Abstract
Simple Summary
Amino acid transporters play a multifaceted role in tumor initiation, progression, and therapy resistance. They are critical to cover the high energetic and biosynthetic needs of fast-growing tumors associated with increased proliferation rates and nutrient-poor environments. Many amino acid transporters are highly expressed in tumors compared to the adjacent normal tissues, and their expression correlates with tumor progression, clinical outcome, and treatment resistance. Tumor growth is driven by epigenetic and metabolic reprogramming and is associated with excessive production of reactive oxygen species causing the damage of vital macromolecules, including DNA. This review describes the role of the amino acid transporters in maintaining tumor redox homeostasis, DNA integrity, and epigenetic landscape under stress conditions and discusses them as potential targets for tumor imaging and treatment.
Abstract
Tumorigenesis is driven by metabolic reprogramming. Oncogenic mutations and epigenetic alterations that cause metabolic rewiring may also upregulate the reactive oxygen species (ROS). Precise regulation of the intracellular ROS levels is critical for tumor cell growth and survival. High ROS production leads to the damage of vital macromolecules, such as DNA, proteins, and lipids, causing genomic instability and further tumor evolution. One of the hallmarks of cancer metabolism is deregulated amino acid uptake. In fast-growing tumors, amino acids are not only the source of energy and building intermediates but also critical regulators of redox homeostasis. Amino acid uptake regulates the intracellular glutathione (GSH) levels, endoplasmic reticulum stress, unfolded protein response signaling, mTOR-mediated antioxidant defense, and epigenetic adaptations of tumor cells to oxidative stress. This review summarizes the role of amino acid transporters as the defender of tumor antioxidant system and genome integrity and discusses them as promising therapeutic targets and tumor imaging tools.
Keywords: amino acid transporters, oxidative stress, reactive oxygen species (ROS), glutathione (GSH), α-ketoglutarate (αKG), SLC7A11/xCT, SLC7A5/LAT1, SLC1A5/ASCT2, epigenetic regulation, Enhancer of zeste homolog 2 (EZH2)
1. Introduction
Cancer is a metabolic disease, and tumor development is driven by metabolic reprogramming [1,2]. Tumorigenesis is associated with the accumulation of genetic mutations and epigenetic alterations that drive cancer cell proliferation and increase tumor nutrient demands. Tumor cells must increase the uptake of glucose and amino acids to cover the energetic and biosynthetic needs associated with high proliferation rates and environmental changes. An increased rate of glucose consumption by tumor cells, even in the presence of oxygen (aerobic glycolysis), was first described in the 1920s by Otto Warburg [3]. Later, elevated glucose uptake has been confirmed for a wide variety of tumor types and employed to develop fluorodeoxyglucose-based (18F)-positron emission tomography (18F-FDG PET), a clinical tumor imaging for diagnostics, staging, and analysis of treatment response. Although 18F-FDG PET is commonly used for the evaluation of cancer patients, it cannot be applied to all tumor entities; thus, alternative precise and accurate imaging approaches are being established based on the amino acid consumption and high expression levels of the amino acid transporters in tumor cells. The high demand of tumor cells for amino acid glutamine (Gln) was first highlighted in the 1950s by Harry Eagle. He noticed that HeLa cells have about ten-fold increased Gln consumption compared to fibroblast culture and cannot survive without Gln supplementation [4]. Although Gln is not considered an essential amino acid (EAA), many tumor types are Gln-addicted and need to acquire Gln from external sources to grow [5,6]. In addition to glucose, Gln is a principal nutrient in fast-growing tumors serving as a source of energy and building intermediates to synthesize critical macromolecules such as DNA and proteins [7]. Gln also regulates the uptake of EAAs, which cannot be produced de novo, by antiport mechanisms [8].
Metabolic reprogramming driven by hypoxia and tumor-associated genetic mutations can induce excessive production of reactive oxygen species (ROS) in cancer cells. Consequently, high tumor-produced ROS levels affect the metabolic and proliferative properties of cancer-associated fibroblasts and inhibit the anti-tumor immune response [9,10]. ROS are mainly generated by the electron transport chain in mitochondria, upon oxidative protein folding in the endoplasmic reticulum (ER) and during some enzymatic reactions involving, e.g., cyclooxygenases, lipoxygenases, xanthine oxidases, and nicotinamide-adenine dinucleotide phosphate (NADPH) oxidases [11,12,13,14]. At fine-tuned levels, ROS-mediated redox signaling is essential for the regulation of many cellular processes in normal tissues, including cell proliferation, growth, migration, and differentiation, as reviewed elsewhere [15,16]. Although ROS play an essential role as intracellular second messengers, increased, ROS production causes the damage of vital macromolecules, including DNA mutations, protein oxidation, and lipid peroxidation [11,12,13,14]. ROS-dependent DNA damage and dysregulation of intracellular signaling such as upregulation of hypoxia response genes may lead to genomic instability, carcinogenesis, and the development of other pathologies.
To counter the harmful effect of high ROS levels, cells are equipped with ROS scavenging molecules such as glutathione (GSH) and thioredoxins, as well as ROS catabolic enzymes such as glutathione peroxidase (GPX), catalase (CAT), and superoxide dismutase (SOD) to overcome the increased ROS production.
The reduced form of GSH is an essential free radical scavenger that plays a critical role in cancer progression and treatment resistance [17]. GSH is synthesized in cytosol from cysteine (Cys) and glutamate (Glu) and then distributed into different organelles, including mitochondria, ER, and nucleus. Intracellular GSH levels can be regulated by modulating the uptake of the precursor amino acids, including Cys, Gln, Glu, and glycine (Gly), by specific amino acid transporters. Gln is converted in the cytosol to Glu by glutaminase (GLS). The reduced form of GSH is oxidized to glutathione disulfide (GSSG) by GPX to convert hydrogen peroxide (H202) into water. GSSG is reduced back to GSH by glutathione reductase (GSR) [18]. GSH levels were found to be high in many tumor types that have important implications for patient survival [19,20]. Increased antioxidant capacity of tumor cells forces their resistance to the prooxidative radio- and chemotherapy [20,21,22]. Amino acid availability contributes to the maintenance of the redox homeostasis by regulating the mammalian target of rapamycin (mTOR)-mediated antioxidant defense, ER stress, and unfolded protein response (UPR) signaling [23,24] (Figure 1). Furthermore, recent findings suggest epigenetic mechanisms to overcome oxidative stress triggered by amino acid uptake and amino acid transporter expression. Amino acid transporters are upregulated in the different types of cancer, and many of them, such as CD98hc/SCL3A2, LAT1/SLC7A5, ASCT2/SLC1A5, emerged as promising molecular biomarkers, therapeutic targets, and novel tools for tumor imaging.
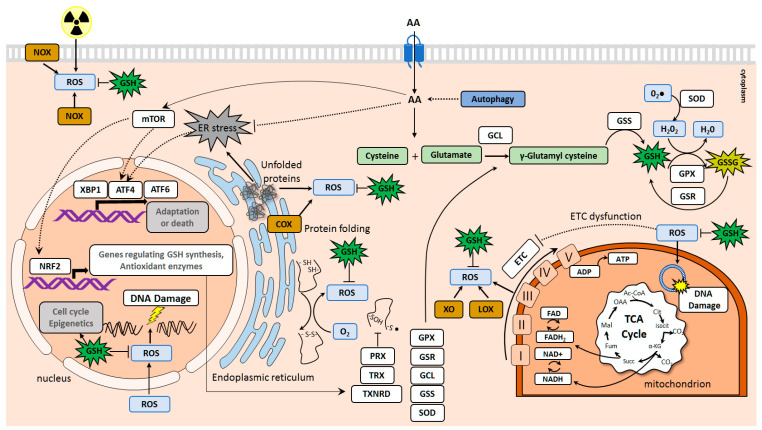
Figure 1.
Amino acid transporters in the maintenance of redox homeostasis. The reactive oxygen species (ROS) are generated in the cells in different ways, including the electron transport chain (ETC) in mitochondria, oxidative protein folding in the endoplasmic reticulum (ER), and enzymatic reactions involving, e.g., cyclooxygenases (COX), lipoxygenases (LOX), xanthine oxidases (XO), and nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX). An increased ROS production contributes to the damaging of DNA, lipids, and proteins. Amino acid transporters regulate the redox homeostasis by driving glutathione (GSH) synthesis, inducing mammalian target of rapamycin (mTOR)-mediated antioxidant defense, and preventing endoplasmic reticulum (ER) stress. Autophagy also contributes to the maintenance of the intracellular free amino acid pool. GSH is synthesized in cytosol from Cys and Glu by glutamate-cysteine ligase (GCL) and glutathione synthetase (GSS). The reduced form of glutathione, GSH, is oxidized to glutathione disulfide (GSSG) by glutathione peroxidase (GPX) to neutralize hydrogen peroxide (H202) and is reduced back to GSH by glutathione reductase (GSR). The mTOR mechanism of antioxidant defense includes activation of the nuclear factor-erythroid 2 -related factor 2 (NRF2), a master transcription factor. NRF2 regulates the expression of many genes involved in the GSH synthesis, including GCL, GSS, GPX, GSR, other genes involved in the regulation of ROS levels, such as superoxide dismutase (SOD) enzyme which converts superoxide (O2•−) to H202, genes encoding thiol-specific antioxidant proteins thioredoxin (TRX) and thioredoxin reductase (TXNRD) as well as peroxiredoxin (PRX). mTOR also triggers ATF4-mediated gene expression of the heterodimeric transmembrane amino acid transporters such as SLC7A5 (LAT1), SLC3A2 (CD98hc), and SLC7A11 (xCT).
This review summarizes the multifaceted role of the amino acid transporters in the maintenance of tumor redox homeostasis, DNA integrity, and epigenetic landscape under stress conditions and discusses them as potential targets for tumor imaging and treatment.
2. Amino Acid Transporters Upregulated in Tumors and Their Role in Cell Protection against Oxidative Damage and DNA Injury
Expression of the amino acid transporters is upregulated in many tumor malignancies and enhances tumor aggressiveness, drug resistance, and oxidative damage protection. Hence, targeting amino acid transporters as an Achilles’ heel of the cancer cells may offer a wide range of opportunities to tackle oxidative stress and sensitize tumor cells to anticancer treatments (Figure 2).
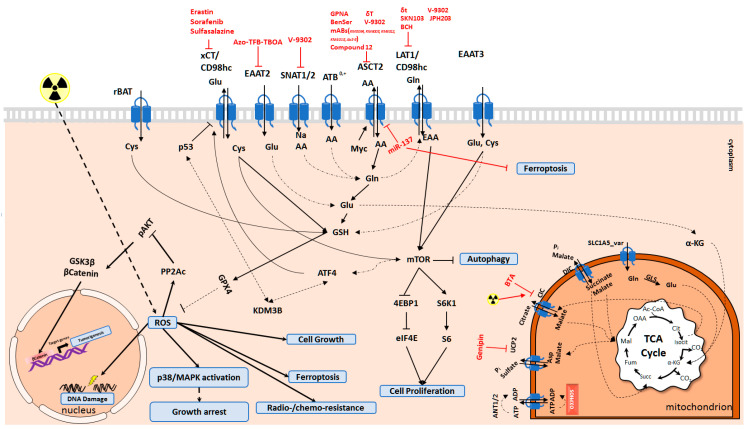
Figure 2.
Molecular mechanisms activated by amino acid transporters. Amino acid transporters are vital to balance the intracellular amino acid pool for many cellular functions. Depending on the amino acid transporter activity, ferroptosis, apoptosis, autophagy, and proliferation of cells is regulated. Cys and Glu are the key amino acids for the synthesis of GSH. Cells deal with ROS species through GSH to decrease DNA damage, protein oxidation, and lipid peroxidation. GLS: Glutaminase; GSH: Glutathione; Cys: Cysteine; Glu: Glutamate; Gln: Glutamine; Asp: Aspartate; AA: Amino acids; EAA: Essential amino acids; αKG: alpha-ketoglutarate.
2.1. SLC1 Family of Amino Acid Transporters
The Solute Carrier 1 (SLC1) family of amino acid transporters is comprised of seven members, which are essential not only to balance extracellular glutamate concentration under neurotoxic levels in the central nervous system but also contribute to the inter-organ Gln flux and nitrogen metabolism in peripheral tissues [25]. While five members of this family (SLC1A1, SLC1A2, SLC1A3, SLC1A6, and SLC1A7) function as high-affinity Glu transporters, the remaining two (SLC1A4 and SLC1A5) work as neutral amino acid transporters [25]. In general, members of this family are essential for activation of the mTOR pathway to maintain cell growth and proliferation as reviewed in [26], and for coping with oxidative stress due to their contribution to the synthesis of GSH [27,28,29].
Excitatory amino acid transporter 3 (EAAT3/SLC1A1) is a critical regulator of GSH synthesis and oxidative stress in neurons [30,31] and an important contributor to tumorigenesis [32]. SLC1A1 expression, which is essential for Glu and Cys import, is sustained by Sortilin Related VPS10 Domain Containing Receptor 2 (SorCS2) [30]. The shortage of SorCS2 leads to the decreased surface expression of SLC1A1 due to its targeting to the late endosomes and results in oxidative brain damage and enhanced neuronal cell death [30]. The serine/threonine mTOR kinase strongly upregulates SLC1A1 expression [33]. Reciprocally, a high level of SLC1A1 augments expression of activating transcription factor 4 (ATF4) and Cys/Glu antiporter xCT/SLC7A11 as well as phosphorylation of mTOR, the mTOR Substrate S6 Kinase 1 (S6K1), and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) [28]. Henceforth, SLC1A1 may activate the mTOR pathway to elevate intracellular Cys levels through increased ATF4 and SLC7A11 expression to cope with the oxidative stress [28].
Alanine, serine, cysteine-preferring transporter 2 (ASCT2), encoded by SLC1A5 in humans, is a member of SLC family proteins that mediates uptake of Gln, which becomes a conditional EAA in the highly proliferative cancer cells [5,6] and supports intracellular amino acid homeostasis maintaining the activity of mTOR signaling pathway [6,34]. SLC1A5 expression is regulated by several transcription factors such as MYC, retinoblastoma/E2F transcription factor pathway (RB/E2F), androgen receptor (AR), and ATF4 [35,36,37,38,39,40]. SLC1A5 depletion leads to an amino acid starvation response in 143B osteosarcoma cells. Depletion of SLC1A5 expression upregulates sodium neutral amino acid transporters from the SLC38 family, sodium-coupled neutral amino acid transporter SNAT1/ SLC38A1 and SNAT2/SLC38A2 to compensate for the lack of SLC1A5 due to their similarity in substrate specificity [34,41,42]. Furthermore, Gln metabolism is promoted by the AR signaling, which upregulates SLC1A4/ASCT1 and SLC1A5 expression to maintain cell growth under serum starvation conditions via mTOR signaling [27]. Consistent with this observation, SLC1A5 knockdown reduces androgen-mediated Gln uptake and growth of androgen-sensitive prostate cancer (PCa) cell lines [27]. Also, a variant of SLC1A5, called SLC1A5_var, promotes metabolic switch by augmenting Gln metabolism in cancer cells [43]. Unlike SLC1A5, SLC1A5_var is localized to the mitochondrial membrane because of its N-terminal mitochondrial targeting signal and may mediate the Gln influx in mitochondrial respiration. The expression of SLC1A5_var is triggered by hypoxia and plays an essential role in the induction of the oxidative phosphorylation (OXPHOS) and increased ROS scavenging attributed to the Gln-derived GSH synthesis [43].
lncRNA X-inactive specific transcript (lncRNA-XIST) is a long non-coding RNA that promotes glioblastoma progression by sponging microRNA (miRNA) miR-137, which targets SLC1A5 expression. Knockdown of lncRNA-XIST reduces SLC1A5 levels and inhibits tumorigenesis of glioma cells in vivo [44]. Correspondingly, lncRNA-XIST knockdown in glioma promotes ROS production and apoptosis and inhibits cell proliferation in SLC1A5/miR-137 dependent fashion, which can be reversed by cell supplementation with N-acetyl-L-Cysteine (NAC), a scavenger of ROS [44]. miR-137 also reduces Gln uptake by directly targeting SLC1A5 in melanoma cells and triggers ROS generation and ferroptosis, a form of the non-apoptotic, iron-dependent mode of cell death associated with the accretion of lipid ROS [45]. Normally, GSH decreases reactive oxygen and nitrogen species (RONS) through glutathione peroxidase (GPX) activity. On the contrary, as a result of a reduction in the intracellular GSH levels and glutathione peroxidase 4 (GPX4) activity, lipid peroxidation cannot be controlled anymore, leading to increased ROS levels and cell death [45]. All in all, these findings suggest that targeting SLC1A5 might offer a new therapeutic strategy for the treatment of different types of malignancies [46].
2.2. SLC3 and SLC7 Families of Heteromeric Amino Acid Transporters
SLC7 family is composed of L-type amino acid transporters (LATs, SLC7A5-13, and SLC7A15) and cationic amino acid transporters (SLC7A1-4, and SLC7A14) [47]. LATs are the light subunits of the heterodimeric transmembrane amino acid transporter complexes (HATC) forming the heterodimers with the heavy subunits of SLC3 family: SLC3A1 and SLC3A2 [47]. In general, members of SLC3 and SLC7 families are essential for mTOR pathway activation as reviewed in [26], and for the regulation of autophagy and ferroptosis by balancing intracellular AA and ROS levels [48,49,50,51,52,53,54].
rBAT is encoded by SLC3A1 and is overexpressed in breast cancer cells promoting tumorigenesis via rBAT mediated Cys uptake [48]. Elevated Cys uptake reduced the activity and stability of serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform (PP2Ac) via ROS level reduction; therefore, it induces activation of AKT signaling [48].
CD98hc/SLC3A2 acts as a part of the transporter complexes for the branched-chain amino acids (BCAA) and aromatic amino acids (AAA). It serves as a regulator of mTOR signaling and enhancer of integrin signaling. The amino acids transported by SLC3A2 HATCs are essential for synthesizing purine and pyrimidine nucleotides and cell cycle progression. The SLC3A2 depletion results in the critical decrease of nucleotide availability and induces replicative stress [55]. SLC3A2 functions as a chaperon for the light subunits and is important for their stability and membrane localization [49,56,57,58,59]. SLC3A2 can form HATCs with xCT (encoded by SLC7A11), LAT1 (encoded by SLC7A5), and y+LAT2 (encoded by SLC7A6).
The depletion of SLC3A2 HATCs results in a reduced intracellular pool of amino acids, including leucine (Leu) and arginine (Arg), potent activators of the mTOR kinase [59,60]. The intracellular amino acid deficiency results in the mTOR signaling inhibition and induces activation of general amino acid control non-derepressible 2 (GCN2), which phosphorylates the subunit of eukaryotic translation initiation factor 2A (eIF2α). These mechanisms repress protein synthesis and trigger the expression of ATF4, which regulates the transcription of multiple targets involved in amino acid metabolism and stress response [61,62]. In particular, ATF4 is essential for the expression of SLC7A5, SLC7A11, and SLC3A2 and, therefore, for the replenishment of the intracellular GSH precursors [51,62,63,64,65]. The ATF4 knockout (KO) cells possess an impaired amino acid import and GSH biosynthesis and are highly predisposed to oxidative stress [62]. The adaptive signaling pathway triggered by ATF4 to recover cellular homeostasis is called the integrated stress response (ISR). ISR leads to the inhibition of the global mRNA translation and is one of the key cell survival strategies in the face of different stresses, including ER stress. The ER is an organelle responsible for protein folding and degradation. Hydrogen peroxide is formed as a byproduct of the disulfide bond formation [66]. The ER consumes cellular GSH that buffers the ER against these hyperoxidizing conditions [67]. The ER stress is induced by the accumulation of unfolded proteins and the formation of improper disulfide bonds. During this process, the released electrons transferred to molecular oxygen result in ROS accumulation as a byproduct and lead to the depleting of the intracellular GSH pool [67,68]. The accumulation of unfolded proteins in ER triggers UPR signaling, which might serve either as a pro-survival or pro-apoptotic process. The exact mechanisms determining the cell fate in response to UPR remains to be yet investigated, although it has been suggested that mitochondrial respiration substantially contributes to the lethal ROS accumulation during ER stress and UPR activation [68,69]. SLC3A2 plays a role in ER stress-related UPR as knockdown of SLC3A2 expression inhibited expression of three key UPR proteins ATF4, ATF6, and XBP1, indicating that the ER stress-mediated UPR is interrupted as a result of SLC3A2 inhibition [70].
The ER stress and UPR - mediated activation of the nuclear factor kappa B (NF-κB) and p38/MAPK (mitogen-activated protein kinase) signaling results in the inhibition of Akt/mTOR/p70S6K pathway and activation of autophagy [71,72,73]. Autophagy plays a dual role as a mechanism that either induces or inhibits cell death depending on the tissue context and environmental cues [74]. Pro-survival autophagy promotes the removal and degradation of unfolded proteins and damaged organelles while providing starved cells with amino acids for protein biosynthesis and energy replenishment [75].
Downregulation of SLC3A2 expression results in depletion of the reduced form of GSH, increased oxidative stress, DNA damage, and radiosensitivity in head and neck squamous carcinoma (HNSCC) cells [49]. Consequently, high expression of SLC3A2 and SLC7A5 contributes to the increased radioresistance in HNSCC in vitro and correlates with a worse outcome in patients with HNSCC treated with radiochemotherapy. Simultaneously, SLC3A2 depletion increases pro-survival autophagy in HNSCC, which helps tumor cells to survive on nutrient shortage and stress conditions. Inhibition of autophagy with bafilomycin A treatment or ATG5 knockdown sensitizes SLC3A2 KO HNSCC cells to ionizing radiation (IR).
SLC7A11 is a subunit of the glutamate/cystine antiporter system xc-. The SLC3A2/ SLC7A11 HATC is essential for the transport of cystine, the oxidized form of Cys, and thus for GSH biosynthesis and the maintaining of cellular redox homeostasis [56,76]. Oxidative stress conditions induce SLC7A11 mRNA expression mediated by transcription factor Nuclear Factor-Erythroid 2-related factor 2 (NRF2), and ATF4. NRF2 and ATF4 bind to the antioxidant response element (ARE) and amino acid response element (AARE), respectively, in the promoter regions of SLC7A11 gene [63,77]. xCT encoded by SLC7A11 is essential for cancer cell survival and tumor formation in vivo. The upregulation of SLC7A11 expression by oncogenic KRAS pathway has been shown to contribute to RAS transformation via regulation of redox balance [64,78,79]. The genetic disruption or chemical inhibition of SLC3A2/ SLC7A11 HATC leads to autophagy and ferroptosis in the different types of tumor cells, including endometrial, colorectal, pancreatic cancer, and hepatocellular carcinoma (HCC) through inhibition of mTOR/p70S6K signaling [45,51,56,57,78,80,81]. On the other hand, SLC7A11 is reciprocally regulated by the mTOR pathway. Mammalian target of rapamycin complex 2 (mTORC2) phosphorylates cytosolic N terminus of SLC7A11 at Serine26 (Ser26) and therefore inhibits its activity [52]. mTOR also induces the phosphorylation of eukaryotic initiation factor 2 alpha (eIF2a). eIF2a triggers ATF4 translation and ATF4-mediated gene expression to maintain the supply of amino acids for GSH and protein biosynthesis [62,82]. These ATF4 target genes include the members of SLC3A2 HATCs such as SLC7A5 (LAT1), SLC3A2 (CD98hc) and SLC7A11 (xCT) [51,62,63,64,65,83].
Mitochondrial dysfunction associated with DNA mutations activates a retrograde mitochondria-to-nucleus response inducing stress defense and metabolic reprogramming [84]. This retrograde signaling contributed to mitochondrial dysfunction-induced cisplatin resistance in a gastric cancer model through activation of the eIF2α-ATF4 pathway and consequent induction of SLC7A11 expression [85]. Pharmacological inhibition of SLC7A11 with sulfasalazine (SSZ) or erastin, genetic silencing of SLC7A11 expression, or inhibition of GSH synthesis with buthionine sulphoximine (BSO) restores cisplatin sensitivity in gastric cancer cells [85]. Moreover, SLC7A11 expression is essential for the anti-cancer toxicity of temozolomide (TMZ), a DNA alkylating agent used to treat malignant gliomas. Tumor cells with SLC7A11 knockdown exhibit higher vulnerability towards TMZ treatment and SLC7A11 inhibitors such as erastin and sorafenib potentiate a toxic impact of TMZ by induction of ferroptosis [86]. Reduction of the intracellular GSH in response to SLC7A11 inhibition with erastin sensitizes breast cancer cells to γ-radiation by induction of complex DNA damage [87].
Therapy resistance has been attributed to the populations of the tumor-initiating cells called cancer stem cells (CSCs) identified in most tumor entities [88,89,90]. A cell surface adhesion protein Cluster of Differenation 44 (CD44) was characterized as one of the CSC markers for different tumors [91]. A CD44 variant (CD44v) interacts with and stabilizes SLC7A11 at the plasma membrane, and therefore positively regulates the GSH levels and ROS scavenging in tumor cells, and in particular, in CD44-expressing colorectal and gastrointestinal CSCs [92,93]. Activation of the ROS scavenging system in CSCs is associated with chemo- and radioresistance [94,95], and targeting SLC7A11 may be a promising approach to eliminate therapy-resistant CSCs [92,93].
The ubiquitin-proteasome system (UPS) is one of the major ways to degrade and remove the oxidatively damaged and misfolded proteins and recycle the resulting amino acids. The disruption of this pathway by proteasome inhibitors induces ER stress and ROS production [96,97]. Proteasome activity is elevated in many human cancers, and CSCs are shown to be highly sensitive to proteasome inhibition [98,99]. Proteasome inhibition with bortezomib induces SLC7A11 expression by the ATF4 and NRF2-dependent mechanisms to increase the intracellular Cys pool and GSH biosynthesis. Genetic depletion of SLC7A11 or pharmacological inhibition of its function increases tumor cell sensitivity to the proteasome inhibition [63].
Although downregulation of SLC7A11 inhibits GSH synthesis and induces oxidative stress, it leads to the increased expression of the efflux transporter P-glycoprotein (P-gp), also called multidrug resistance protein 1, MDR1, and therefore induces a drug-resistant phenotype in breast cancer cells. The study by Ge et al. suggests that reversing the P-gp overexpression and potential collateral sensitivity of P-gp expressing cells to certain chemical drugs might be utilized to increase the anti-cancer potential of the SLC7A11-targeting agents [100,101].
2.3. SLC38 Family of Sodium-Dependent Neutral Amino Acid Transporters
SLC38 family includes 11 Na+-dependent transporters of neutral amino acids [102]. The activity of SLC38 family members is pH-dependent and notably elevated at alkaline pH conditions [102]. SNAT2/SLC38A2 is a rescue amino acid transporter responsible for the Gln uptake under stress conditions [42]. Amino acid deprivation triggers an integrated stress response by cyclin-dependent kinase 7 (CDK7)-mediated SLC38A2 upregulation [103]. Besides, SLC38A2 expression is induced by hypoxia and estrogen receptor alpha (ERα) and contributes to the resistance to anti-endocrine therapy in breast cancer [104]. High SLC38A2 expression is associated with poor clinical prognosis in breast cancer patients, including triple-negative breast cancer (TNBC) [104]. TNBC cells highly depend on Gln uptake to support Cys transport by the SLC7A11 antiporter [105]. SLC38A2 knockdown reduces Gln transport and increases ROS levels upon Gln deprivation [104]. SLC38A3/SNAT3 transports Gln, histidine (His), and aspartate (Asp) [106]. SLC38A3 and NRF2 expression in the kidney tissues is elevated during metabolic acidosis [107]. NRF2 directly induces SLC38A3 mRNA expression upon metabolic acidosis to increase Gln influx and maintain pH homeostasis. Besides, the Nrf2 KO mice exhibited acidosis-induced enhancement of oxidative stress and failed to enhance Slc38a3 expression [107].
2.4. SLC25 Family of Mitochondrial Transporters
SLC25 is the largest family of solute transporters in humans. It includes 53 members and is composed of 24 evolutionary well-conserved subfamilies. Most SLC25 transporters function as monomers and are responsible for the transport of their substrates across the inner mitochondria membrane [108,109]. These transporters function as a bridge between metabolic reactions in mitochondria and cytosol by transporting a wide variety of solutes, including nucleotides, amino acids, vitamins, inorganic ions, fatty acids, dicarboxylate, and citrate. The defects of mitochondrial carriers are associated with dysregulation of the mitochondrial functions and cellular metabolism [108,109]. SLC25 transporters were also characterized as regulators of intracellular ROS levels. The dicarboxylate carrier, DIC encoded by the SLC25A10 gene, regulates mitochondrial GSH transport. Knockdown of SLC25A10 induces sensitivity to Gln deprivation, decreases NADP and NAPDH levels, and is associated with low expression of key genes involved in the antioxidant system including thioredoxin 2 (TXN2) and thioredoxin reductase 2 (TXNRD2). Consequently, the knockdown of SLC25A10 increases sensitivity to oxidative stress and cisplatin treatment [110].
3. The Role of Amino Acid Transporters in the Epigenetic Regulation
Epigenetic regulation of amino acid transporters has been frequently shown in different cancer types [111,112,113,114]. Recently, the reciprocal regulation between amino acid transporters and epigenetic alterations has drawn attention. The altered metabolism reprograms the cancer epigenome, which is involved in cancer development and progression (Figure 3). To date, DNA methylation reactions and histone modifications are among the most broadly studied epigenetic alterations. These epigenetic signatures are dependent on specific regulators that require different metabolites as substrates and cofactors. The availability of most of these metabolites is dependent on amino acid transporter activity [115,116,117,118].
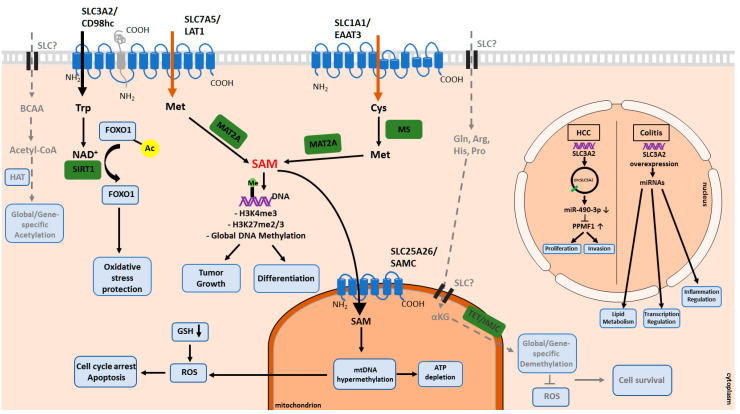
Figure 3.
Epigenetic modifications controlled by amino acid transporters. Amino acid transporters play an important role in global or gene-specific epigenetic regulations. The LAT1(SLC7A5)/CD98hc (SLC3A2) complex is among the amino acid transporters responsible for Trp and Met uptake. SAM is produced from intracellular Met by MAT2A and is used as a methyl donor for methylation reactions. SLC1A1/EAAT3 is among the regulators of methylation reactions through Cys uptake, which is subsequently converted to Met by MS. SAM can be transported into mitochondria by SAMC, a mitochondrial carrier which was shown to be important for mtDNA hypermethylation and subsequently intracellular ROS levels. Intracellular tryptophan, which is transported by SLC7A5, is used for de novo NAD+ production and intracellular NAD+ levels control the activity of HDACs. In the presence of NAD+, SIRT1 removes the acetyl group and inactivates FOXO1, a transcription factor, and a critical cellular redox sensor protecting the cell from oxidative stress. In colitis, SLC3A2 overexpression results in increased miRNA-132 expression levels and consequently, downregulation of its target genes that are involved in different pathways, including lipid metabolism, transcriptional regulation and inflammation regulation. circSLC3A2, a circular RNA encoded by SLC3A2, induces proliferation and invasion in HCC by sponging miR-490-3p and regulating PPM1F expression. Amino acid transporters regulating the demethylation or acetylation reactions remain to be determined. Acetyl-CoA: Acetyl Coenzyme A; Ac: Acetylation; αKG: Alpha-ketoglutarate; Arg: Arginine; Cys: Cysteine; Gln: Glutamine; GSH: Glutathione; HAT: Histone Acetyltransferase; His: Histidine; Met: Methionine; MS: Methionine Synthase; NAD+: Nicotinamide Adenine Dinucleotide; Pro: Proline; ROS: Reactive Oxygen Species; SAM: S-Adenosyl methionine; TET: Ten-Eleven Translocation; Trp: Tryptophan.
3.1. DNA and Histone Methylation and Demethylation Processes
The methylation reaction, a covalent addition of methyl group, is catalyzed by three DNA methyltransferase isoforms (DNMT1, DNMT3A, DNMT3B) and by various histone methyltransferases (HMTs). Methyl groups are added to cytosine nucleotides on DNA to form 5-methylcytosine at CpG locations (5mC), a well-known transcriptional repressive mark. Methylation of histone H3 and H4 at lysine (Lys) and Arg residues can be associated with either repressive or activating transcriptional effect, depending on the location and the number of methyl groups. All cellular methyltransferases require S-adenosylmethionine (SAM) as the universal methyl donor. SAM is produced from methionine (Met) by methionine adenosyltransferase (MAT) in the methionine cycle pathway [119]. Thus, alterations in the Met metabolism influencing the intracellular SAM levels can directly affect methylation levels and, subsequently, gene expression. The amino acid transporter SLC7A5/LAT1, with its obligate chaperone and dimerization partner SLC3A2/CD98hc, has been shown to have a high affinity for Met [120]. SLC7A5 and SLC3A2 expression is highly upregulated in many tumor types, such as HNSCC, prostate, lung, breast, and pancreatic cancers [49,121,122,123,124,125].
Gene knockdown experiments revealed a critical role of SLC7A5 for the transport of Met and maintenance of the intracellular SAM levels in lung cancer [126]. The reduction in the SLC7A5 levels decreases the activity of enhancer of zeste homolog 2 (EZH2) and levels of EZH2-dependent di- and tri-methylation of H3K27. The regulation between SLC7A5 and EZH2 is reciprocal, and EZH2 controls the expression levels of SLC7A5 through epigenetic inhibition of RXRa. Apart from the findings in lung cancer, a recent report showed that triggering naïve T cells via antigen receptors increases SLC7A5 expression and bioavailability of Met and results in increased levels of H3K4me3 and H3K27me3 in vitro and in vivo. These epigenetic alterations drive effector T lymphocyte differentiation [127,128]. The influence of amino acid transporters on epigenetic regulations has been shown not only on the nuclear DNA but also on the mitochondrial DNA. SLC25A26 gene encodes the mitochondrial carrier responsible for the import of SAM into the mitochondrial matrix. Menga et al. revealed that SLC25A26 is downregulated in cervical cancer due to promoter hypermethylation [113]. Overexpression of SLC25A26 increases the uptake of cytosolic SAM into the mitochondria. Methylation analyses showed that the mitochondrial transcription controller region, displacement loop (D-loop), is hypermethylated due to higher mitochondrial SAM levels. SLC25A26 overexpression is associated with hypermethylation of mitochondrial DNA, reduced oxidative phosphorylation, and, subsequently, lower mitochondrial ATP levels. Also, increased mitochondrial SAM availability reduces levels of GSH, leading to oxidative stress, cell cycle arrest, and apoptosis. Another study by Hodgson et al. showed that SLC1A1, which is responsible for Cys influx, is essential for redox balance. Blocking this transporter leads to oxidative stress, and methionine synthase (MS) inhibition subsequently decreases global DNA methylation levels in neuroblastoma cells [129]. Like methyltransferases, demethylases also require metabolites as substrates and cofactors to erase the methyl groups from DNA and histones. α-ketoglutarate (αKG) is one of the major metabolites utilized by demethylases. It can be produced from isocitrate in the citric acid cycle in mitochondria and also can be synthesized from different amino acids such as Gln, Arg, His, and proline (Pro) [130,131,132,133]. In glioma and cholangiocarcinoma, αKG can be a precursor for oncometabolite that leads to DNA and histone methylation aberrations resulting in stem-like phenotype switch [134,135,136]. Thus, the amino acid transporters responsible for intracellular Gln transport, as well as amino acids contributing to the αKG synthesis, can be potential epigenetic regulators through this metabolic pathway. However, the additional links between amino-acid transporters, DNA and histone demethylation processes, and their role in tumor development remain to be determined.
3.2. Histone Acetylation and Deacetylation Mechanisms
Histone acetylation is the addition of a negatively charged acetyl group to the Lys residues of the histone proteins. It subsequently reduces the binding between histones and DNA and induces the recruitment of transcriptional activators [137]. Histone acetylation and deacetylation are mediated by histone acetyltransferases (HAT) and histone deacetylases (HDAC), respectively. They are profoundly affected by cellular acetyl-coenzyme A (CoA) abundance, as a major acetylation source [115,138,139]. Acetyl-CoA can be derived from BCAAs (Leu, isoleucine (Ile), valine (Val) and Lys), which can be further utilized by HATs [140]. Transporters regulating the intracellular levels of these amino acids may play a role in controlling the Acetyl-CoA availability, therefore global or gene-specific acetylation levels.
On the other hand, deacetylation reactions require nicotinamide adenine dinucleotide (NAD+), an oxidizing agent. NAD+ serves as a cofactor for the class III HDAC enzymes known as sirtuins [141] and can be synthesized de novo from tryptophan (Trp). Therefore, intracellular NAD+ availability can regulate the catalytic activity of sirtuins, mostly SIRT1. In addition to histones, other epigenetic regulators such as FOXO1, a transcriptional factor, and a critical cellular redox sensor, can also be regulated by SIRT1-dependent deacetylation. In particular, studies in lung cancer showed that de novo synthesis of NAD+ from Trp is essential for resistance to oxidative stress [142]. The inhibition of SLC7A5 leads to the disruption of Trp uptake, depleting the NAD+ pool, and SIRT1 activity. Consequently, it leads to the accumulation of acetylated FOXO1, which is then translocated to the nucleus and activates downstream pro-apoptotic genes. This study shows that in tumor tissues, increased levels of SLC7A5 may represent an adaptation mechanism for resistance to oxidative stress in cancer cells through regulating epigenetic modifications. These findings reinforce the potential roles of amino acid transporters as metabolic signaling components in the regulation of the cellular epigenetic landscape.
3.3. Other Chromatin Modifications and Epigenetic Regulations
Apart from the epigenetic regulations described above, amino acid transporters influence different chromatin modifications, including the expression of miRNAs. In both in vitro and in vivo conditions, SLC3A2 is highly expressed during intestinal inflammation, which is a risk factor for developing colorectal cancer (CRC) [143,144]. SLC3A2 overexpression in intestinal epithelial cells under inflammatory conditions induces miRNA-132 expression. Consequently, it leads to the downregulation of the miRNA-132-target genes, which are involved in lipid metabolism and transcriptional regulation. Similar gene expression changes also occur in patients with inflammatory bowel disease [145]. Furthermore, by regulating colonic miRNAs, SLC3A2 may impact tight junction proteins, which play a role in colonic barrier dysfunction during intestinal colitis [146]. The study by Han et al. showed that SLC3A2 overexpression induces colon inflammation and increases the risk of colitis-associated cancer via dysregulating broad miRNA expression profile in both tissue villus and crypt cells [147]. Wang et al. revealed that SLC3A2-encoding circular RNA (circRNA) is a critical regulator of tumor growth. circRNA is a new class of non-coding RNAs that has a closed-loop structure and higher conservation between the species. circSLC3A2 regulates cellular proliferation and invasion in HCC by sponging miR-490-3p and upregulating its target, protein phosphatase, Mg2+/Mn2+ dependent 1F (PPM1F) [148]. This study shows that circSLC3A2 is a potential oncogenic factor in HCC, and by modulating downstream players, it may lead to tumor progression. Taken together, these studies demonstrate the possible importance of amino acid transporters for the progress of carcinogenesis through epigenetic modifications.
4. Amino Acid Transporters as Targets for Tumor Treatment
In contrast to normal tissues, many malignancies depend on exogenous supplies. Multiple tumor-promoting genetic mutations cause metabolic reprogramming to provide a growth advantage to highly proliferating cells over healthy cells. Thus, cancer cells upregulate different amino acid transporters to elevate the uptake of nutrients and sustain bioenergetic and biosynthetic processes. Therefore, the lack of certain nutrients due to the targeting of amino acid transporters may cause cancer cell death. On the other hand, normal cells under the same conditions have a better chance of surviving due to their less addiction to non-essential nutrients that can be synthesized from other metabolic intermediates. Unlike normal cells, for instance, acute lymphoblastic leukemia (ALL) cells cannot synthesize asparagine due to the lack of asparagine synthase (ASNS) expression, making them heavily dependent on exogenous asparagine to survive. Thus, L-asparaginase (ASNase) treatment in ALL patients catalyzes the conversion of plasma L-asparagine to aspartic acid and ammonia, leading to the death of cancer cells due to the inhibited global protein synthesis. Similarly, deterioration of asparagine uptake might also cause the death of ALL but not normal cells [149,150,151,152].
There are several strategies to alter the amino acid balance in cancer cells. One approach is to apply amino acid starvation to decrease cancer cell growth [153,154]. Secondly, some transporters might be recruited to selectively increase the concentration of specific amino acids [155,156,157]. Last but not least, transporters might be blocked to prevent amino acid influx or efflux [158,159,160], Table 1. The preclinical inhibition of Gln transporters, which contribute to the anti-oxidative cell defense, in particular, SLC1A5/ASCT2, SLC7A5/LAT1, and cystine/glutamate antiporter SLC7A11/xCT attracted the most attention and will be discussed in more detail.
Table 1.
Targeting of the amino acid transporters.
| Chemical Inhibition | |||||
|---|---|---|---|---|---|
| Transporter | Inhibitor | Experimental Model | Tissue Type | Refs | |
| SLC1A1 | 2-(furan-2-yl)-8-methyl-N-(o-tolyl)imidazo[1,2-a]pyridin-3-amine | In vitro (Cell line) | Human Embryonic Kidney cells | [161] | |
| SLC1A2 | Azo-TFB-TBOA | In vitro (Xenopus laevis oocytes) | Xenopus laevis oocytes | [162] | |
| SLC1A5, SLC38A2, SLC7A5 | V-9302 | In vitro (Cell line) | A panel of human cancer cell lines [163] Head and Neck Squamous Cell Carcinoma [29] | [29,163] | |
| In vivo (Cell line derived-xenograft) | patient-derived xenografts (PDX) model [163], Head and Neck Squamous Cell Carcinoma cell line xenograft [29] | ||||
| SLC1A5 | L-g-glutamyl-p-nitroanilide (GPNA) | In vitro (Cell line) | Colorectal Carcinoma [164], Endometrial Carcinoma [165] | [164,165] | |
| In vivo (Cell line derived-xenograft) | |||||
| SLC1A5 | Benzylserine (BenSer) | In vitro (Cell line) | Endometrial Carcinoma [165], Melanoma [166] | [165,166] | |
| SLC1A5 | Benzyloxybenzyl analogues | In vitro (Cell line) | Human Embryonic Kidney cells, C6 Rat cells | [167] | |
| SLC1A5 | KM8094 mAB | In vivo (Patient derived-xenograft) | Patient-derived Gastric cancer tissue | [168] | |
| SLC1A5 | KM4008 mAB | In vitro (Cell line) | A panel of cell lines | [169] | |
| SLC1A5 | KM4012 mAB | In vitro (Cell line) | A panel of cell lines | [169] | |
| SLC1A5 | KM4018 mAB | In vitro (Cell line) | A panel of cell lines | [169] | |
| SLC1A5 | Ab3-8 mAb | In vitro (Cell line) | Colorectal Carcinoma | [170] | |
| SLC1A5, SLC7A5 | Delta-tocotrienol (δT) | In vitro (Cell line) | Non-Small Cell Lung Cancer | [171] | |
| SLC6A1 | Photoswitchable inhibitor | In vitro (Cell line) | Human Embryonic Kidney cells and Dentate gyrus granule cells | [172] | |
| SLC6A14 | α-methyltryptophan | In vitro (Cell line) | Colon cancer [173], Pancreatic cancer [174,175] | [173,174,175] | |
| In vivo (Cell line derived-xenograft) | |||||
| SLC7A5 | SKN103 | In vitro (Cell line) | A panel of human cancer cell lines | [176] | |
| SLC7A5 | BCH | In vitro (Cell line) | Melanoma | [166] | |
| SLC7A5 | JPH203 | In vitro (Cell line) | Anaplastic thyroid cancer [177], Medulloblastoma [178], Head and Neck Squamous Cell Carcinoma [179], T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia [180] |
[177,178,179,180] | |
| In vivo (Cell line derived-xenograft) | |||||
| SLC7A11 | Sulfasalazine (SSZ) | In vitro (Cell line) | Non-Small Cell Lung Cancer [181], A panel of human cancer cell lines [182], Lung Adenocarcinoma [183], Head and Neck Squamous Cell Carcinoma [184], Bladder Cancer [185], Lymphoma [186,187] | [181,182,183,184,185,186,187] | |
| In vivo (Cell line derived-xenograft) | |||||
| In vivo (KRAS Mouse model) | |||||
| In vivo (Murine metastasis model) | |||||
| SLC25A8 | Genipin | In vitro (Cell line) | Breast Cancer [188], Ovarian Cancer [189] | [189] | |
| SLC43A1 | ESK246 | In vitro (Cell line) | Prostate Cancer | [190] | |
| Drug Delivery | |||||
| Through which Transporter | Molecule | Delivery | Experimental Model | Tissue Type | Refs |
| SLC1A5 | GLN-CD (conjugation of Gln with β-cyclodextrin) | Doxorubicin | In vitro (Cell line) | Triple-negative Breast Cancer | [191] |
| SLC1A5 | Polyglutamine (PGS) | various agents including siRNAs | In vitro (Cell line) | Lung Cancer | [192] |
4.1. Targeting SLC1 Transporter Family
The expression of SLC1A5 is frequently upregulated in the different types of cancer with high Gln demand, and its increased expression is associated with worse clinical prognosis. The preclinical studies using in vitro and in vivo models demonstrated the importance of SLC1A5-mediated Gln uptake for tumor growth, making it an attractive therapeutic target [193]. Schulte et al. developed V-9032, an antagonist of transmembrane Gln flux. V-9032 specifically and effectively targets SLC1A5 and thus leads to the attenuation of proliferation and growth of cancer cells as well as elevation of oxidative stress and cell death, contributing to anti-tumor responses both in vivo and in vitro [163]. Furthermore, either short hairpin RNA (shRNA) or miR-137-mediated silencing of SLC1A5 and pharmacological inhibition of SLC38A2 and SLC1A5 by V-9302 abolish intracellular Gln levels as well as Gln metabolism that eventually affects GSH production [29]. Overall, these effects lead to an increase in apoptosis, autophagy, and oxidative stress and suppression of the mTORC1 signaling and cell proliferation in HNSCC, further boosting tumor cell sensitivity to cetuximab treatment [29]. Inhibition of SLC1A5 in CRC cells by shRNAs or L-g-glutamyl-p-nitroanilide (GPNA) reduces the growth of tumor cells, induces DNA damage, and augments the efficacy of cetuximab on cell proliferation [164]. Additionally, GPNA or benzylserine (BenSer)-mediated inhibition of SLC1A5 markedly reduces the growth of endometrial cancer cells, recapitulating the effect from SLC1A5 knockdown [165]. Also, pharmacological or genetic inhibition of SLC1A5 in NSCLC cells reduces Gln consumption, induces autophagy, and apoptosis as well as reduces tumor growth in NSCLC xenografts [194]. Bröer et al. tested the specificity of 2-amino-4-bis(aryloxybenzyl)aminobutanoic acids (AABA) group of inhibitors, compound 12 [167], and V-9302 [163], in human cancer cell lines with KO of SLC1A5 expression [34]. Interestingly, while these compounds suspend Gln transport in SLC1A5 KO cells but not in the wild type (WT) cells, SLC1A5 KO and WT cells exhibit equal sensitivity to these inhibitors [27]. Both amino acid ablation and SLC1A5 depletion augment SLC38A2 expression, although AABA compounds inhibit the activity of SLC38A2 [34]. Additionally, AABA compounds inhibit Ile uptake via SLC7A5 [34]. Bröer et al. demonstrated that compound 12 or V-9302 do not inhibit SLC1A5 but rather blocks SLC38A2 and SLC7A5, thereby resultant amino acid imbalance leads to the biological effects also described in other studies [34,163,167]. Treatment with an approved anti-epidermal growth factor receptor (EGFR) therapeutic antibody, cetuximab, may increase the susceptibility of HNSCC cells to oxidative stress by decreasing intracellular GSH levels because of reduced Gln uptake [195,196]. Incorporating cetuximab with oxidative therapy might be a useful strategy to eliminate cetuximab-resistant and EGFR-overexpressing cancers [195,196]. On the other hand, targeting SLC1A5 even without combination with cetuximab may be sufficient to sensitize HNSCC cells to ROS-induced apoptosis [29]. Hara et al. developed a monoclonal antibody (mAB) against the extracellular domain of SLC1A5 that decreases intracellular Glu transport, phosphorylation of AKT and ERK as well as abrogates KRAS-mutant tumor growth in athymic mice in vivo [170]. Kasai et al. assessed the anti-tumor potency of novel anti- SLC1A5 humanized KM8094 mAB in gastric cancer patient-derived xenografts (PDXs) [168]. KM8094 mAB blocks Gln uptake, induces oxidative stress and apoptosis, and suppresses the growth of gastric cancer cells [197]. By a cell-based screen, Suzuki et al. established several mABs against SLC1A5 called KM4008, KM4012, and KM4018 and demonstrated that these mABs abolish the Gln-dependent growth of WiDr CRC cells [169]. Moreover, Zhou et al. developed a novel glutamine-β-cyclodextrin (GLN-CD) to deliver doxorubicin into TNBC tumors through SLC1A5 [191]. The doxorubicin-GLN-CD complex engenders G2/M arrest and apoptosis in vitro and markedly accumulates in tumors in vivo with minimum toxicity in main organs compared to doxorubicin itself [198]. Additionally, GW501516, a nuclear receptor peroxisome proliferator-activated receptor δ (PPARδ) agonist, significantly elevates GLUT1, and SLC1A5 expression, thereby promoting tumor growth in several cancer cell lines [199]. On the other hand, Metformin inhibits tumor growth through the reduction of glucose and Gln flux by inhibition of GLUT1 and SLC1A5 [199]. Wang et al. characterized a Gln analog called polyglutamine (PGS) to deliver therapeutic agents to Gln-addicted cancer cells and showed the importance of SLC1A5 for the cellular internalization of the small interfering RNA (siRNA)-PGS complexes [192]. Delta-tocotrienol (δT) markedly inhibits Gln uptake and leads to the reduction of Glu, GSH, and some EAAs in A549 and H1299 NSCLC cell lines by targeting SLC1A5 and SLC7A5 [171]. Thus, inhibition by δT suspends Gln metabolism, cell proliferation, and mTOR pathway, as well as induces apoptosis through the downregulation of mTOR signaling [171]. Moreover, chemical inhibition of SLC7A5 by BCH does not inhibit melanoma cell growth, whereas SLC1A5 inhibition by BenSer reduces 2D and 3D cell proliferation and cell cycle progression. This effect of BenSer is attributed to the inhibited cyclin-dependent kinase 1 (CDK1) and ubiquitin Conjugating Enzyme E2 C (UBE2C) expression as a result of the reduced Gln and Leu transport and downregulated mTORC1 signaling [166].
Wu et al. identified 2-(furan-2-yl)-8-methyl-N-(o-tolyl)imidazo(1,2-a)pyridin-3-amine as selective inhibitor of another member of SLC1 family, EAAT3/ SLC1A1 [161]. Moreover, Cheng et al. developed a photoswitchable inhibitor, Azo-TFB-TBOA, against EAAT family of Glu transporters, which could be reversibly altered from high-affinity to low-affinity configuration depending on the excitation [162]. While Azo-TFB-TBOA selectively inhibits EAAT2/ SLC1A2 in the dark, exposure to the light at 350 nm alters the configuration of Azo-TFB-TBOA and decreases its affinity against SLC1A2 [162]. Combination of EAAT1/ SLC1A3 inhibition with asparaginase (ASNase) treatment results in cell cycle arrest or apoptosis associated with inhibition of TCA cycle, energy production, redox homeostasis, and biosynthesis of lipids and nucleotides [200]. Although inhibition of EAAT activity can be a promising approach to treat certain types of malignancies such as lung and gastric cancer [32,201], EAAT transporters play a critical role in the maintenance Glu homeostasis in the brain. Therefore EAAT inhibition can be potentially associated with neuronal toxicity [30]. To reduce extracellular Glu levels and thus a possibility of excitotoxicity, EAAT inhibition can be potentially combined with inhibition of SLC7A11/xCT antiporter exchanging intracellular glutamate for extracellular cystine [202].
4.2. Targeting SLC7 Transporter Family
The L-type amino acid transporters LAT1/SLC7A5 and xCT/SLC7A11 are overexpressed in a wide variety of human malignancies and emerged as promising therapeutic targets. Genetic or pharmaceutical inhibition of SLC7A5 leads to amino acid stress response via GCN2 and ATF4 activation and mTORC1 inhibition, causing a growth arrest of tumor cells in vitro and in vivo [59]. Of note, SLC3A2 KO cancer cells become more sensitive to SLC7A5 depletion [59], although the genetic silencing of SLC3A2 itself exhibits cell-context-dependent effects on tumor growth and mTORC1 signaling activation [49,59]. Based on the structure of known SLC7A5 inhibitor triiodothyronine (T3), Kongpracha et al. developed a series of SLC7A5 inhibitors. One of them, called SKN103 is an efficient inhibitor of the SLC7A5-mediated Leu transport [176]. Additionally, SKN103 inhibits mTOR activity and growth of cancer cells. This inhibitory effect is markedly enhanced in combination with cisplatin treatment [176]. Huttunen et al. developed a selective and slowly reversible inhibitor that reduces SLC7A5 -mediated Leu uptake and breast cancer cell growth and also markedly augments the inhibitory effect of cisplatin and bestatin on tumor cell viability [203]. This inhibitor has high metabolic stability and selectivity against SLC7A5, but can dissociate from the blood-brain barrier and cell surface, meaning that the inhibition may be reversed gradually [203]. Another SLC7A5 inhibitor called JPH203 was shown to have an anti-cancer effect in a variety of tumor models. JPH203 inhibits thymic carcinoma cell growth in vitro [204], constrains in vitro cell migration, invasion, and proliferation of renal cell carcinoma (RCC) cell lines [205], and suppresses anaplastic thyroid cancer (ATC) cell proliferation by abolishing mTOR signaling and deterring the cell cycle [177]. Cormeiras et al. showed that JPH203 circumvents mTORC1 activity, amino acid homeostasis, and proliferation of medulloblastoma cells without causing drug resistance after long-term usage [178]. Furthermore, combinational use of metformin and JPH203 hinders HNSCC cell proliferation in vitro and in vivo much more dramatically than the mono treatments [179]. JPH203 reduces the proliferation and viability of leukemic cells, triggers autophagy and apoptosis in vitro, and inhibits the growth of xenografted PTEN deficient cells in nude mice. Additionally, chemical SLC7A5 inhibition hampers mTORC1 and AKT activations, reduces c-MYC expression, and activates UPR [180].
Preclinical studies also suggest that SLC7A5 inhibition can be more toxic for tumor cells than for normal tissues. Yun et al. showed that JPH203 suspends the proliferation of oral carcinoma YD-38 cells in a time and dose-dependent manner. In contrast, it was far less toxic for normal human oral keratinocytes (NHOK) proliferation [206]. Furthermore, YD-38 cells possess a high LAT1 expression, whereas NHOK cells have a high LAT2 and a weak LAT1 expression, which underlines lower sensitivity of normal cells to JPH203 mediated inhibition of LAT1 [206]. Similarly, JPH203 exhibits selective cytotoxicity to medulloblastoma and low toxicity towards normal tissues [178].
SLC7A11 protein is another attractive target for anti-cancer therapy due to its role in the GSH synthesis. However, the sensitivity to the SLC7A11 inhibition can be altered by metabolic reprogramming. Therefore, Otsuki et al. applied a combination of SLC7A11 inhibitor SSZ with Oxyfedrine (OXY), a vasodilator, and a β adrenoreceptor agonist, to sensitize a panel of cancer cell lines to the ROS-induced therapy [182]. The combination of OXY and SSZ treatments enhances cytotoxic aldehyde 4-hydroxynonenal (4-HNE) accumulation and induces necrotic cell death in SSZ-resistant cancers in vitro and in vivo [182]. Additionally, inhibition of aldehyde dehydrogenase (ALDH) enzyme by OXY sensitizes cancer cells to GSH deficiency in response to radiotherapy in vitro [182]. Furthermore, chemical or genetic inhibition of SLC7A11 inhibits tumor growth in vivo. It triggers death in KRAS-mutant cancer cells, suggesting that SLC7A11 may be an Achilles’ heel for KRAS-mutant lung adenocarcinoma (LUAD) [183]. While SSZ leads to selective killing in a panel of KRAS-mutant LUAD in vitro and inhibits tumor growth in vivo, inhibition by HG106, a potent inhibitor of SLC7A11, notably reduces Cys uptake, GSH biosynthesis and demonstrates selective cytotoxicity to KRAS-mutant LUAD cells through augmenting ER-stress and oxidative stress-mediated cell death [183]. SSZ-mediated SLC7A11 inhibition efficiently reduces the NSCLC cell proliferation and invasion in vivo and in vitro [181]. SSZ impairs GSH synthesis, as well as augments intracellular αKG levels, mitochondrial metabolism, and ROS accumulation in CD44vhigh undifferentiated stem-like HNSCC cells [184]. Besides, SSZ fine-tunes the CD44v9-SLC7A11 system to hamper the metastatic potential of bladder cancer. It increases cisplatin-induced cytotoxicity through inhibition of GSH levels and production of ROS [185]. A combination of cisplatin and SSZ leads to severe cytotoxicity in MBT-2V cells through upregulation of phospho-p38 MAPK and inhibition of CD44v9 [185]. Targeting SLC7A11 with SSZ induces apoptosis of primary effusion lymphoma (PEL) cells and precludes tumor progression in xenograft models [187]. SSZ-treated PEL cells deregulate genes involved in oxidative stress, cell death, and UPR [186]. All in all, these preclinical studies demonstrate that inhibition of amino acid transporters might open a new avenue for cancer treatment by inhibition of the oxidative cell defense and sensitizing tumor cells to the conventional therapies.
5. Molecular Imaging by Using Amino Acid Transporters
Positron emission tomography (PET) imaging uses a radiolabeled glucose analog 18F-2-fluoro-2-deoxyglucose (18F-FDG) and is widely applied in clinics for detection, staging, and monitoring responsiveness to treatment for most tumor types based on high glucose uptake by tumor tissues. Despite a high sensitivity of the FPG PET, there are several drawbacks of 18F-FDG imaging. First, some malignant tumors with low glycolytic activity, such as early-stage PCa, cannot be detected by FDG uptake [9,207]. Second, 18F-FDG imaging in some cancer types such as brain tumors and renal carcinoma has a poor sensitivity due to low contrast between malignant and normal tissues [208,209]. Further, increased 18F-FDG accumulation has been shown for some normal tissues such as the brain, muscles, gastrointestinal tract, brown adipose tissues, inflammation or infection sites, etc. [210,211,212], resulting in false-positive findings. For this reason, alternative non-FDG PET imaging approaches are being established for precise and accurate detection of tumor tissues, including PET imaging with amino acid transporter-based radiotracers (Table 2).
Table 2.
Molecular imaging by using amino acid transporters.
| Transporter | Tracer | Experimental Model | Tissue Type | References |
|---|---|---|---|---|
| SLC1A1 | N-(2-(18F)fluoropropionyl)-L-glutamate ((18F)FPGLU) | In vitro (Cell line) | Rat C6 glioma cell line, SPC-A-1 lung adenocarcinoma cell line | [213] |
| In vivo (Cell line derived-xenograft) | ||||
| SLC1A5 | 18F-Ala-BF3 | In vivo (Cell line derived-xenograft) | Gastric cancer cell line xenograft | [214] |
| SLC1A5 | 4-(18F)Fluoro-Gln | In vivo (Cell line derived-xenograft) | Non-Small Cell Lung Cancer and Colorectal Carcinoma xenografts | [215] |
| In vivo (EGFR-mutant Mouse model) | ||||
| SLC1A5, SLC7A5 | (18F)fluciclovine | In vitro (Cell line) | Castration-resistant prostate cancer | [216,217] |
| SLC3A2 | Anticalin | Primary prostate cancer tissue | Primary prostate cancer tissue | [218] |
| SLC7A5 | 2-(18F)FELP | In vitro (Cell line) | Glioblastoma [219,220] | [219,220] |
| In vivo (Cell line derived-xenograft) | ||||
| SLC7A5 | [18F]FET | In vitro (Cell line) | Glioblastoma [219,220,221] | [219,220,221] |
| In vivo (Cell line derived-xenograft) | ||||
| SLC7A5 | 18F-FIMP | In vivo (Cell line derived-xenograft) | Glioblastoma | [222] |
| SLC7A5 | 18F-FBPA | In vivo (Cell line derived-xenograft) | C6 Glioma and MIA PaCa-2 xenografts | [223] |
| SLC7A5 | 18F-FAMT | In vitro (Cell line) | C6 Glioma and MIA PaCa-2 xenografts [223], Xenopus oocytes [224] | [223,224] |
| In vivo (Cell line derived-xenograft) | ||||
| In vitro (Xenopus laevis oocytes) | ||||
| SLC7A5 | P(NIPAAm-co-DMAAm) | In vitro (Cell line) | Cervical carcinoma (HeLa cells) | [225] |
| SLC7A5 | (18F)-FDOPA | In vitro (Cell line) | Non tumoral brain tissues and brain metastases [226], Glioblastoma [227] | [226,227] |
| Brain tumor tissue |
5.1. SLC1A5/ASCT2-Dependent Tumor Imaging
The N-(2-(18F)fluoropropionyl)-L-glutamate ((18F)FPGLU) is a promising amino acid tracer for PET imaging of tumors with a high expression of extracellular Glu transporter excitatory amino acid carrier 1 (EAAC1) such as glioma and lung adenocarcinoma [213]. A 18F labeled alanine trifluoroborate (18F-Ala-BF3) is a selective SLC1A5 marker for cancer imaging in the gastric cancer xenograft models. Compared to FDG-PET, 18F-Ala-BF3 based imaging gives a high tumor-to-background contrast and low accumulation in the inflammation tissues [214]. Preclinical evaluation of another SLC1A5 marker, 4-[18F]Fluoro-Gln in lung cancer xenograft models showed a tumor-specific accumulation compared to normal lung tissues. In contrast to 18F-FDG, 4-(18F)Fluoro-Gln uptake by tumor cells positively correlates with SLC1A5 expression [215]. High demand for Gln is a feature of the highly aggressive neuroblastoma, and 4-(18F)Fluoro-Gln has been shown to have higher tumor uptake and tumor-to-muscle ratio than 18F-FDG tracer [228]. 4-(18F)Fluoro-Gln uptake in neuroblastoma cells is mainly mediated by SLC1A5, and overexpression of SLC1A5 is associated with poor clinical prognosis [37]. A preclinical study using PCa cell lines showed that upregulation of SLC1A5 expression in response to dihydrotestosterone (DHT) treatment correlates with increased uptake of [14C]fluciclovine, a tracer for the imaging of PCa [216]. However, analysis of (18F)fluciclovine PET scans of hormone naïve PCa patients demonstrates that (18F)fluciclovine uptake does not correlate with SLC1A5 expression [217]. Given the emerging role of SLC1A5 as a promising cancer biomarker, SLC1A5-specific tracers could be further developed into precision PET imaging diagnostic tools.
5.2. SLC3 and SLC7-Dependent Tumor Imaging
The levels of CD98hc/SLC3A2 and its light chain subunits are upregulated in tumors, and its high expression is associated with tumor progression and treatment resistance [49,229]. It makes these amino acid transporters an attractive target for tumor imaging.
O-(2-(18F)fluoroethyl)-l-tyrosine, (18F)FET is one of the first and most widespread 18F labeled tracers for L-type amino acid transporters [230]. LAT1/SLC7A5 is one of the amino acid transporters mediating (18F)FET accumulation in tumor cells [221,230]. (18F)FET is employed for brain tumor imaging, and is a powerful tool to discriminate recurrent glioblastoma from the posttreatment brain changes and to predict overall survival in glioblastoma patients [231]. Verhoeven et al. reported 2-(18F)-2-fluoroethyl-l-phenylalanine, 2-(18F)FELP as a new SLC7A5-targeting PET tracer for glioblastoma that is predominantly transported by the SLC7A5 and can discriminate glioblastoma from the radiation necrosis even better than (18F)FET [219,220]. Nozaki et al. identified SLC7A5 specific PET tracer, (S)-2-amino-3-[3-(2-18F-fluoroethoxy)-4-iodophenyl]-2-methylpropanoic acid, or 18F-FIMP. 18F-FIMP shows better discrimination between glioblastoma tumor tissue and inflammation lesions in xenograft mice models than other PET probes such as 18F-FET, 11C-MET, and 18F-FDG [222]. Another SLC7A5-specific tracer, 18F-fluoro-phenylalaine (18F-FBPA), has low accumulation in the inflammatory regions and high uptake by tumor tissues in the glioma xenograft model [232]. Aoki et al. suggested that the total distribution of 18F-FBPA and 18F-FAMT in glioma and pancreatic tumor xenografts does not depend on the tumor blood flow but might reflect the high level of SLC7A5 expression in tumor tissues [223]. SLC7A5 has upregulated expression in recurrent brain metastases as compared to healthy brain tissues. The SLC7A5 overexpression is associated with high uptake of (18F)-FDOPA, whereas radionecrosis regions with low SLC7A5 expression are characterized by an absence of (18F)-FDOPA signal [226]. The level of SLC7A5 expression significantly correlates with (18F)fluciclovine uptake by tumor tissues in patients with hormone naïve PCa, and tumors with high Gleason grade have higher SLC7A5 expression and 18F-fluciclovine signal [217].
In addition to the SLC7A5-specific radiotracers, fluorescent tracers are also under development. Matsuura et al. synthesized fluorescent polymer probes based on poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) conjugated to Tyr. This probe is specifically recognized by SLC7A5 and can be accumulated in cells in response to temperature increase, which improves probe hydrophilicity [225].
The probes specific for SLC3A2 imaging are also of high clinical importance. Deuschle et al. developed a high-affinity anticalin protein against human SLC3A2 ectodomain using phage display selection and protein design. 89Zr-labeled anticalin protein is characterized by strong and tumor-specific signals in PET imaging of xenograft tumor models for Burkitt′s lymphoma and metastatic PCa [218].
6. Conclusions
Amino acid transporters play a multifaceted role in tumor initiation, progression, and therapy resistance. They are critical to cover the energetic and biosynthetic demands of fast-growing tumors associated with high proliferation rates and nutrient-poor environments. Many amino acid transporters are highly expressed in tumors compared to the adjacent normal tissues, and their expression correlates with tumor progression, clinical outcome, and treatment resistance [57,154]. The deregulated levels of transporter proteins may be caused by frequent mutations of oncogenes and tumor suppressors. The transcription factor MYC, one of the most frequently amplified genes in human malignancies [233], is a master-regulator of amino acid metabolism promoting the expression of transporter proteins such as SLC1A5, SLC7A5 and SLC43A1 and thus, driving cancer growth by effective amino acid delivery [36,234,235]. In some tumors like PCa, upregulation and the interplay between MYC, AR, and the mTOR mutations cause Gln addiction when Gln becomes a conditional EAA for tumor growth and survival. These oncogenes increase Gln uptake by upregulation of SLC1A4 and SLC1A5 transporters [27].
A high expression of amino acid transporters in tumor cells is also critical for maintaining tumor redox homeostasis by regulating the intracellular GSH levels, ER stress, UPR signaling, and mTOR-mediated antioxidant defense. Amino acid transporters also play a critical role in the epigenetic adaptations of tumor cells to oxidative stress. These findings highlight the role of acid transporters as the defender of tumor antioxidant systems and genome integrity.
Although functional characterization of amino acid transporters in different human malignancies received considerable attention in the last years, research on their potential employment as molecular biomarkers, therapeutic targets, and tools for tumor imaging is still in its infancy. Improvement in therapeutic targeting of amino acid transporters will require a better understanding of their plasticity and redundancy in the individual tumors. Recent studies showed that tumor cells dynamically upregulate redundant amino acid transporters and rapidly harmonize the intracellular amino acid pool in response to the amino acid deficiency in the tumor environment or transporter inhibition. Therefore, tumor cells with reduced transporter plasticity may be more sensitive to the inhibition of amino acid transporters than cells, which can quickly compensate for the ablation of transporters [42].
Another challenge for the therapeutic targeting of amino acid transporters is the activation of the compensatory pro-survival mechanisms. In response to the amino acid depletion, many tumors activate autophagy to overcome nutrient stress. Cell death and sensitivity to conventional treatment in response to the inhibition of amino acid uptake was substantially enhanced in autophagy-deficient tumor cells [49,236,237]. The ongoing clinical trials combine autophagy inhibition with chemotherapy or radiotherapy in patients with solid tumors. However, the role of autophagy as a tumor target is still debatable as it might promote or suppress tumor growth depending on tumor type and stage of tumor development [57,238].
Additionally, the affinity between amino acids and their transporters, availability of the amino acids, transporter expression levels, and redox homeostasis can be substantially influenced by microenvironmental conditions such as hypoxia, acidosis, and reactive stroma [239,240,241]. The research efforts over the past decade have advanced the experimental models for the analysis of tumor metabolism to recapitulate these microenvironmental factors, including patient tumor-derived organoid co-cultures, sliced tissue models, and in vivo stable isotope labeling [242,243,244].
While many potential preclinical approaches to inhibit amino acid transporters are available, none of them entered clinical trials. The development of novel metabolic drug targets requires multidisciplinary approaches to understand the physiological role and regulatory modes for the individual amino acid transporters in the different tumor and normal tissues. It includes multi-omics analyses in response to the transporter ablation combining genome, proteome, and metabolome levels, followed by data integration and computational modeling. Availability of the X-ray structures facilitates the identification of novel transporter inhibitors by structure-based design approach and virtual screening of millions of chemical molecules. Large-scale analysis of the patients’ omics data might help to elucidate the function of the individual transporters in cancer progression [242,245,246]. Finally, recent technological advances in the study of cancer metabolism that help to accurately detect metabolites in high-throughput scale with spatial and temporal resolution [242] and further development of the experimental models, which more accurately recapitulate tumor microenvironment, will play a pivotal role in the development of new efficient drugs and specific tracers toward amino acid transporters.
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| 4EBP1 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| 5mC | 5-methylcytosine |
| 18F-Ala-BF3 | 18F labeled alanine trifluoroborate |
| 18F-FBPA | 18F-fluoro-phenylalaine |
| 18F-FDG PET | (18F)-Fluorodeoxyglucose Positron Emission Tomography |
| αKG | α-ketoglutarate |
| AA | Amino acid |
| AAA | Aromatic amino acid |
| AABA | 2-amino-4-bis(aryloxybenzyl)aminobutanoic acid |
| AARE | Amino acid response element |
| Acetyl-CoA | Acetyl-Coenzyme A |
| Ac | Acetylation |
| AKT | AKT Serine/Threonine Kinase |
| ALDH | Aldehyde dehydrogenase |
| ALL | Acute lymphoblastic leukemia |
| AMPK | AMP-activated protein kinase |
| AR | Androgen Receptor |
| ARE | Antioxidant response element |
| Arg | Arginine |
| ASCT2/ SLC1A5 | Alanine, serine, cysteine-preferring transporter 2 |
| ASNase | Asparaginase |
| ASNS | asparagine synthase |
| Asp | Aspartate |
| ATC | Anaplastic thyroid cancer |
| ATF4 | Activating Transcription Factor 4 |
| ATF6 | Activating Transcription Factor 6 |
| ATG5 | Autophagy Related 5 |
| BCAA | Branched-chain amino acid |
| BenSer | Benzylserine |
| BSO | Buthionine sulphoximine |
| BTA | 1,2,3-benzene-tricarboxylic acid |
| CAT | Catalase |
| CD44 | Cluster of differentiation 44 |
| CD44v | Cluster of differentiation 44 variant |
| CDK1 | Cycling-Dependent Kinase 1 |
| CDK7 | Cyclin-Dependent Kinase 7 |
| CIC | Citrate carrier |
| circRNA | Circular RNA |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| CSC | Cancer stem cells |
| Cys | Cysteine |
| δT | Delta-tocotrienol |
| D-loop | Displacement loop |
| DNA | Deoxyribonucleic Acid |
| DNMT | DNA Methyltransferase |
| EAA | Essential amino acid |
| EAAC1 | Excitatory Amino Acid Carrier 1 |
| EGFR | Epidermal Growth Factor Receptor |
| eIF2α | Eukaryotic Translation Initiation Factor 2A |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| Erα | Estrogen receptor alpha |
| ETC | Electron transport chain |
| EZH2 | Enhancer of zeste homolog 2 |
| GCL | Glutamate-cysteine ligase |
| GCN2 | General amino acid control non-derepressible 2 |
| Gln | Glutamine |
| GLN-CD | Glutamine-β-cyclodextrin |
| GSL | Glutaminase |
| Gly | Glycine |
| GPNA | l-g-glutamyl-p-nitroanilide |
| GPXs | Glutathione Peroxidases |
| GPX4 | Glutathione Peroxidase 4 |
| GSH | Glutathione |
| GSR | Glutathione reductase |
| GSS | Glutathione synthetase |
| GSSG | Glutathione disulfide |
| H2O2 | Hydrogen Peroxide |
| HAT | Histone Acetyltransferase |
| HATC | Heterodimeric transmembrane amino acid transporter complex |
| HCC | Hepatocellular Carcinoma |
| HDAC | Histone Deacetylase |
| HIF1α | Hypoxia Inducible Factor 1 Subunit Alpha |
| HIF2α | Hypoxia Inducible Factor 2 Subunit Alpha |
| His | Histidine |
| HMT | Histone Methyltransferase |
| HNSCC | Head and neck squamous cell carcinoma |
| Ile | Isoleucine |
| IMCA | 2-imino-6-methoxy-2H-chromene-3-carbothioamide |
| IR | Ionizing radiation |
| ISR | Integrated Stress Response |
| JDP2 | Jun Dimerization Protein 2 |
| KDM3B | Lysine Demethylase 3B |
| KO | Knockout |
| KRAS | KRAS Proto-Oncogene, GTPase |
| LAT1/SLC7A5 | L-type Amino Acid Transporter 1 |
| Leu | Leucine |
| lncRNA-XIST | Long Non-Coding RNA (lncRNA) X-Inactive Specific Transcript (XIST) |
| LOX | Lipoxygenase |
| LUAD | Lung adenocarcinoma |
| Lys | Lysine |
| mAB | monoclonal antibody |
| MAPK | Mitogen-activated protein kinase |
| MAT | Methionine Adenosyltransferase |
| MDR1 | Multidrug resistance protein 1 |
| Met | Methionine |
| miRNA | MicroRNA |
| MS | Methionine Synthase |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| mTORC2 | Mammalian target of rapamycin complex 2 |
| NAC | N-acetyl-l-Cysteine |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NF-kB | Nuclear Factor kappa B |
| NOX | NADPH Oxidase |
| Nrf2 | Nuclear factor-erythroid 2 -related factor 2 |
| OXPHOS | Oxidative Phosphorylation |
| OXY | Oxyfedrine |
| PEL | Primary Effusion Lymphoma |
| P-gp | P-glycoprotein |
| p70S6k | The 70-kDa ribosomal protein S6 kinase |
| PCa | Prostate Cancer |
| PDH | Pyruvate dehydogenase |
| PDX | Patient-derived xenograft |
| PET | Positron emission tomography |
| PGS | Polyglutamine |
| PP2Ac | Protein Phosphatase 2 Catalytic Subunit Alpha |
| PPARδ | Peroxisome Proliferator Activated Receptor Delta |
| PPM1F | Protein phosphatase, Mg2+/Mn2+ dependent 1F |
| Pro | Proline |
| PRX | Peroxiredoxin |
| RB | Retinoblastoma protein |
| RNA | Ribonucleic acid |
| RCC | Renal Cell Carcinoma |
| RONS | Reactive oxygen and nitrogen species |
| ROS | Reactive oxygen species |
| S6K1 | The mTOR Substrate S6 Kinase 1 |
| SAM | S-Adenosylmethionine |
| Ser26 | Serine26 |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| SLC | Solute carrier |
| SLC1A1 | Solute Carrier Family 1 Member 1 |
| SLC1A2 | Solute Carrier Family 1 Member 2 |
| SLC1A3 | Solute Carrier Family 1 Member 3 |
| SLC1A4 | Solute Carrier Family 1 Member 4 |
| SLC1A5 | Solute Carrier Family 1 Member 5 |
| SLC1A6 | Solute Carrier Family 1 Member 6 |
| SLC1A7 | Solute Carrier Family 1 Member 7 |
| SLC25A1 | Solute Carrier Family 25 Member 1 |
| SLC25A4 | Solute Carrier Family 25 Member 4 |
| SLC25A5 | Solute Carrier Family 25 Member 5 |
| SLC25A8 | Solute Carrier Family 25 Member 8 |
| SLC25A10 | Solute Carrier Family 25 Member 10 |
| SLC25A23 | Solute Carrier Family 25 Member 23 |
| SLC25A26 | Solute Carrier Family 25 Member 26 |
| SLC25A32 | Solute Carrier Family 25 Member 32 |
| SLC38A1 | Solute Carrier Family 38 Member 1 |
| SLC38A2 | Solute Carrier Family 38 Member 2 |
| SLC38A3 | Solute Carrier Family 38 Member 3 |
| SLC3A1 | Solute Carrier Family 3 Member 1 |
| SLC3A2 | Solute Carrier Family 3 Member 2 |
| SLC6A1 | Solute Carrier Family 6 Member 1 |
| SLC6A14 | Solute Carrier Family 6 Member 14 |
| SLC7A1 | Solute Carrier Family 7 Member 1 |
| SLC7A2 | Solute Carrier Family 7 Member 2 |
| SLC7A3 | Solute Carrier Family 7 Member 3 |
| SLC7A4 | Solute Carrier Family 7 Member 4 |
| SLC7A5 | Solute Carrier Family 7 Member 5 |
| SLC7A6 | Solute Carrier Family 7 Member 6 |
| SLC7A7 | Solute Carrier Family 7 Member 7 |
| SLC7A8 | Solute Carrier Family 7 Member 8 |
| SLC7A9 | Solute Carrier Family 7 Member 9 |
| SLC7A10 | Solute Carrier Family 7 Member 10 |
| SLC7A11 | Solute Carrier Family 7 Member 11 |
| SLC7A12 | Solute Carrier Family 7 Member 12 |
| SLC7A13 | Solute Carrier Family 7 Member 13 |
| SLC7A14 | Solute Carrier Family 7 Member 14 |
| SLC7A15 | Solute Carrier Family 7 Member 15 |
| SNAT1/ SLC38A1 | Sodium-coupled neutral amino acid transporter 1 |
| SNAT2/SLC38A2 | Sodium-coupled neutral amino acid transporter 2 |
| SOD | superoxide dismutase |
| SorCS2 | Sortilin Related VPS10 Domain Containing Receptor 2 |
| SSZ | Sulfasalazine |
| T3 | Triiodothyronine |
| TCA cycle | Tricarboxylic acid cycle |
| TET | Ten-Eleven Translocation |
| TMZ | Temozolomide |
| TNBC | Triple-negative breast cancer |
| Trp | Tryptophan |
| TRX | Thioredoxin |
| TXN2 | Thioredoxin 2 |
| TXNRD | Thioredoxin Reductase |
| UBE2C | Ubiquitin Conjugating Enzyme E2 C |
| UPR | Unfolded protein response |
| UPS | Ubiquitin-Proteasome System |
| Val | Valine |
| VPS10 | Vacuolar protein sorting 10 |
| VSMC | Vascular smooth muscle cells |
| WT | Wild type |
| XO | Xanthine oxidase |
Funding
Work in the Anna Dubrovskalab was partially supported by grants from Deutsche Forschungsgemeinschaft (DFG) (Grant numbers 416001651 and 401326337, SPP2084 µBone).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pavlova N.N., Thompson C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coller H.A. Is cancer a metabolic disease? Am. J. Pathol. 2014;184:4–17. doi: 10.1016/j.ajpath.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBerardinis R.J., Chandel N.S. We need to talk about the Warburg effect. Nat. Metab. 2020;2:127–129. doi: 10.1038/s42255-020-0172-2. [DOI] [PubMed] [Google Scholar]
- 4.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 5.Kekuda R., Prasad P.D., Fei Y.J., Torres-Zamorano V., Sinha S., Yang-Feng T.L., Leibach F.H., Ganapathy V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J. Biol. Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 6.Van Geldermalsen M., Wang Q., Nagarajah R., Marshall A.D., Thoeng A., Gao D., Ritchie W., Feng Y., Bailey C.G., Deng N., et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35:3201–3208. doi: 10.1038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise D.R., Thompson C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pochini L., Scalise M., Galluccio M., Indiveri C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014;2:61. doi: 10.3389/fchem.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peitzsch C., Gorodetska I., Klusa D., Shi Q., Alves T.C., Pantel K., Dubrovska A. Metabolic regulation of prostate cancer heterogeneity and plasticity. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg F., Ramnath N., Nagrath D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers. 2019;11:1191. doi: 10.3390/cancers11081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumacker P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Henriquez-Olguin C., Knudsen J.R., Raun S.H., Li Z., Dalbram E., Treebak J.T., Sylow L., Holmdahl R., Richter E.A., Jaimovich E., et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019;10:4623. doi: 10.1038/s41467-019-12523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brieger K., Schiavone S., Miller F.J., Jr., Krause K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 16.Milkovic L., Cipak Gasparovic A., Cindric M., Mouthuy P.A., Zarkovic N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells. 2019;8:793. doi: 10.3390/cells8080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal A., Simon M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018;217:2291–2298. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sanchez-Perez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamcsik M.P., Kasibhatla M.S., Teeter S.D., Colvin O.M. Glutathione levels in human tumors. Biomarkers. 2012;17:671–691. doi: 10.3109/1354750X.2012.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunes S.C., Serpa J. Glutathione in Ovarian Cancer: A Double-Edged Sword. Int. J. Mol. Sci. 2018;19:1182. doi: 10.3390/ijms19071882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim W., Lee S., Seo D., Kim D., Kim K., Kim E., Kang J., Seong K.M., Youn H., Youn B. Cellular Stress Responses in Radiotherapy. Cells. 2019;8:1105. doi: 10.3390/cells8091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.J., Kim H.S., Seo Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell. Longev. 2019;2019:5381692. doi: 10.1155/2019/5381692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen O., Manig F., Lehmann L., Sorour N., Lock S., Yu Z., Dubrovska A., Baumann M., Kessler B.M., Stasyk O., et al. Dual role of ER stress in response to metabolic co-targeting and radiosensitivity in head and neck cancer cells. Cell. Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo Y., Lee H.J., Jung Y.M., Jung Y.J. mTOR-Mediated Antioxidant Activation in Solid Tumor Radioresistance. J. Oncol. 2019;2019:5956867. doi: 10.1155/2019/5956867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanai Y., Clemencon B., Simonin A., Leuenberger M., Lochner M., Weisstanner M., Hediger M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 2013;34:108–120. doi: 10.1016/j.mam.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Cormerais Y.V., Vučetić M., Parks S.K., Pouyssegur J. Amino Acid Transporters Are a Vital Focal Point in the Control of mTORC1 Signaling and Cancer. Int. J. Mol. Sci. 2021;22:23. doi: 10.3390/ijms22010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White M.A., Lin C., Rajapakshe K., Dong J., Shi Y., Tsouko E., Mukhopadhyay R., Jasso D., Dawood W., Coarfa C., et al. Glutamine Transporters Are Targets of Multiple Oncogenic Signaling Pathways in Prostate Cancer. Mol. Cancer Res. 2017;15:1017–1028. doi: 10.1158/1541-7786.MCR-16-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellsague X., Alemany L., Quer M., Halec G., Quiros B., Tous S., Clavero O., Alos L., Biegner T., Szafarowski T., et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Liu R., Shuai Y., Huang Y., Jin R., Wang X., Luo J. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br. J. Cancer. 2020;122:82–93. doi: 10.1038/s41416-019-0637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik A.R., Szydlowska K., Nizinska K., Asaro A., van Vliet E.A., Popp O., Dittmar G., Fritsche-Guenther R., Kirwan J.A., Nykjaer A., et al. SorCS2 Controls Functional Expression of Amino Acid Transporter EAAT3 and Protects Neurons from Oxidative Stress and Epilepsy-Induced Pathology. Cell Rep. 2019;26:2792–2804.e6. doi: 10.1016/j.celrep.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoyama K., Suh S.W., Hamby A.M., Liu J., Chan W.Y., Chen Y., Swanson R.A. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 32.Guo W., Li K., Sun B., Xu D., Tong L., Yin H., Liao Y., Song H., Wang T., Jing B., et al. Dysregulated Glutamate Transporter SLC1A1 Propels Cystine Uptake via Xc- for Glutathione Synthesis in Lung Cancer. Cancer Res. 2020 doi: 10.1158/0008-5472.CAN-20-0617. [DOI] [PubMed] [Google Scholar]
- 33.Almilaji A., Pakladok T., Guo A., Munoz C., Foller M., Lang F. Regulation of the glutamate transporter EAAT3 by mammalian target of rapamycin mTOR. Biochem. Biophys. Res. Commun. 2012;421:159–163. doi: 10.1016/j.bbrc.2012.03.109. [DOI] [PubMed] [Google Scholar]
- 34.Broer A., Fairweather S., Broer S. Disruption of Amino Acid Homeostasis by Novel ASCT2 Inhibitors Involves Multiple Targets. Front. Pharmacol. 2018;9:785. doi: 10.3389/fphar.2018.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q., Hardie R.A., Hoy A.J., van Geldermalsen M., Gao D., Fazli L., Sadowski M.C., Balaban S., Schreuder M., Nagarajah R., et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 2015;236:278–289. doi: 10.1002/path.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise D.R., DeBerardinis R.J., Mancuso A., Sayed N., Zhang X.Y., Pfeiffer H.K., Nissim I., Daikhin E., Yudkoff M., McMahon S.B., et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren P., Yue M., Xiao D., Xiu R., Gan L., Liu H., Qing G. ATF4 and N-Myc coordinate glutamine metabolism in MYCN-amplified neuroblastoma cells through ASCT2 activation. J. Pathol. 2015;235:90–100. doi: 10.1002/path.4429. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds M.R., Lane A.N., Robertson B., Kemp S., Liu Y., Hill B.G., Dean D.C., Clem B.F. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene. 2014;33:556–566. doi: 10.1038/onc.2012.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q., Tiffen J., Bailey C.G., Lehman M.L., Ritchie W., Fazli L., Metierre C., Feng Y.J., Li E., Gleave M., et al. Targeting amino acid transport in metastatic castration-resistant prostate cancer: Effects on cell cycle, cell growth, and tumor development. J. Natl. Cancer Inst. 2013;105:1463–1473. doi: 10.1093/jnci/djt241. [DOI] [PubMed] [Google Scholar]
- 40.Qi J., Tripathi M., Mishra R., Sahgal N., Fazli L., Ettinger S., Placzek W.J., Claps G., Chung L.W., Bowtell D., et al. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell. 2013;23:332–346. doi: 10.1016/j.ccr.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broer A., Rahimi F., Broer S. Deletion of Amino Acid Transporter ASCT2 (SLC1A5) Reveals an Essential Role for Transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to Sustain Glutaminolysis in Cancer Cells. J. Biol. Chem. 2016;291:13194–13205. doi: 10.1074/jbc.M115.700534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broer A., Gauthier-Coles G., Rahimi F., van Geldermalsen M., Dorsch D., Wegener A., Holst J., Broer S. Ablation of the ASCT2 (SLC1A5) gene encoding a neutral amino acid transporter reveals transporter plasticity and redundancy in cancer cells. J. Biol. Chem. 2019;294:4012–4026. doi: 10.1074/jbc.RA118.006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo H.C., Park S.J., Nam M., Kang J., Kim K., Yeo J.H., Kim J.K., Heo Y., Lee H.S., Lee M.Y., et al. A Variant of SLC1A5 Is a Mitochondrial Glutamine Transporter for Metabolic Reprogramming in Cancer Cells. Cell Metab. 2020;31:267–283.e212. doi: 10.1016/j.cmet.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y., Lv B., Zhang X. Knock-down of LncRNA-XIST induced glioma cell death and inhibited tumorigenesis by regulating miR-137/SLC1A5 axis-mediated ROS production. Naunyn Schmiedebergs Arch. Pharmacol. 2020 doi: 10.1007/s00210-020-01831-3. [DOI] [PubMed] [Google Scholar]
- 45.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo M., Wu L., Zhang K., Wang H., Zhang T., Gutierrez L., O’Connell D., Zhang P., Li Y., Gao T., et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25:1457–1472. doi: 10.1038/s41418-017-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fotiadis D., Kanai Y., Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013;34:139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y., Cao Y., Wang Y., Li W., Liu X., Lv Y., Li X., Mi J. Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis. Theranostics. 2017;7:1036–1046. doi: 10.7150/thno.18005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Digomann D., Kurth I., Tyutyunnykova A., Chen O., Lock S., Gorodetska I., Peitzsch C., Skvortsova I.-I., Negro G., Aschenbrenner B., et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity. Clin. Cancer Res. 2019;25:3152–3163. doi: 10.1158/1078-0432.CCR-18-2951. [DOI] [PubMed] [Google Scholar]
- 50.Chiduza G.N., Johnson R.M., Wright G.S.A., Antonyuk S.V., Muench S.P., Hasnain S.S. LAT1 (SLC7A5) and CD98hc (SLC3A2) complex dynamics revealed by single-particle cryo-EM. Acta Crystallogr. D Struct. Biol. 2019;75:660–669. doi: 10.1107/S2059798319009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L., Liu W., Liu F., Wang Q., Song M., Yu Q., Tang K., Teng T., Wu D., Wang X., et al. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxid. Med. Cell. Longev. 2020;2020:1675613. doi: 10.1155/2020/6901472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu Y., Albuquerque C.P., Braas D., Zhang W., Villa G.R., Bi J., Ikegami S., Masui K., Gini B., Yang H., et al. mTORC2 Regulates Amino Acid Metabolism in Cancer by Phosphorylation of the Cystine-Glutamate Antiporter xCT. Mol. Cell. 2017;67:128–138.e127. doi: 10.1016/j.molcel.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer A.R., Engevik A.C., Willet S.G., Williams J.A., Zou Y., Massion P.P., Mills J.C., Choi E., Goldenring J.R. Cystine/Glutamate Antiporter (xCT) Is Required for Chief Cell Plasticity After Gastric Injury. Cell. Mol. Gastroenterol. Hepatol. 2019;8:379–405. doi: 10.1016/j.jcmgh.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goji T., Takahara K., Negishi M., Katoh H. Cystine uptake through the cystine/glutamate antiporter xCT triggers glioblastoma cell death under glucose deprivation. J. Biol. Chem. 2017;292:19721–19732. doi: 10.1074/jbc.M117.814392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cano-Crespo S., Chillaron J., Junza A., Fernandez-Miranda G., Garcia J., Polte C., Laura R., Ignatova Z., Yanes Ó., Zorzano A., et al. CD98hc (SLC3A2) sustains amino acid and nucleotide availability for cell cycle progression. Sci. Rep. 2019;9:14065. doi: 10.1038/s41598-019-50547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De la Ballina L.R., Cano-Crespo S., Gonzalez-Munoz E., Bial S., Estrach S., Cailleteau L., Tissot F., Daniel H., Zorzano A., Ginsberg M.H., et al. Amino Acid Transport Associated to Cluster of Differentiation 98 Heavy Chain (CD98hc) Is at the Cross-road of Oxidative Stress and Amino Acid Availability. J. Biol. Chem. 2016;291:9700–9711. doi: 10.1074/jbc.M115.704254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Digomann D., Linge A., Dubrovska A. SLC3A2/CD98hc, autophagy and tumor radioresistance: A link confirmed. Autophagy. 2019;15:1850–1851. doi: 10.1080/15548627.2019.1639302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanagida O., Kanai Y., Chairoungdua A., Kim D.K., Segawa H., Nii T., Cha S.H., Matsuo H., Fukushima J., Fukasawa Y., et al. Human L-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta. 2001;1514:291–302. doi: 10.1016/S0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 59.Cormerais Y., Giuliano S., LeFloch R., Front B., Durivault J., Tambutte E., Massard P.A., de la Ballina L.R., Endou H., Wempe M.F., et al. Genetic Disruption of the Multifunctional CD98/LAT1 Complex Demonstrates the Key Role of Essential Amino Acid Transport in the Control of mTORC1 and Tumor Growth. Cancer Res. 2016;76:4481–4492. doi: 10.1158/0008-5472.CAN-15-3376. [DOI] [PubMed] [Google Scholar]
- 60.Torrents D., Estevez R., Pineda M., Fernandez E., Lloberas J., Shi Y.B., Zorzano A., Palacin M. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J. Biol. Chem. 1998;273:32437–32445. doi: 10.1074/jbc.273.49.32437. [DOI] [PubMed] [Google Scholar]
- 61.Averous J., Lambert-Langlais S., Mesclon F., Carraro V., Parry L., Jousse C., Bruhat A., Maurin A.C., Pierre P., Proud C.G., et al. GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci. Rep. 2016;6:27698. doi: 10.1038/srep27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M., Sadri N., Yun C., Popko B., Paules R., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 63.Ye P., Mimura J., Okada T., Sato H., Liu T., Maruyama A., Ohyama C., Itoh K. Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol. Cell. Biol. 2014;34:3421–3434. doi: 10.1128/MCB.00221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim J.K.M., Delaidelli A., Minaker S.W., Zhang H.F., Colovic M., Yang H., Negri G.L., von Karstedt S., Lockwood W.W., Schaffer P., et al. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc. Natl. Acad. Sci. USA. 2019;116:9433–9442. doi: 10.1073/pnas.1821323116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park Y., Reyna-Neyra A., Philippe L., Thoreen C.C. mTORC1 Balances Cellular Amino Acid Supply with Demand for Protein Synthesis through Post-transcriptional Control of ATF4. Cell Rep. 2017;19:1083–1090. doi: 10.1016/j.celrep.2017.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Enyedi B., Varnai P., Geiszt M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxid. Redox Signal. 2010;13:721–729. doi: 10.1089/ars.2009.2880. [DOI] [PubMed] [Google Scholar]
- 67.Cuozzo J.W., Kaiser C.A. Competition between glutathione and protein thiols for disulphide-bond formation. Nat. Cell Biol. 1999;1:130–135. doi: 10.1038/11047. [DOI] [PubMed] [Google Scholar]
- 68.Haynes C.M., Titus E.A., Cooper A.A. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Z., Zhang L., Zhou L., Lei Y., Zhang Y., Huang C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019;25:101047. doi: 10.1016/j.redox.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu C., Li X., Li C., Zhang Z., Gao X., Jia Z., Chen H., Jia Q., Zhao X., Liu J., et al. SLC3A2 is a novel endoplasmic reticulum stress-related signaling protein that regulates the unfolded protein response and apoptosis. PLoS ONE. 2018;13:e0208993. doi: 10.1371/journal.pone.0208993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W., Zhu J., Dou J., She H., Tao K., Xu H., Yang Q., Mao Z. Phosphorylation of LAMP2A by p38 MAPK couples ER stress to chaperone-mediated autophagy. Nat. Commun. 2017;8:1763. doi: 10.1038/s41467-017-01609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu X., Huang L., Gong J., Shi C., Wang Z., Ye B., Xuan A., He X., Long D., Zhu X., et al. NF-kappaB pathway link with ER stress-induced autophagy and apoptosis in cervical tumor cells. Cell Death Discov. 2017;3:17059. doi: 10.1038/cddiscovery.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng X., Li Y., Zhao R., Yan F., Ma Y., Zhao L., Qiao H. xCT deficiency induces autophagy via endoplasmic reticulum stress activated p38-mitogen-activated protein kinase and mTOR in sut melanocytes. Eur. J. Cell Biol. 2016;95:175–181. doi: 10.1016/j.ejcb.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Singh S.S., Vats S., Chia A.Y., Tan T.Z., Deng S., Ong M.S., Arfuso F., Yap C.T., Goh B.C., Sethi G., et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37:1142–1158. doi: 10.1038/s41388-017-0046-6. [DOI] [PubMed] [Google Scholar]
- 75.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 76.Sato H., Shiiya A., Kimata M., Maebara K., Tamba M., Sakakura Y., Makino N., Sugiyama F., Yagami K., Moriguchi T., et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 77.Habib E., Linher-Melville K., Lin H.X., Singh G. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015;5:33–42. doi: 10.1016/j.redox.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daher B., Parks S.K., Durivault J., Cormerais Y., Baidarjad H., Tambutte E., Pouyssegur J., Vucetic M. Genetic Ablation of the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. Cancer Res. 2019;79:3877–3890. doi: 10.1158/0008-5472.CAN-18-3855. [DOI] [PubMed] [Google Scholar]
- 79.Arensman M.D., Yang X.S., Leahy D.M., Toral-Barza L., Mileski M., Rosfjord E.C., Wang F., Deng S., Myers J.S., Abraham R.T., et al. Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc. Natl. Acad. Sci. USA. 2019;116:9533–9542. doi: 10.1073/pnas.1814932116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo W., Zhao Y., Zhang Z., Tan N., Zhao F., Ge C., Liang L., Jia D., Chen T., Yao M., et al. Disruption of xCT inhibits cell growth via the ROS/autophagy pathway in hepatocellular carcinoma. Cancer Lett. 2011;312:55–61. doi: 10.1016/j.canlet.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 81.Sugiyama A., Ohta T., Obata M., Takahashi K., Seino M., Nagase S. xCT inhibitor sulfasalazine depletes paclitaxel-resistant tumor cells through ferroptosis in uterine serous carcinoma. Oncol. Lett. 2020;20:2689–2700. doi: 10.3892/ol.2020.11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Torrence M.E., MacArthur M.R., Hosios A.M., Valvezan A., Asara J.M., Mitchell J.R., Manning B.D. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. bioRxiv. 2020 doi: 10.1101/2020.10.03.324186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quiros P.M., Mottis A., Auwerx J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 2016;17:213–226. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- 85.Wang S.F., Chen M.S., Chou Y.C., Ueng Y.F., Yin P.H., Yeh T.S., Lee H.C. Mitochondrial dysfunction enhances cisplatin resistance in human gastric cancer cells via the ROS-activated GCN2-eIF2alpha-ATF4-xCT pathway. Oncotarget. 2016;7:74132–74151. doi: 10.18632/oncotarget.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sehm T., Rauh M., Wiendieck K., Buchfelder M., Eyupoglu I.Y., Savaskan N.E. Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget. 2016;7:74630–74647. doi: 10.18632/oncotarget.11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cobler L., Zhang H., Suri P., Park C., Timmerman L.A. xCT inhibition sensitizes tumors to gamma-radiation via glutathione reduction. Oncotarget. 2018;9:32280–32297. doi: 10.18632/oncotarget.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cojoc M., Mabert K., Muders M.H., Dubrovska A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin. Cancer Biol. 2015;31:16–27. doi: 10.1016/j.semcancer.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Peitzsch C., Nathansen J., Schniewind S.I., Schwarz F., Dubrovska A. Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications. Cancers. 2019;11:616. doi: 10.3390/cancers11050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schulz A., Meyer F., Dubrovska A., Borgmann K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers. 2019;11:862. doi: 10.3390/cancers11060862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peitzsch C., Tyutyunnykova A., Pantel K., Dubrovska A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 2017;44:10–24. doi: 10.1016/j.semcancer.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 92.Ishimoto T., Nagano O., Yae T., Tamada M., Motohara T., Oshima H., Oshima M., Ikeda T., Asaba R., Yagi H., et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 93.Ju H.Q., Lu Y.X., Chen D.L., Tian T., Mo H.Y., Wei X.L., Liao J.W., Wang F., Zeng Z.L., Pelicano H., et al. Redox Regulation of Stem-like Cells Though the CD44v-xCT Axis in Colorectal Cancer: Mechanisms and Therapeutic Implications. Theranostics. 2016;6:1160–1175. doi: 10.7150/thno.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diehn M., Cho R.W., Lobo N.A., Kalisky T., Dorie M.J., Kulp A.N., Qian D., Lam J.S., Ailles L.E., Wong M., et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nagano O., Okazaki S., Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. 2013;32:5191–5198. doi: 10.1038/onc.2012.638. [DOI] [PubMed] [Google Scholar]
- 96.Wu H.M., Chi K.H., Lin W.W. Proteasome inhibitors stimulate activator protein-1 pathway via reactive oxygen species production. FEBS Lett. 2002;526:101–105. doi: 10.1016/S0014-5793(02)03151-4. [DOI] [PubMed] [Google Scholar]
- 97.Paniagua Soriano G., De Bruin G., Overkleeft H.S., Florea B.I. Toward understanding induction of oxidative stress and apoptosis by proteasome inhibitors. Antioxid. Redox Signal. 2014;21:2419–2443. doi: 10.1089/ars.2013.5794. [DOI] [PubMed] [Google Scholar]
- 98.Yoo Y.D., Lee D.H., Cha-Molstad H., Kim H., Mun S.R., Ji C., Park S.H., Sung K.S., Choi S.A., Hwang J., et al. Glioma-derived cancer stem cells are hypersensitive to proteasomal inhibition. EMBO Rep. 2017;18:150–168. doi: 10.15252/embr.201642360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Monticone M., Biollo E., Fabiano A., Fabbi M., Daga A., Romeo F., Maffei M., Melotti A., Giaretti W., Corte G., et al. z-Leucinyl-leucinyl-norleucinal induces apoptosis of human glioblastoma tumor-initiating cells by proteasome inhibition and mitotic arrest response. Mol. Cancer Res. 2009;7:1822–1834. doi: 10.1158/1541-7786.MCR-09-0225. [DOI] [PubMed] [Google Scholar]
- 100.Callaghan R., Luk F., Bebawy M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy? Drug Metab. Dispos. 2014;42:623–631. doi: 10.1124/dmd.113.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ge C., Cao B., Feng D., Zhou F., Zhang J., Yang N., Feng S., Wang G., Aa J. The down-regulation of SLC7A11 enhances ROS induced P-gp over-expression and drug resistance in MCF-7 breast cancer cells. Sci. Rep. 2017;7:3791. doi: 10.1038/s41598-017-03881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Broer S. The SLC38 family of sodium-amino acid co-transporters. Pflug. Arch. 2014;466:155–172. doi: 10.1007/s00424-013-1393-y. [DOI] [PubMed] [Google Scholar]
- 103.Stretton C., Lipina C., Hyde R., Cwiklinski E., Hoffmann T.M., Taylor P.M., Hundal H.S. CDK7 is a component of the integrated stress response regulating SNAT2 (SLC38A2)/System A adaptation in response to cellular amino acid deprivation. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:978–991. doi: 10.1016/j.bbamcr.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morotti M., Bridges E., Valli A., Choudhry H., Sheldon H., Wigfield S., Gray N., Zois C.E., Grimm F., Jones D., et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc. Natl. Acad. Sci. USA. 2019;116:12452–12461. doi: 10.1073/pnas.1818521116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Timmerman L.A., Holton T., Yuneva M., Louie R.J., Padro M., Daemen A., Hu M., Chan D.A., Ethier S.P., van ‘t Veer L.J., et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24:450–465. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gu S., Roderick H.L., Camacho P., Jiang J.X. Identification and characterization of an amino acid transporter expressed differentially in liver. Proc. Natl. Acad. Sci. USA. 2000;97:3230–3235. doi: 10.1073/pnas.97.7.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lister A., Bourgeois S., Imenez Silva P.H., Rubio-Aliaga I., Marbet P., Walsh J., Shelton L.M., Keller B., Verrey F., Devuyst O., et al. NRF2 regulates the glutamine transporter Slc38a3 (SNAT3) in kidney in response to metabolic acidosis. Sci. Rep. 2018;8:5629. doi: 10.1038/s41598-018-24000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Palmieri F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Asp. Med. 2013;34:465–484. doi: 10.1016/j.mam.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 109.Kunji E.R.S., King M.S., Ruprecht J.J., Thangaratnarajah C. The SLC25 Carrier Family: Important Transport Proteins in Mitochondrial Physiology and Pathology. Physiology. 2020;35:302–327. doi: 10.1152/physiol.00009.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou X., Paredes J.A., Krishnan S., Curbo S., Karlsson A. The mitochondrial carrier SLC25A10 regulates cancer cell growth. Oncotarget. 2015;6:9271–9283. doi: 10.18632/oncotarget.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Y., Shi J., Liu X., Feng L., Gong Z., Koppula P., Sirohi K., Li X., Wei Y., Lee H., et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018;20:1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saad A., Bijian K., Qiu D., da Silva S.D., Marques M., Chang C.H., Nassour H., Ramotar D., Damaraju S., Mackey J., et al. Insights into a novel nuclear function for Fascin in the regulation of the amino-acid transporter SLC3A2. Sci. Rep. 2016;6:36699. doi: 10.1038/srep36699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Menga A., Palmieri E.M., Cianciulli A., Infantino V., Mazzone M., Scilimati A., Palmieri F., Castegna A., Iacobazzi V. SLC25A26 overexpression impairs cell function via mtDNA hypermethylation and rewiring of methyl metabolism. FEBS J. 2017;284:967–984. doi: 10.1111/febs.14028. [DOI] [PubMed] [Google Scholar]
- 114.Infantino V., Dituri F., Convertini P., Santarsiero A., Palmieri F., Todisco S., Mancarella S., Giannelli G., Iacobazzi V. Epigenetic upregulation and functional role of the mitochondrial aspartate/glutamate carrier isoform 1 in hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:38–47. doi: 10.1016/j.bbadis.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 115.Lee J.V., Carrer A., Shah S., Snyder N.W., Wei S., Venneti S., Worth A.J., Yuan Z.F., Lim H.W., Liu S., et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014;20:306–319. doi: 10.1016/j.cmet.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cohen H.M., Griffiths A.D., Tawfik D.S., Loakes D. Determinants of cofactor binding to DNA methyltransferases: Insights from a systematic series of structural variants of S-adenosylhomocysteine. Org. Biomol. Chem. 2005;3:152–161. doi: 10.1039/b415446k. [DOI] [PubMed] [Google Scholar]
- 117.Pritchard J.B. Intracellular alpha-ketoglutarate controls the efficacy of renal organic anion transport. J. Pharmacol. Exp. Ther. 1995;274:1278–1284. [PubMed] [Google Scholar]
- 118.Mentch S.J., Mehrmohamadi M., Huang L., Liu X., Gupta D., Mattocks D., Gomez Padilla P., Ables G., Bamman M.M., Thalacker-Mercer A.E., et al. Histone Methylation Dynamics and Gene Regulation Occur through the Sensing of One-Carbon Metabolism. Cell Metab. 2015;22:861–873. doi: 10.1016/j.cmet.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mentch S.J., Locasale J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016;1363:91–98. doi: 10.1111/nyas.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mastroberardino L., Spindler B., Pfeiffer R., Skelly P.J., Loffing J., Shoemaker C.B., Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 121.Kaira K., Sunose Y., Arakawa K., Ogawa T., Sunaga N., Shimizu K., Tominaga H., Oriuchi N., Itoh H., Nagamori S., et al. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br. J. Cancer. 2012;107:632–638. doi: 10.1038/bjc.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Furuya M., Horiguchi J., Nakajima H., Kanai Y., Oyama T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382–389. doi: 10.1111/j.1349-7006.2011.02151.x. [DOI] [PubMed] [Google Scholar]
- 123.Yazawa T., Shimizu K., Kaira K., Nagashima T., Ohtaki Y., Atsumi J., Obayashi K., Nagamori S., Kanai Y., Oyama T., et al. Clinical significance of coexpression of L-type amino acid transporter 1 (LAT1) and ASC amino acid transporter 2 (ASCT2) in lung adenocarcinoma. Am. J. Transl. Res. 2015;7:1126–1139. [PMC free article] [PubMed] [Google Scholar]
- 124.Xu M., Sakamoto S., Matsushima J., Kimura T., Ueda T., Mizokami A., Kanai Y., Ichikawa T. Up-Regulation of LAT1 during Antiandrogen Therapy Contributes to Progression in Prostate Cancer Cells. J. Urol. 2016;195:1588–1597. doi: 10.1016/j.juro.2015.11.071. [DOI] [PubMed] [Google Scholar]
- 125.Honjo H., Kaira K., Miyazaki T., Yokobori T., Kanai Y., Nagamori S., Oyama T., Asao T., Kuwano H. Clinicopathological significance of LAT1 and ASCT2 in patients with surgically resected esophageal squamous cell carcinoma. J. Surg. Oncol. 2016;113:381–389. doi: 10.1002/jso.24160. [DOI] [PubMed] [Google Scholar]
- 126.Dann S.G., Ryskin M., Barsotti A.M., Golas J., Shi C., Miranda M., Hosselet C., Lemon L., Lucas J., Karnoub M., et al. Reciprocal regulation of amino acid import and epigenetic state through Lat1 and EZH2. EMBO J. 2015;34:1773–1785. doi: 10.15252/embj.201488166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sinclair L.V., Rolf J., Emslie E., Shi Y.B., Taylor P.M., Cantrell D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sinclair L.V., Howden A.J., Brenes A., Spinelli L., Hukelmann J.L., Macintyre A.N., Liu X., Thomson S., Taylor P.M., Rathmell J.C., et al. Antigen receptor control of methionine metabolism in T cells. Elife. 2019;8 doi: 10.7554/eLife.44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hodgson N., Trivedi M., Muratore C., Li S., Deth R. Soluble oligomers of amyloid-beta cause changes in redox state, DNA methylation, and gene transcription by inhibiting EAAT3 mediated cysteine uptake. J. Alzheimers Dis. 2013;36:197–209. doi: 10.3233/JAD-130101. [DOI] [PubMed] [Google Scholar]
- 130.Campbell B., Roberts M., Kerksick C., Wilborn C., Marcello B., Taylor L., Nassar E., Leutholtz B., Bowden R., Rasmussen C., et al. Pharmacokinetics, safety, and effects on exercise performance of L-arginine alpha-ketoglutarate in trained adult men. Nutrition. 2006;22:872–881. doi: 10.1016/j.nut.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 131.Xiao D., Zeng L., Yao K., Kong X., Wu G., Yin Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids. 2016;48:2067–2080. doi: 10.1007/s00726-016-2254-8. [DOI] [PubMed] [Google Scholar]
- 132.Lemire J., Milandu Y., Auger C., Bignucolo A., Appanna V.P., Appanna V.D. Histidine is a source of the antioxidant, alpha-ketoglutarate, in Pseudomonas fluorescens challenged by oxidative stress. FEMS Microbiol. Lett. 2010;309:170–177. doi: 10.1111/j.1574-6968.2010.02034.x. [DOI] [PubMed] [Google Scholar]
- 133.Allison M.J., Robinson I.M. Biosynthesis of alpha-ketoglutarate by the reductive carboxylation of succinate in Bacteroides ruminicola. J. Bacteriol. 1970;104:50–56. doi: 10.1128/JB.104.1.50-56.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Figueroa M.E., Abdel-Wahab O., Lu C., Ward P.S., Patel J., Shih A., Li Y., Bhagwat N., Vasanthakumar A., Fernandez H.F., et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Borger D.R., Goyal L., Yau T., Poon R.T., Ancukiewicz M., Deshpande V., Christiani D.C., Liebman H.M., Yang H., Kim H., et al. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clin. Cancer Res. 2014;20:1884–1890. doi: 10.1158/1078-0432.CCR-13-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu W., Yang H., Liu Y., Yang Y., Wang P., Kim S.H., Ito S., Yang C., Wang P., Xiao M.T., et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 138.Shi L., Tu B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015;33:125–131. doi: 10.1016/j.ceb.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang X.J., Seto E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 140.Violante S., Ijlst L., Ruiter J., Koster J., van Lenthe H., Duran M., de Almeida I.T., Wanders R.J., Houten S.M., Ventura F.V. Substrate specificity of human carnitine acetyltransferase: Implications for fatty acid and branched-chain amino acid metabolism. Biochim. Biophys. Acta. 2013;1832:773–779. doi: 10.1016/j.bbadis.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 141.Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., Guarente L.P., Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu H., Xing R., Cheng X., Li Q., Liu F., Ye H., Zhao M., Wang H., Wang G., Hao H. De-novo NAD+ synthesis regulates SIRT1-FOXO1 apoptotic pathway in response to NQO1 substrates in lung cancer cells. Oncotarget. 2016;7:62503–62519. doi: 10.18632/oncotarget.11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yan Y., Dalmasso G., Sitaraman S., Merlin D. Characterization of the human intestinal CD98 promoter and its regulation by interferon-gamma. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G535–G545. doi: 10.1152/ajpgi.00385.2006. [DOI] [PubMed] [Google Scholar]
- 144.Nguyen H.T., Dalmasso G., Yan Y., Laroui H., Dahan S., Mayer L., Sitaraman S.V., Merlin D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J. Biol. Chem. 2010;285:1479–1489. doi: 10.1074/jbc.M109.057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Charania M.A., Ayyadurai S., Ingersoll S.A., Xiao B., Viennois E., Yan Y., Laroui H., Sitaraman S.V., Merlin D. Intestinal epithelial CD98 synthesis specifically modulates expression of colonic microRNAs during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G1282–G1291. doi: 10.1152/ajpgi.00401.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Amasheh S., Fromm M., Gunzel D. Claudins of intestine and nephron—A correlation of molecular tight junction structure and barrier function. Acta Physiol. 2011;201:133–140. doi: 10.1111/j.1748-1716.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- 147.Han M.K., Baker M., Zhang Y., Yang C., Zhang M., Garg P., Viennois E., Merlin D. Overexpression of CD98 in intestinal epithelium dysregulates miRNAs and their targeted proteins along the ileal villus-crypt axis. Sci. Rep. 2018;8:16220. doi: 10.1038/s41598-018-34474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang H., Chen W., Jin M., Hou L., Chen X., Zhang R., Zhang J., Zhu J. CircSLC3A2 functions as an oncogenic factor in hepatocellular carcinoma by sponging miR-490-3p and regulating PPM1F expression. Mol. Cancer. 2018;17:165. doi: 10.1186/s12943-018-0909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garcia-Bermudez J., Williams R.T., Guarecuco R., Birsoy K. Targeting extracellular nutrient dependencies of cancer cells. Mol. Metab. 2020;33:67–82. doi: 10.1016/j.molmet.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tanabe A., Sahara H. The Metabolic Heterogeneity and Flexibility of Cancer Stem Cells. Cancers. 2020;12:2780. doi: 10.3390/cancers12102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ward P.S., Thompson C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Clarkson B., Krakoff I., Burchenal J., Karnofsky D., Golbey R., Dowling M., Oettgen H., Lipton A. Clinical results of treatment with E. coli L-asparaginase in adults with leukemia, lymphoma, and solid tumors. Cancer. 1970;25:279–305. doi: 10.1002/1097-0142(197002)25:2<279::AID-CNCR2820250205>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 153.Wang Q., Holst J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015;5:1281–1294. [PMC free article] [PubMed] [Google Scholar]
- 154.Bhutia Y.D., Babu E., Ramachandran S., Ganapathy V. Amino Acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75:1782–1788. doi: 10.1158/0008-5472.CAN-14-3745. [DOI] [PubMed] [Google Scholar]
- 155.Fumagalli E., Funicello M., Rauen T., Gobbi M., Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur. J. Pharmacol. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 156.Carbone M., Duty S., Rattray M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem. Int. 2012;60:31–38. doi: 10.1016/j.neuint.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dall’Igna O.P., Bobermin L.D., Souza D.O., Quincozes-Santos A. Riluzole increases glutamate uptake by cultured C6 astroglial cells. Int. J. Dev. Neurosci. 2013;31:482–486. doi: 10.1016/j.ijdevneu.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 158.El-Gebali S., Bentz S., Hediger M.A., Anderle P. Solute carriers (SLCs) in cancer. Mol. Asp. Med. 2013;34:719–734. doi: 10.1016/j.mam.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 159.Rask-Andersen M., Masuram S., Fredriksson R., Schioth H.B. Solute carriers as drug targets: Current use, clinical trials and prospective. Mol. Asp. Med. 2013;34:702–710. doi: 10.1016/j.mam.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 160.Johnston R.A., Rawling T., Chan T., Zhou F., Murray M. Selective inhibition of human solute carrier transporters by multikinase inhibitors. Drug Metab. Dispos. 2014;42:1851–1857. doi: 10.1124/dmd.114.059097. [DOI] [PubMed] [Google Scholar]
- 161.Wu P., Bjorn-Yoshimoto W.E., Staudt M., Jensen A.A., Bunch L. Identification and Structure-Activity Relationship Study of Imidazo[1,2-a]pyridine-3-amines as First Selective Inhibitors of Excitatory Amino Acid Transporter Subtype 3 (EAAT3) ACS Chem. Neurosci. 2019;10:4414–4429. doi: 10.1021/acschemneuro.9b00447. [DOI] [PubMed] [Google Scholar]
- 162.Cheng B., Shchepakin D., Kavanaugh M.P., Trauner D. Photoswitchable Inhibitor of a Glutamate Transporter. ACS Chem. Neurosci. 2017;8:1668–1672. doi: 10.1021/acschemneuro.7b00072. [DOI] [PubMed] [Google Scholar]
- 163.Schulte M.L., Fu A., Zhao P., Li J., Geng L., Smith S.T., Kondo J., Coffey R.J., Johnson M.O., Rathmell J.C., et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med. 2018;24:194–202. doi: 10.1038/nm.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma H., Wu Z., Peng J., Li Y., Huang H., Liao Y., Zhou M., Sun L., Huang N., Shi M., et al. Inhibition of SLC1A5 sensitizes colorectal cancer to cetuximab. Int. J. Cancer. 2018;142:2578–2588. doi: 10.1002/ijc.31274. [DOI] [PubMed] [Google Scholar]
- 165.Marshall A.D., van Geldermalsen M., Otte N.J., Lum T., Vellozzi M., Thoeng A., Pang A., Nagarajah R., Zhang B., Wang Q., et al. ASCT2 regulates glutamine uptake and cell growth in endometrial carcinoma. Oncogenesis. 2017;6:e367. doi: 10.1038/oncsis.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Q., Beaumont K.A., Otte N.J., Font J., Bailey C.G., van Geldermalsen M., Sharp D.M., Tiffen J.C., Ryan R.M., Jormakka M., et al. Targeting glutamine transport to suppress melanoma cell growth. Int. J. Cancer. 2014;135:1060–1071. doi: 10.1002/ijc.28749. [DOI] [PubMed] [Google Scholar]
- 167.Schulte M.L., Khodadadi A.B., Cuthbertson M.L., Smith J.A., Manning H.C. 2-Amino-4-bis(aryloxybenzyl)aminobutanoic acids: A novel scaffold for inhibition of ASCT2-mediated glutamine transport. Bioorg. Med. Chem. Lett. 2016;26:1044–1047. doi: 10.1016/j.bmcl.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kasai N., Sasakawa A., Hosomi K., Poh T.W., Chua B.L., Yong W.P., So J., Chan S.L., Soong R., Kono K., et al. Anti-tumor efficacy evaluation of a novel monoclonal antibody targeting neutral amino acid transporter ASCT2 using patient-derived xenograft mouse models of gastric cancer. Am. J. Transl. Res. 2017;9:3399–3410. [PMC free article] [PubMed] [Google Scholar]
- 169.Suzuki M., Toki H., Furuya A., Ando H. Establishment of monoclonal antibodies against cell surface domains of ASCT2/SLC1A5 and their inhibition of glutamine-dependent tumor cell growth. Biochem. Biophys. Res. Commun. 2017;482:651–657. doi: 10.1016/j.bbrc.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 170.Hara Y., Minami Y., Yoshimoto S., Hayashi N., Yamasaki A., Ueda S., Masuko K., Masuko T. Anti-tumor effects of an antagonistic mAb against the ASCT2 amino acid transporter on KRAS-mutated human colorectal cancer cells. Cancer Med. 2020;9:302–312. doi: 10.1002/cam4.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rajasinghe L.D., Hutchings M., Gupta S.V. Delta-Tocotrienol Modulates Glutamine Dependence by Inhibiting ASCT2 and LAT1 Transporters in Non-Small Cell Lung Cancer (NSCLC) Cells: A Metabolomic Approach. Metabolites. 2019;9:50. doi: 10.3390/metabo9030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quandt G., Hofner G., Pabel J., Dine J., Eder M., Wanner K.T. First photoswitchable neurotransmitter transporter inhibitor: Light-induced control of gamma-aminobutyric acid transporter 1 (GAT1) activity in mouse brain. J. Med. Chem. 2014;57:6809–6821. doi: 10.1021/jm5008566. [DOI] [PubMed] [Google Scholar]
- 173.Sikder M.O.F., Sivaprakasam S., Brown T.P., Thangaraju M., Bhutia Y.D., Ganapathy V. SLC6A14, a Na+/Cl—Coupled amino acid transporter, functions as a tumor promoter in colon and is a target for Wnt signaling. Biochem. J. 2020;477:1409–1425. doi: 10.1042/BCJ20200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Coothankandaswamy V., Cao S., Xu Y., Prasad P.D., Singh P.K., Reynolds C.P., Yang S., Ogura J., Ganapathy V., Bhutia Y.D. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol. 2016;173:3292–3306. doi: 10.1111/bph.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cai A., Zheng H., Chen Z., Lin X., Li C., Yao Q., Bhutia Y.D., Ganapathy V., Chen R., Kou L. Synergism between SLC6A14 blockade and gemcitabine in pancreactic cancer: A 1H-NMR-based metabolomic study in pancreatic cancer cells. Biochem. J. 2020 doi: 10.1042/BCJ20200275. [DOI] [PubMed] [Google Scholar]
- 176.Kongpracha P., Nagamori S., Wiriyasermkul P., Tanaka Y., Kaneda K., Okuda S., Ohgaki R., Kanai Y. Structure-activity relationship of a novel series of inhibitors for cancer type transporter L-type amino acid transporter 1 (LAT1) J. Pharmacol. Sci. 2017;133:96–102. doi: 10.1016/j.jphs.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 177.Enomoto K., Sato F., Tamagawa S., Gunduz M., Onoda N., Uchino S., Muragaki Y., Hotomi M. A novel therapeutic approach for anaplastic thyroid cancer through inhibition of LAT1. Sci. Rep. 2019;9:14616. doi: 10.1038/s41598-019-51144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cormerais Y., Pagnuzzi-Boncompagni M., Schrotter S., Giuliano S., Tambutte E., Endou H., Wempe M.F., Pages G., Pouyssegur J., Picco V. Inhibition of the amino-acid transporter LAT1 demonstrates anti-neoplastic activity in medulloblastoma. J. Cell. Mol. Med. 2019;23:2711–2718. doi: 10.1111/jcmm.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ueno S., Kimura T., Yamaga T., Kawada A., Ochiai T., Endou H., Sakurai H. Metformin enhances anti-tumor effect of L-type amino acid transporter 1 (LAT1) inhibitor. J. Pharmacol. Sci. 2016;131:110–117. doi: 10.1016/j.jphs.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 180.Rosilio C., Nebout M., Imbert V., Griessinger E., Neffati Z., Benadiba J., Hagenbeek T., Spits H., Reverso J., Ambrosetti D., et al. L-type amino-acid transporter 1 (LAT1): A therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia. 2015;29:1253–1266. doi: 10.1038/leu.2014.338. [DOI] [PubMed] [Google Scholar]
- 181.Ji X., Qian J., Rahman S.M.J., Siska P.J., Zou Y., Harris B.K., Hoeksema M.D., Trenary I.A., Heidi C., Eisenberg R., et al. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene. 2018;37:5007–5019. doi: 10.1038/s41388-018-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Otsuki Y., Yamasaki J., Suina K., Okazaki S., Koike N., Saya H., Nagano O. Vasodilator oxyfedrine inhibits aldehyde metabolism and thereby sensitizes cancer cells to xCT-targeted therapy. Cancer Sci. 2020;111:127–136. doi: 10.1111/cas.14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hu K., Li K., Lv J., Feng J., Chen J., Wu H., Cheng F., Jiang W., Wang J., Pei H., et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J. Clin. Investig. 2020;130:1752–1766. doi: 10.1172/JCI124049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Okazaki S., Umene K., Yamasaki J., Suina K., Otsuki Y., Yoshikawa M., Minami Y., Masuko T., Kawaguchi S., Nakayama H., et al. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019;110:3453–3463. doi: 10.1111/cas.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ogihara K., Kikuchi E., Okazaki S., Hagiwara M., Takeda T., Matsumoto K., Kosaka T., Mikami S., Saya H., Oya M. Sulfasalazine could modulate the CD44v9-xCT system and enhance cisplatin-induced cytotoxic effects in metastatic bladder cancer. Cancer Sci. 2019;110:1431–1441. doi: 10.1111/cas.13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dai L., Cao Y., Chen Y., Kaleeba J.A., Zabaleta J., Qin Z. Genomic analysis of xCT-mediated regulatory network: Identification of novel targets against AIDS-associated lymphoma. Oncotarget. 2015;6:12710–12722. doi: 10.18632/oncotarget.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dai L., Cao Y., Chen Y., Parsons C., Qin Z. Targeting xCT, a cystine-glutamate transporter induces apoptosis and tumor regression for KSHV/HIV-associated lymphoma. J. Hematol. Oncol. 2014;7:30. doi: 10.1186/1756-8722-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pons D.G., Nadal-Serrano M., Torrens-Mas M., Valle A., Oliver J., Roca P. UCP2 inhibition sensitizes breast cancer cells to therapeutic agents by increasing oxidative stress. Free Radic. Biol. Med. 2015;86:67–77. doi: 10.1016/j.freeradbiomed.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 189.Kawanishi M., Fukuda T., Shimomura M., Inoue Y., Wada T., Tasaka R., Yasui T., Sumi T. Expression of UCP2 is associated with sensitivity to platinum-based chemotherapy for ovarian serous carcinoma. Oncol. Lett. 2018;15:9923–9928. doi: 10.3892/ol.2018.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Q., Grkovic T., Font J., Bonham S., Pouwer R.H., Bailey C.G., Moran A.M., Ryan R.M., Rasko J.E., Jormakka M., et al. Monoterpene glycoside ESK246 from Pittosporum targets LAT3 amino acid transport and prostate cancer cell growth. ACS Chem. Biol. 2014;9:1369–1376. doi: 10.1021/cb500120x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou P., Liang X., Zhou C., Qin J., Hou C., Zhu Z., Zhang W., Wang S., Zhong D. Glutamine-beta-cyclodextrin for targeted doxorubicin delivery to triple-negative breast cancer tumors via the transporter ASCT2. J. Mater. Chem. B. 2019;7:5363–5375. doi: 10.1039/C9TB01225G. [DOI] [PubMed] [Google Scholar]
- 192.Wang C., Wu J., Wang Z., Yang Z., Li Z., Deng H., Li L., Peng X., Feng M. Glutamine addiction activates polyglutamine-based nanocarriers delivering therapeutic siRNAs to orthotopic lung tumor mediated by glutamine transporter SLC1A5. Biomaterials. 2018;183:77–92. doi: 10.1016/j.biomaterials.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 193.Liu Y., Zhao T., Li Z., Wang L., Yuan S., Sun L. The role of ASCT2 in cancer: A review. Eur. J. Pharmacol. 2018;837:81–87. doi: 10.1016/j.ejphar.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 194.Hassanein M., Qian J., Hoeksema M.D., Wang J., Jacobovitz M., Ji X., Harris F.T., Harris B.K., Boyd K.L., Chen H., et al. Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int. J. Cancer. 2015;137:1587–1597. doi: 10.1002/ijc.29535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lu H., Li X., Lu Y., Qiu S., Fan Z. ASCT2 (SLC1A5) is an EGFR-associated protein that can be co-targeted by cetuximab to sensitize cancer cells to ROS-induced apoptosis. Cancer Lett. 2016;381:23–30. doi: 10.1016/j.canlet.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tao X., Lu Y., Qiu S., Wang Y., Qin J., Fan Z. AP1G1 is involved in cetuximab-mediated downregulation of ASCT2-EGFR complex and sensitization of human head and neck squamous cell carcinoma cells to ROS-induced apoptosis. Cancer Lett. 2017;408:33–42. doi: 10.1016/j.canlet.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Osanai-Sasakawa A., Hosomi K., Sumitomo Y., Takizawa T., Tomura-Suruki S., Imaizumi M., Kasai N., Poh T.W., Yamano K., Yong W.P., et al. An anti-ASCT2 monoclonal antibody suppresses gastric cancer growth by inducing oxidative stress and antibody dependent cellular toxicity in preclinical models. Am. J. Cancer Res. 2018;8:1499–1513. [PMC free article] [PubMed] [Google Scholar]
- 198.Zhuang X., Tong H., Ding Y., Wu L., Cai J., Si Y., Zhang H., Shen M. Long noncoding RNA ABHD11-AS1 functions as a competing endogenous RNA to regulate papillary thyroid cancer progression by miR-199a-5p/SLC1A5 axis. Cell Death Dis. 2019;10:620. doi: 10.1038/s41419-019-1850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ding J., Gou Q., Jin J., Shi J., Liu Q., Hou Y. Metformin inhibits PPARdelta agonist-mediated tumor growth by reducing Glut1 and SLC1A5 expressions of cancer cells. Eur. J. Pharmacol. 2019;857:172425. doi: 10.1016/j.ejphar.2019.172425. [DOI] [PubMed] [Google Scholar]
- 200.Sun J., Nagel R., Zaal E.A., Ugalde A.P., Han R., Proost N., Song J.Y., Pataskar A., Burylo A., Fu H., et al. SLC1A3 contributes to L-asparaginase resistance in solid tumors. EMBO J. 2019;38:e102147. doi: 10.15252/embj.2019102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu L., Chen J., Jia L., Chen X., Awaleh Moumin F., Cai J. SLC1A3 promotes gastric cancer progression via the PI3K/AKT signalling pathway. J. Cell. Mol. Med. 2020;24:14392–14404. doi: 10.1111/jcmm.16060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 202.Lewerenz J., Maher P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015;9:469. doi: 10.3389/fnins.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huttunen K.M., Gynther M., Huttunen J., Puris E., Spicer J.A., Denny W.A. A Selective and Slowly Reversible Inhibitor of l-Type Amino Acid Transporter 1 (LAT1) Potentiates Antiproliferative Drug Efficacy in Cancer Cells. J. Med. Chem. 2016;59:5740–5751. doi: 10.1021/acs.jmedchem.6b00190. [DOI] [PubMed] [Google Scholar]
- 204.Hayashi K., Jutabha P., Maeda S., Supak Y., Ouchi M., Endou H., Fujita T., Chida M., Anzai N. LAT1 acts as a crucial transporter of amino acids in human thymic carcinoma cells. J. Pharmacol. Sci. 2016;132:201–204. doi: 10.1016/j.jphs.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 205.Higuchi K., Sakamoto S., Ando K., Maimaiti M., Takeshita N., Okunushi K., Reien Y., Imamura Y., Sazuka T., Nakamura K., et al. Characterization of the expression of LAT1 as a prognostic indicator and a therapeutic target in renal cell carcinoma. Sci. Rep. 2019;9:16776. doi: 10.1038/s41598-019-53397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yun D.W., Lee S.A., Park M.G., Kim J.S., Yu S.K., Park M.R., Kim S.G., Oh J.S., Kim C.S., Kim H.J., et al. JPH203, an L-type amino acid transporter 1-selective compound, induces apoptosis of YD-38 human oral cancer cells. J. Pharmacol. Sci. 2014;124:208–217. doi: 10.1254/jphs.13154FP. [DOI] [PubMed] [Google Scholar]
- 207.Skvortsov S., Skvortsova I.-I., Tang D.G., Dubrovska A. Concise Review: Prostate Cancer Stem Cells: Current Understanding. Stem Cells. 2018;36:1457–1474. doi: 10.1002/stem.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Strauss L.G. Positron Emission Tomography: Current Role for Diagnosis and Therapy Monitoring in Oncology. Oncologist. 1997;2:381–388. doi: 10.1634/theoncologist.2-6-381. [DOI] [PubMed] [Google Scholar]
- 209.Liu Y. The Place of FDG PET/CT in Renal Cell Carcinoma: Value and Limitations. Front. Oncol. 2016;6:201. doi: 10.3389/fonc.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li S., Zheng Q., Ma Y., Wang Y., Feng Y., Zhao B., Yang Y. Implications of false negative and false positive diagnosis in lymph node staging of NSCLC by means of (1)(8)F-FDG PET/CT. PLoS ONE. 2013;8:e78552. doi: 10.1371/journal.pone.0078552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kumar R., Rani N., Patel C., Basu S., Alavi A. False-Negative and False-Positive Results in FDG-PET and PET/CT in Breast Cancer. PET Clin. 2009;4:289–298. doi: 10.1016/j.cpet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 212.Chang J.M., Lee H.J., Goo J.M., Lee H.Y., Lee J.J., Chung J.K., Im J.G. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J. Radiol. 2006;7:57–69. doi: 10.3348/kjr.2006.7.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tang C., Pan Q., Gao S., Sun A., Wen F., Tang G. Excitatory glutamate transporter EAAC1 as an important transporter of N-(2-[(18)F]fluoropropionyl)-L-glutamate in oncology PET imaging. Nucl. Med. Biol. 2020;84–85:55–62. doi: 10.1016/j.nucmedbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 214.Liu H., Han Y., Li J., Qin M., Fu Q., Wang C., Liu Z. (18)F-Alanine Derivative Serves as an ASCT2 Marker for Cancer Imaging. Mol. Pharm. 2018;15:947–954. doi: 10.1021/acs.molpharmaceut.7b00884. [DOI] [PubMed] [Google Scholar]
- 215.Hassanein M., Hight M.R., Buck J.R., Tantawy M.N., Nickels M.L., Hoeksema M.D., Harris B.K., Boyd K., Massion P.P., Manning H.C. Preclinical Evaluation of 4-[18F]Fluoroglutamine PET to Assess ASCT2 Expression in Lung Cancer. Mol. Imaging Biol. 2016;18:18–23. doi: 10.1007/s11307-015-0862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ono M., Oka S., Okudaira H., Nakanishi T., Mizokami A., Kobayashi M., Schuster D.M., Goodman M.M., Shirakami Y., Kawai K. [(14)C]Fluciclovine (alias anti-[(14)C]FACBC) uptake and ASCT2 expression in castration-resistant prostate cancer cells. Nucl. Med. Biol. 2015;42:887–892. doi: 10.1016/j.nucmedbio.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 217.Saarinen I., Jambor I., Kim M., Kuisma A., Kemppainen J., Merisaari H., Eskola O., Koskenniemi A.R., Perez I.M., Bostrom P., et al. Correlation between (18)F-1-amino-3-fluorocyclobutane-1-carboxylic acid ((18)F-fluciclovine) uptake and expression of alanine-serine-cysteine-transporter 2 (ASCT2) and L-type amino acid transporter 1 (LAT1) in primary prostate cancer. EJNMMI Res. 2019;9:50. doi: 10.1186/s13550-019-0518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Deuschle F.C., Schiefner A., Skerra A. Structural differences between the ectodomains of murine and human CD98hc. Proteins. 2019;87:693–698. doi: 10.1002/prot.25686. [DOI] [PubMed] [Google Scholar]
- 219.Verhoeven J., Baguet T., Piron S., Pauwelyn G., Bouckaert C., Descamps B., Raedt R., Vanhove C., De Vos F., Goethals I. 2-[(18)F]FELP, a novel LAT1-specific PET tracer, for the discrimination between glioblastoma, radiation necrosis and inflammation. Nucl. Med. Biol. 2020;82–83:9–16. doi: 10.1016/j.nucmedbio.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 220.Verhoeven J., Hulpia F., Kersemans K., Bolcaen J., De Lombaerde S., Goeman J., Descamps B., Hallaert G., Van den Broecke C., Deblaere K., et al. New fluoroethyl phenylalanine analogues as potential LAT1-targeting PET tracers for glioblastoma. Sci. Rep. 2019;9:2878. doi: 10.1038/s41598-019-40013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Habermeier A., Graf J., Sandhofer B.F., Boissel J.P., Roesch F., Closs E.I. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET) Amino Acids. 2015;47:335–344. doi: 10.1007/s00726-014-1863-3. [DOI] [PubMed] [Google Scholar]
- 222.Nozaki S., Nakatani Y., Mawatari A., Shibata N., Hume W.E., Hayashinaka E., Wada Y., Doi H., Watanabe Y. (18)F-FIMP: A LAT1-specific PET probe for discrimination between tumor tissue and inflammation. Sci. Rep. 2019;9:15718. doi: 10.1038/s41598-019-52270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Aoki M., Watabe T., Nagamori S., Naka S., Ikeda H., Kongpracha P., Horitsugi G., Kanai Y., Shimosegawa E., Kanai Y., et al. Distribution of LAT1-targeting PET tracer was independent of the tumor blood flow in rat xenograft models of C6 glioma and MIA PaCa-2. Ann. Nucl. Med. 2019;33:394–403. doi: 10.1007/s12149-019-01346-9. [DOI] [PubMed] [Google Scholar]
- 224.Wei L., Tominaga H., Ohgaki R., Wiriyasermkul P., Hagiwara K., Okuda S., Kaira K., Oriuchi N., Nagamori S., Kanai Y. Specific transport of 3-fluoro-l-alpha-methyl-tyrosine by LAT1 explains its specificity to malignant tumors in imaging. Cancer Sci. 2016;107:347–352. doi: 10.1111/cas.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Matsuura M., Ohshima M., Hiruta Y., Nishimura T., Nagase K., Kanazawa H. LAT1-Targeting Thermoresponsive Fluorescent Polymer Probes for Cancer Cell Imaging. Int. J. Mol. Sci. 2018;19:1646. doi: 10.3390/ijms19061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Papin-Michault C., Bonnetaud C., Dufour M., Almairac F., Coutts M., Patouraux S., Virolle T., Darcourt J., Burel-Vandenbos F. Study of LAT1 Expression in Brain Metastases: Towards a Better Understanding of the Results of Positron Emission Tomography Using Amino Acid Tracers. PLoS ONE. 2016;11:e0157139. doi: 10.1371/journal.pone.0157139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dadone-Montaudie B., Ambrosetti D., Dufour M., Darcourt J., Almairac F., Coyne J., Virolle T., Humbert O., Burel-Vandenbos F. [18F] FDOPA standardized uptake values of brain tumors are not exclusively dependent on LAT1 expression. PLoS ONE. 2017;12:e0184625. doi: 10.1371/journal.pone.0184625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li C., Huang S., Guo J., Wang C., Huang Z., Huang R., Liu L., Liang S., Wang H. Metabolic Evaluation of MYCN-Amplified Neuroblastoma by 4-[(18)F]FGln PET Imaging. Mol. Imaging Biol. 2019;21:1117–1126. doi: 10.1007/s11307-019-01330-9. [DOI] [PubMed] [Google Scholar]
- 229.Cantor J.M., Ginsberg M.H. CD98 at the crossroads of adaptive immunity and cancer. J. Cell Sci. 2012;125:1373–1382. doi: 10.1242/jcs.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Langen K.J., Hamacher K., Weckesser M., Floeth F., Stoffels G., Bauer D., Coenen H.H., Pauleit D. O-(2-[18F]fluoroethyl)-L-tyrosine: Uptake mechanisms and clinical applications. Nucl. Med. Biol. 2006;33:287–294. doi: 10.1016/j.nucmedbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 231.Bashir A., Mathilde Jacobsen S., Molby Henriksen O., Broholm H., Urup T., Grunnet K., Andree Larsen V., Moller S., Skjoth-Rasmussen J., Skovgaard Poulsen H., et al. Recurrent glioblastoma versus late posttreatment changes: Diagnostic accuracy of O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography (18F-FET PET) Neuro Oncol. 2019;21:1595–1606. doi: 10.1093/neuonc/noz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Watabe T., Ikeda H., Nagamori S., Wiriyasermkul P., Tanaka Y., Naka S., Kanai Y., Hagiwara K., Aoki M., Shimosegawa E., et al. (18)F-FBPA as a tumor-specific probe of L-type amino acid transporter 1 (LAT1): A comparison study with (18)F-FDG and (11)C-Methionine PET. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:321–331. doi: 10.1007/s00259-016-3487-1. [DOI] [PubMed] [Google Scholar]
- 233.Kalkat M., De Melo J., Hickman K.A., Lourenco C., Redel C., Resetca D., Tamachi A., Tu W.B., Penn L.Z. MYC Deregulation in Primary Human Cancers. Genes. 2017;8:151. doi: 10.3390/genes8060151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yue M., Jiang J., Gao P., Liu H., Qing G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis. Cell Rep. 2017;21:3819–3832. doi: 10.1016/j.celrep.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 235.Venkateswaran N., Lafita-Navarro M.C., Hao Y.H., Kilgore J.A., Perez-Castro L., Braverman J., Borenstein-Auerbach N., Kim M., Lesner N.P., Mishra P., et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019;33:1236–1251. doi: 10.1101/gad.327056.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang N., Yang X., Yuan F., Zhang L., Wang Y., Wang L., Mao Z., Luo J., Zhang H., Zhu W.G., et al. Increased Amino Acid Uptake Supports Autophagy-Deficient Cell Survival upon Glutamine Deprivation. Cell Rep. 2018;23:3006–3020. doi: 10.1016/j.celrep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 237.Zhang N., Yang X., Yuan F., Zhu W.G., Zhao Y. Autophagy-deficient tumor cells rely on extracellular amino acids to survive upon glutamine deprivation. Autophagy. 2018;14:1652–1653. doi: 10.1080/15548627.2018.1493314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Amaravadi R.K., Lippincott-Schwartz J., Yin X.M., Weiss W.A., Takebe N., Timmer W., DiPaola R.S., Lotze M.T., White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 2011;17:654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Broer S., Fairweather S.J. Amino Acid Transport Across the Mammalian Intestine. Compr. Physiol. 2018;9:343–373. doi: 10.1002/cphy.c170041. [DOI] [PubMed] [Google Scholar]
- 240.Bouthelier A., Aragones J. Role of the HIF oxygen sensing pathway in cell defense and proliferation through the control of amino acid metabolism. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118733. doi: 10.1016/j.bbamcr.2020.118733. [DOI] [PubMed] [Google Scholar]
- 241.Mishra R., Haldar S., Placencio V., Madhav A., Rohena-Rivera K., Agarwal P., Duong F., Angara B., Tripathi M., Liu Z., et al. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J. Clin. Investig. 2018;128:4472–4484. doi: 10.1172/JCI99397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kang Y.P., Ward N.P., DeNicola G.M. Recent advances in cancer metabolism: A technological perspective. Exp. Mol. Med. 2018;50:31. doi: 10.1038/s12276-018-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Silva-Almeida C., Ewart M.A., Wilde C. 3D gastrointestinal models and organoids to study metabolism in human colon cancer. Semin. Cell Dev. Biol. 2020;98:98–104. doi: 10.1016/j.semcdb.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 244.Yuan M., Kremer D.M., Huang H., Breitkopf S.B., Ben-Sahra I., Manning B.D., Lyssiotis C.A., Asara J.M. Ex vivo and in vivo stable isotope labelling of central carbon metabolism and related pathways with analysis by LC-MS/MS. Nat. Protoc. 2019;14:313–330. doi: 10.1038/s41596-018-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Singh N., Scalise M., Galluccio M., Wieder M., Seidel T., Langer T., Indiveri C., Ecker G.F. Discovery of Potent Inhibitors for the Large Neutral Amino Acid Transporter 1 (LAT1) by Structure-Based Methods. Int. J. Mol. Sci. 2018;20:27. doi: 10.3390/ijms20010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.O’Brien E.J., Monk J.M., Palsson B.O. Using Genome-scale Models to Predict Biological Capabilities. Cell. 2015;161:971–987. doi: 10.1016/j.cell.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]