Abstract
There are abundant sources of anticancer drugs in nature that have a broad prospect in anticancer drug discovery. Natural compounds, with biological activities extracted from plants and marine and microbial metabolites, have significant antitumor effects, but their mechanisms are various. In addition to providing energy to cells, mitochondria are involved in processes, such as cell differentiation, cell signaling, and cell apoptosis, and they have the ability to regulate cell growth and cell cycle. Summing up recent data on how natural products regulate mitochondria is valuable for the development of anticancer drugs. This review focuses on natural products that have shown antitumor effects via regulating mitochondria. The search was done in PubMed, Web of Science, and Google Scholar databases, over a 5-year period, between 2015 and 2020, with a keyword search that focused on natural products, natural compounds, phytomedicine, Chinese medicine, antitumor, and mitochondria. Many natural products have been studied to have antitumor effects on different cells and can be further processed into useful drugs to treat cancer. In the process of searching for valuable new drugs, natural products such as terpenoids, flavonoids, saponins, alkaloids, coumarins, and quinones cover the broad space.
Keywords: natural products, mitochondria, cancer, cell death
1. Introduction
Cancer is a threat to human health and is the leading cause of premature death; thus, it reduces the productivity of a country. Cancer rates are rising, driven by unhealthy lifestyles, business interests, and an aging society. According to the Global Cancer Observatory (GLOBOCAN) 2018 database, compiled by the International Agency for Research on Cancer, 18.1 million people were diagnosed with cancer and 9.6 million died in 2018. The most common types of diagnosed cancers are lung cancer, female breast cancer, prostate cancer, colorectal cancer, stomach cancer, and liver cancer [1,2]. Despite tremendous efforts to implement new cancer chemotherapy methods, cancer remains a major problem worldwide. Therefore, it is necessary to find new therapeutic drugs that have specific effects on various cancer cells.
Natural products are important sources of lead compounds and new drugs, which include the components or metabolites of plants, animals, insects, marine organisms, microorganisms, as well as many endogenous chemical constituents in humans and animals [3]. Natural products also include water or alcohol extracts of plants, animals, and fungi, etc. [4,5]. This article mainly discusses individual compounds. In drug discovery and development, natural products have played an important role, especially for anticancer drugs. A great quantity of anticancer medicines are natural products or derivatives of them [6]. Taxol, isolated from Taxus baccata, is the most successful antitumor drug that has been found. It has been widely used in the clinical treatment of breast cancer, ovarian cancer, some head and neck cancers, as well as lung cancer [7,8]. Vincristine is another great anticancer natural product, derived from Catharanthus roseus, and often used for the treatment of acute lymphocytic leukemia [9,10]. Natural products, as a source of anticancer drugs, are a vast area worth exploring.
2. The Role of Mitochondria in Cancer Cells
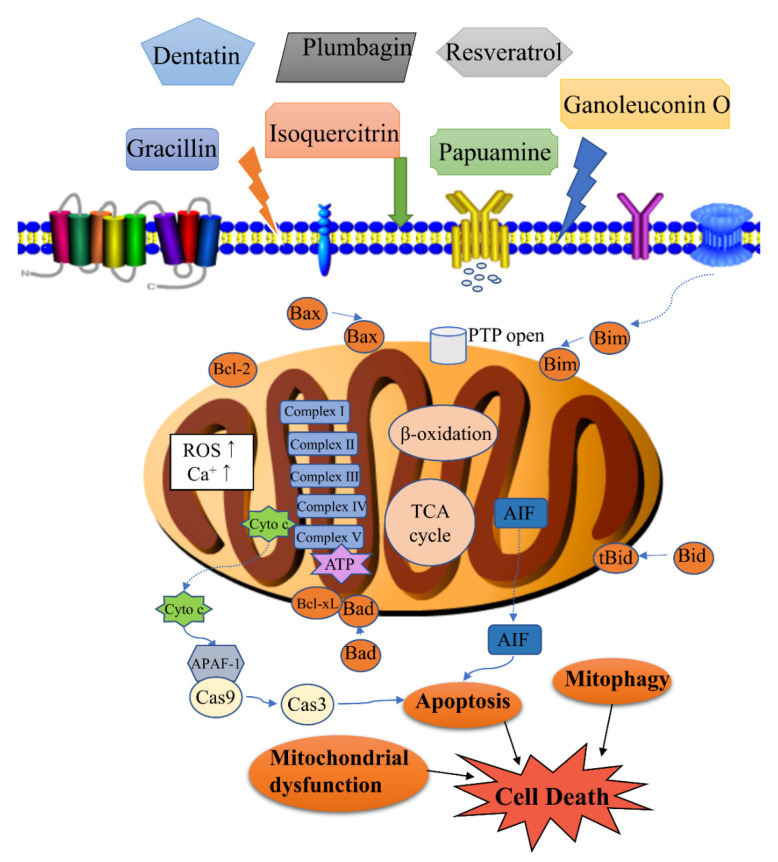
Mitochondria are energy-producing structures and the main site for aerobic respiration in cells, and are therefore called the “powerhouse of the cell” [11,12]. Mitochondria are associated with many diseases, such as Parkinson’s disease [13], diabetic nephropathy [14], acute kidney injury [15], and Down syndrome [16]. Mitochondria also play an important role for cell signaling, apoptosis regulation, and energy metabolism in drug-induced cancer cells death; therefore, they are considered a significant target in cancer chemotherapy [17]. Some scholars have reviewed the mitochondrion as a target of anticancer therapy over the years [18,19,20,21]. Moreover, modulation of mitochondrial-dependent pathways by natural compounds is diverse (Figure 1). However, few researchers have reviewed natural products that regulate mitochondrial pathway in cancers.
Figure 1.
Modulation of mitochondrial-related cell death by natural products. Cell death associated with the activity of natural products includes apoptosis, mitophagy, mitochondrial dysfunction, etc. Apoptosis is regulated by the levels of Bcl-2 (B-cell lymphoma-2) family proteins, release of cytochrome c, and caspase activation. Mitophagy is the targeted phagocytosis and destruction of mitochondria by the autophagy machinery, and it is generally considered as the main mechanism of mitochondrial quality control. A decrease in energy production, an increase of reactive oxygen species (ROS) and permeability transition pore (PTP) opening can lead to mitochondrial dysfunction.
3. Mitochondrial Control of Apoptosis
Mitochondrial involvement is an important pathway in the process of apoptosis. The Bcl-2 protein family regulates apoptosis by controlling mitochondrial permeability. Anti-apoptotic proteins B-cell lymphoma-2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-xL) reside in the outer membrane of mitochondria and inhibit the release of cytochrome c. Pro-apoptotic proteins Bax, Bad, Bid, and Bim can reside in the cytoplasm, translocating to mitochondria after receiving a death signal, and promote cytochrome c release into the cytoplasm. Released cytochrome c binds to apoptotic protease activating factor-1 (Apaf-1) to form apoptosome, amplifying the apoptotic cascade [22,23,24].
Necrotic stimulation leads to increased mitochondrial Ca2+ uptake and ROS production. High levels of Ca2+ and ROS induce the opening of the Cyclophilin-D (Cyp-D) sensitive permeability transition pore (PTP), leading to matrix swelling and Ca2+ release. Swelling damages the outer membrane and releases Ca2+ activating proteases, phosphatases, and nucleases, leading to necrotic degradation [12].
Fission or fusion rates may change under different growth conditions, and result in an increase or decrease in the number of mitochondria. When mitochondria become damaged, their connectivity is reduced, and mitochondria become shorter and rounder. The change from highly branched to fragmented morphologies may be induced by altered fission or fusion rates. At the early stage of apoptosis, the transition from a mitochondrial network to vesicular punctiform mitochondria was detected [25]. Mitochondrial fragmentation occurs in parallel to the formation of apoptotic bodies, increasing the number of the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positive nuclei and cleavage of the caspase substrate polymerase (PARP) [26].
4. Mitochondrial Control of Energy Metabolism
Mitochondria provide considerable flexibility for the growth and survival of tumor cells, and play a key role in harsh conditions, such as nutrient depletion and hypoxia. The rapid proliferation of cancer cells requires more mitochondria than normal cells. Therefore, the development of chemotherapeutic drugs for mitochondria is a breakthrough in the fight against cancer. Many scholars have clarified that the mechanical drive of mitochondrial respiration involves the tricarboxylic acid (TCA) cycle, and fatty acid β-oxidation enzymes in the mitochondrial matrix that generate electron donors to fuel respiration and electron transport chain (ETC) complexes, and ATP synthase in the inner mitochondrial membrane (IMM) that carry out oxidative phosphorylation [27]. Some natural products inhibit electron transport chain complexes. Four such complexes are NADH-ubiquinone reductase(complex I), succinate-ubiquinone reductase (complex II), ubiquinol-cytochrome c reductase (complex III), and cytochrome c oxidase (complex IV) [28]. Complex V, which is called ATP synthase, together with the above four complexes, completes oxidative phosphorylation to produce ATP. Inhibition of mitochondrial ETC complex activity can lead to significant mitochondrial dysfunction.
Cardiolipin, which consists of two phosphatidyl residues linked by a glycerol bridge, is a unique phospholipid dimer in the inner mitochondrial membrane in all eukaryotes. Cardiolipins play an important role in preserving mitochondrial structure and function. They support membrane dynamics and stabilize the lateral organization of protein-rich membranes in mitochondria [29]. Cardiolipins are involved in mitochondrial cristae morphology and stability [30], mitochondrial quality control, and dynamics by fission and fusion [31,32] and mitophagy [33]. They can also serve as a binding platform to recruit apoptotic factors in the apoptotic process [34,35]. However, it is still not clear how these events are interconnected and cooperate. In addition, cardiolipins are very susceptible to damage from ROS because of their high content of unsaturated acyl chains. Thus, the stability and function of mitochondria can be impaired by the biophysical properties of the membranes that are altered [36].
In this paper, we attempt to summarize the mechanisms through which natural products exert anticancer effects, as published in the past five years, by using a structural classification, with emphasis on the molecular mechanisms of mitochondrial involvement. Through all the reports, we found that most natural products regulate a series of proteins, such as Bax, Bcl-2, and caspases-3 and -9. Moreover, inhibitors of electron transport chain complexes can also exert anticancer activity. Details can be found in Table 1.
Table 1.
Natural products (1–81) regulated mitochondria by different mechanisms in cancer cells.
| No. | Isolated Compound | Origin | Cell Line | Mechanism | Reference |
|---|---|---|---|---|---|
| Terpenoids | |||||
| 1 | Ganoleuconin O | Ganoderma leucocontextum | Huh7.5 | Fatty acid immobilization, loss of the mitochondrial lipid cardiolipin | [30] |
| 2 | Lupeol | Bombax ceiba | SK-RC-45 | Mitochondrial hyper fission | [31] |
| 3 | Betulinic acid | Betula alba | HeLa | Cardiolipin modification, ROS generation, Bad, caspase 9 | [32,33] |
| 4 | Alisol B-23-acetate | Alisma orientale | A549, NCI-H292 | ROS generation, Bcl-2↓, Bax↑, activation of caspase-3, -9, release of cytochrome c/AIF | [34] |
| 5 | Genipin | Gardenia jasminoides | N18TG2 | Activation of dicarboxylate carrier, decreased activity of UCP1, UCP3, and complex III of the respiratory chain, UCP2 inhibition | [35] |
| 6 | Alternol | Yew tree | PC-3 | Decrease of mitochondrial respiration, isocitric acid, fumaric acid and malic acid, ATP production | [36,37] |
| 7 | Cyathin Q | Cyathus africanus | HCT116 | Bcl-2↓, Bax↑, Bcl-xL↓, ROS generation, release of cytochrome c | [38] |
| 8 | 3α-hydroxy-19α-hydrogen-29-aldehyde-27-lupanoic acid | Potentilla discolor | HepG2 | Bcl-2↓, Bax↑, release of cytochrome c | [39] |
| 9 | Uvedafolin | Smallanthus sonchifolius | HeLa | MMP loss, release of cytochrome c | [40] |
| 10 | Heteronemin | Hippospongia sp. | Molt4 | ROS generation | [41] |
| 11 | Jatrogossone A | Jatropha gossypiifolia | KOPN-8 | MMP loss, ROS generation | [42] |
| 12 | Walsuronoid B | Walsura robusta | Bel-7402, HepG2 | ROS generation, mitochondrial and lysosomal dysfunction | [43] |
| 13 | Ferruginol | Podocarpus ferruginea | MDA-T32 | ROS generation, MMP loss, Bcl-2↓ | [44,45] |
| 14 | Lobocrassin B | Lobophytum crassum | CL1-5, H520, BEAS-2B |
Bcl-2↓, Bax↑, ROS generation, MMP loss, release of cytochrome c, activation of caspase-3 | [46] |
| 15 | Aellinane | Euphorbia aellenii | Caov-4 | Bcl-2↓, Bax↑, ROS generation, MMP loss | [47] |
| 16 | Tingenin B | Maytenus sp. | MCF-7s | Bcl-2↓, Bax↑, MMP loss | [48] |
| 17 | 3-O-trans-p-coumaroyl alphitolic acid | Ziziphus jujuba | PC-3 | ROS generation | [49] |
| 18 | Zerumbone | Zingiber zerumbet | PC-3, DU-145 | Tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult | [50,51] |
| Flavonoids | |||||
| 19 | Isoquercitrin | Hibiscus cannabinus | MDA-MB-231 | LSD1-induced mitochondrial-mediated apoptosis pathway | [52,53] |
| 20 | Luteolin | Cauliflower, peanut, and carrot | SW1990 | Inhibitor of Bcl-2, mitochondrial permeabilization | [54] |
| 21 | Dihydromyricetin | Ampelopsis grossedentata | HepG2 | Akt/Bad signal pathway, mitochondrial apoptotic pathway, Bax↑, Bad↑, inhibition of the phosphorylation of Bad at Ser136 and Ser112 | [55,56] |
| 22 | Artonin E | Artocarpus elasticus | SKOV-3 | Release of cytochrome c, Activation of caspases-3, -8, and -9, Bax↑, Bcl-2↓, HSP70↓, survivin↓ | [57] |
| 23 | Myricetin | Fruits and vegetables | SNU-80 | Bax/Bcl-2↑, release of AIF | [58] |
| 24 | Xanthones | Garcinia xanthochymus | HepG2 | Bax↑, Bcl-2↓, Bcl-xL↓, Mcl-1↓, and survivin↓ | [59] |
| 25 | Cycloartobiloxanthone | Artocarpus gomezianus | H460 | Bax↑, Bcl-2↓, Mcl-1↓ | [60] |
| 26 | Paratocarpin E | Euphorbia humifusa | MCF-7 | Bax↑, Bcl-2↓, release of cytochrome c | [61] |
| 27 | Puerarin 6′’-O-xyloside | Pueraria lobata | SW480 | Bax↑, Bad↑, Bcl-2↓, caspase-3 and -9 activation | [62] |
| 28 | α-mangostin | Cratoxylum arborescens | HeLa | ROS generation, MMP loss, release of cytochrome c | [63] |
| 29 | Chrysin | Honey and propolis | Mitochondria isolated from hepatocytes of HCC rats |
ROS generation, MMP loss, release of cytochrome c, swelling in mitochondria | [64,65] |
| 30 | Fisetin | Strawberries, apples, grapes, onions, and cucumbers | SCC-4 | ROS generation, Ca2+ production, MMP loss, Bcl-2↓, Bax↑, Bid↑, release of cytochrome c, AIF, and Endo G | [66,67] |
| 31 | Baicalein | Scutellaria baicalensis, Scutellaria radix | A2780 | Combination therapy with baicalein and taxol had much higher antitumor effects compared with the monotherapy. Release of cytochrome c, and caspase-3 and -9 activation |
[68,69] |
| 32 | Alpinetin | Zingiberaceous plants |
A549 | Bcl-2↓, Bax↑, Bcl-xL↓, XIAP↓, PI3K/Akt signaling pathway, sensitized drug-resistant lung cancer cells | [70,71] |
| 33 | Chamaejasmin B | Stellerachamaejasme | KB, KBV200 | Bcl-2↓, Bax↑, MMP loss, release of cytochrome c and AIF | [72] |
| 34 | Mensacarcin | Streptomyces bacteria | SK-Mel-28, SK-Mel-5, HCT-116 | Release of cytochrome c, energy production and mitochondrial function rapidly disturbed | [73] |
| Saponins | |||||
| 35 | Gracillin | Dioscorea gracillima | H226B, H460 | Targeting mitochondrial complex II, suppressing ATP synthesis, ROS generation | [74] |
| 36 | Polyphyllin I | Paris polyphylla | MDA-MB-231 | Mitochondrial translocation of DRP1, mitochondrial fission, release of cytochrome c, mitochondrial PTEN-induced kinase 1↑ | [75,76] |
| 37 | Frondoside A | Cucumaria frondosa | CA46 | Bcl-2↓, survivin↓, release of HtrA2/Omi and cytochrome c, ROS generation | [77] |
| 38 | 3β-O-α-l- arabinopyranoside |
Clematis ganpiniana | MCF-7, MDA-MB-231 | Release of cytochrome c and Apaf-1, upregulation of caspase-9 and caspase-3 | [78] |
| 39 | Sakuraso-saponin | Aegiceras corniculatum | LNcaP, 22RV-1, C4-2 | Bcl-xL↓ | [79,80] |
| 40 | Ginsenoside compound K | Panax ginseng | SK-N-BE(2), SH-SY5Y | Bcl-2↓, Bcl-xL↓ | [81] |
| 41 | Escin | Aesculus hippocastanum | 786-O, Caki-1 | G2/M arrest and ROS-modulated mitochondrial pathways | [82] |
| 42 | α-Hederin | Hedera helix | SW620 | NF-κB signaling pathway, Bcl-2↓, Bax↑, release of cytochrome c | [83,84] |
| Alkaloids | |||||
| 43 | Cathachunine | Catharanthus roseus | HL60 | ROS-dependent mitochondria-mediated intrinsic pathway, Bcl-2/Bax↓, ROS generation, MMP loss, release of cytochrome c | [85] |
| 44 | Berberine | Rhizoma coptidis | T98G, LN18 | ERK1/2-mediated impairment of mitochondrial aerobic respiration | [86,87] |
| 45 | Papuamine | Haliclona sp. | H1299 | Intracellular ATP depleted by causing mitochondrial dysfunction, mitochondrial superoxide production | [88] |
| 46 | Bis (2-ethyl hexyl) 1H-pyrrole-3, 4-dicarboxylate | Tinospora cordifolia | MDA-MB-231 | ROS generation, increase in intracellular calcium, phosphorylation of p53, mitochondrial membrane depolarization, MPTP, and cardiolipin peroxidation, Bcl-2↓, Bax↑, release of cytochrome c, caspase activation, DNA fragmentation | [89] |
| 47 | Unantimycin A | Found in the fraction library of microbial metabolites | Semi-intact cells with specific substrates for each complex of the mitochondrial electron transport chain |
Targeted inhibition of mitochondrial complex I | [90] |
| 48 | NPL40330 | Found in chemical library | Semi-intact cells with specific substrates for each complex of the mitochondrial electron transport chain |
Targeted inhibition of mitochondrial complex III | [90] |
| 49 | Boholamide A | Marine mollusks | U87MG | Influx of Ca2+ | [91] |
| 50 | Cernumidine | Solanum cernuum | T24 | Cytotoxicity and chemosensitizing effect of cernumidine to cisplatin. Bcl-2↓, Bax↑, MMP loss | [92] |
| 51 | Lycorine | Amaryllidaceae plant family | HepG2 | mPTP opening, MMP loss, ATP depletion, release of Ca2+ and cytochrome c, caspase activation | [93] |
| 52 | Lagunamides A | Lyngbya majuscule | A549 | MMP loss, ROS generation | [94] |
| 53 | Cordycepin | Cordyceps | OVCAR-3 | Downregulation of mitochondrial function and limitation of energy production; metastasis and migration suppressed | [95,96] |
| Coumarins | |||||
| 54 | 2,3-Dihydro-7- hydroxy-2R*,3R*- dimethyl-2-[4,8-dimethyl-3(E),7- nonadienyl]-furo[3,2-c]coumarin |
Ferula ferulaeoides | C6 | MMP loss, Bcl-xL↓, Bcl-2↓, Bax↑, cleavage of Bid, FAS↑, FADD↑ | [97] |
| 55 | Dentatin | Clausena excavate | HepG2 | Bcl-xL↓, Bcl-2↓, Bax↑, release of cytochrome c | [98,99] |
| 56 | Aesculetin | Cortex Fraxini | THP-1 | Bcl-2↓, Bax↑ | [100] |
| Quinones | |||||
| 57 | Quambalarine B | Quambalaria cyanescens | Jurkat E6.1 | Inhibition of mitochondrial complex I and II, inhibition of mitochondrial respiration, metabolism reprogramming | [101,102] |
| 58 | Plumbagin | Plumbago zeylanica | MG63 | ROS generation, Bcl-2↓, Bax↑, Bcl-xL↓, and Bak↓, endoplasmic reticulum stress | [103] |
| 59 | Shikonin | Lithospermum erythrorhizon | HGC-27 | Bcl-2↓, Bax↑, survivin↓ | [104] |
| 60 | 2,7-dihydroxy-3-methylanthraquinone | Hedyotis diffusa | SGC-7901 | Bcl-xl↓, Bcl-2↓, Bax↑, Bad↑, release of cytochrome c | [105] |
| 61 | 3-hydroxy-1,5,6-trimethoxy-2-methyl-9,10-anthraquinone | Prismatomeris connate | A549, H1299 | Bcl-2↓, Mcl-1↓, Bax↑ | [106] |
| 62 | Thymoquinone | Nigella sativa | T24, 253J | Bcl-2↓, Bax↑, release of cytochrome c and AIF | [107] |
| Miscellanea | |||||
| 63 | Methylsulfonylmethane | Fruits and vegetables | YD-38 | Bcl-xL↓, Bcl-2↓, Bax↑, release of cytochrome c, MMP loss | [108,109] |
| 64 | Parameritannin A-2 | Urceola huaitingii | HGC27 | Enhanced doxorubicin-induced mitochondria-dependent apoptosis, inhibition of the PI3K/Akt, ERK1/2 and p38 pathways, Bcl-2↓, Bcl-xl↓, Bax↑, Bid↑, release of cytochrome c, caspase activation | [110] |
| 65 | Resveratrol |
Polygonum cuspidatum,
Veratrum nigrum, Cassia obtusifolia |
H838, H520; K562 |
Enhanced antitumor activities of cisplatin; Induced apoptosis |
[111,112] |
| 66 | Oleuropein | Olea europaea | H1299 | Bcl-2/Bax↓, release of cytochrome c, activation of caspase-3 | [113,114] |
| 67 | Homoisoflavanone-1 | Polygonatum odoratum | A549 | Mitochondria-caspase-dependent and ER stress pathways, Bcl-2/ Bak↓ | [115] |
| 68 | Gallic acid | Green tea, grapes, red wine |
H446 | ROS-dependent mitochondrial apoptotic pathway | [116] |
| 69 | Hierridin b | Cyanobium sp. | HT-29 | Proteomics identified 21 differentially expressed proteins belonging to the categories protein folding/synthesis and cell structure and reduced mitochondrial activity and as confirmed by morphological analysis of mitochondrial parameters |
[117,118] |
| 70 | Deoxyarbutin | Ecklonia cava | B16F10 | MMP loss, ATP depletion and ROS overload generation | [119] |
| 71 | Magnolol | Magnolia officinalis | OS-RC-2, 786-O | P53, Bcl-2/Bax↓, release of cytochrome c, caspase activation, ROS generation | [120] |
| 72 | Oblongifolin C | Garcinia yunnanensis | QBC939 | Mitochondrial dysfunction | [121] |
| 73 | Amorfrutin C | Glycyrrhiza foetida | HT-29 | mPTP opening, mitochondrial oxygen consumption and extracellular acidification increased | [122] |
| 74 | Allyl isothiocyanate | Cruciferous vegetables | MCF-7, MDA-MB-231 | ROS and Ca2+ production, MMP loss, release of cytochrome c, AIF, and Endo G, Bcl-2↓, Bax↑ | [123,124] |
| 75 | α-conidendrin | Taxus yunnanensis | MCF-7 and MDA-MB-231 | ROS generation, p53↑, Bax↑, Bcl-2↓, MMP loss, release of cytochrome c, activation of caspases-3 and -9 | [125] |
| 76 | Dehydrobruceine B | Brucea javanica | A549, NCI-H292 | MMP loss, release of cytochrome c, cleavage of caspase-9, caspase-3, and poly (ADP-ribose) polymerase (PARP) | [126] |
| 77 | Frugoside | Calotropis procera | M14, A375 | ROS generation | [127,128] |
| 78 | Methyl caffeate | Solanum torvum | MCF-7 | Bcl-2↓, Bax↑, Bid↑, p53↑, cleavage of caspase-3 and PARP, release of cytochrome c | [129] |
| 79 | Tetrahydrocurcumin | Curcuma longa | MCF-7 | ROS generation, Bcl-2↓, PARP↓, Bax↑, release of cytochrome c, MMP loss | [130] |
| 80 | Phloretin | Apple tree leaves and Manchurian apricot | EC-109 | Bcl-2↓, Bax↑ | [131] |
| 81 | Sesamol | Sesame seeds | HepG2 | Bcl-2↓, Bax↑, MMP loss, H2O2 production, PI3K Class III/Belin-1 pathway | [132] |
5. Natural Products Induce Cancer Cell Death through a Mitochondrial Pathway
5.1. Terpenoids
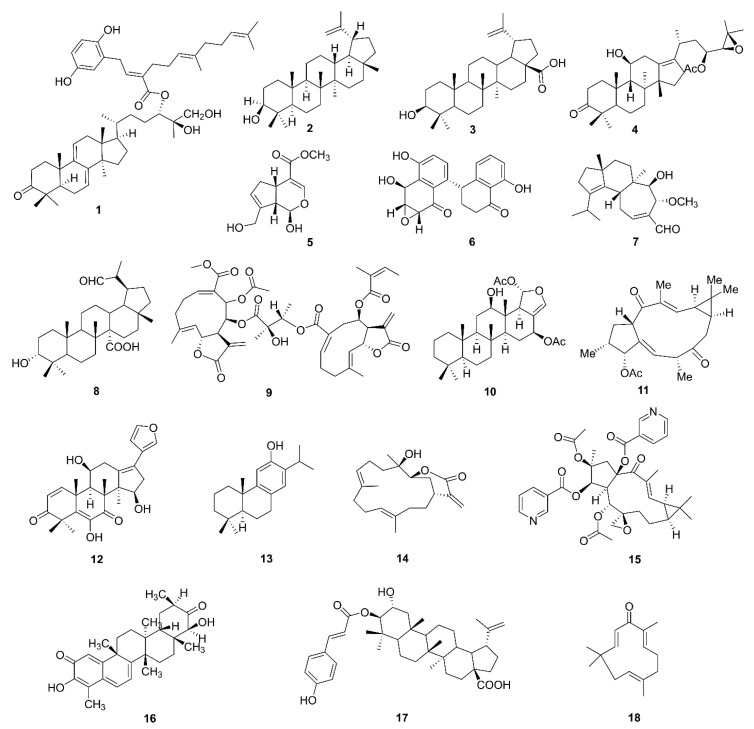
Terpenoids represent a diverse group of compounds consisting of isoprene or isopentane units linked by various connections. Terpenoids have a wide variety and complex structure throughout the plant kingdom, including monoterpenes (myrcene), sesquiterpenes (artemisinin, gossypol), diterpenes (triptolide), triterpenes (oleanolic acid), tetraterpenes (β-carotene), and polyterpenoids (gutta-percha). Due to their different properties, physiological activities are varied, such as anti-malarial [37], anti-fertility [38], insecticidal activities [39], etc. Here, we will summarize the mechanisms through which terpenoids regulate mitochondrial function to stimulate anticancer effects that have been discovered in recent years (Figure 2).
Figure 2.
Chemical structures of terpenoids (1–18).
Ganoleuconin O (GL22) (1), a triterpenoid, is obtained from Ganoderma leucocontextum. After liver cancer cell line Huh7.5 was treated with GL22, it was observed by transmission electron microscopy that the shape and size of mitochondria were changed, and mitochondrial cristae were fragmented. ATP production of Huh7.5 cells with GL22 treatment was decreased in a dose- and time-dependent manner. The amount of cardiolipin, which has vital structural and metabolic functions in mitochondria, was also decreased. The levels of P53 and Bax were upregulated, while Bcl-2 was downregulated. The dissipation of mitochondrial membrane potential (MMP) resulted in release of cytochrome c from mitochondria to the cytosol and caspase-9 activation, eventually triggering apoptosis [40].
Lupeol (lup-20(29)-en-3β-ol) (2), a pentacyclic triterpenoid, is found in fruits, such as strawberries and grapes, and medicinal plants, such as Bombax ceiba. It could affect viability of renal cell carcinoma SK-RC-45 cells by altering mitochondrial dynamics. The study showed the lupeol tilted mitochondrial dynamics towards fission in a dynamic balance between fusion and fission, which ultimately led to apoptosis. Mitochondrial morphometric parameters were evaluated by Fiji (ImageJ v1.52e) using the MiNA macro. It was observed that the defined morphological properties networks, mean length, mean network size, and mitochondrial footprint, were decreased in lupeol treated cells compared to control. In addition, anti-apoptotic protein Bcl-2 knockout enhanced the effect of Lupeol, causing mitochondrial fission and cell death [41].
Betulinic acid (BetA) (3), a lupane-type triterpenoid, derived from Betula alba and other plants has been described to kills tumor cells depending on mitochondrial permeability transition-pore opening. A study found that BetA induced changes of mitochondrial morphology in HeLa cells. The saturation level of cardiolipin can be affected rapidly and directly by BetA. Cardiolipin can regulate mitochondria-dependent cell death with important structural and metabolic functions. Because cardiolipin saturation in mitochondria was enhanced, the mitochondria underwent ultrastructural changes, and then cytochrome c was released inducing cell death [42]. Another study reported that BetA induced production of ROS and decline of MMP in HeLa cells. The protein expression of Bax and caspase 9 was increased. The results indicated ROS was the key factor for regulating the mitochondrial pathway of apoptosis [43]. Wang et al. reported that ROS was increased, MMP was lost, cytochrome c was released, and caspase-3 was activated after treatment with betulinic acid in PC12 cells, while the apoptosis could be reduced significantly by treating with antioxidants [44]. Similarly, Yang et al. reported that betulinic acid induced mitochondria-mediated apoptosis with downregulation of Bcl-2, ROS production and MMP loss in 786-O and ACHN renal cancer cells [45].
Alisol B-23-acetate (4), a tetracyclic triterpenoid, is a compound from Alisma orientale. In human lung cancer NCI-H292 and A549 cells, it reduced MMP, increased ROS level, and the Bax/Bcl-2 ratio. Caspase-3, caspase-9 and PARP were cleaved. Furthermore, cytochrome c was released into the cytoplasm and apoptotic inducing factor was translocated into nuclei [46].
Genipin (5) from Gardenia jasminoides was applied as an inhibitor of proton transport mediated by mitochondrial uncoupling protein 2 (UCP2). The scholars indicated that after treatment with genipin, dicarboxylate carrier was activated and activity of UCP1, UCP3, and complex III were decreased. UCP2 was inhibited in planar lipid bilayer membranes reconstituted with recombinant UCP2 or isolated mitochondrial proteins from N18TG2 cells [47].
Alternol (6), a fermentation product of a microorganism found in the bark of the yew tree [48], reduced the levels of mitochondrial respiration, isocitric acid, fumaric acid, and malic acid. Alternol also remarkably decreased ATP production in PC-3 prostate cancer cells in vitro and in xenograft tissues [49].
Cyathin Q (7), derived from Cyathus africanus, regulated proteins of the Bcl-2 family, increased ROS generation, and released cytochrome c in HCT116 cells [50]. These anticancer mechanisms may also be used by the compounds 3α-hydroxy-19α-hydrogen-29-aldehyde-27-lupanoic acid (8) [51] and uvedafolin (9) [133]. Heteronemin (10), a secondary metabolite in the sponge Hippospongia sp. could induce ROS production in Molt4 cells [134].
Jatrogossone A (11), found in Jatropha gossypiifolia, is a special class of macrocyclic compound featuring a trans-bicyclo [10.3.0] pentadecane framework. It was reported that it affected MMP and induced ROS generation in KONP-8 human leukemic cells, while ROS generation was minimal in non-cancer cells [135]. A limonoid small molecule Walsuronoid B (12), isolated from Walsurarobusta increased the level of ROS generation and induced mitochondrial and lysosomal dysfunction in Bel-7402 and HepG2 liver cancer cells [136].
There are many other compounds isolated from natural products that exert anticancer effects through regulating mitochondria. They induce production of ROS and reduce the expression of Bcl-2 in various tumor cells. These compounds include ferruginol (13) [52,53,137], lobocrassin B (14) [53], aellinane (15) [54], tingenin B (16) [55], 3-O-trans-p-Coumaroyl alphitolic acid (17) [56], and zerumbone (18) [57,58].
5.2. Flavonoids
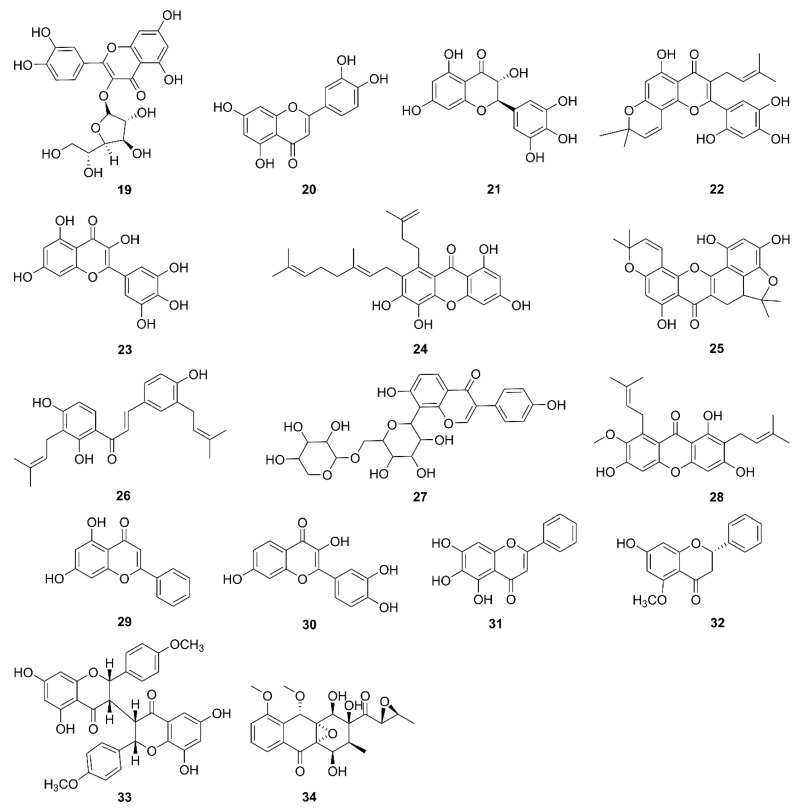
Flavonoids are compounds that exist widely in nature—in vegetables, fruits [59], and Chinese medicine. The basic backbone of flavonoids is two benzene rings connected by three carbon atoms. Flavonoids are divided into flavones, flavonols, isoflavones, flavanols, anthocyanidins, and isoflavones [60]. Flavonoids have a wide range of health benefits, such as gut health [61], and antioxidant, anti-neuroinflammatory [62], and anticancer [63] properties. They have been developed for use in nutraceuticals, cosmetics, and medical drugs. Chemical structures of flavonoids in recent research are displayed in Figure 3.
Figure 3.
Chemical structures of flavonoids (19–34).
Isoquercitrin (19), a flavone-based natural product, induced the expression of key proteins in the mitochondrial-mediated apoptosis pathway, and it also caused apoptosis in the breast cancer cell line MDA-MB-231 by inhibition of lysine-specific demethylase 1 (LSD1), which can regulate mitochondrial functions [64,65,66], and has recently become a therapeutic target for cancer. The study found the mitochondrial transmembrane potential and ratio of Bcl-2/Bax was lower in the isoquercitrin + LSD1 siRNA-treated group than in the control group and the LSD1 siRNA-treated, isoquercitrin groups [67,68].
Luteolin (20), a dietary compound, can be found in fruits and vegetables including cauliflower, peanuts, and carrots. A study reported it was an inhibitor of Bcl-2 by using structure-based virtual ligand screening. The result of a three-dimensional (3D) molecular docking model showed it has a significant ability to interact with the key residues in the hydrophobic pocket of the Bcl-2 protein. Through the microscale thermophoresis (MST) experiment in SW1990 cancer cells, the Kd value of BH3 peptide bound to Bcl-2 was lower than that of luteolin bound to Bcl-2. Besides, luteolin did not bind to the BH3 domain of Bax [69].
Dihydromyricetin (21), a plant flavonol, isolated from Ampelopsis grossedentata, induced apoptosis in HepG2 cells through a mitochondrial pathway in a recent report. Expression of proteins Bax and Bad was upregulated. The phosphorylation of Bad at Ser112 and Ser136 was inhibited. Expression of Akt and its phosphorylation at Ser473 were reduced. The authors concluded that HepG2 apoptosis might be induced by dihydromyricetin by inhibiting the Akt/Bad signaling pathway and stimulating mitochondrial apoptotic pathways [70,71].
There are some other compounds that induce cancer cell death or an anti-proliferative effect, such as artonin E (22) [72], myricetin (23) [73], xanthones (24) [138], cycloartobiloxanthone (25) [139], paratocarpin E (26) [74], and puerarin 6′’-O-xyloside (27) by regulating the Bcl-2 family proteins [75]. Moreover, others induced the overproduction of ROS, such as α-mangostin (28) [76], chrysin (29) [77,78], and fisetin (30) [79,80].
A strong antitumor ability was suggested by the release of cytochrome c into the cytoplasm in the combination treatment with baicalein (31) and taxol in A2780 cells [81,82]. Alpinetin (32), mainly from zingiberaceous plants, increased the resistance of A549 lung cancer cells to cis-diammineddichloridoplatium. It regulated the expression of Bcl-2 family proteins, XIAP and cytochrome c [83,84]. Chamaejasmin B (33), from Stellera chamaejasme exerted an anti-multidrug resistance effect by regulating Bcl-2/Bax ratio, MMP loss, and release of cytochrome c [86]. Mensacarcin (34), extracted from Streptomyces bacteria could quickly disturb mitochondrial function and energy production [87].
5.3. Saponins
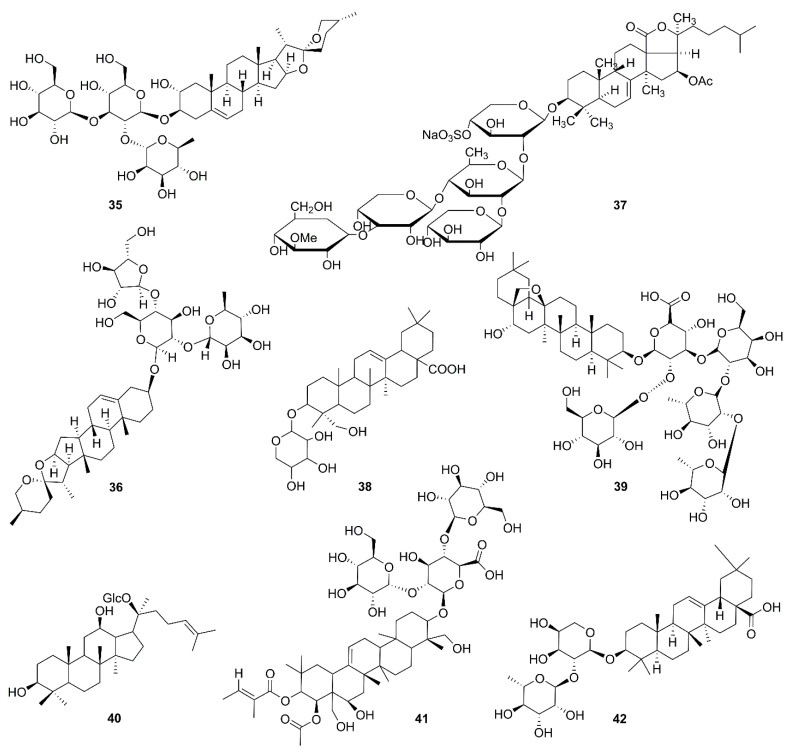
Saponins are a kind of complex glycoside synthesized in the plant kingdom, and are composed of sapogenin and sugar chain(s). The saponins can be divided into two groups according to the structure of their sapogenin: triterpenoid or steroidal saponins [88]. Triterpenoid saponins are mainly distributed in Araliaceae, Leguminosae, Campanulaceae, and other plants. Steroidal saponins are commonly reported in Liliaceae, Dioscoreaceae, Amaryllidaceae, etc. [85]. Chemical structures of saponins in recent research are displayed in Figure 4.
Figure 4.
Chemical structures of saponins (35–42).
Gracillin (35), a diosgenin glycoside, is a steroidal saponin. Hye-Young Min et al. reported it exerted anticancer ability by targeting mitochondrial complex II in H226B and H460 cells. Thus, it reduced mitochondria-mediated cellular bioenergetics by inhibiting ATP synthesis and ROS production. It inhibited complex II function by disabling succinic dehydrogenase activity without affecting the succinate: quinone reductase. The cell death induced by gracillin was enhanced by thenoyltrifluoroacetone or 3-nitropropionic acid, which inhibited complex II by binding to the succinate dehydrogenase complex subunit A (SDHA) active site, or the ubiquinone binding site, respectively [89].
Polyphyllin I (36), a steroidal saponin, extracted from Paris polyphylla rhizomes was reported to induce MDA-MB-231 cells apoptosis through regulating mitochondrial PTEN (Phosphatase and tensin homolog deleted on chromosome ten)-induced kinase 1 (PINK1) levels. PINK1 is localized at the mitochondria as it contains a mitochondrial targeting sequence. Polyphyllin I induced mitochondrial translocation of dynamin-related protein 1 (DRP1) by dephosphorylation of DRP1 at the Ser637 site, resulting in mitochondrial fission, release of cytochrome c, and finally cell apoptosis. It also enhanced stability of the full-length PINK1 on the mitochondrial surface, resulting in the recruitment of microtubule-associated protein light chain 3 beta (LC3B-II), ubiquitin, P62, and PARK2 (a RING domain-containing E3 ubiquitin ligase that can be activated through autoubiquitination) to mitochondria for mitophagy. The knockdown of PINK1 significantly inhibited the mitophagy induced by polyphyllin I and enhanced mitochondrial fission and apoptosis [90,91].
Frondoside A (37), a triterpene glycoside, is a marine product first exacted from Cucumaria frondosa. After treatment of frondoside A in multiresistant CA46 cells, levels of antiapoptotic Bcl-2 and survivin were decreased. Apoptosis-inducing factor, HtrA2/Omi and cytochrome c were released. It induced production of ROS. Frondoside A targeted mitochondria, which was not dependent on p53 and caspases [92].
Clematis hederagenin saponin (hederagenin 3β-O-α-l-arabinopyranoside, (38) is a triterpenoid saponin of Clematis ganpiniana, which was reported to induce apoptosis through the mitochondrial pathway with release of cytochrome c and Apaf-1 and activation of caspase-9 and caspase-3 [93]. In addition, sakuraso-saponin (39) from Aegiceras corniculatum could regulate expression of Bcl-xL [94,95]. Ginsenoside compound K (40) [96] and escin (41) [140] induced ROS-mediated apoptosis, and α-Hederin (42) from Hedera helix induced mitochondrial apoptosis through blocking the NF-κB signaling pathway through the regulation of the levels of Bcl-2, Bax, and cytochrome c [141,142].
5.4. Alkaloids
Alkaloids generally refer to a class of nitrogen-containing natural products, most of which have complex heterocyclic structure, physiological activity, and alkalinity. Morphine isolated from opium has an analgesic effect. Codeine has antitussive effects. Ephedrine has an antiasthmatic effect. Berberine has antibacterial and anti-inflammatory effects. Chemical structures of alkaloids in recent studies are displayed in Figure 5.
Figure 5.
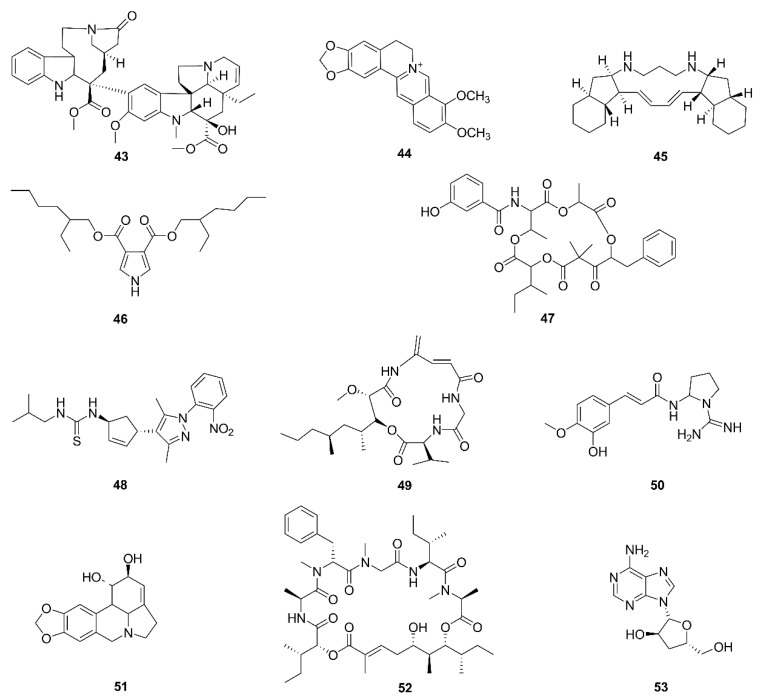
Chemical structures of alkaloids (43–53).
Berberine (44), extracted from Rhizomacoptidis, has long been used as an antimicrobial agent with antitumor abilities in China [97]. In glioma cells, berberine could inhibit the aerobic oxidation and reduce the energy production efficiency of mitochondria, and reduce the metabolic activity by decreasing the activity of extracellular signal-regulated kinase 1/2 (ERK1/2). After treatment with berberine, the ridges and membrane of mitochondria were damaged, the level of ATP dropped rapidly, the ROS scavenger l-Glutathione (GSH) decreased, and NADPH decreased. The authors indicated that inhibition of ERK1/2 activity induced mitochondrial dysregulation by the reduced abundance of p-ERK after treatment of T98G cells [98].
Papuamine (45) is a pentacyclic alkaloid extracted from marine natural products including Haliclona sp. Intracellular ATP was depleted by papuamine through causing dysfunction of mitochondria in H1299 lung cancer cells as MMP was lost and production of mitochondrial superoxide was increased. The study suggested papuamine, by causing mitochondrial dysfunction, thus reducing the generation of cellular energy, induced cell apoptosis, so as to exert its anticancer effect [99].
Cathachunine (43), is a bisindole alkaloid derived from Catharanthus roseus. The apoptosis induced by cathachunine relied on the Bcl-2 protein family through an ROS- dependent mitochondria-mediated intrinsic pathway in HL60 cells. The ratio of Bcl-2/Bax was dysregulated, MMP was lost, cytochrome c was released, and production of ROS was increased [100].
A pyrrole based compound, Bis (2-ethyl hexyl) 1H-pyrrole-3, 4-dicarboxylate (46), from Tinosporacordifolia induced production of ROS, increased intracellular calcium levels, phosphorylated p53, downregulated Bcl-2/Bax ratio and led to cardiolipin peroxidation and mitochondrial membrane depolarization. Thereupon cytochrome c was released and caspases were activated, resulting in MDA-MB-231 cell apoptosis [101].
Unantimycin A (47), found in a fractionated chemical library of microbial metabolites, and NPL40330 (48), found in a chemical library, targeted and inhibited the activity of mitochondrial complexes I and III, respectively. Thus, they played a role in inhibiting mitochondrial respiration [102].
A 4-amido-2,4-pentadieneoate (APD)-class peptide named boholamide A (49) from a bacterial extract (Nocardiopsis sp.) from marine mollusks (Truncatella sp.), directly regulated intracellular Ca2+ in U87MG cells. Natural products of the APD-class have hypoxia-activated cytotoxins, targeting mitochondria [103]. Cernumidine (50) is a guanidinic alkaloid, which exerted antitumor effects through mitochondria by downregulating the Bcl-2/Bax ratio and causing MMP loss in the combination treatment with cisplatin in T24 cells [104]. Lycorine (51), extracted from plants of the Amaryllidaceae family, induced apoptosis in HepG2 cells through mPTP opening, ATP depletion, MMP loss, and mitochondrial Ca2+ and cytochrome c release [105]. Lagunamides A (52) from Lyngbya majuscule caused A549 cell death accompanied by MMP loss, ROS overproduction, mitochondrial dysfunction, and changes in the levels of Bcl-2 family proteins [106]. Cordycepin (53), isolated from Cordyceps, downregulated mitochondrial function and limited energy production, thus inhibiting metastasis and migration in OVCAR-3 cells [107,143].
5.5. Coumarins
Coumarin compounds are widespread in the plant kingdom, with a few coming from animals and microorganisms. Their basic backbone contains a fused benzene and α-pyrone ring [108]. They are widely present in Umbelliferae, Leguminosae, Rutaceae, Solanaceae, and Asteraceae [109] and are found in many traditional Chinese medicines. Coumarin has extensive pharmacological activities, such as anti-inflammatory, antihyperlipidemia, antihypertensive, and antitumor [110]. Chemical structures of coumarin in recent studies are displayed in Figure 6.
Figure 6.
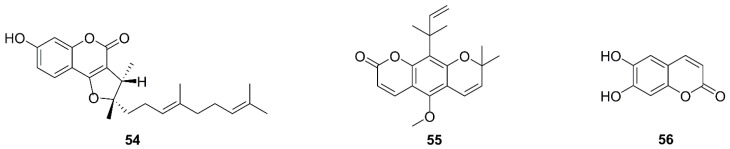
Chemical structures of coumarins (54–56).
The 2,3-Dihydro-7-hydroxy-2R*,3R*-dimethyl-2-[4,8-dimethyl-3(E),7-nonadienyl]-furo[3,2-c]coumarin (54), named DAW22, is a sesquiterpene coumarin extracted from Ferula ferulaeoides. In C6 glioma cells, apoptosis induced by DAW22 is mediated by the death receptor pathway and mitochondrial pathway. It reduced MMP in a time-dependent manner. Results showed that the expression of Bax significantly increased, whereas that of Bcl-2 and Bcl-xL decreased, and the cleavage of Bid was stimulated. Moreover, the level of FAS (recombinant factor related apoptosis) and FADD (Fas-associated protein with death domain) were elevated markedly [111].
Dentatin (55), isolated from Clausena excavate, could increase the level of cytoplasmic cytochrome c and Bax, and down-regulate Bcl-2 and Bcl-xL in HepG2 cells [112,113]. Aesculetin (56), a natural coumarin derivative of intramolecular cyclization produced by a cinnamic acid exerted antitumor effects via mitochondrial mediated apoptosis in THP-1 macrophage cells with upregulating Bax and downregulating Bcl-2 [114].
5.6. Quinones
Natural quinones, the compounds containing a six-member cyclic conjugated unsaturated diketone structure, mainly include four types, benzoquinone, naphthoquinone, phenanthrenequinone, and anthraquinone. Anthraquinone and its derivatives are particularly important in traditional Chinese medicine. Chemical structures of quinines in recent studies are displayed in Figure 7.
Figure 7.
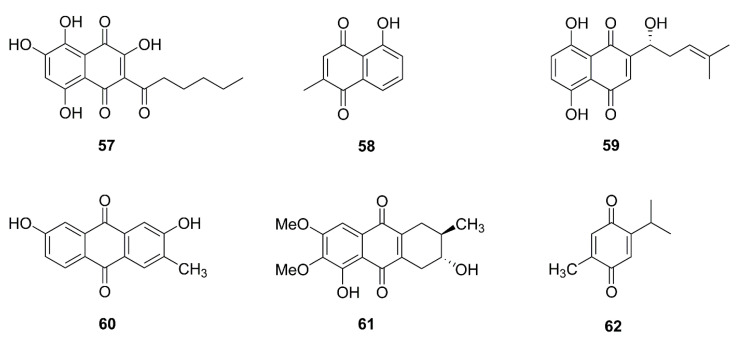
Chemical structures of quinines (57–62).
Quambalarine B (57) is a natural naphthoquinonic compound from Quambalariac yanescens [115]. It inhibited the activity of mitochondrial complex I and II and reduced the metabolism of aspartic acid and folic acid as therapeutic targets in Jurkat cells. Inhibition of mitochondrial respiration by quambalarine B triggered a reprogramming of leukemic cell metabolism, including an imbalance of glycolysis, inhibition of protein o-glycosylation, increased activity of pyruvate kinase, and stimulation of glycine synthesis pathways, and inhibition of aspartate synthesis. This led to increased pyruvic acid and decreased lactic acid levels. To inhibit mitochondrial complex I activity, quambalarine B inhibits folic acid metabolism, reducing the production of formate. In addition, several amino acids were increased at the cellular level [116].
Plumbagin (58), a naphthoquinone, is derived from Plumbago zeylanica. After treatment with plumbagin in MG63 cells for 24 h, production of ROS was increased, the protein levels of Bcl-2, Bax, Bcl-xL, and Bak were altered [117]. The naphthoquinone pigments shikonin (59) of Lithospermum erythrorhizon had a similar mechanism in HGC-27 cells, like that of plumbagin in MG63 cells [118].
The 2,7-dihydroxy-3-methylanthraquinone (60), isolated from Hedyotisdiffusa, decreased the expression of Bcl-xL and Bcl-2, increased Bax and Bad, released cytochrome c, and activated caspase-3 and -9 in SGC-7901 cells [119]. Moreover, 3-hydroxy-1,5,6-trimethoxy-2-methyl-9,10-anthraquinone (61) derived from Prismatomeris connate reduced expression of Bcl-2 and Mcl-1, and increased Bax in A549 and H1299 cells [120]. They are similar to thymoquinone (62), a compound of the black seed oil from Nigella sativa [121].
5.7. Miscellanea
Other natural products, different from the above structures (Figure 8), had an antitumor effect by regulating mitochondria.
Figure 8.
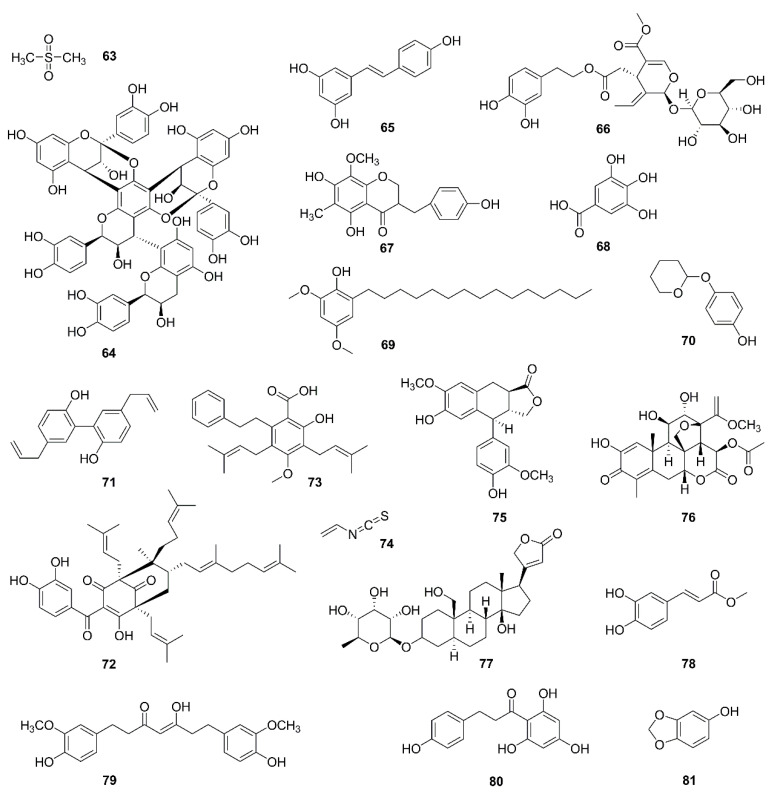
Chemical structures of other compounds isolated from natural products (63–81).
For example, interesting research found that macrocyclic lipodepsipeptides containing 4-amido-2,4-pentadienoate had cytotoxic selectivity for hypoxic cancer cells by inducing a rapid loss of mitochondrial ultrastructure and function [122].
Methylsulfonylmethane (63), a natural organic sulfur-containing compound found in fruits and vegetables, decreased Bcl-2 and Bcl-xL levels and MMP, increased Bax level, and released cytochrome c into the cytosol in YD-38 gingival cancer cells [123]. Recombinant buckwheat trypsin inhibitor, extracted from tartary buckwheat, induced mitophagy, depolarized mitochondria, and increased ROS in HepG2 cells [124].
Some phenols regulate mitochondria. Parameritannin A-2 (64), isolated from Urceolahuaitingii, enhances doxorubicin-induced mitochondria-dependent apoptosis in HGC27 gastric cancer cells, in part by inhibiting the PI3K/Akt, ERK1/2, and p38 pathways. After combination treatment with parameritannin A-2 and doxorubicin, protein levels of Bcl-2 and Bcl-xL were decreased, and Bax and Bid were increased more significantly than after the single treatments. Cytochrome c was released and caspases were activated [125]. Similarly, resveratrol (65) enhanced antitumor activities of cisplatin on H838 and H520 cancer cells [126]. Another study showed resveratrol induced apoptosis in K562 cells [127]. In addition, phenols like oleuropein (66) [128,129], homoisoflavanone-1 (67) [130], gallic acid (68) [131], hierridin b (69) [132,144], and deoxyarbutin (70) [145] could all induce mitochondrial dysfunction.
Magnolol (71), a phenylpropanoid, derived from Magnolia officinalis induced apoptosis in OS-RC-2 and 786-O cell lines by regulation of Bcl-2, Bax and p53. ROS generation, cytochrome c release and caspase activation were also observed [146].
Oblongifolin C (72), a polycyclic polyprenylated acylphloroglucinol (PPAP) compound, isolated from Garcinia yunnanensis induced mitochondrial dysfunction and apoptosis in QBC939 human cholangiocarcinoma cells [147].
Amorfrutin C (73), belongs to the amorfrutin benzoic acid class of compounds found in Glycyrrhiza foetida. Treatment with amorfrutin C disrupted the mitochondrial integrity and permanently opened mPTP, leading to increased mitochondrial oxygen consumption and extracellular acidification in HT-29 cells [148].
There are some other natural compounds exerting antitumor effect via mitochondria with ROS generation, cytochrome c release, MMP loss, altered expression of Bcl-2 family members, and caspase activation, such as allyl isothiocyanate (74) [149,150], α-conidendrin (75) [151], dehydrobruceine B (76) [152], frugoside (77) [153,154], methyl caffeate (78) [155], tetrahydrocurcumin (79) [156], phloretin (80) [157], and sesamol (81) [158].
6. Natural Products and Anticancer Agents in Combination
Natural products have attracted much attention because they are relatively easy to obtain and cause few side effects. Many scholars have studied their activities in combination with cancer drugs in vitro and vivo. Xia et al. showed that there was a protective effect of magnolol on oxaliplatin-induced intestinal injury in mice [159]. Magnolol significantly improved weight loss, diarrhea, and other adverse reactions after oxaliplatin administration.
In addition, some natural products can increase the activity of anticancer agents. They can be used—not only as a complementary and alternative therapy—but also to enhance the efficacy of resistant cell lines. Combination with cisplatin and chrysin promotes the apoptosis of HepG2 cells by upregulating p53 [160], polyphyllin I enhances apoptosis and suppresses the CIP2A/AKT/mTOR signaling pathway in A549/DDP cells [161]. Combining ginsenoside compound K with cisplatin produced a better effect on the apoptosis and epithelial mesenchymal transition through the PI3K/Akt pathway in MCF-7 cells [162]. The combined effect of α-Hederin and cisplatin was better than both compounds alone on apoptosis by increasing ROS and decreasing MMP in vitro and vivo [163]. Berberine [164], cernumidine [144], shikonin [165], gallic acid [131,166], and dehydrobruceine b [167] also had a chemosensitizing effect when added together with cisplatin.
Combination treatment of sorafenib and luteolin enhanced JNK activation and apoptosis in Hep3B and SMMC-7721 hepatocellular carcinoma cells [168]. Sorafenib and luteolin combination synergistically inhibited proliferation of AsPC-1, BxPC-3, and Capan-1 pancreatic ductal adenocarcinoma cells by targeting the P13K/Akt and MAPK signaling pathways [169]. Combination of α-mangostin and sorafenib enhanced apoptosis by inhibition of the activated Akt and Erk pathways in SK-MEL-2 cells and SK-MEL-30 cells [170].
Lupeol and 5-fluorouracil combination exerted a better effect on inhibition of tumor weight on BGC823 xenograft mouse [171]. Combination treatment with 5-fluorouracil and other natural compounds, such as fisetin [172], frondoside a [173], or esculetin [174] led to more significant effects on cancer cells. Similarly, there are highly efficacious co-treatments that use combinations of baicalein and taxol [81], thymoquinone and gemcitabine [175], parameritannin A-2 and doxorubicin [125], gallic acid and paclitaxel [176], and allyl isothiocyanate and celecoxib [177].
7. Conclusions
We focused on natural compounds that have been identified in the last five years with anticancer activity targeting mitochondria and their origin and structural classification. Phytochemistry accounts for most of the composition, with a small amount of marine and microbial metabolites. Most of these compounds are terpenoids, phenols, and flavonoids. Some of these compounds regulate the expression level of Bcl-2 family proteins, some induce ROS production, some alter metabolism, and some target mitochondrial complexes. This review shows that large and varied classes of plant-derived and other natural products can exert anticancer activity by regulating mitochondria. However, some natural products are non-toxic, show poor efficacy, or have special mitochondrial targeting, so it is of great significance to develop further structural modifications and derivatives based on the natural structures.
Author Contributions
Y.Y. contributed to literature search and writing the manuscript. P.-Y.H. and Y.Z. contributed to the table and figures. N.L. contributed to the conception and design of the review. Authorship must be limited to those who have contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (31670353) and key research and development projects in Anhui Province (202004a07020035).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Lancet GLOBOCAN 2018: Counting the toll of cancer. Lancet. 2018;392:985. doi: 10.1016/S0140-6736(18)32252-9. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y., He P., Li N. The Antitumor Potential of Extract of the Oak Bracket Medicinal Mushroom Inonotus baumii in SMMC-7721 Tumor Cells. Evid. Based Complement. Alternat. Med. 2019;2019:1242784. doi: 10.1155/2019/1242784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y., Zhang L., Chen Q., Lu W.L., Li N. Antitumor Effects of Extract of the Oak Bracket Medicinal Mushroom, Phellinus baumii (Agaricomycetes), on Human Melanoma Cells A375 In Vitro and In Vivo. Int. J. Med. Mushrooms. 2020;22:197–209. doi: 10.1615/IntJMedMushrooms.2020033687. [DOI] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M., Snader K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaou K.C., Yang Z., Liu J.J., Ueno H., Nantermet P.G., Guy R.K., Claiborne C.F., Renaud J., Couladouros E.A., Paulvannan K., et al. Total synthesis of taxol. Nature. 1994;367:630–634. doi: 10.1038/367630a0. [DOI] [PubMed] [Google Scholar]
- 8.Wall M.E., Wani M.C. Camptothecin and taxol: Discovery to clinic—Thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1995;55:753–760. [PubMed] [Google Scholar]
- 9.Vilpo J., Vilpo L. Selective toxicity of vincristine against chronic lymphocytic leukaemia in vitro. The Tampere CLL Group. Lancet. 1996;347:1491–1492. doi: 10.1016/S0140-6736(96)91730-4. [DOI] [PubMed] [Google Scholar]
- 10.Kano Y., Ohnuma T., Okano T., Holland J.F. Effects of vincristine in combination with methotrexate and other antitumor agents in human acute lymphoblastic leukemia cells in culture. Cancer Res. 1988;48:351–356. [PubMed] [Google Scholar]
- 11.Bhandary B., Marahatta A., Kim H.R., Chae H.J. Mitochondria in relation to cancer metastasis. J. Bioenerg. Biomembr. 2012;44:623–627. doi: 10.1007/s10863-012-9464-x. [DOI] [PubMed] [Google Scholar]
- 12.Peixoto P.M., Ryu S.Y., Kinnally K.W. Mitochondrial ion channels as therapeutic targets. FEBS Lett. 2010;584:2142–2152. doi: 10.1016/j.febslet.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunewald A., Kumar K.R., Sue C.M. New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 2019;177:73–93. doi: 10.1016/j.pneurobio.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Yang S., Han Y., Liu J., Song P., Xu X., Zhao L., Hu C., Xiao L., Liu F., Zhang H., et al. Mitochondria: A Novel Therapeutic Target in Diabetic Nephropathy. Curr. Med. Chem. 2017;24:3185–3202. doi: 10.2174/0929867324666170509121003. [DOI] [PubMed] [Google Scholar]
- 15.Ishimoto Y., Inagi R. Mitochondria: A therapeutic target in acute kidney injury. Nephrol. Dial. Transplant. 2016;31:1062–1069. doi: 10.1093/ndt/gfv317. [DOI] [PubMed] [Google Scholar]
- 16.Valenti D., Braidy N., De Rasmo D., Signorile A., Rossi L., Atanasov A.G., Volpicella M., Henrion-Caude A., Nabavi S.M., Vacca R.A. Mitochondria as pharmacological targets in Down syndrome. Free Radic. Biol. Med. 2018;114:69–83. doi: 10.1016/j.freeradbiomed.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Grad J.M., Cepero E., Boise L.H. Mitochondria as targets for established and novel anti-cancer agents. Drug Resist. Updat. 2001;4:85–91. doi: 10.1054/drup.2001.0192. [DOI] [PubMed] [Google Scholar]
- 18.Costantini P., Jacotot E., Decaudin D., Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J. Natl. Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- 19.Wen S., Zhu D., Huang P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med. Chem. 2013;5:53–67. doi: 10.4155/fmc.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D., Wang X., Sun H. The role of mitochondria in cellular toxicity as a potential drug target. Cell Biol. Toxicol. 2018;34:87–91. doi: 10.1007/s10565-018-9425-1. [DOI] [PubMed] [Google Scholar]
- 21.Bhat T.A., Kumar S., Chaudhary A.K., Yadav N., Chandra D. Restoration of mitochondria function as a target for cancer therapy. Drug Discov. Today. 2015;20:635–643. doi: 10.1016/j.drudis.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner D., Mak T.W. Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 2009;21:871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay J., Esposti M.D., Gilmore A.P. Bcl-2 proteins and mitochondria—Specificity in membrane targeting for death. Biochim. Biophys. Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Chalah A., Khosravi-Far R. The mitochondrial death pathway. Adv. Exp. Med. Biol. 2008;615:25–45. doi: 10.1007/978-1-4020-6554-5_3. [DOI] [PubMed] [Google Scholar]
- 25.Karbowski M., Youle R.J. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 26.Mancini M., Anderson B.O., Caldwell E., Sedghinasab M., Paty P.B., Hockenbery D.M. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J. Cell Biol. 1997;138:449–469. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vyas S., Zaganjor E., Haigis M.C. Mitochondria and Cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernster L., Schatz G. Mitochondria: A historical review. J. Cell Biol. 1981;91:227s–255s. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren M., Phoon C.K., Schlame M. Metabolism and function of mitochondrial cardiolipin. Prog. Lipid Res. 2014;55:1–16. doi: 10.1016/j.plipres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Ikon N., Ryan R.O. Cardiolipin and mitochondrial cristae organization. Biochim. Biophys. Acta Biomembr. 2017;1859:1156–1163. doi: 10.1016/j.bbamem.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dudek J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell Dev. Biol. 2017;5:90. doi: 10.3389/fcell.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kameoka S., Adachi Y., Okamoto K., Iijima M., Sesaki H. Phosphatidic Acid and Cardiolipin Coordinate Mitochondrial Dynamics. Trends Cell Biol. 2018;28:67–76. doi: 10.1016/j.tcb.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlattner U., Tokarska-Schlattner M., Epand R.M., Boissan M., Lacombe M.L., Kagan V.E. NME4/nucleoside diphosphate kinase D in cardiolipin signaling and mitophagy. Lab. Investig. 2018;98:228–232. doi: 10.1038/labinvest.2017.113. [DOI] [PubMed] [Google Scholar]
- 34.Ott M., Zhivotovsky B., Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- 35.Lucken-Ardjomande S., Montessuit S., Martinou J.C. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ. 2008;15:929–937. doi: 10.1038/cdd.2008.9. [DOI] [PubMed] [Google Scholar]
- 36.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells. 2019;8:728. doi: 10.3390/cells8070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong L.Y., Tan R.X. Artemisinin, a miracle of traditional Chinese medicine. Nat. Prod. Rep. 2015;32:1617–1621. doi: 10.1039/C5NP00133A. [DOI] [PubMed] [Google Scholar]
- 38.Ding G.S. Important Chinese herbal remedies. Clin. Ther. 1987;9:345–357. [PubMed] [Google Scholar]
- 39.Benelli G., Pavela R., Cianfaglione K., Sender J., Danuta U., Maslanko W., Canale A., Barboni L., Petrelli R., Zeppa L., et al. Ascaridole-rich essential oil from marsh rosemary (Ledum palustre) growing in Poland exerts insecticidal activity on mosquitoes, moths and flies without serious effects on non-target organisms and human cells. Food Chem. Toxicol. 2020;138:111184. doi: 10.1016/j.fct.2020.111184. [DOI] [PubMed] [Google Scholar]
- 40.Liu G., Wang K., Kuang S., Cao R., Bao L., Liu R., Liu H., Sun C. The natural compound GL22, isolated from Ganoderma mushrooms, suppresses tumor growth by altering lipid metabolism and triggering cell death. Cell Death Dis. 2018;9:689. doi: 10.1038/s41419-018-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha K., Chowdhury S., Banerjee S., Mandal B., Mandal M., Majhi S., Brahmachari G., Ghosh J., Sil P.C. Lupeol alters viability of SK-RC-45 (Renal cell carcinoma cell line) by modulating its mitochondrial dynamics. Heliyon. 2019;5:e02107. doi: 10.1016/j.heliyon.2019.e02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potze L., Di Franco S., Grandela C., Pras-Raves M.L., Picavet D.I., van Veen H.A., van Lenthe H., Mullauer F.B., van der Wel N.N., Luyf A., et al. Betulinic acid induces a novel cell death pathway that depends on cardiolipin modification. Oncogene. 2016;35:427–437. doi: 10.1038/onc.2015.102. [DOI] [PubMed] [Google Scholar]
- 43.Xu T., Pang Q., Wang Y., Yan X. Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells. Int. J. Mol. Med. 2017;40:1669–1678. doi: 10.3892/ijmm.2017.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J., Li H., Wang X., Shen T., Wang S., Ren D. Alisol B-23-acetate, a tetracyclic triterpenoid isolated from Alisma orientale, induces apoptosis in human lung cancer cells via the mitochondrial pathway. Biochem. Biophys. Res. Commun. 2018;505:1015–1021. doi: 10.1016/j.bbrc.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Lu X., Zhu R., Zhang K., Li S., Chen Z., Li L. Betulinic Acid Induces Apoptosis in Differentiated PC12 Cells Via ROS-Mediated Mitochondrial Pathway. Neurochem. Res. 2017;42:1130–1140. doi: 10.1007/s11064-016-2147-y. [DOI] [PubMed] [Google Scholar]
- 46.Yang C., Li Y., Fu L., Jiang T., Meng F. Betulinic acid induces apoptosis and inhibits metastasis of human renal carcinoma cells in vitro and in vivo. J. Cell. Biochem. 2018;119:8611–8622. doi: 10.1002/jcb.27116. [DOI] [PubMed] [Google Scholar]
- 47.Kreiter J., Rupprecht A., Zimmermann L., Moschinger M., Rokitskaya T.I., Antonenko Y.N., Gille L., Fedorova M., Pohl E.E. Molecular Mechanisms Responsible for Pharmacological Effects of Genipin on Mitochondrial Proteins. Biophys. J. 2019;117:1845–1857. doi: 10.1016/j.bpj.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X., Wang J., Sun B., Zhang Y., Zhu J., Li C. Cell growth inhibition, G2M cell cycle arrest, and apoptosis induced by the novel compound Alternol in human gastric carcinoma cell line MGC803. Investig. New Drugs. 2007;25:505–517. doi: 10.1007/s10637-007-9057-4. [DOI] [PubMed] [Google Scholar]
- 49.Li C., He C., Xu Y., Xu H., Tang Y., Chavan H., Duan S., Artigues A., Forrest M.L., Krishnamurthy P., et al. Alternol eliminates excessive ATP production by disturbing Krebs cycle in prostate cancer. Prostate. 2019;79:628–639. doi: 10.1002/pros.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He L., Han J., Li B., Huang L., Ma K., Chen Q., Liu X., Bao L., Liu H. Identification of a new cyathane diterpene that induces mitochondrial and autophagy-dependent apoptosis and shows a potent in vivo anti-colorectal cancer activity. Eur. J. Med. Chem. 2016;111:183–192. doi: 10.1016/j.ejmech.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J., Liu C., Huang R.Z., Chen H.F., Liao Z.X., Sun J.Y., Xia X.K., Wang F.X. Three new C-27-carboxylated-lupane-triterpenoid derivatives from Potentilla discolor Bunge and their in vitro antitumor activities. PLoS ONE. 2017;12:e0175502. doi: 10.1371/journal.pone.0175502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bispo de Jesus M., Zambuzzi W.F., Ruela de Sousa R.R., Areche C., Santos de Souza A.C., Aoyama H., Schmeda-Hirschmann G., Rodriguez J.A., Monteiro de Souza Brito A.R., Peppelenbosch M.P., et al. Ferruginol suppresses survival signaling pathways in androgen-independent human prostate cancer cells. Biochimie. 2008;90:843–854. doi: 10.1016/j.biochi.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Lin M.X., Lin S.H., Li Y.R., Chao Y.H., Lin C.H., Su J.H., Lin C.C. Lobocrassin B Induces Apoptosis of Human Lung Cancer and Inhibits Tumor Xenograft Growth. Mar. Drugs. 2017;15:378. doi: 10.3390/md15120378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nabatchian F., Moradi A., Aghaei M., Ghanadian M., Jafari S.M., Tabesh S. New 6(17)-epoxylathyrane diterpene: Aellinane from Euphorbia aellenii induces apoptosis via mitochondrial pathway in ovarian cancer cell line. Toxicol. Mech. Methods. 2017;27:622–630. doi: 10.1080/15376516.2017.1347735. [DOI] [PubMed] [Google Scholar]
- 55.Cevatemre B., Botta B., Mori M., Berardozzi S., Ingallina C., Ulukaya E. The plant-derived triterpenoid tingenin B is a potent anticancer agent due to its cytotoxic activity on cancer stem cells of breast cancer in vitro. Chem. Biol. Interact. 2016;260:248–255. doi: 10.1016/j.cbi.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Shin M., Lee B.M., Kim O., Tran H.N.K., Lee S., Hwangbo C., Min B.S., Lee J.H. Triterpenoids from Ziziphus jujuba induce apoptotic cell death in human cancer cells through mitochondrial reactive oxygen species production. Food Funct. 2018;9:3895–3905. doi: 10.1039/C8FO00526E. [DOI] [PubMed] [Google Scholar]
- 57.Chan M.L., Liang J.W., Hsu L.C., Chang W.L., Lee S.S., Guh J.H. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015;388:1223–1236. doi: 10.1007/s00210-015-1152-z. [DOI] [PubMed] [Google Scholar]
- 58.Prasannan R., Kalesh K.A., Shanmugam M.K., Nachiyappan A., Ramachandran L., Nguyen A.H., Kumar A.P., Lakshmanan M., Ahn K.S., Sethi G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012;84:1268–1276. doi: 10.1016/j.bcp.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Assini J.M., Mulvihill E.E., Huff M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013;24:34–40. doi: 10.1097/MOL.0b013e32835c07fd. [DOI] [PubMed] [Google Scholar]
- 60.Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 61.Pei R., Liu X., Bolling B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020;61:153–159. doi: 10.1016/j.copbio.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 62.Li Q., Wang Y., Mai Y., Li H., Wang Z., Xu J., He X. Health Benefits of the Flavonoids from Onion: Constituents and Their Pronounced Antioxidant and Anti-Neuroinflammatory Capacities. J. Agric. Food Chem. 2020;68:799–807. doi: 10.1021/acs.jafc.9b07418. [DOI] [PubMed] [Google Scholar]
- 63.Androutsopoulos V.P., Papakyriakou A., Vourloumis D., Tsatsakis A.M., Spandidos D.A. Dietary flavonoids in cancer therapy and prevention: Substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol. Ther. 2010;126:9–20. doi: 10.1016/j.pharmthera.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Duteil D., Metzger E., Willmann D., Karagianni P., Friedrichs N., Greschik H., Gunther T., Buettner R., Talianidis I., Metzger D., et al. LSD1 promotes oxidative metabolism of white adipose tissue. Nat. Commun. 2014;5:4093. doi: 10.1038/ncomms5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hino S., Sakamoto A., Nagaoka K., Anan K., Wang Y., Mimasu S., Umehara T., Yokoyama S., Kosai K., Nakao M. FAD-dependent lysine-specific demethylase-1 regulates cellular energy expenditure. Nat. Commun. 2012;3:758. doi: 10.1038/ncomms1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sakamoto A., Hino S., Nagaoka K., Anan K., Takase R., Matsumori H., Ojima H., Kanai Y., Arita K., Nakao M. Lysine Demethylase LSD1 Coordinates Glycolytic and Mitochondrial Metabolism in Hepatocellular Carcinoma Cells. Cancer Res. 2015;75:1445–1456. doi: 10.1158/0008-5472.CAN-14-1560. [DOI] [PubMed] [Google Scholar]
- 67.Xu X., Peng W., Liu C., Li S., Lei J., Wang Z., Kong L., Han C. Flavone-based natural product agents as new lysine-specific demethylase 1 inhibitors exhibiting cytotoxicity against breast cancer cells in vitro. Bioorg. Med. Chem. 2019;27:370–374. doi: 10.1016/j.bmc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 68.Valentova K., Vrba J., Bancirova M., Ulrichova J., Kren V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014;68:267–282. doi: 10.1016/j.fct.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 69.Li Z., Zhang Y., Chen L., Li H. The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Funct. 2018;9:3018–3027. doi: 10.1039/C8FO00033F. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z., Zhang H., Chen S., Xu Y., Yao A., Liao Q., Han L., Zou Z., Zhang X. Dihydromyricetin induces mitochondria-mediated apoptosis in HepG2 cells through down-regulation of the Akt/Bad pathway. Nutr. Res. 2017;38:27–33. doi: 10.1016/j.nutres.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 71.Hou X.L., Tong Q., Wang W.Q., Shi C.Y., Xiong W., Chen J., Liu X., Fang J.G. Suppression of Inflammatory Responses by Dihydromyricetin, a Flavonoid from Ampelopsis grossedentata, via Inhibiting the Activation of NF-κB and MAPK Signaling Pathways. J. Nat. Prod. 2015;78:1689–1696. doi: 10.1021/acs.jnatprod.5b00275. [DOI] [PubMed] [Google Scholar]
- 72.Rahman M.A., Ramli F., Karimian H., Dehghan F., Nordin N., Ali H.M., Mohan S., Hashim N.M. Artonin E Induces Apoptosis via Mitochondrial Dysregulation in SKOV-3 Ovarian Cancer Cells. PLoS ONE. 2016;11:e0151466. doi: 10.1371/journal.pone.0151466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jo S., Ha T.K., Han S.H., Kim M.E., Jung I., Lee H.W., Bae S.K., Lee J.S. Myricetin Induces Apoptosis of Human Anaplastic Thyroid Cancer Cells via Mitochondria Dysfunction. Anticancer Res. 2017;37:1705–1710. doi: 10.21873/anticanres.11502. [DOI] [PubMed] [Google Scholar]
- 74.Gao S., Sun D., Wang G., Zhang J., Jiang Y., Li G., Zhang K., Wang L., Huang J., Chen L. Growth inhibitory effect of paratocarpin E, a prenylated chalcone isolated from Euphorbia humifusa Wild. by induction of autophagy and apoptosis in human breast cancer cells. Bioorg. Chem. 2016;69:121–128. doi: 10.1016/j.bioorg.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X.L., Wang B.B., Mo J.S. Puerarin 6″-O-xyloside possesses significant antitumor activities on colon cancer through inducing apoptosis. Oncol. Lett. 2018;16:5557–5564. doi: 10.3892/ol.2018.9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Habbash A.I., Mohd Hashim N., Ibrahim M.Y., Yahayu M., Omer F.A.E., Abd Rahman M., Nordin N., Lian G.E.C. In vitro assessment of anti-proliferative effect induced by alpha-mangostin from Cratoxylum arborescens on HeLa cells. PeerJ. 2017;5:e3460. doi: 10.7717/peerj.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seydi E., Rahimpour Z., Salimi A., Pourahmad J. Selective toxicity of chrysin on mitochondria isolated from liver of a HCC rat model. Bioorg. Med. Chem. 2019;27:115163. doi: 10.1016/j.bmc.2019.115163. [DOI] [PubMed] [Google Scholar]
- 78.Khoo B.Y., Chua S.L., Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010;11:2188–2199. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su C.H., Kuo C.L., Lu K.W., Yu F.S., Ma Y.S., Yang J.L., Chu Y.L., Chueh F.S., Liu K.C., Chung J.G. Fisetin-induced apoptosis of human oral cancer SCC-4 cells through reactive oxygen species production, endoplasmic reticulum stress, caspase-, and mitochondria-dependent signaling pathways. Environ. Toxicol. 2017;32:1725–1741. doi: 10.1002/tox.22396. [DOI] [PubMed] [Google Scholar]
- 80.Murtaza I., Adhami V.M., Hafeez B.B., Saleem M., Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-κB. Int. J. Cancer. 2009;125:2465–2473. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan Q., Xue M., Xiao S.S., Wan Y.J., Xu D.B. A Combination Therapy with Baicalein and Taxol Promotes Mitochondria-Mediated Cell Apoptosis: Involving in Akt/beta-Catenin Signaling Pathway. DNA Cell Biol. 2016;35:646–656. doi: 10.1089/dna.2016.3312. [DOI] [PubMed] [Google Scholar]
- 82.Kong E.K., Yu S., Sanderson J.E., Chen K.B., Huang Y., Yu C.M. A novel anti-fibrotic agent, baicalein, for the treatment of myocardial fibrosis in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011;658:175–181. doi: 10.1016/j.ejphar.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 83.Wu L., Yang W., Zhang S.N., Lu J.B. Alpinetin inhibits lung cancer progression and elevates sensitization drug-resistant lung cancer cells to cis-diammined dichloridoplatium. Drug Des. Dev. Ther. 2015;9:6119–6127. doi: 10.2147/DDDT.S92702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Umehara K., Nemoto K., Kimijima K., Matsushita A., Terada E., Monthakantirat O., De-Eknamkul W., Miyase T., Warashina T., Degawa M., et al. Estrogenic constituents of the heartwood of Dalbergia parviflora. Phytochemistry. 2008;69:546–552. doi: 10.1016/j.phytochem.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 85.Makkar H.P.S., Siddhuraju P., Becker K. In: Plant Secondary Metabolites. Walker J.M., editor. Volume 393. Publisher; Stuttgart, Germany: 2007. pp. 1–122. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y.J., Li Q., Xiao H.B., Li Y.J., Yang Q., Kan X.X., Chen Y., Liu X.N., Weng X.G., Chen X., et al. Chamaejasmin B exerts anti-MDR effect in vitro and in vivo via initiating mitochondria-dependant intrinsic apoptosis pathway. Drug Des. Dev. Ther. 2015;9:5301–5313. doi: 10.2147/DDDT.S89392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plitzko B., Kaweesa E.N., Loesgen S. The natural product mensacarcin induces mitochondrial toxicity and apoptosis in melanoma cells. J. Biol. Chem. 2017;292:21102–21116. doi: 10.1074/jbc.M116.774836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Del Hierro J.N., Cueva C., Tamargo A., Nunez-Gomez E., Moreno-Arribas M.V., Reglero G., Martin D. In Vitro Colonic Fermentation of Saponin-Rich Extracts from Quinoa, Lentil, and Fenugreek. Effect on Sapogenins Yield and Human Gut Microbiota. J. Agric. Food Chem. 2020;68:106–116. doi: 10.1021/acs.jafc.9b05659. [DOI] [PubMed] [Google Scholar]
- 89.Min H.Y., Jang H.J., Park K.H., Hyun S.Y., Park S.J., Kim J.H., Son J., Kang S.S., Lee H.Y. The natural compound gracillin exerts potent antitumor activity by targeting mitochondrial complex II. Cell Death Dis. 2019;10:810. doi: 10.1038/s41419-019-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li G.B., Fu R.Q., Shen H.M., Zhou J., Hu X.Y., Liu Y.X., Li Y.N., Zhang H.W., Liu X., Zhang Y.H., et al. Polyphyllin I induces mitophagic and apoptotic cell death in human breast cancer cells by increasing mitochondrial PINK1 levels. Oncotarget. 2017;8:10359–10374. doi: 10.18632/oncotarget.14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang J., Wang H., Wang X., Zhao Y., Zhao D., Wang C., Li Y., Yang Z., Lu S., Zeng Q., et al. Molecular mechanisms of Polyphyllin I-induced apoptosis and reversal of the epithelial-mesenchymal transition in human osteosarcoma cells. J. Ethnopharmacol. 2015;170:117–127. doi: 10.1016/j.jep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 92.Dyshlovoy S.A., Rast S., Hauschild J., Otte K., Alsdorf W.H., Madanchi R., Kalinin V.I., Silchenko A.S., Avilov S.A., Dierlamm J., et al. Frondoside A induces AIF-associated caspase-independent apoptosis in Burkitt lymphoma cells. Leuk. Lymphoma. 2017;58:2905–2915. doi: 10.1080/10428194.2017.1317091. [DOI] [PubMed] [Google Scholar]
- 93.Cheng L., Shi L., Wu J., Zhou X., Li X., Sun X., Zhu L., Xia T.S., Ding Q. A hederagenin saponin isolated from Clematis ganpiniana induces apoptosis in breast cancer cells via the mitochondrial pathway. Oncol. Lett. 2018;15:1737–1743. doi: 10.3892/ol.2017.7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song I.S., Jeong Y.J., Kim J., Seo K.H., Baek N.I., Kim Y., Kim C.S., Jang S.W. Pharmacological inhibition of androgen receptor expression induces cell death in prostate cancer cells. Cell. Mol. Life Sci. 2020;77:4663–4673. doi: 10.1007/s00018-019-03429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vinh L.B., Nguyet N.T.M., Yang S.Y., Kim J.H., Thanh N.V., Cuong N.X., Nam N.H., Minh C.V., Hwang I., Kim Y.H. Cytotoxic triterpene saponins from the mangrove Aegiceras corniculatum. Nat. Prod. Res. 2019;33:628–634. doi: 10.1080/14786419.2017.1402320. [DOI] [PubMed] [Google Scholar]
- 96.Oh J.M., Kim E., Chun S. Ginsenoside Compound K Induces Ros-Mediated Apoptosis and Autophagic Inhibition in Human Neuroblastoma Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2019;20:4279. doi: 10.3390/ijms20174279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zou K., Li Z., Zhang Y., Zhang H.Y., Li B., Zhu W.L., Shi J.Y., Jia Q., Li Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017;38:157–167. doi: 10.1038/aps.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun Y., Yu J., Liu X., Zhang C., Cao J., Li G., Liu X., Chen Y., Huang H. Oncosis-like cell death is induced by berberine through ERK1/2-mediated impairment of mitochondrial aerobic respiration in gliomas. Biomed. Pharmacother. 2018;102:699–710. doi: 10.1016/j.biopha.2018.03.132. [DOI] [PubMed] [Google Scholar]
- 99.Min H.Y., Jung Y., Park K.H., Lee H.Y. Papuamine Inhibits Viability of Non-small Cell Lung Cancer Cells by Inducing Mitochondrial Dysfunction. Anticancer Res. 2020;40:323–333. doi: 10.21873/anticanres.13956. [DOI] [PubMed] [Google Scholar]
- 100.Wang X.D., Li C.Y., Jiang M.M., Li D., Wen P., Song X., Chen J.D., Guo L.X., Hu X.P., Li G.Q., et al. Induction of apoptosis in human leukemia cells through an intrinsic pathway by cathachunine, a unique alkaloid isolated from Catharanthus roseus. Phytomedicine. 2016;23:641–653. doi: 10.1016/j.phymed.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Rashmi K.C., Harsha Raj M., Paul M., Girish K.S., Salimath B.P., Aparna H.S. A new pyrrole based small molecule from Tinospora cordifolia induces apoptosis in MDA-MB-231 breast cancer cells via ROS mediated mitochondrial damage and restoration of p53 activity. Chem. Biol. Interact. 2019;299:120–130. doi: 10.1016/j.cbi.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Futamura Y., Muroi M., Aono H., Kawatani M., Hayashida M., Sekine T., Nogawa T., Osada H. Bioenergetic and proteomic profiling to screen small molecule inhibitors that target cancer metabolisms. Biochim. Biophys. Acta Proteins Proteom. 2019;1867:28–37. doi: 10.1016/j.bbapap.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Torres J.P., Lin Z., Fenton D.S., Leavitt L.U., Niu C., Lam P.Y., Robes J.M., Peterson R.T., Concepcion G.P., Haygood M.G., et al. Boholamide A, an APD-Class, Hypoxia-Selective Cyclodepsipeptide. J. Nat. Prod. 2020;83:1249–1257. doi: 10.1021/acs.jnatprod.0c00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miranda M.A., Mondal A., Sachdeva M., Cabral H., Neto Y., Khan I., Groppo M., McChesney J.D., Bastos J.K. Chemosensitizing Effect of Cernumidine Extracted from Solanum cernuum on Bladder Cancer Cells In Vitro. Chem. Biodivers. 2019;16:e1900334. doi: 10.1002/cbdv.201900334. [DOI] [PubMed] [Google Scholar]
- 105.Liu W.Y., Tang Q., Zhang Q., Hu C.P., Huang J.B., Sheng F.F., Liu Y.L., Zhou M., Lai W.J., Li G.B., et al. Lycorine Induces Mitochondria-Dependent Apoptosis in Hepatoblastoma HepG2 Cells Through ROCK1 Activation. Front. Pharmacol. 2019;10:651. doi: 10.3389/fphar.2019.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang X., Huang W., Li L., Sun X., Song S., Xu Q., Zhang L., Wei B.G., Deng X. Structure Determinants of Lagunamide A for Anticancer Activity and Its Molecular Mechanism of Mitochondrial Apoptosis. Mol. Pharm. 2016;13:3756–3763. doi: 10.1021/acs.molpharmaceut.6b00564. [DOI] [PubMed] [Google Scholar]
- 107.Wang C.W., Hsu W.H., Tai C.J. Antimetastatic effects of cordycepin mediated by the inhibition of mitochondrial activity and estrogen-related receptor alpha in human ovarian carcinoma cells. Oncotarget. 2017;8:3049–3058. doi: 10.18632/oncotarget.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kostova I. Studying plant-derived coumarins for their pharmacological and therapeutic properties as potential anticancer drugs. Expert Opin. Drug Discov. 2007;2:1605–1618. doi: 10.1517/17460441.2.12.1605. [DOI] [PubMed] [Google Scholar]
- 109.Venugopala K.N., Rashmi V., Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013;2013:963248. doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng X.M., Damu G.L., Zhou C. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013;19:3884–3930. doi: 10.2174/1381612811319210013. [DOI] [PubMed] [Google Scholar]
- 111.Zhang L., Tong X., Zhang J., Huang J., Wang J. DAW22, a natural sesquiterpene coumarin isolated from Ferula ferulaeoides (Steud.) Korov. that induces C6 glioma cell apoptosis and endoplasmic reticulum (ER) stress. Fitoterapia. 2015;103:46–54. doi: 10.1016/j.fitote.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 112.Andas A.R., Abdul A.B., Rahman H.S., Sukari M.A., Abdelwahab S.I., Samad N.A., Anasamy T., Arbab I.A. Dentatin from Clausena excavata Induces Apoptosis in HepG2 Cells via Mitochondrial Mediated Signaling. Asian Pac. J. Cancer Prev. 2015;16:4311–4316. doi: 10.7314/APJCP.2015.16.10.4311. [DOI] [PubMed] [Google Scholar]
- 113.Arbab I.A., Abdul A.B., Sukari M.A., Abdullah R., Syam S., Kamalidehghan B., Ibrahim M.Y., Taha M.M., Abdelwahab S.I., Ali H.M., et al. Dentatin isolated from Clausena excavata induces apoptosis in MCF-7 cells through the intrinsic pathway with involvement of NF-κB signaling and G0/G1 cell cycle arrest: A bioassay-guided approach. J. Ethnopharmacol. 2013;145:343–354. doi: 10.1016/j.jep.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Gong J., Zhang W.G., Feng X.F., Shao M.J., Xing C. Aesculetin (6,7-dihydroxycoumarin) exhibits potent and selective antitumor activity in human acute myeloid leukemia cells (THP-1) via induction of mitochondrial mediated apoptosis and cancer cell migration inhibition. J. BUON. 2017;22:1563–1569. [PubMed] [Google Scholar]
- 115.Grobarova V., Valis K., Talacko P., Pavlu B., Hernychova L., Novakova J., Stodulkova E., Flieger M., Novak P., Cerny J. Quambalarine B, a Secondary Metabolite from Quambalaria cyanescens with Potential Anticancer Properties. J. Nat. Prod. 2016;79:2304–2314. doi: 10.1021/acs.jnatprod.6b00362. [DOI] [PubMed] [Google Scholar]
- 116.Valis K., Grobarova V., Hernychova L., Buganova M., Kavan D., Kalous M., Cerny J., Stodulkova E., Kuzma M., Flieger M., et al. Reprogramming of leukemic cell metabolism through the naphthoquinonic compound Quambalarine B. Oncotarget. 2017;8:103137–103153. doi: 10.18632/oncotarget.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chao C.C., Hou S.M., Huang C.C., Hou C.H., Chen P.C., Liu J.F. Plumbagin induces apoptosis in human osteosarcoma through ROS generation, endoplasmic reticulum stress and mitochondrial apoptosis pathway. Mol. Med. Rep. 2017;16:5480–5488. doi: 10.3892/mmr.2017.7222. [DOI] [PubMed] [Google Scholar]
- 118.Hou Y., Xu J., Liu X., Xia X., Li N., Bi X. Shikonin induces apoptosis in the human gastric cancer cells HGC-27 through mitochondria-mediated pathway. Pharmacogn. Mag. 2015;11:250–256. doi: 10.4103/0973-1296.153074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu H., Zheng Z., Zhang J., Liu X., Liu Y., Yang W., Liu Y., Zhang T., Zhao Y., Liu Y., et al. Anticancer effect of 2,7-dihydroxy-3-methylanthraquinone on human gastric cancer SGC-7901 cells in vitro and in vivo. Pharm. Biol. 2016;54:285–292. doi: 10.3109/13880209.2015.1033563. [DOI] [PubMed] [Google Scholar]
- 120.Feng S., Wang Z., Zhang M., Zhu X., Ren Z. HG30, a tetrahydroanthraquinone compound isolated from the roots of Prismatomeris connate, induces apoptosis in human non-small cell lung cancer cells. Biomed. Pharmacother. 2018;100:124–131. doi: 10.1016/j.biopha.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Zhang M., Du H., Huang Z., Zhang P., Yue Y., Wang W., Liu W., Zeng J., Ma J., Chen G., et al. Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chem. Biol. Interact. 2018;292:65–75. doi: 10.1016/j.cbi.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 122.Jacobsen K.M., Villadsen N.L., Torring T., Nielsen C.B., Salomon T., Nielsen M.M., Tsakos M., Sibbersen C., Scavenius C., Nielsen R., et al. APD-Containing Cyclolipodepsipeptides Target Mitochondrial Function in Hypoxic Cancer Cells. Cell Chem. Biol. 2018;25:1337–1349.e12. doi: 10.1016/j.chembiol.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 123.Nipin S.P., Kang D.Y., Kim B.J., Joung Y.H., Darvin P., Byun H.J., Kim J.G., Park J.U., Yang Y.M. Methylsulfonylmethane Induces G1 Arrest and Mitochondrial Apoptosis in YD-38 Gingival Cancer Cells. Anticancer Res. 2017;37:1637–1646. doi: 10.21873/anticanres.11494. [DOI] [PubMed] [Google Scholar]
- 124.Wang Z., Li S., Ren R., Li J., Cui X. Recombinant Buckwheat Trypsin Inhibitor Induces Mitophagy by Directly Targeting Mitochondria and Causes Mitochondrial Dysfunction in Hep G2 Cells. J. Agric. Food Chem. 2015;63:7795–7804. doi: 10.1021/acs.jafc.5b02644. [DOI] [PubMed] [Google Scholar]
- 125.Liang L., Amin A., Cheung W.Y., Xu R., Yu R., Tang J., Yao X., Liang C. Parameritannin A-2 from Urceola huaitingii enhances doxorubicin-induced mitochondria-dependent apoptosis by inhibiting the PI3K/Akt, ERK1/2 and p38 pathways in gastric cancer cells. Chem. Biol. Interact. 2020;316:108924. doi: 10.1016/j.cbi.2019.108924. [DOI] [PubMed] [Google Scholar]
- 126.Ma L., Li W., Wang R., Nan Y., Wang Q., Liu W., Jin F. Resveratrol enhanced anticancer effects of cisplatin on non-small cell lung cancer cell lines by inducing mitochondrial dysfunction and cell apoptosis. Int. J. Oncol. 2015;47:1460–1468. doi: 10.3892/ijo.2015.3124. [DOI] [PubMed] [Google Scholar]
- 127.Wang B., Liu J., Gong Z. Resveratrol induces apoptosis in K562 cells via the regulation of mitochondrial signaling pathways. Int. J. Clin. Exp. Med. 2015;8:16926–16933. [PMC free article] [PubMed] [Google Scholar]
- 128.Wang W., Wu J., Zhang Q., Li X., Zhu X., Wang Q., Cao S., Du L. Mitochondria-mediated apoptosis was induced by oleuropein in H1299 cells involving activation of p38 MAP kinase. J. Cell. Biochem. 2019;120:5480–5494. doi: 10.1002/jcb.27827. [DOI] [PubMed] [Google Scholar]
- 129.Shamshoum H., Vlavcheski F., Tsiani E. Anticancer effects of oleuropein. Biofactors. 2017;43:517–528. doi: 10.1002/biof.1366. [DOI] [PubMed] [Google Scholar]
- 130.Ning D., Jin M., Xv T., Sun J., Li M. Homoisoflavanone-1 isolated from Polygonatum odoratum arrests the cell cycle and induces apoptosis in A549 cells. Oncol. Lett. 2018;16:3545–3554. doi: 10.3892/ol.2018.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang R., Ma L., Weng D., Yao J., Liu X., Jin F. Gallic acid induces apoptosis and enhances the anticancer effects of cisplatin in human small cell lung cancer H446 cell line via the ROS-dependent mitochondrial apoptotic pathway. Oncol. Rep. 2016;35:3075–3083. doi: 10.3892/or.2016.4690. [DOI] [PubMed] [Google Scholar]
- 132.Freitas S., Martins R., Costa M., Leao P.N., Vitorino R., Vasconcelos V., Urbatzka R. Hierridin B Isolated from a Marine Cyanobacterium Alters VDAC1, Mitochondrial Activity, and Cell Cycle Genes on HT-29 Colon Adenocarcinoma Cells. Mar. Drugs. 2016;14:158. doi: 10.3390/md14090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kitai Y., Zhang X., Hayashida Y., Kakehi Y., Tamura H. Induction of G2/M arrest and apoptosis through mitochondria pathway by a dimer sesquiterpene lactone from Smallanthus sonchifolius in HeLa cells. J. Food Drug Anal. 2017;25:619–627. doi: 10.1016/j.jfda.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen Y.C., Lu M.C., El-Shazly M., Lai K.H., Wu T.Y., Hsu Y.M., Lee Y.L., Liu Y.C. Breaking down Leukemia Walls: Heteronemin, a Sesterterpene Derivative, Induces Apoptosis in Leukemia Molt4 Cells through Oxidative Stress, Mitochondrial Dysfunction and Induction of Talin Expression. Mar. Drugs. 2018;16:212. doi: 10.3390/md16060212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ling T., Lang W.H., Craig J., Potts M.B., Budhraja A., Opferman J., Bollinger J., Maier J., Marsico T.D., Rivas F. Studies of Jatrogossone A as a Reactive Oxygen Species Inducer in Cancer Cellular Models. J. Nat. Prod. 2019;82:1301–1311. doi: 10.1021/acs.jnatprod.8b01087. [DOI] [PubMed] [Google Scholar]
- 136.Geng Y.D., Zhang C., Lei J.L., Yu P., Xia Y.Z., Zhang H., Yang L., Kong L.Y. Walsuronoid B induces mitochondrial and lysosomal dysfunction leading to apoptotic rather than autophagic cell death via ROS/p53 signaling pathways in liver cancer. Biochem. Pharmacol. 2017;142:71–86. doi: 10.1016/j.bcp.2017.06.134. [DOI] [PubMed] [Google Scholar]
- 137.Luo G., Zhou J., Li G., Hu N., Xia X., Zhou H. Ferruginol Diterpenoid Selectively Inhibits Human Thyroid Cancer Growth by Inducing Mitochondrial Dependent Apoptosis, Endogenous Reactive Oxygen Species (ROS) Production, Mitochondrial Membrane Potential Loss and Suppression of Mitogen-Activated Protein Kinase (MAPK) and PI3K/AKT Signaling Pathways. Med. Sci. Monit. 2019;25:2935–2942. doi: 10.12659/MSM.914348. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Jin S., Shi K., Liu L., Chen Y., Yang G. Xanthones from the Bark of Garcinia xanthochymus and the Mechanism of Induced Apoptosis in Human Hepatocellular Carcinoma HepG2 Cells via the Mitochondrial Pathway. Int. J. Mol. Sci. 2019;20:4803. doi: 10.3390/ijms20194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Losuwannarak N., Sritularak B., Chanvorachote P. Cycloartobiloxanthone Induces Human Lung Cancer Cell Apoptosis via Mitochondria-dependent Apoptotic Pathway. In Vivo. 2018;32:71–78. doi: 10.21873/invivo.11206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yuan S.Y., Cheng C.L., Wang S.S., Ho H.C., Chiu K.Y., Chen C.S., Chen C.C., Shiau M.Y., Ou Y.C. Escin induces apoptosis in human renal cancer cells through G2/M arrest and reactive oxygen species-modulated mitochondrial pathways. Oncol. Rep. 2017;37:1002–1010. doi: 10.3892/or.2017.5348. [DOI] [PubMed] [Google Scholar]
- 141.Sun D., Shen W., Zhang F., Fan H., Tan J., Li L., Xu C., Zhang H., Yang Y., Cheng H. α-Hederin Arrests Cell Cycle at G2/M Checkpoint and Promotes Mitochondrial Apoptosis by Blocking Nuclear Factor-κB Signaling in Colon Cancer Cells. Biomed. Res. Int. 2018;2018:2548378. doi: 10.1155/2018/2548378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schulte-Michels J., Wolf A., Aatz S., Engelhard K., Sieben A., Martinez-Osuna M., Haberlein F., Haberlein H. α-Hederin inhibits G protein-coupled receptor kinase 2-mediated phosphorylation of β2-adrenergic receptors. Phytomedicine. 2016;23:52–57. doi: 10.1016/j.phymed.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 143.Wang X.A., Xiang S.S., Li H.F., Wu X.S., Li M.L., Shu Y.J., Zhang F., Cao Y., Ye Y.Y., Bao R.F., et al. Cordycepin induces S phase arrest and apoptosis in human gallbladder cancer cells. Molecules. 2014;19:11350–11365. doi: 10.3390/molecules190811350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salvador-Reyes L.A., Luesch H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015;32:478–503. doi: 10.1039/C4NP00104D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ma L., Xu Y., Wei Z., Xin G., Xing Z., Niu H., Huang W. Deoxyarbutin displays antitumour activity against melanoma in vitro and in vivo through a p38-mediated mitochondria associated apoptotic pathway. Sci. Rep. 2017;7:7197. doi: 10.1038/s41598-017-05416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wen H., Zhou S., Song J. Induction of apoptosis by magnolol via the mitochondrial pathway and cell cycle arrest in renal carcinoma cells. Biochem. Biophys. Res. Commun. 2019;508:1271–1278. doi: 10.1016/j.bbrc.2018.12.087. [DOI] [PubMed] [Google Scholar]
- 147.Zhang A., He W., Shi H., Huang X., Ji G. Natural compound oblongifolin C inhibits autophagic flux, and induces apoptosis and mitochondrial dysfunction in human cholangiocarcinoma QBC939 cells. Mol. Med. Rep. 2016;14:3179–3183. doi: 10.3892/mmr.2016.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weidner C., Rousseau M., Micikas R.J., Fischer C., Plauth A., Wowro S.J., Siems K., Hetterling G., Kliem M., Schroeder F.C., et al. Amorfrutin C Induces Apoptosis and Inhibits Proliferation in Colon Cancer Cells through Targeting Mitochondria. J. Nat. Prod. 2016;79:2–12. doi: 10.1021/acs.jnatprod.5b00072. [DOI] [PubMed] [Google Scholar]
- 149.Bo P., Lien J.C., Chen Y.Y., Yu F.S., Lu H.F., Yu C.S., Chou Y.C., Yu C.C., Chung J.G. Allyl Isothiocyanate Induces Cell Toxicity by Multiple Pathways in Human Breast Cancer Cells. Am. J. Chin. Med. 2016;44:415–437. doi: 10.1142/S0192415X16500245. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010;54:127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hafezi K., Hemmati A.A., Abbaszadeh H., Valizadeh A., Makvandi M. Anticancer activity and molecular mechanisms of α-conidendrin, a polyphenolic compound present in Taxus yunnanensis, on human breast cancer cell lines. Phytother. Res. 2020;34:1397–1408. doi: 10.1002/ptr.6613. [DOI] [PubMed] [Google Scholar]
- 152.Zhao L., Wen Q., Yang G., Huang Z., Shen T., Li H., Ren D. Apoptosis induction of dehydrobruceine B on two kinds of human lung cancer cell lines through mitochondrial-dependent pathway. Phytomedicine. 2016;23:114–122. doi: 10.1016/j.phymed.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 153.Song I.S., Jeong Y.J., Kim J.E., Shin J., Jang S.W. Frugoside Induces Mitochondria-Mediated Apoptotic Cell Death through Inhibition of Sulfiredoxin Expression in Melanoma Cells. Cancers. 2019;11:854. doi: 10.3390/cancers11060854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ibrahim S.R., Mohamed G.A., Shaala L.A., Moreno L., Banuls Y., Kiss R., Youssef D.T. Proceraside A, a new cardiac glycoside from the root barks of Calotropis procera with in vitro anticancer effects. Nat. Prod. Res. 2014;28:1322–1327. doi: 10.1080/14786419.2014.901323. [DOI] [PubMed] [Google Scholar]
- 155.Balachandran C., Emi N., Arun Y., Yamamoto Y., Ahilan B., Sangeetha B., Duraipandiyan V., Inaguma Y., Okamoto A., Ignacimuthu S., et al. In vitro anticancer activity of methyl caffeate isolated from Solanum torvum Swartz. fruit. Chem. Biol. Interact. 2015;242:81–90. doi: 10.1016/j.cbi.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 156.Han X., Deng S., Wang N., Liu Y., Yang X. Inhibitory effects and molecular mechanisms of tetrahydrocurcumin against human breast cancer MCF-7 cells. Food Nutr. Res. 2016;60:30616. doi: 10.3402/fnr.v60.30616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duan H., Wang R., Yan X., Liu H., Zhang Y., Mu D., Han J., Li X. Phloretin induces apoptosis of human esophageal cancer via a mitochondria-dependent pathway. Oncol. Lett. 2017;14:6763–6768. doi: 10.3892/ol.2017.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu Z., Ren B., Wang Y., Zou C., Qiao Q., Diao Z., Mi Y., Zhu D., Liu X. Sesamol Induces Human Hepatocellular Carcinoma Cells Apoptosis by Impairing Mitochondrial Function and Suppressing Autophagy. Sci. Rep. 2017;7:45728. doi: 10.1038/srep45728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xia T., Zhang J., Han L., Jin Z., Wang J., Li X., Man S., Liu C., Gao W. Protective effect of magnolol on oxaliplatin-induced intestinal injury in mice. Phytother. Res. 2019;33:1161–1172. doi: 10.1002/ptr.6311. [DOI] [PubMed] [Google Scholar]
- 160.Li X., Huang J.M., Wang J.N., Xiong X.K., Yang X.F., Zou F. Combination of chrysin and cisplatin promotes the apoptosis of Hep G2 cells by up-regulating p53. Chem. Biol. Interact. 2015;232:12–20. doi: 10.1016/j.cbi.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 161.Feng F., Cheng P., Wang C., Wang Y., Wang W. Polyphyllin I and VII potentiate the chemosensitivity of A549/DDP cells to cisplatin by enhancing apoptosis, reversing EMT and suppressing the CIP2A/AKT/mTOR signaling axis. Oncol. Lett. 2019;18:5428–5436. doi: 10.3892/ol.2019.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang K., Li Y. Effects of ginsenoside compound K combined with cisplatin on the proliferation, apoptosis and epithelial mesenchymal transition in MCF-7 cells of human breast cancer. Pharm. Biol. 2016;54:561–568. doi: 10.3109/13880209.2015.1101142. [DOI] [PubMed] [Google Scholar]
- 163.Deng H., Ma J., Liu Y., He P., Dong W. Combining α-Hederin with cisplatin increases the apoptosis of gastric cancer in vivo and in vitro via mitochondrial related apoptosis pathway. Biomed. Pharmacother. 2019;120:109477. doi: 10.1016/j.biopha.2019.109477. [DOI] [PubMed] [Google Scholar]
- 164.Liu L., Fan J., Ai G., Liu J., Luo N., Li C., Cheng Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019;52:37. doi: 10.1186/s40659-019-0243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.He G., He G., Zhou R., Pi Z., Zhu T., Jiang L., Xie Y. Enhancement of cisplatin-induced colon cancer cells apoptosis by shikonin, a natural inducer of ROS in vitro and in vivo. Biochem. Biophys. Res. Commun. 2016;469:1075–1082. doi: 10.1016/j.bbrc.2015.12.100. [DOI] [PubMed] [Google Scholar]
- 166.Zhang T., Ma L., Wu P., Li W., Li T., Gu R., Dan X., Li Z., Fan X., Xiao Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in nonsmall cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019;41:1779–1788. doi: 10.3892/or.2019.6976. [DOI] [PubMed] [Google Scholar]
- 167.Huang Z., Yang G., Shen T., Wang X., Li H., Ren D. Dehydrobruceine B enhances the cisplatin-induced cytotoxicity through regulation of the mitochondrial apoptotic pathway in lung cancer A549 cells. Biomed. Pharmacother. 2017;89:623–631. doi: 10.1016/j.biopha.2017.02.055. [DOI] [PubMed] [Google Scholar]
- 168.Feng X.Q., Rong L.W., Wang R.X., Zheng X.L., Zhang L., Zhang L., Lin Y., Wang X., Li Z.P. Luteolin and sorafenib combination kills human hepatocellular carcinoma cells through apoptosis potentiation and JNK activation. Oncol. Lett. 2018;16:648–653. doi: 10.3892/ol.2018.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kutkowska J., Strzadala L., Rapak A. Sorafenib in Combination with Betulinic Acid Synergistically Induces Cell Cycle Arrest and Inhibits Clonogenic Activity in Pancreatic Ductal Adenocarcinoma Cells. Int. J. Mol. Sci. 2018;19:3234. doi: 10.3390/ijms19103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xia Y., Li Y., Westover K.D., Sun J., Chen H., Zhang J., Fisher D.E. Inhibition of Cell Proliferation in an NRAS Mutant Melanoma Cell Line by Combining Sorafenib and alpha-Mangostin. PLoS ONE. 2016;11:e0155217. doi: 10.1371/journal.pone.0155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y., Bi T., Dai W., Wang G., Qian L., Shen G., Gao Q. Lupeol enhances inhibitory effect of 5-fluorouracil on human gastric carcinoma cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016;389:477–484. doi: 10.1007/s00210-016-1221-y. [DOI] [PubMed] [Google Scholar]
- 172.Khan N., Jajeh F., Eberhardt E.L., Miller D.D., Albrecht D.M., Van Doorn R., Hruby M.D., Maresh M.E., Clipson L., Mukhtar H., et al. Fisetin and 5-fluorouracil: Effective combination for PIK3CA-mutant colorectal cancer. Int. J. Cancer. 2019;145:3022–3032. doi: 10.1002/ijc.32367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Attoub S., Arafat K., Khalaf T., Sulaiman S., Iratni R. Frondoside A Enhances the Anti-Cancer Effects of Oxaliplatin and 5-Fluorouracil on Colon Cancer Cells. Nutrients. 2018;10:560. doi: 10.3390/nu10050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yan L., Yu H.H., Liu Y.S., Wang Y.S., Zhao W.H. Esculetin enhances the inhibitory effect of 5-Fluorouracil on the proliferation, migration and epithelial-mesenchymal transition of colorectal cancer. Cancer Biomark. 2019;24:231–240. doi: 10.3233/CBM-181764. [DOI] [PubMed] [Google Scholar]
- 175.Bashmail H.A., Alamoudi A.A., Noorwali A., Hegazy G.A., AJabnoor G., Choudhry H., Al-Abd A.M. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci. Rep. 2018;8:11674. doi: 10.1038/s41598-018-30046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aborehab N.M., Osama N. Effect of Gallic acid in potentiating chemotherapeutic effect of Paclitaxel in HeLa cervical cancer cells. Cancer Cell Int. 2019;19:154. doi: 10.1186/s12935-019-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bhattacharya A., Li Y., Shi Y., Zhang Y. Enhanced inhibition of urinary bladder cancer growth and muscle invasion by allyl isothiocyanate and celecoxib in combination. Carcinogenesis. 2013;34:2593–2599. doi: 10.1093/carcin/bgt280. [DOI] [PMC free article] [PubMed] [Google Scholar]