Abstract
Lung cancer is still the leading cause of cancer death in the world. For this reason, novel approaches for early and more accurate diagnosis are needed. Computer-aided decision (CAD) can be an interesting option for a noninvasive tumour characterisation based on thoracic computed tomography (CT) image analysis. Until now, radiomics have been focused on tumour features analysis, and have not considered the information on other lung structures that can have relevant features for tumour genotype classification, especially for epidermal growth factor receptor (EGFR), which is the mutation with the most successful targeted therapies. With this perspective paper, we aim to explore a comprehensive analysis of the need to combine the information from tumours with other lung structures for the next generation of CADs, which could create a high impact on targeted therapies and personalised medicine. The forthcoming artificial intelligence (AI)-based approaches for lung cancer assessment should be able to make a holistic analysis, capturing information from pathological processes involved in cancer development. The powerful and interpretable AI models allow us to identify novel biomarkers of cancer development, contributing to new insights about the pathological processes, and making a more accurate diagnosis to help in the treatment plan selection.
Keywords: lung cancer assessment, tumour characterisation, personalised medicine, computer-aided decision, computed tomography analysis
1. Introduction
Lung cancer is still the leading cause of cancer death in the world as a result of high incidence combined with low 5-year survival rates [1,2]. For these reasons, lung cancer deserves special attention from the medicine, biology, and scientific communities in order to develop novel solutions to increase the early diagnosis, assist in treatment decisions, and monitor responses to improve patient outcomes. The molecular profile of the tumour tissues enables the identification of driver mutations, and targeted therapies can be used for particular genotypes. Traditional chemotherapy works by killing all cells, without discriminating between normal and cancerous cells. Instead, targeted therapy acts in specific elements, interfering with the cancer driver genes and stopping or slowing the growth of tumour cells.
Epidermal growth factor receptors (EGFRs) and Kirsten rat sarcoma viral oncogenes (KRASs) are the most frequently mutated genes present in non-small-cell lung cancer (NSCLC) [3,4,5], which is a major sub-type of lung cancer [6]. Activating mutations in EGFRs (namely exon 19 deletions or exon 21 L858R point mutations) benefit from treatment with EGFR tyrosine kinase inhibitors (TKIs) [7,8,9]. This gene is responsible for multiple biological processes and is useful to determine the clinical outcomes in many lung diseases. Abnormalities in EGFR pathways cause abnormal EGFR signalling and are associated with cancer, lung fibrosis, and numerous airway diseases [10]. Targeted therapies have been studied in recent years, with encouraging results for EGFRs [11,12], improving progression-free survival for patients with advanced NSCLC who were selected on the basis of EGFR mutations [12,13,14,15]. EGFR-dedicated therapies are currently used as first- and second-line lung cancer treatments [16], and several others are in development [17]. On the other hand, mutant KRAS has a wide spectrum of other co-occurring genetic alterations and a high biological heterogeneity, including diverse KRAS point mutations, which hinder the development of new target therapies [18]. For mutated KRAS, there are no current clinically approved targeted therapies, but there are several KRAS inhibitors in clinical trials [19,20,21]. Additionally, another target therapy of NSCLC has emerged—immunotherapy. This therapy relies on the use of immune checkpoint inhibitors to release the patient’s immune cells to fight the cancer [22]. Although it has demonstrated significant patient improvement, only a small portion of patients benefit from this therapy (20%) [23]. This is attributed to the low performance of the current predictive biomarkers of response to immune checkpoint blockade therapy, which rely on detection of programmed death ligand 1 (PD-L1) in cancer tissue [24]. Tumour-infiltrating immune cells are a key population of the tumour microenvironment and mediate the antitumor effects of immunotherapy [25]. The classification of the different immune cells helps to better define the immunogenic potency of NSCLC [26]. Despite the evident benefits, with the increased use of these personalised therapies in oncology, new side effects have emerged, causing important clinical challenges in the management of lung cancer patients. In fact, although the majority of these events are mild, some of them can be severe and potentially life-threatening [27].
Tissue biopsy is the traditional method to identify the main biomarkers of the tumour [28]; however, it is an invasive procedure with clinical implications such as pneumothorax, pain, and complications like infection, haemorrhage, and damage to surrounding tissues [29]. Due to the importance of tumour characterisation, less invasive, easier, and faster techniques to access the genotype of the tumour are needed. Computed tomography (CT) plays a key role in lung cancer management from initial diagnosis and staging to treatment response assessment [30]. CT is more sensitive than chest radiography in lung cancer screening [31,32,33]. Moreover, it allows for a three-dimensional (3D) thorax characterisation as each nodule is assessed, and information about other lung structures can be retrieved. The application of artificial intelligence (AI) solutions for lung cancer imaging has been dedicated to reproducing the radiology procedure. The traditional computer-aided decision (CAD) support system approaches based on a CT scan start with nodule detection and segmentation for further analysis, e.g., malignancy and subtype classification [32,34,35]. AI-based solutions dedicated to predicting the risk of LCa showed high-performance results and represent an opportunity to optimise the screening process, reducing the false positives and false negatives on assessments performed by radiologists [35]. On the genotype characterisation, previous studies using the radiomic features from CT images have shown that it is possible to use the imaging information to predict the gene mutation status related to cancer development [36,37,38,39,40]. However, the majority of these studies are focused on the nodule, which cannot capture the extension and complexity of the pathophysiological phenomena that occur in the other structures, but that can be related to the cancer development—and could introduce insights useful for the diagnosis and prognosis of the patient.
The general idea for AI-based solutions is to follow the same procedure but improve the accuracy of the diagnosis by trying to detect missed nodules, reducing the time and effort of clinicians during the evaluation process, and creating a measurable impact on clinical management and patient outcomes. Furthermore, AI-based solutions enable the identification of radiomic information from the lung structures on the CT images that are not visible to the naked eyes of radiologists, producing a more accurate genotype characterisation. In fact, this comprehensive perspective has not yet been explored for CADs in lung cancer. Recent works studied other lung structure abnormalities and found relevant relations with the presence of lung cancer. The current work identifies the studies of the most important pathophysiological processes in the lung related to lung cancer and opens the discussion about what kind of information can be decisive for these novel and comprehensive radiomic approaches. The next generation of CADs aim to integrate more representative information about the relevant biological changes in the lung to produce predictive models that can improve the accuracy of the tumour characterisation (avoiding the need for biopsy) in order to help therapeutic decisions and leverage personalised medicine—selecting patients who will definitely benefit from targeted therapies and avoiding superfluous therapy-related side effects.
2. Pathophysiologic Features
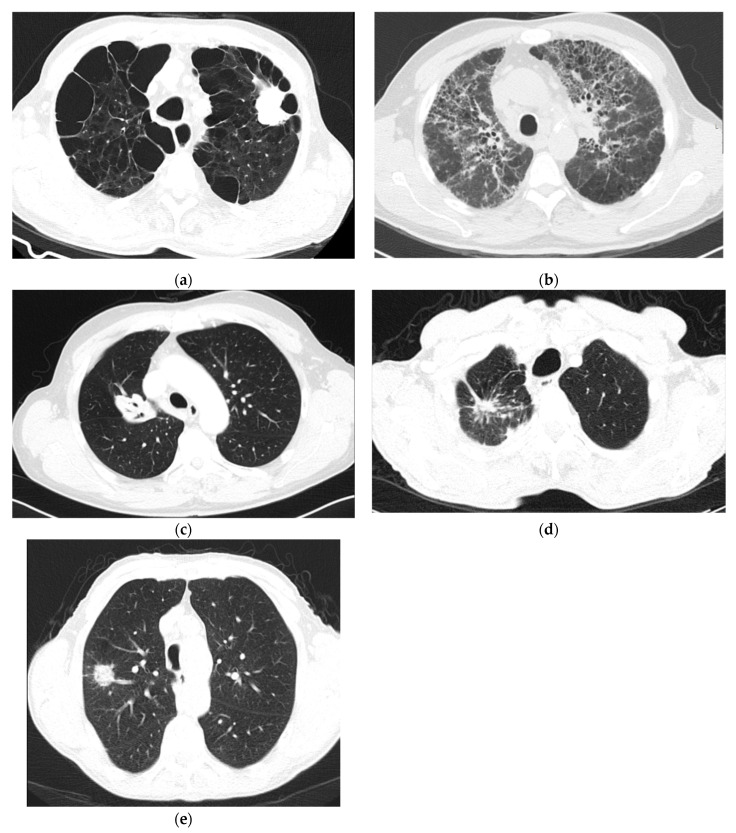
Genotype characterisation, as an essential step for treatment decision, may benefit if more information about the simultaneous pathophysiological processes that occur is combined with traditionally used nodule information. Recent studies have identified other relevant biological structures beyond the nodule that can help the tumour characterisation and contribute to a better understanding of cancer development [41,42]. In fact, lung cancer has been studied as a more extensive clinicopathological phenomenon that involves several other lung structure alterations, and their relationship with cancer development has been identified. The main pathophysiological changes related to lung cancer can be identified on specific findings in the CT images. The most relevant examples are represented in Figure 1: emphysema, pulmonary fibrosis, air bronchogram, pleural retraction, and vascular convergence.
Figure 1.
A set of axial computed tomography (CT) images with findings related with lung cancer pathogenesis: (a) centrilobular emphysema; (b) pulmonary-fibrosis; (c) air bronchogram; (d) pleural retraction; and, (e) vascular convergence.
Emphysema causes damage to the alveoli, and, as a consequence, there is a reduction in the gas exchange efficiency [43]. On CT images, emphysema is characterised by a compartment of air seen at extremely low attenuation areas (Figure 1a) [44]. Chronic inflammation in the airways has been shown to be important to the pathogenesis of both emphysema and lung cancer [45,46,47,48,49], and it is recommended to consider emphysema when assessing lung cancer risk [45,46].
Pulmonary fibrosis affects the tissue surrounding the alveoli (interstitium), and this condition occurs when lung tissue becomes thick and stiff [50]. The three specific findings for fibrosis are: traction bronchiectasis, loss of volume, and honeycomb (Figure 1b) [51]. Fibrosis might contribute to carcinogenesis due to the occurrence of atypical or dysplastic epithelial changes that progress to invasive malignancy [52].
Air bronchogram is characterised by a pattern of air-filled bronchi on the background of a nodular opacity [53]. The airways appear in the CT images as air-filled structures that originate an opacification of the surrounding alveoli (Figure 1c) [54]. The correlation between air bronchogram and lung cancer has been studied, and CT air bronchogram is an important malignant feature to predict the invasiveness of lung cancer [55,56].
Pleural retraction consists of pulling the visceral pleura toward the invading neoplastic tissue [57], and can be identified in the CT images as millimetre-thin lines of spun pleura (Figure 1d). Pleural retraction is correlated with lung cancer [58], and with the EGFR mutation [59,60].
Vascular convergence is verified when the vessels converge to a nodule without adjoining or contacting the edge of the nodule (Figure 1e) [57,61,62]. This phenomenon reflects angiogenesis [57].
Angiogenesis is essential for tumour growth and metastasis; maybe, for this reason, the convergence of vasculature towards or surrounding a nodule is related to lung cancer stage and pathology [61].
3. Comprehensive Perspective for the Next Generation of CADs
The correlation between several pathophysiological changes in the lung has been studied, since those phenomena do not occur in isolation and usually share pathways and/or functional mechanisms with lung cancer development. EGFR regulates several biological processes, and the correlations between different lung pathologies have been suggested. This is an important point and can expand the region of interest (ROI) for predictive models.
Radiomics in lung cancer have mostly been based on nodule assessment [32,63]. Recently, a few approaches have tried to use features from other lung structures; however, this information came from semantic annotations provided by radiologists [41,42,59]. As AI has shown to be able to extract and use relevant information, the quantitative analysis of the lung cancer performed by radiomic analysis could improve the performance on tumour characterisation—if the data from relevant structures of the lung were taken into consideration and selected by automatic feature learning methods, avoiding the human effort required for semantic annotations and making the process less subjective. The next generation of CADs should be able to use large lung regions for analysis and feature learning in order to capture more information associated with cancer pathogenesis. The most powerful learning methods are based on deep learning techniques, which allow for the capture of information that is not visible to the naked eye and avoid ad hoc feature extractions, depending on the feature engineering processes used for the task. Furthermore, those methods allow us to cope with the wide heterogeneities of clinical data using massive databases. Based on these advantages, the AI-based models for novel and comprehensive CADs would be mainly based on deep learning algorithms. Additionally, explainable AI, based on activation maps, can identify which part in the medical image was used to contribute to the final classification from the learning model [64,65,66]. AI solutions will move from “black boxes” to interpretable models that will help clinicians to understand which are the regions and features that contribute to the final decision, build trust in the methods to use in the clinical context, and create a deeper understanding of pathological features that will facilitate the management of lung cancer [67].
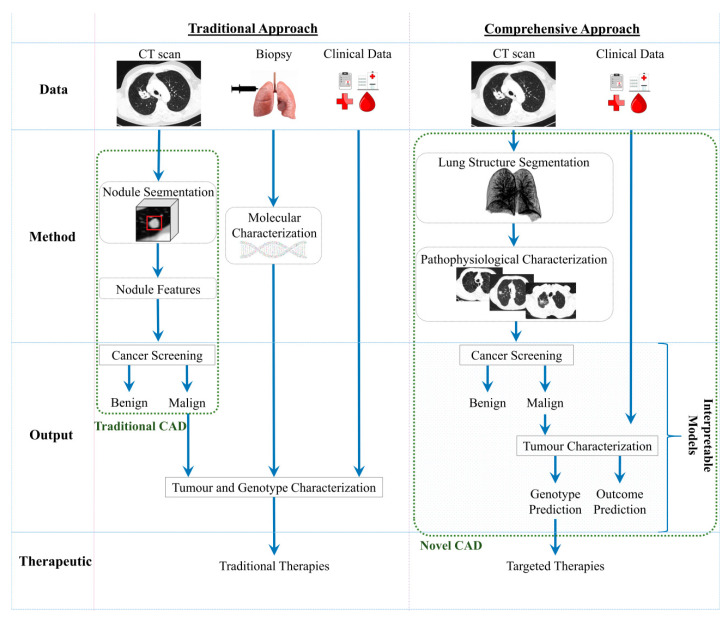
Figure 2 represents the main differences between the traditional CADs and the novel and comprehensive approach of next-generation CADs. The current solutions for automatic imaging analysis are: focus on the nodule assessment, mainly for screening; and the need of a biopsy for the tumour characterisation. Novel CADs should be able to detect the malignancy and characterise the lung tumour based on CT images and clinical data, using explainable models that help clinicians to understand the choice made by the model. However, despite not being represented in Figure 2, even the new AI-based solutions will still need to use biopsy as a backup method for cases in which radiomics would not be conclusive on tumour characterisation.
Figure 2.
Conceptual diagram of the traditional and comprehensive approaches based on CADs. CT, computed tomography; CAD, computer-aided decision.
There are three main levels of actions for CADs: screening, diagnosis/characterisation, and treatment assignment. On treatment planning, the comprehensive approach would assist clinicians in choosing the optimal treatment, with clear and transparent explanations for recommendations. Currently, recommendations are clear about the need for biopsy to determine the mutational status of the tumour in order to select the best available treatment [68]. However, learning from a comprehensive approach offers the possibility of capturing correspondences between processes and gaining an in-depth understanding of pathophysiological changes. This would allow for the selection of a personalised treatment that will improve effectiveness and efficiency while diminishing avoidable therapy-related adverse events. The AI-based approach would stratify the patient groups according to their singular properties in order to more effectively evaluate the potential efficacy of treatments, recommend the sequence of therapies, and predict the effects of specific drugs and clinical outcomes. This strategy may be particularly helpful in elderly or unfit patients who are at higher risk of procedure-related complications. In these patients, such risks may interfere with a proper diagnosis or treatment. For example: since inoperable early stage lung cancer patients may benefit from stereotactic radiotherapy [69], a noninvasive diagnosis strategy in this group of patients could diminish the risks, prevent complications associated with traditional methods, and allow for prompt treatment, thus leading to better disease control and survival rates. Moreover, CADs would use aggregated knowledge of many patients with matching results, history, biomarkers, physiological characteristics, and behavioural risk to present clinicians with the most efficacious treatment option. In addition to preventing toxicities, personalised treatment recommendations can reduce time loss and costs.
The two approaches represented in Figure 2 have pros and cons based on the specific features of those approaches and the stage of development (Table 1). The traditional lung cancer characterisation (biopsy) is an invasive procedure that limits repetition for treatment response evaluation, and automatic solutions are not currently significantly used in the screening process. The comprehensive lung cancer characterisation using imagiological data represents a noninvasive option, avoiding all the complications associated with an invasive procedure. For this reason, it can be repeated several times in order to assess the treatment response and personalise a treatment plan. The novel approaches are in development with the emergence of powerful AI-based models that can give interpretable information that can be helpful when identifying relations between pathophysiological processes that occur during cancer development. Novel radiomic approaches will allow for the identification of the main biomarkers and will study the relationship between the imagiological findings and the lung cancer development, creating a comprehensive analysis of pathophysiological processes.
Table 1.
Pros and Cons of traditional and comprehensive approaches for lung cancer diagnosis.
| Traditional Approach | Comprehensive Approach | |
|---|---|---|
| Pros | -Clinically validated | -Non-invasive assessment, faster and with lower costs; -Safety repeated; -Leverage the personalised medicine; -Interpretable models; -Comprehensive perspective |
| Cons | -Invasive and with clinical implications; -Restriction for the repetitions of the procedure; -AI based solutions with residual help in the diagnosis |
-In development; -Requirement large datasets to train the predictive models |
The biggest challenge for these comprehensive models, which will use information from multiple lung structures, comes from the size of the dataset used for training. Due to the high degree of variability that can be found in lung structures, there is a need for a massive amount of data that covers all heterogeneities, in order to create a good representation of the population [70]. Only with large datasets will it be possible to capture the relevant information, correlate pathophysiological phenomena between structures, and create a better understanding of the processes and mechanisms involved in cancer development. The analysis based on multiple structures has not yet proven conclusive, but certainly deserves additional studies since multiple pathophysiological processes share common pathways and have been shown to be related [47].
The need for large datasets is a transverse limitation on AI-based solutions in healthcare. The ImageNet, composed of 14 million natural images, allowed for the training of complex and powerful neural networks and consequently revolutionised image classification [71]. Currently, the biomedical field is struggling with data size limitations and attempting to build robust models to help clinicians with diagnoses. Large medical datasets are extremely difficult to obtain due to privacy and security issues, annotation efforts by experts, and the huge investment required to collect, store, and maintain the data. The reuse of clinical data in data banks will allow for an important improvement in deep learning solutions for healthcare. The LIDC Data Collection Process for Nodule Detection and Annotation was used in multiple publications [72], allowing for the development of the most relevant radiomic studies for lung cancer screening using CT images [63]. This shows the importance of large datasets in leveraging the development of AI tools. With large datasets, which cover all of the heterogeneities in the population, it will be possible to study the importance of other lung structures for lung cancer characterisation.
4. Conclusions
Several previous works showed the relationship between pathophysiological changes in lung structures and lung cancer development, which suggests that there are common biological pathways that can be captured by CT images and used by comprehensive and automatic systems to characterise lung cancer. The dataset size (under-representative of the population) is still the biggest limitation on the development of powerful methods to cope with the heterogeneities of all lung structures. Some recent works have already tried to include more information than the nodule features; however, the small datasets (hundreds of patients) were not representative of all of the variabilities. For this reason, they did not achieve relevant performance improvement. Even so, they confirm the relevance of those other structures. These relations can be used to study the mechanism of cancer development. For radiomic-based solutions, the integration of novel information will allow for the development of a more comprehensive assessment and more accurate models, leading to better tumour characterisation and personalised treatment plans. Understanding the mechanisms that drive cancer processes with other pulmonary diseases—along with better disease models—is essential for the development of new targeted treatments.
Author Contributions
T.P., J.M., F.S., A.C. and H.P.O. conceived the presented perspective; C.F. and V.H. gave the pneumology insights; E.N., B.F.d.L., M.C.d.S., I.R. and A.J.M. gave the radiology insights and selected the C.F. images; and J.L.C. gave the molecular biology insights. T.P. drafted the manuscript. All authors provided critical feedback and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financed by the ERDF–European Regional Development Fund through the Operational Programme for Competitiveness and Internationalisation–COMPETE 2020 Programme and by National Funds through the Portuguese funding agency, FCT–Fundação para a Ciência e a Tecnologia within project POCI-01-0145-FEDER-030263.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin L., Yan L., Liu Y., Yuan F., Li H., Ni J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J. Hematol. Oncol. 2019;12:96. doi: 10.1186/s13045-019-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu T., Yang X., Huang Y., Zhao M., Li M., Ma K., Yin J., Zhan C., Wang Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019;11:943–953. doi: 10.2147/CMAR.S187317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shigematsu H., Lin L., Takahashi T., Nomura M., Suzuki M., Wistuba I.I., Fong K.M., Lee H., Toyooka S., Shimizu N., et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Q.-G., Zhang S.-M., Ding X.-X., He B., Zhang H.-Q. Driver genes in non-small cell lung cancer: Characteristics, detection methods, and targeted therapies. Oncotarget. 2017;8:57680–57692. doi: 10.18632/oncotarget.17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakra T., Mehta A., Bal A., Nambirajan A., Mishra D., Midha D., Gupta N., Arora N., Gupta P., Gupta P., et al. Epidermal growth factor receptor mutation status in pulmonary adenocarcinoma: Multi-institutional data discussion at national conference of “Lung Cancer Management in Indian context”. Curr. Probl. Cancer. 2020;44:100561. doi: 10.1016/j.currproblcancer.2020.100561. [DOI] [PubMed] [Google Scholar]
- 6.Tas F., Ciftci R., Kilic L., Karabulut S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol. Lett. 2013;6:1507–1513. doi: 10.3892/ol.2013.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 8.Paez J.G., Jänne P.A., Lee J.C., Tracy S., Greulich H., Gabriel S., Herman P., Kaye F.J., Lindeman N., Boggon T.J., et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 9.Pao W., Miller V., Zakowski M., Doherty J., Politi K., Sarkaria I., Singh B., Heelan R., Rusch V., Fulton L., et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallath S., Hynds R.E., Succony L., Janes S.M., Giangreco A. Targeting EGFR signalling in chronic lung disease: Therapeutic challenges and opportunities. Eur. Respir. J. 2014;44:513–522. doi: 10.1183/09031936.00146413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C., Leighl N.B., Wu Y.-L., Zhong W.-Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019;12:1–24. doi: 10.1186/s13045-019-0731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I., et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 13.Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., Seto T., Satouchi M., Tada H., Hirashima T., et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C., Wu Y.-L., Chen G., Feng J., Liu X.-Q., Wang C., Zhang S., Wang J., Zhou S., Ren S., et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 15.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 16.Holleman M.S., Al M.J., Zaim R., Groen H.J.M., Uyl-de Groot C.A. Cost-effectiveness analysis of the first-line EGFR-TKIs in patients with non-small cell lung cancer harbouring EGFR mutations. Eur. J. Health Econ. 2020;21:153–164. doi: 10.1007/s10198-019-01117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu M.J., Johnson D.E., Grandis J.R. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017;36:463–473. doi: 10.1007/s10555-017-9687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H., Liang S.-Q., Schmid R.A., Peng R.-W. New horizons in KRAS-mutant lung cancer: Dawn after darkness. Front. Oncol. 2019;9:953. doi: 10.3389/fonc.2019.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 20.Adderley H., Blackhall F.H., Lindsay C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine. 2019;41:711–716. doi: 10.1016/j.ebiom.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullard A. Cracking KRAS. Nat. Rev. Drug Discov. 2019;18:887–891. doi: 10.1038/d41573-019-00195-5. [DOI] [PubMed] [Google Scholar]
- 22.Dine J., Gordon R., Shames Y., Kasler M.K., Barton-Burke M. Immune checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pac. J. Oncol. Nurs. 2017;4:127–135. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P. Immune checkpoint therapy and the search for predictive biomarkers. Cancer J. 2016;22:68–72. doi: 10.1097/PPO.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backman M., La Fleur L., Kurppa P., Djureinovic D., Elfving H., Brunnström H., Mattsson J.S.M., Pontén V., Eltahir M., Mangsbo S., et al. Characterization of patterns of immune cell infiltration in NSCLC. J. Thorac. Oncol. 2020 doi: 10.1016/j.jtho.2019.12.127. [DOI] [PubMed] [Google Scholar]
- 27.Haanen J., Carbonnel F., Robert C., Kerr K., Peters S., Larkin J., Jordan K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 28.Tuzi A., Bolzacchini E., Suter M.B., Giaquinto A., Passaro A., Gobba S., Vallini I., Pinotti G. Biopsy and re-biopsy in lung cancer: The oncologist requests and the role of endobronchial ultrasounds transbronchial needle aspiration. J. Thorac. Dis. 2017;9:S405–S409. doi: 10.21037/jtd.2017.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C.C., Maher M.M., Shepard J.-A.O. Complications of CT-guided percutaneous needle biopsy of the chest: Prevention and management. Am. J. Roentgenol. 2011;196:W678–W682. doi: 10.2214/AJR.10.4659. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhry A., Gul M., Chaudhry A. Utility of computed tomography lung cancer screening and the management of computed tomography screen-detected findings. J. Thorac. Dis. 2018;10:1352–1355. doi: 10.21037/jtd.2018.03.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The National Lung Screening Trial Research Team. Church T.R., Black W.C., Aberle D.R., Berg C.D., Clingan K.L., Duan F., Fagerstrom R.M., Gareen I.L., Gierada D.S., et al. Results of initial low-dose computed tomographic screening for lung cancer. N. Engl. J. Med. 2013;368:1980–1991. doi: 10.1056/nejmoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Mohammad B., Brennan P., Mello-Thoms C. A review of lung cancer screening and the role of computer-aided detection. Clin. Radiol. 2017;72:433–442. doi: 10.1016/j.crad.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 33.de Koning H.J., Van Der Aalst C.M., De Jong P.A., Scholten E.T., Nackaerts K., Heuvelmans M.A., Lammers J.-W.J., Weenink C., Yousaf-Khan U., Horeweg N., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 34.El-Baz A., Beache G.M., Gimel’Farb G., Suzuki K., Okada K., Elnakib A., Soliman A., Abdollahi B. Computer-aided diagnosis systems for lung cancer: Challenges and methodologies. Int. J. Biomed. Imaging. 2013;2013:942353. doi: 10.1155/2013/942353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ardila D., Kiraly A.P., Bharadwaj S., Choi B., Reicher J.J., Peng L., Tse D., Etemadi M., Ye W., Corrado G., et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019;25:954–961. doi: 10.1038/s41591-019-0447-x. [DOI] [PubMed] [Google Scholar]
- 36.Li S., Ding C., Zhang H., Song J., Wu L. Radiomics for the prediction of EGFR mutation subtypes in non-small cell lung cancer. Med. Phys. 2019;46:4545–4552. doi: 10.1002/mp.13747. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Kim J., Balagurunathan Y., Li Q., Garcia A.L., Stringfield O., Ye Z., Gillies R.J. Radiomic features are associated with EGFR mutation status in lung adenocarcinomas. Clin. Lung Cancer. 2016;17:441–448.e6. doi: 10.1016/j.cllc.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Kong C., Xu W., Yang S., Shi D., Zhang J., Du M., Wang S., Bai Y., Zhang T., et al. Decoding tumor mutation burden and driver mutations in early stage lung adenocarcinoma using CT-based radiomics signature. Thorac. Cancer. 2019;10:1904–1912. doi: 10.1111/1759-7714.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia T.-Y., Xiong J.-F., Li X.-Y., Yu W., Xu Z.-Y., Cai X.-W., Ma J.-C., Ren Y.-C., Larsson R., Zhang J., et al. Identifying EGFR mutations in lung adenocarcinoma by noninvasive imaging using radiomics features and random forest modeling. Eur. Radiol. 2019;29:4742–4750. doi: 10.1007/s00330-019-06024-y. [DOI] [PubMed] [Google Scholar]
- 40.Tu W., Sun G., Fan L., Wang Y., Xia Y., Guan Y., Li Q., Zhang D., Liu S., Li Z. Radiomics signature: A potential and incremental predictor for EGFR mutation status in NSCLC patients, comparison with CT morphology. Lung Cancer. 2019;132:28–35. doi: 10.1016/j.lungcan.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 41.Gevaert O., Echegaray S., Khuong A., Hoang C.D., Shrager J.B., Jensen K.C., Berry G.J., Guo H.H., Lau C., Plevritis S.K., et al. Predictive radiogenomics modeling of EGFR mutation status in lung cancer. Sci. Rep. 2017;7:41674. doi: 10.1038/srep41674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinheiro G., Pereira T., Dias C., Freitas C., Hespanhol V., Costa J.L., Cunha A., Oliveira H.P. Identifying relationships between imaging phenotypes and lung cancer-related mutation status: EGFR and KRAS. Sci. Rep. 2020;10:3625. doi: 10.1038/s41598-020-60202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah P.L., Herth F.J., Van Geffen W.H., Deslee G., Slebos D.-J. Lung volume reduction for emphysema. Lancet Respir. Med. 2017;5:147–156. doi: 10.1016/S2213-2600(16)30221-1. [DOI] [PubMed] [Google Scholar]
- 44.Friedman P.J. Imaging Studies in Emphysema. Proc. Am. Thorac. Soc. 2008;5:494–500. doi: 10.1513/pats.200708-128ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson D.O., Weissfeld J.L., Balkan A., Schragin J.G., Fuhrman C.R., Fisher S.N., Wilson J., Leader J.K., Siegfried J.M., Shapiro S.D., et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am. J. Respir. Crit. Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bae K., Jeon K.N., Lee S.J., Kim H.C., Ha J.Y., Park S.E., Baek H.J., Choi B.H., Cho S.B., Moon J.I. Severity of pulmonary emphysema and lung cancer. Medicine. 2016;95:e5494. doi: 10.1097/MD.0000000000005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hohberger L.A., Schroeder D.R., Bartholmai B.J., Yang P., Wendt C.H., Bitterman P.B., Larsson O., Limper A.H. Correlation of regional emphysema and lung cancer: A lung tissue research consortium-based study. J. Thorac. Oncol. 2014;9:639–645. doi: 10.1097/JTO.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wille M.M.W., Thomsen L.H., Petersen J., De Bruijne M., Dirksen A., Pedersen J.H., Shaker S.B. Visual assessment of early emphysema and interstitial abnormalities on CT is useful in lung cancer risk analysis. Eur. Radiol. 2016;26:487–494. doi: 10.1007/s00330-015-3826-9. [DOI] [PubMed] [Google Scholar]
- 49.Nishio M., Kubo T., Togashi K. Estimation of lung cancer risk using homology-based emphysema quantification in patients with lung nodules. PLoS ONE. 2019;14:e0210720. doi: 10.1371/journal.pone.0210720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King T.E., Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 51.Gotway M.B., Freemer M.M., E King T., Jr. Challenges in pulmonary fibrosis. 1: Use of high resolution CT scanning of the lung for the evaluation of patients with idiopathic interstitial pneumonias. Thorax. 2007;62:546–553. doi: 10.1136/thx.2004.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karampitsakos T., Tzilas V., Tringidou R., Steiropoulos P., Aidinis V., Papiris S.A., Bouros D., Tzouvelekis A. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2017;45:1–10. doi: 10.1016/j.pupt.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Hansell D., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 54.Raju S., Ghosh S., Mehta A.C. Chest CT signs in pulmonary disease: A pictorial review. Chest. 2017;151:1356–1374. doi: 10.1016/j.chest.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 55.Xiang W., Xing Y., Jiang S., Chen G., Mao H., Labh K., Jia X., Sun X. Morphological factors differentiating between early lung adenocarcinomas appearing as pure ground-glass nodules measuring ≤10 mm on thin-section computed tomography. Cancer Imaging. 2014;14:33. doi: 10.1186/s40644-014-0033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiang J., Zhou K., Lu G., Wang Q., Ye X., Xu S., Tan L. The relationship between solitary pulmonary nodules and bronchi: Multi-slice CT–pathological correlation. Clin. Radiol. 2004;59:1121–1127. doi: 10.1016/j.crad.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 57.Snoeckx A., Reyntiens P., Desbuquoit D., Spinhoven M.J., Van Schil P.E., Van Meerbeeck J.P., Parizel P.M. Evaluation of the solitary pulmonary nodule: Size matters, but do not ignore the power of morphology. Insights Imaging. 2018;9:73–86. doi: 10.1007/s13244-017-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu Z.-G., Zhang Y., Li W.-J., Li Q., Zheng Y.-N., Lv F.-J. Primary solid lung cancerous nodules with different sizes: Computed tomography features and their variations. BMC Cancer. 2019;19 doi: 10.1186/s12885-019-6274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rizzo S., Raimondi S., De Jong E.E.C., Van Elmpt W., De Piano F., Petrella F., Bagnardi V., Jochems A., Bellomi M., Dingemans A.M., et al. Genomics of non-small cell lung cancer (NSCLC): Association between CT-based imaging features and EGFR and K-RAS mutations in 122 patients—An external validation. Eur. J. Radiol. 2019;110:148–155. doi: 10.1016/j.ejrad.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y., Kim J., Qu F., Liu S., Wang H., Balagurunathan Y., Ye Z., Gillies R.J. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology. 2016;280:271–280. doi: 10.1148/radiol.2016151455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu H., Wang Q., Tang H., Xiong L., Lin Q. Multi-slice computed tomography characteristics of solitary pulmonary ground-glass nodules: Differences between malignant and benign. Thorac. Cancer. 2016;7:80–87. doi: 10.1111/1759-7714.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J., Wang H., Geng C., Dai Y., Ji J. Advances in intelligent diagnosis methods for pulmonary ground-glass opacity nodules. Biomed. Eng. Online. 2018;17:20. doi: 10.1186/s12938-018-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaukat F., Raja G., Frangi A.F. Computer-aided detection of lung nodules: A review. J. Med. Imaging. 2019;6:02090. doi: 10.1117/1.JMI.6.2.020901. [DOI] [Google Scholar]
- 64.Holzinger A., Biemann C., Pattichis C.S., Kell D.B. What do we need to build explainable AI systems for the medical domain? arXiv. 20171712.09923 [Google Scholar]
- 65.Carvalho D.V., Pereira E.M., Cardoso J.S. Machine Learning Interpretability: A Survey on Methods and Metrics. Electronics. 2019;8:832. doi: 10.3390/electronics8080832. [DOI] [Google Scholar]
- 66.Kawagishi M., Kubo T., Sakamoto R., Yakami M., Fujimoto K., Aoyama G., Emoto Y., Sekiguchi H., Sakai K., Iizuka Y., et al. Automatic inference model construction for computer-aided diagnosis of lung nodule: Explanation adequacy, inference accuracy, and experts’ knowledge. PLoS ONE. 2018;13:e0207661. doi: 10.1371/journal.pone.0207661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akhtar N., Bansal J.G. Risk factors of Lung Cancer in nonsmoker. Curr. Probl. Cancer. 2017;41:328–339. doi: 10.1016/j.currproblcancer.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Lindeman N.I., Cagle P.T., Aisner D.L., Arcila M.E., Beasley M.B., Bernicker E.H., Colasacco C., Dacic S., Hirsch F.R., Kerr K., et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the college of American pathologists, the international association for the study of lung cancer, and the association for molecular pathology. Arch. Pathol. Lab. Med. 2018;142:321–346. doi: 10.5858/arpa.2017-0388-cp. [DOI] [PubMed] [Google Scholar]
- 69.Videtic G.M.M., Donington J., Giuliani M., Heinzerling J., Karas T.Z., Kelsey C.R., Lally B.E., Latzka K., Lo S.S.-M., Moghanaki D., et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pr. Radiat. Oncol. 2017;7:295–301. doi: 10.1016/j.prro.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Najafabadi M.M., Villanustre F., Khoshgoftaar T.M., Seliya N., Wald R., Muharemagic E. Deep learning applications and challenges in big data analytics. J. Big Data. 2015;2 doi: 10.1186/s40537-014-0007-7. [DOI] [Google Scholar]
- 71.Russakovsky O., Deng J., Su H., Krause J., Satheesh S., Ma S., Huang Z., Karpathy A., Khosla A., Bernstein M., et al. ImageNet large scale visual recognition challenge. Int. J. Comput. Vis. 2015;115:211–252. doi: 10.1007/s11263-015-0816-y. [DOI] [Google Scholar]
- 72.McNitt-Gray M.F., Armato S.G., Meyer C.R., Reeves A.P., McLennan G., Pais R.C., Freymann J., Brown M.S., Engelmann R.M., Bland P.H., et al. The Lung Image Database Consortium (LIDC) data collection process for nodule detection and annotation. Acad. Radiol. 2007;14:1464–1474. doi: 10.1016/j.acra.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]