dudefoi W., Terrisse H., Richard-Plouet M., Gautron E., Popa F., Humbert B., Ropers M.H. Criteria to define a more relevant reference sample of titanium dioxide in the context of food: A multiscale approach. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017;34:653–665. doi: 10.1080/19440049.2017.1284346. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y., Doudrick K., Bi X., Hristovski K., Herckes P., Westerhoff P., Kaegi R. Characterization of food-grade titanium dioxide: The presence of nanosized particles. Environ. Sci. Technol. 2014;48:6391–6400. doi: 10.1021/es500436x. [DOI] [PubMed] [Google Scholar]
- 49.Rompelberg C., Heringa M.B., van Donkersgoed G., Drijvers J., Roos A., Westenbrink S., Peters R., van Bemmel G., Brand W., Oomen A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology. 2016;10:1404–1414. doi: 10.1080/17435390.2016.1222457. [DOI] [PubMed] [Google Scholar]
- 50.Wu F., Hicks A.L. Estimating human exposure to titanium dioxide from personal care products through a social survey approach. Integr. Environ. Assess. Manag. 2020;16:10–16. doi: 10.1002/ieam.4197. [DOI] [PubMed] [Google Scholar]
- 51.Bachler G., von Goetz N., Hungerbuhler K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology. 2015;9:373–380. doi: 10.3109/17435390.2014.940404. [DOI] [PubMed] [Google Scholar]
- 52.Sprong C., Bakker M., Niekerk M., Vennemann M. Exposure Assessment of the Food Additive Titanium Dioxide (E 171) Based on Use Levels Provided by the Industry. National Institute for Public Health and the Environment; Bilthoven, The Netherlands: 2016. [Google Scholar]
- 53.Christensen F.M., Christensen T.B., Vogel U., Jensen K.A., Lassen C. Better Control of Nanomaterials—Summary of the 4-Year Danish Initiative on Nanomaterials. The Danish Environmental Protection Agency; København, Denmark: 2015. [Google Scholar]
- 54.Powell J.J., Faria N., Thomas-McKay E., Pele L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010;34:J226–J233. doi: 10.1016/j.jaut.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Golja V., Drazic G., Lorenzetti M., Vidmar J., Scancar J., Zalaznik M., Kalin M., Novak S. Characterisation of food contact non-stick coatings containing TiO2 nanoparticles and study of their possible release into food. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017;34:421–433. doi: 10.1080/19440049.2016.1269954. [DOI] [PubMed] [Google Scholar]
- 56.Teubl B.J., Leitinger G., Schneider M., Lehr C.M., Frohlich E., Zimmer A., Roblegg E. The buccal mucosa as a route for TiO2 nanoparticle uptake. Nanotoxicology. 2015;9:253–261. doi: 10.3109/17435390.2014.921343. [DOI] [PubMed] [Google Scholar]
- 57.Teubl B.J., Schimpel C., Leitinger G., Bauer B., Frohlich E., Zimmer A., Roblegg E. Interactions between nano-TiO2 and the oral cavity: Impact of nanomaterial surface hydrophilicity/hydrophobicity. J. Hazard. Mater. 2015;286:298–305. doi: 10.1016/j.jhazmat.2014.12.064. [DOI] [PubMed] [Google Scholar]
- 58.Pedata P., Ricci G., Malorni L., Venezia A., Cammarota M., Volpe M.G., Iannaccone N., Guida V., Schiraldi C., Romano M., et al. In vitro intestinal epithelium responses to titanium dioxide nanoparticles. Food Res. Int. 2019;119:634–642. doi: 10.1016/j.foodres.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 59.Deciga-Alcaraz A., Delgado-Buenrostro N.L., Ispanixtlahuatl-Meraz O., Freyre-Fonseca V., Flores-Flores J.O., Ganem-Rondero A., Vaca-Paniagua F., Pilar Ramos-Godinez M.D., Morales-Barcenas R., Sanchez-Perez Y., et al. Irreversible disruption of the cytoskeleton as induced by non-cytotoxic exposure to titanium dioxide nanoparticles in lung epithelial cells. Chem. Biol. Interact. 2020;323:109063. doi: 10.1016/j.cbi.2020.109063. [DOI] [PubMed] [Google Scholar]
- 60.Brun E., Barreau F., Veronesi G., Fayard B., Sorieul S., Chaneac C., Carapito C., Rabilloud T., Mabondzo A., Herlin-Boime N., et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014;11:13. doi: 10.1186/1743-8977-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Comera C., Cartier C., Gaultier E., Catrice O., Panouille Q., El Hamdi S., Tirez K., Nelissen I., Theodorou V., Houdeau E. Jejunal villus absorption and paracellular tight junction permeability are major routes for early intestinal uptake of food-grade TiO2 particles: An in vivo and ex vivo study in mice. Part. Fibre Toxicol. 2020;17:26. doi: 10.1186/s12989-020-00357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kreyling W.G., Holzwarth U., Haberl N., Kozempel J., Hirn S., Wenk A., Schleh C., Schaffler M., Lipka J., Semmler-Behnke M., et al. Quantitative biokinetics of titanium dioxide nanoparticles after intravenous injection in rats: Part 1. Nanotoxicology. 2017;11:434–442. doi: 10.1080/17435390.2017.1306892. [DOI] [PubMed] [Google Scholar]
- 63.Demir E. An in vivo study of nanorod, nanosphere, and nanowire forms of titanium dioxide using Drosophila melanogaster: Toxicity, cellular uptake, oxidative stress, and DNA damage. J Toxicol. Environ. Health A. 2020;83:456–469. doi: 10.1080/15287394.2020.1777236. [DOI] [PubMed] [Google Scholar]
- 64.Wang J., Zhou G., Chen C., Yu H., Wang T., Ma Y., Jia G., Gao Y., Li B., Sun J., et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007;168:176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Shinohara N., Danno N., Ichinose T., Sasaki T., Fukui H., Honda K., Gamo M. Tissue distribution and clearance of intravenously administered titanium dioxide (TiO2) nanoparticles. Nanotoxicology. 2014;8:132–141. doi: 10.3109/17435390.2012.763001. [DOI] [PubMed] [Google Scholar]
- 66.Tassinari R., Cubadda F., Moracci G., Aureli F., D’Amato M., Valeri M., De Berardis B., Raggi A., Mantovani A., Passeri D., et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology. 2014;8:654–662. doi: 10.3109/17435390.2013.822114. [DOI] [PubMed] [Google Scholar]
- 67.Donner E.M., Myhre A., Brown S.C., Boatman R., Warheit D.B. In vivo micronucleus studies with 6 titanium dioxide materials (3 pigment-grade & 3 nanoscale) in orally-exposed rats. Regul. Toxicol. Pharmacol. 2016;74:64–74. doi: 10.1016/j.yrtph.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Bockmann J., Lahl H., Eckert T., Unterhalt B. Blood titanium levels before and after oral administration titanium dioxide. Pharmazie. 2000;55:140–143. [PubMed] [Google Scholar]
- 69.Jones K., Morton J., Smith I., Jurkschat K., Harding A.H., Evans G. Human in vivo and in vitro studies on gastrointestinal absorption of titanium dioxide nanoparticles. Toxicol. Lett. 2015;233:95–101. doi: 10.1016/j.toxlet.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Pele L.C., Thoree V., Bruggraber S.F., Koller D., Thompson R.P., Lomer M.C., Powell J.J. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part. Fibre Toxicol. 2015;12:26. doi: 10.1186/s12989-015-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geraets L., Oomen A.G., Krystek P., Jacobsen N.R., Wallin H., Laurentie M., Verharen H.W., Brandon E.F., de Jong W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014;11:30. doi: 10.1186/1743-8977-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jia Y., Wang Y., Dong L., Huang J., Zhang Y., Su J., Zang J. A hybrid of titanium nitride and nitrogen-doped amorphous carbon supported on SiC as a noble metal-free electrocatalyst for oxygen reduction reaction. Chem. Commun. 2015;51:2625–2628. doi: 10.1039/C4CC08007F. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Chen Z., Ba T., Pu J., Chen T., Song Y., Gu Y., Qian Q., Xu Y., Xiang K., et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small. 2013;9:1742–1752. doi: 10.1002/smll.201201185. [DOI] [PubMed] [Google Scholar]
- 74.Hummel T.Z., Kindermann A., Stokkers P.C., Benninga M.A., ten Kate F.J. Exogenous pigment in Peyer patches of children suspected of having IBD. J. Pediatr. Gastroenterol. Nutr. 2014;58:477–480. doi: 10.1097/MPG.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 75.Powell J.J., Ainley C.C., Harvey R.S., Mason I.M., Kendall M.D., Sankey E.A., Dhillon A.P., Thompson R.P. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut. 1996;38:390–395. doi: 10.1136/gut.38.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heringa M.B., Peters R.J.B., Bleys R., van der Lee M.K., Tromp P.C., van Kesteren P.C.E., van Eijkeren J.C.H., Undas A.K., Oomen A.G., Bouwmeester H. Detection of titanium particles in human liver and spleen and possible health implications. Part. Fibre Toxicol. 2018;15:15. doi: 10.1186/s12989-018-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guillard A., Gaultier E., Cartier C., Devoille L., Noireaux J., Chevalier L., Morin M., Grandin F., Lacroix M.Z., Comera C., et al. Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO2 nanoparticles in an ex vivo placental perfusion model. Part. Fibre Toxicol. 2020;17:51. doi: 10.1186/s12989-020-00381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho W.S., Kang B.C., Lee J.K., Jeong J., Che J.H., Seok S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013;10:9. doi: 10.1186/1743-8977-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Da Silva A.B., Miniter M., Thom W., Hewitt R.E., Wills J., Jugdaohsingh R., Powell J.J. Gastrointestinal Absorption and Toxicity of Nanoparticles and Microparticles: Myth, Reality and Pitfalls explored through Titanium Dioxide. Curr. Opin. Toxicol. 2020;19:112–120. doi: 10.1016/j.cotox.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamas B., Martins Breyner N., Houdeau E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: Potential consequences for host health. Part. Fibre Toxicol. 2020;17:19. doi: 10.1186/s12989-020-00349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.NTP . Bioassay of Titanium Dioxide for Possible Carcinogenicity. National Cancer Institute; Bathesda, MD, USA: 1979. [PubMed] [Google Scholar]
- 82.Bartsch R., Brinkmann B., Jahnke G., Laube B., Lohmann R., Michaelsen S., Neumann I., Greim H. Human relevance of follicular thyroid tumors in rodents caused by non-genotoxic substances. Regul. Toxicol. Pharmacol. 2018;98:199–208. doi: 10.1016/j.yrtph.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 83.Warheit D.B., Brown S.C., Donner E.M. Acute and subchronic oral toxicity studies in rats with nanoscale and pigment grade titanium dioxide particles. Food Chem. Toxicol. 2015;84:208–224. doi: 10.1016/j.fct.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 84.Warheit D.B., Boatman R., Brown S.C. Developmental toxicity studies with 6 forms of titanium dioxide test materials (3 pigment-different grade & 3 nanoscale) demonstrate an absence of effects in orally-exposed rats. Regul. Toxicol. Pharmacol. 2015;73:887–896. doi: 10.1016/j.yrtph.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 85.Chen Z., Zhou D., Han S., Zhou S., Jia G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019;16:48. doi: 10.1186/s12989-019-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moradi A., Ziamajidi N., Ghafourikhosroshahi A., Abbasalipourkabir R. Effects of vitamin A and vitamin E on attenuation of titanium dioxide nanoparticles-induced toxicity in the liver of male Wistar rats. Mol. Biol. Rep. 2019;46:2919–2932. doi: 10.1007/s11033-019-04752-4. [DOI] [PubMed] [Google Scholar]
- 87.Orazizadeh M., Fakhredini F., Mansouri E., Khorsandi L. Effect of glycyrrhizic acid on titanium dioxide nanoparticles-induced hepatotoxicity in rats. Chem. Biol. Interact. 2014;220:214–221. doi: 10.1016/j.cbi.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Hu H., Li L., Guo Q., Zong H., Yan Y., Yin Y., Wang Y., Oh Y., Feng Y., Wu Q., et al. RNA sequencing analysis shows that titanium dioxide nanoparticles induce endoplasmic reticulum stress, which has a central role in mediating plasma glucose in mice. Nanotoxicology. 2018;12:341–356. doi: 10.1080/17435390.2018.1446560. [DOI] [PubMed] [Google Scholar]
- 89.Ruiz P.A., Moron B., Becker H.M., Lang S., Atrott K., Spalinger M.R., Scharl M., Wojtal K.A., Fischbeck-Terhalle A., Frey-Wagner I., et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut. 2017;66:1216–1224. doi: 10.1136/gutjnl-2015-310297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trouiller B., Reliene R., Westbrook A., Solaimani P., Schiestl R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009;69:8784–8789. doi: 10.1158/0008-5472.CAN-09-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chakrabarti S., Goyary D., Karmakar S., Chattopadhyay P. Exploration of cytotoxic and genotoxic endpoints following sub-chronic oral exposure to titanium dioxide nanoparticles. Toxicol. Ind. Health. 2019;35:577–592. doi: 10.1177/0748233719879611. [DOI] [PubMed] [Google Scholar]
- 92.Proquin H., Jetten M.J., Jonkhout M.C.M., Garduno-Balderas L.G., Briede J.J., de Kok T.M., Chirino Y.I., van Loveren H. Gene expression profiling in colon of mice exposed to food additive titanium dioxide (E171) Food Chem. Toxicol. 2018;111:153–165. doi: 10.1016/j.fct.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 93.Sycheva L.P., Zhurkov V.S., Iurchenko V.V., Daugel-Dauge N.O., Kovalenko M.A., Krivtsova E.K., Durnev A.D. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutat. Res. 2011;726:8–14. doi: 10.1016/j.mrgentox.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Jensen D.M., Lohr M., Sheykhzade M., Lykkesfeldt J., Wils R.S., Loft S., Moller P. Telomere length and genotoxicity in the lung of rats following intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Mutagenesis. 2019;34:203–214. doi: 10.1093/mutage/gez003. [DOI] [PubMed] [Google Scholar]
- 95.Hu H., Zhang B., Li L., Guo Q., Yang D., Wei X., Fan X., Liu J., Wu Q., Oh Y., et al. The toxic effects of titanium dioxide nanoparticles on plasma glucose metabolism are more severe in developing mice than in adult mice. Environ. Toxicol. 2020;35:443–456. doi: 10.1002/tox.22880. [DOI] [PubMed] [Google Scholar]
- 96.Fadoju O., Ogunsuyi O., Akanni O., Alabi O., Alimba C., Adaramoye O., Cambier S., Eswara S., Gutleb A.C., Bakare A. Evaluation of cytogenotoxicity and oxidative stress parameters in male Swiss mice co-exposed to titanium dioxide and zinc oxide nanoparticles. Environ. Toxicol. Pharmacol. 2019;70:103204. doi: 10.1016/j.etap.2019.103204. [DOI] [PubMed] [Google Scholar]
- 97.Louro H., Tavares A., Vital N., Costa P.M., Alverca E., Zwart E., de Jong W.H., Fessard V., Lavinha J., Silva M.J. Integrated approach to the in vivo genotoxic effects of a titanium dioxide nanomaterial using LacZ plasmid-based transgenic mice. Environ. Mol. Mutagen. 2014;55:500–509. doi: 10.1002/em.21864. [DOI] [PubMed] [Google Scholar]
- 98.Riedle S., Pele L.C., Otter D.E., Hewitt R.E., Singh H., Roy N.C., Powell J.J. Pro-inflammatory adjuvant properties of pigment-grade titanium dioxide particles are augmented by a genotype that potentiates interleukin 1beta processing. Part. Fibre Toxicol. 2017;14:51. doi: 10.1186/s12989-017-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Z., Zhou D., Zhou S., Jia G. Gender difference in hepatic toxicity of titanium dioxide nanoparticles after subchronic oral exposure in Sprague-Dawley rats. J. Appl. Toxicol. 2019;39:807–819. doi: 10.1002/jat.3769. [DOI] [PubMed] [Google Scholar]
- 100.Bu Q., Yan G., Deng P., Peng F., Lin H., Xu Y., Cao Z., Zhou T., Xue A., Wang Y., et al. NMR-based metabonomic study of the sub-acute toxicity of titanium dioxide nanoparticles in rats after oral administration. Nanotechnology. 2010;21:125105. doi: 10.1088/0957-4484/21/12/125105. [DOI] [PubMed] [Google Scholar]
- 101.Waller T., Chen C., Walkerm L. Food and industrial grade titanium dioxide impacts gut microbiota. Environ. Eng. Sci. 2017;34:537–550. doi: 10.1089/ees.2016.0364. [DOI] [Google Scholar]
- 102.Mao Z., Li Y., Dong T., Zhang L., Zhang Y., Li S., Hu H., Sun C., Xia Y. Exposure to Titanium Dioxide Nanoparticles During Pregnancy Changed Maternal Gut Microbiota and Increased Blood Glucose of Rat. Nanoscale Res. Lett. 2019;14:26. doi: 10.1186/s11671-018-2834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mu W., Wang Y., Huang C., Fu Y., Li J., Wang H., Jia X., Ba Q. Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019;67:9382–9389. doi: 10.1021/acs.jafc.9b02391. [DOI] [PubMed] [Google Scholar]
- 104.Pinget G., Tan J., Janac B., Kaakoush N.O., Angelatos A.S., O’Sullivan J., Koay Y.C., Sierro F., Davis J., Divakarla S.K., et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019;6:57. doi: 10.3389/fnut.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Flemer B., Lynch D.B., Brown J.M., Jeffery I.B., Ryan F.J., Claesson M.J., O’Riordain M., Shanahan F., O’Toole P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66:633–643. doi: 10.1136/gutjnl-2015-309595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saus E., Iraola-Guzman S., Willis J.R., Brunet-Vega A., Gabaldon T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol. Asp. Med. 2019;69:93–106. doi: 10.1016/j.mam.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sobhani I., Bergsten E., Couffin S., Amiot A., Nebbad B., Barau C., de’Angelis N., Rabot S., Canoui-Poitrine F., Mestivier D., et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc. Natl. Acad. Sci. USA. 2019;116:24285–24295. doi: 10.1073/pnas.1912129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grissa I., ElGhoul J., Mrimi R., Mir L.E., Cheikh H.B., Horcajada P. In deep evaluation of the neurotoxicity of orally administered TiO2 nanoparticles. Brain Res. Bull. 2020;155:119–128. doi: 10.1016/j.brainresbull.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 109.Grissa I., Guezguez S., Ezzi L., Chakroun S., Sallem A., Kerkeni E., Elghoul J., El Mir L., Mehdi M., Cheikh H.B., et al. The effect of titanium dioxide nanoparticles on neuroinflammation response in rat brain. Environ. Sci. Pollut. Res. Int. 2016;23:20205–20213. doi: 10.1007/s11356-016-7234-8. [DOI] [PubMed] [Google Scholar]
- 110.Heidari Z., Mohammadipour A., Haeri P., Ebrahimzadeh-Bideskan A. The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran. J. Basic Med. Sci. 2019;22:745–751. doi: 10.22038/ijbms.2019.33611.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jensen D.M., Christophersen D.V., Sheykhzade M., Skovsted G.F., Lykkesfeldt J., Munter R., Roursgaard M., Loft S., Moller P. Vasomotor function in rat arteries after ex vivo and intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Part. Fibre Toxicol. 2018;15:12. doi: 10.1186/s12989-018-0248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jensen D.M., Skovsted G.F., Lykkesfeldt J., Dreier R., Berg J.O., Jeppesen J.L., Sheykhzade M., Loft S., Moller P. Vasomotor dysfunction in human subcutaneous arteries exposed ex vivo to food-grade titanium dioxide. Food Chem. Toxicol. 2018;120:321–327. doi: 10.1016/j.fct.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 113.Karimi S., Khorsandi L., Nejaddehbashi F. Protective effects of Curcumin on testicular toxicity induced by titanium dioxide nanoparticles in mice. JBRA Assist. Reprod. 2019;23:344–351. doi: 10.5935/1518-0557.20190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shimizu M., Tainaka H., Oba T., Mizuo K., Umezawa M., Takeda K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part. Fibre Toxicol. 2009;6:20. doi: 10.1186/1743-8977-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shahin N.N., Mohamed M.M. Nano-Sized titanium dioxide toxicity in rat prostate and testis: Possible ameliorative effect of morin. Toxicol. Appl. Pharmacol. 2017;334:129–141. doi: 10.1016/j.taap.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 116.Wang J., Zhu X., Zhang X., Zhao Z., Liu H., George R., Wilson-Rawls J., Chang Y., Chen Y. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO(2) nanoparticles. Chemosphere. 2011;83:461–467. doi: 10.1016/j.chemosphere.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 117.Ma H., Lenz K.A., Gao X., Li S., Wallis L.K. Comparative toxicity of a food additive TiO2, a bulk TiO2, and a nano-sized P25 to a model organism the nematode C. elegans. Environ. Sci. Pollut. Res. Int. 2019;26:3556–3568. doi: 10.1007/s11356-018-3810-4. [DOI] [PubMed] [Google Scholar]
- 118.Jovanovic B., Cvetkovic V.J., Mitrovic T. Effects of human food grade titanium dioxide nanoparticle dietary exposure on Drosophila melanogaster survival, fecundity, pupation and expression of antioxidant genes. Chemosphere. 2016;144:43–49. doi: 10.1016/j.chemosphere.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 119.Jovanovic B., Jovanovic N., Cvetkovic V.J., Matic S., Stanic S., Whitley E.M., Mitrovic T.L. The effects of a human food additive, titanium dioxide nanoparticles E171, on Drosophila melanogaster—A 20 generation dietary exposure experiment. Sci. Rep. 2018;8:17922. doi: 10.1038/s41598-018-36174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim D.H., Kundu J., Chae I.G., Lee J.K., Heo J.S., Chun K.S. Titanium dioxide nanoparticles induce COX-2 expression through ROS generation in human periodontal ligament cells. J. Toxicol. Sci. 2019;44:335–345. doi: 10.2131/jts.44.335. [DOI] [PubMed] [Google Scholar]
- 121.Gerloff K., Fenoglio I., Carella E., Kolling J., Albrecht C., Boots A.W., Forster I., Schins R.P. Distinctive toxicity of TiO2 rutile/anatase mixed phase nanoparticles on Caco-2 cells. Chem Res. Toxicol. 2012;25:646–655. doi: 10.1021/tx200334k. [DOI] [PubMed] [Google Scholar]
- 122.Dorier M., Beal D., Marie-Desvergne C., Dubosson M., Barreau F., Houdeau E., Herlin-Boime N., Carriere M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology. 2017;11:751–761. doi: 10.1080/17435390.2017.1349203. [DOI] [PubMed] [Google Scholar]
- 123.Dorier M., Brun E., Veronesi G., Barreau F., Pernet-Gallay K., Desvergne C., Rabilloud T., Carapito C., Herlin-Boime N., Carriere M. Impact of anatase and rutile titanium dioxide nanoparticles on uptake carriers and efflux pumps in Caco-2 gut epithelial cells. Nanoscale. 2015;7:7352–7360. doi: 10.1039/C5NR00505A. [DOI] [PubMed] [Google Scholar]
- 124.Dorier M., Tisseyre C., Dussert F., Beal D., Arnal M.E., Douki T., Valdiglesias V., Laffon B., Fraga S., Brandao F., et al. Toxicological impact of acute exposure to E171 food additive and TiO2 nanoparticles on a co-culture of Caco-2 and HT29-MTX intestinal cells. Mutat. Res. 2019;845:402980. doi: 10.1016/j.mrgentox.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Cao X., DeLoid G.M., Bitounis D., De La Torre-Roche R., White J.C., Zhang Z., Ho C.G., Ng K.W., Eitzer B.D., Demokritou P. Co-exposure to the food additives SiO2 (E551) or TiO2 (E171) and the pesticide boscalid increases cytotoxicity and bioavailability of the pesticide in a tri-culture small intestinal epithelium model: Potential health implications. Environ. Sci. Nano. 2019;6:2786–2800. doi: 10.1039/C9EN00676A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li W., Jia M.X., Deng J., Wang J.H., Zuberi Z., Yang S., Ba J., Chen Z. MicroRNA Response and toxicity of potential pathways in human colon cancer cells exposed to titanium dioxide nanoparticles. Cancers. 2020;12:1236. doi: 10.3390/cancers12051236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ghosh M., Chakraborty A., Mukherjee A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J. Appl. Toxicol. 2013;33:1097–1110. doi: 10.1002/jat.2863. [DOI] [PubMed] [Google Scholar]
- 128.Dai X., Liu R., Li N., Yi J. Titanium dioxide nanoparticles induce in vitro autophagy. Hum. Exp. Toxicol. 2019;38:56–64. doi: 10.1177/0960327118777849. [DOI] [PubMed] [Google Scholar]
- 129.Shukla R.K., Kumar A., Gurbani D., Pandey A.K., Singh S., Dhawan A. TiO(2) nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology. 2013;7:48–60. doi: 10.3109/17435390.2011.629747. [DOI] [PubMed] [Google Scholar]
- 130.Shi Z., Niu Y., Wang Q., Shi L., Guo H., Liu Y., Zhu Y., Liu S., Liu C., Chen X., et al. Reduction of DNA damage induced by titanium dioxide nanoparticles through Nrf2 in vitro and in vivo. J. Hazard. Mater. 2015;298:310–319. doi: 10.1016/j.jhazmat.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 131.Brzicova T., Sikorova J., Milcova A., Vrbova K., Klema J., Pikal P., Lubovska Z., Philimonenko V., Franco F., Topinka J., et al. Nano-TiO2 stability in medium and size as important factors of toxicity in macrophage-like cells. Toxicol. In Vitro. 2019;54:178–188. doi: 10.1016/j.tiv.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 132.Popp L., Tran V., Patel R., Segatori L. Autophagic response to cellular exposure to titanium dioxide nanoparticles. Acta Biomater. 2018;79:354–363. doi: 10.1016/j.actbio.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 133.Botelho M.C., Costa C., Silva S., Costa S., Dhawan A., Oliveira P.A., Teixeira J.P. Effects of titanium dioxide nanoparticles in human gastric epithelial cells in vitro. Biomed. Pharmacother. 2014;68:59–64. doi: 10.1016/j.biopha.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 134.An H., Ling C., Xu M., Hu M., Wang H., Liu J., Song G., Liu J. Oxidative damage induced by nano-titanium dioxide in rats and mice: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2020;194:184–202. doi: 10.1007/s12011-019-01761-z. [DOI] [PubMed] [Google Scholar]
- 135.Canli E.G., Atli G., Canli M. Response of the antioxidant enzymes of the erythrocyte and alterations in the serum biomarkers in rats following oral administration of nanoparticles. Environ. Toxicol. Pharmacol. 2017;50:145–150. doi: 10.1016/j.etap.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 136.Hassanein K.M., El-Amir Y.O. Protective effects of thymoquinone and avenanthramides on titanium dioxide nanoparticles induced toxicity in Sprague-Dawley rats. Pathol. Res. Pract. 2017;213:13–22. doi: 10.1016/j.prp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 137.Hassanein K.M.A., El-Amir Y.O. Ameliorative effects of thymoquinone on titanium dioxide nanoparticles induced acute toxicity in rats. Int. J. Vet. Sci. Med. 2018;6:16–21. doi: 10.1016/j.ijvsm.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pelclova D., Zdimal V., Kacer P., Komarc M., Fenclova Z., Vlckova S., Zikova N., Schwarz J., Makes O., Navratil T., et al. Markers of lipid oxidative damage among office workers exposed intermittently to air pollutants including nanoTiO2 particles. Rev. Environ. Health. 2017;32:193–200. doi: 10.1515/reveh-2016-0030. [DOI] [PubMed] [Google Scholar]
- 139.Charles S., Jomini S., Fessard V., Bigorgne-Vizade E., Rousselle C., Michel C. Assessment of the in vitro genotoxicity of TiO2 nanoparticles in a regulatory context. Nanotoxicology. 2018;12:357–374. doi: 10.1080/17435390.2018.1451567. [DOI] [PubMed] [Google Scholar]
- 140.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.He Y., Hara H., Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Winter M., Beer H.D., Hornung V., Kramer U., Schins R.P., Forster I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011;5:326–340. doi: 10.3109/17435390.2010.506957. [DOI] [PubMed] [Google Scholar]
- 143.Baron L., Gombault A., Fanny M., Villeret B., Savigny F., Guillou N., Panek C., Le Bert M., Lagente V., Rassendren F., et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015;6:e1629. doi: 10.1038/cddis.2014.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yu L.C. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. J. Biomed. Sci. 2018;25:79. doi: 10.1186/s12929-018-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Karimipour M., Zirak Javanmard M., Ahmadi A., Jafari A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int. J. Reprod. Biomed. 2018;16:397–404. doi: 10.29252/ijrm.16.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jin C., Tang Y., Fan X.Y., Ye X.T., Li X.L., Tang K., Zhang Y.F., Li A.G., Yang Y.J. In vivo evaluation of the interaction between titanium dioxide nanoparticle and rat liver DNA. Toxicol. Ind. Health. 2013;29:235–244. doi: 10.1177/0748233713479898. [DOI] [PubMed] [Google Scholar]
- 147.Latif M.A., Jabeen F., Ali M., Rasul A., Naz S., Akram M. Neurotoxic effects of titanium dioxide nanoparticles on the brain of male sprague dawley rats. Pak. J. Pharm. Sci. 2019;32:2311–2316. [PubMed] [Google Scholar]
- 148.Kazimirova A., Baranokova M., Staruchova M., Drlickova M., Volkovova K., Dusinska M. Titanium dioxide nanoparticles tested for genotoxicity with the comet and micronucleus assays in vitro, ex vivo and in vivo. Mutat. Res. 2019;843:57–65. doi: 10.1016/j.mrgentox.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 149.Kim H., Jeon D., Oh S., Nam K., Son S., Gye M.C., Shin I. Titanium dioxide nanoparticles induce apoptosis by interfering with EGFR signaling in human breast cancer cells. Environ. Res. 2019;175:117–123. doi: 10.1016/j.envres.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 150.Montalvo-Quiros S., Luque-Garcia J.L. Combination of bioanalytical approaches and quantitative proteomics for the elucidation of the toxicity mechanisms associated to TiO2 nanoparticles exposure in human keratinocytes. Food Chem. Toxicol. 2019;127:197–205. doi: 10.1016/j.fct.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 151.Du X., Gao S., Hong L., Zheng X., Zhou Q., Wu J. Genotoxicity evaluation of titanium dioxide nanoparticles using the mouse lymphoma assay and the Ames test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019;838:22–27. doi: 10.1016/j.mrgentox.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 152.Freyre-Fonseca V., Tellez-Medina D.I., Medina-Reyes E.I., Cornejo-Mazon M., Lopez-Villegas E.O., Alamilla-Beltran L., Ocotlan-Flores J., Chirino Y.I., Gutierrez-Lopez G.F. Morphological and physicochemical characterization of agglomerates of titanium dioxide nanoparticles in cell culture media. J. Nanomater. 2016;2016:5937932. doi: 10.1155/2016/5937932. [DOI] [Google Scholar]
- 153.Kobayashi K., Kubota H., Hojo R., Miyagawa M. Effective dispersal of titanium dioxide nanoparticles for toxicity testing. J. Toxicol. Sci. 2019;44:515–521. doi: 10.2131/jts.44.515. [DOI] [PubMed] [Google Scholar]
- 154.JRC Titanium dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and physico-chemical properties. JRC. 2014 doi: 10.2788/79554. [DOI] [Google Scholar]
- 155.Action N.J. Facilitating the Safety Evaluation of Manufactured Nanomaterials by Characterising Their Potential Genotoxic Hazard. French Agency for Food, Environmental and Occupational Health & Safety (ANSES); Maisons-Alfort, France: 2013. NANOGENOTOX Final Report. [Google Scholar]
- 156.Jensen K., Kembouche Y., Christiansen E., Jacobsen N., Wallin H., Guiot C., Spalla O., Witschger O. The Generic NANOGENOTOX Dispersion Protocol—Standard Operation Procedure (SOP) Volume 3 French Agency for Food, Environmental and Occupational Health & Safety (ANSES); Maisons-Alfort, France: 2011. NANOGENOTOX Deliverable Report. [Google Scholar]
- 157.Donovan A.R., Adams C.D., Ma Y., Stephan C., Eichholz T., Shi H. Single particle ICP-MS characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment. Chemosphere. 2016;144:148–153. doi: 10.1016/j.chemosphere.2015.07.081. [DOI] [PubMed] [Google Scholar]
- 158.Brodkorb A., Egger L., Alminger M., Alvito P., Assuncao R., Ballance S., Bohn T., Bourlieu-Lacanal C., Boutrou R., Carriere F., et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019;14:991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- 159.Abdelkhaliq A., van der Zande M., Undas A.K., Peters R.J.B., Bouwmeester H. Impact of in vitro digestion on gastrointestinal fate and uptake of silver nanoparticles with different surface modifications. Nanotoxicology. 2020;14:111–126. doi: 10.1080/17435390.2019.1675794. [DOI] [PubMed] [Google Scholar]
- 160.Sohal I.S., Cho Y.K., O’Fallon K.S., Gaines P., Demokritou P., Bello D. Dissolution Behavior and Biodurability of Ingested Engineered Nanomaterials in the Gastrointestinal Environment. ACS Nano. 2018;12:8115–8128. doi: 10.1021/acsnano.8b02978. [DOI] [PubMed] [Google Scholar]
- 161.Sohal I.S., O’Fallon K.S., Gaines P., Demokritou P., Bello D. Ingested engineered nanomaterials: State of science in nanotoxicity testing and future research needs. Part. Fibre Toxicol. 2018;15:29. doi: 10.1186/s12989-018-0265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bettencourt A., Goncalves L.M., Gramacho A.C., Vieira A., Rolo D., Martins C., Assuncao R., Alvito P., Silva M.J., Louro H. Analysis of the characteristics and cytotoxicity of titanium dioxide nanomaterials following simulated In Vitro digestion. Nanomaterials. 2020;10:1516. doi: 10.3390/nano10081516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Terrisse H.M.-B.M. Titanium Dioxide as Food Additive. INTECH; London, UK: 2017. [Google Scholar]
- 164.Hwang J.S., Yu J., Kim H.M., Oh J.M., Choi S.J. Food additive titanium dioxide and its fate in commercial foods. Nanomaterials. 2019;9:1175. doi: 10.3390/nano9081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Noben M., Vanhove W., Arnauts K., Santo Ramalho A., Van Assche G., Vermeire S., Verfaillie C., Ferrante M. Human intestinal epithelium in a dish: Current models for research into gastrointestinal pathophysiology. United Eur. Gastroenterol. J. 2017;5:1073–1081. doi: 10.1177/2050640617722903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Augustyniak J., Bertero A., Coccini T., Baderna D., Buzanska L., Caloni F. Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol. 2019;39:1610–1622. doi: 10.1002/jat.3815. [DOI] [PubMed] [Google Scholar]
- 167.Kijanska M., Kelm J. In vitro 3D spheroids and microtissues: ATP-based cell viability and toxicity assays. In: Sittampalam G.S., Grossman A., Brimacombe K., Arkin M., Auld D., Austin C.P., Baell J., Bejcek B., Caaveiro J.M.M., Chung T.D.Y., et al., editors. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing; Bethesda, MD, USA: 2004. [PubMed] [Google Scholar]
- 168.Michels B.E., Mosa M.H., Grebbin B.M., Yepes D., Darvishi T., Hausmann J., Urlaub H., Zeuzem S., Kvasnicka H.M., Oellerich T., et al. Human colon organoids reveal distinct physiologic and oncogenic Wnt responses. J. Exp. Med. 2019;216:704–720. doi: 10.1084/jem.20180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Onozato D., Yamashita M., Nakanishi A., Akagawa T., Kida Y., Ogawa I., Hashita T., Iwao T., Matsunaga T. Generation of intestinal organoids suitable for pharmacokinetic studies from human induced pluripotent stem cells. Drug Metab. Dispos. 2018;46:1572–1580. doi: 10.1124/dmd.118.080374. [DOI] [PubMed] [Google Scholar]
- 170.Dinse G.E., Peddada S.D., Harris S.F., Elmore S.A. Comparison of NTP historical control tumor incidence rates in female Harlan Sprague Dawley and Fischer 344/N Rats. Toxicol. Pathol. 2010;38:765–775. doi: 10.1177/0192623310373777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Swindle M.M., Makin A., Herron A.J., Clubb F.J., Jr., Frazier K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012;49:344–356. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 172.Bode G., Clausing P., Gervais F., Loegsted J., Luft J., Nogues V., Sims J., Steering Group of the RETHINK Project The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Methods. 2010;62:196–220. doi: 10.1016/j.vascn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 173.Henze L.J., Koehl N.J., O’Shea J.P., Kostewicz E.S., Holm R., Griffin B.T. The pig as a preclinical model for predicting oral bioavailability and in vivo performance of pharmaceutical oral dosage forms: A PEARRL review. J. Pharm. Pharmacol. 2019;71:581–602. doi: 10.1111/jphp.12912. [DOI] [PubMed] [Google Scholar]