Abstract
(1) Background: The endothelial glycocalyx is a primary target during the early phase of sepsis. We previously reported a newly developed recombinant non-fucosylated antithrombin has protective effects in vitro. We further evaluated the effects of this recombinant antithrombin on the glycocalyx damage in an animal model of sepsis. (2) Methods: Following endotoxin injection, in Wistar rats, circulating levels of hyaluronan, syndecan-1 and other biomarkers were evaluated in low-dose or high-dose recombinant antithrombin-treated animals and a control group (n = 7 per group). Leukocyte adhesion and blood flow were evaluated with intravital microscopy. The glycocalyx was also examined using side-stream dark-field imaging. (3) Results: The activation of coagulation was inhibited by recombinant antithrombin, leukocyte adhesion was significantly decreased, and flow was better maintained in the high-dose group (both p < 0.05). Circulating levels of syndecan-1 (p < 0.01, high-dose group) and hyaluronan (p < 0.05, low-dose group; p < 0.01, high-dose group) were significantly reduced by recombinant antithrombin treatment. Increases in lactate and decreases in albumin levels were significantly attenuated in the high-dose group (p < 0.05, respectively). The glycocalyx thickness was reduced over time in control animals, but the derangement was attenuated and microvascular perfusion was better maintained in the high-dose group recombinant antithrombin group (p < 0.05). (4) Conclusions: Recombinant antithrombin maintained vascular integrity and the microcirculation by preserving the glycocalyx in this sepsis model, effects that were more prominent with high-dose therapy.
Keywords: antithrombin, endothelium, glycocalyx, syndecan-1, hyaluronan
1. Introduction
Coagulation activation in sepsis is recognized as a natural event for host-defense that prevents pathogen dissemination and localizes infection [1,2]. However, the resulting inflammatory injury can cause a microcirculatory disturbance that leads to tissue ischemia, organ failure, and death [3]. Therefore, the goal of anticoagulant therapy in sepsis is to limit excessive coagulopathy while treating the underlying cause along with adjunctive therapy including antibiotics.
Antithrombin is one of the most important physiological anticoagulants. It provides host protection as an anticoagulant, but also through inhibiting acute inflammatory proteases such as thrombin and elastase [4,5,6]. Antithrombin is known to increase its anticoagulant activity by binding to heparan sulfate on the endothelial glycocalyx [7]. A unique feature of antithrombin is its capability to attenuate glycocalyx injury [8]. Antithrombin reportedly binds to heparan sulfate and contributes to glycocalyx stabilization [8,9]. Since antithrombin levels decrease dramatically in sepsis with coagulopathy [10], the supplementation of antithrombin for septic-associated disseminated intravascular coagulation (DIC) is a potential therapeutic approach [11]. Despite the lack of robust evidence, the antithrombin repletion in sepsis-associated DIC empirically administered in Japan is based on guidelines [12]. However, it has been suggested that the dose (intended to recover 80% of the activity) used in Japan is insufficient [13].
To overcome this problem, a recombinant non-fucosylated antithrombin was developed, and this novel antithrombin was named AT-γ. AT-γ is produced using the CHO DG44 cell line, which is deficient in α-1,6-fucosyltransferase (FUT8), and has four oligosaccharides (α-isoform) but lacks a core fucose, giving it a similar bioactivity and half-life to those of plasma-derived antithrombin [14,15]. In healthy volunteers, a dose of 72 IU/kg of AT-γ was shown to be bioequivalent to 60 IU/kg of plasma-derived antithrombin (data on file; Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan). Furthermore, a multicenter open-label, randomized, active-controlled, phase 3 study has demonstrated an equivalent DIC resolution rate and mortality [16]. We previously reported the protective effects of AT-γ on cultured endothelial cells in vitro [17], and the effects of AT-γ on the glycocalyx and microcirculation were examined in an animal model in the present study. As a result, AT-γ administration facilitated the maintenance of the glycocalyx and tissue circulation and suppressed organ injury. We also confirmed that these effects were more prominent with high-dose AT-γ administration. In the present study, we investigated the protective effects of AT-γ on the glycocalyx in an animal model of sepsis.
2. Results
2.1. Changes in Leukocyte Adhesion and Blood Flow
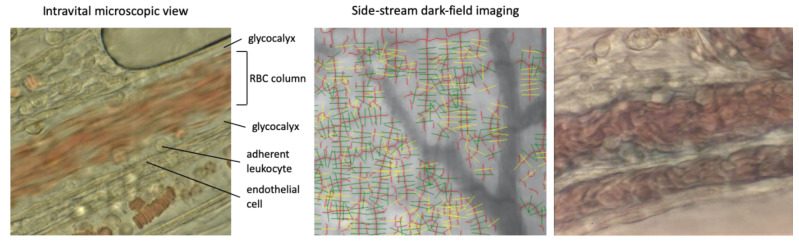
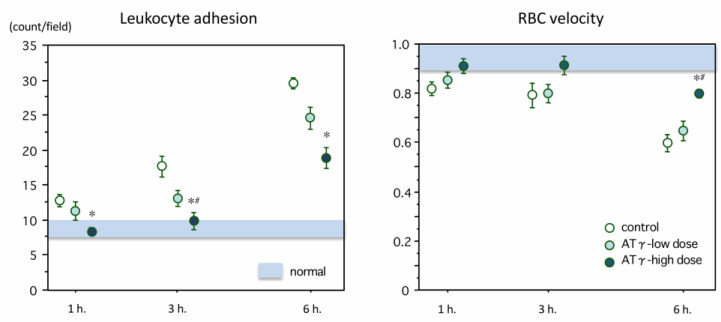
One hour after lipopolysaccharide (LPS) administration, endothelial leukocyte adhesion had begun. As shown in Figure 1, left, the intact endothelium appeared as a smooth homogeneous monolayer of endothelial cells, while the glycocalyx appeared as a gap between the red blood cell (RBC) column and the endothelium. Following injection of LPS, multiple structural changes in the endothelium were observed including disruption, irregularities, and thickening as well as loss of the glycocalyx layer and an increase in leukocytes adhering to the endothelium. The number of leukocytes adhering to the endothelial layer was 17.6 ± 1.46/field at 3 h, increasing to 29.6 ± 1.81/field at 6 h in the control group. Leukocyte adhesion was suppressed in the high-dose AT-γ group, and the differences were significant at 3 and 6 h after LPS administration (p < 0.05, respectively) (Figure 2, left). Decreases in RBC velocity compared to the control group occurred due to leukocyte adhesion, accumulation, and microcirculatory plugging. These changes were suppressed in the high-dose AT-γ group at 6 h (p < 0.05) (Figure 2, right). Significant differences in leukocyte adhesion and RBC velocity were recognized between the high-dose and low-dose AT-γ groups at 3 and 6 h, respectively.
Figure 1.
View of microvessels using intravital microscopy and image acquisition using the GlycoCheck™ System. Upper left: At baseline, the endothelium is visible as a smooth, thin layer, and the red blood cells (RBCs) are moving fluently. Some leukocytes have become stuck on the endothelium, but most of them are rolling. The glycocalyx layer is shown as a gap between the red cell column and the endothelial surface. 40× objective lens
Figure 2.
Changes in leukocyte adhesion and RBC velocity. Left: The adhesion of leukocytes onto the mesenteric vascular endothelium increased over time after lipopolysaccharide (LPS) administration in the control group, and the incidence of adherent leukocytes was significantly reduced in the high-dose AT-γ (novel antithrombin) group at 1, 3 and 6 h (n = 7 in each group). Right: The red blood cell (RBC) velocity in the mesenteric venule gradually decreased until 6 h after lipopolysaccharide administration. The velocity was significantly better maintained in the high-dose AT-γ group at 6 h. * p < 0.05, compared with the control group, # p < 0.05, compared with the low-dose group.
Bottom left: at 6 h, endothelial cells were thickened and the surface became irregular. The glycocalyx layer was lost and the blood cell contacted directly to endothelium. The leukocytes adherent to the endothelium increased and the blood flow decreased. Upper right: representative image of the microvessels in the intestinal wall. Quality checks were automatically performed using the GlycoCheck™ software. Invalid vascular segments are marked in yellow and were automatically discarded, while all the valid vascular segments (green lines) were further analyzed. The valid vascular segments were then visualized as the valid microvasculature (red lines).
2.2. Changes in Perfused Boundary Region (PBR) and Red Blood Cell (RBC) Filling Percentage
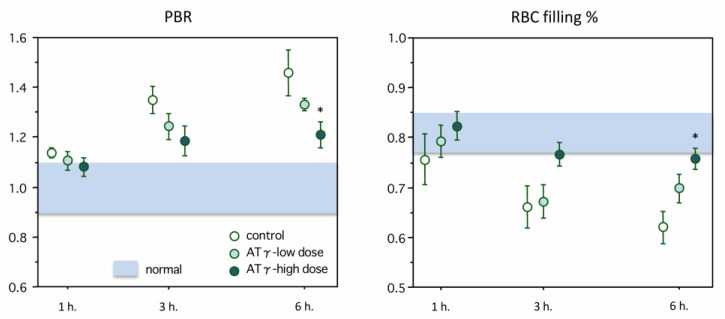
Figure 1 (right panel) shows representative vessel images obtained using side-stream dark-field microscopy. The accompanying software automatically discriminated valid vascular segments (green lines) from invalid segments (yellow lines). Then, the valid vascular segments were visualized as valid microvasculature (red lines). Figure 3 (left and right panels) showed the changes in the perfused boundary region (PBR) and the RBC filling percentage. The data are shown as the ratios relative to the normal values. The PBR began to increase at 1 h after LPS administration and reached 1.46 ± 0.09-fold of the normal value at 6 h. This increase was significantly suppressed in the high-dose AT-γ group (p < 0.05). Meanwhile, the RBC filling percentage began to decrease at 1 h after LPS administration, and the difference between the control group and the high-dose AT-γ group was also significant at 6 h (p < 0.05).
Figure 3.
Changes in the perfused boundary region (PBR) and RBC filling percentage. Left: Table 1. 4-fold of the baseline value at 6 h after lipopolysaccharide administration. The increase in the PBR was significantly suppressed in the high-dose AT-γ group at 6 h (n = 7 in each group). Right: The changes in the red blood cell (RBC) filling percentage in the mesenteric microvessels are shown. The RBC filling percentage started to decrease after lipopolysaccharide administration and reached 62% of the baseline value at 6 h. The RBC filling percentage was significantly maintained better in the high-dose AT-γ group at 6 h. * p < 0.05, compared with the control group.
2.3. Laboratory Data
The laboratory data are summarized in Table 1. Antithrombin activity was significantly elevated by treatment with low-dose or high-dose AT-γ (p < 0.05, respectively) and recovered to within the normal range in the high-dose AT-γ group. The platelet count was decreased to 17 × 103/mm3 in the control group, and this decrease was attenuated by treatment with both low-dose and high-dose AT-γ (p < 0.05, respectively). Coagulation abnormalities represented by a prolongation of the activated partial thromboplastin time (APTT) and a decrease in fibrinogen were attenuated by the treatment with high-dose AT-γ (p < 0.05, respectively). Organ damage markers such as ALT and BUN were elevated at 6 h after LPS administration, and treatment with AT-γ significantly reduced the elevation of BUN (p < 0.05, in both groups) but did not have a significant effect on the ALT level. A decrease in albumin and an elevation in lactate were recognized in the control group, and these changes were significantly attenuated in the high-dose AT-γ group (p < 0.05, respectively).
Table 1.
Laboratory findings of the endotoxemic rats treated with or without antithrombin.
| Parameters | Platelet count (×103/mm3) |
APTT (second) |
Fibrinogen (mg/dL) |
AT activity (%) |
ALT (IU/L) |
BUN (mg/dL) |
Albumin (mg/dL) | Lactate (mmol/L) |
|---|---|---|---|---|---|---|---|---|
| Normal group | 110–120 | 16.1–16.7 | 210–220 | 100–120 | 15–32 | 12.7–14.5 | 4.7–5.1 | 0.8–0.9 |
| Control group | 17 ± 2 | 33 ± 3 | 82 ± 10 | 41 ± 10 | 144 ± 21 | 52 ± 3 | 2.3 ± 0.1 | 4.6 ± 0.3 |
| Low-dose group | 27 ± 3 * | 31 ± 3 | 109 ± 11 | 82 ± 8 * | 100 ± 13 | 35 ± 3 * | 2.8 ± 0.1 | 3.6 ± 0.3 |
| High-dose group | 27 ± 3 * | 24 ± 3 * | 122 ± 12 * | 101 ± 11 * | 93 ± 16 | 37 ± 4 * | 3.1 ± 0.2 * | 3.4 ± 0.3 * |
APTT activated partial thromboplastin time, AT antithrombin, ALT alanine aminotransferase, BUN blood urea nitrogen; * significant difference from the control group at p < 0.05.
2.4. Hyaluronan and Syndecan-1 Measurements
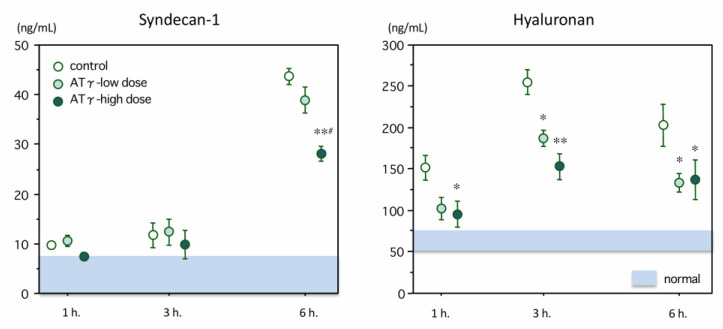
Changes in syndecan-1 levels is shown in Figure 4 (left panel). In normal rats, the plasma levels were <5.0 ng/mL. The syndecan-1 levels increased over time and reached 42.81 ± 1.35 ng/mL at 6 h in the control group. The syndecan-1 levels were significantly lower in the high-dose group (28.07 ± 1.50 ng/mL, p = 0.001) at 6 h. The plasma level of hyaluronan in the normal rats was below 50.0 ng/mL, and it increased to 255.3 ± 15.5 ng/mL at 3 h after LPS administration and then decreased at 6 h. The plasma hyaluronan level was significantly lower in both the low-dose group (187.6 ± 10.1 ng/mL, p = 0.012) and the high-dose group (153.3 ± 15.9 ng/mL, p < 0.001) at 3 h (Figure 4, right).
Figure 4.
Changes in plasma levels of syndecan-1 and hyaluronan. Left: The syndecan-1 level was elevated at 6 h after lipopolysaccharide administration. The syndecan-1 level in the high-dose AT-γ group was significantly lower than that in the control group (n = 7 in each group). Right: The plasma level of hyaluronan peaked at 3 h after lipopolysaccharide administration. The hyaluronan levels were significantly suppressed in the AT-γ groups. * p < 0.05 compared with the control group, ** p < 0.01 compared with the control group, # p < 0.05 compared with the low-dose group.
3. Discussion
Endothelial glycocalyx is a critical aspect of the vascular endothelium that regulates vascular permeability, flow, cellular interactions, and anticoagulation [8,18,19,20]. However, following vascular injury due to causes that include hypoxia [21], trauma [22], and infection [23], the glycocalyx is easily disrupted, and major components including syndecans and glycosaminoglycans (hyaluronan) are released that provide potential biomarkers for evaluating injury [24]. In septic patients with acute lung injury, respiratory distress syndrome [25] or DIC [26], syndecan-1 and hyaluronan are elevated, and may serve as biomarkers to follow for evaluating therapies to improve vascular and endothelial dysfunction.
Potential pharmacologic approaches to protect the glycocalyx include corticosteroids [27] and heparinoids [28], however, they have not been investigated in clinical settings of sepsis [29]. Plasma including albumin and antithrombin also are reported to protect the glycocalyx and reduce shedding in an animal model of ischemia and reperfusion [19,30,31]. We have also reported the ability of purified antithrombin to preserve vascular integrity [32], and similar results also observed using a novel recombinant non-fucosylated antithrombin namely AT-γ in vitro [17]. In the present study, we intended to confirm the effects of AT-γ in the animal model.
Decreased glycocalyx thickness is reported to correlate with the severity of sepsis [33]. The combination of side-stream dark-field imaging and an automated computer calculation system enable the evaluation of the glycocalyx thickness and microperfusion. Theoretically, the deeper penetration of RBCs into the glycocalyx, as expressed by an increased PBR, should be associated with a reduced thickness of the glycocalyx. Thus, an increased PBR should represent a reduction in endothelial barrier properties for RBC accessibility (the reciprocal of glycocalyx thickness) [34]. The RBC filling percentage was monitored as a marker of microvascular perfusion. Lee et al. [4] reported a negative correlation between PBR and the RBC filling percentage. Similarly, the PBR increased over time after LPS administration, while the RBC filling percentage decreased over time in our study; both of these changes were significantly suppressed by treatment with high-dose AT-γ.
As described above, the glycocalyx is important in preserving vascular integrity but is easily damaged in sepsis. Following injury, glycocalyx fragments including syndecan-1 and hyaluronan, have been studied as vascular injury biomarkers. Recent clinical studies have also reported that these biomarkers can be used as diagnostic or potential prognostic indicators [35,36]. In the present study, the syndecan-1 level increased over time, whereas the hyaluronan level peaked at 3 h after LPS administration, and a beneficial effect of AT-γ was observed at 6 h for syndecan-1 and at 3 h for hyaluronan. Smart et al. [37] measured the syndecan-1 and hyaluronan levels in septic patients and reported that the syndecan-1 concentration increased, while the hyaluronan concentration peaked at an earlier timing. This phenomenon can be explained by the difference in the locations of these components. Syndecan-1 is a membrane-attached glycoprotein, while hyaluronan is mainly distributed on the surface of the glycocalyx [38]. Thus, hyaluronan is released beginning at an earlier stage of sepsis, and hyaluronan release is then followed by the shedding of syndecans.
The plasma albumin level has also been used to evaluate vascular integrity. Due to its amphoteric nature, albumin binds to the glycocalyx to decreases the vascular hydraulic conductivity [29]. The glycocalyx also has a negative charge that facilitates the repulsion of albumin. Overall, injury and destruction of the endothelial glycocalyx increases permeability to facilitate intravascular albumin loss. In our current study, albumin levels were better preserved in the high-dose group we believe to be a result of glycocalyx preservation.
Limitations
The present study has several limitations. First, our sepsis model was LPS injection to the rat. Unfortunately, only the short-term observation can be possible with this model, and the long-term effect and the effect on mortality should also be examined in an additional experiment utilizing cecal ligation and puncture (CLP). However, a CLP model is not suitable for the intravital microscopic observation. Second, the detailed mechanism responsible for the action of AT-γ was not characterized in this study. Other than the direct protection of the glycocalyx, AT-γ may help to maintain the glycocalyx by improving anticoagulation. In the same manner, thrombin-mediated inflammatory reactions expressed via the activation of protease-activated receptors (PARs) might be involved [39]. Other mechanisms, such as the stimulation of prostacyclin production, should also be considered [40]. In addition, since this experiment does not have the formulation buffer of AT-γ group and, therefore, the buffer effect cannot be excluded. Although the observation period of our present study was limited, intravital microscopic observation or side-stream dark-field imaging are not suitable for long periods of observation.
4. Materials and Methods
4.1. Lipopolysaccharide Administration and the Sepsis Model
All animal procedures were performed in accordance with the guidelines for animal experimentation of the Japanese Association for Laboratory Animal Science. Therefore, the Ethical Committee for Animal Experiments of Juntendo University waived the need for the approval of the institutional review board. Wistar rats (Tokyo Laboratory Animals Science Co., Tokyo, Japan) that were 10–12 weeks old were studied. Following intraperitoneal administration of sodium thiopental (100 mg/kg, Pentothal; Sigma Chemical Co., St. Louis, MO, USA), lipopolysaccharide (LPS, E. coli O55-B5; Difco Laboratories, Detroit, MI, USA) was diluted with 0.15 mL of sterile saline and a dose of 8.0 mg/kg of was administered intravenously (IV), followed by IV 250 IU/kg (low dose) or 500 IU/kg (high-dose) of AT-γ (Japan Blood Products Organization, Tokyo, Japan). Control animals (vehicle) group received IV LPS and saline.
4.2. Evaluation of the Microcirculation Using Intravital Microscopy
Following a median incision under anesthesia, the abdomen was opened to expose the mesentery, and immobilized on a warmed stand. Intravital microscopy (n = 7 in each group) was used to examine the mesenteric microcirculation using the Eclipse Pol™ microscopic system (Nikon Co., Tokyo, Japan) at 1, 3, and 6 h following LPS administration. A total of 6 successive fields were selected for each animal that was recorded at 30 frames/second for 5 min using a high-vision recording system (α7 III; SONY Co., Tokyo, Japan). Two independent blinded examiners were used to count adherent leukocytes in each field that was defined as adhered to a venule and stationary for 30 s. To analyze red blood cell (RBC) velocity, venules of 20–50 micrometers were localized, and a high-speed camera (Memrecam GX-1; Nac Image Technology Inc., Tokyo, Japan) recorded images at 1, 3 and 6 h after the LPS injection. Particle image velocimetry (Digimo Co., Tokyo, Japan) was used to determined RBC velocity, with baseline considered as 1.0, and decreases in the ratio determined.
4.3. Glycocalyx Evaluation Using Side-Stream Dark-Field Imaging
Glycocalyx thickness in intestinal microvessels was evaluated using side-stream dark-field imaging with the GlycoCheck™ System (Microvascular Health Solutions Inc., Salt Lake City, UT, USA) (n = 7 in each group). We have previously reported the measurement protocol [41]. The GlycoCheck™ System automatically records images and evaluates vascular segments at 10-micrometer intervals recording a total of 40 frames that include 300 major vascular segments. We selected 6 successive fields for every animal, moving the camera to a different location after ≥3000 vascular segments had been evaluated. Also, the perfused boundary region (PBR) and the RBC filling percentage were calculated.
4.4. Blood Sampling and Measurement
Six hours following LPS infusion, 7 animals per group for each timing were sacrificed in an ether chamber. Blood samples, obtained from the inferior vena cava, were collected in citrate, centrifuged, and stored at −80 °C until assayed. Three additional no treated rats were used as controls for blood sampling. Laboratory testing included platelet count, APTT, fibrinogen, antithrombin activity, alanine aminotransferase (ALT), blood urea nitrogen (BUN), and albumin levels using standard analytic techniques. A blood gas analyzer (ABL715; Radiometer, Københaven, Denmark) was used to determine lactate levels. An enzyme-linked immunosorbent assay (ELISA) kit was used to measure syndecan-1 (Cloud-Clone Corp., Katy, TX, USA), and a hyaluronan Quantikine™ ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
4.5. Statistical Analysis
Data were analyzed using a one-way analysis of variance (ANOVA) with the Dunnett post-hoc test using statistical software (StatView IITM, Abacus Concepts, Piscataway, NJ, USA). Data were presented as the mean ± standard error (SE). Differences were considered statistically significant at p < 0.05.
5. Conclusions
AT-γ attenuated damage to the glycocalyx in a rat model of sepsis. The shedding of syndecan-1 and hyaluronan from the vascular endothelial surface was suppressed by AT-γ, and these effects were more effective in animals treated with a high dose of AT-γ. The observed effects were thought to have led to the preservation of the glycocalyx, maintenance of the microcirculation, and the prevention of subsequent organ dysfunction. Thrombin is thought to be the key mediator in sepsis [42], and the observed effects were considered to be expressed through inhibition of the coagulation cascades since APTT and fibrinogen levels were better maintained in the high-dose group. However, as the direct evidence of thrombin inhibition was not proven in this study, it should be further confirmed. Finally, the present experiment examined only the short-term effect of AT-γ and we cannot exclude that the effects are transient and thrombin inhibition does not have a significant impact on disease evolution.
Acknowledgments
A part of this study was presented at the 64th Annual Scientific and Standardization Committee meeting of International Society on Thrombosis and Haemostasis.
Abbreviations
| DIC | disseminated intravascular coagulation |
| AT-γ | recombinant antithrombin |
| RBC | red blood cell |
| PBR | perfused boundary region |
| APTT | activated partial thromboplastin time |
| ALT | alanine aminotransferase |
| BUN | blood urea nitrogen |
| ELISA | enzyme-linked immunosorbent assay |
| FUT8 | α-1,6-fucosyltransferase |
Author Contributions
T.I., J.H.L., and K.A., and K.K. conceived of the study and participated in its design. T.I., and K.S. performed the experiment. H.T., K.S., and I.N. analyzed the data. T.I. and J.H.L. are involved in drafting and revising of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Strategic Research Foundation at Private Universities 2019.
Institutional Review Board Statement
The Institutional Review Board did not require ethical approval since this study followed the standard procedure conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Not applicable
Data Availability Statement
Data sharing not applicable
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 2.Esmon C.T., Xu J., Lupu F. Innate immunity and coagulation. J. Thromb. Haemost. 2011;9:182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons J., Pittet J.F. The coagulopathy of acute sepsis. Curr. Opin. Anaesthesiol. 2015;28:227–236. doi: 10.1097/ACO.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iba T., Levy J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018;16:231–241. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 5.Lee D.H., Dane M.J., van den Berg B.M., Boels M.G., van Teeffelen J.W., de Mutsert R., den Heijer M., Rosendaal F.R., van der Vlag J., van Zonneveld A.J., et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE. 2014;9:e96477. doi: 10.1371/journal.pone.0096477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iba T., Nakarai E., Takayama T., Nakajima K., Sasaoka T., Ohno Y. Combination effect of antithrombin and recombinant human soluble thrombomodulin in a lipopolysaccharide induced rat sepsis model. Crit. Care. 2009;13:R203. doi: 10.1186/cc8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobczak A.I.S., Pitt S.J., Stewart A.J. Glycosaminoglycan Neutralization in Coagulation Control. Arterioscler. Thromb. Vasc. Biol. 2018;38:1258–1270. doi: 10.1161/ATVBAHA.118.311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker B.F., Jacob M., Leipert S., Salmon A.H., Chappell D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015;80:389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers G.J., Wegner J. Endothelial Glycocalyx and Cardiopulmonary Bypass. J. Extra Corpor. Technol. 2017;49:174–181. [PMC free article] [PubMed] [Google Scholar]
- 10.Iba T., Di Nisio M., Thachil J., Wada H., Asakura H., Sato K., Kitamura N., Saitoh D. Revision of the Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) diagnostic criteria using antithrombin activity. Crit. Care. 2016;20:287. doi: 10.1186/s13054-016-1468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagami T., Matsui H., Horiguchi H., Fushimi K., Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: An observational nationwide study. J. Thromb. Haemost. 2014;12:1470–1479. doi: 10.1111/jth.12643. [DOI] [PubMed] [Google Scholar]
- 12.Wada H., Asakura H., Okamoto K., Iba T., Uchiyama T., Kawasugi K., Koga S., Mayumi T., Koike K., Gando S., et al. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb. Res. 2010;125:6–11. doi: 10.1016/j.thromres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Iba T., Saitoh D., Wada H., Asakura H. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: A secondary survey. Crit. Care. 2014;18:497. doi: 10.1186/s13054-014-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirose M., Tsukada M., Hirayama F., Kubo Y., Kajii M., Mochizuki S., Hamato N., Ohi H. Recombinant human antithrombin expressed in Chinese hamster ovary cells shows in vivo efficacy on rat DIC model smilarly to plasma-derived antithrombin regardless of different N-glycosylation. Thromb. Res. 2007;119:631–641. doi: 10.1016/j.thromres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Yamane-Ohnuki N., Kinoshita S., Inoue-Urakubo M., Kusunoki M., Iida S., Nakano R., Wakitani M., Niwa R., Sakurada M., Uchida K., et al. Establishment of FUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependentcellular cytotoxicity. Biotechnol. Bioeng. 2004;87:614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 16.Endo S., Shimazaki R. An open-label, randomized, phase 3 study of the efficacy and safety of antithrombin gamma in patients with sepsis-induced disseminated intravascular coagulation syndrome. J. Intensiv. Care. 2018;6:75. doi: 10.1186/s40560-018-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iba T., Hirota T., Sato K., Nagaoka I. Protective effect of a newly developed fucose-deficient recombinant antithrombin against histone-induced endothelial damage. Int. J. Hematol. 2018;107:528–534. doi: 10.1007/s12185-018-2402-x. [DOI] [PubMed] [Google Scholar]
- 18.Woodcock T.E., Woodcock T.M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 2012;108:384–394. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 19.Becker B.F., Chappell D., Bruegger D., Annecke T., Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: Acute deficits, but great potential. Cardiovasc. Res. 2010;87:300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 20.Chappell D., Dörfler N., Jacob M., Rehm M., Welsch U., Conzen P., Becker B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34:133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 21.Grundmann S., Fink K., Rabadzhieva L., Bourgeois N., Schwab T., Moser M., Bode C., Busch H.J. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83:715–720. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Johansson P.I., Stensballe J., Rasmussen L.S., Ostrowski S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 23.Holzmann M.S., Winkler M.S., Strunden M.S., Izbicki J.R., Schoen G., Greiwe G., Pinnschmidt H.O., Poppe A., Saugel B., Daum G., et al. Syndecan-1 as a biomarker for sepsis survival after major abdominal surgery. Biomark. Med. 2018;12:119–127. doi: 10.2217/bmm-2017-0231. [DOI] [PubMed] [Google Scholar]
- 24.Cerny V., Astapenko D., Brettner F., Benes J., Hyspler R., Lehmann C., Zadak Z. Targeting the endothelial glycocalyx in acute critical illness as a challenge for clinical and laboratory medicine. Crit. Rev. Clin. Lab. Sci. 2017;54:343–357. doi: 10.1080/10408363.2017.1379943. [DOI] [PubMed] [Google Scholar]
- 25.Murphy L.S., Wickersham N., McNeil J.B., Shaver C.M., May A.K., Bastarache J.A., Ware L.B. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann. Intensiv. Care. 2017;7:102. doi: 10.1186/s13613-017-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda M., Matsumoto H., Ogura H., Hirose T., Shimizu K., Yamamoto K., Maruyama I., Shimazu T. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J. Crit. Care. 2018;43:48–53. doi: 10.1016/j.jcrc.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 27.Chappell D., Hofmann-Kiefer K., Jacob M., Rehm M., Briegel J., Welsch U., Conzen P., Becker B.F. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res. Cardiol. 2009;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 28.Song J.W., Zullo J.A., Liveris D., Dragovich M., Zhang X.F., Goligorsky M.S. Therapeutic Restoration of Endothelial Glycocalyx in Sepsis. J. Pharmacol. Exp. Ther. 2017;361:115–121. doi: 10.1124/jpet.116.239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alphonsus C.S., Rodseth R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia. 2014;69:777–784. doi: 10.1111/anae.12661. [DOI] [PubMed] [Google Scholar]
- 30.Schött U., Solomon C., Fries D., Bentzer P. The endothelial glycocalyx and its disruption, protection and regeneration: A narrative review. Scand. J. Trauma Resusc. Emerg. Med. 2016;24:48. doi: 10.1186/s13049-016-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chappell D., Jacob M., Hofmann-Kiefer K., Rehm M., Welsch U., Conzen P., Becker B.F. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc. Res. 2009;83:388–396. doi: 10.1093/cvr/cvp097. [DOI] [PubMed] [Google Scholar]
- 32.Iba T., Sasaki T., Ohshima T., Sato K., Nagaoka I., Thachil J. The comparison of the protective effects of a and β-antithrombin against vascular endothelial cell damage induced by histone in vitro. Thromb. Haemost. Open. 2017;1:e3–e10. doi: 10.1055/s-0037-1603926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldecoa C., Llau J.V., Nuvials X., Artigas A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: A review. Ann. Intensiv. Care. 2020;10:85. doi: 10.1186/s13613-020-00697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlahu C.A., Lemkes B.A., Struijk D.G., Koopman M.G., Krediet R.T., Vink H. Damage of the endothelial glycocalyx in dialysis patients. J. Am. Soc. Nephrol. 2012;23:1900–1908. doi: 10.1681/ASN.2011121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ushiyama A., Kataoka H., Iijima T. Glycocalyx and its involvement in clinical pathophysiologies. J. Intensiv. Care. 2016;4:59. doi: 10.1186/s40560-016-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand D., Ray S., Srivastava L.M., Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin. Biochem. 2016;49:768–776. doi: 10.1016/j.clinbiochem.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Smart L., Macdonald S.P.J., Burrows S., Bosio E., Arendts G., Fatovich D.M. Endothelial glycocalyx biomarkers increase in patients with infection during Emergency Department treatment. J. Crit. Care. 2017;42:304–309. doi: 10.1016/j.jcrc.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Curry F.E., Adamson R.H. Endothelial glycocalyx: Permeability barrier and mechanosensor. Ann. Biomed. Eng. 2012;40:828–839. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Poll T., Levi M. Crosstalk between inflammation and coagulation: The lessons of sepsis. Curr. Vasc. Pharmacol. 2012;10:632–638. doi: 10.2174/157016112801784549. [DOI] [PubMed] [Google Scholar]
- 40.Iba T., Saitoh D. Efficacy of antithrombin in preclinical and clinical applications for sepsis-associated disseminated intravascular coagulation. J. Intensiv. Care. 2014;2:66. doi: 10.1186/s40560-014-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martens R.J., Vink H., van Oostenbrugge R.J., Staals J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis. 2013;35:451–454. doi: 10.1159/000348854. [DOI] [PubMed] [Google Scholar]
- 42.Conway E.M. Thrombin: Coagulation’s master regulator of innate immunity. J. Thromb. Haemost. 2019;17:1785–1789. doi: 10.1111/jth.14586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable