Abstract
Background
Conversion from minimally invasive to open colorectal surgery remains common and costly. Robotic colorectal surgery is associated with lower rates of conversion than laparoscopy, but institutions and payers remain concerned about equipment and implementation costs. Recognizing that reimbursement reform and bundled payments expand perspectives on cost to include the entire surgical episode, we evaluated the role of minimally invasive conversion in total payments.
Methods
This is an observational study from a linked data registry including clinical data from the Michigan Surgical Quality Collaborative and payment data from the Michigan Value Collaborative between July 2012 and April 2015. We evaluated colorectal resections initiated with open and minimally invasive approaches, and compared reported risk-adjusted and price-standardized 30-day episode payments and their components.
Results
We identified 1061 open, 1604 laparoscopic, and 275 robotic colorectal resections. Adjusted episode payments were significantly higher for open operations than for minimally invasive procedures completed without conversion ($19,489 vs. $15,518, p<0.001). The conversion rate was significantly higher with laparoscopic than robotic operations (15.1 vs. 7.6%, p<0.001). Adjusted episode payments for minimally invasive operations converted to open were significantly higher than for those completed by minimally invasive approaches ($18,098 vs. $15,518, p<0.001). Payments for operations completed robotically were greater than those completed laparoscopically ($16,949 vs. $15,250, p<0.001), but the difference was substantially decreased when conversion to open cases was included ($16,939 vs. $15,699, p = 0.041).
Conclusion
Episode payments for open colorectal surgery exceed both laparoscopic and robotic minimally invasive options. Conversion to open surgery significantly increases the payments associated with minimally invasive colorectal surgery. Because conversion rates in robotic colorectal operations are half of those in laparoscopy, the excess expenditures attributable to robotics are attenuated by consideration of the cost of conversions.
Keywords: Colorectal, Minimally invasive, Cost, Robotic, Laparoscopic
The growth of minimally invasive colorectal surgery was accompanied by concern about the costs of instrumentation. Over time, numerous studies demonstrated significant cost savings attributable to laparoscopic colectomy when compared to the traditional open approach that served to alleviate some of these concerns [1–5]. As the uptake of robotic colorectal operations has increased significantly since 2008, the even greater institutional costs of robotics have precluded wider implementation of this minimally invasive platform in some hospitals [6–11].
However, previous analyses of cost considerations around operative options in colorectal surgery have been limited in two key ways. First, because most studies use administrative procedure codes to classify operations, minimally invasive surgery (MIS) that is converted to open has been misclassified as open, which imparts bias against the open approach [11–14]. Converted operations have longer hospital length of stay and incur more frequent complications when compared to cases that are not converted [15–17]. We have previously observed significantly lower rates of conversion with robotic colorectal operations when compared with the laparoscopic approach, suggesting that administrative-based classifications may not account for cost differences attributable to conversion [18, 19]. Second, most economic studies have evaluated only inpatient in-hospital costs or charges, which do not accurately represent the realized costs of care from the perspective of the payer and society, who are interested in total payments for entire episodes around surgery [11, 12, 14].
Recognizing that the consideration of converted operations may alter the cost comparison between operative approaches to colorectal resections and that the open approach to colorectal surgery is still the most common option utilized, we sought to evaluate overall episode payments for open, laparoscopic, and robotic colorectal surgery. We used a unique, population-based, linked database from two statewide registries, one with rich clinical data, including the occurrence of conversions, and one with detailed price-standardized payments for all services in the surgical episode.
Methods
Data sources
This study is an analysis of colorectal resection cases in a linked data registry that includes clinical metrics and outcomes from the Michigan Surgical Quality Collaborative (MSQC, http://www.msqc.org) and adjudicated complete claims payments from the Michigan Value Collaborative (MVC, http://michiganvalue.org). MSQC is a statewide multicenter collaborative composed of 72 hospitals that maintains a protocol-driven and validated clinical data registry. In the MSQC, trained nurse reviewers conduct detailed standardized medical record abstraction. Operative approach data for colorectal operations include identification of converted cases through detailed surveillance of the operative report dictated by the surgeon. MVC is a statewide 75-hospital consortium whose claims-based registry contains complete episode payment data for Blue Cross Blue Shield of Michigan (BCBSM) Preferred Provider Organization patients and Medicare fee-for-service beneficiaries over age 65. MVC payments are price-standardized, using algorithms developed by researchers for the Dartmouth Atlas of Healthcare to account for intended differences in payments to hospitals associated with, for example, regional wage variation, graduate medical education, and disproportionate share of uncompensated care [20]. All payments are presented in price-standardized, inflation-adjusted dollars according to the 2012 Medicare payment schedule.
For index hospitalizations, we included DRG and outlier payments, when present. We included all hospital payments for readmissions initiated within 90 days of discharge after the index procedure, even when hospital stays extended beyond that time window. To conform with emerging bundled payment programs, we pro-rated payments to home health care and rehabilitation hospitals to the 90-day window. Payments to skilled nursing facilities were based on per diem payments in the 90-day period.
Study sample
For this study, MSQC clinical data were linked to MVC claims data by a patient-level, deterministic match on facility ID, admittance date, discharge date, year of birth, and gender. Surgeon NPI was used to validate a successful linkage on over 95% of matched cases. Case inclusion criteria were patients age ≥18 years undergoing elective colorectal resections that were initiated with an open or a minimally invasive laparoscopic or robotic approach between July 2012 and April 2015. We identified candidates for inclusion based on Current Procedural Terminology (CPT) codes 44140, 44160, 44145, 44146, 45110, 45540, 45550, 44204, 44205, 44207, 44208, 45395, 45400, or 45402. Cases that converted to open were determined through medical record abstraction by trained and certified abstractors. Minimally invasive surgical approaches were categorized in Tables as “Completed” if there was no conversion and “All” to include both completed and converted cases. We excluded patients with American Society of Anesthesiologists’ (ASA) physical status classification of 5 or 6.
Surgical approach
Surgical approach cohorts were defined to distinguish between initial intended approach and completion of the operation. Cases for which the initial approach was open are classified as ‘Open.’ The MSQC database has strict definitions for conversion, including an initial attempt to perform the operation in a minimally invasive approach, followed by a change in approach during the operation. The timing of conversion within the operation is not recorded. Laparoscopic and robotic cases that converted to open were classified as ‘MIS-Converted’; while those that did not require conversion were classified as ‘MIS-Completed.’ The ‘MIS-All’ group consisted of ‘MIS-Converted’ and ‘MIS-Completed’ combined.
Outcome and explanatory variables
Outcome variables of interest included risk-adjusted, price-standardized 30-day payments for the overall episode, index hospitalization, post-discharge, readmission, and physician services. Explanatory variables included demographics (age, sex, race), general health factors (BMI, smoker, alcohol, functional status, ASA class), comorbidities (diabetes, ventilator, hypertension, CHF, PVD, ascites, cancer, chronic steroid use, body weight loss, and bleeding disorder), ostomy, and postoperative diagnosis.
Statistical analysis
Unadjusted differences in patient characteristics, operative factors, and utilization were compared for selected pairwise combinations of surgical approach using the Pearson’s Chisquare test or the Fisher’s exact test.
Payment differences between surgical approaches were compared using adjusted means estimated by generalized linear models. The full set of explanatory variables was modeled to adjust for case mix. Clustering on provider was not modeled due to sample size limitations. Payment data showed a positively skewed distribution and were log transformed before modeling for unbiased estimation of adjustment means. Model estimates were obtained using a likelihood-based approach in SAS PROC GLIMMIX. Statistical significance for pairwise adjusted mean differences was reported using p values for T tests. All statistical analyses were performed using SAS software (SAS version 9.4; SAS Institute, Cary, NC).
Results
Patients and procedures
We identified 2940 colorectal resections meeting inclusion criteria, including 1061 open, 1604 laparoscopic, and 275 robotic operations. Patient characteristics, care processes, postoperative occurrences, and utilization with statistical significance for unadjusted associations with surgical approach cohorts are detailed in Table 1. Cases in the ‘Open’ cohort were older, more frequently on Medicare, of black race, had poorer health status, had more postoperative occurrences, and had more readmissions, Emergency Department (ED) visits, and extended hospital length of stay (LOS) than were cases in the ‘MIS-All’ cohort. In the ‘MIS-Completed’ cohort, there were few statistically significant differences between the laparoscopic and robotic cases. Among completed MIS operations, patients having laparoscopic operations, as compared to robotic operations, were more likely to be white, have an ASA class of 3–4, chronic steroid use, procedure duration <100 min, or inflammatory bowel disease, but did not show statistically significant differences for postoperative occurrences, readmissions, ED visits, extended hospital LOS, or reoperations. Comparing laparoscopic versus robotic among all MIS operations showed that the laparoscopic cases were more likely to have an ASA class of 3–4, chronic steroid use, and an operative duration <100 min; they were less likely to have a postoperative diagnosis of diverticular disease or fistula, but more likely to have inflammatory bowel disease. Finally, in this ‘MIS-All’ cohort, laparoscopic cases were more likely to have experienced an extended LOS as compared to robotic cases.
Table 1.
Patient characteristics, care processes, postop-occurrences, and utilization by surgical approach cohorts
| Open versus MIS-A11 | MIS-Completed | MIS-A11 | MIS-Converted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic, n (%) | Open | MIS-All | p value | Laparoscopic | Robotic | p value | Laparoscopic | Robotic | p value | Laparoscopic | Robotic | p value |
| Cases, n | 1061 | 1879 | 1361 | 254 | 1604 | 275 | 243 | 21 | ||||
| Demographics | ||||||||||||
| Insurance: medicare | 503 (47.4) | 583 (31.0) | <0.001 | 413 (30.3) | 68 (26.8) | 0.253 | 508 (31.7) | 75 (27.3) | 0.145 | 95 (39.1) | 7 (33.3) | 0.774 |
| Insurance: blue cross blue shield michigan | 558 (52.6) | 1296 (69.0) | <0.001 | 948 (69.7) | 186 (73.2) | 0.253 | 1096 (68.3) | 200 (72.7) | 0.145 | 148 (60.9) | 14 (66.7) | 0.774 |
| Age: <45 | 119 (11.2) | 221 (11.8) | 0.657 | 165 (12.1) | 32 (12.6) | 0.832 | 189 (11.8) | 32 (11.6) | 0.944 | 24 (9.9) | 0 (0.0) | 0.233 |
| Age: 45–64 | 501 (47.2) | 1142 (60.8) | <0.001 | 831 (61.1) | 159 (62.6) | 0.644 | 969 (60.4) | 173 (62.9) | 0.433 | 138 (56.8) | 14 (66.7) | 0.517 |
| Age: 65+ | 441 (41.6) | 516 (27.5) | <0.001 | 365 (26.8) | 63 (24.8) | 0.504 | 446 (27.8) | 70 (25.5) | 0.42 | 81 (33.3) | 7 (33.3) | 1.000 |
| Gender: male | 456 (43.0) | 837 (44.5) | 0.411 | 598 (43.9) | 109 (42.9) | 0.763 | 723 (45.1) | 114 (41.5) | 0.264 | 125 (51.4) | 5 (23.8) | 0.028 |
| Race: white | 911 (85.9) | 1632 (86.9) | 0.45 | 1205 (88.5) | 210 (82.7) | 0.009 | 1401 (87.3) | 231 (84.0) | 0.129 | 196 (80.7) | 21 (100.0) | 0.032 |
| Race: black | 111 (10.5) | 133 (7.1) | 0.001 | 82 (6.0) | 22 (8.7) | 0.116 | 111 (6.9) | 22 (8.0) | 0.519 | 29 (11.9) | 0 (0.0) | 0.143 |
| Race: other | 39 (3.7) | 114 (6.1) | 0.005 | 74 (5.4) | 22 (8.7) | 0.046 | 92 (5.7) | 22 (8.0) | 0.146 | 18 (7.4) | 0 (0.0) | 0.376 |
| General health factors | ||||||||||||
| Obese | 383 (36.1) | 728 (38.7) | 0.155 | 500 (36.7) | 101 (39.8) | 0.36 | 621 (38.7) | 107 (38.9) | 0.952 | 121 (49.8) | 6 (28.6) | 0.101 |
| Tobacco use | 204 (19.2) | 374 (19.9) | 0.657 | 267 (19.6) | 47 (18.5) | 0.681 | 323 (20.1) | 51 (18.5) | 0.541 | 56 (23.0) | 4 (19.0) | 0.792 |
| Alcohol use | 27 (2.5) | 56 (3.0) | 0.494 | 35 (2.6) | 8 (3.1) | 0.599 | 46 (2.9) | 10 (3.6) | 0.489 | 11 (4.5) | 2 (9.5) | 0.277 |
| Functional status not independent | 54 (5.1) | 31 (1.6) | <0.001 | 24 (1.8) | 3 (1.2) | 0.789 | 27 (1.7) | 4 (1.5) | 0.999 | 3 (1.2) | 1 (4.8) | 0.284 |
| ASA class: 3–4 | 615 (58.0) | 739 (39.3) | <0.001 | 532 (39.1) | 80 (31.5) | 0.022 | 649 (40.5) | 90 (32.7) | 0.015 | 117 (48.1) | 10 (47.6) | 1.000 |
| Comorbidities | ||||||||||||
| Diabetes | 198 (18.7) | 279 (14.8) | 0.007 | 187 (13.7) | 35 (13.8) | 0.987 | 239 (14.9) | 40 (14.5) | 0.879 | 52 (21.4) | 5 (23.8) | 1.000 |
| Ventilator | 4 (0.4) | 1 (0.1) | 0.06 | 0 (0.0) | 0 (0.0) | 0.789 | 1 (0.1) | 0 (0.0) | 0.999 | 1 (0.4) | 0 (0.0) | 1.000 |
| Hypertension | 603 (56.8) | 901 (48.0) | <0.001 | 632 (46.4) | 126 (49.6) | 0.353 | 767 (47.8) | 134 (48.7) | 0.78 | 135 (55.6) | 8 (38.1) | 0.189 |
| Congestive heart failure | 8 (0.8) | 7 (0.4) | 0.163 | 6 (0.4) | 0 (0.0) | 0.598 | 7 (0.4) | 0 (0.0) | 0.603 | 1 (0.4) | 0 (0.0) | 1.000 |
| Peripheral vascular disease | 41 (3.9) | 19 (1.0) | <0.001 | 13 (1.0) | 5 (2.0) | 0.184 | 14 (0.9) | 5 (1.8) | 0.181 | 1 (0.4) | 0 (0.0) | 1.000 |
| Cancer | 26 (2.5) | 15 (0.8) | 0.002 | 11 (0.8) | 0 (0.0) | 0.231 | 15 (0.9) | 0 (0.0) | 0.148 | 4 (1.6) | 0 (0.0) | 1.000 |
| Chronic steroid use | 61 (5.7) | 105 (5.6) | 0.856 | 82 (6.0) | 5 (2.0) | 0.006 | 98 (6.1) | 7 (2.5) | 0.015 | 16 (6.6) | 2 (9.5) | 0.643 |
| Loss of >10% body weight | 41 (3.9) | 29 (1.5) | <0.001 | 19 (1.4) | 7 (2.8) | 0.114 | 22 (1.4) | 7 (2.5) | 0.145 | 3 (1.2) | 0 (0.0) | 1.000 |
| Bleeding disorder | 53 (5.0) | 38 (2.0) | <0.001 | 26 (1.9) | 2 (0.8) | 0.296 | 34 (2.1) | 4 (1.5) | 0.643 | 8 (3.3) | 2 (9.5) | 0.184 |
| Care processes | ||||||||||||
| Ostomy | 14 (1.3) | 7 (0.4) | 0.003 | 6 (0.4) | 1 (0.4) | 0.999 | 6 (0.4) | 1 (0.4) | 0.999 | 0 (0.0) | 0 (0.0) | 1.000 |
| Surgical site: pelvis | 323 (30.4) | 471 (25.1) | 0.002 | 319 (23.4) | 71 (28.0) | 0.123 | 396 (24.7) | 75 (27.3) | 0.361 | 77 (31.7) | 4 (19.0) | 0.325 |
| Procedure duration <100 min | 355 (33.5) | 343 (18.3) | <0.001 | 285 (20.9) | 22 (8.7) | <0.001 | 321 (20.0) | 22 (8.0) | <.001 | 36 (14.8) | 0 (0.0) | 0.089 |
| Estimated blood loss <300 mL | 862 (81.2) | 1764 (93.9) | <0.001 | 1316 (96.7) | 243 (95.7) | 0.413 | 1503 (93.7) | 261 (94.9) | 0.441 | 187 (77.0) | 18 (85.7) | 0.428 |
| Postoperative ICD9 diagnoses | ||||||||||||
| Colorectal cancer | 358 (33.7) | 565 (30.1) | 0.039 | 417 (30.6) | 79 (31.1) | 0.883 | 481 (30.0) | 84 (30.5) | 0.852 | 64 (26.3) | 5 (23.8) | 1.000 |
| Colorectal adenomas/polyps | 101 (9.5) | 263 (14.0) | <0.001 | 193 (14.2) | 33 (13.0) | 0.616 | 229 (14.3) | 34 (12.4) | 0.398 | 36 (14.8) | 1 (4.8) | 0.327 |
| Other neoplasms | 39 (3.7) | 57 (3.0) | 0.347 | 42 (3.1) | 5 (2.0) | 0.419 | 52 (3.2) | 5 (1.8) | 0.255 | 10 (4.1) | 0 (0.0) | 1.000 |
| Diverticular Dz and fistulas | 349 (32.9) | 821 (43.7) | <0.001 | 591 (43.4) | 127 (50.0) | 0.053 | 685 (42.7) | 136 (49.5) | 0.037 | 94 (38.7) | 9 (42.9) | 0.886 |
| Inflammatory bowel Dz | 45 (4.2) | 78 (4.2) | 0.907 | 60 (4.4) | 2 (0.8) | 0.004 | 74 (4.6) | 4 (1.5) | 0.013 | 14 (5.8) | 2 (9.5) | 0.370 |
| Other | 169 (15.9) | 95 (5.1) | <0.001 | 58 (4.3) | 8 (3.1) | 0.412 | 83 (5.2) | 12 (4.4) | 0.571 | 25 (10.3) | 4 (19.0) | 0.264 |
| Postoperative occurrences | ||||||||||||
| Mortality | 20 (1.9) | 9 (0.5) | 0.0002 | 7 (0.5) | 2 (0.8) | 0.639 | 7 (0.4) | 2 (0.7) | 0.628 | 0 (0.0) | 0 (0.0) | 1.000 |
| Any morbidity | 229 (21.6) | 213 (11.3) | <0.001 | 130 (9.6) | 27 (10.6) | 0.594 | 180 (11.2) | 33 (12.0) | 0.707 | 50 (20.6) | 6 (28.6) | 0.561 |
| SSI-total | 104 (9.8) | 103 (5.5) | <0.001 | 58 (4.3) | 8 (3.1) | 0.411 | 93 (5.8) | 10 (3.6) | 0.146 | 35 (14.4) | 2 (9.5) | 0.748 |
| Utilization | ||||||||||||
| Readmission | 116 (10.9) | 151 (8.0) | 0.009 | 92 (6.8) | 25 (9.8) | 0.082 | 121 (7.5) | 30 (10.9) | 0.058 | 29 (11.9) | 5 (23.8) | 0.223 |
| ED visit | 110 (10.4) | 115 (6.1) | <0.001 | 68 (5.0) | 18 (7.1) | 0.173 | 95 (5.9) | 20 (7.3) | 0.388 | 27 (11.1) | 2 (9.5) | 1.000 |
| Extended LOS | 94 (8.9) | 83 (4.4) | <0.001 | 51 (3.7) | 4 (1.6) | 0.09 | 79 (4.9) | 4 (1.5) | 0.007 | 28 (11.5) | 0 (0.0) | 0.142 |
| Reoperation | 54 (5.1) | 87 (4.6) | 0.576 | 53 (3.9) | 12 (4.7) | 0.537 | 70 (4.4) | 17 (6.2) | 0.185 | 17 (7.0) | 5 (23.8) | 0.024 |
Conversion to open surgery occurred in 15.1% of operations initiated with a laparoscopic approach (243/1361), compared with 7.6% of those initiated robotically (21/254, p<0.001). Converted operations, as compared to completed, were significantly more likely to result in extended LOS (10.6%[28/264] vs. 3.4%[55/1560], p<0.001), readmission (12.9%[34/264] vs. 7.2%[117/1615], p = 0.002), ED visit (11.0%[29/264] vs. 5.3%[86/1615], p<0.001), or reoperation (8.3%[22/264] vs. 4.0%[65/1615], p = 0.003).
Operative approach and total episode payments
Figure 1 shows adjusted total episode payments by surgical approach. Compared with open operations, those completed with MIS incurred significantly lower total payments ($15,518 vs. $19,489, p<0.001). Payments for converted operations were significantly greater than for those completed with MIS ($18,098 vs. $15,518, p<0.001). Even including converted cases among the MIS group, however, operations initiated with MIS still had significantly lower average total episode spending when compared to open operations ($15,880 vs. $19,489, p<0.001).
Fig. 1.
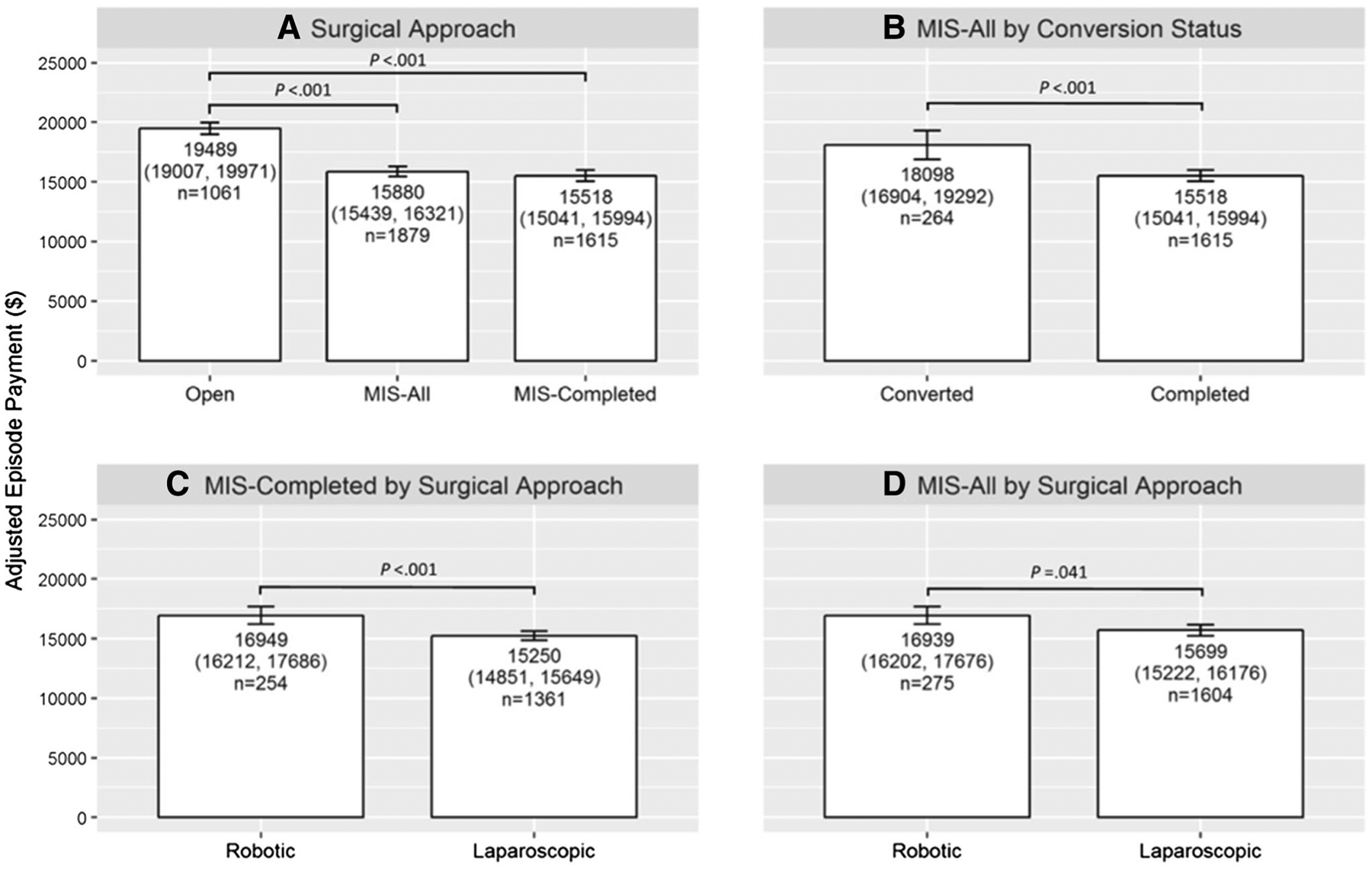
Adjusted episode payment with 95% confidence intervals for surgical approach cohorts as Open, minimally invasive surgery (MIS) All, and MIS-Completed (A); MIS-All cohort by conversion status (B); by MIS-Completed cohort by surgical approach (C); and by MIS-All cohort by surgical approach (D)
Within MIS, total episode payments for completed robotic operations were significantly higher than completed laparoscopic operations ($16,949 vs. $15,250, p<0.001). When accounting for converted operations, this difference was attenuated but remained statistically significant ($16,939 vs. $15,699, p = 0.041).
Episode payment components
Episode payment components are depicted in Tables 2, 3, and 4. Index hospitalization and Post Discharge payments were significantly higher in the Open group when compared to MIS-Completed operations (Index hospitalization $16,194 vs. $13,743, p<0.001; Post Discharge $930 vs. $58, p<0.001) and MIS-All operations (Index Hospitalization $16,194 vs. 14,012, p<0.001, Post Discharge $930 vs. $101, p<0.001), while there was no significant difference in readmission and professional payments between Open and both MIS-Completed and MIS-All groups. Index hospitalization payments for Converted operations were significantly higher than for the Completed group ($15,660 vs. 13,743, p<0.001). Index hospitalization ($14,422 vs. $13,616, p = 0.41) and Professional payments ($471 vs. $382, p = 0.007) were significantly higher for completed robotic operations when compared to completed laparoscopic operations. Professional payments were significantly higher for all robotic operations (completed and converted) when compared to all laparoscopic operations ($461 vs. $382, p = 0.012).
Table 2.
Payments by open and minimally invasive surgery (MIS) Completed and All stratums as adjusted means and differences with 95% confidence intervals
| Payment category | Open (n = 1061) | MIS-A11 (n = 1879) | MIS-Completed (n = 1615) | Open versus MIS-A11, p value | Open versus MIS-Completed, p value |
|---|---|---|---|---|---|
| Total episode | 19,489 (19,007, 19,971) | 15,880 (15,439, 16,321) | 15,518 (15,041, 15,994) | <0.001 | <0.001 |
| Index | 16,194 (15,849, 16,540) | 14,012 (13,750, 14,273) | 13,743 (13,463, 14,023) | <0.001 | <0.001 |
| Post discharge | 930 (643, 1216) | 101 (−107, 309) | 58 (−174, 291) | <0.001 | <0.001 |
| Readmission | 568 (331, 806) | 305 (129, 480) | 296 (103, 489) | 0.074 | 0.079 |
| Professional | 411 (381, 442) | 394 (371, 416) | 396 (371, 421) | 0.351 | 0.456 |
Table 3.
Payments by laparoscopic and robotic on minimally invasive surgery (MIS) Completed and All stratums as adjusted means and differences with 95% confidence intervals
| Payment category | MIS-Completed | MIS-A11 | ||||
|---|---|---|---|---|---|---|
| Robotic (n = 254) | Laparoscopic (n = 1361) | p Value | Robotic (n = 275) | Laparoscopic (n = 1604) | p Value | |
| Total episode | 16,949 (16,212, 17,686) | 15,250 (14,851, 15,649) | <0.001 | 16,939 (16,202, 17,676) | 15,699 (15,222, 16,176) | 0.041 |
| Index | 14,422 (13,715, 15,129) | 13,616 (13,310, 13,921) | 0.041 | 14,392 (13,709, 15,074) | 13,946 (13,664, 14,229) | 0.236 |
| Post discharge | 346 (−220, 913) | 4 (−240, 249) | 0.278 | 374 (−170, 919) | 54 (−171, 280) | 0.287 |
| Readmission | 546 (68, 1023) | 249 (43, 456) | 0.262 | 547 (88, 1005) | 263 (73, 453) | 0.263 |
| Professional | 471 (410, 532) | 382 (355, 408) | 0.007 | 461 (402, 520) | 382 (358, 406) | 0.012 |
Table 4.
Payments by completed and converted on the minimally invasive surgery (MIS) stratum as adjusted means and differences with 95% confidence intervals
| Payment category | Converted (n = 264) | Completed (n = 1615) | p Value |
|---|---|---|---|
| Total episode | 18,098 (16,904, 19,292) | 15,518 (15,041, 15,994) | <0.001 |
| Index | 15,660 (14,967, 16,353) | 13,743 (13,463, 14,023) | <0.001 |
| Post discharge | 365 (−190, 920) | 58 (−174, 291) | 0.216 |
| Readmission | 357 (−111, 825) | 296 (103, 489) | 0.771 |
| Professional | 382 (322, 442) | 396 (371, 421) | 0.614 |
Discussion
This analysis of linked regional clinical outcomes and claims registries revealed that episode payments for open colorectal surgery are higher than for laparoscopic and robotic options and that conversion to open surgery significantly increased third-party payments associated with minimally invasive colon and rectal resections. This study replicated the finding from our previous work and other studies demonstrating that conversion to open surgery incurs significantly worse clinical outcomes and that conversion rates are significantly lower for operations initiated with a robotic approach than with a laparoscopic approach [18, 21–23]. Expanding on those observations, we found in this study that the difference in conversion rates substantially changed the cost comparison between the two approaches. While robotic operations completed without conversion incurred an average of $1699 more in services than those completed by the laparoscopic approach, the difference in conversion eliminated 27% of this payment difference between robotic and laparoscopic groups when converted cases were included in an intention-to-treat model.
It has been previously observed that conversion increases postoperative length of stay, opioid requirements, and in-hospital complications, as well as adverse events after discharge [16, 24–27]. In this study, we expand on these observations, and found that conversion significantly increased expenditures for the entire surgical episode, attributable mainly to payments for the index hospitalization. Furthermore, this study shows that even after accounting for the costs and consequences of conversion, minimally invasive laparoscopic or robotic operations incur lower payments than open procedures. The payment differences between minimally invasive and open cases are most evident in the index hospitalization and post-discharge care. The differences between the laparoscopic and robotic payments are seen primarily in the index hospitalization and in professional billing. Recognizing that robotic surgery most often substitutes for open surgery, rather than substituting robotics for laparoscopy, the comparisons of payments between each MIS approach and open surgery are most important for clinical decision-makers.
The population-based, validated hospital registries that provide the data are the strength of this study and resolve a key shortcoming of previous investigations. In administrative data, as well as most other surgical registries, converted cases are pooled with open operations, significantly biasing comparisons in favor of minimally invasive approaches. The protocol-driven MSQC database stratifies laparoscopic and robotic cases in an intention-to-treat manner thereby allowing reliable identification of converted cases that are inaccurately considered open cases in other studies that rely on administrative databases. [11, 12, 14] This enabled insights in the role of conversion in the cost considerations around colorectal resections.
Further, the adjusted claims-based MVC database allows evaluation of real-time total episode payments rather than merely costs or charges associated with in-hospital care. Most cost studies to date have centered only on direct hospital costs associated with conducting the operative intervention [1, 10–13]. This study evaluates conversion and surgical approach from the payer and society perspective recognizing the total realized cost of surgical care both in and out of the hospital. The consideration of the costs of conversion adds more detailed understanding to these claims-based comparisons, because converted-to-open cases result in higher costs than unconverted cases due to the utilization of more instruments, longer operative times, the associated longer hospital length of stay, and higher complication rates for converted cases [11, 15–17]. Administrative datasets and most other clinical registries have not clearly defined converted operations, and thus, conversions are typically coded with open procedural CPT codes, potentially biasing results against open operations. We found that, on average, converted cases incurred nearly 17% increased expenditures in the 30-day episode around surgery.
Just as there is currently attention to the cost of robotics, the cost of laparoscopic colorectal surgery was a national concern early in the evolution of this modality [28–31]. With time, laparoscopy became cost effective for those conquering the learning curve, because of favorable outcomes with respect to hospital length of stay, postoperative pain management, surgical site infections, and long-term morbidities including incisional hernias [1–4]. With the implementation of federal reimbursement reform and bundled payment initiatives based on quality metrics, patients and payers will be incentivized to identify surgical options that provide the most clinical value with respect to clinical outcomes and cost.
Other studies have similarly addressed clinical value in the context of quality outcomes and cost. In a claims-based analysis of 4615 colectomies, Keller et al. found that the net episode cost of care that included post-discharge and readmission costs was significantly higher for open colectomy when compared to the laparoscopic approach. Although they utilized a different claims database, these authors similarly concluded that health plans and employers would garner financial benefits by transitioning from open to minimally invasive colectomy [32]. In an ACSNSQIP study, Hollis et al., matched laparoscopic and robotic colorectal cases 1:1 with open cases and found that the median hospital costs were similar between laparoscopic and open approaches, and between robotic and open surgery. These authors also determined that minimally invasive options compare favorably to the open approach. Their study differs from ours in that it is a cost comparison from an institutional viewpoint rather than the payer perspective [14].
Our study has several limitations, including the inherent biases of any retrospective database review. Although we accounted for most recognized contributors to episode payment variation using validated, audited clinical registries, we cannot exclude the possibility of residual confounding due to unmeasured characteristics. The MSQC data source does not account for selection bias with respect to which patients are chosen for laparoscopic or robotic options. However, there is expected to be less confounding by indication when comparing laparoscopic to robotic approaches than when comparing either minimally invasive option to open, since the indications and contraindications for laparoscopic and robotic options are similar. Rectal resections are typically associated with higher conversion rates and cost than colectomies, and thus the higher use of robotics in pelvic colorectal operations could bias the results against robotic, as compared with laparoscopic operations, in ways that are not measured [18]. We adjusted for colon versus rectal resection in the statistical model based on CPT codes in the MSQC database. However, the MVC database includes specific clinical data, such as could be obtained from chart review, and thus a more clinically specific subgroup analysis of rectal versus colon resections is not possible.
The databases in this study do not allow assessment of specific surgeons utilizing different surgical approaches, possible variations in surgeon volumes with surgical approaches, and other care processes that may affect outcomes and cost like Enhanced Recovery Pathways. A recent MSQC study by our group showed varying degrees of low-volume and high-volume surgeons within the collaborative for minimally invasive and open colorectal surgery options [33]. The MSQC data for this study are stratified, and though we do not know individual surgeon volumes, it is unlikely that this study is biased by high-volume surgeons in one group and low-volume surgeons in another group. Of the 75 MSQC hospitals, only four have long established Enhanced Recovery Pathways and it is likely that perioperative protocols that may impact patient care and outcomes are evenly distributed amongst open, laparoscopic, and robotic groups.
While conversion to open is strictly defined in the MSQC database, there is no distinction between early and late conversions, the latter of which may be associated with worse outcomes [34]. Further, we chose to evaluate payments, rather than hospital expenses or charges, in order to best represent the societal perspective of the cost of care. Still, this does not account for other measures of cost, such as the direct expenses for patients. Because complications and prolonged recovery incur significant personal financial burden on patients, the reduced morbidity from fewer conversions in robotic surgery may impart additional personal economic benefits to patients beyond what we measured in this study [35].
The value considerations around open, laparoscopic, and robotic colorectal surgery are likely dependent on surgeon skill sets, comfort levels, and perceived advantages [36]. Rather than substituting for laparoscopy, robotics seems to have instead displaced open operations, especially for some surgeons performing pelvic operations for rectal cancer, in which laparoscopic penetration has reached only 10–20% [7–10, 14, 37, 38]. Recognizing that open and converted operations incur substantially greater spending than minimally invasive completed surgery, providers motivated to reduce total episode costs will continue to favor approaches that maximize the likelihood of completing an operation with a minimally invasive technique. From the hospital perspective, these considerations may depend on the number of cases, the number of disciplines with different minimally invasive needs, institutional surgeon complication rates, and how many expert laparoscopic surgeons are already providers at the individual institution. From both a clinical and economic perspective, the reduction in conversions to open surgery with robotics at those institutions that have significant differences in minimally invasive conversion rates, may thus offer benefit in carefully selected cases.
In conclusion, this study demonstrates substantially increased episode payments associated with open and minimally invasive converted-to-open colorectal operations. Although we observe greater payments associated with robotic operations, the reduction in rates of conversion in robotic surgery offsets some of the increased expenditures. If experience with robotic surgery continues to increase and allows a decrease in open surgery, it will be important to continue to assess the relative outcomes and costs associated with surgical techniques from an intention-to-treat perspective that accounts for the costs of conversion on payers, providers, and patients.
Footnotes
Disclosures Dr. Cleary reports personal fees from Intuitive Surgical, outside the submitted work. Mr. Mullard reports grants from Blue Cross Blue Shield of Michigan, during the conduct of the study. Dr. Regenbogen is supported by the American Society of Colon and Rectal Surgeons Career Development Award CDG-015, National Institute on Aging Grants for Early Medical/Surgical Specialists Transition to Aging Research R03-AG047860, and National Institute on Aging K08-AG047252. Ms. Ferraro and Dr. Regenbogen have nothing to disclose.
References
- 1.Thompson BS, Coory MD, Gordon LG, Lumley JW (2014) Cost savings for elective laparoscopic resection compared with open resection for colorectal cancer in a region of high uptake. Surg Endosc 28:1515–1521 [DOI] [PubMed] [Google Scholar]
- 2.Jordan J, Dowson H, Gage H, Jackson D, Rockall T (2014) Laparoscopic versus open colorectal resection for cancer and polyps: a cost-effectiveness study. CEOR 6:414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva-Velazco J, Dietz DW, Stocchi L, Costedio M, Gorgun E,Kalady MF, Kessler H, Lavery IC, Remzi FH (2016) Considering value in rectal cancer surgery: an analysis of costs and outcomes on the open, laparoscopic, and robotic approach for proctectomy. Ann Surg. doi: 10.1097/SLA0000000000001815 [DOI] [PubMed] [Google Scholar]
- 4.Harr JN, Juo YY, Luka S, Agarwal S, Brody F, Obias V (2016) Incisional and port-site hernias following robotic colorectal surgery. Surg Endosc 30:3505–3510 [DOI] [PubMed] [Google Scholar]
- 5.Sheetz KH, Norton EC, Regenbogen SE, Dimick JB (2017) An instrumental variable analysis comparing Medicare expenditures for laparoscopic versus open colectomy. JAMA Surg. doi: 10.1001/jamasurg.2017.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox J, Gross CP, Longo W, Reddy V (2012) Laparoscopic colectomy for the treatment of cancer has been widely adopted in the United States. Dis Colon Rectum 55:501–508 [DOI] [PubMed] [Google Scholar]
- 7.Damle RN, Macomber CW, Flahive JM, Davids JS, Sweeney WB, Sturrock PR, Maykel JA, Santry HP, Alavi K (2014) Surgeon volume and elective resection for colon cancer: an analysis of outcomes and use of laparoscopy. J Am Coll Surg 218:1223–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halabi WJ, Kang CY, Nguyen VQ, Carmichael JC, Mills S, Stamos MJ, Pigazzi A (2013) Robotic-assisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg 37:2782–2790 [DOI] [PubMed] [Google Scholar]
- 9.Moghadamyeghaneh Z, Phelan M, Smith BR, Stamos MJ (2015) Variations in laparoscopic colectomy utilization in the United States. Dis Colon Rectum 58:950–956 [DOI] [PubMed] [Google Scholar]
- 10.Keller DS, Senagore AJ, Lawrence JK, Champagne BJ, Delaney CP (2014) Comparative effectiveness of laparoscopic versus robot-assisted colorectal resection. Surg Endosc 28:212–221 [DOI] [PubMed] [Google Scholar]
- 11.Tyler JA, Fox JP, Desai MM, Perry WB, Glasgow SC (2013) Outcomes and costs associated with robotic colectomy in the minimally invasive era. Dis Colon Rectum 56:458–466 [DOI] [PubMed] [Google Scholar]
- 12.Rawlings AL, Woodland JH, Vegunta RK, Crawford DL (2007) Robotic versus laparoscopic colectomy. Surg Endosc 21:1701–1708 [DOI] [PubMed] [Google Scholar]
- 13.Salman M, Bell T, Martin J, Bhuva K, Grim R, Ahuja V (2013) Use, cost, complications, and mortality of robotic versus non-robotic general surgery procedures based on a nationwide database. Am Surg 79:553–560 [PubMed] [Google Scholar]
- 14.Hollis RH, Cannon JA, Singletary BA, Korb ML, Hawn MT, Heslin MJ (2016) Understanding the value of both laparoscopic and robotic approaches compared to open approach in colorectal surgery. J Laparoendosc Adv Surg Tech A 26:850–856 [DOI] [PubMed] [Google Scholar]
- 15.The Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopic assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059 [DOI] [PubMed] [Google Scholar]
- 16.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97:1638–1645 [DOI] [PubMed] [Google Scholar]
- 17.Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100:75–82 [DOI] [PubMed] [Google Scholar]
- 18.Bhama AR, Obias V, Welch KB, Vandewarker JF, Cleary RK (2016) A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons—National Surgical Improvement Program (ACS-NSQIP) database. Surg Endosc 30:1576–1584 [DOI] [PubMed] [Google Scholar]
- 19.Tam MS, Kaoutzanis C, Mullard AJ, Regenbogen SE, Franz MG, Hendren S, Kraphol G, Vandewarker JF, Lampman RM, Cleary RK (2016) A population-based study comparing laparoscopic and robotic outcomes in colorectal surgery. Surg Endosc 30:455–463 [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb DJ, Zhou W, Song Y, Andrews KG, Skinner JS, Sutherland JM (2010) Prices don’t drive regional Medicare spending variations. Health Aff (Millwood) 29(3):537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolejs SC, Waters JA, Ceppa EP, Zarzaur BL (2016) Laparoscopic versus robotic colectomy: a national surgical quality improvement project analysis. Surg Endosc. doi: 10.1007/s00464-016-5239-5 [DOI] [PubMed] [Google Scholar]
- 22.Feinberg AE, Elnahas A, Bashir S, Cleghorn MC, Quereshy FA (2016) Comparison of robotic and laparoscopic colorectal resections with respect to 30-day perioperative morbidity. Can J Surg 59:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Wei ZQ, Bie M, Peng XD, Chen C (2016) Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: a meta-analysis. Surg Endosc 30:5601–5614 [DOI] [PubMed] [Google Scholar]
- 24.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AMH, Heath RM, Brown JM, for the MRC CLASICC trial group (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multi-centre, randomized trial. Lancet 365:1718–1726 [DOI] [PubMed] [Google Scholar]
- 25.Chan ACY, Poon JTC, Fan JK, Lo SH, Law WL (2008) Impact of conversion on the long-term outcome in laparoscopic resection of colorectal cancer. Surg Endosc 22:2625–2630 [DOI] [PubMed] [Google Scholar]
- 26.Chew MH, Ng KH, Fook-Chong MC, Eu KW (2011) Redefining conversion in laparoscopic colectomy and its influence on outcome analysis of 418 cases from a single institution. World J Surg 35:178–185 [DOI] [PubMed] [Google Scholar]
- 27.Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H, Clinical Outcomes of Surgical Therapy Study Group (2007) Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST study group trial. Ann Surg 246:655–664 [DOI] [PubMed] [Google Scholar]
- 28.Pfeifer J, Wexner SD, Reissman O, Bernstein M, Nogueras JJ, Singh S, Weiss E (1995) Laparoscopic versus open colon surgery. Costs and outcome. Surg Endosc 12:1322–1326 [PubMed] [Google Scholar]
- 29.Leung KL, Kwok SPY, Lam SCW, Lee JFY, Yiu RYC, Ng SSM, Lai PBS, Lau WY (2004) Laparoscopic resection of rectosigmoid carcinoma: prospective randomized trial. Lancet 363:1187–1192 [DOI] [PubMed] [Google Scholar]
- 30.Dowson HM, Gage H, Jackson D, Qiao Y, Williams P, Rockall TA (2012) Laparoscopic and open colorectal surgery: a prospective cost analysis. Colorectal Dis 14:1424–1430 [DOI] [PubMed] [Google Scholar]
- 31.Dowson HM, Huang A, Soon Y, Gage H, Lovell DP, Rockall TA (2007) Systematic review of the costs of laparoscopic colorectal surgery. Dis Colon Rectum 50:908–919 [DOI] [PubMed] [Google Scholar]
- 32.Keller DS, Senagore AJ, Fitch K, Bochner A, Haas EM (2016) A new perspective on the value of minimally invasive colorectal surgery—payer, provider, and patient benefits. Surg Endosc. doi: 10.1007/s00464-016-5295-x [DOI] [PubMed] [Google Scholar]
- 33.Healy MA, Regenbogen SE, Kanters AE, Suwanabol PA, Varban OA, Campbell DA, Dimick JB, Byrn JC (2017) Surgeon variation in complications with minimally invasive and open colectomy: results from the Michigan Surgical Quality Collaborative. JAMA Surg. doi: 10.1001/jamasurg.2017.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolfschoten NE, van Leersum NJ, Gooiker GA, van de Mheen PJM, Eddes EH, Kievit J, Brand R, Tanis PJ, Bemelman WA, Tollenaar RAEM (2013) Successful and safe introduction of laparoscopic colorectal cancer surgery in Dutch hospitals. Ann Surg 257:916–921 [DOI] [PubMed] [Google Scholar]
- 35.Regenbogen SE, Veenstra CM, Hawley ST, Banerjee M, Ward KC, Kato I, Morris AM (2014) The personal financial burden of complications after colorectal cancer surgery. Cancer 120:3073–3081 [DOI] [PubMed] [Google Scholar]
- 36.Morelli L, Guadagni S, Lorenzoni V, DiFranco G, Cobuccio L, Palmeri M, Caprili G, D’Isidoro C, Moglia A, Ferrari V, DiCandio G, Mosca F, Turchetti G (2016) Robot-assisted versus laparoscopic rectal resection for cancer in a single surgeon’s experience: a cost analysis covering the initial 50 robotic cases with the daVinci Si. Int J Colorectal Dis 31:1639–1648 [DOI] [PubMed] [Google Scholar]
- 37.Yeo HL, Isaacs AJ, Abelson JS, Milsom JW, Sedrakyan A (2016) Comparison of open, laparoscopic, and robotic colectomies using a large national database: outcomes and trends related to surgery center volume. Dis Colon Rectum 59:535–542 [DOI] [PubMed] [Google Scholar]
- 38.Schootman M, Hendren A, Ratnapradipa K, Stringer L, Davidson NO (2016) Adoption of robotic technology for treating colorectal cancer. Dis Colon Rectum 59:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]


